Published online Nov 26, 2018. doi: 10.12998/wjcc.v6.i14.830
Peer-review started: August 4, 2018
First decision: September 11, 2018
Revised: September 15, 2018
Accepted: October 31, 2018
Article in press: November 1, 2018
Published online: November 26, 2018
Processing time: 114 Days and 23.2 Hours
Osteofibrous dysplasia (OFD) is a developmental skeletal disorder, and cases with a giant affected area in the pelvis are rare.
In this case report, a 48-year-old man presented with a large tumor in the right iliac region that turned out to be OFD. The patient had rebound tenderness in his right hip. After radiography examination, magnetic resonance imaging examinations and some physical examination, extensive bone destruction in the right ilium was confirmed. Moreover, changes in bone mineral density and peripheral cortical bone sclerosis with surrounding soft tissue swelling were observed. Thus, this patient was considered to have giant monostotic OFD of the ilium. The tumor-related area was removed completely by surgery, and the remaining cavity was filled by artificial bones from the opposite ilium. According to the results of follow-up, the patient had normal flexion and extension activities of the right hip joint, and there was no evidence of recurrence of the tumor.
Suture of iliopsoas and gluteus medius muscle following focus curettage and bone grafting is a promising and effective method to treat giant OFD of the ilium. It is a feasible way to fill a large cavity after removing a lesion like the one is this case.
Core tip: Osteofibrous dysplasia is a developmental skeletal disorder, and cases involving the pelvis with a large affected area are rare. This report is the first case, to our knowledge, of a 48-year-old man with a huge tumor in the right iliac that turned out to be osteofibrous dysplasia. With the assistance of computed tomography and magnetic resonance imaging, the tumor was completely removed, and the left empty cavity was reasonably filled by pulling and suturing nearby muscles and using some artificial bone.
- Citation: Liu YB, Zou TM. Giant monostotic osteofibrous dysplasia of the ilium: A case report and review of literature. World J Clin Cases 2018; 6(14): 830-835
- URL: https://www.wjgnet.com/2307-8960/full/v6/i14/830.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i14.830
Osteofibrous dysplasia (OFD) is a developmental skeletal disorder and is characterized by substitution of normal bone with benign cellular fibrous connective tissue[1]. Currently, OFD can be classified as monostotic, polyostotic and McCune Albright syndrome[2]. Most cases of monostotic lesions present no significant symptoms and are often found incidentally on radiography taken for other symptoms[3]. Monostotic fibrous dysplasia mainly affects patients in their third decade of life[4]. Generally, monostotic OFD involves the ribs, the proximal femur, and craniofacial bones[5]. Cases involving the pelvis are uncommon, and cases with a large affected area are even rarer in clinics. Here, we report one patient with giant monostotic OFD of the ilium who was treated by tumor curettage and bone grafting. In the surgical procedure, we sutured the iliopsoas and gluteus medius muscle together and filled the remaining empty cavity to reduce the dead cavity volume and achieve a good outcome. We report here the diagnosis and treatment of giant OFD of the ilium.
A 48-year-old man who was diagnosed with a right iliac tumor more than 2 mo ago during a general physical examination.
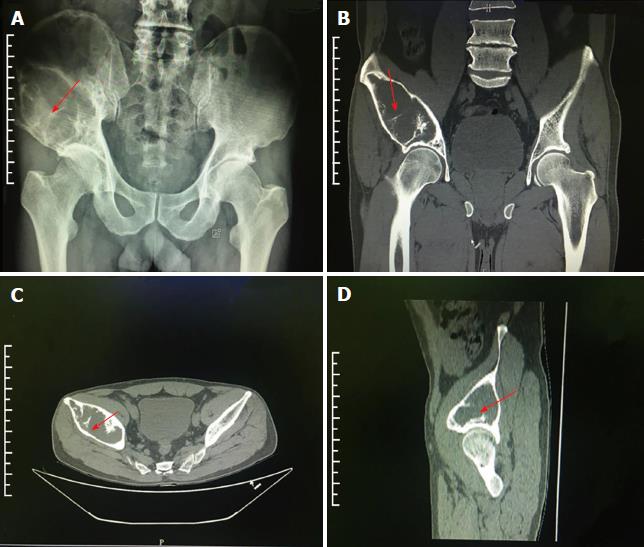
The use of painkillers, like aspirin, did not alleviate his discomfort in the right hip. Radiography examination showed giant bone destruction in the right ilium (Figure 1A).
Past and family medical history was unremarkable.
On physical examination, the patient had deep tenderness in the right hip, and the flexion and extension activities of the bilateral hip joints were normal.
The results of chest radiography examination and other laboratory examinations were all normal.
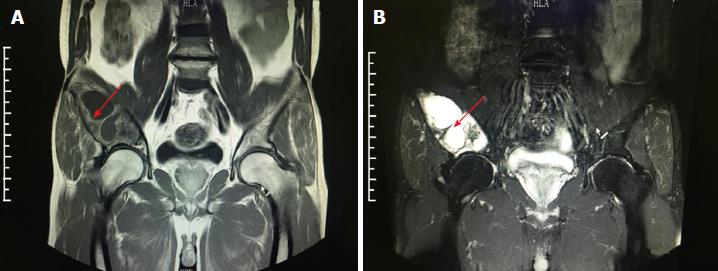
Subsequent computed tomography (CT) and magnetic resonance imaging (MRI) examinations indicated extensive bone destruction of the right ilium, with a size of about 10 cm × 5 cm × 5 cm. Bone mineral density was changed in this area, and peripheral cortical bone sclerosis with surrounding soft tissue swelling was noted (Figures 1 and 2).
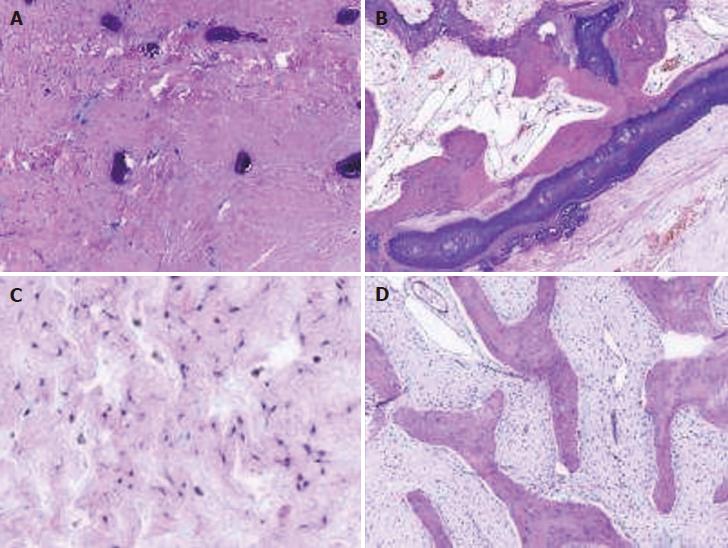
Postoperative pathological examination confirmed the diagnosis of OFD (Figure 3).
Intraoperative findings showed that the bone cortex in front of the focus in the right iliac bone was thin. A bone knife was used to chisel open the medial thin bone cortex, and the gray-looking tumor-like tissue was visualized. The texture was tenacious; its boundary was clear, without invasion of surrounding soft tissues. After the tumor was completely curetted, there remained a giant empty cavity. There was an even larger empty cavity after the giant empty cavity was filled with bone harvested from the opposite ilium and 20 g artificial bone. During the operation, a bone knife was used to chisel open a part of the external iliac plate in the thin bone cortex at the posterior-lateral side of the empty cavity. After the window was opened, parts of muscle bellies of the anterior iliopsoas and posterior gluteus medius muscle were pulled together and sutured to fill up the empty cavity.
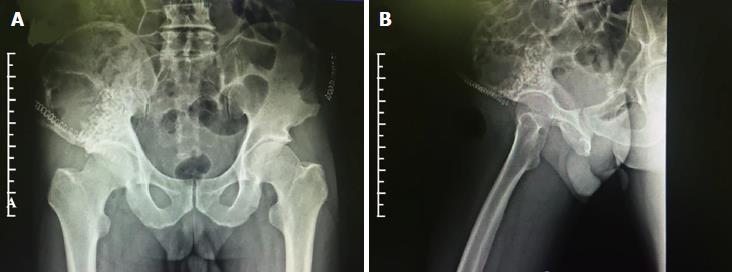
Post-operative radiography reexamination (Figure 4) showed that the artificial bone and autogenous bone in the weight-bearing area of the acetabulum top were in place, and the coverage was satisfactory.
A follow-up at 6 mo showed that the visual analogous scale pain score was 0, and the patient had normal flexion and extension activities of the right hip joint. There was no evidence of recurrence of the tumor.
In 1966, the lesion described in this case was named “ossifying fibroma” by Kempson[6]. In 1976, OFD was commendably categorized by Campanacci[7]. Subsequently, Campanacci and Laus[8] reported 35 cases from their facility; and 22 additional cases were also reviewed in the literature. OFD is a kind of fibro-osseous process that is commonly found in the diaphysis of the tibia. According to a series of 80 OFD cases reported by Park et al[9], 77 were tibia-related and only three appeared in the fibula. Moreover, nine involved of both the tibia and fibula on the ipsilateral side. In other case reports, the ulna and the radius were also common sites of involvement.
OFD is a kind of benign deformity-inducing fibro-osseous lesion that usually occurs during childhood and often involves the mid-shaft of the tibia with or without involvement of the fibula. OFD is regarded as a common and benign skeletal disorder[10]. OFD appears generally as a localized mass, with the potential of pseudoarthrosis caused by bowed tibia, with or without pain. As the patient feels no special discomfort, misdiagnosis and occasional pathological fracture may occur[11]. The case reported here is extremely rare, not only because the large tumor was found in the right iliac of a middle-aged male but also because this tumor turned out to be OFD.
In immunohistochemical studies, single or strands of keratin-positive cells are found in most of cases of OFD. In a series of immunohistochemical studies, cytokeratin-positive cells were identified in 80% of 85 OFD patients[12-15]. According to a case report by Kahn[16], isolated cytokeratin-positive cells were distinguishable as mast cells and not epithelial cells in corresponding hematoxylin and eosin and Giemsa stained preparations. This discovery aided an issue concerning the histogenesis of all isolated cytokeratin-positive cells in previous studies. The OFD in long bones should not be confused with the entity gnathic ossifying fibroma with well-circumscribed mass that occurs in jaw. Additionally, the expression of cytokeratin (CK) 19 was demonstrated in OFD, whereas the expression of CK8 and CK18 was negative[15]. Similar findings, including a high incidence of CK1 positivity and a basal cell phenotype, were reported for both entities[17]. Furthermore, Bovée et al[18] demonstrated that epidermal and fibroblast growth factor type 2 and its receptor were expressed in OFD. In this study, related laboratory diagnosis was performed: complete blood count and electrolytes were normal, and C-reactive protein and alkaline phosphatase were slightly elevated.
OFD is often identified with radiography examination, where it is typically manifested as glass-like change. The bone cortex grows expansively to become thinner, and the boundary is clear without periosteal reaction[19]. In clinic, radiography examination combined with CT and MRI examinations is conducive to determine the surgical procedure[20]. In this case, the imaging examination combined with the medical history of the patient indicated that there was extensive bone destruction in the right ilium, the focus had a clear boundary, and the bone cortex was extensive and thin and showed a ground glass-like change, without significant periosteal reaction. The affected area was considered to be a benign tumor-like lesion, with a high possibility of it being OFD. The involving range of tumor was large, and a part of the focus was located in the non-weight-bearing area of the acetabulum top. Thus, it was necessary to carry out bone grafting to reduce damage to the functional structures in the weight-bearing area. Complete curettage of the tumor led to a large residual cavity, which could easily form a dead cavity and increase the risk of infection. Therefore, a part of the external iliac plate in the thin cortical bone was chiseled open, and the anterior iliopsoas and posterior gluteus medius muscle were pulled together and sutured. This method not only effectively reduces the dead cavity, but it also minimizes the surgical trauma and does not affect the overall stability of the pelvic ring, making it conducive to postoperative recovery.
At present, the major treatment strategies for OFD are conservative treatment and surgical treatment[21]. Although it is a benign lesion with no symptoms, it is progressive and may lead to severe defects and lesions in bone and skin[2]. The surgical methods for OFD mainly include focus curettage and bone grafting combined with or without external fixation[22-25]. Curettage is a common treatment method for benign lesions, aggressive lesions, some cartilaginous malignant lesions and bone metastases[26]. However, the bone cavity created after curettage often needs to be filled with filling substances, such as acrylic cement or bone grafts, to guarantee its mechanical stability[27-29]. In this case, the allogeneic bone mixed with autogeneous bone was implanted in the weight-bearing area of the acetabulum. There is a large amount of cancellous substance in the ilium, and it has a good osteogenic activity. Compared to other materials, it has a faster creeping replacement. In addition, under the condition that the stability of the pelvic ring is unaffected, simple windowing in the external iliac plate and suturing between muscles can reduce the dead cavity and avoid internal fixation. Therefore, this method not only reduces the operation time and the operating difficulty but also lowers the cost for the patients.
In conclusion, the case recorded in this report was rare because most OFD occur in the mid-shaft of tibia with or without involvement of the fibula. Furthermore, the surgical methods utilized were effective without influencing the stability of pelvic ring. Moreover, the cavity generated by curettage was treated by filling up with mixture of autograft and allograft. Finally, the patient had noticeable recovery, specifically as normal flexion and extension activities of the right hip joint were observed in the patient without any evidence of tumor recurrence. Therefore, it is reasonable to believe that performing focus curettage and bone grafting with suture of iliopsoas and gluteus medius muscle will provide a promising and effective method in treating giant OFD of the ilium. This method not only reduces operation time and the operating difficulty but also lowers the cost for the patients. To our limited knowledge, it is the first case report to describe a large OFD tumor in the right iliac, its removal, and the filling of the empty cavity created after tumor removal by surrounding muscles and artificial bone. A limitation in this case is that the long-term efficacy needs further observation in the following period.
Some experiences and lessons were shared in this case, specifically: (1) combination of MRI and CT examinations to make precise diagnosis; (2) the allogeneic bone mixed with autogeneous bone was implanted in the weight-bearing area of the acetabulum to fill the hole caused by surgery; and (3) parts of muscle bellies of the anterior iliopsoas and posterior gluteus medius muscle were pulled together and sutured to fill up the empty cavity. Above-mentioned processes in this case not only reduce the operation time and the operating difficulty but also avoid the utilization of internal fixation and lower the expense.
Conflict of interest statement: Neither of the authors has any conflict of interest related to the article.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chen YK, Dinc B S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Song H
| 1. | Nadaf A, Radhika M, Paremala K, Srinath N. Monostostic fibrous dysplasia with nonspecific cystic degeneration: A case report and review of literature. J Oral Maxillofac Pathol. 2013;17:274-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Basaran R, Kaksi M, Gur E, Efendioglu M, Balkuv E, Sav A. Monostotic fibrous dysplasia involving occipital bone: a case report and review of literature. Pan Afr Med J. 2014;19:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | DiCaprio MR, Enneking WF. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint Surg Am. 2005;87:1848-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 212] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 4. | Feller L, Wood NH, Khammissa RA, Lemmer J, Raubenheimer EJ. The nature of fibrous dysplasia. Head Face Med. 2009;5:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Parekh SG, Donthineni-Rao R, Ricchetti E, Lackman RD. Fibrous dysplasia. J Am Acad Orthop Surg. 2004;12:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Kempson RL. Ossifying fibroma of the long bones. A light and electron microscopic study. Arch Pathol. 1966;82:218-233. [PubMed] |
| 7. | Campanacci M. Osteofibrous dysplasia of long bones a new clinical entity. Ital J Orthop Traumatol. 1976;2:221-237. [PubMed] |
| 8. | Campanacci M, Laus M. Osteofibrous dysplasia of the tibia and fibula. J Bone Joint Surg Am. 1981;63:367-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 104] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Park YK, Unni KK, McLeod RA, Pritchard DJ. Osteofibrous dysplasia: clinicopathologic study of 80 cases. Hum Pathol. 1993;24:1339-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Gwark JY, Jeong JH, Hwang SC, Nam DC, Lee JH, Na JB, Kim DH. Monostotic fibrous dysplasia in the proximal tibial epiphysis: a case report. J Med Case Rep. 2014;8:452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Gajjar SM, Walsh HP. Leg swelling in a newborn. Postgrad Med J. 2005;81:e13, e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Sweet DE, Vinh TN, Devaney K. Cortical osteofibrous dysplasia of long bone and its relationship to adamantinoma. A clinicopathologic study of 30 cases. Am J Surg Pathol. 1992;16:282-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Ueda Y, Blasius S, Edel G, Wuisman P, Böcker W, Roessner A. Osteofibrous dysplasia of long bones a reactive process to adamantinomatous tissue. J Cancer Res Clin Oncol. 1992;118:152-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Ishida T, Iijima T, Kikuchi F, Kitagawa T, Tanida T, Imamura T, Machinami R. A clinicopathological and immunohistochemical study of osteofibrous dysplasia, differentiated adamantinoma, and adamantinoma of long bones. Skeletal Radiol. 1992;21:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Benassi MS, Campanacci L, Gamberi G, Ferrari C, Picci P, Sangiorgi L, Campanacci M. Cytokeratin expression and distribution in adamantinoma of the long bones and osteofibrous dysplasia of tibia and fibula. An immunohistochemical study correlated to histogenesis. Histopathology. 1994;25:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Kahn LB. Adamantinoma, osteofibrous dysplasia and differentiated adamantinoma. Skeletal Radiol. 2003;32:245-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Maki M, Saitoh K, Kaneko Y, Fukayama M, Morohoshi T. Expression of cytokeratin 1, 5, 14, 19 and transforming growth factors-beta1, beta2, beta3 in osteofibrous dysplasia and adamantinoma: A possible association of transforming growth factor-beta with basal cell phenotype promotion. Pathol Int. 2000;50:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Bovée JV, van den Broek LJ, de Boer WI, Hogendoorn PC. Expression of growth factors and their receptors in adamantinoma of long bones and the implication for its histogenesis. J Pathol. 1998;184:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Guler I, Nayman A, Gedik GK, Koplay M, Sari O. Fibrous dysplasia mimicking vertebral bone metastasis on 18F-FDG PET/computed tomography in a patient with tongue cancer. Spine J. 2015;15:1501-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Adetayo OA, Salcedo SE, Borad V, Richards SS, Workman AD, Ray AO. Fibrous dysplasia: an overview of disease process, indications for surgical management, and a case report. Eplasty. 2015;15:e6. [PubMed] |
| 21. | Hakim DN, Pelly T, Kulendran M, Caris JA. Benign tumours of the bone: A review. J Bone Oncol. 2015;4:37-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 22. | Guille JT, Kumar SJ, MacEwen GD. Fibrous dysplasia of the proximal part of the femur. Long-term results of curettage and bone-grafting and mechanical realignment. J Bone Joint Surg Am. 1998;80:648-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 89] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Ippolito E, Caterini R, Farsetti P, Potenza V. Surgical treatment of fibrous dysplasia of bone in McCune-Albright syndrome. J Pediatr Endocrinol Metab. 2002;15 Suppl 3:939-944. [PubMed] |
| 24. | O’Sullivan M, Zacharin M. Intramedullary rodding and bisphosphonate treatment of polyostotic fibrous dysplasia associated with the McCune-Albright syndrome. J Pediatr Orthop. 2002;22:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Enneking WF, Gearen PF. Fibrous dysplasia of the femoral neck. Treatment by cortical bone-grafting. J Bone Joint Surg Am. 1986;68:1415-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 63] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Perisano C, Barone C, Stomeo D, Di Giacomo G, Vasso M, Schiavone Panni A, Maccauro G. Indications for prophylactic osteosynthesis associated with curettage in benign and low-grade malignant primitive bone tumors of the distal femur in adult patients: a case series. J Orthop Traumatol. 2016;17:377-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Ogilvie CM, Fox EJ, Lackman RD. Current surgical management of bone metastases in the extremities and pelvis. Semin Oncol. 2008;35:118-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Gibbs CP, Lewis VO, Peabody T. Beyond bone grafting: techniques in the surgical management of benign bone tumors. Instr Course Lect. 2005;54:497-503. [PubMed] |
| 29. | Maccauro G, Liuzza F, Scaramuzzo L, Milani A, Muratori F, Rossi B, Waide V, Logroscino G, Logroscino CA, Maffulli N. Percutaneous acetabuloplasty for metastatic acetabular lesions. BMC Musculoskelet Disord. 2008;9:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |