Published online Aug 16, 2025. doi: 10.12998/wjcc.v13.i23.105762
Revised: April 9, 2025
Accepted: May 7, 2025
Published online: August 16, 2025
Processing time: 118 Days and 3.2 Hours
Chronic psychological stress (CPS) is increasingly recognized for its detrimental effects on systemic and oral health, yet its impact on peri-implantitis remains underexplored.
To evaluate the evidence linking CPS to peri-implantitis.
This systematic review was conducted according to the PRISMA guidelines. Publications searching PubMed, EMBASE, MEDLINE, Cochrane Library, and ClinicalTrials.gov for human studies published in English from 1983 to December 2024. Additionally, quality assessment of selected full-text articles were performed using the modified Newcastle–Ottawa Scale.
From an initial total of 3964 studies, 4 cross-sectional studies comprising 432 participants met the inclusion criteria and consistently demonstrated a positive association between CPS and peri-implantitis. However, the findings are compromised by small sample sizes, study design limitations, methodological heterogeneity, and inadequate adjustment for critical confounders such as smoking and prior periodontitis.
Cortisol levels in peri-implant sulcus fluid were linearly correlated with probing depth, with evidence suggesting this relationship may be independent of hyperglycemia. Depression emerged as the most significant CPS subtype associated with peri-implantitis. Additionally, CPS may amplify peri-implantitis inflammation by modulating cytokine expression effects. Long-term studies with larger, more diverse patient populations and careful control of confounding variables are needed to establish causality and understand the underlying mechanisms. Including psychological evaluations and stress management techniques in peri-implant care protocols could improve treatment outcomes and patient health.
Core Tip: The bidirectional oral-brain axis has been recently proposed. Numerous contemporary studies have highlighted the association between psychological distress and periodontitis. However, the relationship between psychological distress and peri-implantitis remains unclear. Although the current evidence is limited, all human studies to date suggest that psychological distress increases the risk of peri-implantitis, similar to its impact on periodontitis.
- Citation: Chang YL, Lin GM, Lin SY, Huang RY, Kuo PJ, Chang NNS, Tsai KZ. Evidence for the association between psychological stress and peri-implant health among middle-aged and elderly adults: A systemic review. World J Clin Cases 2025; 13(23): 105762
- URL: https://www.wjgnet.com/2307-8960/full/v13/i23/105762.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i23.105762
The human body continuously adapts to internal and external changes to maintain stability, a process known as homeostasis. This dynamic equilibrium relies on a complex interplay of physiological mechanisms, particularly stress response pathways regulated by hormones such as cortisol, adrenaline, and noradrenaline[1]. These hormones activate the autonomic and central nervous systems, enabling the body to respond effectively to stressors in a process called allostasis[1,2]. While allostasis facilitates adaptation to fluctuating environmental conditions[2], chronic activation of stress response pathways can result in "allostatic load", a physiological dysregulation linked to pathological conditions, e.g. cardiovascular dysfunction, metabolic derangements, and impaired glucose homeostasis[3-5]. These adverse out
CPS has been shown to significantly affect oral health, particularly in the development and progression of periodontal diseases[8,9]. Chronic activation of the hypothalamic-pituitary-adrenal (HPA) axis leads to prolonged glucocorticoid hormone release, result in immune suppression[6,8]. This immunosuppressive state weakens host defenses against oral pathogens, exacerbates inflammatory processes, and accelerates tissue destruction. Clinical and experimental evidence consistently demonstrates that CPS heightens inflammatory processes, delays wound healing, and contributes to periodontal tissue deterioration, underscoring its critical role in periodontal health[6,8-10].
Peri-implantitis, an inflammatory condition characterized by the progressive destruction of peri-implant tissues, shares similar pathophysiological mechanisms with periodontitis but presents unique challenges due to the implant-tissue interface[11]. Despite the well- established link between CPS and periodontitis, the association between stress-induced immune modulation and peri-implantitis remains inadequately explored[12-14]. Investigating this relationship is crucial for understanding the broader impact of CPS on oral health and for developing targeted therapeutic and preventive strategies to mitigate peri-implant tissue destruction. This systematic review aims to bridge this knowledge gap by examining the pathophysiological links between CPS and peri-implantitis in human models, advancing our un
This systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[15]. A systemic and comprehensive literature search was conducted across multiple databases, including PubMed, EMBASE, MEDLINE, Cochrane Library and the ClinicalTrials.gov, with a focus on English-language publications. Furthermore, reference lists of retrieved articles and relevant reviews were manually screened to identify additional studies. Search terms were constructed using specific keywords combined with Boolean operators, including [(heart rate) OR (blood pressure) OR (cytokines) OR (cortisol) OR (catecholamines) OR (copeptin) OR (salivary amylase) OR (interleukin-6) OR (C-reactive protein)] OR [(psychological health) OR (mental health) OR (psychological stress) OR (mental stress) OR (psychological disorders) OR (mental disorders)] AND [(peri-implant health) OR (peri-implantitis) OR (peri-implant mucositis) OR (osseointegration)]. The final update of the search was performed on December 10, 2024.
Two independent reviewers (Yen-Lan Chang and Shih-Ying Lin) conducted eligibility assessments and data extraction using standardized forms. Titles and abstracts were initially screened against predefined criteria. Full-text reviews were conducted for studies meeting eligibility requirements, and disagreements were resolved through discussion or consultation with a third reviewer (Kun-Zhe Tsai). The review exclusively included clinical investigations in human samples, such as randomized and non-randomized controlled trials and cohort studies examining the association between CPS and peri-implantitis prevalence. Systematic and narrative reviews, case reports, letters, commentaries, in vitro studies, and animal experiments were excluded.
Efforts were made to address missing or unclear data by contacting corresponding authors. In cases of data redundancy across publications, the most comprehensive dataset was extracted. The quality and risk of bias (ROB) were evaluated using a modified Newcastle–Ottawa Scale for cross-sectional studies (Supplementary Table 1), ensuring a rigorous evaluation of study reliability and validity[16,17]. The review focused on human studies directly examining dental implants, specifically excluding pediatric studies and studies centered on natural teeth.
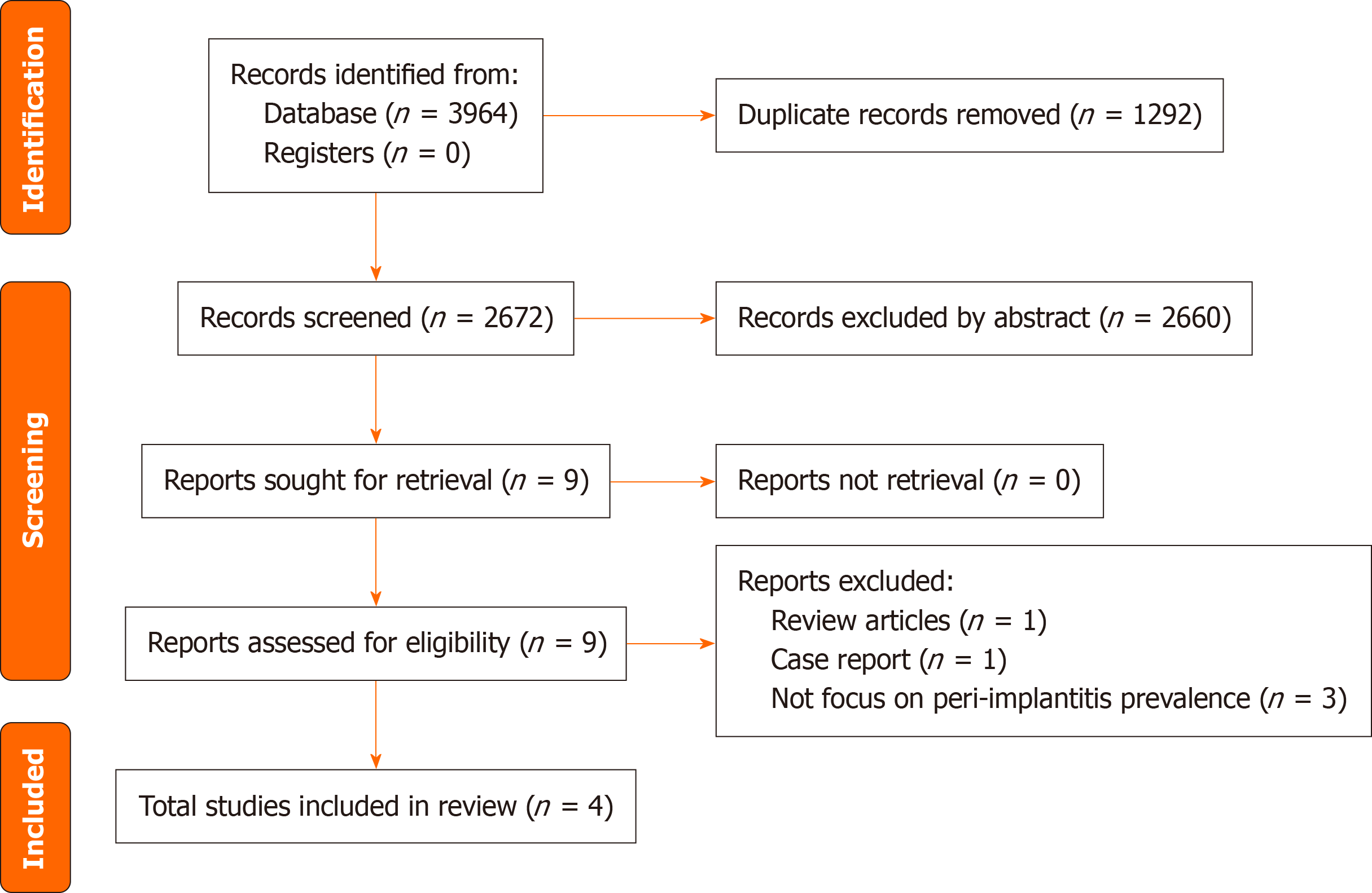
Figure 1 presents the selection process in a PRISMA flow diagram. A total of 3964 studies were identified after database searches, with 1292 duplicates subsequently excluded. After screening titles and abstracts, 9 studies were retrieved for full-text evaluation. During this stage, 5 studies were excluded for specific reasons detailed in Supplementary Table 2. Ultimately, 4 cross-sectional studies comprising 432 participants fulfilled the stringent inclusion criteria and were comprehensively analyzed.
Table 1 reveals the quality assessment of included studies. Among these four eligible studies, one had a low ROB[18], two showed a moderate ROB[19,20], and one was assessed as having a high ROB[21]. All studies exhibited selection bias due to the non-random selection of participants and small sample sizes. Only one study explicitly excluded individuals with a history of periodontitis[21], while another study excluded those with active periodontal disease[20]. One study noted that participants had no active periodontal disease at the time of implant placement[18], and another did not report whether periodontal conditions were considered during participant selection[19]. Moreover, one study failed to adjust for key confounding factors[19], such as smoking and blood glucose, which may affect cortisol levels in bodily fluids[22,23].
| Ref. | ROB | Selection | Study design | Outcome assessment | Confounding factor assessment | Data analysis | Study results | ||||||||
| Representative sample | Adequate sample size | Accuracy of exposure/risk factors | Data quality control | Definition of cases and controls | Selection of control group | Accuracy of outcome | Timing of outcome assessment | Control of confounding factors | Stratification or multivariate analysis | Appropriateness of statistical methods | Reporting of results | Consistency of results | Openness and reproducibility | ||
| Soysal et al[21], 2024 | Medium | + | + | + | + | + | + | + | + | ||||||
| Strooker et al[20], 2022 | Low | + | + | + | + | + | + | + | + | + | + | + | |||
| Ali et al[19], 2022 | High | + | + | ||||||||||||
| Alresayes et al[18], 2021 | Medium | + | + | + | + | + | + | + | |||||||
Table 2 illustrates the major characteristics of these four studies. CPS was assessed through cortisol levels in peri-implant sulcular fluid (PISF) in two studies[19,21], via questionnaires in one study[18], and through a combination of questionnaires with measurements of glucocorticoid receptor-α GRα concentration in another[20]. While one study explicitly reported no antipsychotic drug use among participants[20], the remaining three studies did not address medication status, making it uncertain about the potential effect of antipsychotic drugs on the results[18,19,21]. Peri-implantitis definitions varied across the studies. Two studies[19,20] adhered to the 2017 World Workshop consensus criteria, which included probing depth ≥ 6 mm, bleeding on probing/suppuration, and marginal bone loss ≥ 3 mm[11]. One study employed less stringent criteria, defining peri-implantitis as probing depth ≥ 4 mm, bleeding on probing/suppuration, and marginal bone loss ≥ 2 mm[18]. Another study did not provide the definition of peri-implantitis[21].
| Ref. | Country | Design | Participants | Study groups and age (mean ± SD) | Smoker | Psychological health assessment | Antipsychotic drugs | Definition of peri-implantitis |
| Soysal et al[21], 2024 | Turkey | Cross-sectional | 50 (F 16/M 34) | Healthy implant & high stress: 53.06 ± 11.44 years; Healthy implant & low stress: 52.11 ± 16.70 years; Peri-implantitis & high stress: 56.00 ± 6.71 years; Peri-implantitis & low stress: 59.80 ± 7.24 years | No | HAD and STAI questionnaires; GR-α levels of saliva | No use | PD ≥ 6 mm, BoP/suppuration, and BL ≥ 3 mm |
| Strooker et al[20], 2022 | Netherlands | Cross-sectional | 230 (F 137/M 93) | No peri-implantitis: 64.4 ± 11.3 years; Peri-implantitis: 62.9 ± 10.8 years | 10% | SCL-90 questionnaire | Not available | PD ≥ 4 mm, BoP/suppuration, and BL ≥ 2 mm |
| Ali et al[19], 2022 | Kuwait | Cross-sectional | 64 | T2DM with peri-implantitis: 53.8 ± 5.6 years; T2DM without peri-implantitis: 52.5 ± 3.2 years; Non-DM with peri-implantitis: 52.7 ± 1.6 years; Non-DM without peri-implantitis: 52.2 ± 1.2 years | No | Cortisol level in peri-implant sulcular fluid | Not available | Not available |
| Alresayes et al[18], 2021 | Saudi Arabia | Cross-sectional | 88 (F 42/M 46) | Peri-implantitis: 65.3 ± 5.6 years; No peri-implantitis: 63.8 ± 4.4 years | No | Cortisol level in peri-implant sulcular fluid | Not available | PD ≥ 6 mm, BoP/suppuration, and BL ≥ 3 mm |
Three studies[18,20,21] identified a potential association of CPS with peri-implantitis, with one study[21] revealing this relationship persisted independently of hyperglycemia. Notably, although one study similarly identified a positive result, the authors cautioned that while elevated PISF cortisol levels may indicate CPS, the measurements alone could not definitively confirm chronic stress levels in affected patients[19]. Additionally, in absences of controlling for established confounding factors, such as smoking and blood glucose concentrations, a conclusive relationship between CPS and peri-implantitis cannot be established[19]. Since the heterogeneity of the data and the variety of studies included, a further meta-analysis could not be conducted.
Cortisol, the primary glucocorticoid synthesized in the adrenal cortex's zona fasciculata, has been extensively studied for its role in systemic stress responses[24,25]. Recent studies have reported significant positive associations between cortisol levels and probing depth in peri-implantitis, with the strongest correlations observed in individuals without Type 2 diabetes (T2DM)[19,21]. Alresayes et al[19] demonstrated linear relationships between cortisol levels, probing depth, and crestal bone loss, while Ali et al[19] additionally observed this association only in participants without T2DM. In contrast to cortisol-focused studies, Strooker et al[18] utilized the Symptom Checklist-90 (SCL-90) to assess psychological distress across eight subdomains, identifying depression as the only psychological variable significantly associated with peri-implantitis in univariate analyses [odds ratio (OR) = 2.150, 95% confidence interval (CI) = 1.147–4.032]. Even after adjusting for confounders such as smoking, medical treatments, and lung conditions during a five-year follow-up, depression remained an independent risk factor (OR = 2.036, 95%CI = 1.067–3.884)[18]. Building upon the evidence presented in previous studies, Soysal et al[20] offer another approach to exploring the relationship between CPS and peri-implantitis by integrating questionnaire-based assessments with biomarker analyses. CPS was evaluated using the Hospital Anxiety and Depression Scale and the State-Trait Anxiety Inventory, alongside measurements of salivary GRα mRNA expression, a stress-related biomarker. Although no significant association was observed between GRα levels and peri-implantitis, their analysis of salivary cytokines suggested that CPS might exacerbate peri-implantitis-related inflammation by influencing cytokine expression[20].
Clinical data linking the psychological stress to peri-implantitis are limited. While CPS has been extensively studied in relation to periodontitis, offering valuable insights, these findings can only serve as a reference due to fundamental differences between the two conditions. Peri-implantitis is an immune-mediated complication driven by bacterial biofilms on implant surfaces, with unique pathophysiological mechanisms distinct from periodontitis[26]. Unlike mineralized hydroxyapatite of teeth, implant surfaces—typically composed of titanium dioxide or other alloys—alter initial bacterial adhesion through differing electrostatic forces and ionic interactions[26]. These differences may lead to distinct biofilm characteristics and expose peri-implant tissues to implant degradation products, which can affect microbiota behavior and immune responses[26]. Further investigation into the relationship between peri-implantitis and CPS should build on the established understanding of periodontitis and stress to uncover both shared and distinct pathways. Nevertheless, the binary oral-brain axis concept, originally proposed in the context of periodontitis, remains a valuable framework for reference.
Many observational studies have reported an association between periodontitis and CPS, regardless of the source of stress, including financial[27], occupational[28], marital[29], or military-related stress[30-32]. However, the evidences are not entirely consistent, as not all studies have demonstrated a clear link between CPS and periodontal tissue destruction. A systematic review conducted in 2007 assessed stress and psychological factors as potential risk factors for periodontitis, with 57.1% of these studies reporting a positive association, 28.5% observing mixed outcomes (positive for some characteristics and negative for others), and 14.2% finding no association[33].
Among neuropsychiatric disorders, major depression is the most extensively studied for periodontitis. Three systematic reviews with meta-analyses have examined the association[34-36]. Findings from cross-sectional studies alone did not reveal a significant relationship between periodontitis and depression[34-36]. However, analyses incorporating case-control studies demonstrated a significant association between periodontitis and depression. Liu et al[34], conducted a meta-analysis combining cross-sectional and case-control studies, reporting a significant association (OR = 1.61, 95%CI = 1.16–2.23). Similarly, a subgroup analysis in Zheng et al’s study[35] focusing on severe periodontitis showed a higher likelihood of depression (OR = 1.43, 95%CI = 1.05–1.93).
Emerging evidence suggests that the relationship between periodontal health and depression may be bidirectional[37]. A cohort study by Hsu et al[38] examined 12708 patients with newly diagnosed periodontitis and 50832 periodontally healthy matched controls over a follow-up period of 5 to 11 years. After adjusting for sex, age, and comorbidities, individuals with periodontitis exhibited a 1.73-fold increased risk of developing depression compared to controls. Additionally, a three-year longitudinal study of 7656 older adults in Japan found that edentulous individuals had a higher risk of developing depressive symptoms[39]. Regarding to peri-implantitis, Strooker et al[18] reported that individuals with depression have approximately twice the odds of having peri-implantitis compared to those without depression.
Periodontitis is increasingly recognized as a potential contributor to CPS and central nervous system (CNS) dysfunction through diverse mechanistic pathways. Periodontal pathogens such as Porphyromonas gingivalis can access the CNS via hematogenous spread, cranial nerves, or circumventricular organs, compromising the integrity of the blood-brain barrier (BBB) and enabling bacterial invasion[40]. These pathogens activate microglia and trigger immune responses, with lipopolysaccharides (LPS) engaging toll-like receptors (TLRs) and factor nuclear kappa B (NF-κB) signaling to generate reactive oxygen species and pro-inflammatory cytokines[41-44]. These processes disrupt CNS homeostasis and may allow intracellular survival of pathogens via mechanisms like the Trojan horse strategy[45,46]. The neuroinflammation underscores an association between oral dysbiosis and CNS pathology[47].
In addition to vascular and neural routes, evidence suggests a potential lymphatic pathway connecting the oral cavity to the brain. Microorganisms such as oral Treponema have been identified in cerebrospinal fluid and the fourth cerebral ventricle, implicating the lymphatic system in the dissemination of oral bacteria[48,49]. Tooth loss and reduced chewing efficiency may impair venous and lymphatic return, facilitating bacterial entry into lymphatic circulation and subsequent systemic dissemination[48,50,51]. Although regional lymph nodes contain antigen-presenting cells to neutralize pathogens, certain bacteria evade phagosome-lysosome fusion, exploiting host cell migration to reach cerebral sites, including the III and IV ventricles[48].
Systemic inflammation associated with periodontitis further exacerbates neuroinflammation through endothelial NF-κB activation, macrophage recruitment, and sustained microglial activation. The lymphatic system’s drainage into the bloodstream may amplify bacteremia, particularly in the context of weakened BBB integrity[52]. Additionally, exosomes, nanoscale vesicles involved in cellular communication, have been implicated in disease progression. Alterations in salivary exosomal markers and the identification of immune-related proteins linked to severe periodontitis underscore the connection between oral dysbiosis and systemic immune modulation[40,53].
Under acute psychological stress, the body's initial response is driven by the sympathoadrenal medullary (SAM) system, which activates rapidly to prepare for immediate action—the "fight or flight" response[54]. The SAM system promptly triggers the release of catecholamines, particularly adrenaline and noradrenaline, from the adrenal medulla[55]. Following this immediate SAM system response, the HPA axis is engaged to sustain the stress response over a longer period. When activated, the HPA axis initiates the release of corticotropin-releasing hormone from the hypothalamus, which in turn stimulates the anterior pituitary to secrete adrenocorticotropic hormone (ACTH)[6,8,56]. ACTH then prompts the adrenal cortex to release cortisol, a glucocorticoid that initially enhances immune activity by boosting natural killer (NK) cell function and promoting pro-inflammatory cytokines, such as interleukin-6 and tumor necrosis factor (TNF)-α[56]. However, chronic cortisol exposure due to CPS could lead to immune dysregulation, reducing T cell proliferation and weakening adaptive immune responses, which may increase susceptibility to infections[57]. In parallel, the sympathetic nervous system activation causes short-term catecholamine release that mobilizes immune cells and enhances innate immunity through NK cells and macrophages. However, chronic exposure to catecholamines might suppress adaptive immunity by downregulating T cell receptors and inhibiting T cell activation[56,58]. In experimental studies on mice, thought acute norepinephrine release boosts macrophage activity, CPS ultimately impairs B cell function and antibody production, thereby compromising humoral immunity[59]. In addition to cortisol, other neuroendocrine mediators—particularly adrenaline and noradrenaline may activate both autonomic and central nervous system responses and are intimately involved in the body’s acute and adaptive mechanisms for managing stress[1]. Their functions are encapsulated within the concept of allostasis, a dynamic process that facilitates physiological stability in the face of environmental challenges. However, chronic activation of these stress pathways may lead to allostatic load, a maladaptive state marked by systemic dysregulation. Allostatic load has been implicated in a range of adverse health outcomes, including cardiovascular disease, metabolic disturbances, and impaired glucose homeostasis[1]. These pathophysiological consequences may stem from both direct neuroendocrine effects and indirect behavioral changes associated with chronic stress, thereby highlighting the complex and multifactorial impact of sustained psychological stress on systemic and oral health.
Upon initial exposure to a stressor, central catecholamine levels surge rapidly that trigger an immediate physiological response, and brain corticosteroid levels rise more gradually that can sustain the response over time. Catecholamines initiate fast-acting effects, while corticosteroids exert a dual action: Rapid non-genomic effects followed by delayed genomic responses[54]. These effects overlap within an early window post-stress onset, with successive waves of stress-related neurotransmitters and hormones affecting diverse brain regions[54]. At the cellular level, catecholamines interact with corticosteroids’ early non-genomic effects, while cortisol’s genomic actions typically emerge approximately 60 minutes after stress initiation[60].
Periodontitis/peri-implantitis, a chronic inflammatory condition, has been increasingly linked to systemic inflammation and the development of non-communicable diseases such as cardiovascular diseases, diabetes, and respiratory disorders[61]. The condition arises from an imbalance between oral microorganisms and host immune defenses, leading to periodontal tissue destruction and systemic effects[62,63]. Systemic chronic low-grade inflammation is increasingly recognized as a shared risk factor for psychiatric conditions, including anxiety disorders, mood disorders, and trauma-related disorders[64-66].
CPS also indirectly contributes to periodontal/peri-implant tissue destruction by promoting maladaptive behaviors and impairing oral hygiene. Stress-related structural and functional changes in the hippocampus, as revealed by imaging, post-mortem, and rodent studies, lead to reduced motivation, disorganization, and poor adherence to personal care routines[67-70]. These behaviors manifest as decreased brushing frequency, poor brushing quality, and reduced compliance with dental maintenance, which elevate the risk of periodontal diseases[71-73]. Patients with severe periodontitis, inadequate plaque control, and irregular post-implant care are particularly vulnerable to peri-implantitis[11]. Additional factors, such as poor nutrition, substance misuse (e.g., alcohol, betel nut, and tobacco), limited access to dental care, and xerostomia from psychotropic medications, further exacerbate oral health issues in stressed individuals[74].
Psychiatric disorders and their associated psychotropic medications present significant challenges to bone health and dental outcomes. Conditions such as schizophrenia, depression, and bipolar disorder, when treated with medications like lithium (Li), antidepressants, and antipsychotics can compromise bone metabolism through multiple complex mechanisms. These pharmacological interventions can induce substantial changes in physiological processes, resulting in reduced bone mineral density and an increased risks of osteoporosis and dental implant failure[75-78].
The underlying pathophysiological mechanisms are multifaceted. Medication-induced hormonal disruptions, including hyperprolactinemia, hypercortisolemia, and hypogonadism, directly impact bone remodeling. Moreover, the interaction of serotonin with bone metabolism accelerates bone resorption and compromises osseointegration[75]. Compounding these pharmaceutical effects, lifestyle factors such as active smoking, malnutrition, and sedentary behavior further amplify the potential for adverse bone and dental implant outcomes[79-84].
Of particular concern are selective serotonin reuptake inhibitors (SSRIs), especially sertraline, which demonstrate a notable correlation with increased implant failure rates[76,77]. However, the existing research landscape is nuanced. Current studies exhibit methodological limitations, including inconsistent drug standardization and variable research protocols[76,78]. Consequently, while preliminary evidence suggests a potential risk of SSRIs, the scientific community has not yet established a definitive, unequivocal association between antidepressant use and dental implant complications.
Nevertheless, a materials science investigation uncovered intriguing insights into the interactions between lithium and bone regeneration in dental implant surfaces. When Li was incorporated into a sandblasted, large-grit, acid-etched (SLA) surface, it showed significant inhibitory effects on osteoclastogenesis—the process of bone cell breakdown[85]. The researchers found that the SLA-Li surface activated the wingless-related integration site (Wnt)/β-catenin signaling pathway and modulated the receptor activator of nuclear factor-κB ligand (RANKL)/osteoprotegerin (OPG) signaling axis, which are critical molecular mechanisms in bone metabolism[85-91]. Notably, in vivo experiments revealed that the SLA-Li surface substantially enhanced bone formation and osseointegration during the crucial early stages of dental implant surgery, suggesting promising therapeutic potential for improving implant success rates[85].
Sex-specific considerations warrant greater attention. In particular, the male-to-female ratios reported in the studies and the influence of hormonal changes associated with menopause should be carefully evaluated. Postmenopausal women may be especially susceptible to the effects of CPS, and recent evidence suggests that depression—a prevalent and severe subtype of chronic stress—may be significantly associated with peri-implantitis in this population[8]. This finding underscores the complex and bidirectional relationship between mental health and peri-implant disease, and reinforces the importance of adopting an integrative framework that accounts for psychological, physiological, and demographic factors in future research.
This systematic review is subject to several noteworthy limitations that constrain the interpretability and generalizability of its findings. First, all four included studies employed cross-sectional designs, which inherently limit the ability to draw causal inferences. Second, the relatively small total sample size, coupled with the absence of randomization in participant selection, introduces the potential for selection bias and undermines the internal validity of the findings. Third, considerable methodological heterogeneity across studies may weaken the strength of the conclusions. CPS was assessed by diverse modalities —including cortisol concentrations in peri-implant sulcular fluid, self-reported questionnaires, and glucocorticoid receptor expression—while the diagnostic criteria for peri-implantitis varied substantially. Only a subset of the studies adhered to the standardized 2017 World Workshop consensus, thereby limiting comparability and the consistency of pooled results. Fourth, although some studies excluded individuals with active periodontal disease or a history of periodontitis, others did not adequately control for critical confounders, e.g., smoking and hyperglycemia—both of which are independently associated with peri-implant disease and stress biomarkers. The failure to account for these factors may lead to a bias into the observed associations. Furthermore, the potential influence of antipsychotic medications—known to modulate HPA axis function and, consequently, affect both cortisol levels and peri-implant tissue health—was largely overlooked, with three of the four studies failing to consider or report medication use. Fifth, given the paucity of research in this area, interpretations for the mechanisms linking CPS to peri-implantitis should be made with caution, underscoring the need for rigorous, longitudinal cohort studies. Future investigations should incorporate larger, more diverse populations; adopt standardized diagnostic criteria and validated stress assessment tools; and employ robust analytical approaches that comprehensively control for confounding factors. Such methodological rigor is essential for elucidating the complex interplay between chronic psychological stress and peri-implant disease pathogenesis.
Previous research has identified a link between chronic CPS and periodontitis, introducing the concept of a bilateral oral-brain axis. Even current evidence also suggests a potential association between CPS and peri-implantitis in the general population, and this relationship may be independent of the effects of hyperglycemia. Additionally, CPS may amplify peri-implantitis inflammation through its modulating effects on cytokine expression. However, the existing evidence remains weak, constrained by small sample sizes, high heterogeneity, and failure to account for key confounding variables. These limitations preclude definitive conclusions regarding causality or even a robust association. Future research should focus on well-designed longitudinal cohort studies with larger, more diverse populations to strengthen the evidence base. Such studies should also consider potential confounding factors, such as psychotropic medication use or therapeutic interventions, and provide comprehensive assessments of peri-implant tissue health, including bleeding on probing, probing depth, and clinical attachment levels, before and after CPS interventions.
| 1. | O'Connor DB, Thayer JF, Vedhara K. Stress and Health: A Review of Psychobiological Processes. Annu Rev Psychol. 2021;72:663-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 376] [Article Influence: 75.2] [Reference Citation Analysis (1)] |
| 2. | Guidi J, Lucente M, Sonino N, Fava GA. Allostatic Load and Its Impact on Health: A Systematic Review. Psychother Psychosom. 2021;90:11-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 675] [Cited by in RCA: 545] [Article Influence: 109.0] [Reference Citation Analysis (1)] |
| 3. | Manolis TA, Manolis AA, Manolis AS. Cognitive Behavioral Therapy in Cardiovascular Disease. Curr Vasc Pharmacol. 2025;23:77-97. [PubMed] [DOI] [Full Text] |
| 4. | Lihua M, Kaipeng Z, Xiyan M, Yaowen C, Tao Z. Systematic review and meta-analysis of stress management intervention studies in patients with metabolic syndrome combined with psychological symptoms. Medicine (Baltimore). 2023;102:e35558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Mehdi S, Wani SUD, Krishna KL, Kinattingal N, Roohi TF. A review on linking stress, depression, and insulin resistance via low-grade chronic inflammation. Biochem Biophys Rep. 2023;36:101571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 6. | Gupta A, Agarwal V. Inflammation as a shared mechanism of chronic stress related disorders with potential novel therapeutic targets. Naunyn Schmiedebergs Arch Pharmacol. 2024;397:8383-8394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Hendry E, McCallister B, Elman DJ, Freeman R, Borsook D, Elman I. Validity of mental and physical stress models. Neurosci Biobehav Rev. 2024;158:105566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Ball J, Darby I. Mental health and periodontal and peri-implant diseases. Periodontol 2000. 2022;90:106-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 9. | Decker AM, Kapila YL, Wang HL. The psychobiological links between chronic stress-related diseases, periodontal/peri-implant diseases, and wound healing. Periodontol 2000. 2021;87:94-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Ganesan A, Kumar G, Gauthaman J, Lakshmi KC, Kumbalaparambil YA. Exploring the Relationship between Psychoneuroimmunology and Oral Diseases: A Comprehensive Review and Analysis. J Lifestyle Med. 2024;14:13-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, Camargo PM, Chen S, Cochran D, Derks J, Figuero E, Hämmerle CHF, Heitz-Mayfield LJA, Huynh-Ba G, Iacono V, Koo KT, Lambert F, McCauley L, Quirynen M, Renvert S, Salvi GE, Schwarz F, Tarnow D, Tomasi C, Wang HL, Zitzmann N. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol. 2018;45 Suppl 20:S286-S291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 703] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 12. | Albandar JM, Rams TE. Global epidemiology of periodontal diseases: an overview. Periodontol 2000. 2002;29:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 279] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 13. | Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42 Suppl 16:S158-S171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 787] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 14. | Lee CT, Huang YW, Zhu L, Weltman R. Prevalences of peri-implantitis and peri-implant mucositis: systematic review and meta-analysis. J Dent. 2017;62:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 334] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 15. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021;18:e1003583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1310] [Cited by in RCA: 1793] [Article Influence: 448.3] [Reference Citation Analysis (0)] |
| 16. | Moskalewicz A, Oremus M. No clear choice between Newcastle-Ottawa Scale and Appraisal Tool for Cross-Sectional Studies to assess methodological quality in cross-sectional studies of health-related quality of life and breast cancer. J Clin Epidemiol. 2020;120:94-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 188] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 17. | Deng J, Qin Y. From meta-analysis to Mendelian randomization: Unidirectional perspectives on the association of glaucoma with depression and anxiety. PLoS One. 2024;19:e0310985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Strooker H, de Waal YCM, Bildt MM. Psychological risk indicators for peri-implantitis: A cross-sectional study. J Clin Periodontol. 2022;49:980-987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 19. | Alresayes S, Al-Askar M, Mokeem SA, Javed F, Vohra F, Abduljabbar T. Cortisol levels in the peri-implant sulcular fluid among patients with and without peri-implantitis. J Periodontal Res. 2021;56:746-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Soysal F, Unsal B, Isler SC, Akca G, Bakirarar B, Ozcan M. Evaluation of salivary stress markers and inflammatory cytokine levels in peri-implantitis patients. Clin Oral Investig. 2024;28:290. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Ali D, Baskaradoss JK, Joseph BK. Cortisol Levels in the Peri-implant Sulcular Fluid of Type-2 Diabetic and Non-diabetic Patients with Peri-implantitis. Oral Health Prev Dent. 2022;20:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Gonzalez-Bono E, Rohleder N, Hellhammer DH, Salvador A, Kirschbaum C. Glucose but not protein or fat load amplifies the cortisol response to psychosocial stress. Horm Behav. 2002;41:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | LaFond M, DeAngelis B, al'Absi M. Hypothalamic pituitary adrenal and autonomic nervous system biomarkers of stress and tobacco relapse: Review of the research. Biol Psychol. 2024;192:108854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1055] [Cited by in RCA: 1130] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 25. | Rogerson O, Wilding S, Prudenzi A, O'Connor DB. Effectiveness of stress management interventions to change cortisol levels: a systematic review and meta-analysis. Psychoneuroendocrinology. 2024;159:106415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 26. | Kotsakis GA, Olmedo DG. Peri-implantitis is not periodontitis: Scientific discoveries shed light on microbiome-biomaterial interactions that may determine disease phenotype. Periodontol 2000. 2021;86:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 27. | Genco RJ, Ho AW, Grossi SG, Dunford RG, Tedesco LA. Relationship of stress, distress and inadequate coping behaviors to periodontal disease. J Periodontol. 1999;70:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 296] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 28. | Marcenes WS, Sheiham A. The relationship between work stress and oral health status. Soc Sci Med. 1992;35:1511-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Hugoson A, Ljungquist B, Breivik T. The relationship of some negative events and psychological factors to periodontal disease in an adult Swedish population 50 to 80 years of age. J Clin Periodontol. 2002;29:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (4)] |
| 30. | Vered Y, Soskolne V, Zini A, Livny A, Sgan-Cohen HD. Psychological distress and social support are determinants of changing oral health status among an immigrant population from Ethiopia. Community Dent Oral Epidemiol. 2011;39:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Spalj S, Plancak D, Bozic D, Kasaj A, Willershausen B, Jelusic D. Periodontal conditions and oral hygiene in rural population of post-war Vukovar region, Croatia in correlation to stress. Eur J Med Res. 2008;13:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Tsai KZ, Tsai SC, Lin KH, Chang YC, Lin YP, Lin GM. Associations of decayed teeth and localized periodontitis with mental stress in young adults: CHIEF oral health study. Sci Rep. 2022;12:19139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 33. | Peruzzo DC, Benatti BB, Ambrosano GM, Nogueira-Filho GR, Sallum EA, Casati MZ, Nociti FH Jr. A systematic review of stress and psychological factors as possible risk factors for periodontal disease. J Periodontol. 2007;78:1491-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 34. | Liu F, Wen YF, Zhou Y, Lei G, Guo QY, Dang YH. A meta-analysis of emotional disorders as possible risk factors for chronic periodontitis. Medicine (Baltimore). 2018;97:e11434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Zheng DX, Kang XN, Wang YX, Huang YN, Pang CF, Chen YX, Kuang ZL, Peng Y. Periodontal disease and emotional disorders: A meta-analysis. J Clin Periodontol. 2021;48:180-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 36. | Araújo MM, Martins CC, Costa LC, Cota LO, Faria RL, Cunha FA, Costa FO. Association between depression and periodontitis: a systematic review and meta-analysis. J Clin Periodontol. 2016;43:216-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Karimi P, Zojaji S, Fard AA, Nateghi MN, Mansouri Z, Zojaji R. The impact of oral health on depression: A systematic review. Spec Care Dentist. 2025;45:e13079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 38. | Hsu CC, Hsu YC, Chen HJ, Lin CC, Chang KH, Lee CY, Chong LW, Kao CH. Association of Periodontitis and Subsequent Depression: A Nationwide Population-Based Study. Medicine (Baltimore). 2015;94:e2347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Yamamoto T, Aida J, Kondo K, Fuchida S, Tani Y, Saito M, Sasaki Y. Oral Health and Incident Depressive Symptoms: JAGES Project Longitudinal Study in Older Japanese. J Am Geriatr Soc. 2017;65:1079-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 40. | Martínez M, Postolache TT, García-Bueno B, Leza JC, Figuero E, Lowry CA, Malan-Müller S. The Role of the Oral Microbiota Related to Periodontal Diseases in Anxiety, Mood and Trauma- and Stress-Related Disorders. Front Psychiatry. 2021;12:814177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 41. | Diogenes A, Ferraz CC, Akopian AN, Henry MA, Hargreaves KM. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res. 2011;90:759-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 219] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 42. | Kaewpitak A, Bauer CS, Seward EP, Boissonade FM, Douglas CWI. Porphyromonas gingivalis lipopolysaccharide rapidly activates trigeminal sensory neurons and may contribute to pulpal pain. Int Endod J. 2020;53:846-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Díaz-Zúñiga J, Monasterio G, Alvarez C, Melgar-Rodríguez S, Benítez A, Ciuchi P, García M, Arias J, Sanz M, Vernal R. Variability of the dendritic cell response triggered by different serotypes of Aggregatibacter actinomycetemcomitans or Porphyromonas gingivalis is toll-like receptor 2 (TLR2) or TLR4 dependent. J Periodontol. 2015;86:108-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Go M, Kou J, Lim JE, Yang J, Fukuchi KI. Microglial response to LPS increases in wild-type mice during aging but diminishes in an Alzheimer's mouse model: Implication of TLR4 signaling in disease progression. Biochem Biophys Res Commun. 2016;479:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 45. | Santiago-Tirado FH, Onken MD, Cooper JA, Klein RS, Doering TL. Trojan Horse Transit Contributes to Blood-Brain Barrier Crossing of a Eukaryotic Pathogen. mBio. 2017;8:e02183-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 174] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 46. | Santiago-Tirado FH, Doering TL. False friends: Phagocytes as Trojan horses in microbial brain infections. PLoS Pathog. 2017;13:e1006680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 47. | Tessarin GWL, Toro LF, Pereira RF, Dos Santos RM, Azevedo RG. Peri-implantitis with a potential axis to brain inflammation: an inferential review. Odontology. 2024;112:1033-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 48. | Sansores-España LD, Melgar-Rodríguez S, Olivares-Sagredo K, Cafferata EA, Martínez-Aguilar VM, Vernal R, Paula-Lima AC, Díaz-Zúñiga J. Oral-Gut-Brain Axis in Experimental Models of Periodontitis: Associating Gut Dysbiosis With Neurodegenerative Diseases. Front Aging. 2021;2:781582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 49. | Riviere GR, Riviere KH, Smith KS. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer's disease. Oral Microbiol Immunol. 2002;17:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 257] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 50. | Tsutsui K, Kaku M, Motokawa M, Tohma Y, Kawata T, Fujita T, Kohno S, Ohtani J, Tenjoh K, Nakano M, Kamada H, Tanne K. Influences of reduced masticatory sensory input from soft-diet feeding upon spatial memory/learning ability in mice. Biomed Res. 2007;28:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science. 2020;370:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 574] [Article Influence: 114.8] [Reference Citation Analysis (1)] |
| 52. | Breslin JW, Yang Y, Scallan JP, Sweat RS, Adderley SP, Murfee WL. Lymphatic Vessel Network Structure and Physiology. Compr Physiol. 2018;9:207-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 216] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 53. | Huang X, Hu X, Zhao M, Zhang Q. Analysis of salivary exosomal proteins in young adults with severe periodontitis. Oral Dis. 2020;26:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 54. | Sarmiento LF, Ríos-Flórez JA, Rincón Uribe FA, Rodrigues Lima R, Kalenscher T, Gouveia A Jr, Nitsch FJ. Do stress hormones influence choice? A systematic review of pharmacological interventions on the HPA axis and/or SAM system. Soc Cogn Affect Neurosci. 2024;19:nsae069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 55. | Tank AW, Lee Wong D. Peripheral and central effects of circulating catecholamines. Compr Physiol. 2015;5:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 148] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 56. | Alotiby A. Immunology of Stress: A Review Article. J Clin Med. 2024;13:6394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 57. | Seiler A, Fagundes CP, Christian LM. The Impact of Everyday Stressors on the Immune System and Health. In: Choukèr A, (eds). Stress Challenges and Immunity in Space. Cham: Springer, 2020. [DOI] [Full Text] |
| 58. | Lei Y, Liao F, Tian Y, Wang Y, Xia F, Wang J. Investigating the crosstalk between chronic stress and immune cells: implications for enhanced cancer therapy. Front Neurosci. 2023;17:1321176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 59. | Pedersen AF, Zachariae R, Bovbjerg DH. Psychological stress and antibody response to influenza vaccination: a meta-analysis. Brain Behav Immun. 2009;23:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 60. | Hermans EJ, Henckens MJ, Joëls M, Fernández G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014;37:304-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 615] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 61. | Herrera D, Sanz M, Shapira L, Brotons C, Chapple I, Frese T, Graziani F, Hobbs FDR, Huck O, Hummers E, Jepsen S, Kravtchenko O, Madianos P, Molina A, Ungan M, Vilaseca J, Windak A, Vinker S. Periodontal diseases and cardiovascular diseases, diabetes, and respiratory diseases: Summary of the consensus report by the European Federation of Periodontology and WONCA Europe. Eur J Gen Pract. 2024;30:2320120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 62. | Manoil D, Parga A, Bostanci N, Belibasakis GN. Microbial diagnostics in periodontal diseases. Periodontol 2000. 2024;95:176-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 63. | Meyle J, Chapple I. Molecular aspects of the pathogenesis of periodontitis. Periodontol 2000. 2015;69:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 433] [Article Influence: 54.1] [Reference Citation Analysis (1)] |
| 64. | Leclercq S, Forsythe P, Bienenstock J. Posttraumatic Stress Disorder: Does the Gut Microbiome Hold the Key? Can J Psychiatry. 2016;61:204-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 65. | Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T. Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology. 2017;42:254-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 489] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 66. | Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1671] [Cited by in RCA: 2421] [Article Influence: 269.0] [Reference Citation Analysis (0)] |
| 67. | Arnone D, McKie S, Elliott R, Juhasz G, Thomas EJ, Downey D, Williams S, Deakin JF, Anderson IM. State-dependent changes in hippocampal grey matter in depression. Mol Psychiatry. 2013;18:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 68. | Duric V, Banasr M, Stockmeier CA, Simen AA, Newton SS, Overholser JC, Jurjus GJ, Dieter L, Duman RS. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int J Neuropsychopharmacol. 2013;16:69-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 237] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 69. | Ferland CL, Schrader LA. Regulation of histone acetylation in the hippocampus of chronically stressed rats: a potential role of sirtuins. Neuroscience. 2011;174:104-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 70. | Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1077] [Cited by in RCA: 1113] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 71. | Laforgia A, Corsalini M, Stefanachi G, Pettini F, Di Venere D. Assessment of Psychopatologic Traits in a Group of Patients with Adult Chronic Periodontitis: Study on 108 Cases and Analysis of Compliance during and after Periodontal Treatment. Int J Med Sci. 2015;12:832-839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Umaki TM, Umaki MR, Cobb CM. The psychology of patient compliance: a focused review of the literature. J Periodontol. 2012;83:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 73. | Warren KR, Postolache TT, Groer ME, Pinjari O, Kelly DL, Reynolds MA. Role of chronic stress and depression in periodontal diseases. Periodontol 2000. 2014;64:127-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 74. | Bardow A, Nyvad B, Nauntofte B. Relationships between medication intake, complaints of dry mouth, salivary flow rate and composition, and the rate of tooth demineralization in situ. Arch Oral Biol. 2001;46:413-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 152] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 75. | Misra M, Papakostas GI, Klibanski A. Effects of psychiatric disorders and psychotropic medications on prolactin and bone metabolism. J Clin Psychiatry. 2004;65:1607-18; quiz 1590, 1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 76. | Silva CCG, Dos Santos MS, Monteiro JLGC, de Aguiar Soares Carneiro SC, do Egito Vasconcelos BC. Is there an association between the use of antidepressants and complications involving dental implants? A systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2021;50:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 77. | Harutyunyan L, Lieuw K, Yang B, Lee E, Yeh YT, Chen HH, Lin GH. The Effect of Antidepressants on Dental Implant Failure: A Systematic Review and Meta-analysis. Int J Oral Maxillofac Implants. 2024;39:665-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 78. | Altay MA, Sindel A, Özalp Ö, Yildirimyan N, Kader D, Bilge U, Baur DA. Does the Intake of Selective Serotonin Reuptake Inhibitors Negatively Affect Dental Implant Osseointegration? A Retrospective Study. J Oral Implantol. 2018;44:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 79. | Apatzidou DA. The role of cigarette smoking in periodontal disease and treatment outcomes of dental implant therapy. Periodontol 2000. 2022;90:45-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 80. | Banerjee U, Dhawan P, Rani S, Jain N. Evidence-Based Critical Assessment of the Success Rate of Dental Implants in Smokers: An Umbrella Systematic Review. Cureus. 2024;16:e70067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 81. | Werny JG, Sagheb K, Diaz L, Kämmerer PW, Al-Nawas B, Schiegnitz E. Does vitamin D have an effect on osseointegration of dental implants? A systematic review. Int J Implant Dent. 2022;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 82. | Choukroun J, Khoury G, Khoury F, Russe P, Testori T, Komiyama Y, Sammartino G, Palacci P, Tunali M, Choukroun E. Two neglected biologic risk factors in bone grafting and implantology: high low-density lipoprotein cholesterol and low serum vitamin D. J Oral Implantol. 2014;40:110-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 83. | Rodriguez-Fernandez I, Bretschneider T, Menzel A, Suljevic O, Sommer NG, Weinberg AM, Appel C, Liebi M, Diaz A, Pircher L, Hellmich C, Schwarze UY, Lichtenegger HC, Grünewald TA. Physical exercise impacts bone remodeling around bio-resorbable magnesium implants. Acta Biomater. 2025;193:623-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 84. | Li A, Chen Y, Du M, Deng K, Cui X, Lin C, Tjakkes GE, Zhuang X, Hu S. Healthy lifestyles ameliorate an increased risk of periodontitis associated with polycyclic aromatic hydrocarbons. Chemosphere. 2024;364:143086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 85. | Huang TB, Li YZ, Yu K, Yu Z, Wang Y, Jiang ZW, Wang HM, Yang GL. Effect of the Wnt signal-RANKL/OPG axis on the enhanced osteogenic integration of a lithium incorporated surface. Biomater Sci. 2019;7:1101-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 86. | Karner CM, Long F. Wnt signaling and cellular metabolism in osteoblasts. Cell Mol Life Sci. 2017;74:1649-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 224] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 87. | Li L, Peng X, Qin Y, Wang R, Tang J, Cui X, Wang T, Liu W, Pan H, Li B. Acceleration of bone regeneration by activating Wnt/β-catenin signalling pathway via lithium released from lithium chloride/calcium phosphate cement in osteoporosis. Sci Rep. 2017;7:45204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 88. | Arioka M, Takahashi-Yanaga F, Sasaki M, Yoshihara T, Morimoto S, Hirata M, Mori Y, Sasaguri T. Acceleration of bone regeneration by local application of lithium: Wnt signal-mediated osteoblastogenesis and Wnt signal-independent suppression of osteoclastogenesis. Biochem Pharmacol. 2014;90:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 89. | Chen Y, Whetstone HC, Lin AC, Nadesan P, Wei Q, Poon R, Alman BA. Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med. 2007;4:e249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 309] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 90. | Sağlam M, Köseoğlu S, Hatipoğlu M, Esen HH, Köksal E. Effect of sumac extract on serum oxidative status, RANKL/OPG system and alveolar bone loss in experimental periodontitis in rats. J Appl Oral Sci. 2015;23:33-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 91. | Wu Z, Duan S, Li M, Zhang A, Yang H, Luo J, Cheng R, Hu T. Autophagy regulates bone loss via the RANKL/RANK/OPG axis in an experimental rat apical periodontitis model. Int Endod J. 2024;57:1315-1325. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |