Published online Aug 6, 2025. doi: 10.12998/wjcc.v13.i22.104924
Revised: March 22, 2025
Accepted: April 14, 2025
Published online: August 6, 2025
Processing time: 127 Days and 23.1 Hours
Hepatic epithelioid hemangioendothelioma (HEHE) is a rare malignant vascular liver tumor diagnosed by histopathological evaluation. Standardized treatment is challenging because of its rarity; hepatectomy is preferred for solitary lesions and multiple transplantations. There is no consensus on the optimal treatment for HEHE; however, surgical excision is often considered effective. This report presents a case of initially suspected cholangiocarcinoma or renal cell carcinoma (RCC) metastasis, which was later confirmed as HEHE, with no recurrence during follow-up.
A 52-year-old man with a history of left nephrectomy for RCC presented with an incidentally detected liver mass and nonspecific abdominal discomfort. Imaging revealed a 3-cm centripetal enhancing lesion in the right hepatic dome with indeterminate malignant potential. The patient underwent a laparoscopic right anterior sectionectomy and remained recurrence-free without complications during the 3-year follow-up period.
Managing HEHE is challenging. Accurate diagnosis and surgical options, such as resection or transplantation, are essential with tailored multidisciplinary follow-up. The authors have read the CARE Checklist (2016) and the manuscript was prepared and revised according to the CARE Checklist (2016).
Core Tip: Hepatic epithelioid hemangioendothelioma (HEHE) is a rare vascular tumor often misdiagnosed as other malignancies. This case highlights the role of surgical resection not only for definitive diagnosis but also for effective management of resectable HEHE, achieving long-term recurrence-free outcomes and ensuring safe follow-up.
- Citation: Shin SH, Koh YS, Song S. Hepatic epithelioid hemangioendothelioma managed with minimally invasive surgery: A case report. World J Clin Cases 2025; 13(22): 104924
- URL: https://www.wjgnet.com/2307-8960/full/v13/i22/104924.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i22.104924
Hepatic tumors are classified as benign or malignant. Hepatic hemangioma is the most common benign liver tumor, with an estimated prevalence of 0.4% and 7.3%[1]. In contrast, hepatocellular carcinoma (HCC) constitutes 75%-85% of all malignant liver tumors and is the leading cause of liver cancer-related morbidity and mortality[2]. Among rare hepatic malignancies, hepatic epithelioid hemangioendothelioma (HEHE) is a low-grade malignant vascular tumor first described by Weiss and Enzinger in 1982[3].
HEHE is an uncommon condition with an incidence of approximately 1-2 cases per million individuals, it is an uncommon condition[4-6]. HEHE primarily affects adults in the third to fifth decades of life, with a slight predominance among women[5,7].
Clinically, HEHE presents differently, ranging from asymptomatic cases to cases with symptoms such as right upper abdominal pain, hepatomegaly, and non-specific weight loss[4,8,9]. Imaging studies, including ultrasound, computed tomography (CT), Magnetic resonance imaging (MRI), and positron emission tomography-CT (PET-CT), are crucial for identifying HEHE and differentiating it from other hepatic malignancies[4,10]. However, radiological findings often overlap with more common conditions, such as cholangiocarcinoma and metastatic disease, necessitating histopathological confirmation. Immunohistochemical analysis of biopsy specimens has demonstrated that endothelial markers such as ERG, CD31, and CD34 are critical for the diagnosis[11,12].
The management of HEHE remains challenging owing to its rarity and variable disease course. Given the absence of standardized treatment protocols, individualized therapeutic strategies using multidisciplinary approaches are essential to optimize patient outcomes.
Surgical resection is the treatment of choice for localized resectable lesions, and offers excellent survival outcomes. Among the surgical methods, minimally invasive surgery (MIS), particularly laparoscopic hepatectomy, has been gaining increasing attention owing to its advantages over open surgery, including shorter hospital stay, lower morbidity, and fewer minor postoperative complications[13]. Additionally, liver transplantation has emerged as a viable option[4,5,8].
Non-surgical treatments include radiotherapy, chemotherapy, transcatheter arterial chemoembolization (TACE), antiangiogenic drugs, and locoregional ablation. TACE combined with immunotherapy may have a synergistic effect. Currently, chemotherapy and vascular endothelial growth factor targeted therapies are under investigation[4].
This case report presents a patient initially suspected of having cholangiocarcinoma or metastatic RCC, in whom laparoscopic liver resection enabled both definitive diagnosis and successful treatment of HEHE. It underscores the importance of a multidisciplinary approach involving radiology, pathology, surgery, and oncology, as well as the need for further multicenter studies and genetic analyses to establish more effective treatment strategies.
A 52-year-old man presented to our hospital with a 3 cm hepatic mass that was incidentally detected during routine surveillance following nephrectomy for RCC.
The patient was asymptomatic at presentation and underwent routine follow-up evaluations for RCC at a local hospital. Laboratory findings were unremarkable. However, abdominal CT revealed a suspicious hepatic lesion, which prompted further evaluation using abdominal MRI. PET-CT was performed to assess systemic disease and distant metastasis, confirming a localized hepatic mass. The patient was then referred to our hospital for further evaluation and manage
The patient was diagnosed with hypertension and diabetes mellitus 5 years ago. Hypertension was well controlled with antihypertensive medications (mean systolic blood pressure, 140 mmHg), and diabetes was adequately managed with oral hypoglycemic agents (HbA1c, 5.9%). In addition, the patient had a history of left nephrectomy for RCC.
The patient has a family history of hypertension and diabetes mellitus.
On examination, the patient’s abdomen was soft and non-tender with no palpable abnormalities.
Routine laboratory tests revealed normal findings.
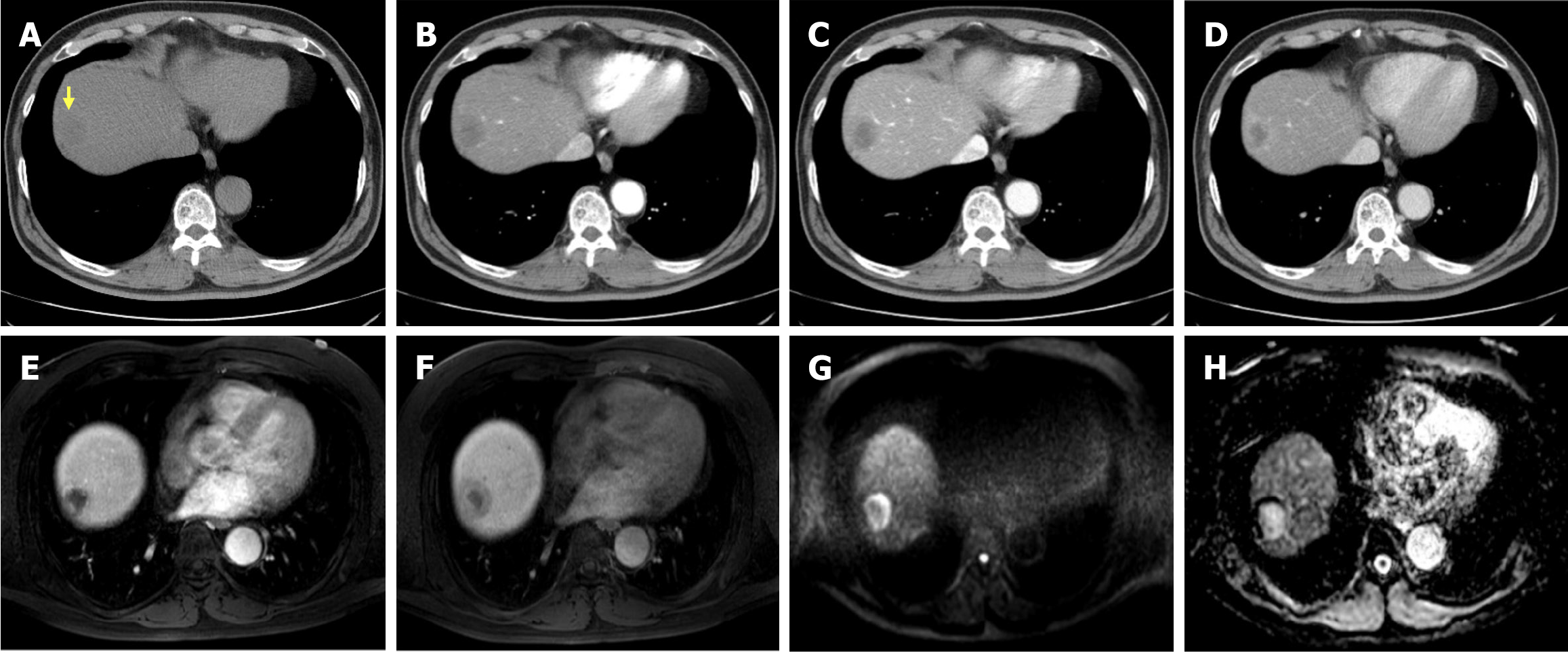
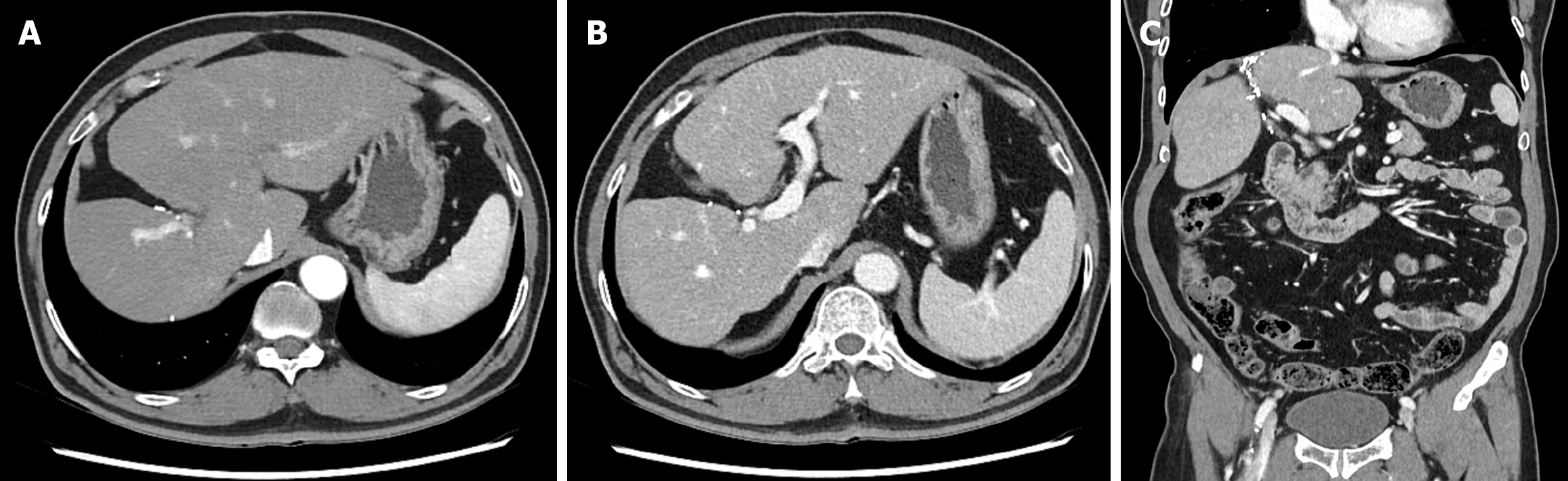
Abdominal contrast-enhanced CT and MRI demonstrated a 3 cm centripetal enhancing lesion located in the right hepatic dome, raising the suspicion of a malignant lesion such as cholangiocarcinoma or recurrent RCC metastasis. The lesion exhibited ill-defined margins without any evidence of major vessel invasion or distant metastasis (Figure 1).
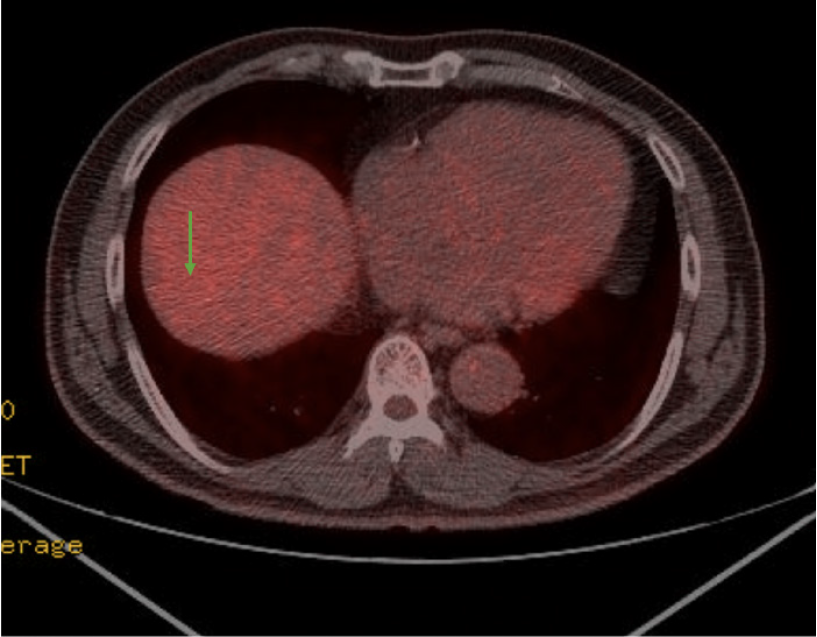
Torso PET-CT findings were more suggestive of a benign mass than a malignancy, with no metabolic evidence of local recurrence or regional lymph node involvement (Figure 2).
The initial preoperative diagnosis was either cholangiocarcinoma or recurrent RCC carcinoma. However, the posto
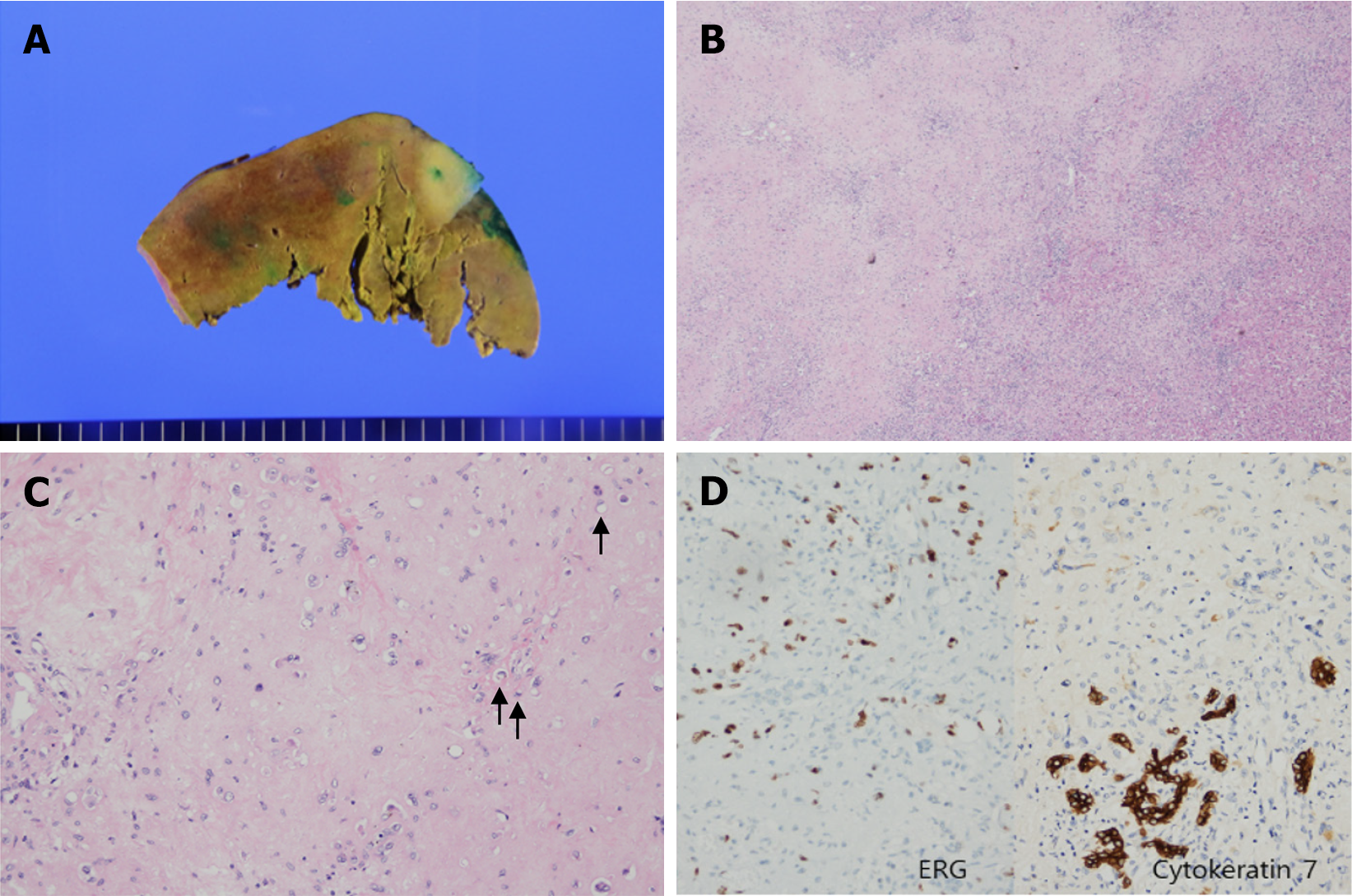
Although genetic analysis could not be performed due to insurance limitations, immunohistochemical staining of the tissue biopsy supported the diagnosis. The tumor tested positive for vascular endothelial markers (ERG, CD31, CD34, and FLI1), confirming its vascular origin. Additionally, CK7 positivity suggested a resemblance to the intrahepatic bile ducts. Focal positivity for TFE3 raised the possibility of YAP-TFE3 fusion. Based on these findings, a diagnosis of HEHE was considered appropriate.
Based on imaging findings, a 3 cm liver mass was identified in segment 8 (Figures 1 and 2), with no evidence of distant metastasis to the abdomen or chest. Based on these findings, a radical resection was performed. Considering the advantages of minimally invasive surgery, a laparoscopic right anterior sectionectomy was performed.
To achieve safe and effective resection, a thorough understanding of the key surgical principles and meticulous techniques is essential. Laparoscopic right anterior sectionectomy involves resection of segments V and VIII and requires precise vascular and biliary control. The key surgical points include proper liver mobilization, identification and ligation of the right anterior Glissonian pedicle, and meticulous parenchymal transection while preserving the right posterior sector and hepatic veins. Crucial steps involve intraoperative ultrasonography for vascular mapping, maintenance of low central venous pressure to minimize bleeding, and ensuring bile duct integrity to prevent postoperative bile leaks. Excessive traction of the liver, misidentification of the right anterior pedicle, and inadvertent injury to the middle hepatic vein prevent hemorrhage and functional impairment of the remaining liver.
On postoperative day 1, the patient experienced acute myocardial infarction and underwent coronary angiography with stent placement, which required anticoagulation therapy.
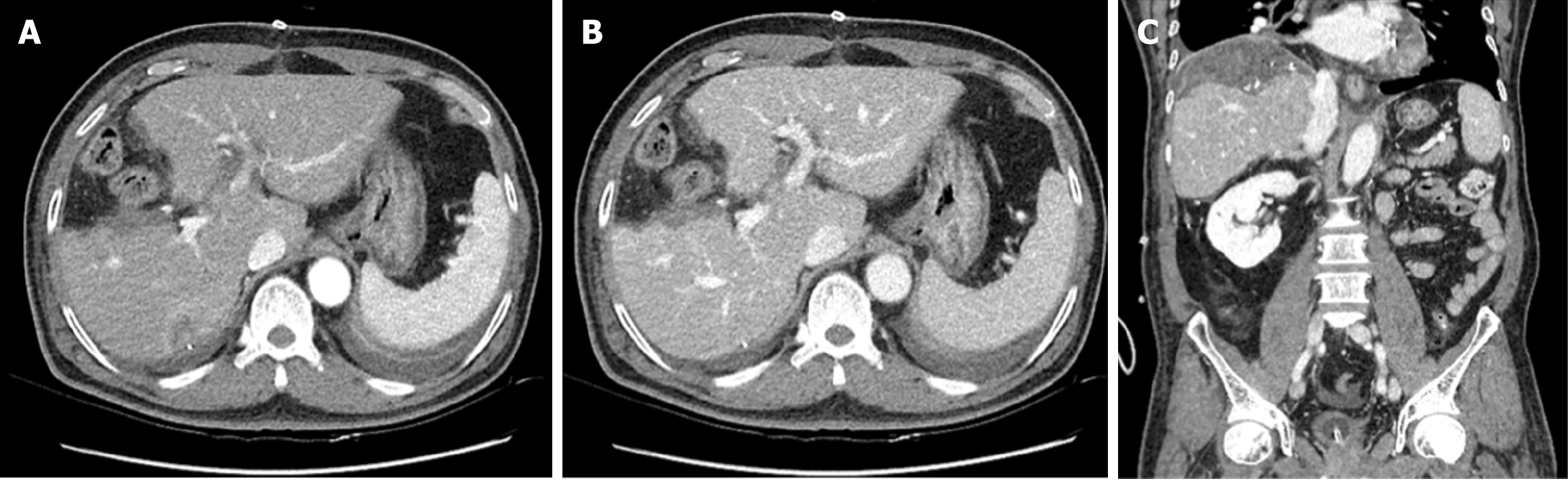
On postoperative day 5, follow-up abdominal dynamic CT revealed typical postoperative changes after laparoscopic anterior sectionectomy (Figure 4).
The patient developed wound pain, along with mild pulmonary and peripheral edema, which was successfully managed with analgesics and diuretics. No significant complications were noted and the patient recovered well. Histopathological examination confirmed the diagnoses of HEHE and fatty liver disease. The patient was discharged in a stable condition on postoperative day 12.
Following discharge, the patient was monitored with regular follow-ups, including CT and laboratory tests, performed every 3-4 months during the first year and subsequently at 6-month intervals. Over the 3-year follow-up period, there was no evidence of liver recurrence or postoperative complications (Figure 5), and the patient remained in good health.
HEHE is a rare malignancy of vascular origin that presents challenges in diagnosis and management because of its clinical heterogeneity and rarity[6]. Accurate diagnosis relies on imaging techniques, including ultrasonography, CT, MRI, and PET-CT, which help identify multifocal intrahepatic lesions, centripetal enhancement, and intratumoral calcifications[8,10]. However, radiological overlap with conditions such as cholangiocarcinoma or metastatic disease necessitates histopathological confirmation, particularly when endothelial markers such as ERG, CD31, and CD34[12,14].
Given the diagnostic complexity of HEHE, distinguishing it from other hepatic tumors is essential. Hepatic tumors exhibit distinct imaging features, immunohistochemical (IHC) markers, and molecular characteristics. HEHE typically presents with target sign, lollipop sign, and capsular retraction on imaging. IHC staining was positive for CD31, CD34, CD10, vimentin, and Factor VIII antigens, while molecular analysis revealed WWTR1-CAMTA1 and YAP1-TFE3 fusion genes. Similarly, angiosarcoma is characterized by heterogeneous centripetal enhancement on imaging and IHC positivity for CD31, CD34, and Factor VIII antigens. Molecular studies have identified mutations in HRAS, KRAS, NRAS, and PTPRB. Cholangiocarcinomas typically present with biliary duct dilation and capsular retraction on imaging, with pan-cytokeratin as a key IHC marker. Additionally, HCC exhibits hyperechoic enhancement in the arterial phase and hypoechoic enhancement in the portal and delayed phases, with HepPar-1 and pan-cytokeratin as commonly used IHC markers. These imaging and molecular characteristics play crucial roles in distinguishing hepatic tumors, aiding in accurate diagnosis and guiding appropriate management strategies[15].
Treatment strategies for HEHE are not yet standardized and are broadly divided into surgical approaches, such as liver resection (LR) and liver transplantation (LT), and non-surgical options, including chemotherapy and observation.
Complete tumor resection is the preferred treatment for localized HEHE, with reported 1-year and 5-year overall survival (OS) rates of 86.6% and 75.2%, respectively. However, oncological resection is often unfeasible because of multicenter lesions and anatomical challenges. In such cases, LT offers an alternative, with 1-year and 5-year OS rates of 96% and 54.5%, respectively[16].
Although surgical resection is the preferred treatment, Kaltenmeier et al[17], analyzed treatment outcomes for HEHE in the United States from 2004 to 2018, comparing LT and LR. LT resulted in a longer median survival (111 months) than LR (69 months) and was associated with a significant survival benefit (HR: 0.61, P = 0.035).
Chahrour et al[16], analyzed 353 patients with HEHE from the SEER database (2004–2016) and found that surgical treatment significantly improved survival outcomes compared with non-surgical management. The 5-year overall survival rates were 75.2% for surgical and 37.4% for nonsurgical groups, respectively, with surgery identified as a strongly favorable prognostic factor (HR: 0.404, P = 0.005). Conversely, advanced age (> 65 years) and tumor size (> 10 cm) were associated with poor outcomes. These findings highlight the critical role of surgical intervention in the management.
Furthermore, Sawma et al[18] from the Mayo Clinic emphasized the superiority of surgical treatments, including LR and LT, in achieving better long-term survival than non-surgical management. While previous studies have suggested that extrahepatic metastasis is not a contraindication for surgery, bone metastasis has been identified as a significant predictor of poor outcomes and proposed as a contraindication for surgical interventions, unlike non-bony metastases confined to the lungs.
Recent advancements in MIS such as laparoscopic and robot-assisted hepatectomy have expanded the treatment options for HEHE. MIS offers significant advantages over open surgery including reduced intraoperative blood loss, shorter recovery periods, and improved postoperative outcomes[19-21]. Although data remain limited due to the rarity of the disease, this case demonstrates the successful application of laparoscopic anterior sectionectomy, highlighting the feasibility and evolving role of MIS in the management of HEHE.
For patients unsuitable for surgery, alternative therapies, including TACE and systemic treatment with agents such as interferon-alpha (IFN), sunitinib, sorafenib, and bevacizumab, have shown promise. The combination of TACE and IFN may synergistically inhibit angiogenesis and slow tumor progression[4,12]. However, these approaches remain under investigation and require further validation in larger studies.
In one reported case, a patient with unresectable HEHE underwent TACE combined with systemic therapy, which resulted in partial tumor regression and stabilization of disease progression. These findings highlight the potential utility of non-surgical therapies when surgery is not feasible; however, further studies are required to optimize treatment sequencing and combination strategies[22].
Cao et al[23], highlighted the potential role of the initial follow-up period in managing HEHE, emphasizing its indolent nature. Their study observed that patients who underwent surveillance without immediate treatment for approximately 1-3 months showed stable disease and favorable outcomes, suggesting that initial observation may be an effective strategy for assessing tumor biology before deciding on further interventions. If progression is noted during follow-up, definitive treatments such as liver resection or transplantation can be pursued based on the disease extent and patient condition.
Given the marked heterogeneity of HEHE, a multidisciplinary approach involving hepatologists, surgeons, radio
This study is limited by its single-case nature, which may not fully represent the broader patient population with HEHE. Furthermore, the lack of a multidisciplinary approach in the diagnostic and therapeutic decision-making process represents an additional limitation. Future multicenter studies with larger cohorts and integrated multidisciplinary collaboration are necessary to validate and expand upon the findings presented here.
The management of HEHE remains challenging because of its rarity and diverse clinical presentations. While stan
| 1. | Mocchegiani F, Vincenzi P, Coletta M, Agostini A, Marzioni M, Baroni GS, Giovagnoni A, Guerrieri M, Marmorale C, Risaliti A, Vivarelli M. Prevalence and clinical outcome of hepatic haemangioma with specific reference to the risk of rupture: A large retrospective cross-sectional study. Dig Liver Dis. 2016;48:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Qiu S, Cai J, Yang Z, He X, Xing Z, Zu J, Xie E, Henry L, Chong CR, John EM, Cheung R, Ji F, Nguyen MH. Trends in Hepatocellular Carcinoma Mortality Rates in the US and Projections Through 2040. JAMA Netw Open. 2024;7:e2445525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 3. | Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer. 1982;50:970-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 4. | Zhao M, Yin F. Hepatic epithelioid hemangioendothelioma: Clinical characteristics, diagnosis, treatment, and prognosis. World J Clin Cases. 2022;10:5606-5619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Virarkar M, Saleh M, Diab R, Taggart M, Bhargava P, Bhosale P. Hepatic Hemangioendothelioma: An update. World J Gastrointest Oncol. 2020;12:248-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 6. | Mehrabi A, Kashfi A, Fonouni H, Schemmer P, Schmied BM, Hallscheidt P, Schirmacher P, Weitz J, Friess H, Buchler MW, Schmidt J. Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer. 2006;107:2108-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 308] [Article Influence: 16.2] [Reference Citation Analysis (1)] |
| 7. | Sardaro A, Bardoscia L, Petruzzelli MF, Portaluri M. Epithelioid hemangioendothelioma: an overview and update on a rare vascular tumor. Oncol Rev. 2014;8:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 8. | Lerut JP, Orlando G, Adam R, Schiavo M, Klempnauer J, Mirza D, Boleslawski E, Burroughs A, Sellés CF, Jaeck D, Pfitzmann R, Salizzoni M, Söderdahl G, Steininger R, Wettergren A, Mazzaferro V, Le Treut YP, Karam V; European Liver Transplant Registry. The place of liver transplantation in the treatment of hepatic epitheloid hemangioendothelioma: report of the European liver transplant registry. Ann Surg. 2007;246:949-57; discussion 957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Miller WJ, Dodd GD 3rd, Federle MP, Baron RL. Epithelioid hemangioendothelioma of the liver: imaging findings with pathologic correlation. AJR Am J Roentgenol. 1992;159:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 124] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Ganeshan D, Pickhardt PJ, Morani AC, Javadi S, Lubner MG, Elmohr MM, Duran C, Elsayes KM. Hepatic hemangioendothelioma: CT, MR, and FDG-PET-CT in 67 patients-a bi-institutional comprehensive cancer center review. Eur Radiol. 2020;30:2435-2442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Makhlouf HR, Ishak KG, Goodman ZD. Epithelioid hemangioendothelioma of the liver: a clinicopathologic study of 137 cases. Cancer. 1999;85:562-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Stacchiotti S, Miah AB, Frezza AM, Messiou C, Morosi C, Caraceni A, Antonescu CR, Bajpai J, Baldini E, Bauer S, Biagini R, Bielack S, Blay JY, Bonvalot S, Boukovinas I, Bovee JVMG, Boye K, Brodowicz T, Callegaro D, De Alava E, Deoras-Sutliff M, Dufresne A, Eriksson M, Errani C, Fedenko A, Ferraresi V, Ferrari A, Fletcher CDM, Garcia Del Muro X, Gelderblom H, Gladdy RA, Gouin F, Grignani G, Gutkovich J, Haas R, Hindi N, Hohenberger P, Huang P, Joensuu H, Jones RL, Jungels C, Kasper B, Kawai A, Le Cesne A, Le Grange F, Leithner A, Leonard H, Lopez Pousa A, Martin Broto J, Merimsky O, Merriam P, Miceli R, Mir O, Molinari M, Montemurro M, Oldani G, Palmerini E, Pantaleo MA, Patel S, Piperno-Neumann S, Raut CP, Ravi V, Razak ARA, Reichardt P, Rubin BP, Rutkowski P, Safwat AA, Sangalli C, Sapisochin G, Sbaraglia M, Scheipl S, Schöffski P, Strauss D, Strauss SJ, Sundby Hall K, Tap WD, Trama A, Tweddle A, van der Graaf WTA, Van De Sande MAJ, Van Houdt W, van Oortmerssen G, Wagner AJ, Wartenberg M, Wood J, Zaffaroni N, Zimmermann C, Casali PG, Dei Tos AP, Gronchi A. Epithelioid hemangioendothelioma, an ultra-rare cancer: a consensus paper from the community of experts. ESMO Open. 2021;6:100170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 113] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 13. | Kasai M, Cipriani F, Gayet B, Aldrighetti L, Ratti F, Sarmiento JM, Scatton O, Kim KH, Dagher I, Topal B, Primrose J, Nomi T, Fuks D, Abu Hilal M. Laparoscopic versus open major hepatectomy: a systematic review and meta-analysis of individual patient data. Surgery. 2018;163:985-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 14. | Nudo CG, Yoshida EM, Bain VG, Marleau D, Wong P, Marotta PJ, Renner E, Watt KD, Deschênes M. Liver transplantation for hepatic epithelioid hemangioendothelioma: the Canadian multicentre experience. Can J Gastroenterol. 2008;22:821-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Kou K, Chen YG, Zhou JP, Sun XD, Sun DW, Li SX, Lv GY. Hepatic epithelioid hemangioendothelioma: Update on diagnosis and therapy. World J Clin Cases. 2020;8:3978-3987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (3)] |
| 16. | Chahrour MA, Khachfe HH, Habib JR, El-Asmar R, Saifi O, Jamali FR. Treatment and Prognosis of Hepatic Epithelioid Hemangioendothelioma: A SEER Database Analysis. World J Surg. 2021;45:2886-2894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Kaltenmeier C, Stacchiotti S, Gronchi A, Sapisochin G, Liu H, Ashwat E, Gunabushanam V, Reddy D, Thompson A, Geller D, Tohme S, Zureikat A, Molinari M. Treatment modalities and long-term outcomes of hepatic hemangioendothelioma in the United States. HPB (Oxford). 2022;24:1688-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Sawma T, Sultan A, Abdulmoneim S, Grotz T, Rosen CB, Taner T, Heimbach JK, Warner SG, Siontis BL, Ho TP, Robinson SI, Thiels CA. Management and Long-Term Outcomes of Patients With Hepatic Epithelioid Hemangioendothelioma. J Surg Oncol. 2024;130:1062-1069. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Xu J, Hu S, Li S, Wang W, Zhou X, Wu Y, Su Z, Cheng X, Gao Y, Zheng Q. Laparoscopic resection of hepatic epithelioid hemangioendothelioma: report of eleven rare cases and literature review. World J Surg Oncol. 2020;18:282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Hu L, Yao L, Li X, Jin P, Yang K, Guo T. Effectiveness and safety of robotic-assisted versus laparoscopic hepatectomy for liver neoplasms: A meta-analysis of retrospective studies. Asian J Surg. 2018;41:401-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Stewart C, Fong Y. Robotic liver surgery—advantages and limitations. Eur Surg. 2021;53:149-157. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Lau A, Malangone S, Green M, Badari A, Clarke K, Elquza E. Combination capecitabine and bevacizumab in the treatment of metastatic hepatic epithelioid hemangioendothelioma. Ther Adv Med Oncol. 2015;7:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Cao L, Hong J, Zhou L, Ye Y, Liu Y, Yu J, Zheng S. Selection of treatment for hepatic epithelioid hemangioendothelioma: a single-center experience. World J Surg Oncol. 2019;17:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 24. | Atyah MM, Sun Y, Yang Z. The challenges of hepatic epithelioid hemangioendothelioma: the diagnosis and current treatments of a problematic tumor. Orphanet J Rare Dis. 2024;19:449. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |