Published online Aug 6, 2025. doi: 10.12998/wjcc.v13.i22.104643
Revised: March 21, 2025
Accepted: April 14, 2025
Published online: August 6, 2025
Processing time: 138 Days and 22 Hours
Serous carcinoma of the uterine cervix (USCC) represents a rare subtype of cervical adenocarcinoma, classified into human papillomavirus (HPV)-inde
A 58-year-old Chinese woman presented with painless vaginal bleeding after sexual intercourse, which appeared as droplets. HPV testing and histopathological analysis confirmed the diagnosis of HPV-associated primary serous carcinoma of the USCC. The patient underwent radical hysterectomy and was diagnosed with primary serous carcinoma of the uterine cervix, stage III C2 (FIGO 2018). A multimodal treatment approach, including surgery, radiotherapy, and chemotherapy, was administered. After additional concurrent chemoradiotherapy and three cycles of chemotherapy, the patient showed no evidence of disease progression and achieved long-term survival for 53 months.
USCC is a rare and aggressive malignancy. Upon diagnosis, multimodal treat
Core Tip: A 58-year-old Chinese woman presented with painless vaginal bleeding that appeared as droplets after sexual intercourse. Based on human papillomavirus (HPV) testing and pathology results after radical hysterectomy, the patient was diagnosed with primary advanced HPV-associated primary serous carcinoma of the uterine cervix (USCC). A multimodal treatment approach incorporating surgery, radiotherapy, and chemotherapy was used. The patient showed no evidence of disease progression and achieved a long-term survival of 53 months. USCC is a rare, invasive disease; moreover, comprehensive treatment can prolong patient survival and improve prognosis.
- Citation: Huang HQ, Yang L, Li QL, Sun CT, Gong FM. Human papillomavirus associated serous carcinoma of the uterine cervix in a patient with long-term survival: A case report. World J Clin Cases 2025; 13(22): 104643
- URL: https://www.wjgnet.com/2307-8960/full/v13/i22/104643.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i22.104643
Serous carcinoma of the uterine cervix (USCC) is a rare subtype of cervical adenocarcinoma, accounting for approximately 0.4% of all cervical cancers[1]. It is characterized by a rapid growth rate and poor outcomes, particularly in the advanced stages of the disease. USCC is usually regarded as a morphological variant of human papillomavirus (HPV)-associated endocervical adenocarcinoma or as a metastasis from serous carcinoma of the upper genital tract, including the fallopian tubes, ovaries, endometrium, and peritoneum[2]. In our case, no malignant lesions were observed in the endometrium or adnexa; therefore, the patient was diagnosed with primary USCC. This report, outlines the pathological diagnosis and the detailed treatment of a patient with USCC who achieved long-term survival of 53 months.
In April 2020, a 58-year-old Chinese woman was admitted to our hospital with painless vaginal bleeding after sexual intercourse and presented with droplets.
The patient has been menopausal for 8 years.
She reported no history of hypertension or any other medical conditions.
No personal or family history was available.
During the gynecological examination, the patient presented with vulvar hypopigmentation, atrophy, and a smooth vaginal mucosa. Examination of the uterine cervix revealed a small, cauliflower-like mass measuring approximately 4 cm in diameter. The cervix was mobile and had a crisp texture, with atrophy noted in the shallow vaginal fornix. No palpable abnormalities were detected in the bilateral adnexal regions.
HPV testing was positive for HPV types 18 and 68. Histopathological examination of a cervical biopsy revealed a poorly differentiated malignant cervical tumor. Serum cancer antigen test results were negative. The patient’s laboratory data on admission, including blood counts and kidney and liver function, were within normal limits.
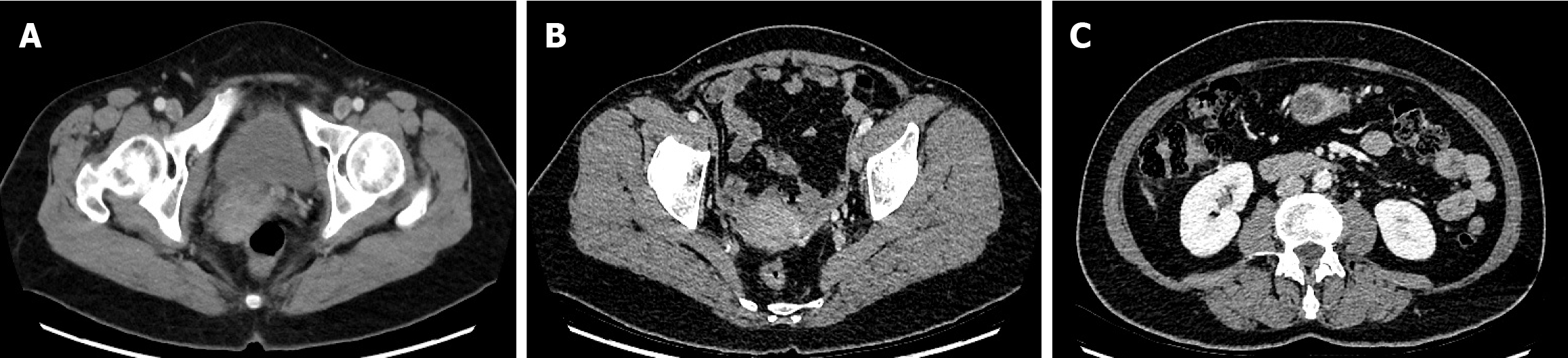
Pelvic computed tomography with intravenous contrast revealed a slightly enlarged cervix (3.3 cm in diameter), with an uneven reinforcement area and blurred margins, but no metastases to distant organs or lymph nodes (Figure 1).
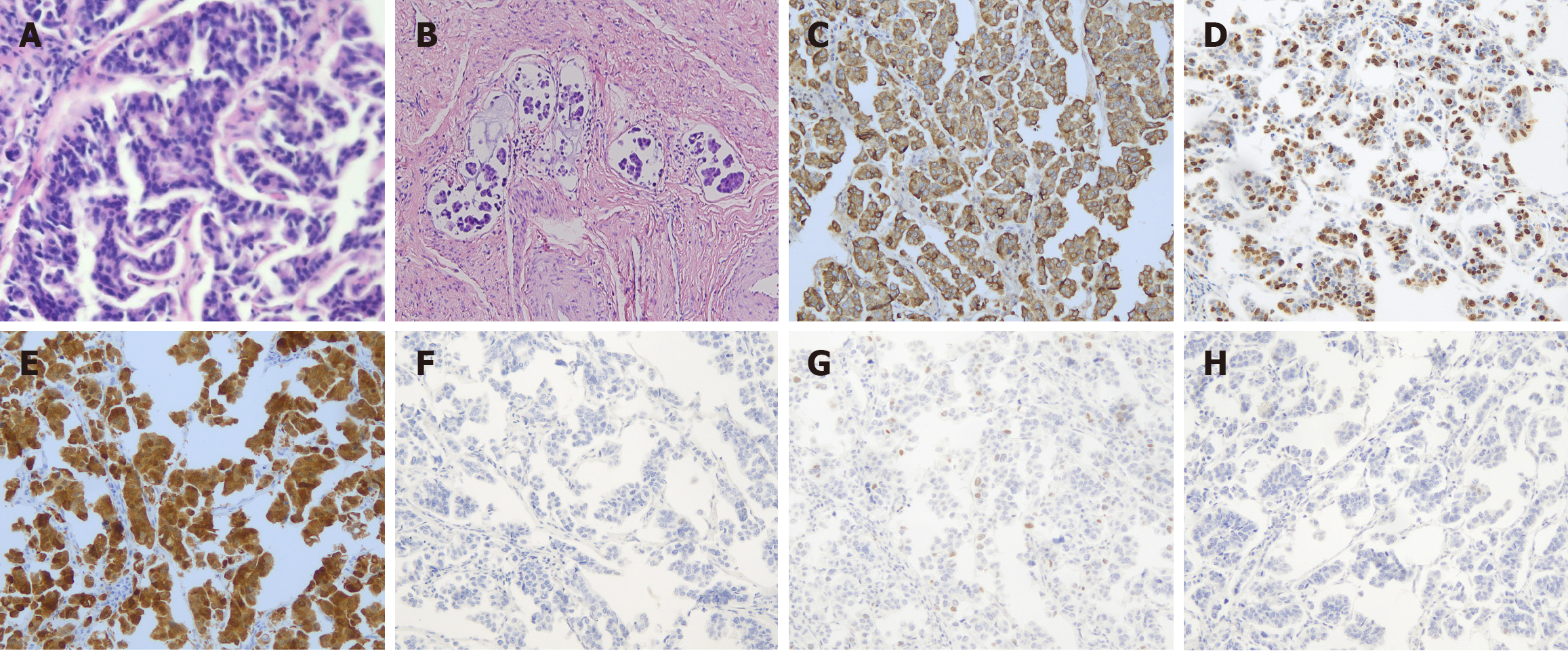
On June 12, 2020, the patient underwent a radical hysterectomy, bilateral oophorectomy, pelvic lymph node dissection, and abdominal aortic lymph node sampling. Hematoxylin and eosin staining revealed a high-grade serous carcinoma with atypical epithelial cells arranged in an adenoidal or micropapillary configuration. The tumor cells were epithelioid, round, or polygonal; had an abundant cytoplasm; were eosinophilic or transparent; and contained vacuoles and indistinct cell borders. The nuclei were round or polygonal with thickened nuclear membranes, had unevenly distributed chromatin blocks, and contained single or multiple prominent nucleoli. Micropapillary components form numerous cancer emboli within the blood vessels. Immunohistochemistry revealed diffuse positive staining of P16, CK7, CEA, CA125, CD15, and Ki67. However, immunohistochemistry for WT-1, P53, CK20, Pax-8, Vimentin, ER, PR, Napsin-A, and HNF-1β yielded negative results. In the micropapillary region, EMA expression was enhanced at the periphery of cell clusters (Figure 2). The final pathological diagnosis of the surgical specimen revealed a tumor measuring 3 cm in diameter with invasion into the deep one-third layer of the cervical stroma, lymphovascular space invasion, and positive margins in the vaginal cuff and pelvic wall, along with pelvic and para-aortic lymph node metastases. According to the FIGO 2018 stage revision, the diagnoses was serous carcinoma of the USCC, stage III C2.
The patient received adjuvant treatment with concurrent chemoradiotherapy. External pelvic radiotherapy was administered at a dose of 45 Gy, fractionated into 25 doses of 1.8 Gy each, targeting the entire pelvis and para-aortic regions. The dose to the vaginal cuff and pelvic wall was 60 Gy, divided into 27 fractions (2.2 Gy), using an intensity-modulated radiation therapy technique with concurrent weekly 40 mg/m2 cisplatin chemotherapy. Intravaginal brachytherapy was administered using high-dose-rate iridium-192 (192Ir) at a total dose of 12 Gy, fractionated into two doses of 6 Gy each. Following concurrent chemoradiotherapy, the patient received three cycles of chemotherapy with carboplatin AUC5 and paclitaxel 175 mg/m2.
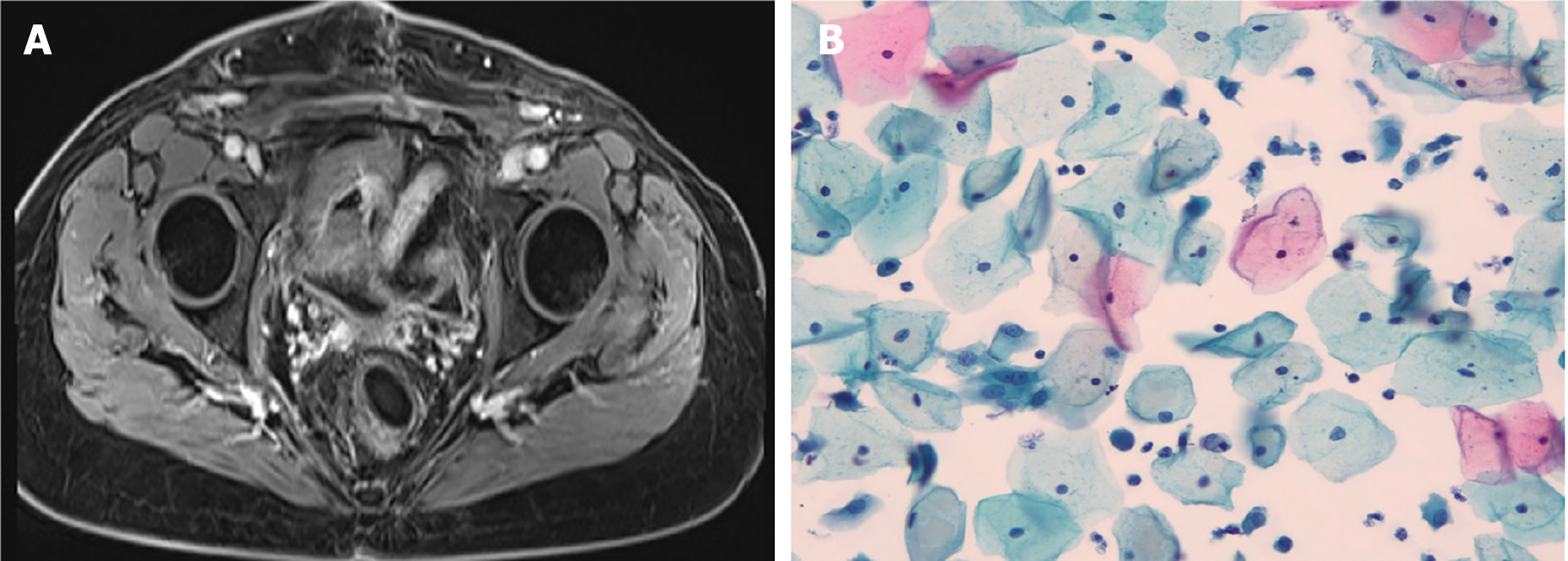
The patient tolerated the radiotherapy well, experiencing only moderate nausea and vomiting, along with grade 4 myelosuppression [according to the Radiation Therapy Oncology Group (RTOG) scale]. At the end of treatment, the patient had grade 1 diarrhea [per the Common Terminology Criteria for Adverse Events (CTCAE)], which required no medication. Six weeks after radiotherapy, the patient was in good condition [Karnofsky Performance Status (KPS) 80%], with all symptoms, including diarrhea, having resolved. The patient experienced tolerable vaginal pain, and bowel movements and defecation have been normal since the completion of radiotherapy. The patient was provided with a vaginal dilator kit and instructed to use it three to four times per week to prevent vaginal stenosis. Follow-up evaluations were conducted every 3 months. The patient remained under clinical follow-up for 53 months without evidence of relapse (Figure 3).
Primary serous carcinoma of the USCC is characterized by morphological features including complex papillary and/or micropapillary structures, as well as high-grade nuclear atypia[3]. USCC was classified in 2020 by the World Health Organization under the Classification of Tumors-Female Genital Tumors, and it was subdivided into HPV-associated and HPV-independent tumors. The age of patients with USCC has two peaks: Before 40 years of age and after the age of 54[4]. The most common symptoms are abnormal vaginal bleeding, polypoid masses, watery vaginal discharge, and no abnormalities on gynecological examination[1].
From a pathological perspective, this type of adenocarcinoma is characterized by extreme polarity reversal. Bright and empty intercellular spaces were observed around cell clusters. Immunohistochemistry has shown that MUC-1 is highly expressed linearly around tumor cell clusters, whereas E-cadherin is absent in the adjacent interstitial part of the tumor cells[5]. In most cases, EMA shows an obvious polarity reversal, i.e. There is no staining in the cytoplasm or between the tumor cells, and it is expressed only in the adjacent interstitial part of the cell clusters, outlining the papillary contour[6]. Serous adenocarcinomas of the female reproductive system are commonly associated with primary lesions of the endometrium and ovaries. Diagnosis based on a combination of history, imaging results, and immunohistochemical expression patterns is not difficult. Serous adenocarcinomas are generally highly invasive and tend to form vascular tumor thrombi and lymph node metastases. The mechanism of tumor development may be related to epithe
Due to limited knowledge regarding the pathology of USCC, treatment guidelines for USCC have yet to be established. Most patients undergo surgery, and radiotherapy is often used as an adjuvant therapy, but not as an initial therapy. Chemotherapy is sometimes selected[8-10]. Primary surgical therapy may be preferable to primary radiotherapy for early-stage USCC[8]. The initial treatment decision depends on the disease status, including primary lesions and lymph node metastasis. USCC may not be an independent prognostic factor when considering stage, tumor size, depth of invasion, and presence of lymph node metastases. USCC without lymph node metastasis may be potentially curable, while lymph node metastasis is often associated with poor prognosis. USCC with lymph node metastasis should be treated using multimodality therapy. Advanced clinical stages of USCC might benefit from a multimodality therapy combining primary surgery, adjuvant radiotherapy for local control and chemotherapy for distant control. In the present case, the patient treated with multimodal therapy had prolonged survival despite pelvic and para-aortic lymph node metastases, and the prognosis was unexpectedly favorable. Nearly all patients with USCC exhibit abnormally strong and diffuse p53 expression, mutant p53 may be an attractive target for therapy in drug form[2] due to the lack of hormone receptor expression in most USCC cases, patients with this entity can be safely cured with hormone replacement therapy[11,12]. Immune checkpoint inhibitors, such as nivolumab and pembrolizumab, may also play important roles in maintenance therapy in patients with recurrent, metastatic, or persistent disease after chemotherapy[13].
In conclusion, we salvaged patients with USCC using multimodal therapy, even those with lymph node metastasis and peritoneal dissemination, with aggressive multimodal therapy. Owing to the extreme rarity of USCC, further accumulation of cases is required.
| 1. | Kitade S, Ariyoshi K, Taguchi K, Maenohara S, Tomita Y, Sonoda K, Okadome M, Saito T. Serous carcinoma of the uterine cervix: Clinicopathological features differing from serous carcinomas of other female organs. J Obstet Gynaecol Res. 2020;46:153-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Shintaku M, Ueda H. Serous papillary adenocarcinoma of the uterine cervix. Histopathology. 1993;22:506-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Höhn AK, Brambs CE, Hiller GGR, May D, Schmoeckel E, Horn LC. 2020 WHO Classification of Female Genital Tumors. Geburtshilfe Frauenheilkd. 2021;81:1145-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 156] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 4. | Khan M, Gilman AD, Nizami S, Barbaryan A, Ali AM, Mirrakhimov AE. Papillary serous carcinoma of the uterine cervix with lung metastasis. Case Rep Oncol Med. 2014;2014:683103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Cserni G. Reversed polarity of the glandular epithelial cells in micropapillary carcinoma of the large intestine and the EMA/MUC1 immunostain. Pathology. 2014;46:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Stewart CJR, Koay MHE, Leslie C, Acott N, Leung YC. Cervical carcinomas with a micropapillary component: a clinicopathological study of eight cases. Histopathology. 2018;72:626-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Gruel N, Benhamo V, Bhalshankar J, Popova T, Fréneaux P, Arnould L, Mariani O, Stern MH, Raynal V, Sastre-Garau X, Rouzier R, Delattre O, Vincent-Salomon A. Polarity gene alterations in pure invasive micropapillary carcinomas of the breast. Breast Cancer Res. 2014;16:R46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Zhou C, Gilks CB, Hayes M, Clement PB. Papillary serous carcinoma of the uterine cervix: a clinicopathologic study of 17 cases. Am J Surg Pathol. 1998;22:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Togami S, Kasamatsu T, Sasajima Y, Onda T, Ishikawa M, Ikeda S, Kato T, Tsuda H. Serous adenocarcinoma of the uterine cervix: a clinicopathological study of 12 cases and a review of the literature. Gynecol Obstet Invest. 2012;73:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Yüksel H, Sezer SD, Küçük M, Riza Obadaşi A, Döger FK. Papillary serous adenocarcinoma of the uterine cervix: a case report. Eur J Gynaecol Oncol. 2011;32:240-242. [PubMed] |
| 11. | Watrowski R, Striepecke E, Jäger C, Bauknecht T, Horst C. Papillary-serous adenocarcinoma of the uterine cervix during tamoxifen therapy after bilateral breast cancer. Anticancer Res. 2012;32:5075-5078. [PubMed] |
| 12. | Yemelyanova A, Ji H, Shih IeM, Wang TL, Wu LS, Ronnett BM. Utility of p16 expression for distinction of uterine serous carcinomas from endometrial endometrioid and endocervical adenocarcinomas: immunohistochemical analysis of 201 cases. Am J Surg Pathol. 2009;33:1504-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Gadducci A, Guerrieri ME. Immune Checkpoint Inhibitors in Gynecological Cancers: Update of Literature and Perspectives of Clinical Research. Anticancer Res. 2017;37:5955-5965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |