Published online Feb 6, 2023. doi: 10.12998/wjcc.v11.i4.866
Peer-review started: September 19, 2022
First decision: December 13, 2022
Revised: December 22, 2022
Accepted: January 9, 2023
Article in press: January 9, 2023
Published online: February 6, 2023
Processing time: 139 Days and 14 Hours
The advent of molecular targeted agents and immune checkpoint inhibitors has greatly improved the treatment of advanced renal cell carcinoma (RCC), thus significantly improving patient survival. The incidence of rare drug-related adverse events has gained increased attention.
We report a patient with advanced RCC treated with multiple lines of molecular targeted agents and immune checkpoint inhibitors, who developed a pulmonary infection after treatment with everolimus in combination with lenvatinib. Dete
Rare pulmonary infections caused by molecular targeted agents are not uncom
Core Tip: The application of molecular targeted agents and immune checkpoint inhibitors have greatly improved the prognosis of advanced renal cell carcinoma (RCC). We report a patient with advanced RCC treated with multiple lines of molecular targeted agents, who developed a Pneumocystis jirovecii pneumonia after treatment with everolimus in combination with lenvatinib. The pathogenic organism was identified by next-generation sequencing (NGS) of bronchoscopic alveolar lavage fluid (BALF) and successfully treated with trimethoprim-sulfamethoxazole. Evaluating BALF with NGS technology might be used to detect pathogens and determine the correct treatment plan for patients with rare infections caused by the use of molecular targeted agents.
- Citation: Cheng QW, Shen HL, Dong ZH, Zhang QQ, Wang YF, Yan J, Wang YS, Zhang NG. Pneumocystis jirovecii diagnosed by next-generation sequencing of bronchoscopic alveolar lavage fluid: A case report and review of literature. World J Clin Cases 2023; 11(4): 866-873
- URL: https://www.wjgnet.com/2307-8960/full/v11/i4/866.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i4.866
Renal cell carcinoma (RCC) is a malignant tumor originating from the renal tubular epithelium and accounts for 80% to 90% of renal malignancies[1]. According to GLOBOCAN 2020 global cancer statistics, RCC was the 14th most prevalent and the 15th most deadly malignancy[2]. Radical surgical resection is the mainstay treatment for localized renal cancer, while a combination of systemic drugs is preferred for advanced renal cancer. Sorafenib was first approved for the treatment of metastatic renal cancer in 2005[3], followed by the approval of targeted drugs such as pegaptanib, sunitinib, axitinib, sorafenib, and everolimus[4-7]; while immune checkpoint inhibitors such as navulizumab, pablizumab, and ipilimumab have also been licensed for use[8-10]. The combination of various molecular targeted drugs and immune checkpoint inhibitors has resulted in a rise in rare adverse effects.
Herein, we report a case of RCC that progressed after multiple lines of therapy and improved with the mammalian target of rapamycin (mTOR) inhibitor, everolimus in combination with lenvatinib; however, the patient complained of a dry cough that developed into a lung infection. Due to difficulty in identifying the infection, empirical anti-bacterial treatment was initially administered, with unsatisfactory results. Eventually, this rare case of Pneumocystis jirovecii infection was confirmed using next-generation sequencing (NGS) of bronchoscopic alveolar lavage fluid (BALF), and the infection improved after appropriate treatment.
A 61-year-old man diagnosed with RCC for nearly 3 years presented to our department because of a dry cough with occasional expectoration on February 22, 2022.
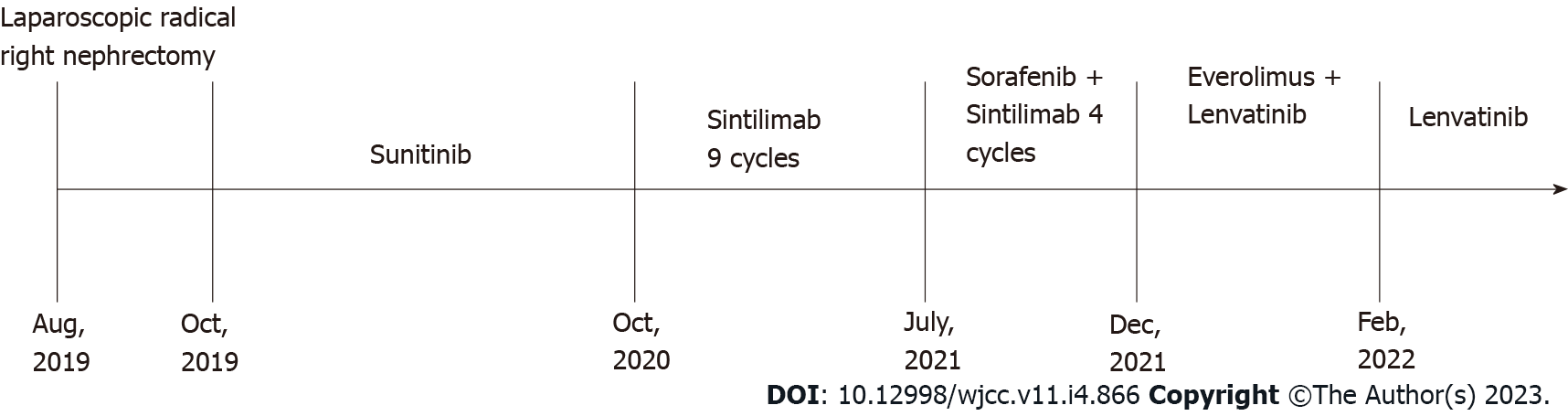
The patient was admitted to our hospital with “hematuria for 7 d” on August 5, 2019. A computed tomography (CT) scan on admission revealed right kidney and right renal pelvis occupancy, suggestive of malignancy. In addition, multiple nodules in both lungs were seen, and metastasis was suspected. A timeline of the episode of treatment is shown in Figure 1. After consultation and discussion, the patient agreed to undergo a laparoscopic radical right nephrectomy. Postoperative pathology revealed clear cell RCC of the right kidney. Following surgery, he was treated with oral sunitinib. A follow-up CT scan after 9 mo revealed metastases in the right adrenal gland, and axitinib was given to the patient. Three months later, CT imaging found that the metastases in the right adrenal gland and both lungs were significantly larger than before. He was thus treated with nine cycles of the immune checkpoint inhibitor sintilimab. However, a subsequent CT scan showed that the right adrenal metastasis was slightly enlarged, with multiple metastases appearing in the abdominal cavity, while the size of the lung metastases was unchanged. On July 19, 2021, four cycles of sorafenib in combination with sintilimab were administered. CT scanning revealed that the lung and abdominal cavity metastases were slightly larger than before, and new subcapsular metastases were detected on the right lobe of the liver. On December 14, 2021, he was switched to everolimus 10 mg once daily in combination with lenvatinib 8 mg once daily. However, on February 22, 2022, the patient developed a dry cough with occasional and little sputum, accompanied by mild dyspnea after activity. No fever or other discomfort was observed.
The patient had no past illness.
The patient had no specific personal and family history.
On physical examination, the patient’s basic vital signs were within normal limits, and his respiratory sounds were rough on both lungs, without dry or wet rales.
The initial blood investigations revealed the following: Leukocytes 5.17 × 109/L (reference range: 4.0-11.0), hemoglobin 121.0 g/L, platelet count 400 × 109/L, lymphocytes 15.9%, monocytes 6.8%, neutrophils 76.3%, eosinophils 0.6%, basophils 0.4%, high sensitivity C-reactive protein 37.0 mg/L (reference range: 0-5 mg/L), and procalcitonin 0.07 ng/mL (normal value < 0.052 ng/mL). Liver and kidney functions were normal, and sputum culture revealed normal oral flora.
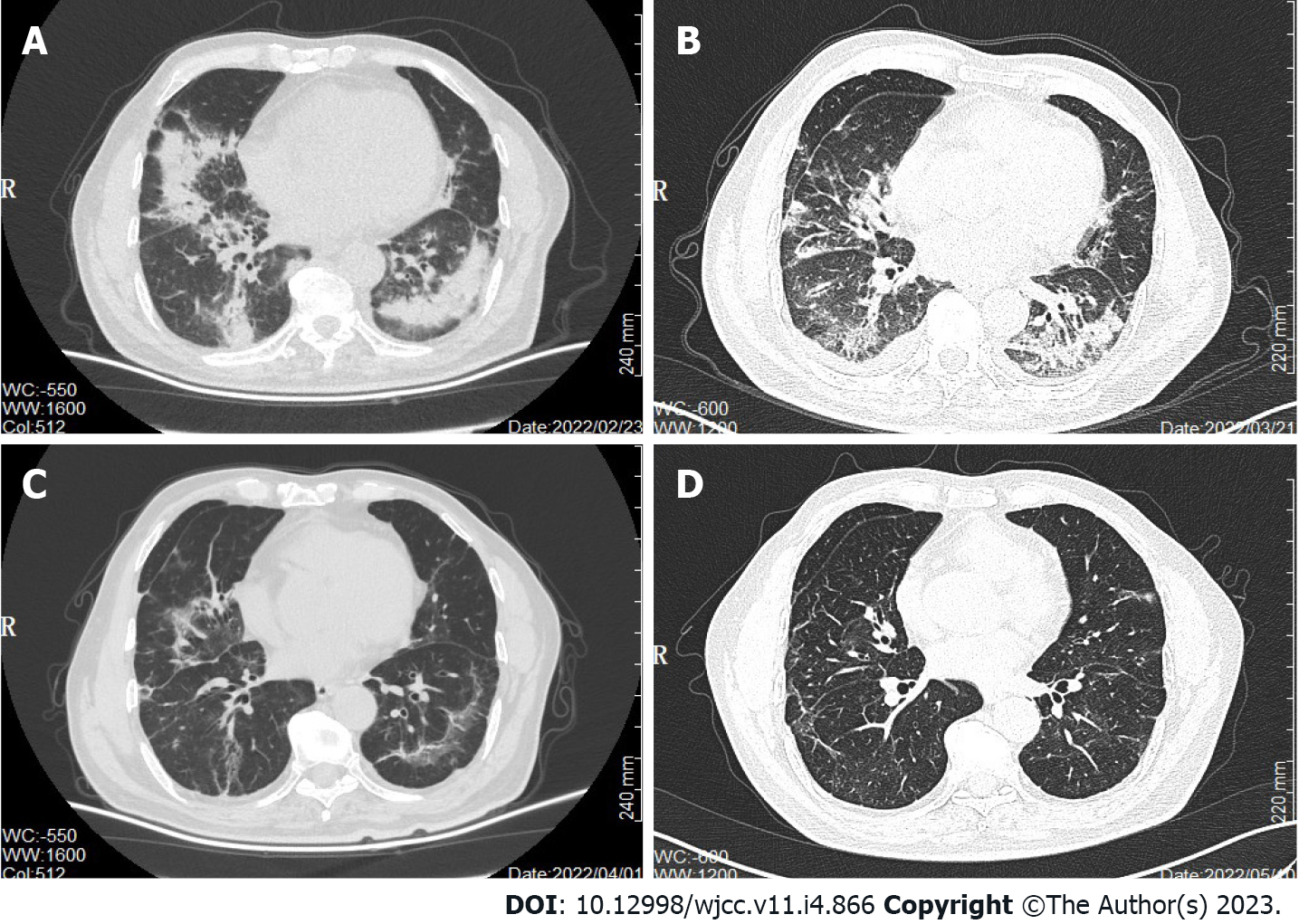
CT scan on February 23, 2022 (Figure 2A) identified multiple patches and ground glass shadows in both lungs and scattered soft tissue nodules of different sizes in both lungs, suggestive of a pulmonary infection. The lung and peritoneal metastases were slightly smaller in size. The infection was treated with cefuroxime sodium, piperacillin sulbactam, and moxifloxacin injections, but the patient’s condition did not improve, and he still had a persistent dry cough. Another CT scan on the March 21, 2022 (Figure 2B) showed little to no resolution of the lung infection.
A bronchoscopy lavage fluid NGS test was performed on March 25, 2022. The patient underwent bronchoscopy, and the fiberoptic bronchoscope reached the trachea and bronchi of both lungs. Irrigation with sterile saline was performed repeatedly in the left lower lobe bronchus and right lower lobe bronchus. The collected samples were then sent to the laboratory for NGS testing and analysis, which revealed Pneumocystis jirovecii infection.
Two cotrimoxazole tablets (sulfamethoxazole 0.4 g, trimethoprim 80 mg/tablet) were given every 6 h and 40 mg methylprednisolone succinate was given twice a day for 5 d, followed by 40 mg once daily for 5 d.
Following treatment, the patient’s cough disappeared. Cotrimoxazole tablets were continued for 3 wk, and the repeat CT on April 1, 2022 (Figure 2C) and May 10, 2022 (Figure 2D) showed significant improvement and resolution in both lungs. There were no treatment-related adverse effects. At present, the patient is well and has not complained of symptoms such as cough and dyspnea.
Kidney cancer is the third most prevalent urogenital tumor in China, accounting for 2%-3% of adult malignant tumors, and its incidence is increasing annually[11]. Targeted therapy is the primary treatment strategy for advanced renal cancer. According to their targets, these drugs can be divided into two main categories: Vascular endothelial growth factor (VEGF)/VEGF receptor inhibitors and mTOR inhibitors. VEGF, a major factor in the angiogenesis process in RCC, is a primary target of anti-angiogenic treatments[12,13]. Additionally, mTOR, which is positioned downstream of phosphoinositide 3-kinase and protein kinase B and is regulated by phosphatase and tensin homolog, is heavily involved in RCC development[14]. Inhibition of the mTOR pathway can inhibit both angiogenesis and tumor cell proliferation. Everolimus, an mTOR inhibitor, has been found to improve survival in metastatic RCC patients after TKI-targeted drug therapy failure[15,16].
Everolimus is not only approved for use in advanced RCC but also for the treatment of advanced breast cancer[17], pancreatic neuroendocrine tumor[18], subependymal giant cell astrocytoma associated with tuberous sclerosis[19], and other tumors. It can also prevent immunological rejection after kidney, liver, or heart transplantation[20]. The adverse reactions of everolimus mainly affect the digestive system, respiratory system, endocrine system, and skin mucosa[21,22], with interstitial lung disease (ILD) being the most common pulmonary associated toxicity[23-25]. The incidence of non-infectious pneumonia of metastatic RCC treated with everolimus can reach up to 14%[16]. Following everolimus administration, our patient developed a dry cough, with the CT scan revealing a pulmonary infection. Repeated sputum culture and sputum smear showed no clear evidence of microbial infection. After empirical anti-bacterial treatment, the therapeutic outcome was poor. We thus suspected an uncommon bacterial or fungal infection, but identifying the pathogen was challenging. Therefore, we resorted to BALF NGS testing and revealed that Pneumocystis jirovecii was the cause of the infection. Prior to the lung infection, the patient had been treated with many lines of targeted treatments and immune checkpoint inhibitors, and his immune function was compromised. Following treatment with everolimus in combination with lenvatinib, he acquired a rare Pneumocystis jirovecii infection. Given that lenvatinib is a multi-targeted anti-angiogenic agent, its common adverse effects include hypertension, fatigue, diarrhea, palmoplantar erythroderma syndrome, proteinuria and hemorrhagic events, while everolimus is immune-suppressive, making patients susceptible to opportunistic pulmonary infections[26]. Loron et al[27] found that the use of everolimus in patients with advanced RCC can lead to rare pathogenic infections, including Pneumocystis jirovecii infections. As a result, we speculate that the Pneumocystis jirovecii infection in this patient was associated with the use of everolimus.
Pneumocystis jirovecii can accumulate on the surface of the respiratory tract of healthy humans and can proliferate when the immune system is weakened, leading to opportunistic infections[28]. In the 1980s, with the emergence of the human immunodeficiency virus (HIV), Pneumocystis jirovecii pneumonia (PJP) became more prevalent in HIV patients[29]. With the increased use of immunosuppressive drugs, the incidence of PJP has gradually increased in non-HIV-infected patients, especially in those with malignant tumors who are treated with immunosuppressants[30,31]. The early stage of infection in non-HIV-infected patients is characterized by a repeated low-grade fever and dry cough without characteristic symptoms, and the disease can advance rapidly to fever and respiratory failure. The mortality rate of non-HIV patients with PJP is 30% to 50%, higher than that of HIV patients[32].
The clinical features of PJP are not specific, and the diagnosis mainly depends on the detection of pathogens. Normal or reduced white blood cells are often seen while serum lactate dehydrogenase and blood fungal (1-3)-β-d-glucan are elevated, and the G test is positive; however, these markers have limited specificity and clinical translational value[33]. X-ray and CT chest examinations lack specificity in the early stage of infection, while after disease progression, CT imaging usually shows diffuse, bilateral pulmonary “ground glass” interstitial infiltrates, which may also appear as pulmonary nodules. Nonetheless, these imaging changes are not specific, especially as they are identical to the presentation of ILD in everolimus-induced interstitial pneumonia, which can easily lead to misdiagnosis[34]. Unlike bacterial and other fungal infections, most non-HIV individuals have a low PJP load, and standard smear microscopy has a low sensitivity; thus, early PJP diagnosis is difficult. Microscopic inspection is commonly utilized to confirm PJP, and clinical specimens typically employed include induced sputum, bronchoalveolar lavage fluid, and lung tissue biopsy, but induced sputum culture has a low positive rate, and lung tissue biopsy is traumatic and difficult to carry out clinically. The BALF technique allows targeted sampling of the lower respiratory tract with a diagnostic positivity rate of 90% to 99% compared to sputum analysis[35]. Polymerase chain reaction (PCR) of alveolar lavage fluid samples is a reliable method for diagnosing PJP, with some studies indicating that PCR has a sensitivity of ≥ 97% and a negative predictive value of ≥ 99%[36]. If the BALF PCR test is negative, PJP can be ruled out, while a positive PCR makes distinguishing between colonization and active infection difficult[37].
Traditional diagnostic methods, such as smear microscopy and induced sputum culture, have a low positive rate for the diagnosis of Pneumocystis jirovecii infection. Lung tissue biopsy is a traumatic examination. BALF test has a higher positive rate. As a new detection method independent of microbial culture, NGS is a second-generation gene sequencing technology with the advantages of high thro
Trimethoprim-sulfamethoxazole (TMP/SMZ) is the preferred drug for the treatment of PJP, and it is emphasized that early and adequate dosage yields the best outcome[44], and the standard course to treat PJP is 3 wk[45]. The main side effects of TMP/SMZ include skin rash, drug fever, leukopenia, renal dysfunction, electrolyte disorder, and hepatotoxicity. Patients with renal insufficiency should lower their TMP dosage according to the creatinine clearance rate. For some patients with ineffective TMP/SMZ treatment or intolerable side effects, in recent years, caspofungin combined with low-dose TMP/SMZ has been used in patients with PJP infection following organ transplant. These two drugs have a synergistic effect, achieving satisfactory efficacy and a low incidence of adverse reactions[44,46].
With the widespread use of anti-tumor immunosuppressants, the risks of lung infection with atypical bacteria or fungi in cancer patients have increased, and existing traditional diagnostic procedures make such infections difficult to diagnose. Therefore, in the event that tumor patients experience lung infection after anti-tumor treatment and the effect of conventional diagnosis and treatment are unsatisfactory, evaluating BALF with NGS technology can be used to detect pathogens and determine the correct treatment plan for such patients. Collectively, we believe that this approach is promising in the early diagnosis of such infections and deserves more clinical attention.
We sincerely thank the patient involved in this report.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS, Spain; Soewondo W, Indonesia S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Cai Q, Chen Y, Qi X, Zhang D, Pan J, Xie Z, Xu C, Li S, Zhang X, Gao Y, Hou J, Guo X, Zhou X, Zhang B, Ma F, Zhang W, Lin G, Xin Z, Niu Y, Wang Y. Temporal trends of kidney cancer incidence and mortality from 1990 to 2016 and projections to 2030. Transl Androl Urol. 2020;9:166-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64456] [Article Influence: 16114.0] [Reference Citation Analysis (176)] |
| 3. | Liu XL, Xue HY, Chu Q, Liu JY, Li J. Comparative efficacy and safety of sunitinib vs sorafenib in renal cell carcinoma: A systematic review and meta-analysis. Medicine (Baltimore). 2020;99:e19570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Cai W, Yuan YC, Li MY, Kong W, Dong BJ, Chen YH, Zhang J, Xue W, Huang YR, Zhou LX, Huang JW. [Comparison of efficacy between sorafenib and sunitinib as first-line therapy for metastatic renal cell carcinoma and analyze prognostic factors for survival]. Zhonghua Zhong Liu Za Zhi. 2018;40:384-389. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Sheng X, Jin J, He Z, Huang Y, Zhou A, Wang J, Ren X, Ye D, Zhang X, Qin S, Zhou F, Wang B, Guo J. Pazopanib versus sunitinib in Chinese patients with locally advanced or metastatic renal cell carcinoma: pooled subgroup analysis from the randomized, COMPARZ studies. BMC Cancer. 2020;20:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, Jassem J, Zolnierek J, Maroto JP, Mellado B, Melichar B, Tomasek J, Kremer A, Kim HJ, Wood K, Dutcus C, Larkin J. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16:1473-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 697] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 7. | Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, Grünwald V, Hutson TE, Kopyltsov E, Méndez-Vidal MJ, Kozlov V, Alyasova A, Hong SH, Kapoor A, Alonso Gordoa T, Merchan JR, Winquist E, Maroto P, Goh JC, Kim M, Gurney H, Patel V, Peer A, Procopio G, Takagi T, Melichar B, Rolland F, De Giorgi U, Wong S, Bedke J, Schmidinger M, Dutcus CE, Smith AD, Dutta L, Mody K, Perini RF, Xing D, Choueiri TK; CLEAR Trial Investigators. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. 2021;384:1289-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 1133] [Article Influence: 283.3] [Reference Citation Analysis (0)] |
| 8. | Ning K, Wu Z, Zou X, Liu H, Wu Y, Xiong L, Yu C, Guo S, Han H, Zhou F, Dong P, Zhang Z. Immune checkpoint inhibitors further aggravate proteinuria in patients with metastatic renal cell carcinoma after long-term targeted therapy. Transl Androl Urol. 2022;11:386-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Motzer RJ, Rini BI, McDermott DF, Arén Frontera O, Hammers HJ, Carducci MA, Salman P, Escudier B, Beuselinck B, Amin A, Porta C, George S, Neiman V, Bracarda S, Tykodi SS, Barthélémy P, Leibowitz-Amit R, Plimack ER, Oosting SF, Redman B, Melichar B, Powles T, Nathan P, Oudard S, Pook D, Choueiri TK, Donskov F, Grimm MO, Gurney H, Heng DYC, Kollmannsberger CK, Harrison MR, Tomita Y, Duran I, Grünwald V, McHenry MB, Mekan S, Tannir NM; CheckMate 214 investigators. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20:1370-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 614] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 10. | Gil L, Alves FR, Silva D, Fernandes I, Fontes-Sousa M, Alves M, Papoila A, Da Luz R. Prognostic Impact of Baseline Neutrophil-to-Eosinophil Ratio in Patients With Metastatic Renal Cell Carcinoma Treated With Nivolumab Therapy in Second or Later Lines. Cureus. 2022;14:e22224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Zheng RS, Zhang SW, Zeng HM, Wang SM, Sun KX, Chen R, Li L, Wei WQ, He J. Cancer incidence and mortality in China, 2016. J Nat Cancer Cent. 2022;2:1-9. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 852] [Cited by in RCA: 946] [Article Influence: 315.3] [Reference Citation Analysis (1)] |
| 12. | Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME, Rusakov IG, Negrier S, Ou YC, Castellano D, Lim HY, Uemura H, Tarazi J, Cella D, Chen C, Rosbrook B, Kim S, Motzer RJ. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1380] [Cited by in RCA: 1433] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 13. | Wolf MM, Kimryn Rathmell W, Beckermann KE. Modeling clear cell renal cell carcinoma and therapeutic implications. Oncogene. 2020;39:3413-3426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 14. | Miricescu D, Balan DG, Tulin A, Stiru O, Vacaroiu IA, Mihai DA, Popa CC, Papacocea RI, Enyedi M, Sorin NA, Vatachki G, Georgescu DE, Nica AE, Stefani C. PI3K/AKT/mTOR signalling pathway involvement in renal cell carcinoma pathogenesis (Review). Exp Ther Med. 2021;21:540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 15. | Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA, Hollaender N, Urbanowitz G, Berg WJ, Kay A, Lebwohl D, Ravaud A; RECORD-1 Study Group. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2365] [Cited by in RCA: 2374] [Article Influence: 139.6] [Reference Citation Analysis (0)] |
| 16. | Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA, Hollaender N, Kay A, Ravaud A; RECORD‐1 Study Group. Phase 3 trial of everolimus for metastatic renal cell carcinoma : final results and analysis of prognostic factors. Cancer. 2010;116:4256-4265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 926] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 17. | Vernieri C, Corti F, Nichetti F, Ligorio F, Manglaviti S, Zattarin E, Rea CG, Capri G, Bianchi GV, de Braud F. Everolimus versus alpelisib in advanced hormone receptor-positive HER2-negative breast cancer: targeting different nodes of the PI3K/AKT/mTORC1 pathway with different clinical implications. Breast Cancer Res. 2020;22:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Ma ZY, Gong YF, Zhuang HK, Zhou ZX, Huang SZ, Zou YP, Huang BW, Sun ZH, Zhang CZ, Tang YQ, Hou BH. Pancreatic neuroendocrine tumors: A review of serum biomarkers, staging, and management. World J Gastroenterol. 2020;26:2305-2322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (5)] |
| 19. | Bakhtiary H, Barzegar M, Shiva S, Poorshiri B, Hajalioghli P, Herizchi Ghadim H. The Effect of Everolimus on Subependymal Giant Cell Astrocytoma (SEGA) in Children with Tuberous Sclerosis Complex. Iran J Child Neurol. 2021;15:15-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Paoletti E, Citterio F, Corsini A, Potena L, Rigotti P, Sandrini S, Bussalino E, Stallone G; ENTROPIA Project. Everolimus in kidney transplant recipients at high cardiovascular risk: a narrative review. J Nephrol. 2020;33:69-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Chang SC, Tsai CY, Liu KH, Wang SY, Hsu JT, Yeh TS, Yeh CN. Everolimus Related Fulminant Hepatitis in Pancreatic Neuroendocrine Tumor With Liver Metastases: A Case Report and Literature Review. Front Endocrinol (Lausanne). 2021;12:639967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Ruiz-Falcó Rojas ML, Feucht M, Macaya A, Wilken B, Hahn A, Maamari R, Hirschberg Y, Ridolfi A, Kingswood JC. Real-World Evidence Study on the Long-Term Safety of Everolimus in Patients With Tuberous Sclerosis Complex: Final Analysis Results. Front Pharmacol. 2022;13:802334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Lee L, Ito T, Jensen RT. Everolimus in the treatment of neuroendocrine tumors: efficacy, side-effects, resistance, and factors affecting its place in the treatment sequence. Expert Opin Pharmacother. 2018;19:909-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 24. | Porta C, Osanto S, Ravaud A, Climent MA, Vaishampayan U, White DA, Creel P, Dickow B, Fischer P, Gornell SS, Meloni F, Motzer RJ. Management of adverse events associated with the use of everolimus in patients with advanced renal cell carcinoma. Eur J Cancer. 2011;47:1287-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 25. | Akata K, Yatera K, Ishimoto H, Kozaki M, Yamasaki K, Nagata S, Nishida C, Yoshida T, Kawanami T, Matsumoto T, Mukae H. Two cases of everolimus-associated interstitial pneumonia in patients with renal cell carcinoma. Intern Med. 2011;50:3013-3017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Mauro C, de Jesus VHF, Barros M, Costa FP, Weschenfelder RF, D'Agustini N, Angel M, Luca R, Nuñez JE, O'Connor JM, Riechelmann RP. Opportunistic and Serious Infections in Patients with Neuroendocrine Tumors Treated with Everolimus: A Multicenter Study of Real-World Patients. Neuroendocrinology. 2021;111:631-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Loron MC, Grange S, Guerrot D, Di Fiore F, Freguin C, Hanoy M, Le Roy F, Poussard G, Etienne I, Legallicier B, Pfister C, Godin M, Bertrand D. Pneumocystis jirovecii pneumonia in everolimus-treated renal cell carcinoma. J Clin Oncol. 2015;33:e45-e47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Braga BP, Prieto-González S, Hernández-Rodríguez J. Pneumocystis jirovecii pneumonia prophylaxis in immunocompromised patients with systemic autoimmune diseases. Med Clin (Barc). 2019;152:502-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Huang YS, Yang JJ, Lee NY, Chen GJ, Ko WC, Sun HY, Hung CC. Treatment of Pneumocystis jirovecii pneumonia in HIV-infected patients: a review. Expert Rev Anti Infect Ther. 2017;15:873-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Avino LJ, Naylor SM, Roecker AM. Pneumocystis jirovecii Pneumonia in the Non-HIV-Infected Population. Ann Pharmacother. 2016;50:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 31. | Echavarria I, Carrión Galindo JR, Corral J, Diz Taín MP, Henao Carrasco F, Iranzo González-Cruz V, Mielgo-Rubio X, Quintanar T, Rivas Corredor C, Pérez Segura P. SEOM clinical guidelines for the prophylaxis of infectious diseases in cancer patients (2021). Clin Transl Oncol. 2022;24:724-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | White PL, Backx M, Barnes RA. Diagnosis and management of Pneumocystis jirovecii infection. Expert Rev Anti Infect Ther. 2017;15:435-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 33. | Bateman M, Oladele R, Kolls JK. Diagnosing Pneumocystis jirovecii pneumonia: A review of current methods and novel approaches. Med Mycol. 2020;58:1015-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 34. | Kloth C, Thaiss WM, Beck R, Haap M, Fritz J, Beer M, Horger M. Potential role of CT-textural features for differentiation between viral interstitial pneumonias, pneumocystis jirovecii pneumonia and diffuse alveolar hemorrhage in early stages of disease: a proof of principle. BMC Med Imaging. 2019;19:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Liu L, Yuan M, Shi Y, Su X. Clinical Performance of BAL Metagenomic Next-Generation Sequence and Serum (1,3)-β-D-Glucan for Differential Diagnosis of Pneumocystis jirovecii Pneumonia and Pneumocystis jirovecii Colonisation. Front Cell Infect Microbiol. 2021;11:784236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 36. | Fan LC, Lu HW, Cheng KB, Li HP, Xu JF. Evaluation of PCR in bronchoalveolar lavage fluid for diagnosis of Pneumocystis jirovecii pneumonia: a bivariate meta-analysis and systematic review. PLoS One. 2013;8:e73099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 37. | Sivaraj V, Cliff P, Douthwaite S, Smith M, Kulasegaram R. Pneumocystis jirovecii pneumonia PCR test on upper respiratory tract swab. HIV Med. 2021;22:321-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 38. | Qian YY, Wang HY, Zhou Y, Zhang HC, Zhu YM, Zhou X, Ying Y, Cui P, Wu HL, Zhang WH, Jin JL, Ai JW. Improving Pulmonary Infection Diagnosis with Metagenomic Next Generation Sequencing. Front Cell Infect Microbiol. 2020;10:567615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 39. | Chen Y, Feng W, Ye K, Guo L, Xia H, Guan Y, Chai L, Shi W, Zhai C, Wang J, Yan X, Wang Q, Zhang Q, Li C, Liu P, Li M. Application of Metagenomic Next-Generation Sequencing in the Diagnosis of Pulmonary Infectious Pathogens From Bronchoalveolar Lavage Samples. Front Cell Infect Microbiol. 2021;11:541092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 40. | Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. 2019;20:341-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 761] [Cited by in RCA: 901] [Article Influence: 150.2] [Reference Citation Analysis (0)] |
| 41. | Wang J, Han Y, Feng J. Metagenomic next-generation sequencing for mixed pulmonary infection diagnosis. BMC Pulm Med. 2019;19:252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 42. | Lu X, Zhang J, Ma W, Xing L, Ning H, Yao M. Pneumocystis Jirovecii Pneumonia Diagnosis via Metagenomic Next-Generation Sequencing. Front Med (Lausanne). 2022;9:812005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 43. | Zhang Y, Ai JW, Cui P, Zhang WH, Wu HL, Ye MZ. A cluster of cases of pneumocystis pneumonia identified by shotgun metagenomics approach. J Infect. 2019;78:158-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 44. | Wu HH, Fang SY, Chen YX, Feng LF. Treatment of Pneumocystis jirovecii pneumonia in non-human immunodeficiency virus-infected patients using a combination of trimethoprim-sulfamethoxazole and caspofungin. World J Clin Cases. 2022;10:2743-2750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Maschmeyer G, Helweg-Larsen J, Pagano L, Robin C, Cordonnier C, Schellongowski P; 6th European Conference on Infections in Leukemia (ECIL-6), a joint venture of The European Group for Blood and Marrow Transplantation (EBMT), The European Organization for Research and Treatment of Cancer (EORTC), the International Immunocompromised Host Society (ICHS) and The European LeukemiaNet (ELN). ECIL guidelines for treatment of Pneumocystis jirovecii pneumonia in non-HIV-infected haematology patients. J Antimicrob Chemother. 2016;71:2405-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 46. | Jin F, Liu XH, Chen WC, Fan ZL, Wang HL. High initial (1, 3) Beta-d-Glucan concentration may be a predictor of satisfactory response of c aspofungin combined with TMP/SMZ for HIV-negative patients with moderate to severe Pneumocystis jirovecii pneumonia. Int J Infect Dis. 2019;88:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |