Published online Jul 16, 2023. doi: 10.12998/wjcc.v11.i20.4903
Peer-review started: February 28, 2023
First decision: April 10, 2023
Revised: April 22, 2023
Accepted: June 13, 2023
Article in press: June 13, 2023
Published online: July 16, 2023
Processing time: 133 Days and 17.5 Hours
Gene mutations in ATP-binding cassette, subfamily B (ABCB4) lead to autosomal recessive disorders. Primary light amyloidosis is a rare and incurable disease. Here, we report a rare case of liver cirrhosis caused by ABCB4 gene mutation combined with primary light amyloidosis.
We report a case of a 25-year-old female who was hospitalized due to recurrent abdominal pain caused by calculous cholecystitis and underwent cholecy
This report presents the diagnosis and treatment of liver cirrhosis, a rare disease that is easily misdiagnosed or missed.
Core Tip: We report a unique case of liver cirrhosis resulting from a mutation in the ATP-binding cassette, subfamily B
- Citation: Cheng N, Qin YJ, Zhang Q, Li H. ABCB4 gene mutation-associated cirrhosis with systemic amyloidosis: A case report. World J Clin Cases 2023; 11(20): 4903-4911
- URL: https://www.wjgnet.com/2307-8960/full/v11/i20/4903.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i20.4903
Multidrug resistance protein (MDR3), encoded by the ABCB4 gene, can transport phospholipids to bile, which plays an important role in normal bile formation. Mutations in ABCB4 can result in bile duct damage and cholestasis. It is an autosomal recessive disorder with clinical indications of gallbladder/intrahepatic bile duct stones and recurrent jaundice, which causes progressive familial intrahepatic cholestasis 3 (PFIC-3). Some patients progress to portal hypertension, liver cirrhosis, or even liver failure. Laboratory examination is characterized by persistent or repeated alkaline phosphatase (ALP) and gamma-glutamyl transpeptidase (GGT) increase[1-2], and ursodeoxycholic acid is the main drug of choice[3].
Primary light chain amyloidosis is an infrequent and untreatable condition. According to previous research, the incidence of this ailment in Europe and America is estimated to be approximately 9-14 individuals per million people per year[4]. The formation of amyloid proteins is attributed to misfolding of the monoclonal immunoglobulin light chain. This protein is deposited in various tissues and organs, leading to deterioration of the tissue structure, organ dysfunction, and gradual advancement. The etiology of this condition is associated with the cytotoxicity of unbound light chain amyloid proteins and gradual deterioration of organ function due to tissue structure degradation[5]. This condition may affect various tissues or organs, including but not limited to the liver, kidney, nerve, heart, and gastrointestinal tract, either successively or concurrently[6-7]. Because of the atypical and intricate symptoms of multiple organs and systems, early diagnosis and therapy are difficult, and the degree of pathological alterations in diverse tissues and organs in most individuals is irreversible at diagnosis.
This study presents a unique example of liver cirrhosis resulting from a mutation in the ABCB4 gene in conjunction with primary light chain amyloidosis. Clinical manifestations of the disease include portal hypertension, recurrent ascites, and jaundice. In addition, intractable proteinuria, peripheral neuropathy, and gradual damage to cardiac function may occur during disease progression. Following an extensive 4-year pursuit of medical intervention, a diagnosis was ultimately established, and the condition improved after treatment with a combination of ursodeoxycholic acid and the cluster of differentiation (CD38) monoclonal antibody daratumumab.
The patient was hospitalized on January 26, 2022, because of abdominal pain, palpitations, and numbness in both lower limbs for a duration of 2 d.
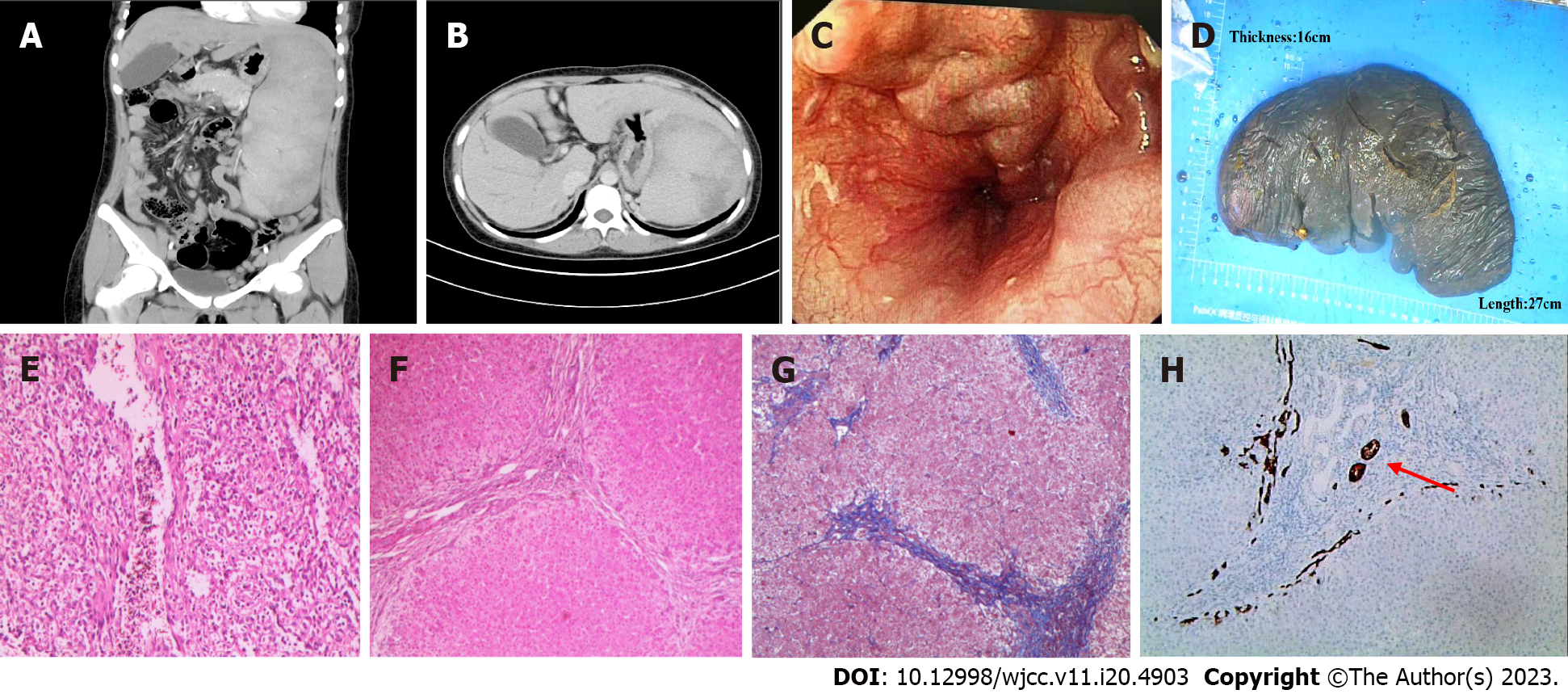
A 25-year-old woman presented with unexplained splenomegaly during a physical examination at 18 years of age. She felt no discomfort so had no further diagnosis and treatment until she was admitted to the hospital in November 2018 because of recurring abdominal pain caused by calculous cholecystitis. We planned to perform cholecystectomy, with preoperative abdominal computed tomography (CT) scan showing splenomegaly and normal liver imaging morphology. Gastroscopy examination indicated esophageal varices and portal hypertensive gastropathy, Blood analyses indicated a significant reduction in platelet count (33 × 109/L). This finding was attributed to hypersplenism resulting from splenomegaly. However, no additional investigation was performed via bone marrow puncture. Selective laparoscopic cholecystectomies and splenectomies were performed. Nodular changes were observed on the surface of the liver during surgery and a small amount of liver tissue was obtained for pathological analyses. The postoperative pathological diagnosis of the liver tissue revealed the presence of liver cirrhosis and bile duct injury (epigastrium CT, gastroscope, liver and spleen pathological tissue pictures are shown in Figure 1). The results of the laboratory tests for hepatitis B surface antigen (HBSAg), hepatitis C virus (HCV) antibody, ceruloplasmin, iron, ferritin, antinuclear antibody (ANA) antibody spectrum, autoantibody against the liver, anticardiolipin antibody, and antineutrophil were negative. The etiology of liver cirrhosis remained unclear. Because of the apparent elevation of ALP and GGT levels in the liver, ursodeoxycholic acid was administered at a dose of 15 mg/kg/d. In July 2020, positive urinary protein was initially detected, followed by the onset of persistent proteinuria and hypoproteinemia. The patient received medical attention from the nephrology department to exclude the possibility of rheumatism and immune-related ailments. However, the etiology of this condition remained unknown. The patient was hospitalized on January 26, 2022, because of abdominal pain, palpitations, and numbness in both lower limbs for a duration of 2 d.
The patient has no history of alcohol consumption, medication, or hepatitis.
The patient refrained from consuming alcohol or any form of drugs. The patient denied any family history of tumors.
The results of the physical examination revealed tenderness in the upper abdominal region, along with the presence of ascites and edema in both lower extremities. No apparent jaundice of the skin or sclera was observed, and the nervous system exhibited normal function.
Peripheral blood tests indicated a significant increase in GGT and ALP levels, which are indicative of impaired liver function. However, renal function was within normal limits. Additionally, the levels of N-brain natriuretic peptide precursor and troponin were significantly elevated. Routine urine analyses indicated a urinary protein level of 3 + and a 24-h urinary protein level of 744.98 mg/24-h. Following the administration of symptomatic treatment, the patient’s symptoms improved. The etiology of liver cirrhosis, including but not limited to HBSAg, HCV antibody, ceruloplasmin, ANA antibody spectrum, and autoantibodies, yielded negative findings.
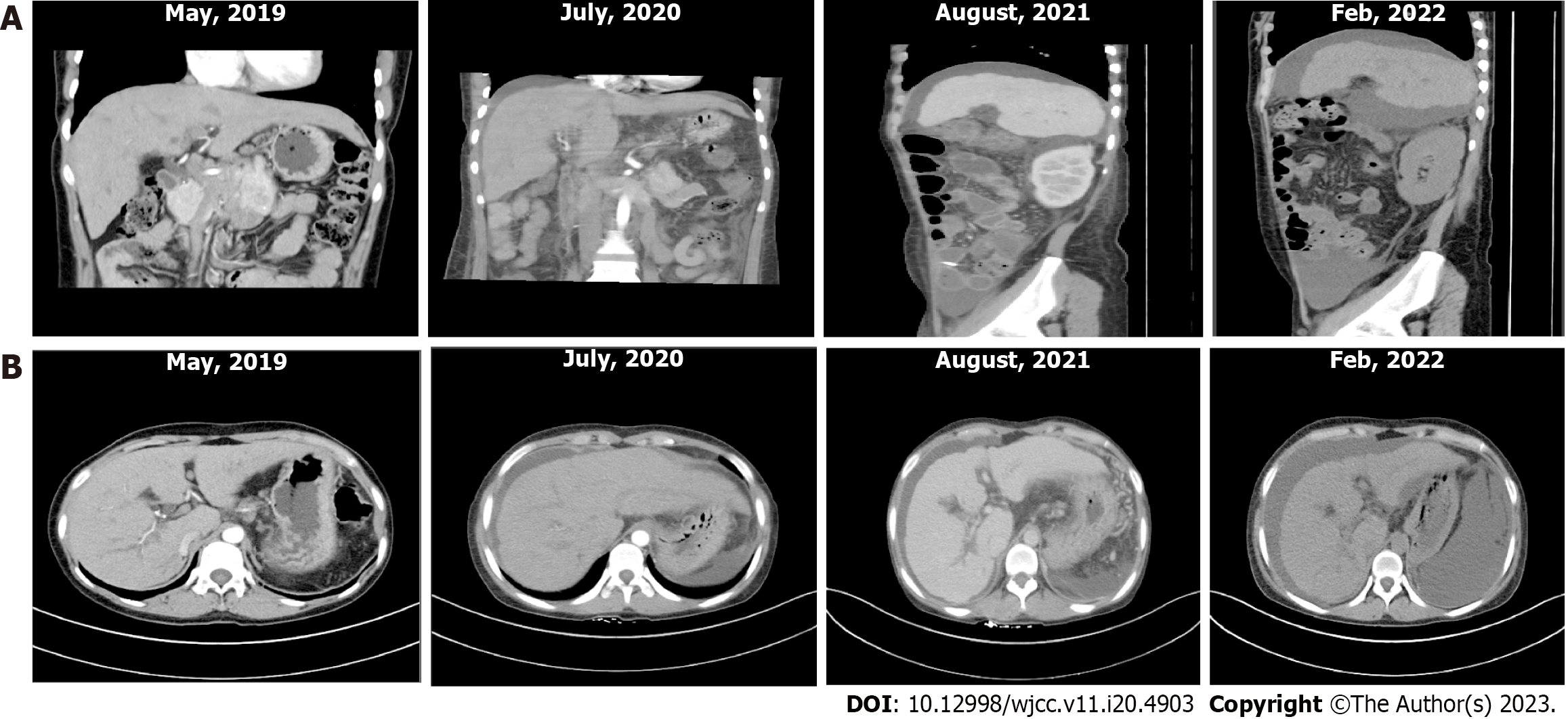
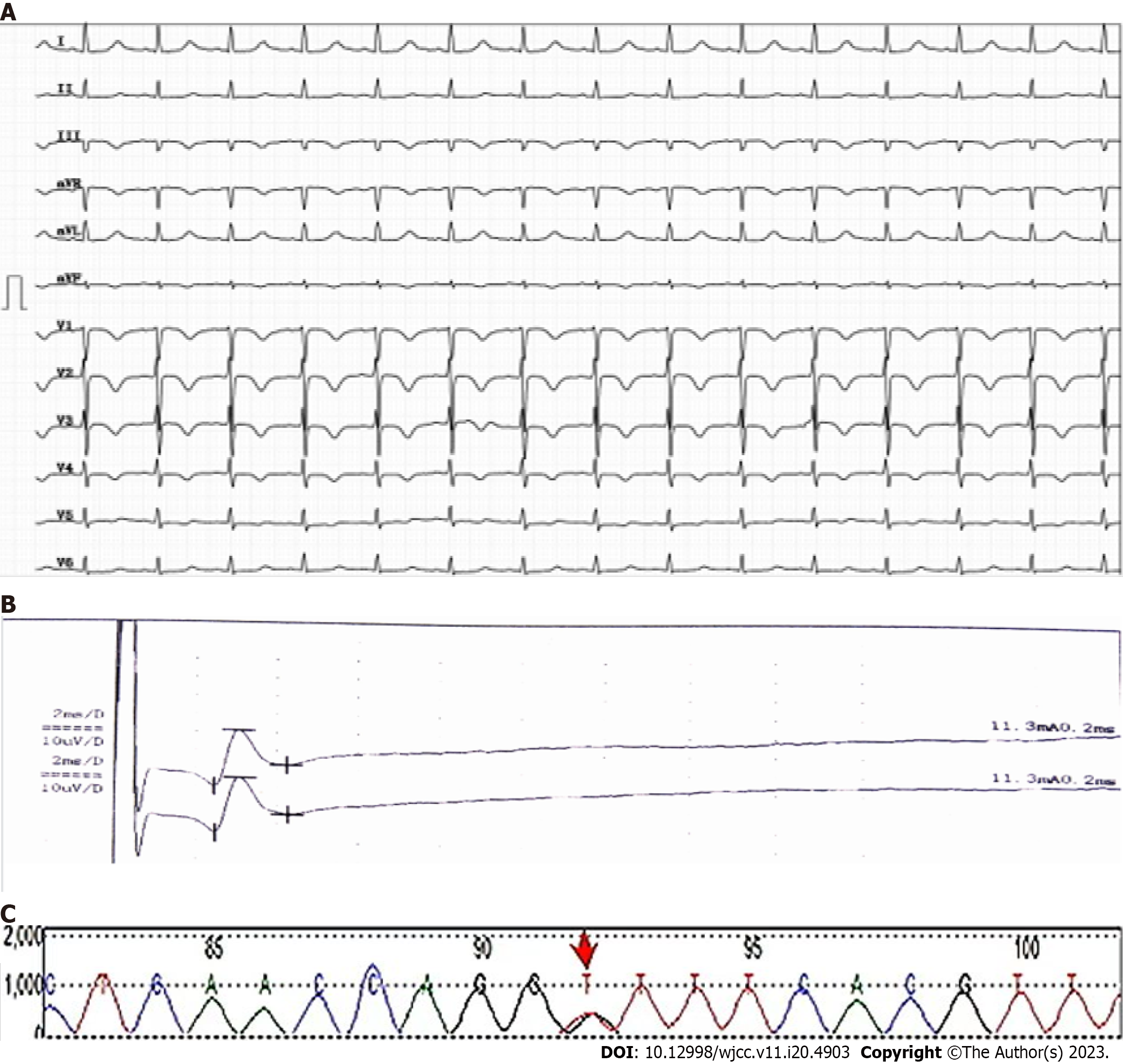
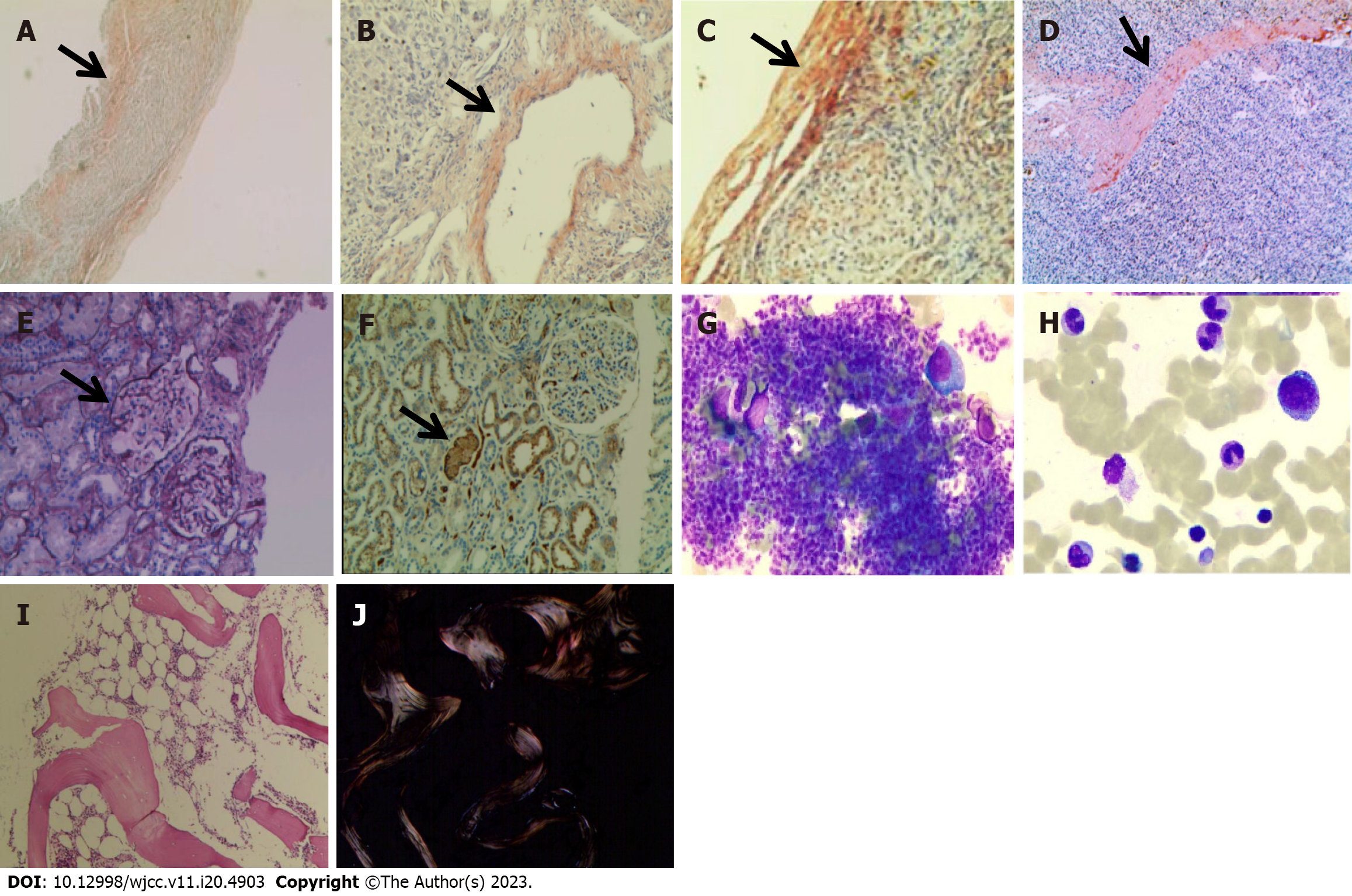
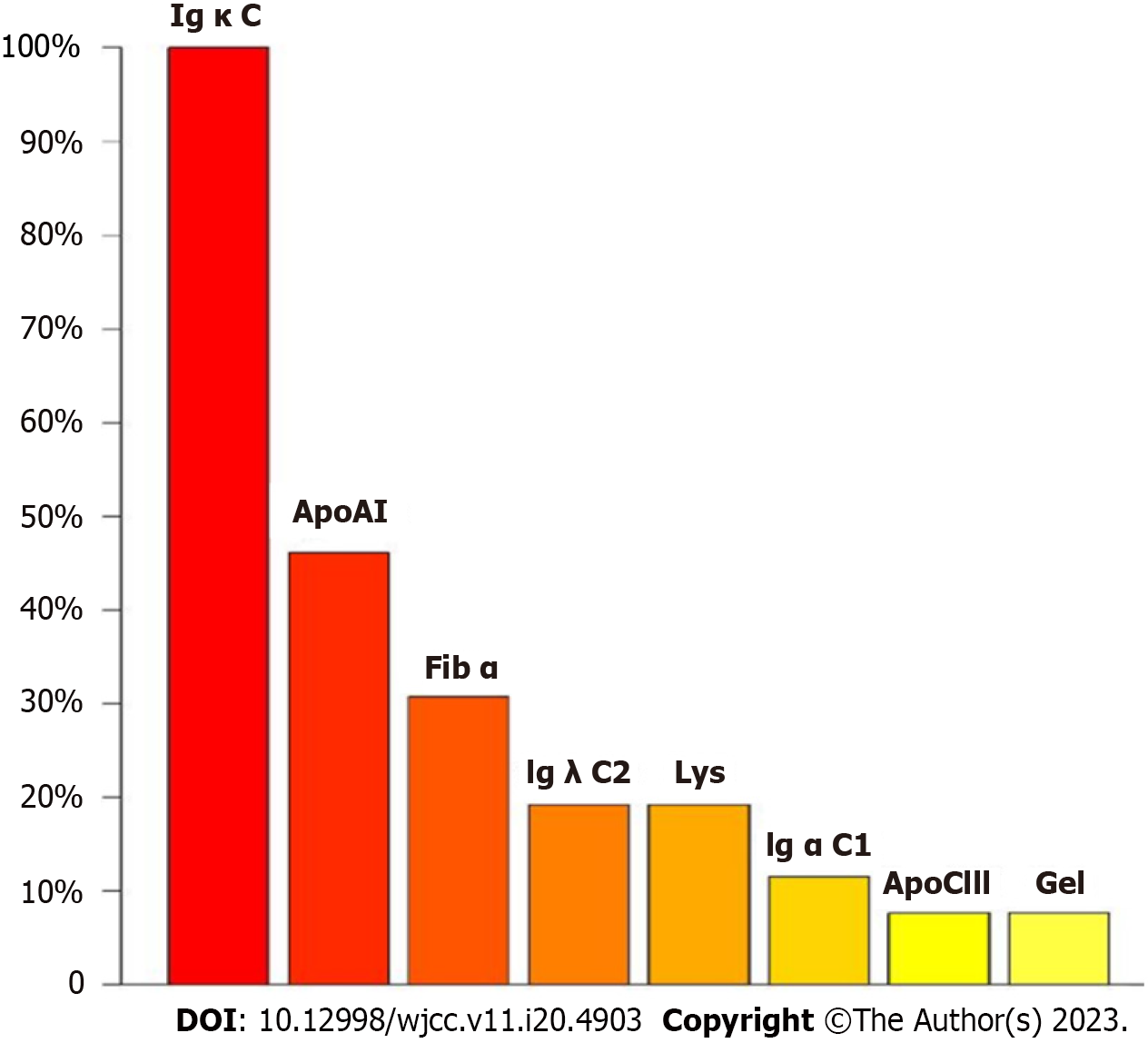
An abnormality was observed on electrocardiography. CT of the upper abdomen revealed ascites attributed to cirrhosis (Figure 2). To investigate the etiology of liver cirrhosis, exons of the entire genome from patients’ peripheral blood were analyzed. These findings revealed the presence of a heterozygous mutation in the ABCB4 c.2318G>T gene (see Figure 3 for the electrocardiography, electromyography, and ABCB4 gene mutation sites). ABCB4 mutations are associated with several clinical manifestations including cholecystolithiasis, bile duct injury, and cholestasis. In some cases, patients may progress to cirrhosis, as evidenced by persistent and significant abnormalities in GGT and ALP liver function tests. However, the development of additional symptoms such as massive proteinuria, abnormal cardiac function indices, and numbness in both lower limbs suggests the presence of other factors that contribute to the development of systemic multiple organ diseases. In light of the patient’s splenomegaly of unknown etiology at the age of 18 years, there was a potential association with hematologic or neoplastic pathologies. Subsequent analyses revealed the presence of hyperplastic anemia on standard bone marrow examination, whereas bone marrow biopsy indicated a significant decrease in hematopoietic tissue proliferation. The serum and urine immunofixation electrophoresis results were negative. Immunohistochemical analyses of hematological tumors (specifically, bone marrow blood) did not detect monoclonal B cells. The results of cardiac ultrasound and cardiac magnetic resonance imaging plus pyrophosphate radionuclide scanning were within normal limits. The positron emission tomography-CT scan yielded negative results for tumor lesions. However, the levels of free κ and λ light chains in serum and urine increased (blood free κ light chain 45.41 mg/L, blood free λ light chain 63.07 mg/L, urine free κ light chain 72.40 mg/L, and urine free λ light chain 65.20 mg/L). Electromyography results indicated a decrease in the conduction velocity of the superficial peroneal nerve. These findings suggested the potential presence of amyloidosis; however, a pathological assessment was required for confirmation. The liver and spleen pathological tissues that were preserved after the 2018 operation, as well as the bone marrow biopsy tissue from the current hospitalization, were subjected to Congo red staining with permission from the patients and their families. The staining findings were uniformly positive. Additionally, further renal puncture examination revealed positive Congo red staining in the pathological tissue, immunohistochemical κ staining positive, λ staining negative. Liver mass spectrometry (MS) analyses also confirmed κ light chain amyloidosis (Figure 4 for histopathological examination of the liver, spleen, kidney, and bone marrow; Figure 5 shows the results of liver MS analyses). Although heart involvement was suspected, the patient and his family refused myocardial biopsy considering the danger, but the diagnosis of primary light chain (κ) amyloidosis was sufficient. At this point, the mystery of splenomegaly with an unknown etiology, persistent cirrhosis, proteinuria, peripheral neuropathy, and cardiac function damage was finally resolved.
ABCB4 gene mutation-associated cirrhosis with systemic amyloidosis was diagnosed.
Following discharge, the patient adhered to the prescribed ursodeoxycholic acid regimen at a dose of 15 mg/kg/d and treatment with CD38 monoclonal antibody daratumumab (intravenous infusion, 900 mg per administration, administered every other week), and underwent regular monitoring.
Urinary protein levels were negative, whereas the free light chains present in both the peripheral blood and urine returned to a standard level. In addition, electrocardiography showed normal readings. The patient’s bilateral lower limb numbness resolved and his clinical status remained stable. The patient is currently undergoing medical treatment and receiving frequent monitoring (Table 1).
| Analyte | November, 2018 | July, 2020 | August, 2021 | December, 2021 | January, 2022 | March, 2022 | April, 2022 | May, 2022 | Reference range |
| WBC as × 109/L | 1.14 | 14.94 | 10.1 | 9.6 | 13.9 | 9.6 | 7.8 | 8.5 | 3.5-9.5 |
| Hb in g/L | 83 | 97 | 96 | 86 | 97 | 91 | 88 | 95 | 115-150 |
| PLT as × 109L | 33 | 359 | 253 | 316 | 267 | 377 | 361 | 345 | 125-350 |
| ALT in U/L | 58.2 | 33.6 | 56.1 | 81.9 | 66.2 | 716 | 42.8 | 31.6 | 7-40 |
| AST in U/L | 136.4 | 108.6 | 175.8 | 236.5 | 315.2 | 298.3 | 211.9 | 125.8 | 13-35 |
| TBIL in μmol/L | 66.1 | 179 | 14.2 | 46.9 | 67.2 | 36.9 | 33.2 | 23.04 | < 23 |
| DBIL in μmol/L | 47.8 | 128.6 | 9 | 31.85 | 39.2 | 26.7 | 18.39 | 13.81 | ≤ 8 |
| ALB in g/L | 35.3 | 24.6 | 16.9 | 25.86 | 22.71 | 26.39 | 25.33 | 27.58 | 40-55 |
| ALP in U/L | 501 | 1174 | 426 | 904 | 887 | 1160 | 875 | 978 | 35-100 |
| GGT in U/L | 268.26 | 1367 | 439.51 | 387.3 | 971.19 | 554 | 382 | 542.22 | 7-45 |
| ChE in U/L | 2796 | 2301 | 3958 | 4623 | 3112 | 4728 | 2738 | 3288 | 5000-12000 |
| CREA in μmol/L | 48 | 49.83 | 56.87 | / | 96.6 | 66 | 68 | 46.9 | 41-73 |
| NT-proBNP in pg/mL | / | 176 | 238.4 | / | 3020 | 475 | / | 110.9 | 41.4-153 |
| cTnT in ng/mL | 0.003 | 0.009 | / | / | 0.645 | 0.465 | / | 0.001 | 0-0.014 |
| LDH in U/L | 136 | 244 | 279 | 426 | 295 | 481 | 364 | 290 | 120-250 |
| uPRO | / | Positive (2 +) | Positive (3 +) | Positive (3 +) | Positive (3 +) | Positive (3 +) | Positive (2 +) | Positive (1 +) | Negative (-) |
| uALB in mg/L | / | 964 | / | / | 744.98 | / | / | 352.17 | < 140 |
| Serum κ light chain in mg/dL | / | / | / | / | 1050 | 1710 | 689 | 649 | 629-1350 |
| Serum λ light chain in mg/dL | / | / | / | / | 537 | 916 | 330 | 249 | 313-723 |
| Urinary κ light chain in mg/dL | / | / | / | / | 52 | 72.4 | / | 4.08 | 0-1.85 |
| Urinary λ light chain in mg/dL | / | / | / | / | 37.9 | 62.5 | / | < 5 | 0-5 |
| Drug | UDCA | UDCA + Daratumumab |
PFIC-3 is an autosomal recessive hereditary disease caused by mutations in the ABCB4 gene. The incidence rate of this condition is notably low, with an average occurrence of 1 in 500000, and it is predominantly sporadic. Mutation of the ABCB4 gene results in impairment of the MDR3 glycoprotein, which is present in the membrane of the capillary bile duct of hepatocytes. This leads to disruption in the metabolism of bile acids, elevation in the formation of cholesterol stones, damage to the bile duct, and the onset of intrahepatic cholestasis. Laboratory analyses indicated a continual elevation in serum GGT along with hyperbilirubinemia, primarily resulting from an increase in conjugated bilirubin. Histopathological examination of the liver revealed nonspecific alterations, including intrahepatic bile duct damage and hyperplasia of the fibrous tissue. Clinical manifestations of PFIC-3 frequently include gallbladder stones and chronic progressive liver damage. In some cases, affected patients may develop cirrhosis during late childhood or adolescence[1,8]. Currently, the administration of ursodeoxycholic acid is primarily oral in nature, with the aim of competing with primary bile acids for reabsorption in the small intestine. This approach is intended to mitigate the harm caused by cholestasis in liver cells. Liver transplantation is typically required in patients with end-stage liver disease[9].
Systemic light chain amyloidosis is a rare disease. European and American countries have reported an incidence rate of 8-10 cases per million person-years. The formation of amyloid proteins is attributed to the misfolding of the monoclonal immunoglobulin light chain, leading to its deposition in tissues and organs. This process results in the destruction of tissue structure, organ dysfunction, and the onset of progressive disease. This condition is primarily associated with the abnormal proliferation of clonal plasma cells, with a minor proportion linked to lymphoproliferative diseases[10]. The amyloid protein exhibits the following characteristics. According to sources[11,12], hematoxylin and eosin staining appears eosinophilic and homogeneous, whereas Congo red staining displays a brick red color. In addition, the use of a polarization microscope results in apple green birefringence. In general, the biopsy positivity rate for symptomatic organs or tissues is > 95%, whereas that for bone marrow ranges from 50%–65%. Routine recommendations for myocardial biopsy are discouraged because of its high risk[13]. Currently, the gold standard for diagnosis is based on histopathological results and protein MS analyses. According to the types of monoclonal light chain deposition, it is divided into λ light chain type and κ light chain type. In clinic, λ light chain type is the main one, accounting for about 85%, and κ light chain type is rare. Due to the different tissues and organs involved, the clinical manifestations of the disease are diverse, causing great difficulties in early diagnosis and treatment. Most patients have an irreversible function in the involved tissues and organs when they are diagnosed. Autologous peripheral blood stem cell transplantation and targeted plasma cell therapy, such as daratumumab, are the main treatments for amyloidosis.
The co-occurrence of PFIC-3 and systemic amyloidosis in a single patient has not been reported, likely because of the rarity of these diseases. The patient initially presented with an unexplained splenomegaly and cholecystolithiasis. Subsequent diagnostic tests, including liver function analyses, abdominal CT, gastroscopy, and liver histopathology, confirmed the diagnosis of cirrhosis. However, its underlying etiology remains unclear. Subsequently, the state of liver cirrhosis progressively declines, with the involvement of numerous organs including the kidney, peripheral nerves, and heart. A heterozygous variation at the ABCB4 c.2318G>T locus was identified using gene sequencing. Pathological biopsy of the liver tissue revealed bile duct injury, cholestasis, and cirrhosis, which was consistent with the cirrhosis caused by PFIC-3, in conjunction with elevated GGT and ALP levels. However, ailments do not account for the engagement of many bodily systems. Through further detection of Congo red staining in the liver, spleen, kidney, bone marrow, and other tissues of the patient, and MS analyses of liver tissue amyloidosis, Igκ was highly expressed. Finally, it was thoroughly and definitively diagnosed as rare PFIC-3 complicated with systemic light chain κ amyloidosis.
For the treatment of liver cirrhosis related to ABCB4 gene mutations, the early use of ursodeoxycholic acid can improve cholestasis, delay the process of fibrosis, and thus improve prognosis[3]. Antiplasma cell therapy is the core treatment for systemic light chain κ amyloidosis. Daratumumab is a humanized IgG1-κ monoclonal antibody targeting CD38 antigen on the plasma cell surface. Studies have shown that this drug can quickly achieve deep hematological and organ remission with good safety[14,15]. The patient's condition worsened after long-term administration of ursodeoxycholic acid for a long period. After daratumumab treatment, the hematuria light chain decreased noticeably, the urine protein level became negative, and symptoms such as numbness and palpitations in both lower limbs disappeared. This treatment had a positive effect.
In conjunction with the aforementioned instance, we made the following observations: (1) The majority of experts tend to have a limited scope of expertise and may overlook the potential existence of alternative illnesses; (2) Patients diagnosed with liver cirrhosis exhibiting elevated levels of ALP and GGT, coupled with unknown bile duct changes in liver biopsy, may benefit from genetic testing to identify rare genetic disorders, such as liver lesions caused by ABCB4 mutations; (3) The involvement of multiple organs, including the liver, kidneys, heart, and peripheral nervous system, in systemic amyloidosis results in complex and inconspicuous clinical manifestations, posing challenges for early diagnosis and treatment. The diagnosis relies upon the utilization of Congo red staining for tissue biopsy and protein MS analyses; and (4) Liver transplantation is the preferred treatment option for patients with liver-limited amyloidosis.
For systemic amyloidosis, which affects multiple tissues and organs throughout the body, the current therapeutic approach involves the use of CD38 monoclonal antibody drug regimens. The administration of anti-plasma cell therapy in the early stages, along with symptomatic treatment, has been found to be beneficial in enhancing clinical symptoms and extending the duration of survival, as per previous research.
We thank the patient for his contribution to this case report. We thank Dr. Li Hong and Dr. Zhang Quan for their scientific guidance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Paparoupa M, Germany; Protopapas AA, Greece S-Editor: Li L L-Editor: Filipodia P-Editor: Ju JL
| 1. | Stättermayer AF, Halilbasic E, Wrba F, Ferenci P, Trauner M. Variants in ABCB4 (MDR3) across the spectrum of cholestatic liver diseases in adults. J Hepatol. 2020;73:651-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 2. | Wang HH, Portincasa P, Liu M, Wang DQ. Genetic Analysis of ABCB4 Mutations and Variants Related to the Pathogenesis and Pathophysiology of Low Phospholipid-Associated Cholelithiasis. Genes (Basel). 2022;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Davit-Spraul A, Gonzales E, Baussan C, Jacquemin E. The spectrum of liver diseases related to ABCB4 gene mutations: pathophysiology and clinical aspects. Semin Liver Dis. 2010;30:134-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 4. | Kumar N, Zhang NJ, Cherepanov D, Romanus D, Hughes M, Faller DV. Global epidemiology of amyloid light-chain amyloidosis. Orphanet J Rare Dis. 2022;17:278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 5. | Merlini G. AL amyloidosis: from molecular mechanisms to targeted therapies. Hematology Am Soc Hematol Educ Program. 2017;2017:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 6. | Cuddy SAM, Falk RH. Amyloidosis as a Systemic Disease in Context. Can J Cardiol. 2020;36:396-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Gertz MA. Immunoglobulin light chain amyloidosis: 2022 update on diagnosis, prognosis, and treatment. Am J Hematol. 2022;97:818-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 8. | Degiorgio D, Corsetto PA, Rizzo AM, Colombo C, Seia M, Costantino L, Montorfano G, Tomaiuolo R, Bordo D, Sansanelli S, Li M, Tavian D, Rastaldi MP, Coviello DA. Two ABCB4 point mutations of strategic NBD-motifs do not prevent protein targeting to the plasma membrane but promote MDR3 dysfunction. Eur J Hum Genet. 2014;22:633-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Saleem K, Cui Q, Zaib T, Zhu S, Qin Q, Wang Y, Dam J, Ji W, Liu P, Jia X, Wu J, Bai J, Fu S, Sun W. Evaluation of a Novel Missense Mutation in ABCB4 Gene Causing Progressive Familial Intrahepatic Cholestasis Type 3. Dis Markers. 2020;2020:6292818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Lin XY, Pan D, Sang LX, Chang B. Primary localized gastric amyloidosis: A scoping review of the literature from clinical presentations to prognosis. World J Gastroenterol. 2021;27:1132-1148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Sinha A, Manjunath GV, Basavaraj V. Primary cutaneous amyloidosis: A clinicopathological, histochemical, and immunohistochemical study. Indian J Pathol Microbiol. 2021;64:323-328. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Picken MM. The Pathology of Amyloidosis in Classification: A Review. Acta Haematol. 2020;143:322-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 13. | Dispenzieri A, Gertz MA, Buadi F. What do I need to know about immunoglobulin light chain (AL) amyloidosis? Blood Rev. 2012;26:137-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Roussel M, Merlini G, Chevret S, Arnulf B, Stoppa AM, Perrot A, Palladini G, Karlin L, Royer B, Huart A, Macro M, Morel P, Frenzel L, Touzeau C, Boyle E, Dorvaux V, Le Bras F, Lavergne D, Bridoux F, Jaccard A. A prospective phase 2 trial of daratumumab in patients with previously treated systemic light-chain amyloidosis. Blood. 2020;135:1531-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 15. | Muchtar E, Dispenzieri A, Gertz MA, Kumar SK, Buadi FK, Leung N, Lacy MQ, Dingli D, Ailawadhi S, Bergsagel PL, Fonseca R, Hayman SR, Kapoor P, Grogan M, Abou Ezzeddine OF, Rosenthal JL, Mauermann M, Siddiqui M, Gonsalves WI, Kourelis TV, Larsen JT, Reeder CB, Warsame R, Go RS, Murray DL, McPhail ED, Dasari S, Jevremovic D, Kyle RA, Lin Y, Lust JA, Russell SJ, Hwa YL, Fonder AL, Hobbs MA, Rajkumar SV, Roy V, Sher T. Treatment of AL Amyloidosis: Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) Consensus Statement 2020 Update. Mayo Clin Proc. 2021;96:1546-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |