Published online May 16, 2023. doi: 10.12998/wjcc.v11.i14.3340
Peer-review started: February 16, 2023
First decision: March 14, 2023
Revised: March 26, 2023
Accepted: April 12, 2023
Article in press: April 12, 2023
Published online: May 16, 2023
Processing time: 88 Days and 20.3 Hours
Early-onset progressive encephalopathy with brain edema and/or leukoencephalopathy-1 (PEBEL1) is a rare autosomal recessive severe neurometabolic disease. The aim of this study was to investigate the clinical characteristics and genetic pathogenicity of PEBEL1 caused by rare NAXE (or APOA1BP)-related defects.
The patient was a girl aged 2 years and 10 mo. She was hospitalized due to walking disorder for > 40 d. The clinical manifestations were ataxia, motor function regression, hypotonia, and eyelid ptosis. Within 1 mo of hospitalization, she developed sigh breathing, respiratory failure, cerebellar edema and brain hernia, and finally she died. Changes were found in cranial imaging, including cerebellar edema accompanied by symmetrical myelopathy. Through whole exome sequencing, we detected NAXE compound heterozygous variation (NM 144772.3) c.733A>C (p. Lys245Gln, dbSNP: rs770023429) and novel variation c.370G>T (p.Gly124Cys) in the germline gene. The clinical features and core phenotypes of this case were consistent with 18 previously reported cases of PEBEL1.
This is the first case of NAXE-related PEBEL1 with severe clinical phenotype in Mainland China. The p.Gly124Cys mutation discovered in this case has enriched the pathogenic variation spectrum of NAXE.
Core Tip: We report a girl with early-onset progressive encephalopathy with brain edema and/or leukoencephalopathy-1 (PEBEL1), and review the reported cases in the literature. The disease has rapid progression with an unfavorable prognosis. Gene detection is the only diagnostic method. We report the first case of PEBEL1 with severe clinical phenotype in Mainland China.
- Citation: Ding L, Huang TT, Ying GH, Wang SY, Xu HF, Qian H, Rahman F, Lu XP, Guo H, Zheng G, Zhang G. De novo mutation of NAXE (APOAIBP)-related early-onset progressive encephalopathy with brain edema and/or leukoencephalopathy-1: A case report. World J Clin Cases 2023; 11(14): 3340-3350
- URL: https://www.wjgnet.com/2307-8960/full/v11/i14/3340.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i14.3340
Early-onset progressive encephalopathy with brain edema and/or leukoencephalopathy 1 (PEBEL1, OMIM: #617186) is a rare autosomal recessive severe neurometabolic disease[1]. At present, more than 10 cases have been reported worldwide since 2016[1-3], but only one case has been reported in China. In this study, the clinical data of a child with PEBEL1 treated in the Department of Neurology, Children’s Hospital Affiliated to Nanjing Medical University in July 2021 were retrospectively analyzed, and the related literature was reviewed to improve the understanding, diagnosis, and treatment of the disease.
The patient was a girl aged 2 years and 10 mo. She was hospitalized because of walking disorder for > 40 d.
Forty days ago, she presented with unstable walking and was suspected of having synovitis and stayed in bed at home. She had an episode of fever 30 d prior to that, and the fever spike was 38°C. Blood examination in the outpatient clinic showed leukocytosis, and the fever subsided after oral administration of cephalosporin for 1 wk. During this period, the patient did not leave bed or walk again. Two weeks ago, she developed binocular movement disorder, slow eyeball pursuit, and slight drooping eyelids. Gradually, the patient had wobbling in sitting, and resisted sitting, and cried when urinating, so she was admitted to hospital.
There was a sudden suspicious episode of choking during a meal 3 mo ago, with gaze, unconsciousness, and clenched teeth for 6-7 min, which was relieved after patting on the back and vomiting. There was no abnormality in computed tomography (CT) examination of the head and chest in the local hospital. She was scratched by a cat 2 mo ago and was injected with rabies vaccine three times in 2 wk. Fifty days ago, she suddenly developed generalized weakness and bowed her head during playing, and then her limbs became stiffer, which lasted for about 1 min, followed by vomiting and incontinence. The follow-up was as usual, and the parents made an appointment for a video electroencephalogram examination, which was not done because the patient was immobilized at home.
There was no special personal history, and the milestone of intellectual and motor development was normal since childhood. Her parents were in good health and denied any consanguinity. The 11-year-old brother also was in good health.
There was no special personal history, and the milestone of intellectual and motor development was normal since childhood. Her parents were in good health and denied any consanguinity. The 11-year-old brother also was in good health.
After admission, blood routine tests were done. Biochemical studies, blood ammonia, blood lactic acid, erythrocyte sedimentation rate, procalcitonin, six items of coagulation, complete set of autoantibodies, cellular immunity, humoral immunity, four items of infectious diseases, and seven items of thyroid function tests were normal. Screening of hereditary metabolic diseases in hematuria was not abnormal. Four items of tumor: nonspecific enolase was 40.33 ng/mL (0–16.3 ng/mL), a-fetoprotein, carcinoembryonic antigen and carbohydrate antigen 19-9 were normal. Cerebrospinal fluid biochemistry showed: protein 0.27 g/L and glucose 4.82 mmol/L. Routine cerebrospinal fluid analysis showed: nucleated cell count 3 × 106/L; cerebrospinal fluid-MP, epidermolysis bullosa, cytomegalovirus and herpes simplex virus deoxyribonucleic acid was negative; cerebrospinal fluid and blood autoimmune encephalitis antibody, central demyelination antibody, and peripheral nerve disease spectrum antibody were negative; and cerebrospinal fluid pathogen macro gene was negative.
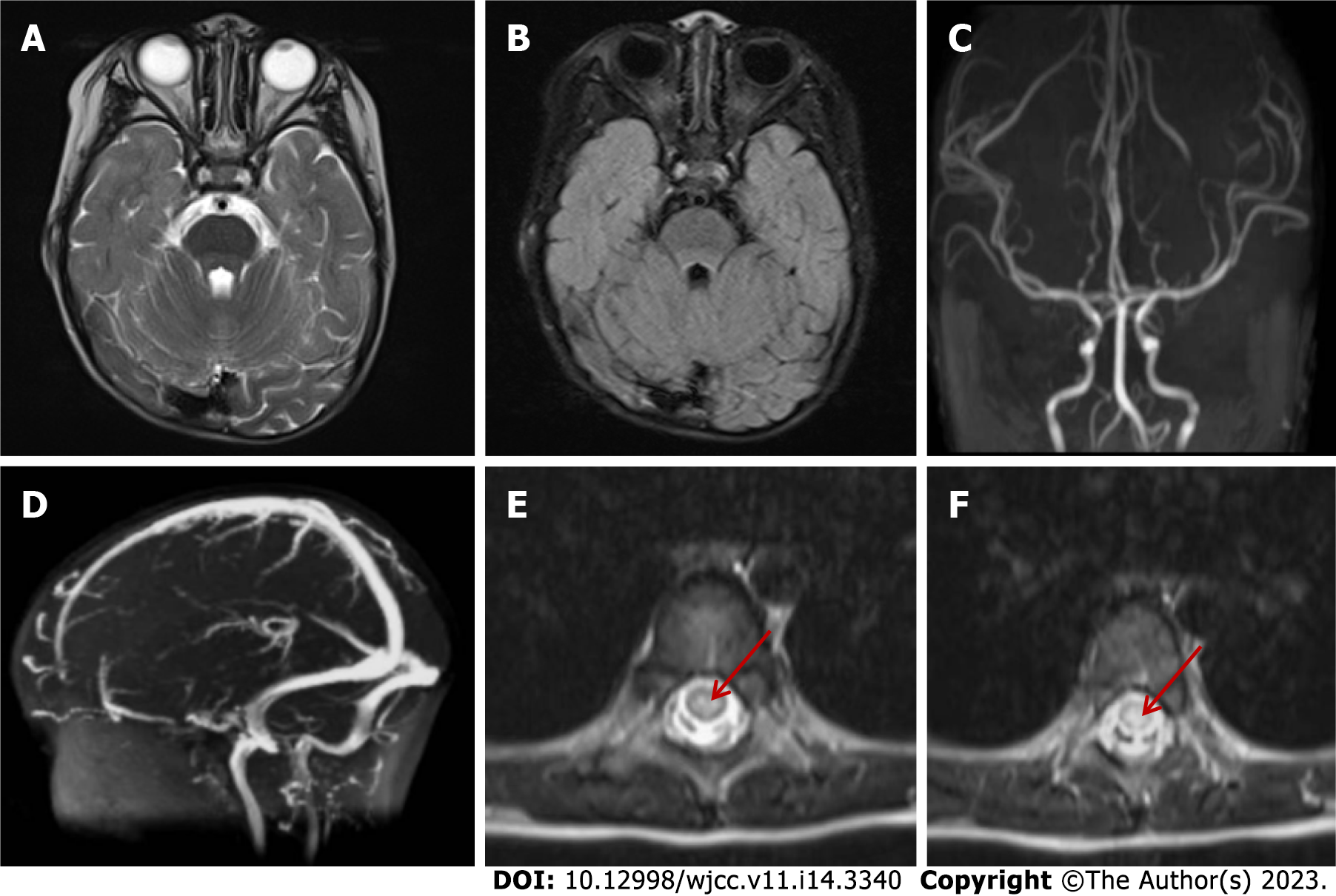
Plain CT of the chest and abdomen showed no abnormalities, with normal abdominal contents and obvious bladder filling. No abnormality was found in skull magnetic resonance imaging (MRI), magnetic resonance (MR) angiography and MR venography on day 2 after admission, and spinal MRI showed T2 high signal in the spinal cord at the T7–10 level (poor coordination, heavy artifact) (Figure 1). Electroencepholography showed background moderation, poor rhythmicity and responsiveness, with spike waves and spike slow waves in bilateral anterior and middle temporal regions during sleep, which were not synchronized between left and right.
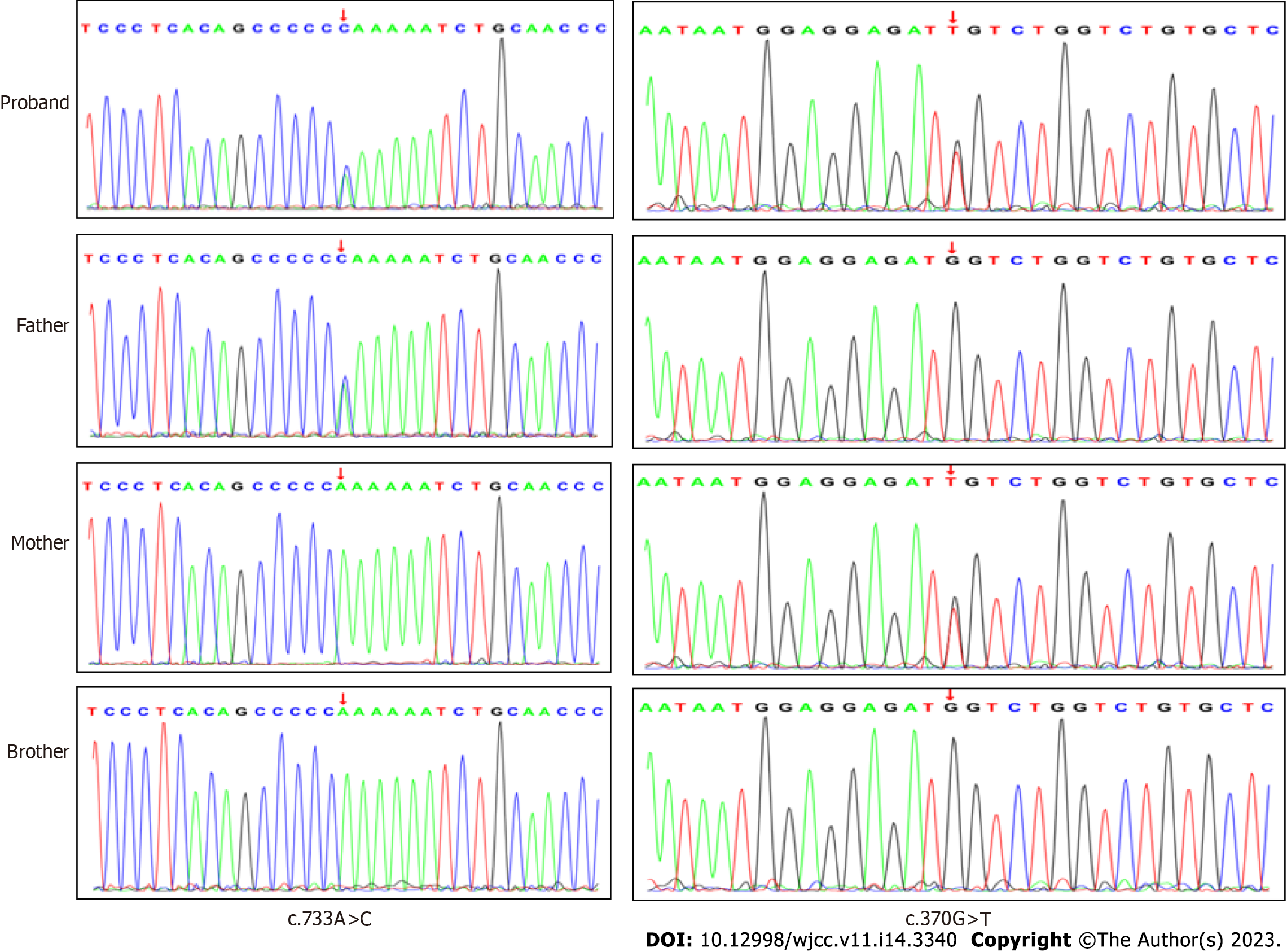
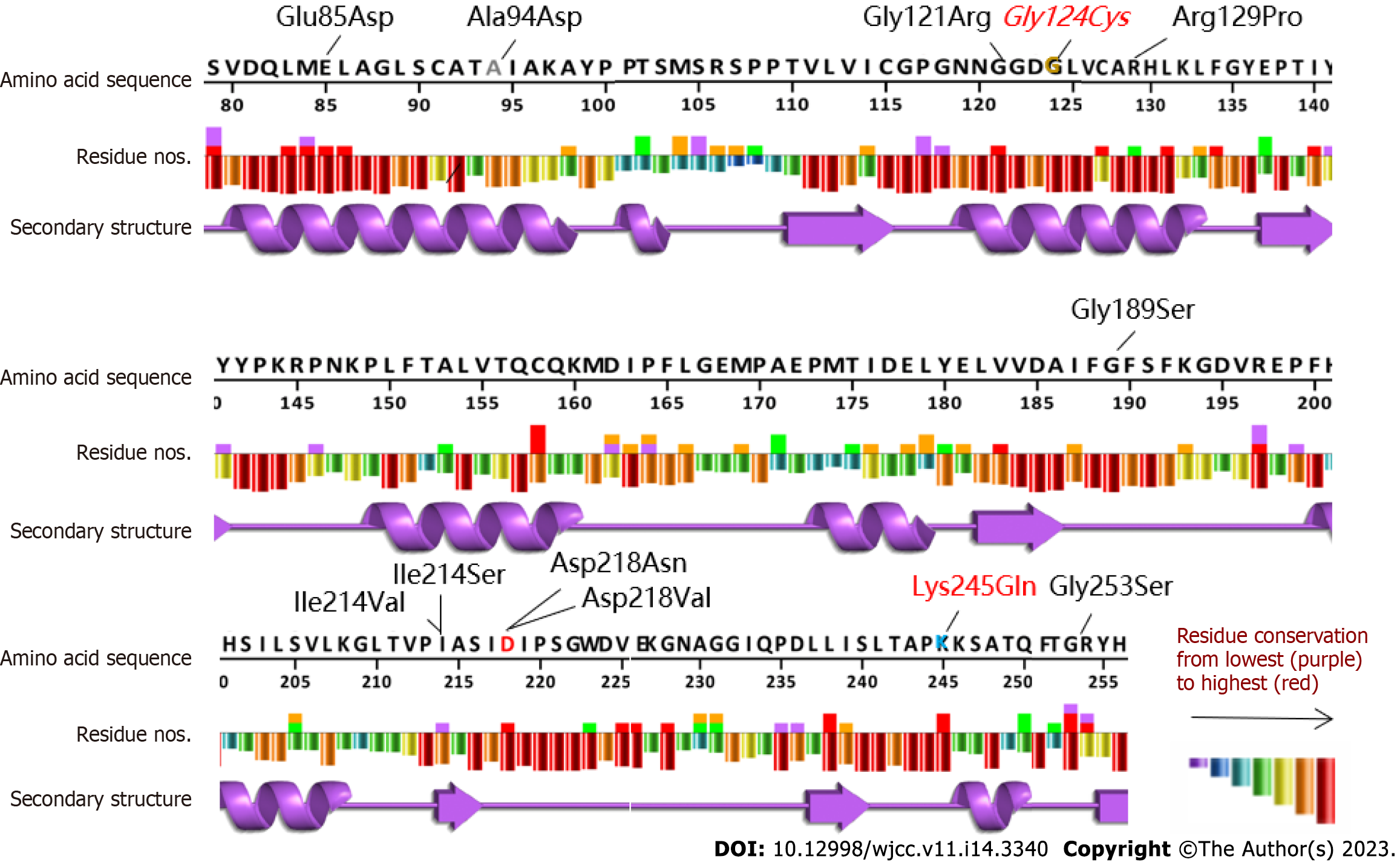
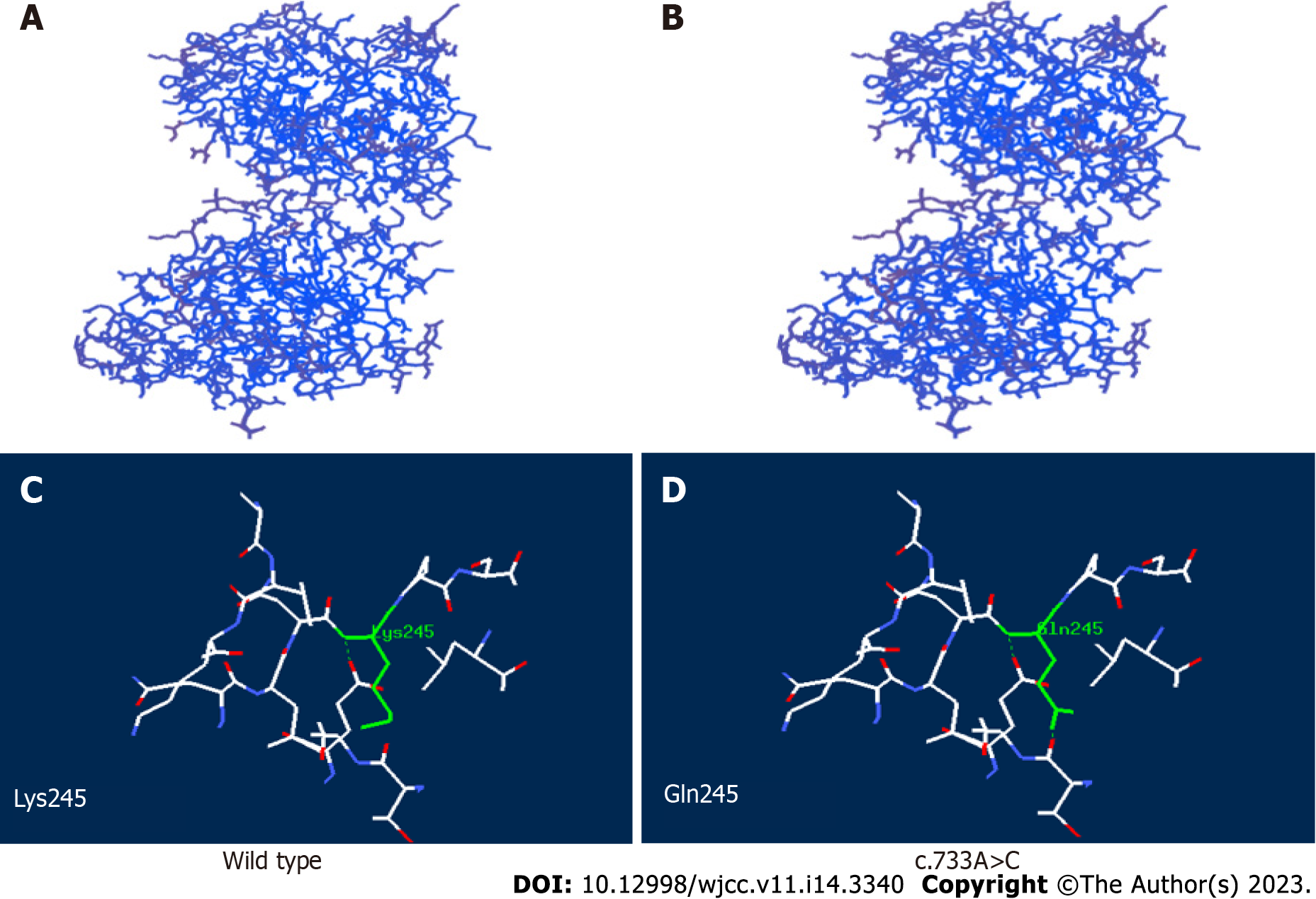
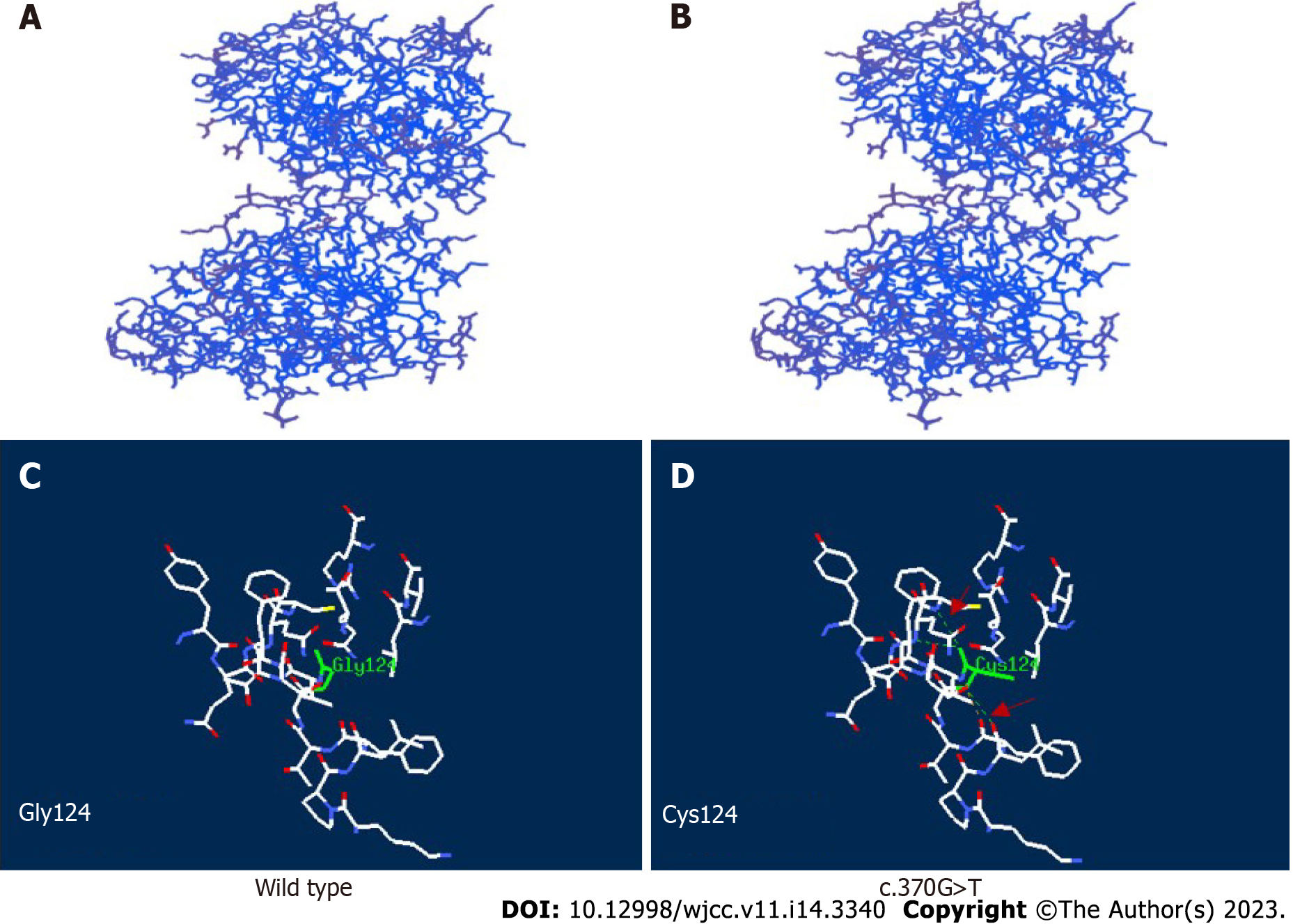
With the approval of the Medical Ethics Committee of the hospital (approval number: 202111116-1) and the informed consent of the guardians of the child, 2 mL peripheral blood from the patient and her parents were taken for whole exome sequencing (Beijing Zhiyin Dongfang). The whole-exome library was constructed using xGen ®Exome Research Panel v1.0 (IDT, United States) capture probe, and the NovaSeq 6000 (Illumina, United States) series sequencer was used for high-throughput sequencing. The compound heterozygous mutations c.733A>C (p.Lys245Gln, dbSNP: rs770023429) and c.370G>T (p.Gly124Cys) in NAXE (APOA1BP) inherited from her parents were identified and verified by Sanger sequencing (Figure 2). According to the American Society of Medical Genetics 2015[3], Lys245Gln (reported in the PS1+PM1, gnomAD East Asia MAF: 0.0019, PEBEL1 case[4]) was likely pathogenic and Gly124Cys (PM1+PM2+PP3, no MAF record) was annotated as unknown pathogenicity variation. The variant amino acid residues were analyzed using VarSite (https://www.ebi.ac.uk/thornton-srv/databases/VarSite); a variant analysis tool provided by European Institute of Bioinformatics, and the two variants were both highly evolutionarily conservative (Figure 3). The conservatism of Lys245Gln in 174 homologous sequences was 0.5, which was lower than that of Gly124Cys in 190 homologous sequences (0.1). Using SWISS-MODEL and Swiss-pdb Viewer software to predict the pathogenicity of the mutation sites, the protein structure, the residues of the mutation site, and the functional sites nearby, it is suggested that the possible pathogenicity, c.733A>C site is shown in Figure 4, c.370G>T mutation site is shown in Figure 5.
The patient was diagnosed with PEBEL1.
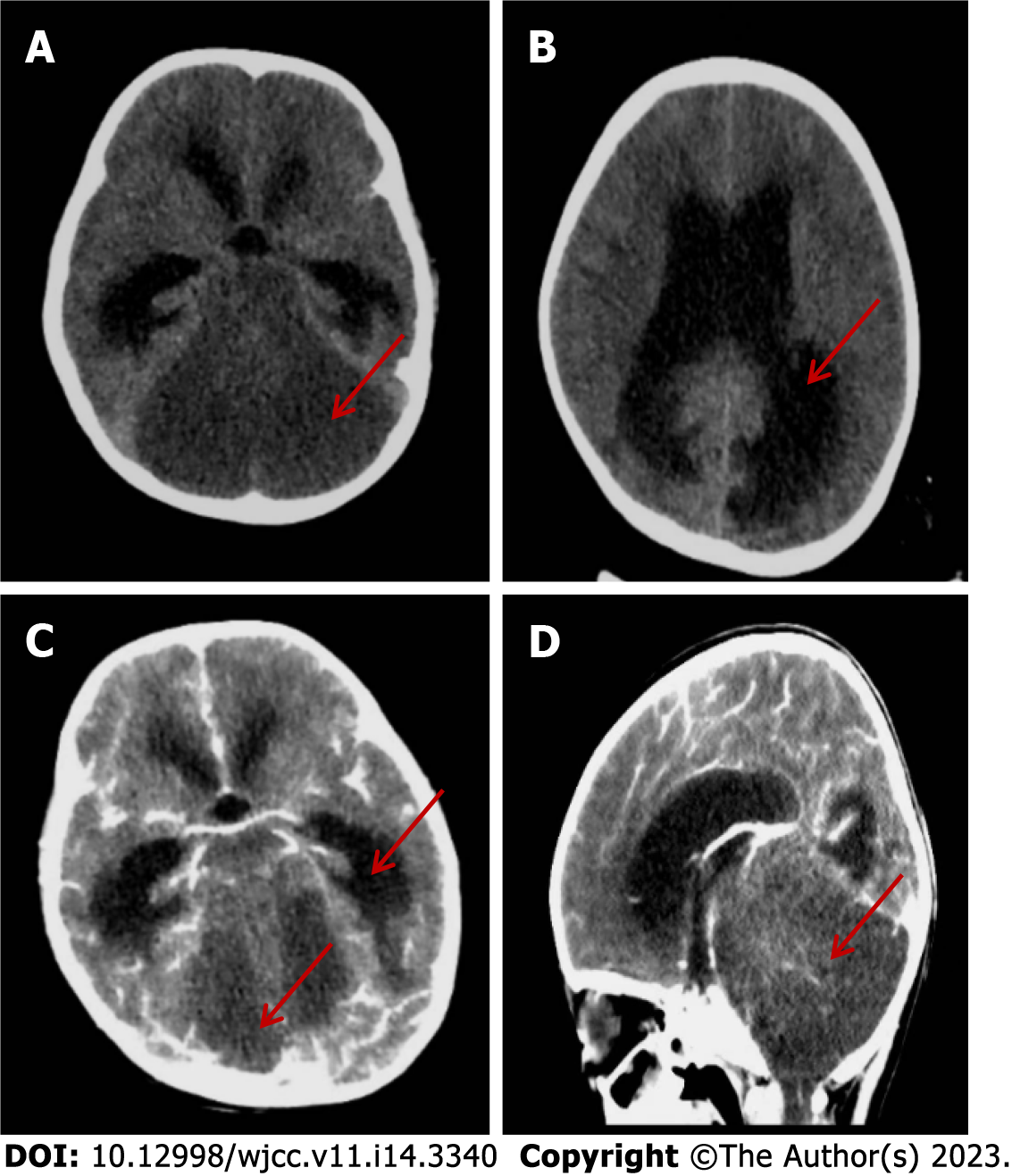
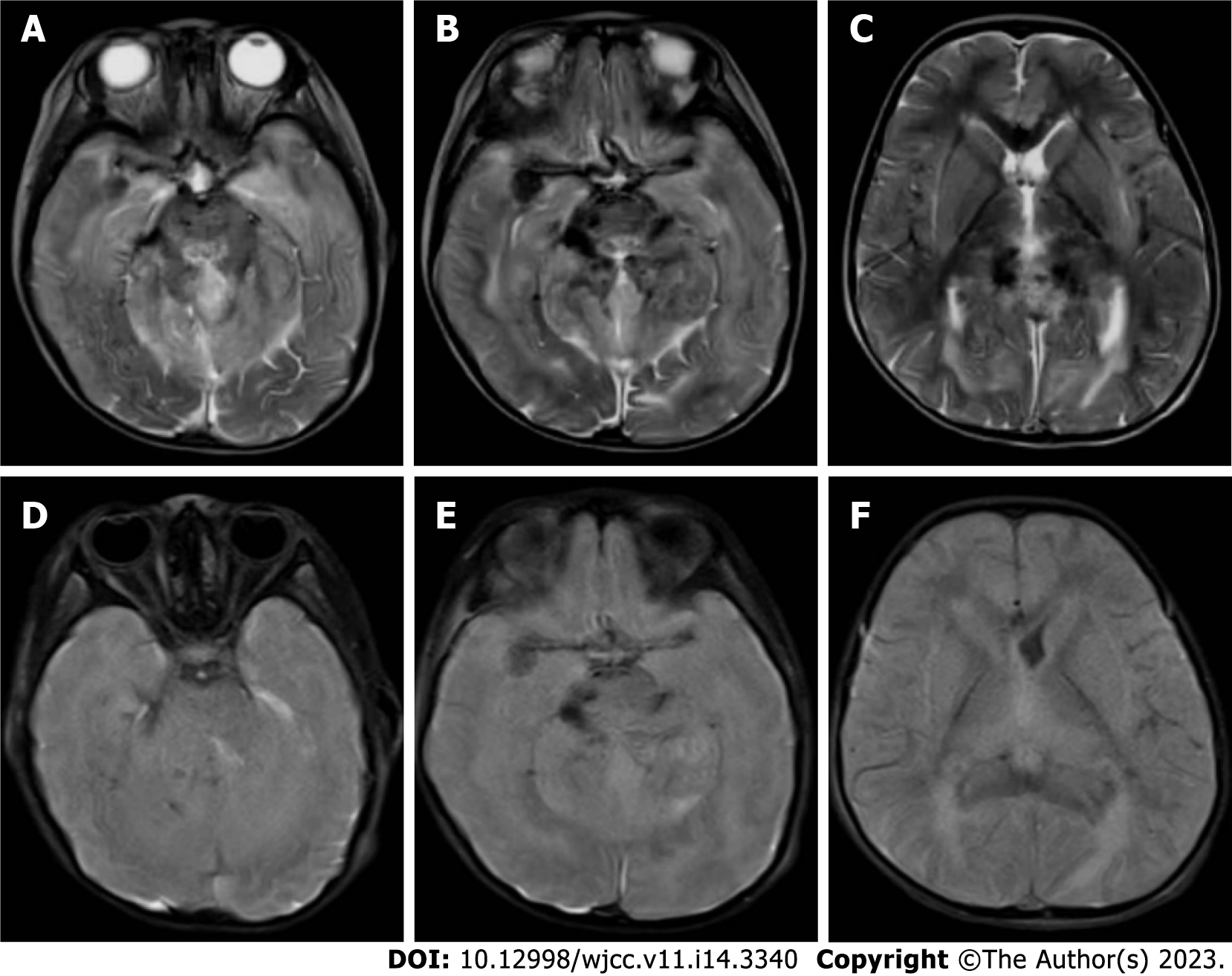
After admission, a detailed examination was done and the patient was treated with acyclovir, dexamethasone (0.5 mg/kg), gammaglobulin (2 g/kg), and other anti-inflammatory drugs. Urinary retention occurred on the day 3 and indwelling catheterization was performed. On day 5, the patient developed confusion, irregular respiratory rhythm, and poor response, and was transferred to the pediatric intensive care unit. This was followed by a decrease in heart rate and blood oxygen saturation, and she received cardiopulmonary resuscitation and mechanical ventilation. Nutritional support with levocarnitine and coenzyme Q10 was given, and simultaneous high-dose methylprednisolone (20 mg/kg) was given for 3 d, and plasma exchange was performed for 2.5 h on day 10. Bilateral pupil dilation, the disappearance of light reflex and deep coma appeared on day 12. Cranial CT suggested diffuse cerebellar swelling with hydrocephalus and ventricular dilatation, and further improvement of cranial CT angiography enhancement suggested an unclear display of straight sinus and no significant parenchymal enhancing mass shadow was seen (Figure 6). On day 14, after multidisciplinary team discussion, intraventricular drilling and drainage, and intracranial pressure probe implantation were performed. About 100 mL cerebrospinal fluid was drained after surgery, and the intracranial pressure was maintained at 20–74 mmHg. On day 17, whole exosome sequencing detected NAXE compound heterozygous mutation, and we added 100 mg nicotinamide intravenous drip. The patient gradually deteriorated and developed a slow heart rate, hypotension, central diabetes insipidus and electrolyte disturbance, and was treated with symptomatic support such as volume expansion, plasma, albumin, electrolyte supplement, blood transfusion, and maintenance of blood pressure using dopamine, norepinephrine, epinephrine, and posterior pituitary hormone. On day 26, cerebral MRI showed cerebellar swelling, possible herniation of the cerebellar curtain notch, and multiple abnormal signals in the brain parenchyma and cervical spinal cord (Figure 7).
After 30 d, the child became brain dead and her parents abandoned treatment.
PEBEL1 is a rare fatal encephalopathy caused by a double allele mutation of NAXE (APOA1BP) on chromosome 1q22. In 2016, Spiegel[2] reported for the first time that five children from Israel, who were near relatives were affected. The age at onset was 6-12 mo, with loss of motor function after infection, bedridden at the age of 2 years, mechanically ventilated, and finally in a vegetative state. Four patients died between 1 and 3 years of age, and one child was supported by a ventilator until 5.5 years of age, and MRI revealed deep white matter lesions. Kremer et al[1] summarized the clinical features of five patients who presented with infantile/early childhood onset, usually caused by fever with rapidly progressive deterioration of neurological function. The patients showed muscular hypotonia, motor development regression, cognitive loss, ataxia, nystagmus, seizures, quadriplegia, and respiratory failure, which eventually led to a vegetative state and brain death. Brain and spinal cord imaging may show white matter abnormalities, cerebral atrophy, cerebellar edema, and myelopathy. It has also been reported[1] that a large area of subacute bullous dermatosis occurred within a few weeks after the onset of neurological symptoms.
The keywords NAXE, APOAIBP, NAD(P)HX epimerase and AIBP were searched in PubMed, ClinVar, Wanfang, and CNKI, and papers with clear clinical data were selected. As of October 2021, 17 reported pathogenic NAXE variants in 12 cases had been collected (Table 1). These included 11 missense mutations (Glu85Asp, Ala94Asp, Gly121Arg, Arg129Pro, Gly189Ser, Ile214Val, Ile214Ser, Asp218Asn, Asp218Val, Lys245Gln and Gly253Ser, 64.7%, 11/17), two splice site mutations (c. 516+1G>A and c. 665-1G>A, 11.8%, 2/17), two nonsense mutations (Tyr59Ter and Gln66Ter,11.8%,2/17), one frameshift mutation (Ala248fs, 5.9%, 1/17), and one deletion/insertion mutation (c.804_807delinsA/Lys270del, 5.9%, 1/17)[1,2,4-7]. The Lys245Gln detected in the present case was the reported homozygous pathogenic variant[4]. The clinical characteristics of the present case and 18 previous cases from 12 families are summarized as follows[1-8]. The age of onset of PEBEL1, except for a German family with two girls who developed the disease at the age of 20 and 22 years, respectively[4], was < 3 years in 89% of the patients (17/19). Most cases were induced by febrile infection (58%, 11/19). The main manifestations were rapidly progressive or recurrent respiratory insufficiency (79%, 15/19), motor cognitive regression (79%, 15/19), decreased muscle strength and muscle tone (79%, 15/19), ataxia (42%, 8/19), nystagmus (32%, 6/19), strabismus (21%, 4/19), seizures (21%,4/19), bilateral blepharoptosis (11%, 2/19), dysarthria and dysphagia (21%, 4/19), and skin erythema rash (11%, 2/19). Eleven of the 19 patients had brain MRI changes, including brain edema (55%, 6/11), brain white matter abnormalities (27%, 3/11), brain atrophy (27%, 3/11), myelopathy (27%, 3/11), cortical and basal ganglia lesions (10%, 1/11), and brain stem and intracranial hemorrhage (10%, 1/11). The cerebrospinal fluid lactic acid test was elevated in seven of the 19 cases, (71%, 5/7), and 12 of 19 cases died of end-stage coma within 3 years of age (63%, 12/19).
| Ref. | Gender | Nationality | NAXEvariation | Age at onset | Age at death | Inducement | Clinical characteristics | MRI features | |
| 1 | This study | F | China | p.Lys245Gln; p.Gly124Cys | 2 yr 10 mo | 3 yr | Fever | Respiratory insufficiency, motor regression, poor eye contact, hypotonia, dysphagia, nystagmus, drowsiness, bilateral ptosis | Cerebellar edema, myelopathy |
| 2 | [1] | M | China | p.Glu85Asp; p.Gly121Arg | 2 yr | Unknown | Fever/infection | Decreased muscle tone of the extremities, weakening abdominal wall reflex and cremaster reflex, unsteady walking, finger-nose test and Romberg sign positive | Brain atrophy |
| 3 | [6] | M | Iran | p.Gly189Ser | 2 yr | 3 yr | Unknown | Neuromotor development and cognitive regression, unclear speech, uncoordinated hand movement, nystagmus, poor coordination, impaired finger–nose test, increased lactic acid in cerebrospinal fluid | Bilateral temporal cortex, basal ganglia lesions |
| 4 | [2] | M | Turkey | p.Arg129Pro; p.Ile214Ser | 1.5 yr | 3 yr | Unknown | Cognitive impairment, axial dystonia, quadriplegia, strabismus, increased deep tendon reflex of lower extremities, ankle clonus, bilateral Babinski sign positive | Not significant |
| 5-1 | [5] | F | Germany | c.665-1G>A; p.Gly253Ser | 22 yr | > 29 yr | Unknown | Recurrent illness, initial headache, respiratory insufficiency, developmental disabilities, seizures, myoclonus, comatose state, cerebellar ataxia, spastic quadriplegia, dysarthria, dysphagia, cervical dystonia, non-infectious fever, psychiatric symptoms | Not significant |
| 5-2 | [5] | F | Germany | c.665-1 G>A; p.Gly254Ser | 20 yr | 22 yr | Alcohol, cannabis | Recurrent illness, initial headache, respiratory insufficiency, comatose state, cerebellar ataxia, cognitive impairment, myoclonus, nystagmus, diplopia, neuropsychiatric symptoms, hypokinesia | Not significant |
| 6 | [5] | M | Saudi Arabia | p.Lys245Gln | Unknown | Unknown | Unknown | Respiratory insufficiency, progressive motor development delay, dystonia, septicemia | Unknown |
| 7 | [5] | M | Jordan | p.Asp218Asn | 1 d | Unknown | Unknown | Respiratory insufficiency, coma, developmental disabilities, hypodystonia, strabismus, bradycardia, decreased serum creatinine, hypoventilation, thrombocytosis, mitral regurgitation | White matter abnormalities, brainstem MRI signal intensity abnormalities, intracranial hemorrhage, brain atrophy |
| 8 | [5] | M | India | p.Ile214Val | Unknown | Unknown | Unknown | Developmental disabilities, elevated lactic acid in cerebrospinal fluid, pigmented retinopathy, elevated serum creatine phosphokinase | |
| 9 | [3] | M | Gambia | p.Tyr59Ter | 20 mo | 21 mo | Fever/infection | Recurrent illness, respiratory insufficiency, coma, ataxia, seizures, bullous dermatosis, elevated lactic acid in cerebrospinal fluid and blood, quadriplegia, nystagmus, torticollis | Cerebral edema, myelopathy |
| 10 | [3] | F | Croatia | p.Gln66*, c.516+1G>A | 15 mo | 2 yr | Fever/infection | Recurrent illness, respiratory insufficiency, coma, delayed psychomotor development, tremor, ataxia, dystonia, edema and erythema rash, elevated lactic acid in cerebrospinal fluid | Cerebral edema, brain atrophy, myelopathy |
| 11 | [3] | M | Germany | p.Lys270del | 16 mo | 18 mo | Fever/infection | Rapid progression, respiratory insufficiency, progressive ataxia, developmental disabilities, coma, elevated cerebrospinal fluid lactic acid, nystagmus, bilateral ptosis | Cerebral edema |
| 12-1 | [3] | M | Poland | p.Asp218Val; p.Ala248fs | 16 mo | 29 mo | Unknown | Recurrent illness, respiratory insufficiency, coma, ataxia, dystonia, focal redness, skin changes in psoriasis of the neck, increased lactic acid in cerebrospinal fluid, dysarthria, nystagmus | Cerebral edema |
| 12-2 | [3] | M | Poland | p.Asp218Val; p.Ala248fs | 8 mo | 2 yr | Unknown | Recurrent illness, respiratory insufficiency, coma, seizures, dystonia, elevated lactic acid in cerebrospinal fluid | Brain dysplasia, acute hydrocephalus |
In the present case, the patient had normal development since childhood and had a history of rabies vaccination in the early stage of onset, and the nervous system symptoms were gradually aggravated after fever. It was possible to be misdiagnosed as an immune inflammatory disease; however, the condition still deteriorated rapidly after active immunotherapy, with respiratory failure, cerebellar edema, and cerebral herniation, which posed major challenges to clinicians. Carefully combing the medical history, we found that the patient had two paroxysmal events within 3 mo of PREBLE1 onset, which were suspected to be epileptic seizures. Physical examination on admission showed that the patient had droopy eyelids, eye movement disorder, and hypotonia of the extremities, and the pathological reflex was positive and the spinal cord lesions were symmetrical. Due to the rapid change of the disease, electromyographic examination was not conducted, and it was necessary to be alert to the possibility of hereditary metabolic diseases (mitochondria), so gene detection was performed as soon as possible. The clinical features, imaging findings, and disease progression of the child were consistent with the characteristics of PEBEL1.
The human nicotinamide nucleotide repair system consists of two chaperone enzymes: NAD(P)HX differential isomerase (NAXE, formerly known as APOA1BP, OMIM:608862), which converts R-NAD(P)HX to S-NAD(P)HX, and the NAD(P)HX dehydratase (NAXD, formerly CARKD, OMIM:615910), which converts SNAD(P)HX back to NAD(P)H[9] in an ATP-dependent manner. The presence of NAD(P)HX repair enzymes in all tissues and species, coupled with the core metabolism of their cofactors which play a protective role, suggests that the repair system is essential for life maintenance[10].
The NAXE (APOAIBP) gene encodes NAD(P)HX differential isomerase or apolipoprotein AI binding protein (AIBP), located on the 1q22 chromosome. There are five transcripts, including six exons (https://www.uniprot.org/uniprot/Q8NCW5), spanning 2.5 kb and composed of 288 amino acids. It contains mitochondrial transport peptide (residues 1–47), NAD(P)H hydrate isomerase (residues 48–288), yjefn terminal domain (residues 65–275), two NAD(P)HX region (residues 119–223, residues 189–195). NAXE protein exists in cerebrospinal fluid and urine, and is widely expressed in the kidneys, heart and liver[11]. In in vitro experiments in fibroblasts from PEBEL1 patients, NAXE deficiency caused a significant increase in circulating NADHX and two NADHX isoforms (S and R isoforms) under normal body temperature (37°C). The elevated levels of circulating NADHX were greater in NAXE-deficient cells after treatment with heat stress (40°C), and the elevation of S- and R-type NADHX isoforms was attenuated. In addition, the activity of intact mitochondria in the muscle tissue of two patients also decreased significantly[1]. These prove that NAXE deficiency leads to the accumulation of the toxic metabolites of the biochemical reaction cofactors NADH and NADPH, circulating NADHX, then affects the oxidative phosphorylation of mitochondria and leads to the deficiency/disorder of mitochondrial energy metabolism. Therefore, it can be predicted that the brain with high demand for energy supply produced by mitochondria is particularly vulnerable to the damage of NAD(P)HX repair, which can also explain the deterioration of neurological symptoms clinically in children after fever. Although NAXE is highly expressed in the human brain (https://gtexportal.org/home/gene/NAXE), the mechanism of its defect in the central nervous system is still unclear.
Vitamin B3 and coenzyme Q are specific treatments for elevated NAD+ levels, so they are also expected to benefit PEBEL1 patients. A 22-year-old female patient reported by TRINH[4] in Germany, in addition to receiving vitaminB3 treatment (niacin 40 mg bid to 2 × 40 mg bid), received anticonvulsant therapy (topiramate 50 mg bid, clomazone 20 mg/d, lamotrigine 300 mg/d, levetiracetam 1000 mg/d, piracetam 2400 mg/d, vitamin D3, esomeprazole, and laxative). The patient survived up to 29 years of age and had significant improvement in spasticity with some recovery in motor abilities. In our case, the patient had acute onset and progressed rapidly. Although nicotinamide (vitamin B3) and coenzyme Q were used on day 17 of onset, they still could not reverse/delay brain edema, and the fatal pathological changes need to be proved by practice.
This is the first case of severe clinical phenotypic PEBEL1 reported in Mainland China, and a compound heterozygous variation of the NAXE gene was detected and a new variant Gly124Cys was identified, which expanded the pathogenicity variation spectrum of PEBEL1. Due to the rapid progress of PEBEL1 and mostly sporadic cases, its early presentation, often characterized by acute onset of fever-induced deterioration of central nervous function or even cerebral edema, is not easy to indicate hereditary diseases, which may be an important reason for the lack of awareness of the disease among clinicians. For patients suspected of PEBEL1, vitamin B3 and coenzyme Q therapy should be tried first, and gene detection should be carried out as soon as possible to make a definite diagnosis. For the family of this case, we suggest that the NAXE mutation test should be used as a necessary part of prenatal genetic screening.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lucke-Wold B, United States; Reis F, Brazil S-Editor: Liu JH L-Editor: A P-Editor: Zhao S
| 1. | Kremer LS, Danhauser K, Herebian D, Petkovic Ramadža D, Piekutowska-Abramczuk D, Seibt A, Müller-Felber W, Haack TB, Płoski R, Lohmeier K, Schneider D, Klee D, Rokicki D, Mayatepek E, Strom TM, Meitinger T, Klopstock T, Pronicka E, Mayr JA, Baric I, Distelmaier F, Prokisch H. NAXE Mutations Disrupt the Cellular NAD(P)HX Repair System and Cause a Lethal Neurometabolic Disorder of Early Childhood. Am J Hum Genet. 2016;99:894-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Spiegel R, Shaag A, Shalev S, Elpeleg O. Homozygous mutation in the APOA1BP is associated with a lethal infantile leukoencephalopathy. Neurogenetics. 2016;17:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19696] [Cited by in RCA: 22434] [Article Influence: 2243.4] [Reference Citation Analysis (0)] |
| 4. | Trinh J, Imhoff S, Dulovic-Mahlow M, Kandaswamy KK, Tadic V, Schäfer J, Dobricic V, Nolte A, Werber M, Rolfs A, Münchau A, Klein C, Lohmann K, Brüggemann N. Novel NAXE variants as a cause for neurometabolic disorder: implications for treatment. J Neurol. 2020;267:770-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Yu D, Zhao FM, Cai XT, Zhou H, Cheng Y. [Clinical and genetic features of early-onset progressive encephalopathy associated with NAXE gene mutations]. Zhongguo Dang Dai Er Ke Za Zhi. 2018;20:524-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Incecık F, Ceylaner S. Early-onset progressive encephalopathy associated with NAXE gene variants: a case report of a Turkish child. Acta Neurol Belg. 2020;120:733-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Mohammadi P, Heidari M, Ashrafi MR, Mahdieh N, Garshasbi M. A novel homozygous missense variant in the NAXE gene in an Iranian family with progressive encephalopathy with brain edema and leukoencephalopathy. Acta Neurol Belg. 2022;122:1201-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Choi SH, Agatisa-Boyle C, Gonen A, Kim A, Kim J, Alekseeva E, Tsimikas S, Miller YI. Intracellular AIBP (Apolipoprotein A-I Binding Protein) Regulates Oxidized LDL (Low-Density Lipoprotein)-Induced Mitophagy in Macrophages. Arterioscler Thromb Vasc Biol. 2021;41:e82-e96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Marbaix AY, Noël G, Detroux AM, Vertommen D, Van Schaftingen E, Linster CL. Extremely conserved ATP- or ADP-dependent enzymatic system for nicotinamide nucleotide repair. J Biol Chem. 2011;286:41246-41252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Marbaix AY, Tyteca D, Niehaus TD, Hanson AD, Linster CL, Van Schaftingen E. Occurrence and subcellular distribution of the NADPHX repair system in mammals. Biochem J. 2014;460:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Ritter M, Buechler C, Boettcher A, Barlage S, Schmitz-Madry A, Orsó E, Bared SM, Schmiedeknecht G, Baehr CH, Fricker G, Schmitz G. Cloning and characterization of a novel apolipoprotein A-I binding protein, AI-BP, secreted by cells of the kidney proximal tubules in response to HDL or ApoA-I. Genomics. 2002;79:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |