Published online Jan 6, 2023. doi: 10.12998/wjcc.v11.i1.233
Peer-review started: October 24, 2022
First decision: November 11, 2022
Revised: November 24, 2022
Accepted: December 15, 2022
Article in press: December 15, 2022
Published online: January 6, 2023
Processing time: 72 Days and 12.5 Hours
Bronchiolar adenoma (BA) and ciliated muconodular papillary tumor are rare tumors that have bilayered cell proliferation and continuous expression of p40 and CK5/6 in the basal cell layer. Diagnosis is difficult because of the limited knowledge of these tumors and their morphological similarities to malignant tumors, including invasive mucinous adenocarcinoma, especially based on the histopathology of intraoperative frozen sections. These tumors are now con
A 57-year-old woman presented with a 17.0 mm × 7.0 mm nodule in the lower lobe of the left lung. Hematoxylin-eosin staining and immunohistochemistry of a surgical specimen were performed. The tumor consisted of a BA area and a muc
The present study indicated that BA may be carcinogenic, and suggests that clinicians should be aware of its potential for malignant transformation.
Core Tip: This study adds to the currently limited information regarding the malignant transformation of pulmonary bronchiolar adenomas into mucinous adenocarcinomas. Our results suggest clinicians should have a high index of suspicion when encountering a lesion that appears to be benign.
- Citation: Liu XL, Miao CF, Li M, Li P. Malignant transformation of pulmonary bronchiolar adenoma into mucinous adenocarcinoma: A case report. World J Clin Cases 2023; 11(1): 233-241
- URL: https://www.wjgnet.com/2307-8960/full/v11/i1/233.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i1.233
Chang et al[1] proposed the term bronchiolar adenoma (BA) in 2018 to describe a putatively benign neoplasm that is histologically similar to the bronchial epithelium. These lesions involve the alveolar lung parenchyma instead of the bronchioles. Gross observations indicate they proliferate along the native alveolar wall and have a papillary or flat architecture; microscopic observations indicate they consist of proliferating bilayered cells with luminal cuboidal and goblet cells, and a continual basal cell layer that expresses p40 or CK5/6. Thus, Chang et al[1] considered BA as a broader category that includes ciliated muconodular papillary tumors (CMPTs).
The increasing utilization of high-resolution computed tomography (CT) has led to the detection of a wide array of pulmonary abnormalities, including nodules with a ground-glass appearance or peripheral mucus-secreting characteristics. The limited knowledge of BA/CMPT and its morphologic similarity to malignant tumors, including invasive mucinous adenocarcinoma (IMA), presents a diagnostic challenge, especially in intraoperative frozen sections. We reviewed the available English language literature from 2002 to 2022 and identified only four cases of BA/CMPT with malignant transformation[2-5]. Thus, much the biological nature and clinical significance of BA/CMPT remain unknown. Herein, we present a patient with mucinous adenocarcinoma (MA) that developed from the malignant transformation of a BA to raise awareness of this disease and improve its diagnosis.
A 57-year-old female patient presented with a two-year history of a space-occupying pulmonary lesion without any other clinical signs or symptoms.
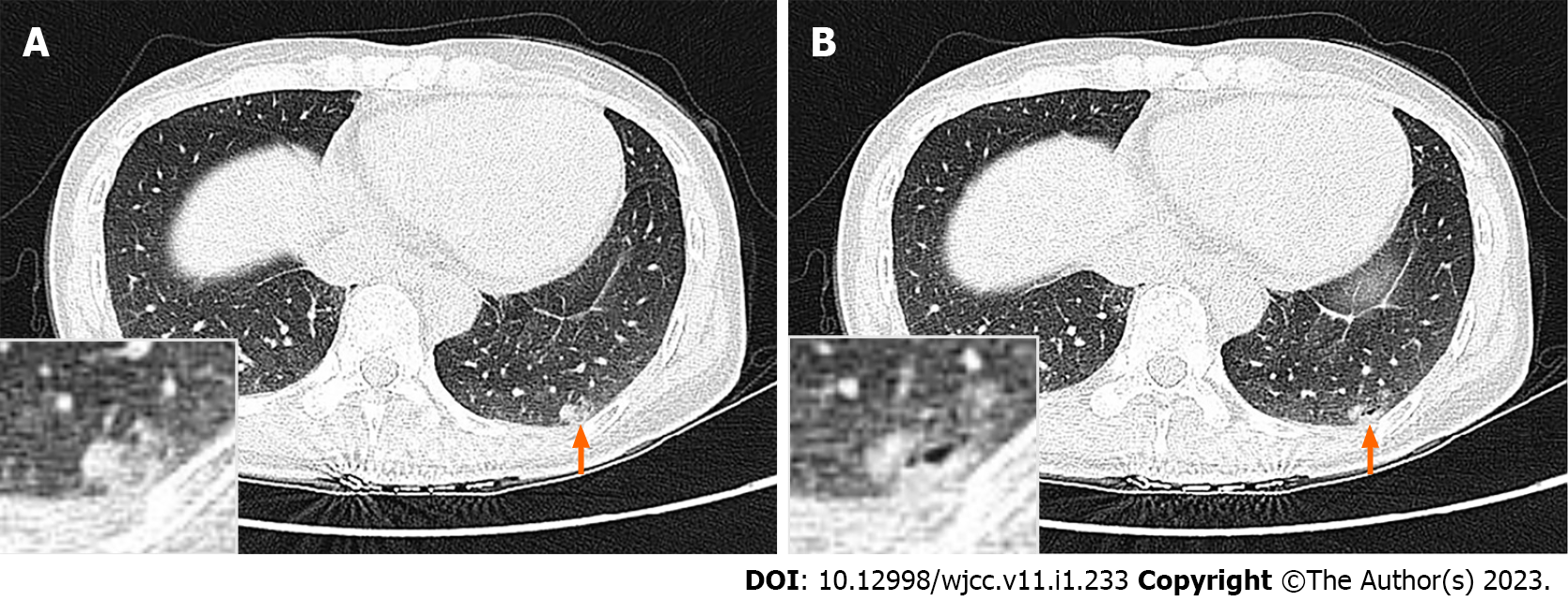
The patient first received a plain chest CT scan during a physical examination on November 3, 2019. The results indicated a few patches in the lower lobe of the left lung, considered to be an inflammatory disease at that time. A CT reexamination on September 15, 2021 showed that the density of these lesions had increased and the margin was slightly blurred, but it was still considered to be an inflammatory disease. We recommended annual follow-ups at that time. On December 22, 2021, the patient received a high-resolution plain chest CT scan. The results indicated a mixed solid and ground-glass nodule in the posterior basal segment of the lower lobe of the left lung that was approximately 17.0 mm × 7.0 mm, irregular in shape, and close to the pleura. We could not exclude a malignant lesion, and recommended surgery. The patient denied the presence of chest tightness, chest pain, cough, or expectoration, and reported no weight loss.
The patient had no relevant medical history, or habits, such as smoking and drinking.
The patient denied any relevant family history.
There were no special physical signs.
Routine laboratory blood results were normal.
A high-resolution chest CT plain scan showed a 17.0 mm × 7.0 mm nodule in the left lower lobe of the lung (Figure 1).
We diagnosed the intraoperative frozen section as a kind of pulmonary epithelial tumor, but it was difficult to differentiate BA from invasive adenocarcinoma, because this requires immunohistochemical examination of paraffin sections. The final diagnosis was mixed BA and MA based on the results of histopathology and immunohistochemistry of the surgical specimen.
A biopsy with wedge-resection of the lung under video-assisted thoracoscopy was performed. The gross appearance of the resected specimen revealed a 17.0 mm firm, isolated, gray-white nodule of moderate hardness in the lung parenchyma adherent to the pleura.
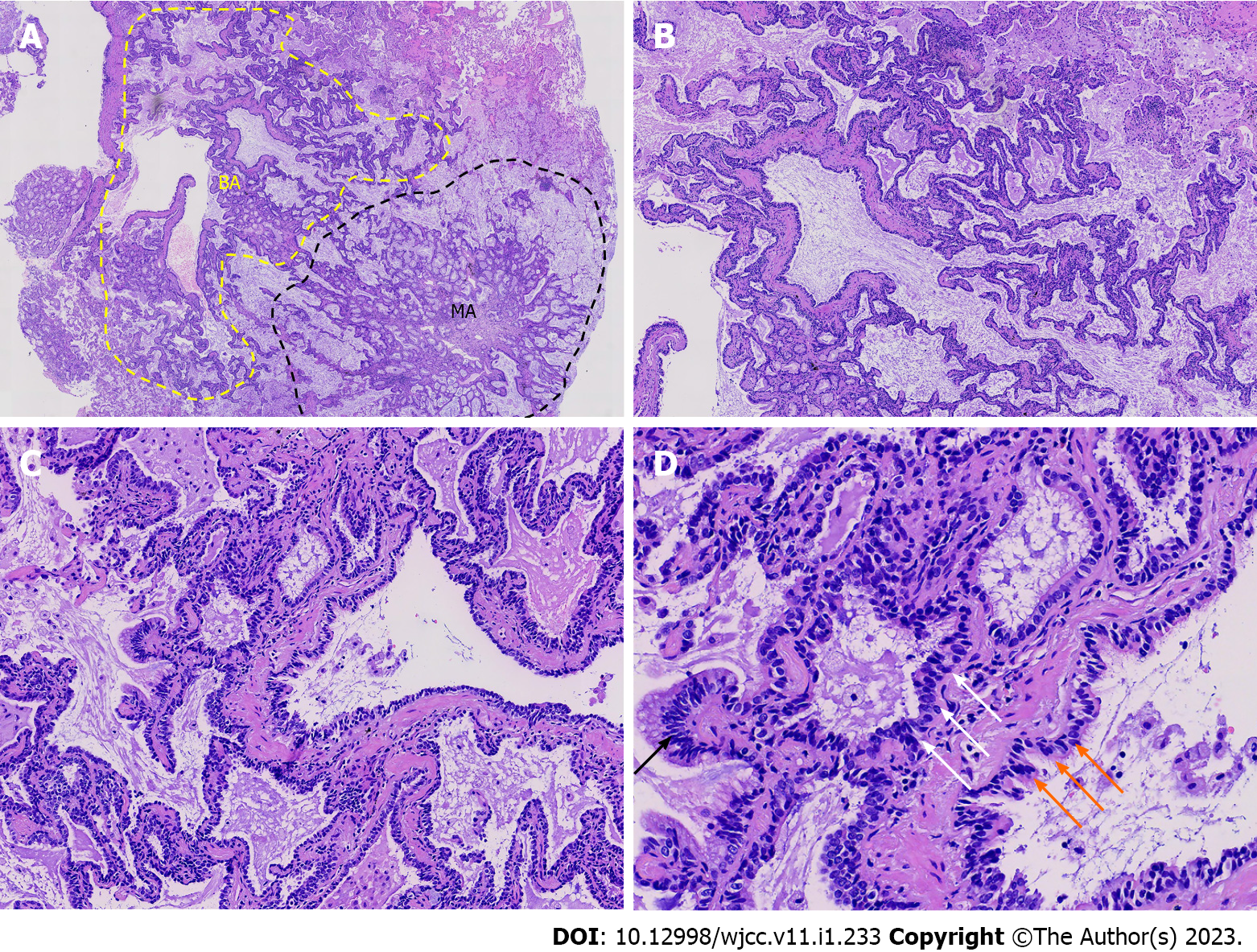
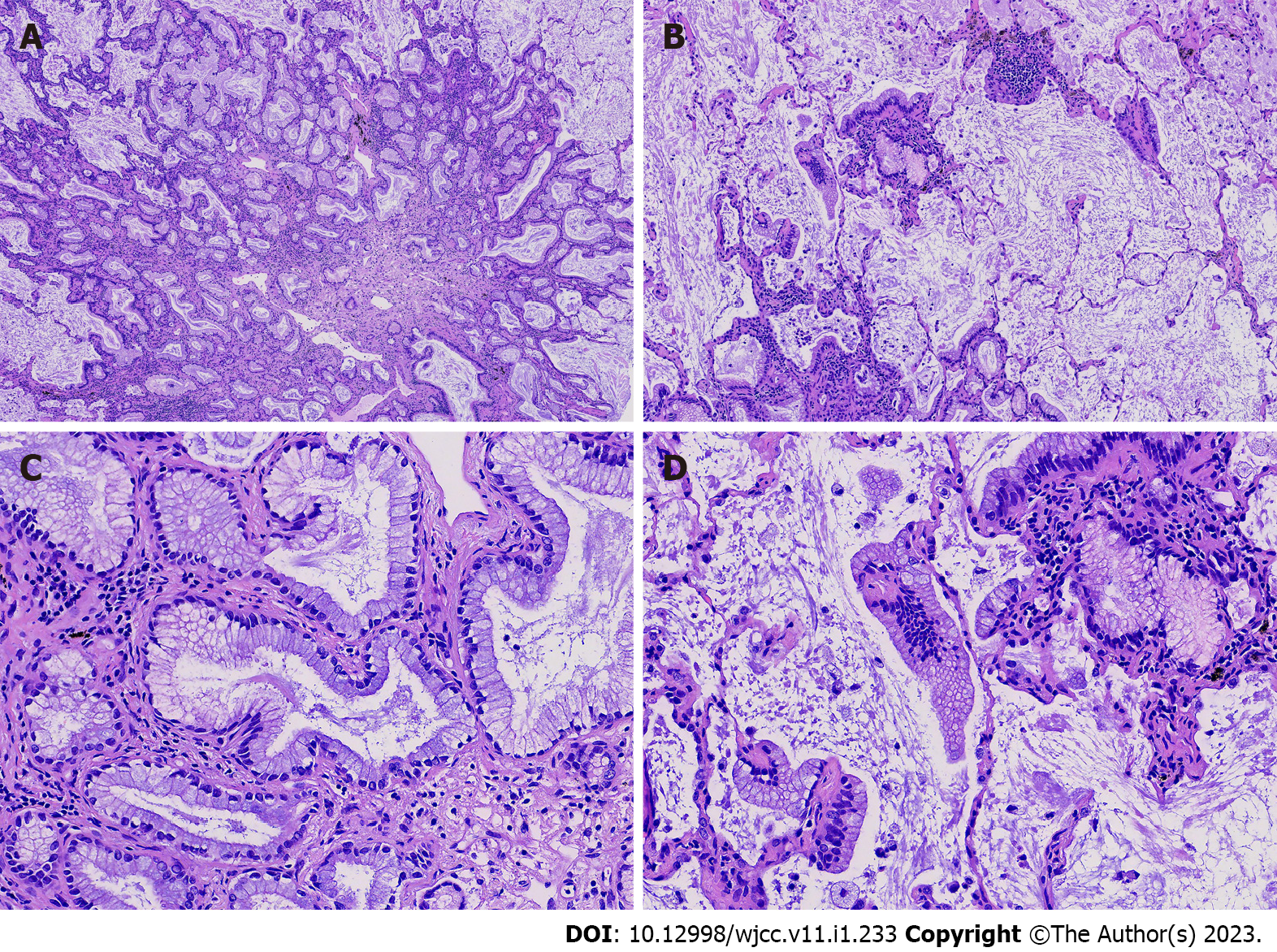
Hematoxylin-eosin (H&E) staining of the postoperative paraffin section showed the tumor was poorly delineated, closely associated with the bronchioles, and had spread to the adjacent alveolar wall (Figure 2A). In the center of the lesion, the presence of a bilayered structure could be observed-it consisted of tripartite cellular components that were analogous to the bronchiolar-type epithelium, and was comprised mainly of basal cells and luminal epithelial cells (Figure 2A, in yellow dotted lines; Figure 2B-D). The luminal layer was predominantly formed of mucinous, ciliated columnar, and cuboidal or low columnar cells in various numbers and proportions (Figure 2D). These cells were arranged continuously in a flat, papillary, and glandular pattern along the preexisting alveolar wall. Skip lesions of tumor cells along the alveolar wall were observed. The tripartite cellular components were devoid of significant atypia, mitosis, or necrosis. Most areas in the center of the lesion had a continuous layer of basal cells, but other regions had no evidence of this layer or papillary fronds. However, part of the tumor periphery had some characteristics of an adenocarcinoma (Figures 2A, in black dotted lines), mainly represented by glandular structures lined with tall columnar mucinous cells that proliferated along the alveolar wall, which had mild atypia and low mitotic activity (Figure 3). There were no ciliated cells, but a few basal cells were observed. Skip lesions remained (Figure 3B and C), with focal areas of large mucinous cells and abundant mucus secretion.
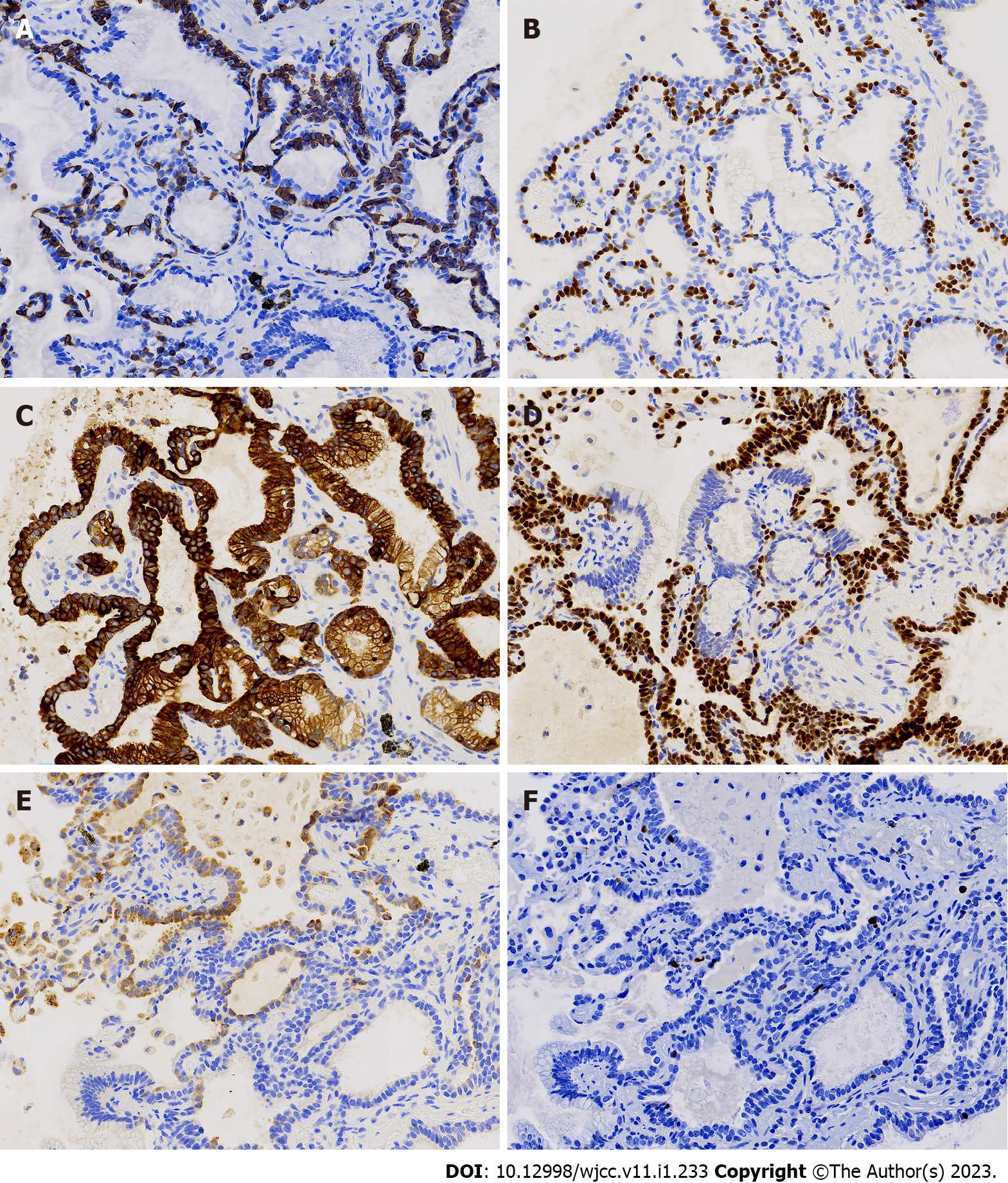
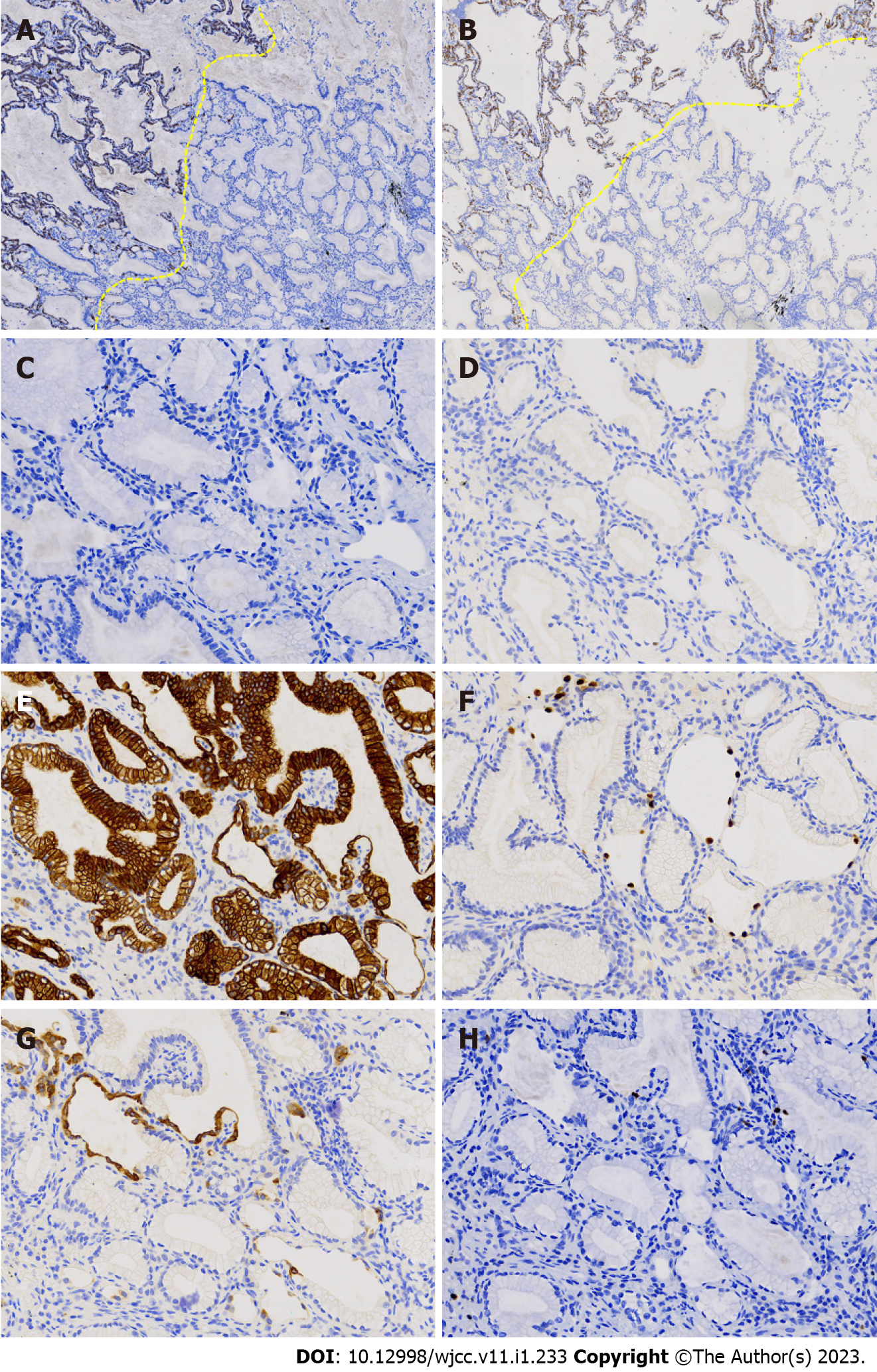
We used immunohistochemical analysis to detect CK7, TTF-1, napsin A, CK5/6, p40, and Ki-67. The results showed that CK5/6 (Figure 4A) and p40 (Figure 4B) had continuous and consistent expression in basal cells, but discontinuous expression in some cells with a skipping pattern. It was hypothesized that the distal bronchioles could potentially function in gas exchange, and that basal layer cells might be discontinuous as they migrate to the alveolar sacs. The absence of basal cell biomarkers (Figure 5A-D) indicated that basal cells were absent in certain glandular areas. CK7 showed diffuse positivity in basal and luminal cells (Figures 4C and 5E). TTF1 was expressed in basal, ciliated, cubic, and low columnar cells (Figure 4D), but not in mucinous cells (Figure 5F). Expression of napsin A was weak (Figure 4E and 5G), and the Ki-67 proliferation index (1%-2%) was low in cubic and low columnar cells (Figure 4F and 5H).
We finally diagnosed the patient with mixed BA and MA based on pathology, and discharged her 3 d after the operation. She was well and stable, and we considered further work-up unnecessary.
The hallmark of BA is a continuous basal cell layer. This feature indicates it is a noncancerous neoplasm[1] and distinguishes it from lung adenocarcinoma. Under low -power magnification, BA may be misdiagnosed as a preinvasive or invasive carcinoma, especially in frozen sections. However, high-power magnification shows ciliated and basal cells, thus preventing this misdiagnosis[6]. In the present case, our frozen epithelial neoplasm sections indicated flat, papillary, and glandular structures, making it difficult to identify it as a benign or malignant lesion.
The diagnosis of BA should be based on morphological and immunohistochemical observations. Chang et al[1] showed that BA has a spectrum of morphologic features that range from the proximal to distal respiratory bronchioles. The proximal type features a bilayered epithelium consisting of continuous basal and luminal epithelial cells (mucinous and ciliated columnar cells); the bilayered bronchiolar proliferation appears as a papillary structure and the luminal cells have no expression or weak expression of TTF-1 and napsin A, and the basal cells have continuous expression of p40 and CK5/6. In contrast, cells in the distal-type BA are not ciliated or mucinous, and a papillary configuration may be absent. Luminal cells express TTF-1 and napsin A, whereas basal cells express p40 and CK5/6 in a skipping pattern.
Our patient’s tumor was 17.0 mm in diameter, and had focal areas with a continuous basal cell layer. Significant ciliated differentiation was observed in the luminal cells, accompanied by abundant mucinous cells. However, the basal cell biomarkers had a skipping pattern and lacked a papillary architecture in certain areas. The luminal cells were cuboidal or low columnar cells, and were positive for TTF-1 and napsin A. Thus, the morphological and immunohistochemical features of the lesion were consistent with proximal to distal bronchiolar changes in the mucosal epithelial cells.
Although the 2021 WHO classification considers BA as a benign tumor, other evidence suggests that BA has malignant potential[1,7-13]. The high prevalence of mutations in certain driver genes (EGFR, BRAF, ALK, and KRAS)[1,8,12,14,15] suggests these lesions are neoplastic rather than reactive or metaplastic. However, the association of these mutated genes with BA pathogenesis and prognosis remains uncertain and requires further investigation.
Four previous publications reported patients who had BA/CMPT with malignant transformation (Table 1)[2-5]. Miyai and collaborators[2] presented a case of CMPT with malignant transformation of basal cells. Chen and colleagues[3] reported a case of MA resulting from CMPT, and speculated that CMPT was a precursor of MA. Han and co-workers[4] presented a unique case with loss of the continuous basal cell layer at the junction between BA and IMA, although the BA and IMA had the same KRAS mutations. Wang et al[5] also described a patient with malignant CMPT. We used H&E staining and immunohistochemistry to diagnose our patient as having MA caused by the malignant transformation of BA, thus providing histological evidence of the malignant potential of BA. Ultimately, a common feature of all these cases is the absence of the basal cell layer.
| Ref. | Age (yr)/Sex | Size (mm) | Location | Diagnosis | Malignant transformation | Gene mutation |
| Miyai et al[2], 2018 | 67/F | 20 | Middle lobe of the right lung | CMPT | Invasive spindle cell carcinoma | / |
| Chen et al[3], 2021 | 61/M | 14 | Inferior lobe of the right lung | CMPT | MA | - |
| Han et al[4], 2021 | 70/M | 15 | Middle lobe of the right lung | BA | MA | KRAS (G12V) |
| Wang et al[5], 2021 | 67/F | 7 | Dorsal segment of the left lung | BA | Adenocarcinoma | CCNE1, HER2 |
| Present study | 56/F | 17 | Inferior lobe of the left lung | BA | MA | / |
Several gene mutations that drive lung adenocarcinoma also occur in BA. The high incidence of BRAF mutations in CMPT (50%)[14] contrasts with EGFR mutations in lung adenocarcinoma. Mutations of EGFR, AKTI, KRAS, and gene rearrangement of ALK can also occur in BA[12,14,15]. Zheng et al[16] used the term “classic CMPT” to refer to a lesion with tripartite cellular constituents, including ciliated columnar cells, mucinous cells, and basal cells with predominantly papillary architecture. Compared with the non-classic CMPT, the frequency of BRAF mutations is higher in classic CMPT. However, Chang and collaborators[13] reported that BRAF mutations were more frequent and common in distal-type BA.
Postoperative pathological findings in our patient confirmed the presence of mixed BA and MA, with the latter’s underlying etiology suspected to be from the malignant potential of BA. Examination of the pathological morphological characteristics of frozen sections makes it difficult to differentiate BA from adenocarcinoma, because it is difficult to observe basal cells in these sections. Therefore, we suggest that pathologists should increase their knowledge of BA to reduce the probability of misdiagnosis and missed diagnosis.
The authors thank the patient for allowing her case to be presented.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pathology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Masyeni S, Indonesia; Yeh YC, Taiwan S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Chang JC, Montecalvo J, Borsu L, Lu S, Larsen BT, Wallace WD, Sae-Ow W, Mackinnon AC, Kim HR, Bowman A, Sauter JL, Arcila ME, Ladanyi M, Travis WD, Rekhtman N. Bronchiolar Adenoma: Expansion of the Concept of Ciliated Muconodular Papillary Tumors With Proposal for Revised Terminology Based on Morphologic, Immunophenotypic, and Genomic Analysis of 25 Cases. Am J Surg Pathol. 2018;42:1010-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 2. | Miyai K, Takeo H, Nakayama T, Obara K, Aida S, Sato K, Matsukuma S. Invasive form of ciliated muconodular papillary tumor of the lung: A case report and review of the literature. Pathol Int. 2018;68:530-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Chen F, Ren F, Zhao H, Xu X, Chen J. Mucinous adenocarcinoma caused by cancerization from a ciliated multinodular papilloma tumor: A case report. Thorac Cancer. 2021;12:1629-1633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Han X, Hao J, Ding S, Wang EH, Wang L. Bronchiolar Adenoma Transforming to Invasive Mucinous Adenocarcinoma: A Case Report. Onco Targets Ther. 2021;14:2241-2246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Wang F, Shen MH, Cao D, Lv JH. Malignant Ciliated Muconodular Papillary Tumors of the Lung: A Case Report. Int J Surg Pathol. 2021;29:520-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Chu HH, Park SY, Cha EJ. Ciliated muconodular papillary tumor of the lung: the risk of false-positive diagnosis in frozen section. Hum Pathol Case Rep. 2017;7:8-10. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Kataoka T, Okudela K, Matsumura M, Mitsui H, Suzuki T, Koike C, Sawazumi T, Umeda S, Tateishi Y, Yamanaka S, Ishikawa Y, Arai H, Tajiri M, Ohashi K. A molecular pathological study of four cases of ciliated muconodular papillary tumors of the lung. Pathol Int. 2018;68:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Kashima J, Hishima T, Tonooka A, Horiguchi SI, Motoi T, Okuma Y, Hosimi Y, Horio H. Genetic and immunohistochemical analyses of ciliated muconodular papillary tumors of the lung: A report of five cases. SAGE Open Med Case Rep. 2019;7:2050313X19830483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Sato S, Koike T, Homma K, Yokoyama A. Ciliated muconodular papillary tumour of the lung: a newly defined low-grade malignant tumour. Interact Cardiovasc Thorac Surg. 2010;11:685-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Hata Y, Yuasa R, Sato F, Otsuka H, Goto H, Isobe K, Mitsuda A, Wakayama M, Shibuya K, Takagi K, Watanabe Y. Ciliated muconodular papillary tumor of the lung: a newly defined low-grade malignant tumor with CT findings reminiscent of adenocarcinoma. Jpn J Clin Oncol. 2013;43:205-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Kon T, Baba Y, Fukai I, Watanabe G, Uchiyama T, Murata T. Ciliated muconodular papillary tumor of the lung: A report of five cases. Pathol Int. 2016;66:633-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Udo E, Furusato B, Sakai K, Prentice LM, Tanaka T, Kitamura Y, Tsuchiya T, Yamasaki N, Nagayasu T, Nishio K, Fukuoka J. Ciliated muconodular papillary tumors of the lung with KRAS/BRAF/AKT1 mutation. Diagn Pathol. 2017;12:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Chang JC, Montecalvo J, Lu S, Wallace D, Sae-Ow W, Borsu L, Kim HR, Ladanyi M, Travis W, Rekhtman N. Pulmonary ciliated muconodular papillary tumor (CMPT) with classic and non-classic morphology: expanded morphologic and molecular spectrum of bilayered lesions with bronchiolar-type differentiation. Seattle: USCAP; 2017.. [RCA] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 14. | Kamata T, Sunami K, Yoshida A, Shiraishi K, Furuta K, Shimada Y, Katai H, Watanabe S, Asamura H, Kohno T, Tsuta K. Frequent BRAF or EGFR Mutations in Ciliated Muconodular Papillary Tumors of the Lung. J Thorac Oncol. 2016;11:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Jin Y, Shen X, Shen L, Sun Y, Chen H, Li Y. Ciliated muconodular papillary tumor of the lung harboring ALK gene rearrangement: Case report and review of the literature. Pathol Int. 2017;67:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Zheng Q, Luo R, Jin Y, Shen X, Shan L, Shen L, Hou Y, Li Y. So-called "non-classic" ciliated muconodular papillary tumors: a comprehensive comparison of the clinicopathological and molecular features with classic ciliated muconodular papillary tumors. Hum Pathol. 2018;82:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |