Published online Mar 16, 2022. doi: 10.12998/wjcc.v10.i8.2543
Peer-review started: August 16, 2021
First decision: November 6, 2021
Revised: November 16, 2021
Accepted: January 27, 2022
Article in press: January 27, 2022
Published online: March 16, 2022
Processing time: 206 Days and 15.5 Hours
IgG4-related disease (IgG4-RD), an immune-mediated chronic progressive fibroinflammatory disease, can affect the functions of several organs. Some common characteristics can be observed in different IgG4-RDs, such as higher prevalence in middle-aged and elderly male patients, raised serum IgG4 levels, abundant infiltration of IgG4-positive cells and fibrosis, diffuse or localized swelling of the affected organs, and good response to glucocorticoids treatment.
A 72-year-old man complained of left upper abdominal pain 3 mo ago, and he was diagnosed with acute onset of chronic cholecystitis and acute pancreatitis in the local hospital. Pain improved after relevant treatment. Several days ago, his abdominal pain worsened, and he was admitted to our hospital for further treatment. Doppler ultrasound showed that the pancreas presented with sausage-like swelling and the parenchymal echo was diffusely reduced. Gallbladder volume was increased, while the wall was rough and thickened with bilateral signs. Furthermore, the left submandibular gland was enlarged, accompanied with significantly increased blood flow signals. Finally, we found that the adventitia of the abdominal aorta and right iliac artery was thickened locally. Serum IgG4 was elevated to 12600 mg/L. Therefore, the patient was diagnosed with IgG4-RD. After treatment with methylprednisolone, he had an uneventful course and was discharged in good condition.
IgG4-RD can involve almost any organs. Ultrasound has a significant role in timely and accurately diagnosis.
Core Tip: Immunoglobulin G4-related disease (IgG4-RD) can involve multiple organs and sites, such as the glands and ductal tissues. However, it is rare that a patient with more than three organs are involved at the same time, as well as the arterial lesions. In our case, we report a patient with the autoimmune inflammation, his four organs and tissues are involved, they are the aorta, pancreas, gallbladder, and submandibular gland. Meanwhile, the involved abdominal aorta and iliac artery presented as IgG4-related periarteritis. Finally, we made a comprehensive diagnosis according to the clinical histology, imaging, serology, and the response to the therapy.
- Citation: An YQ, Ma N, Liu Y. Immunoglobulin G4-related disease involving multiple systems: A case report. World J Clin Cases 2022; 10(8): 2543-2549
- URL: https://www.wjgnet.com/2307-8960/full/v10/i8/2543.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i8.2543
IgG4-related disease (IgG4-RD) is an immune-mediated chronic progressive fibroinflammatory disease, which can involve multiple organs or tissues, such as pancreas, biliary tract, salivary glands, lacrimal glands, lung, kidney, and retroperitoneum[1-4]. IgG4-RD has a higher prevalence in male patients with age over 50 years[5,6] and it is easily confused with malignant tumor, infection, and other autoimmune diseases. Histopathology plays a key role in the diagnosis of IgG4-RD, and the typical pathological manifestations include lymphoplasmacytic infiltration, storiform fibrosis, and obliterative phlebitis[7,8]. The affected organs present as localized or diffused swelling, while the serum IgG4 concentration is often significantly elevated[9]. A comprehensive diagnostic criterion was established by the Japan College of Rheumatology in 2011[9] and the corelative classification criteria for IgG4-RD was also developed by the American College of Rheumatology/European League Against in 2019[10]. Besides, most patients have a good response to the steroids or rituximab therapy within a short time, but it is common for this disease to recur.
Although IgG4-RD can involve multiple organs and sites, it is still rare that a patient with more than three organs are involved at the same time and most of the affected sites are glands and ductal tissues. Meanwhile, the arterial lesions are less common in IgG4-RD. In our case, the autoimmune inflammation involved four organs and tissues, including the aorta, pancreas, gallbladder, submandibular gland, and the abdominal aorta and iliac artery presented with IgG4-related periarteritis. Finally, a comprehensive diagnosis is made, according to the clinical histology, imaging, serology, the appearance of the affected organs, and response to therapy.
A 72-year-old man was admitted to the gastroenterology department with pain in the left upper quadrant for 3 mo.
The patient had left upper abdominal pain 3 mo ago, accompanying with symptoms of anorexia, yellow urine, chills, nausea, vomiting, abdominal distention, and diarrhea, but no cough, expectoration, hemoptysis and other lung symptoms. Thus, he was referred to the local hospital. After the relevant laboratory and imaging examinations, the patient was diagnosed with acute onset of chronic cholecystitis and acute pancreatitis. The pain symptom improved after liver protective and anti-infective treatment, resolving tetany, pain relief, and fluid rehydration. Three days ago, his abdominal pain worsened and he was admitted to the Department of Gastroenterology in our hospital for further treatment.
He has a history of chronic hepatitis B > 30 years and hypertension for 10 years treated with nifedipine. He underwent excision of the right mandible mass 5 mo ago, and pathological results showed massive lymphocyte infiltration with fibrous tissue hyperplasia.
His father and daughter are both carriers of hepatitis B virus.
There was tenderness in the upper abdomen, no rebound pain and muscle tension, and no mass was touched in the whole abdomen. A surgical scar of 3-4 cm was seen in his right mandible. The others showed no obvious abnormality.
Biochemical examinations showed that ESR was 25 mm/h (normal range 0-15 mm/h). Tumor marker carbohydrate antigen 15-3 was 24.3 U/L. Hepatitis B surface antigen (HBsAg) was 1.06 IU/mL, hepatitis Be antigen (HBeAg) was 0.02 S/CO, hepatitis B core antibody (HBcAg) was 10.07 S/CO. Finally, serum IgG4 was elevated to 12600 mg/L. The routine urine tests and renal function tests were all normal.
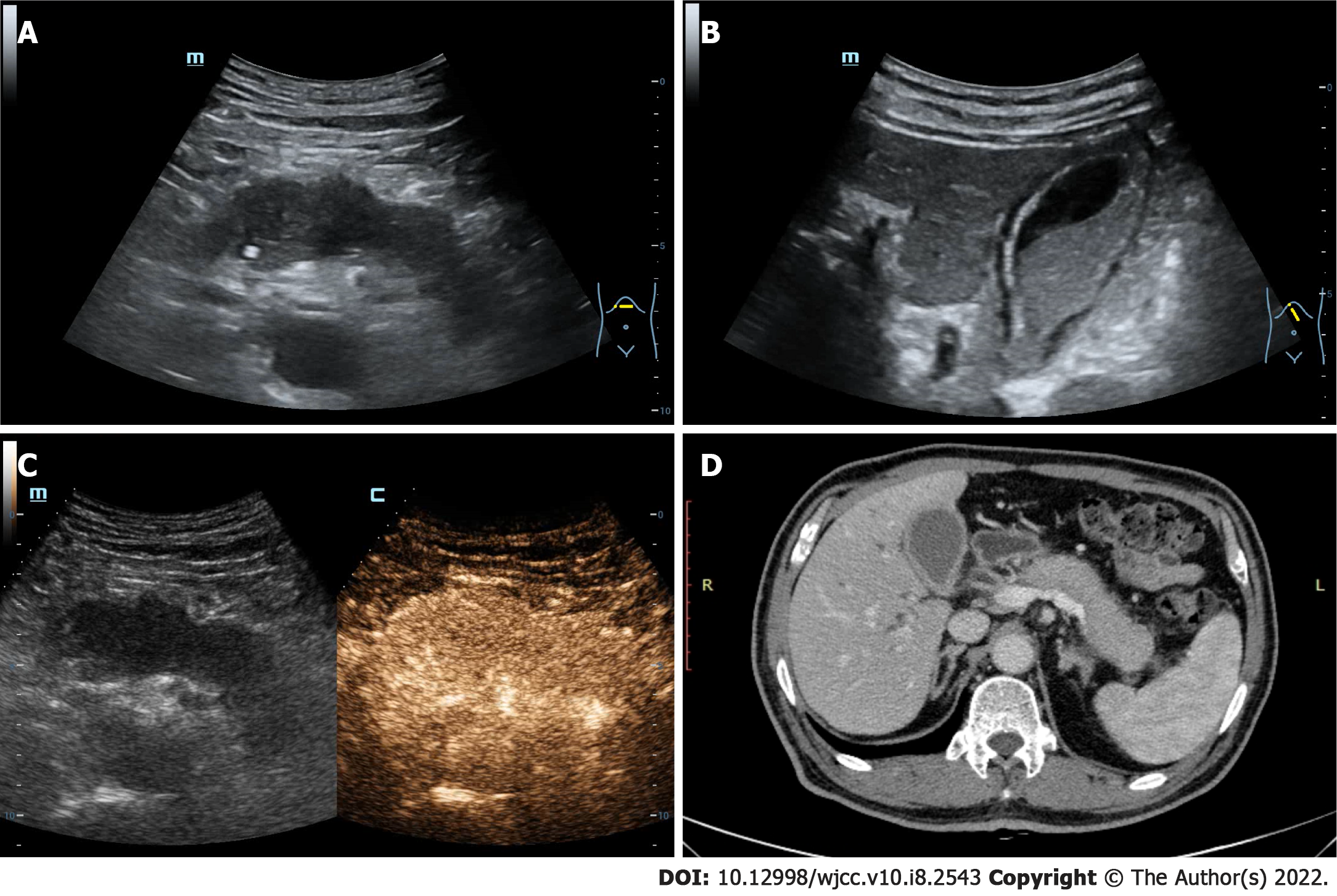
Abdominal ultrasound revealed that the pancreas was diffusely enlarged and sausage-shaped, in which the anteroposterior diameters of the pancreatic head, body and tail were 3.1 cm, 2.7 cm and 2.2 cm, respectively. The pancreatic parenchyma echo was diffusely reduced and the boundary was not clear (Figure 1A). The gallbladder volume was enlarged, and the wall was rough and thickened with bilateral signs. Silt-like deposits were found in the gallbladder with a range of 5.6 cm × 2.0 cm (Figure 1B). Contrast-enhanced ultrasound (CEUS) revealed that the pancreatic lesions were uniformly enhanced in the arterial phase (Figure 1C). Computed tomography scan indicated that the pancreas was enlarged, and its head had spotty, high-density foci. The gallbladder was enlarged and the cyst wall was thickened (Figure 1D). Furthermore, there were no obvious abnormalities in the lung and kidneys. Thus, autoimmune pancreatitis with gallbladder involvement was considered.
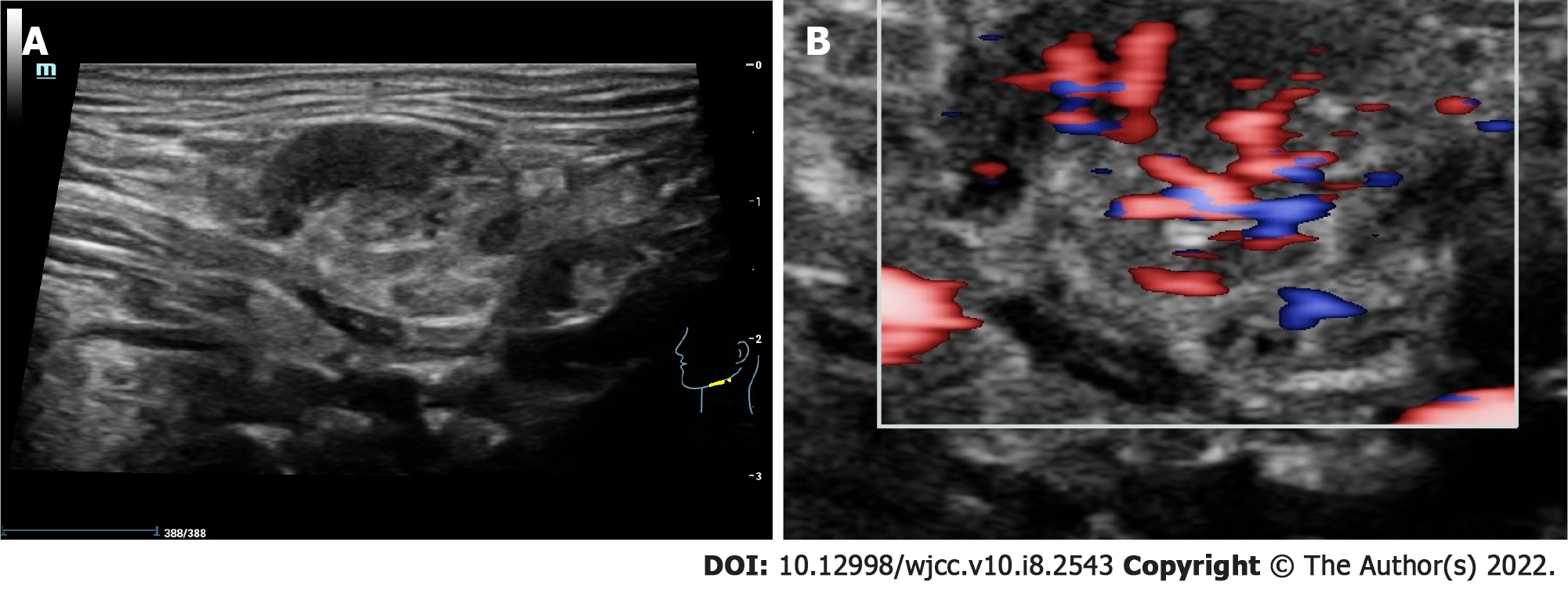
Salivary gland ultrasound showed that the right submandibular gland was almost completely removed, and the left submandibular gland was enlarged with a size of 2.5 cm × 1.4 cm. We found that the parenchymal echo was not uniform, and companied with reticular separation and scattered flake-like hypoecho (Figure 2A). Color doppler flow imaging suggested that the blood flow signal was significantly increased (Figure 2B). There was no obvious abnormality in the bilateral parotid and sublingual glands.
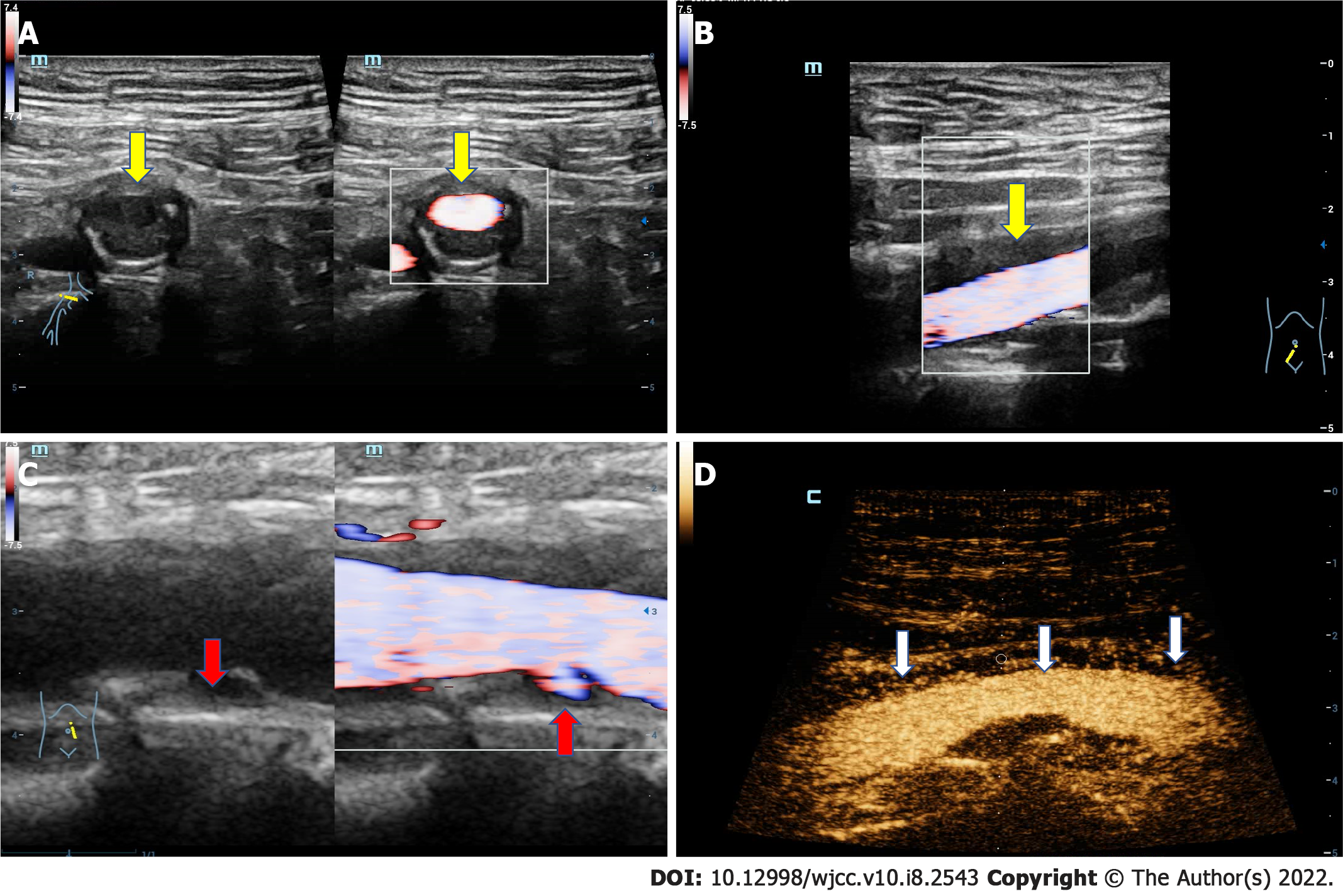
Abdominal arterial ultrasound showed that the adventitia of the abdominal aorta and right iliac artery was locally thickened with a maximum up to 5.0 mm (Figure 3A and B). CEUS demonstrated that extensive new blood vessels were distributed in the adventitia, while the intensity of imaging was evaluated to grade III (Figure 3D). These results were similar to the inflammatory activity of periarteritis. Multiple strong echogenic plaques were seen on the arterial wall, suggesting atherosclerosis of the abdominal aorta and iliac artery. Additionally, ulceration was observed in the plaque of the posterior wall of the abdominal aorta (Figure 3C).
Combined with the clinical manifestations, pathological results, laboratory examinations, and imaging results, IgG4-RD simultaneously affects the pancreas (autoimmune pancreatitis), gallbladder (cholecystitis), salivary gland (submandibular adenitis), and aorta (pancreatitis).
After excluding the relative contraindications for glucocorticoids, such as tumors and tuberculosis, the patient was injected intravenously with methylprednisolone and supplemented with antihypertensive and hypoglycemic treatment, gastric mucosa protection, liver protection and other treatments.
The patient had an uneventful course and was discharged in good general condition. The patient was instructed to take prednisolone on time and closely monitor blood pressure and blood glucose. One month later, there was no obvious abnormality when re-examined by the ultrasound and biochemical tests.
IgG4-RD is a rare disease that can involve multiple organs at the same time[1,11,12], which is similar to many malignant, infectious, and inflammatory diseases[13]. The epidemiological data of IgG4-RD have not been completely established due to rarity and misdiagnosis[14,15]. The clinical manifestations of IgG4-RD mainly depend on the affected organs and lack specificity, which is challengeable for making correct diagnosis[16]. According to the comprehensive diagnostic criteria published in 2012[9], IgG4-RD patients must have (1) A compatible clinical presentation (swelling or masses in single/multiple organs); (2) Serum IgG4 concentration > 135 mg/dL; and (3) Histopathological evidence of marked lymphocytic and plasmocytic infiltration (IgG4-plasma cells/high-power field > 10 with IgG4/IgG-positive cell ratio > 40%). The patient in our case initially presented with epigastric pain similar to pancreatitis and was misdiagnosed with acute pancreatitis in another hospital. However, it was later found that the lesions involved multiple sites, and the volume of the affected organs, such as the pancreas, gallbladder, and submandibular gland increased significantly, and serum IgG4 increased to 12600 mg/L (> 1.35 g/L). The pathological results of the previous operation on the submandibular gland suggested that lymphocyte infiltration was accompanied by fibrous tissue hyperplasia. Our case fulfilled all the comprehensive diagnostic criteria, so we made the diagnosis of multisystem IgG4-RD.
If patients present with any typical clinical, serological or radiographic results, the clinical diagnosis sometimes can be made without pathological biopsy examinations[17]. If the patient has typical imaging findings of IgG4-RD, such as sausage-shaped pancreas[3,18] and periarteritis affecting the aorta below the renal artery, combined with relevant clinical manifestations and serological data, clinical diagnosis of IgG4-RD should be considered[10]. For our patient, most of the characteristic manifestations of IgG4-RD were found (such as the lesion involved the pancreas and salivary gland), along with typical imaging findings (such as sausage-shaped swollen pancreas). In addition, periarteritis affecting the abdominal aorta and iliac artery, with inflammatory thickening of the arterial wall, supported the diagnosis of IgG4-RD[19].
The 72-year-old patient presented with multiple sclerotic plaques in the abdominal aorta and iliac artery. There was ulceration on the surface of the sclerotic plaque. At that time, we suspected that the autoimmune inflammation stimulated the arterial wall for a long time and IgG4-RD caused the sclerotic plaques to rupture and ulcerate. It has been shown that IgG4-RD retroperitoneal fibrosis usually occurs around the aorta, suggesting that the adventitia of the arterial vessels may also be a target of this disease[20]. Doppler ultrasound and CEUS revealed that the adventitia of the abdominal aorta and iliac artery were thickened with multiple neovascularization, which also suggested that IgG4-RD mainly involved the adventitia. It has also been reported that IgG4-RD arteritis can lead to aneurysm formation[20], suggesting that inflammation may stimulate diffuse thickening of the arterial wall, narrow the arterial lumen, and cause aneurysm formation[19]. Therefore, if the multisite lesion does not respond to conventional treatments, IgG4-RD should be considered. More attention should be paid to the lesions involving large vessels and aneurysm formation.
Glucocorticoid therapy is the best choice after diagnosis of IgG4-RD[21], while the surgery, radiotherapy and chemotherapy should be avoided as far as possible. Before using glucocorticoids, contraindications to corticosteroids (such as tuberculosis and tumor) should be excluded[22]. For patients with the arteritis, it is important to be aware of the risk of the artery wall becoming thinner or even rupturing during glucocorticoid therapy, which requires real-time monitoring and appropriate intervention.
In our case, IgG4-RD involved several anatomical sites and multiple tissues and organs. A comprehensive diagnosis of IgG4-RD, including clinical history, imaging results, and pathological features, should be made. Doppler ultrasound as a noninvasive and convenient method plays an important role in the diagnosis of IgG4-RD. Timely and effective diagnosis could prevent serious organ injury, tissue fibrosis, and even death.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Immunology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shao Q S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Okazaki K, Uchida K. Current perspectives on autoimmune pancreatitis and IgG4-related disease. Proc Jpn Acad Ser B Phys Biol Sci. 2018;94:412-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Miyabe K, Zen Y, Cornell LD, Rajagopalan G, Chowdhary VR, Roberts LR, Chari ST. Gastrointestinal and Extra-Intestinal Manifestations of IgG4-Related Disease. Gastroenterology. 2018;155:990-1003.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Chen JH, Deshpande V. IgG4-related Disease and the Liver. Gastroenterol Clin North Am. 2017;46:195-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Zhang Z, Guan W, Lin Q, Yu W. Thoracic paravertebral involvement in patients with IgG4-related disease: CT and MR imaging findings. Rheumatology (Oxford). 2020;59:3878-3885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Masamune A, Kikuta K, Hamada S, Tsuji I, Takeyama Y, Shimosegawa T, Okazaki K; Collaborators. Nationwide epidemiological survey of autoimmune pancreatitis in Japan in 2016. J Gastroenterol. 2020;55:462-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 6. | Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1856] [Cited by in RCA: 1872] [Article Influence: 144.0] [Reference Citation Analysis (83)] |
| 7. | Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385:1460-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 849] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 8. | Okazaki K. Autoimmune Pancreatitis and IgG4-Related Disease: The Storiform Discovery to Treatment. Dig Dis Sci. 2019;64:2385-2394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Yoshino T, Nakamura S, Kawa S, Hamano H, Kamisawa T, Shimosegawa T, Shimatsu A, Ito T, Notohara K, Sumida T, Tanaka Y, Mimori T, Chiba T, Mishima M, Hibi T, Tsubouchi H, Inui K, Ohara H. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 625] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 10. | Wallace ZS, Naden RP, Chari S, Choi HK, Della-Torre E, Dicaire JF, Hart PA, Inoue D, Kawano M, Khosroshahi A, Lanzillotta M, Okazaki K, Perugino CA, Sharma A, Saeki T, Schleinitz N, Takahashi N, Umehara H, Zen Y, Stone JH; Members of the ACR/EULAR IgG4-RD Classification Criteria Working Group. The 2019 American College of Rheumatology/European League Against Rheumatism classification criteria for IgG4-related disease. Ann Rheum Dis. 2020;79:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 395] [Article Influence: 79.0] [Reference Citation Analysis (1)] |
| 11. | Sanges S, Jeanpierre E, Lopez B, Russick J, Delignat S, Carpentier B, Dubois R, Dubucquoi S, Guerrier T, Hachulla É, Hatron PY, Paris C, Susen S, Launay D, Lacroix-Desmazes S, Terriou L. Acquired Hemophilia A in IgG4-Related Disease: Case Report, Immunopathogenic Study, and Review of the Literature. Front Immunol. 2020;11:558811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Mahajan VS, Mattoo H, Deshpande V, Pillai SS, Stone JH. IgG4-related disease. Annu Rev Pathol. 2014;9:315-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 264] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 13. | Roos E, Hubers LM, Coelen RJS, Doorenspleet ME, de Vries N, Verheij J, Beuers U, van Gulik TM. IgG4-Associated Cholangitis in Patients Resected for Presumed Perihilar Cholangiocarcinoma: a 30-Year Tertiary Care Experience. Am J Gastroenterol. 2018;113:765-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | AbdelRazek MA, Venna N, Stone JH. IgG4-related disease of the central and peripheral nervous systems. Lancet Neurol. 2018;17:183-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 15. | Chen LYC, Mattman A, Seidman MA, Carruthers MN. IgG4-related disease: what a hematologist needs to know. Haematologica. 2019;104:444-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 16. | Wallace ZS, Zhang Y, Perugino CA, Naden R, Choi HK, Stone JH; ACR/EULAR IgG4-RD Classification Criteria Committee. Clinical phenotypes of IgG4-related disease: an analysis of two international cross-sectional cohorts. Ann Rheum Dis. 2019;78:406-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 263] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 17. | Sánchez-Oro R, Alonso-Muñoz EM, Martí Romero L. Review of IgG4-related disease. Gastroenterol Hepatol. 2019;42:638-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Thompson A, Whyte A. Imaging of IgG4-related disease of the head and neck. Clin Radiol. 2018;73:106-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Akiyama M, Kaneko Y, Takeuchi T. Characteristics and prognosis of IgG4-related periaortitis/periarteritis: A systematic literature review. Autoimmun Rev. 2019;18:102354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 20. | Matsumoto Y, Kasashima S, Kawashima A, Sasaki H, Endo M, Kawakami K, Zen Y, Nakanuma Y. A case of multiple immunoglobulin G4-related periarteritis: a tumorous lesion of the coronary artery and abdominal aortic aneurysm. Hum Pathol. 2008;39:975-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Zhang YJ, Chen Y, Zhao X, Chang X, Guan S, Tian J, Xia BY, Qu X. Coronary Arteritis and Periaortitis in IgG4-Related Disease. Can J Cardiol. 2020;36:589.e5-589.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Melenotte C, Seguier J, Ebbo M, Kaphan E, Bernit E, Saillier L, Audoin B, Feyeux D, Daniel L, Roche PH, Graillon T, Dufour H, Boutière C, Girard N, Closs-Prophette F, Guillaud C, Tieulié N, Regent A, Harlé JR, Hamidou M, Mekinian A, Grados A, Schleinitz N. Clinical presentation, treatment and outcome of IgG4-related pachymeningitis: From a national case registry and literature review. Semin Arthritis Rheum. 2019;49:430-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |