Published online Dec 26, 2022. doi: 10.12998/wjcc.v10.i36.13264
Peer-review started: August 28, 2022
First decision: October 5, 2022
Revised: October 22, 2022
Accepted: December 5, 2022
Article in press: December 5, 2022
Published online: December 26, 2022
Processing time: 120 Days and 4.5 Hours
Esophageal stenosis is one of the main complications of endoscopic submucosal dissection (ESD) for the treatment of large-area superficial esophageal squamous cell carcinoma and precancerous lesions (≥ 3/4 of the lumen). Oral prednisone is useful to prevent esophageal stenosis, but the curative effect remains controversial.
To share our experience of the precautions against esophageal stenosis after ESD to remove large superficial esophageal lesions.
Between June 2019 and March 2022, we enrolled patients with large superficial esophageal squamous cell carcinoma and high-grade intraepithelial neoplasia experienced who underwent ESD. Prednisone (50 mg/d) was administered orally on the second morning after ESD for 1 mo, and tapered gradually (5 mg/wk) for 13 wk.
In total, 14 patients met the inclusion criteria. All patients received ESD without operation-related bleeding or perforation. There were 11 patients with ≥ 3/4 and < 7/8 of lumen mucosal defects and 1 patient with ≥ 7/8 of lumen mucosal defect and 2 patients with the entire circumferential mucosal defects due to ESD. The longitudinal extension of the esophageal mucosal defect was < 50 mm in 3 patients and ≥ 50 mm in 11 patients. The esophageal stenosis rate after ESD was 0% (0/14). One patient developed esophageal candida infection on the 30th d after ESD, and completely recovered after 7 d of administration of oral fluconazole 100 mg/d. No other adverse events of oral steroids were found.
Oral prednisone (50 mg/d) and prolonged prednisone usage time may effectively prevent esophageal stricture after ESD without increasing the incidence of glucocorticoid-related adverse events. However, further investigation of larger samples is required to warrant feasibility and safety.
Core Tip: Esophageal stenosis is one of the main complications of endoscopic submucosal dissection (ESD) for the remedy of large-area superficial esophageal squamous cell carcinoma and precancerous lesions (≥ 3/4 of the lumen). Oral prednisone (30 mg/d) is one of the most commonly used treatment measures to prevent postoperative stenosis after esophageal ESD; however, several studies have drawn inconsistent conclusions. For the first time, we took a higher dose of prednisone (50 mg/d) orally to prevent esophageal stenosis after esophageal ESD and no stenosis occurred in 14 patients, meanwhile, no significant glucocorticoid-related adverse events occurred.
- Citation: Zhan SG, Wu BH, Li DF, Yao J, Xu ZL, Zhang DG, Shi RY, Tian YH, Wang LS. Oral higher dose prednisolone to prevent stenosis after endoscopic submucosal dissection for early esophageal cancer. World J Clin Cases 2022; 10(36): 13264-13273
- URL: https://www.wjgnet.com/2307-8960/full/v10/i36/13264.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i36.13264
Endoscopic submucosal dissection (ESD) is one of main treatment measures for early esophageal cancer and esophageal high-grade intraepithelial neoplasia[1]. It is minimally invasive and permits en bloc resection of large esophageal lesions without esophagectomy. However, esophageal stenosis generally occurs after ESD resection of esophageal lesions, especially for lesions ≥ 3/4 of the lumen. Multivariate analysis has shown that esophageal mucosal membrane defects over 3/4 of the lumen is an important predictor of stenosis formation. Without prophylactic treatment, the occurrence rate of esophageal strictures can reach 83.3%-94.1%; especially when the lesion affects the whole circumference of the esophagus, the rate of esophageal strictures is even higher[2,3]. This often requires repeated endoscopic balloon dilatation (EBD) to alleviate symptoms; however, the benefit is limited[4].
Recently, it has been reported that hormones, as a preventive treatment, can reduce the occurrence of stricture after esophageal ESD[5,6]. Yamaguchi et al[5] first reported that oral prednisone 30 mg/d can effectively prevent esophageal stenosis after ESD, and the postoperative stenosis rate can be reduced to 5.3% (1/19). A study also showed that oral prednisone 30 mg /d is not effective in preventing esophageal stenosis in patients with an entire circumference or ≥ 7/8 circumferential mucosal defects[7]. Meanwhile, one case reported that a patient with superficial esophageal squamous cell carcinoma received high-dose dexamethasone therapy (40 mg for 4 d) for multiple myeloma on the 9th d after ESD. After three courses of treatment, no esophageal stenosis was found in the follow-up gastroscopy, the histopathological evaluation showed that the submucosa became thinner, and the fibrosis degree of wound scar after ESD was the lowest[8]. In a prospective study by Nakamura, 11 patients with superficial esophageal squamous cell carcinoma with lesions ≥ 3/4 of the circumference were treated with steroid pulse therapy from the next day after ESD (intravenous infusion of sodium methylprednisolone succinate 500 mg/d for 3 consecutive days from the next day after ESD)[9]. It was found that although steroid pulse therapy was safe, steroid pulse therapy had no preventive effect on esophageal stenosis after ESD. Therefore, oral hormone is an effective method to prevent esophageal stenosis after esophageal ESD, but the dose, use time, effectiveness, and safety of the hormone need to be further studied.
Between June 2019 and March 2022, 74 patients with superficial esophageal squamous cell carcinoma or precancerous lesions of esophagus were en bloc resected with ESD at the Digestive Endoscopy Center of Shenzhen People’s Hospital (Guangdong province, China). Of these, 18 patients accepted mucosal resection via ESD, and the mucosal defects involved a 3/4 or larger circumference of the esophageal lumen. However, 1 patient who had received additional chemoradiotherapy (CRT) and 3 patients who had undergone additional surgery were removed from our study. Ultimately, 14 patients were included in this study. The indication criteria were as follows: (1) Before esophageal ESD, the intrapapillary capillary of the lesion mucosa observed by narrow band imaging magnifying endoscopy was type B1; (2) The preoperative lesions of ESD were histologically confirmed as superficial esophageal squamous cell carcinoma or esophageal high-grade intraepithelial neoplasia; (3) Thoracoabdominal enhanced computed tomography (CT) showed no lymph node or distant metastasis; (4) Provision of written informed consent was given; (5) There was no achalasia; and (6) There was no corrosive injury of esophagus. The exclusion criteria were as follows: (1) Patients who could not be followed up for 6 mo or longer; (2) patients whose stenosis formed before esophageal ESD; (3) patients who had prior esophageal cancer with CRT; and (4) patients with additional CRT or additional esophagectomy after non-curative ESD.
The ESD was operated in an operating room. The patients were endotracheal intubated and kept in the left lying position. An endoscope (GIF-Q260J; Olympus Co., Tokyo, Japan) was attached with forward water delivery function and was used with carbon dioxide insufflation. The head end of the endoscope was connected with a pellucid cap (diameter 12.4 mm, length 4 mm, D-201-11804; Olympus Co.). Iodine 0.75% staining was used for identifying the range of the lesion, and a dual knife (KD-650Q; Olympus Co.) marking dots 3 mm away from the edge of the lesion. An electronic surgical workstation (VIO 200D; ERBE, Tübingen, Germany) was used with an operating mode (Forced Coagulation model, effect 2, maximum power 20 W). The 0.9% saline solution containing 0.3% indigo carmine was used for adequate submucosal injection along the marked dots. After circumferential mucosal incision, submucosal dissection was operated by the dual knife (KD-650Q) from the oral side to the anal side of the lesion under an operating mode (Endocut I mode, Forced Coagulation model, effect 2, maximum power 50 W, VIO 200D; ERBE). Bleeding during the operation and visible blood vessels were handled with hemostatic forceps (FD-410LR; Olympus Co.) under a soft coagulating mode (Soft Coagulation mode, effect 4, maximum power 80 W, VIO 200D; ERBE).
Postoperative-related bleeding was defined as bleeding requiring blood transfusion or surgical intervention, or bleeding that resulted in a 2 g/dL decline in hemoglobin levels. Postoperative-related perforation was diagnosed by endoscopy or chest CT[7]. All patients treated with proton pump inhibitor, esomeprazole, with a dose of 20 mg, twice a day after ESD for 28 d[5,9]. After ESD, each patient received two pieces of calcium carbonate and vitamin D3 chewable tablets (each tablet contains 300 mg calcium and 60 IU vitamin D3) per day until the prednisone was stopped to prevent glucocorticoid-induced osteoporosis[10].
Prednisolone was taken orally at a dose of 50 mg/d from the next morning after ESD for 1 mo, and then decreased gradually (45, 40, 35, 30, 25, 20, 15, 10, and 5 mg for 7 d each) until 13 wk later.
Regular endoscopy was examined at 1, 3, and 6 mo after ESD operation, and then annually thereafter. In addition, endoscopic examination was performed whenever the patient had dysphagia to determine whether there was esophageal stenosis. EBD was performed subsequently if esophageal stricture was identified. Any abnormal mucosa required biopsy for pathological evaluation of whether there was local tumor recurrence. Meanwhile, regular physical and blood examinations were carried out to evaluate the side effects of the steroid. Bone mineral density testing was performed before ESD treatment and 6 mo after ESD treatment.
The main outcome measure was incidence of esophageal stenosis. Esophageal stenosis was defined as the inability of 9.9 mm diameter gastroscope (GIF-Q260J; Olympus) to pass through the esophageal stenosis. Secondary observation indicators of glucocorticoid-related adverse events were observed at 1, 3, and 6 mo after ESD such as newly diagnosed diabetes or aggravation of diabetes, pepticulcer, adrenocortical insufficiency, aggravation of osteoporosis or fracture, and corticosteroid-related mental disorders.
End point: The follow-up was terminated if tumor recurrence and serious adverse events of glucocorticoid and procedure-related complications (procedure-related bleeding and procedure-related perforation) occurred.
Continuous variables are presented as the mean ± standard deviation or median (interquartile range, 25%-75%). Categorical variables are expressed by proportion. Date analyses were conducted using SPSS 23.0 software (version 23.0 for Mac).
After ESD surgery, there were 18 patients with mucosal defects more than 3/4 of the esophageal circumference. One patient received additional CRT treatment, and 3 patients received additional surgery and were removed from this study. Eventually, a total of 14 patients met the criteria. Patients and characteristics of lesions are shown in Table 1 and Table 2. Male patients accounted for 64%, with a mean age of 62.1 years (ranging from 45 to 75 years). According to the Paris endoscopic classification,13 cases of endoscopic tumor morphology were classified as type 0-IIa and 1 case was type 0-IIc. The lesions were mainly located in the middle and lower esophagus, and 1 case was located in the upper esophagus. Each patient successfully received esophageal ESD treatment, and postoperative pathology confirmed that the lesion was completely removed. All patients had no procedure-related bleeding and procedure-related perforation after esophageal ESD. The mean resection size was 55.5 mm in diameter (ranging from 47.5 mm to 65.0 mm). According to the range of esophageal mucosal defect, 11 cases involved ≥ 3/4 and < 7/8 circumference, 1 case involved ≥ 7/8 circumference, and 2 cases involved the entire circumference. The longitudinal extension of mucosal defect was < 50 mm in 3 patients and ≥ 50 mm in 11 patients. In 12 cases, the depth of invasion of pathological tissues was limited within the epithelium and lamina propria mucosa, whereas 2 lesions were limited within muscularis mucosa without lymphovascular infiltration.
| Characteristics | |
| Sex | |
| Male | 9 (64) |
| Female | 5 (36) |
| Age in yr, mean (range) | 62.1 (45-75) |
| Tumor location | |
| Cervical esophagus | 0 (0) |
| Upper thoracic esophagus | 1 (7) |
| Middle thoracic esophagus | 7 (50) |
| Lower thoracic esophagus | 6 (43) |
| Macroscopic type | |
| 0-IIa | 13 (93) |
| 0-IIc | 1 (7) |
| Tumor size in mm, mean (range) | 51.7 (43.8-60.7) |
| Resection size in mm, mean (range) | 55.5 (47.5-65.0) |
| Transverse extension of mucosal defect | |
| ≥ 3/4 and < 7/8 circumference | 11 (79) |
| ≥ 7/8 circumference | 1 (7) |
| The entire circumference | 2 (14) |
| Longitudinal extension of the mucosal defect | |
| < 50 mm | 3 (21) |
| ≥ 50 mm | 11 (79) |
| Depth of tumor invasion | |
| M1 or M2 | 12 (86) |
| M3 | 2 (14) |
| Outcome | |
| Stricture rate | 0 (0) |
| Main complication | 1 (7) |
| Procedure-related bleeding | 0 (0) |
| Procedure-related perforation | 0 (0) |
| Newly diagnosed diabetes or aggravation of diabetes | 0 (0) |
| Pepticulcer | 0 (0) |
| Adrenocortical insufficiency | 0 (0) |
| Aggravation of osteoporosis or fracture | 0 (0) |
| Infection | 1 (7) |
| Corticosteroid related mental disorders | 0 (0) |
| Follow-up period (mo), median (range) | 13 (6-28) |
| Residual | 0 (0) |
| Recurrence | 0 (0) |
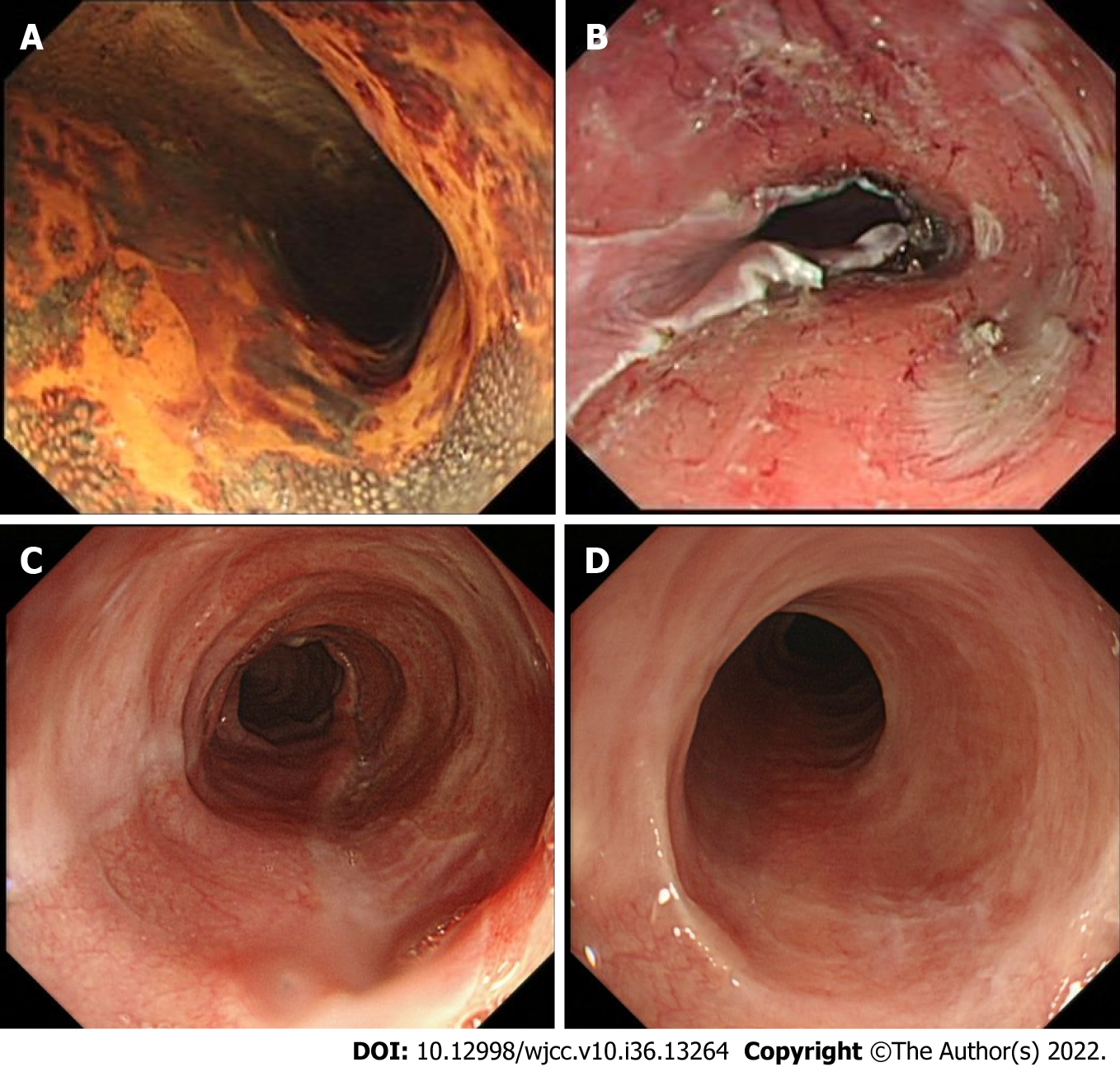
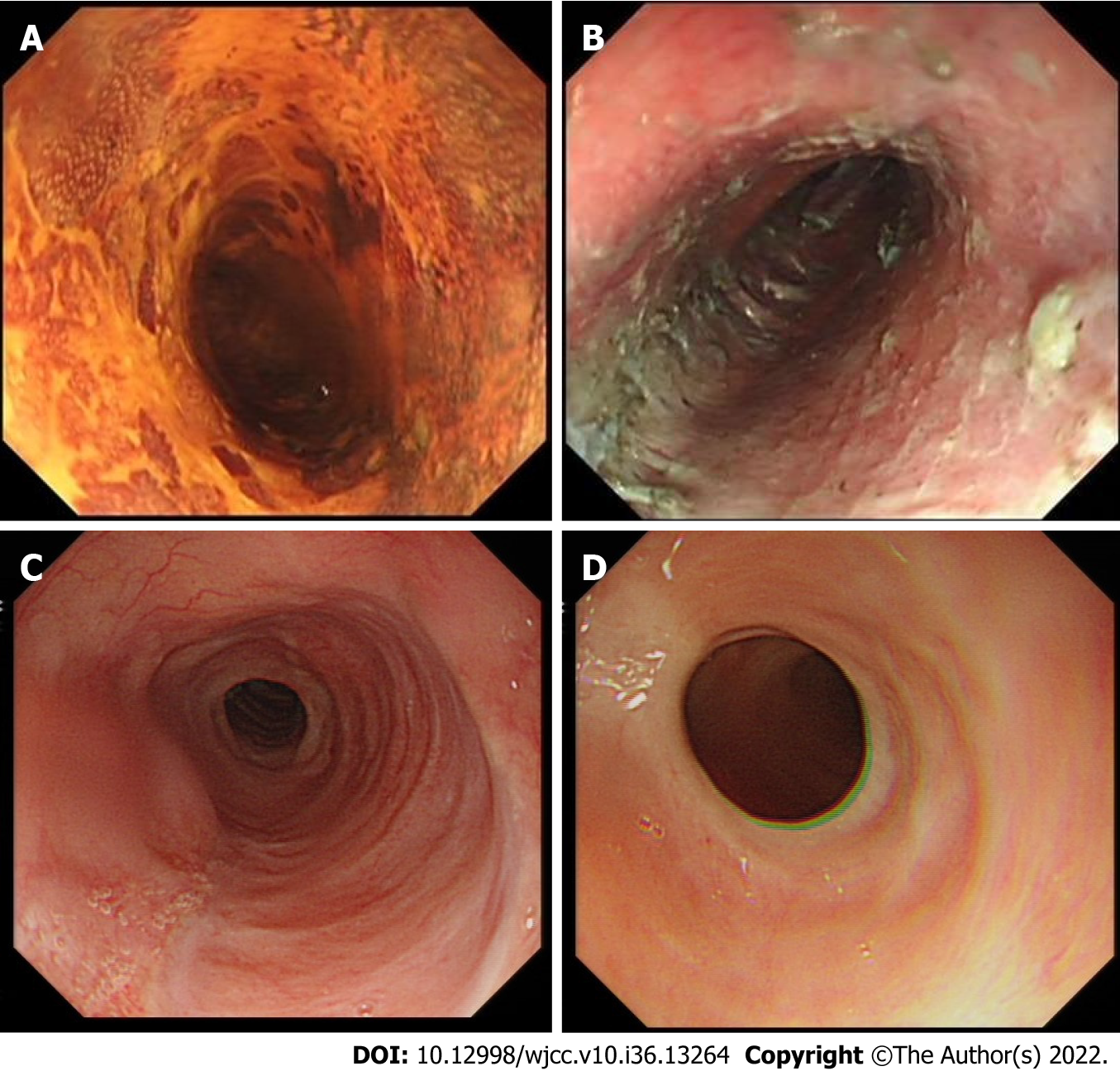
The shortest follow-up time of all cases was 6 mo, the longest follow-up time was 28 mo, and the median follow-up time was 13 mo. During this period, all patients were followed up by endoscopy regularly without dysphagia. The incidence of esophageal stenosis was 0% (0/14) (Table 3). Representative cases are shown in Figures 1 and 2.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 | Case 12 | Case 13 | Case 14 | |
| Sex | Male | Male | Male | Female | Female | Male | Male | Male | Female | Male | Female | Male | Male | Female |
| Age in yr | 66 | 45 | 50 | 75 | 67 | 61 | 63 | 45 | 70 | 72 | 67 | 55 | 66 | 67 |
| Tumor location | Lt | Ut | Lt | Mt | Mt | Mt | Lt | Lt | Mt | Mt | Mt | Lt | Lt | Mt |
| Macroscopic type | 0-IIa | 0-IIa | 0-IIa | 0-IIa | 0-IIc | 0-IIa | 0-IIa | 0-IIa | 0-IIa | 0-IIa | 0-IIa | 0-IIa | 0-IIa | 0-IIa |
| Tumor size in mm | 50.7 | 49.5 | 48.4 | 43.8 | 47.2 | 53.3 | 60.7 | 49.2 | 59.8 | 46.5 | 59.5 | 45.2 | 51.8 | 58.6 |
| Resection size in mm | 56.1 | 52.6 | 52.7 | 47.5 | 50.8 | 57.6 | 65.0 | 53.2 | 64.7 | 50.1 | 63.5 | 47.1 | 54.6 | 61.2 |
| Transverse extension of mucosal defect | ≥ 3/4 and < 7/8 | ≥ 3/4 and < 7/8 | ≥ 3/4 and < 7/8 | ≥ 3/4 and < 7/8 | ≥ 3/4 and < 7/8 | ≥ 3/4 and < 7/8 | Entire | ≥ 3/4 and < 7/8 | Entire | ≥ 3/4 and < 7/8 | ≥ 7/8 | ≥ 3/4 and < 7/8 | ≥ 3/4 and < 7/8 | ≥ 3/4 and < 7/8 |
| Longitudinal extension of the mucosal defect in mm | ≥ 50 | ≥ 50 | < 50 | ≥ 50 | ≥ 50 | ≥ 50 | ≥ 50 | < 50 | ≥ 50 | ≥ 50 | ≥ 50 | < 50 | ≥ 50 | ≥ 50 |
| Depth of tumor invasion | M3 | M2 | M1 | M1 | M2 | M3 | M2 | M1 | M2 | M1 | M2 | M1 | M1 | M2 |
| Stricture | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Follow up time in mo | 9 | 12 | 12 | 12 | 12 | 12 | 15 | 18 | 28 | 28 | 6 | 6 | 7 | 8 |
| Residual | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Recurrence | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
Only 1 patient developed esophageal Candida infection on the 30th d after ESD and recovered completely after 7d of treatment with oral fluconazole 100 mg/d. Glucocorticoid-related adverse events were observed such as newly diagnosed diabetes or aggravation of diabetes, pepticulcer, adrenocortical insufficiency, aggravation of osteoporosis or fracture, and corticosteroid-related mental disorders.
In the current study, increasing the dose of oral hormone (prednisone acetate 50 mg/d) and prolonging the treatment time (13 wk) were effective to prevent esophageal stenosis in patients with mucosal defects ≥ 3/4 circumference after ESD. Studies have shown that the occurrence of esophageal stricture after ESD is related to the infiltration of postoperative inflammatory cells and vascular proliferation[11,12]. At the same time, epithelial cells proliferate and migrate from the edge of the wound after ESD, fibroblasts proliferate continuously, and finally fibrous scar is formed. This process is divided into three stages: acute inflammation, proliferation, and remodeling; however, the duration of this process is unknown. Through the dog model study, Honda found that within about 1 mo after esophageal EMR, the mucosal defect healed and was covered by squamous cells. Although the proper muscle layer was not damaged, muscle fiber atrophy still occurred in the 1st wk after operation, and finally fibrosis was formed[13]. Some clinical studies have also shown that esophageal stenosis mostly occurs within 2 to 4 wk after surgery[3,14], but this was confined to endoscopic observation. Glucocorticoid has a strong anti-inflammatory effect, which not only inhibits the synthesis of collagen but also promotes the decomposition of collagen to inhibit the formation of stenosis. In our study, all cases did not have esophageal stenosis after ESD. We believe that increasing the dose of prednisolone can enhance the anti-inflammatory effect in the acute inflammatory period, especially in the critical period of the 1st mo after ESD. Meanwhile, we speculate that the process of esophageal stenosis may last longer than expected, and prolonging the usage of prednisolone may inhibit the proliferation of fibroblasts steadily to prevent esophageal remodeling and the formation of esophageal stenosis.
There are several reports on the application of steroids to prevent stenosis after ESD operation for large-area superficial esophageal squamous cell carcinoma and precancerous lesions. Yamaguchi et al[5] reported the therapeutic effect of oral prednisolone after esophageal ESD for the first time. In their report, prednisone was taken on the 3rd d after ESD, with an initial dose of 30 mg/d, and then decreased gradually (30, 30, 25, 25, 20, 15, 10, and 5 mg for 1 wk each). The incidence of stenosis after semi-circumferential ESD resection and entire circumferential ESD resection were 6.3% (1/16) and 0% (0/3), respectively[5]. However, for the cases of circumferential esophageal mucosal defect after ESD, Sato et al[15] found that oral prednisone 30 mg/d could not reduce the incidence of postoperative esophageal stenosis, but could decrease the total number of EBD expansions required. In Kadota’s[7] study, the stenosis rate of patients with less than entire circumferential ESD resection and with oral prednisone 30 mg/d administration was similar to the results by Yamaguchi et al[5], whereas patients with entire circumferential ESD resection showed higher stenosis rate (10/14) even with additional local submucosal steroid injections[7]. Meanwhile, two studies of submucosal injection of triamcinolone acetonide within the mucosal defects combined with oral prednisone in the prevention of esophageal stenosis post-ESD for lesions more than 3/4 circumference have obtained completely opposite results. Chu et al[16] reported that after treated with submucosal injection of triamcinolone acetonide within the mucosal defects combined with oral prednisone 30 mg/d, the incidence of esophageal stenosis was only 18.2% (2/11), including lesions with total circumferential resection. Surprisingly, in the Hanaoka et al[17] study of 12 cases with whole circumferential defect, the same steroid submucosal injection combined with oral prednisone 5 mg/d were used for post-ESD treatment; nevertheless, 11 patients failed to avoid postoperative stenosis. This discrepancy may be caused by different doses of orally-taken prednisolone in these studies. A study about short-term usage of oral prednisolone (30, 20, and 10 mg/d for 1 wk each) for mucosal defects ≥ 3/4 circumference, including 3 patients with total circumferential resection that showed a stenosis rate of 18% (3/17), and 1 of 3 patients with total circumferential resection withstood stenosis[18]. Accordingly, we speculate that the prevention of esophageal stenosis after esophageal ESD by oral prednisone was correlated with the dose and the use time. In our study, we increased the dose of prednisone to 50 mg/d and prolonged the treatment time to 13 wk. During our follow-up, 14 patients had no feedback of dysphagia symptoms, and no esophageal stenosis observed by endoscopic examination. Especially the 2 patients with entire circumference mucosal defects, although the esophageal wounds were fibrotic, the 9.9 mm diameter gastroscope (GIF-Q260J; Olympus) could pass, and we did not add EBD treatment. In addition, studies have shown that the injury of the intrinsic muscle layer was one of the risk factors for esophageal stenosis after ESD for early esophageal cancer and precancerous lesions[19,20]. Therefore, we paid more attention to avoid the injury of the intrinsic muscle layer as much as possible during ESD operation, which we think is also helpful for the prevention of postoperative esophageal stenosis.
Furthermore, systemic steroids are associated with adverse events, including newly diagnosed diabetes or aggravation of diabetes, pepticulcer, adrenocortical insufficiency, aggravation of osteoporosis or fracture, and corticosteroid-related mental disorders. Stuck et al[21] showed that when the cumulative dose of oral prednisone exceeded 700 mg, the risk of infectious complications in patients taking prednisone increased with the increase of prednisone dosage. One study also found that even short-term steroid use is related to increased risks of adverse events[22]. However, in our protocol, the accumulated dose of oral steroids was 3075 mg, which was higher than that of other studies, and proton pump inhibitor, oral calcium, and vitamin D3 were taken simultaneously. One patient was found to have esophageal Candida infection on the 30th d after operation, and completely recovered after 7 d of oral fluconazole 100 mg/d therapy, and no patients experienced other adverse incidents related to orally-taken prednisolone. Therefore, we believe that the treatment scheme of increasing the dose of prednisone (50 mg/d) is safe, but still needs long-term follow-up and observation.
This study had several limitations. First, this study was a retrospective analysis in single-centered, and possible bias could not be avoided. Second, the follow-up time was insufficient and could not comprehensively evaluate the feasibility and safety of the hormone. Third, the number of subjects was relatively small, and the control group was lacking, so statistical difference analysis could not be conducted. Due to these limitations, prospective randomized controlled studies should be established to validate the efficacy and safety of prophylactic steroid therapy.
In conclusion, increasing the dose of oral prednisone (50 mg/d) and prolonging the usage time (total 13 wk) may effectively prevent esophageal stenosis after ESD to remove large-area superficial esophageal squamous cell carcinoma or precancerous lesions of esophagus, and does not increase the incidence of glucocorticoid-related adverse events.
Esophageal stenosis is one of the main complications of endoscopic submucosal dissection (ESD) for the treatment of large-area superficial esophageal squamous cell carcinoma and precancerous lesions (≥ 3/4 of the lumen). Oral prednisone is useful to prevent esophageal stenosis, but the curative effect remains controversial.
Explore more effective methods to prevent esophageal stenosis after ESD for early esophageal cancer and precancerous lesions.
We shared our experience of the precautions against esophageal stenosis after ESD to remove large superficial esophageal lesions.
Patients with large superficial esophageal squamous cell carcinoma and high-grade intraepithelial neoplasia experienced ESD were enrolled. Prednisone (50 mg/d) was administered orally on the 2nd d after ESD for 1 mo, and tapered gradually (5 mg/wk) for 13 wk.
According to the range of esophageal mucosal defect, 11 cases involved ≥ 3/4 and < 7/8 circumference, 1 case involved ≥ 7/8 circumference, and 2 cases involved the entire circumference. The incidence of esophageal stenosis was 0% (0/14), and only 1 patient developed esophageal Candida infection on the 30th d after ESD and recovered completely after 7d of treatment with oral fluconazole 100 mg/d.
Further investigation of larger samples is required to warrant feasibility and safety.
In conclusion, increasing the dose of oral prednisone (50 mg/d) and prolonging the usage time (total 13 wk) may effectively prevent esophageal stenosis after ESD removing large-area superficial esophageal squamous cell carcinoma or precancerous lesions of esophagus, and does not increase the incidence of glucocorticoid-related adverse events.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Dilek ON, Turkey; Lim KT, Singapore; Salimi M, Iran; Sumi K, Japan S-Editor: Liu GL L-Editor: Filipodia P-Editor: Liu GL
| 1. | Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 459] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 2. | Shi Q, Ju H, Yao LQ, Zhou PH, Xu MD, Chen T, Zhou JM, Chen TY, Zhong YS. Risk factors for postoperative stricture after endoscopic submucosal dissection for superficial esophageal carcinoma. Endoscopy. 2014;46:640-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 3. | Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy. 2009;41:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 274] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 4. | Yoda Y, Yano T, Kaneko K, Tsuruta S, Oono Y, Kojima T, Minashi K, Ikematsu H, Ohtsu A. Endoscopic balloon dilatation for benign fibrotic strictures after curative nonsurgical treatment for esophageal cancer. Surg Endosc. 2012;26:2877-2883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Yamaguchi N, Isomoto H, Nakayama T, Hayashi T, Nishiyama H, Ohnita K, Takeshima F, Shikuwa S, Kohno S, Nakao K. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc. 2011;73:1115-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 6. | Hashimoto S, Kobayashi M, Takeuchi M, Sato Y, Narisawa R, Aoyagi Y. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest Endosc. 2011;74:1389-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 7. | Kadota T, Yano T, Kato T, Imajoh M, Noguchi M, Morimoto H, Osera S, Yoda Y, Oono Y, Ikematsu H, Ohtsu A, Kaneko K. Prophylactic steroid administration for strictures after endoscopic resection of large superficial esophageal squamous cell carcinoma. Endosc Int Open. 2016;4:E1267-E1274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Ono S, Fujishiro M, Kodashima S, Minatsuki C, Hirano K, Niimi K, Goto O, Yamamichi N, Fukuda T, Seto Y, Koike K. High-dose dexamethasone may prevent esophageal stricture after endoscopic submucosal dissection. Clin J Gastroenterol. 2010;3:155-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Nakamura J, Hikichi T, Watanabe K, Sato M, Obara K, Ohira H. Feasibility of Short-Period, High-Dose Intravenous Methylprednisolone for Preventing Stricture after Endoscopic Submucosal Dissection for Esophageal Cancer: A Preliminary Study. Gastroenterol Res Pract. 2017;2017:9312517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, Humphrey MB, Lane NE, Magrey M, Miller M, Morrison L, Rao M, Robinson AB, Saha S, Wolver S, Bannuru RR, Vaysbrot E, Osani M, Turgunbaev M, Miller AS, McAlindon T. 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Rheumatol. 2017;69:1521-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 354] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 11. | Wick G, Grundtman C, Mayerl C, Wimpissinger TF, Feichtinger J, Zelger B, Sgonc R, Wolfram D. The immunology of fibrosis. Annu Rev Immunol. 2013;31:107-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 262] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 12. | Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annu Rev Pathol. 2013;8:241-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 469] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 13. | Honda M, Nakamura T, Hori Y, Shionoya Y, Yamamoto K, Nishizawa Y, Kojima F, Shigeno K. Feasibility study of corticosteroid treatment for esophageal ulcer after EMR in a canine model. J Gastroenterol. 2011;46:866-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Mizuta H, Nishimori I, Kuratani Y, Higashidani Y, Kohsaki T, Onishi S. Predictive factors for esophageal stenosis after endoscopic submucosal dissection for superficial esophageal cancer. Dis Esophagus. 2009;22:626-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Sato H, Inoue H, Kobayashi Y, Maselli R, Santi EG, Hayee B, Igarashi K, Yoshida A, Ikeda H, Onimaru M, Aoyagi Y, Kudo SE. Control of severe strictures after circumferential endoscopic submucosal dissection for esophageal carcinoma: oral steroid therapy with balloon dilation or balloon dilation alone. Gastrointest Endosc. 2013;78:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Chu Y, Chen T, Li H, Zhou P, Zhang Y, Chen W, Zhong Y, Yao L, Xu M. Long-term efficacy and safety of intralesional steroid injection plus oral steroid administration in preventing stricture after endoscopic submucosal dissection for esophageal epithelial neoplasms. Surg Endosc. 2019;33:1244-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Hanaoka N, Ishihara R, Uedo N, Takeuchi Y, Higashino K, Akasaka T, Kanesaka T, Matsuura N, Yamasaki Y, Hamada K, Iishi H. Refractory strictures despite steroid injection after esophageal endoscopic resection. Endosc Int Open. 2016;4:E354-E359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Kataoka M, Anzai S, Shirasaki T, Ikemiyagi H, Fujii T, Mabuchi K, Suzuki S, Yoshida M, Kawai T, Kitajima M. Efficacy of short period, low dose oral prednisolone for the prevention of stricture after circumferential endoscopic submucosal dissection (ESD) for esophageal cancer. Endosc Int Open. 2015;3:E113-E117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Ezoe Y, Muto M, Horimatsu T, Morita S, Miyamoto S, Mochizuki S, Minashi K, Yano T, Ohtsu A, Chiba T. Efficacy of preventive endoscopic balloon dilation for esophageal stricture after endoscopic resection. J Clin Gastroenterol. 2011;45:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | Miwata T, Oka S, Tanaka S, Kagemoto K, Sanomura Y, Urabe Y, Hiyama T, Chayama K. Risk factors for esophageal stenosis after entire circumferential endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Surg Endosc. 2016;30:4049-4056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 21. | Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11:954-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 557] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 22. | Waljee AK, Rogers MA, Lin P, Singal AG, Stein JD, Marks RM, Ayanian JZ, Nallamothu BK. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 394] [Cited by in RCA: 528] [Article Influence: 66.0] [Reference Citation Analysis (0)] |