Published online Nov 16, 2022. doi: 10.12998/wjcc.v10.i32.11955
Peer-review started: July 13, 2022
First decision: September 25, 2022
Revised: October 1, 2022
Accepted: October 18, 2022
Article in press: October 18, 2022
Published online: November 16, 2022
Processing time: 117 Days and 18.9 Hours
Aplastic anemia (AA) complicated with myocardial infarction (MI) is rare and associated with poor prognosis. Here, we present a case of AA with recurrent acute MI (AMI) in a patient treated with cyclosporine A (CsA) and stanozolol. In this patient, we suspect the long-term use of medication linked to platelets hyperfunction.
In 2017, a 45-year-old man was rushed to the emergency department of China-Japan Union Hospital due to precordial pain for 5 h. Based on his symptoms, medical history, blood tests, and findings from coronary angiography (CAG), the patient was diagnosed with acute anterior wall, ST-segment elevated MI, Killip II grade, AA, and dyslipidemia. In 2021, the patient was readmitted to the hospital for 2 h due to chest pain. Because the patient’s platelet count was 30 × 109/L and he had severe thrombocytopenia, we performed CAG following platelet transfusion. Optical coherence tomography revealed lipid plaque and thrombus mass in his right coronary artery. The antithrombotic approach was adjusted to employ only anticoagulants (factor Xa inhibitors) and adenosine diphosphate inhibitors (clopidogrel) after assessing the risk of bleeding/thrombotic events. Long-term follow-up revealed that the patient had made a good recovery.
Patients with AA should be closely monitored for the risk of thrombosis and cardiovascular events, particularly when taking stanozolol or CsA for an extended period of time.
Core Tip: Acute myocardial infarction combined with aplastic anemia (AA) has been reported but not well studied. The medicine used to treat AA in this patient may have caused coronary artery and left ventricular thrombosis. Coronary intervention, particularly stenting, is still controversial in this rare clinical scenario. Thus far, no specific management regarding this disease, especially for intracoronary findings, has been reported. Early revascularization may be the most important factor in improving patient prognosis.
- Citation: Zhao YN, Chen WW, Yan XY, Liu K, Liu GH, Yang P. What is responsible for acute myocardial infarction in combination with aplastic anemia? A case report and literature review. World J Clin Cases 2022; 10(32): 11955-11966
- URL: https://www.wjgnet.com/2307-8960/full/v10/i32/11955.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i32.11955
Aplastic anemia (AA) is a kind of bone marrow hematopoietic failure syndrome defined by the decreased proliferation of bone marrow hematopoietic cells and peripheral blood cytopenia, which can be caused by a variety of factors[1]. The most prevalent clinical manifestations are anemia, hemorrhage, and infection. AA is treated with immunosuppressive agents such as cyclosporine A (CsA) and anabolic steroids such as stanozolol, with the purpose of stimulating bone marrow hematopoiesis[2]. However, long-term use of these medicines may increase the risk of abnormal blood lipid metabolism and atherosclerosis[3,4]. AA complicated by myocardial infarction (MI) is considered uncommon in the clinic. Herein, we present a case of recurrent acute MI (AMI) in a patient with AA who was receiving long-term CsA and stanozolol treatment. We suspect that this occurrence is linked to platelet hyperfunction and long-term medication usage.
A 45-year-old man was admitted to the hospital with chest pain.
The patient’s chest pain started 5 h prior to presentation while at work; he was transferred to the hospital via ambulance. He described a crushing pain located posterior to the interruption of the sternum, which radiated to the back of the shoulder. The pain was not relieved by sublingual nitroglycerin. The patient complained of persistent pain even after arriving at the hospital.
Four years before this admission, AA was diagnosed based on the patient’s bone marrow biopsy pathology report. CsA 75 mg and stanozolol (Conlone) 2 mg (t.i.d.) were prescribed for AA. The patient had no history of smoking, hypertension, or type 2 diabetes.
His family had no early onset history of cardiovascular diseases.
Physical examinations showed normal blood pressure (120/70 mmHg), and chest auscultation revealed lung rales.
Table 1 shows the blood test result, including blood count, liver function, renal function, and markers of myocardial injury, etc. Column 1 presents the results of blood tests at the first admission.
| Parameters | Result of first in hospital (2017) | Result of second in hospital (2021) | Reference range |
| Hemocyte profile | |||
| White blood cell | 15.52 × 109/L | 5.3 × 109/L | 4-10 × 109/L |
| Hemoglobin | 157 g/L | 149 g/L | 120-160 g/L |
| Platelet count | 85 × 109/L | 30 × 109/L | 100-300 × 109/L |
| Neutrophil | 12.98 × 109/L | 3.31 × 109/L | 2-7 × 109/L |
| Lymphocyte | 1.49 × 109/L | 1.53 × 109/L | 0.8-4.0 × 109/L |
| Monocyte | 1.03 × 109/L | 0.37 × 109/L | 0.12-0.8 × 109/L |
| Neutrophil% | 83.5% | 62.4% | 50%-70% |
| Lymphocyte% | 9.6% | 28.9% | 20%-40% |
| Eosinophil | 0.01 × 109/L | 0.08 × 109/L | 0.05-0.50 × 109/L |
| Basophil | 0.01 × 109/L | 0.01 × 109/L | 0.00-0.10 × 109/L |
| Mean corpuscular volume | 108.6 fL | 113.1 fL | 80-100 fL |
| Mean corpuscular hemoglobin | 36.5 pg | 38.1 pg | 27-34 pg |
| Platelet volume distribution width | 17.4% | 16.1% | 15%-17% |
| Mean platelet volume | 12.3 fL | 10.1 fL | 7-11 fL |
| Hypersensitive troponin Ia | 1.76 ng/mL (0-0.04) | 0.038 ng/mL (0.01-0.023) | |
| Myoglobina | 781.5 ng/mL (0-120) | 48 ng/mL (23-112) | |
| Blood urea nitrogen | 5.67 mmol/L | 5.67 mmol/L | 3.2-7.1 mmol/L |
| Creatinine | 91.91 umol/L | 90.7 umol/L | 58.0-110.0 umol/L |
| Thromboelastography | |||
| Inhibition rate of AA channel | 50.4% | 75.1% | ≥ 50%, with aspirin |
| inhibition rate of ADP channel | 25.9% | 28.3% | ≥ 30% with ADP inhibitor |
| Thrombophilia profile | |||
| Prothrombin time | 10 s | 10.5 s | 9.4-12.5 s |
| Prothrombin activity | 108% | 111% | 70%-140% |
| International normalized ratio | 0.93 | 0.93 | 0.7-1.4 |
| Fibrinogen | 4.1 g/L | 3.24 g/L | 2.00-4.00 g/L |
| D-dimer | NK | 1.16 ug/mL | 0.00-0.50 ug/mL |
| Lipid profile | |||
| Total cholesterol | 8.17 mmol/L | 5.95 mmol/L | 3-5.7 mmol/L |
| Triglycerides | 2.91 mmol/L | 2.36 mmol/L | < 1.7 mmol/L |
| HDL cholesterol | 0.74 mmol/L | 0.77 mmol/L | 1.16-1.42 mmol/L |
| LDL cholesterol | 6.82 mmol/L | 4.14 mmol/L | < 4.3 mmol/L |
| Apolipoprotein A | 0.64 g/L | 0.88 g/L | 0.264-1.362 g/L |
| Apolipoprotein B | 2.81 g/L | 1.36 g/L | 1.05-1.75 g/L |
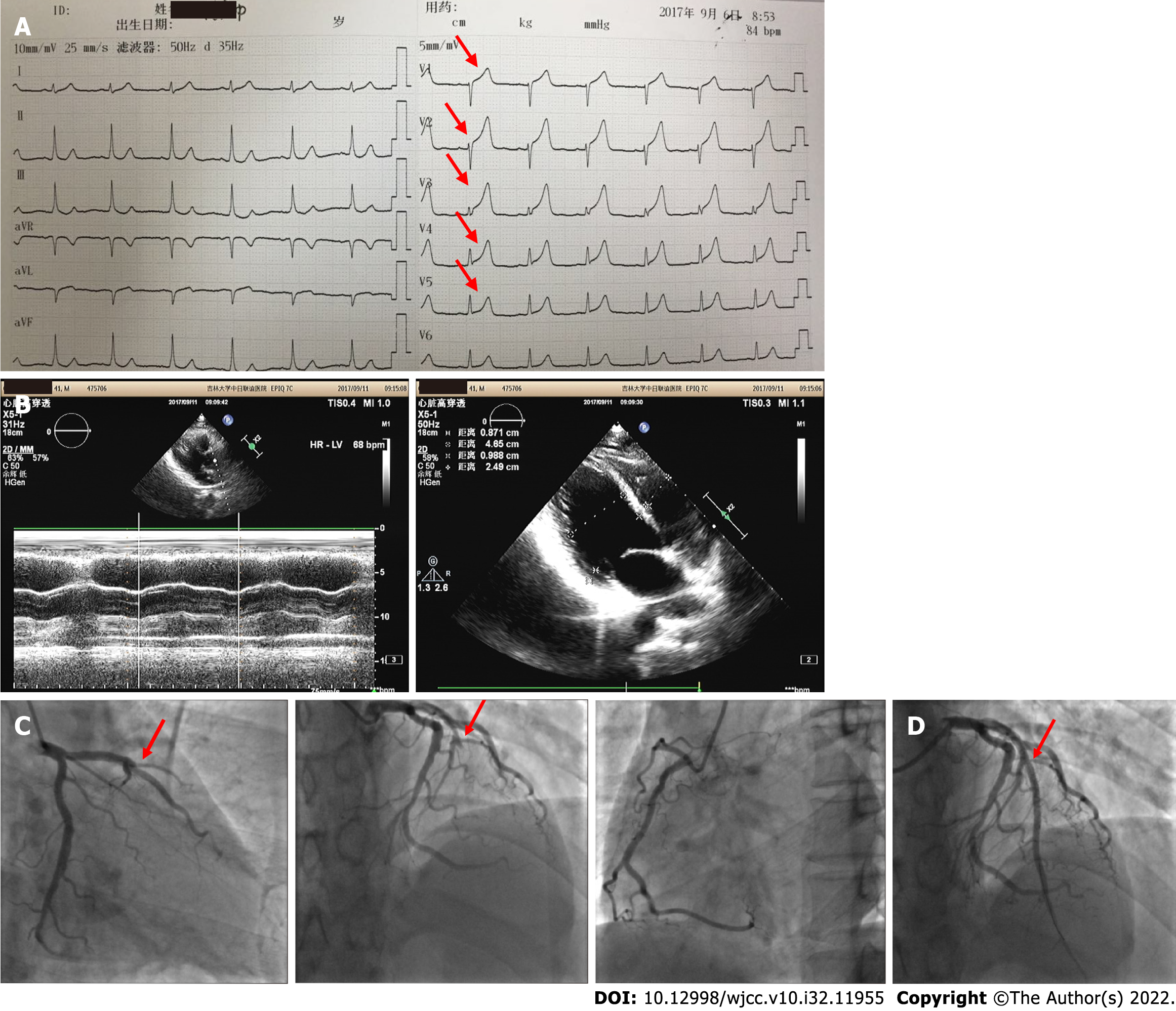
Electrocardiogram (ECG) showed that ST-segment in the V1–V6 Leads was elevated, and the T wave was high and sharp (Figure 1A). According to the ECG data, the motion amplitude of the left ventricular anterior wall and anterior septum was reduced, and the ejection fraction was 60.6% (Figure 1B). An emergency coronary angiography (CAG) revealed that the proximal segment of the anterior descending coronary artery was completely occluded (Figure 1C).
Based on his symptoms, medical history, blood tests, and CAG findings, the patient was diagnosed with acute anterior wall ST-segment elevated MI (STEMI), Killip II grade, AA, and dyslipidemia.
Based on the CAG findings, one stent was implanted in the Left descending coronary to revascularize the artery (Figure 1D). Following the thromboelastography (TEG) results (Table 1) and the risk of bleeding and thrombus, the patient was prescribed clopidogrel 75 mg and rosuvastatin 10 mg, once a day.
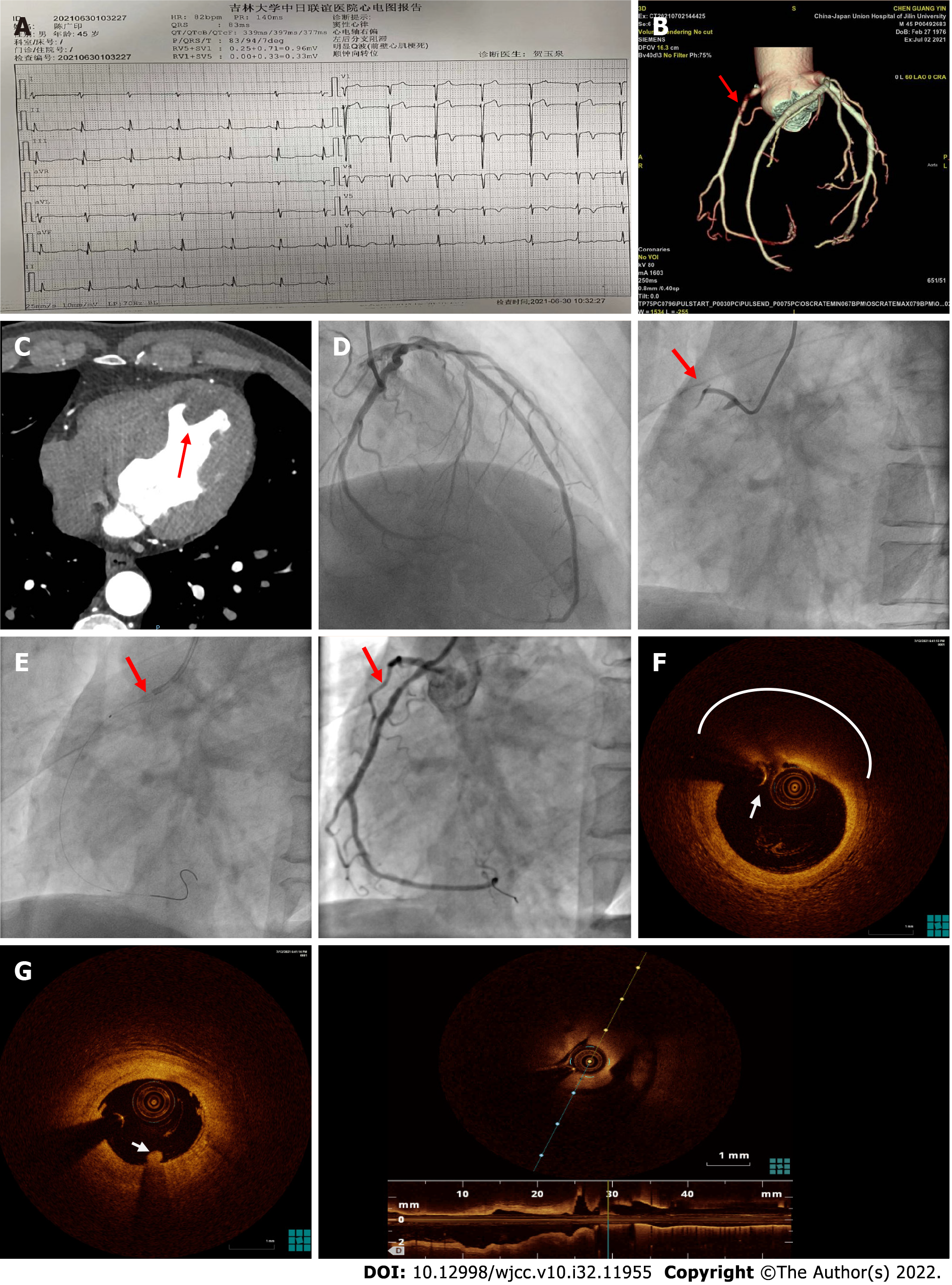
In 2021, the patient was readmitted to the hospital for chest pain for 2 h. The ECG result revealed left posterior branch block and Q-wave formation in the anterior wall lead (Figure 2A). Table 1, Column 2 displays the results of the blood test. The results of coronary artery computed tomographic angiography (CTA) revealed that the thrombus had occluded the proximal segment of the right coronary artery, resulting in moderate-to-severe stenosis of the corresponding lumen (Figure 2B). Meanwhile, thrombosis of the left ventricle was discovered (Figure 2C). Based on his symptoms, medical history, blood test results, and CTA findings, the patient was diagnosed as acute non-STEMI, Killip II grade, AA, and dyslipidemia. Following several guidelines and expert consensus's directions[5], we performed CAG after platelet transfusion because the patient had severe thrombocytopenia (TP). CAG revealed total occlusion of the proximal segment of the right coronary artery (Figure 2D). After percutaneous coronary intervention (PCI) and thrombus aspiration, the coronary blood flow recovered to thrombolysis in MI (TIMI) 3. (Figure 2E). We further performed optical coherence tomography (OCT) to determine the cause of coronary artery occlusion. According to the OCT results, the proximal segment of the right coronary artery displayed a significant attenuation zone with a blurred edge and high dorsal reflection. The OCT revealed a fibrous cap at the surface of the atherosclerotic plaque area, as well as a homogenous high-signal area with minimal attenuation (Figure 2F), and a low-signal zone with diffuse boundaries in the lipid-rich core. Meanwhile, the thrombus mass attached to the surface of the lumen was visible (Figure 2G). The minimum lumen area was 2.6 mm2. The rate of area stenosis was 65%. TEG was utilized to detect platelet function. The result showed that the adenosine diphosphate (ADP) channel inhibition rate was 28.3%, while the arachidonic acid channel inhibition rate was 75.1%. Although the patient’s platelet count was low (30 × 109/L), thrombus could still develop in his coronary artery and left ventricle, which indicated that the platelet count and function likely play independent roles in thrombosis[6,7]. After the risk of bleeding and thrombotic events was evaluated, the antithrombotic strategy was modified to include only anticoagulants (factor Xa inhibitors) and ADP inhibitors (clopidogrel). Long-term follow-up showed that the patient had made a good recovery.
The first documented case of AMI complicating AA was in 1994. Such cases have remained relatively uncommon[8]. Table 2 shows the cases of AA associated with MI that have been published in PubMed[8-12]. During the treatment of AA with steroids and CsA, there is an increased risk of AMI[9]. AA complicated by AMI has a poor prognosis in the majority of patients. This could also be worsened by pulmonary fungal infection as a result of the drop in white blood cell count in most individuals[11]. Literature suggested that revascularization early in the course of these individuals’ treatment improves their prognosis[10]. Taken together, these case reports indicate that using anabolic steroids and immunosuppressive drugs may be one of the risk factors for AMI.
| Ref. | Biographical information | Age of first diagnosis of AA | AA drug therapy and duration | Location of AMI | Coronary artery (by CAG or autopsy) | Therapy | Prognosis |
| [8] | 61-year-old male | 56-year-old | Metenolone enanthate (45mg) | Inferior wall | RCA | NS | NS |
| 59-year-old female | 42-year-old | Metenolone enanthate (100mg) oxymetholone (30 mg) | Inferior wall | RCA | NS | NS | |
| [9] | 16-year-old girl | 3-year-old | BMT; GVHD: cyclosporine A and azathioprine | Inferior wall | RCA | Stent implantation | Discharge and recover well |
| [10] | 76-year-old man | 76-year-old | Cyclosporine A | NSTEMI | LAD,RCA, And LCX | Medical treatment; Recurrent AMI 1 mo later and accepted PCI. Left-sided chronic subdural hematoma after PCI | Heart failure, acute kidney injury, and recurrent AMI |
| [11] | 38-year-old man | 32-year-old | Cyclosporin A, granulocyte colony-stimulating factor, corticosteroids and antithymocyte globulin | Anterior wall | LAD | Medical treatment | Death autopsy revealed disseminated aspergillosis |
| [12] | 52-year-old man | NS | Oxymetholone (100 mg/daily) platelet transfusion after in hospital | Anterior wall | LAD in the portion of the previously inserted stent | Balloon angioplasty | Discharged |
The side effects of anabolic steroids may have an influence on the cardiovascular system. Stanozolol is a non-aromatic steroid synthesized from dihydrotestosterone[13], which is one of the most extensively used steroids worldwide[14,15]. Stanozolol can induce MI, atrial fibrillation[3], and ventricular arrhythmias in those who take a large dose or use it for long periods. Table 3 lists case reports of cardiovascular adverse events caused by stanozolol[16-24]. The following cardiovascular risk factors may be linked to the use of stanozolol.
| Ref. | Biographical information | Drug and dose | Duration of use | Diagnosis | Coronary artery (by CAG or autopsy) | Therapy | Prognosis |
| [16] | 30-year-old male | Orally stanozolol 10 mg daily and intramuscularly 250 mg testosterone twice per week | 2 mo | Anterior wall AMI | LAD | Medical treatment | Discharged |
| [17] | 25-year-old male | Nandrolone 100 mg/wk stanozolol tablets 25 mg/d | 3 wk | Takotsubo cardiomyopathy | N | Medical treatment | Discharged |
| [18] | 26-year-old male | Stanozolol 10 mg daily | 3 mo | Ischemic Stroke | N (Angiography of the Cerebral artery) | Medical treatment and rehabilitation | Discharged with severe disability |
| [19] | 28-year-old male | Stanozolol 280mg weekly | 2 yr | NSTEMI; ventricular tachycardia | LADRCA | Percutaneous translumind coronary angioplasty | Discharged |
| [20] | 22-year-old male | Stanozolol, 10 mg/d and clenbuterol 40 μg/d for 7 dstanozolol, 20 mg/d and clenbuterol 80 μg/d for 3 d. triiodothy-ronine 25ug/d | 10 d | Cardiomyopathy; acute hepatic injury | NS | Medical treatment | Discharged |
| [21] | 29-year-old female | Ephedrine, tadalafil, metandienon, mestanolon, stanozolol | NS | Sudden cardiac death; cardiac arrhythmia | LAD | NA | Sudden cardiac death |
| [22] | 24-year-old male | Stanozolol, testosterone, tamoxifen, mesterolone, and nandrolone | 6 mo | Cardiorespiratoryarrest | The left main trunk and LAD | NA | Sudden cardiac death |
| [23] | 24-year-old male | Stanozolol (40 mgs daily) nandrolone 200 mgs intramuscularly twice weekly, Sustanon 250 (testosterone esters) 1 mL intramuscularly once a week | 6 wk | Anterior wall AMI | NS | Medical treatment | NS |
| [24] | 37-year-old male | Nandrolone Testosterone cypionate stanozolol; oxandrolone | 3-16 wk | Inferior wall AMI | N; Consider spasm | Medical treatment | Discharged |
Influence on blood lipid metabolism: Stanozolol can increase low-density lipoprotein cholesterol (LDL-C) while reducing high-density lipoprotein cholesterol (HDL-C) and serum lipoprotein (a) levels[25]. These changes are reversible with the withdrawal of medication[19]. LDL-C is an independent risk factor for the diagnosis and prognostication of arteriosclerotic cardiovascular disease[26-28].
Influence on blood pressure: Stanozolol can cause sodium and water retention as well as vasospasm, which may further increase in systolic and diastolic blood pressure[29,30].
Influence on platelet aggregation: Stanozolol has been demonstrated to enhance platelet aggregation by increasing thromboxane A2 synthesis while lowering prostacyclin levels[31-34].
Endothelial dysfunction: Endothelial dysfunction is the initial step in atherosclerosis and is significantly related to cardiovascular disease[35]. Endothelial dysfunction is caused by an imbalance between vasoconstrictors and vasodilators, which is disrupted by oxidative stress[36]. Stanozolol inhibits guanylate cyclase, which may increase the aorta's response to vasoconstrictors while reducing the response to vasodilators, resulting in less nitric oxide-mediated arterial relaxation[37,38]. Furthermore, stanozolol has been shown to promote endothelial dysfunction by enhancing oxidative stress via catalase, super oxide dismutase 1, and glutathione peroxidase 4, as well as activating the hemostatic system and increasing fibrinolytic activity[39-41].
Myocardial injury: Left ventricular hypertrophy is the most prevalent pathological sign in postmortem hearts of long-term stanozolol users[42], which might be the consequence of direct action on cardiac androgen receptors[43]. Animal studies have also shown that stanozolol stimulates the immune system and enhances oxidative stress[44-46]. In an in-vitro investigation, stanozolol was shown to promote cardiac cell apoptosis[47]. All these factors play a critical role in the development of atherosclerosis and cardiovascular disease[48].
CsA is a common T lymphocyte immunosuppressant that has been utilized extensively in the treatment of allograft rejection and kidney disease. CsA can inhibit the opening of mitochondrial permeability transition (MPT) pores. The opening of the MPT pore causes a breakdown of membrane, uncoupling of the respiratory chain, and the outflow of cytochrome C and other pro-apoptotic molecules, which can lead to cell apoptosis or necrosis[49]. Whether the usage of CsA prior to myocardial ischemia in animal experiments can reduce the infarction area remains ambiguous[50-53]. According to clinical research, CsA injection prior to PCI did not improve clinical outcomes in terms of left ventricular ejection fraction, mortality, recurrent MI, re-hospitalization, and heart failure[54,55]. Long-term CsA medication has been shown to have adverse effects on the cardiovascular system, including pericarditis[56,57], hyperlipidemia, and increased risk of atherosclerosis. The following cardiovascular risk factors may be linked to the usage of CsA.
Dyslipidemia and arteriosclerosis: CsA lowers the rate of catabolism and the generation of LDL-C[4], reduces liver X receptor ligand synthesis and 27-hydroxycholesterol efflux, which may lead to foam cell macrophage formation and the development of atherosclerotic plaques. Thus, CsA’s ability to suppress lipolysis may contribute to plasma triglyceride accumulation. CsA reduces the levels of lipoprotein lipase (LPL) in adipose tissue and skeletal muscle in rats[58]. LPL activity was consistently observed to be reduced in the plasma of dyslipidemia patients treated with CsA[59].
Endothelial dysfunction: CsA, like stanozolol, can promote endothelial dysfunction by increasing superoxide generation[60].
The patient in this case used stanozolol and CsA for approximately 5 years before suffering an AMI. He did not smoke and had no family history of cardiovascular disease; therefore, he did not have traditional risk factors for atherosclerosis or thrombosis. However, the OCT revealed lipid plaque and thrombus mass in the right coronary artery. Meanwhile, his LDL-C levels were high (6.82 mmol/L) and his HDL-C levels were low (0.74 mmol/L). CTA revealed a ventricular thrombus, indicating abnormal platelet function and coagulation. We cannot be sure as to what caused the ventricular thrombosis; however, a previously published case report showed that long-term, high-dose stanozolol usage was linked to ventricular thrombosis[61]. Based on the available facts from this case report and the literature, we suspected that combined long-term use of stanozolol and CsA are responsible for the risk of atherosclerosis and thrombosis.
AA is characterized by decreased bone marrow hematopoietic cell proliferation and peripheral blood pancytopenia, with bleeding and infection as the most prevalent clinical symptoms. Morphological and functional changes in platelets play an important role in the occurrence and development of coronary heart disease. TP is a rare complication of coronary heart disease that affects around 5.4% patients (platelet count: < 150 × 109/L)[62]. Thompson et al[63] found that large platelets have a greater coagulation capability than small platelets. Ulu et al[64] found that mean platelet volume (MPV) is associated with left ventricular thrombosis. In this instance, the MPV increased, which suggests platelet hyperfunction and necessitates further research on platelet activation status. There were several challenges in balancing antiplatelet therapy with bleeding control in this patient. The European Society of Cardiology has issued a guideline for the management of individuals with acute coronary syndrome and TP. There are three levels of severity based on platelet count: (1) Mild: platelet count, 100-150 × 109/L; (2) Moderate: platelet count, 50-100 × 109/L; and (3) Severe: platelet count, < 50 × 109/L. Mild TP has no effect on antiplatelet therapeutic regimens. For patients with moderate TP, PCI is a viable option. After PCI, patients are given dual antiplatelet therapy for a month before switching to clopidogrel monotherapy. It is also recommended that a proton pump inhibitor be used in combination with any treatment. In patients with severe TP, all antiplatelet medications should be discontinued, and PCI should be avoided[5]. It is important to note that while assessing the risk of antiplatelet therapy for TP patients, platelet function should take precedence over platelet count[65].
The platelet count in our patient was 85 × 109/L during his initial MI, indicating a modest reduction in platelets. His cardiac function was damaged at the time of onset, primarily evidenced by persistent and intense chest pain and rales in both lungs. As a result, immediate PCI and revascularization were required. Simultaneously, clopidogrel was given as an antiplatelet therapy based on the TEG result and relevant guidelines. His platelets further decreased to 30 × 109/L during his second MI in the right coronary artery. Following the recommendations of several guidelines and expert consensus[5], we performed CAG post platelets transfusion. This time, we used OCT to examine the intravascular atherosclerotic plaques in more detail. After weighing the risks of bleeding and thrombosis, the anti-thrombotic strategy was changed to focus on anticoagulants (factor Xa inhibitors) and ADP inhibitors (clopidogrel). Follow-up evaluation of the patient showed that the antithrombotic treatment had been fairly successful, since there had been no bleeding and the patient had recovered.
Here, we reported a case of AA combined with AMI. Coronary artery and left ventricular thrombosis may be linked to medicines used to treat AA in this patient. Doctors should be aware of the risk of thrombosis and cardiovascular problems in patients with AA, especially if using stanozolol with CsA for a prolonged duration. Antiplatelet therapy for patients with coronary artery disease and low platelet counts must also strike a balance between the risks of thrombosis and hemorrhage. Furthermore, in contrast to past examples, timely revascularization may be the most important factor in improving the patient's prognosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abubakar MS, Nigeria; Aldiansyah D, Indonesia; Gupta P, United States S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Young NS. Current concepts in the pathophysiology and treatment of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2013;2013:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 2. | Young NS. Aplastic Anemia. N Engl J Med. 2018;379:1643-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 476] [Article Influence: 68.0] [Reference Citation Analysis (60)] |
| 3. | Lau DH, Stiles MK, John B, Shashidhar, Young GD, Sanders P. Atrial fibrillation and anabolic steroid abuse. Int J Cardiol. 2007;117:e86-e87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (59)] |
| 4. | López-Miranda J, Vilella E, Pérez-Jiménez F, Espino A, Jiménez-Perepérez JA, Masana L, Turner PR. Low-density lipoprotein metabolism in rats treated with cyclosporine. Metabolism. 1993;42:678-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (61)] |
| 5. | McCarthy CP, Steg G, Bhatt DL. The management of antiplatelet therapy in acute coronary syndrome patients with thrombocytopenia: a clinical conundrum. Eur Heart J. 2017;38:3488-3492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (61)] |
| 6. | Karpatkin S. Heterogeneity of human platelets. VI. Correlation of platelet function with platelet volume. Blood. 1978;51:307-316. [PubMed] |
| 7. | Psaila B, Bussel JB, Linden MD, Babula B, Li Y, Barnard MR, Tate C, Mathur K, Frelinger AL, Michelson AD. In vivo effects of eltrombopag on platelet function in immune thrombocytopenia: no evidence of platelet activation. Blood. 2012;119:4066-4072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (61)] |
| 8. | Toyama M, Watanabe S, Kobayashi T, Iida K, Koseki S, Yamaguchi I, Sugishita Y. Two cases of acute myocardial infarction associated with aplastic anemia during treatment with anabolic steroids. Jpn Heart J. 1994;35:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (63)] |
| 9. | Miura T, Izawa A, Kumazaki S, Ishii E, Otagiri K, Aizawa K, Koshikawa M, Kasai H, Tomita T, Miyashita Y, Tsutsui H, Koyama J, Ikeda U. Acute myocardial infarction in a 16-year-old girl with chronic GVHD. Bone Marrow Transplant. 2010;45:1576-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 10. | Nishikawa M, Shiraishi J, Ohshiro M, Yashige M, Hyogo M, Sawada T. Left main crossover stenting in a patient with severe thrombocytopenia due to aplastic anemia. Cardiovasc Interv Ther. 2017;32:409-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 11. | Itoh M, Takahashi M, Mori M, Tamekiyo H, Yoshida H, Yago K, Shimada H, Arai K. Myocardial infarction caused by Aspergillus embolization in a patient with aplastic anemia. Intern Med. 2006;45:547-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 12. | Shin HS, Kang TS. A case of late stent thrombosis following platelet transfusion in a patient with aplastic anemia. Korean Circ J. 2012;42:54-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 13. | Tabatabaee SM, Elahi R, Savaj S. Bile cast nephropathy due to cholestatic jaundice after using stanozolol in 2 amateur bodybuilders. Iran J Kidney Dis. 2015;9:331-334. [PubMed] |
| 14. | Wilson JD. Androgen abuse by athletes. Endocr Rev. 1988;9:181-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 231] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 15. | Piacentino D, Kotzalidis GD, Del Casale A, Aromatario MR, Pomara C, Girardi P, Sani G. Anabolic-androgenic steroid use and psychopathology in athletes. A systematic review. Curr Neuropharmacol. 2015;13:101-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 16. | Christou GA, Christou KA, Nikas DN, Goudevenos JA. Acute myocardial infarction in a young bodybuilder taking anabolic androgenic steroids: A case report and critical review of the literature. Eur J Prev Cardiol. 2016;23:1785-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 17. | Placci A, Sella G, Bellanti G, Margheri M. Anabolic androgenic steroid-induced Takotsubo cardiomyopathy. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 18. | Santamarina RD, Besocke AG, Romano LM, Ioli PL, Gonorazky SE. Ischemic stroke related to anabolic abuse. Clin Neuropharmacol. 2008;31:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 19. | Mewis C, Spyridopoulos I, Kühlkamp V, Seipel L. Manifestation of severe coronary heart disease after anabolic drug abuse. Clin Cardiol. 1996;19:153-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 20. | Li C, Adhikari BK, Gao L, Zhang S, Liu Q, Wang Y, Sun J. Performance-Enhancing Drugs Abuse Caused Cardiomyopathy and Acute Hepatic Injury in a Young Bodybuilder. Am J Mens Health. 2018;12:1700-1704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 21. | Thiblin I, Mobini-Far H, Frisk M. Sudden unexpected death in a female fitness athlete, with a possible connection to the use of anabolic androgenic steroids (AAS) and ephedrine. Forensic Sci Int. 2009;184:e7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 22. | Hernández-Guerra AI, Tapia J, Menéndez-Quintanal LM, Lucena JS. Sudden cardiac death in anabolic androgenic steroids abuse: case report and literature review. Forensic Sci Res. 2019;4:267-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 23. | Kennedy C. Myocardial infarction in association with misuse of anabolic steroids. Ulster Med J. 1993;62:174-176. [PubMed] |
| 24. | Ferenchick GS, Adelman S. Myocardial infarction associated with anabolic steroid use in a previously healthy 37-year-old weight lifter. Am Heart J. 1992;124:507-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | García-Esperón C, Hervás-García JV, Jiménez-González M, Pérez de la Ossa-Herrero N, Gomis-Cortina M, Dorado-Bouix L, López-Cancio Martinez E, Castaño-Duque CH, Millán-Torné M, Dávalos A. [Ingestion of anabolic steroids and ischaemic stroke. A clinical case report and review of the literature]. Rev Neurol. 2013;56:327-331. [PubMed] |
| 26. | Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 5337] [Article Influence: 1067.4] [Reference Citation Analysis (0)] |
| 27. | Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med. 2018;379:2097-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2585] [Cited by in RCA: 2287] [Article Influence: 326.7] [Reference Citation Analysis (0)] |
| 28. | Jin JL, Cao YX, Zhang HW, Sun D, Hua Q, Li YF, Guo YL, Wu NQ, Zhu CG, Gao Y, Dong QT, Liu HH, Dong Q, Li JJ. Lipoprotein(a) and Cardiovascular Outcomes in Patients With Coronary Artery Disease and Prediabetes or Diabetes. Diabetes Care. 2019;42:1312-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 29. | Solberg EE, Borjesson M, Sharma S, Papadakis M, Wilhelm M, Drezner JA, Harmon KG, Alonso JM, Heidbuchel H, Dugmore D, Panhuyzen-Goedkoop NM, Mellwig KP, Carre F, Rasmusen H, Niebauer J, Behr ER, Thiene G, Sheppard MN, Basso C, Corrado D; Sport Cardiology Section of the EACPR of the ESC. Sudden cardiac arrest in sports - need for uniform registration: A Position Paper from the Sport Cardiology Section of the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Prev Cardiol. 2016;23:657-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Gheshlaghi F, Piri-Ardakani MR, Masoumi GR, Behjati M, Paydar P. Cardiovascular manifestations of anabolic steroids in association with demographic variables in body building athletes. J Res Med Sci. 2015;20:165-168. [PubMed] |
| 31. | Pilo R, Aharony D, Raz A. Testosterone potentiation of ionophore and ADP induced platelet aggregation: relationship to arachidonic acid metabolism. Thromb Haemost. 1981;46:538-542. [PubMed] |
| 32. | Johnson M, Ramey E, Ramwell PW. Androgen-mediated sensitivity in platelet aggregation. Am J Physiol. 1977;232:H381-H385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Rosenblum WI, el-Sabban F, Nelson GH, Allison TB. Effects in mice of testosterone and dihydrotestosterone on platelet aggregation in injured arterioles and ex vivo. Thromb Res. 1987;45:719-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Nakao J, Change WC, Murota SI, Orimo H. Testosterone inhibits prostacyclin production by rat aortic smooth muscle cells in culture. Atherosclerosis. 1981;39:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15621] [Cited by in RCA: 15527] [Article Influence: 597.2] [Reference Citation Analysis (0)] |
| 36. | Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009;73:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 557] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 37. | Ammar EM, Said SA, Hassan MS. Enhanced vasoconstriction and reduced vasorelaxation induced by testosterone and nandrolone in hypercholesterolemic rabbits. Pharmacol Res. 2004;50:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Ferrer M, Encabo A, Marín J, Balfagón G. Chronic treatment with the anabolic steroid, nandrolone, inhibits vasodilator responses in rabbit aorta. Eur J Pharmacol. 1994;252:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Ebenbichler CF, Sturm W, Gänzer H, Bodner J, Mangweth B, Ritsch A, Sandhofer A, Lechleitner M, Föger B, Patsch JR. Flow-mediated, endothelium-dependent vasodilatation is impaired in male body builders taking anabolic-androgenic steroids. Atherosclerosis. 2001;158:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Lane HA, Grace F, Smith JC, Morris K, Cockcroft J, Scanlon MF, Davies JS. Impaired vasoreactivity in bodybuilders using androgenic anabolic steroids. Eur J Clin Invest. 2006;36:483-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Skogastierna C, Hotzen M, Rane A, Ekström L. A supraphysiological dose of testosterone induces nitric oxide production and oxidative stress. Eur J Prev Cardiol. 2014;21:1049-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Frati P, Busardò FP, Cipolloni L, Dominicis ED, Fineschi V. Anabolic Androgenic Steroid (AAS) related deaths: autoptic, histopathological and toxicological findings. Curr Neuropharmacol. 2015;13:146-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 43. | Montisci M, El Mazloum R, Cecchetto G, Terranova C, Ferrara SD, Thiene G, Basso C. Anabolic androgenic steroids abuse and cardiac death in athletes: morphological and toxicological findings in four fatal cases. Forensic Sci Int. 2012;217:e13-e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 44. | Dornelles GL, Bueno A, de Oliveira JS, da Silva AS, França RT, da Silva CB, Machado MS, Petry LD, Abdalla FH, Lhamas CL, de Andrade CM. Biochemical and oxidative stress markers in the liver and kidneys of rats submitted to different protocols of anabolic steroids. Mol Cell Biochem. 2017;425:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Inamdar Doddamani LS, Jayamma Y. Acceleration of neutrophil precursors' maturation and immunostimulation of CD3+, CD4+ lymphocytes by stanozolol in mice. J Steroid Biochem Mol Biol. 2012;129:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Pey A, Saborido A, Blázquez I, Delgado J, Megías A. Effects of prolonged stanozolol treatment on antioxidant enzyme activities, oxidative stress markers, and heat shock protein HSP72 Levels in rat liver. J Steroid Biochem Mol Biol. 2003;87:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Zaugg M, Jamali NZ, Lucchinetti E, Xu W, Alam M, Shafiq SA, Siddiqui MA. Anabolic-androgenic steroids induce apoptotic cell death in adult rat ventricular myocytes. J Cell Physiol. 2001;187:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 48. | Tapia-Vieyra JV, Delgado-Coello B, Mas-Oliva J. Atherosclerosis and Cancer; A Resemblance with Far-reaching Implications. Arch Med Res. 2017;48:12-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 49. | Mironova GD, Pavlov EV. Mitochondrial Cyclosporine A-Independent Palmitate/Ca2+-Induced Permeability Transition Pore (PA-mPT Pore) and Its Role in Mitochondrial Function and Protection against Calcium Overload and Glutamate Toxicity. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Skyschally A, Schulz R, Heusch G. Cyclosporine A at reperfusion reduces infarct size in pigs. Cardiovasc Drugs Ther. 2010;24:85-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 51. | Karlsson LO, Zhou AX, Larsson E, Aström-Olsson K, Månsson C, Akyürek LM, Grip L. Cyclosporine does not reduce myocardial infarct size in a porcine ischemia-reperfusion model. J Cardiovasc Pharmacol Ther. 2010;15:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | Karlsson LO, Bergh N, Grip L. Cyclosporine A, 2.5 mg/kg, does not reduce myocardial infarct size in a porcine model of ischemia and reperfusion. J Cardiovasc Pharmacol Ther. 2012;17:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Dow J, Kloner RA. Postconditioning does not reduce myocardial infarct size in an in vivo regional ischemia rodent model. J Cardiovasc Pharmacol Ther. 2007;12:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 54. | Rahman FA, Abdullah SS, Manan WZWA, Tan LT, Neoh CF, Ming LC, Chan KG, Lee LH, Goh BH, Salmasi S, Wu DB, Khan TM. Efficacy and Safety of Cyclosporine in Acute Myocardial Infarction: A Systematic Review and Meta-Analysis. Front Pharmacol. 2018;9:238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D, Bonnefoy-Cudraz E, Guérin P, Elbaz M, Delarche N, Coste P, Vanzetto G, Metge M, Aupetit JF, Jouve B, Motreff P, Tron C, Labeque JN, Steg PG, Cottin Y, Range G, Clerc J, Claeys MJ, Coussement P, Prunier F, Moulin F, Roth O, Belle L, Dubois P, Barragan P, Gilard M, Piot C, Colin P, De Poli F, Morice MC, Ider O, Dubois-Randé JL, Unterseeh T, Le Breton H, Béard T, Blanchard D, Grollier G, Malquarti V, Staat P, Sudre A, Elmer E, Hansson MJ, Bergerot C, Boussaha I, Jossan C, Derumeaux G, Mewton N, Ovize M. Cyclosporine before PCI in Patients with Acute Myocardial Infarction. N Engl J Med. 2015;373:1021-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 540] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 56. | Daoulah A, Alqahtani AA, Ocheltree SR, Alhabib A, Ocheltree AR. Acute myocardial infarction in a 56-year-old female patient treated with sulfasalazine. Am J Emerg Med. 2012;30:638.e1-638.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Jain S, Singh P, Naidu S, Sharma A. Cyclosporine-induced pericarditis: an elusive cause of chest pain in Behçet's disease. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Vaziri ND, Liang K, Azad H. Effect of cyclosporine on HMG-CoA reductase, cholesterol 7alpha-hydroxylase, LDL receptor, HDL receptor, VLDL receptor, and lipoprotein lipase expressions. J Pharmacol Exp Ther. 2000;294:778-783. [PubMed] |
| 59. | Tory R, Sachs-Barrable K, Hill JS, Wasan KM. Cyclosporine A and Rapamycin induce in vitro cholesteryl ester transfer protein activity, and suppress lipoprotein lipase activity in human plasma. Int J Pharm. 2008;358:219-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 60. | Diederich D, Skopec J, Diederich A, Dai FX. Cyclosporine produces endothelial dysfunction by increased production of superoxide. Hypertension. 1994;23:957-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 61. | McCarthy K, Tang AT, Dalrymple-Hay MJ, Haw MP. Ventricular thrombosis and systemic embolism in bodybuilders: etiology and management. Ann Thorac Surg. 2000;70:658-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Yadav M, Genereux P, Kirtane AJ, Madhavan MV, Xu K, Brener S, Mehran R, Stone G. TCT-244 Baseline Thrombocytopenia: A Strong Predictor Of One-Year Adverse Ischemic Events In Patients With ACS Undergoing PCI: A Pooled analysis From ACUITY And HORIZONS-AMI Trials. J Am Coll Cardiol. 2013;62:B80. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 63. | Thompson CB, Eaton KA, Princiotta SM, Rushin CA, Valeri CR. Size dependent platelet subpopulations: relationship of platelet volume to ultrastructure, enzymatic activity, and function. Br J Haematol. 1982;50:509-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 242] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 64. | Ulu SM, Ozkeçeci G, Akci O, Ahsen A, Altug A, Demir K, Acartürk G. Mean platelet volume, in predicting severity of mitral regurgitation and left atrial appendage thrombosis. Blood Coagul Fibrinolysis. 2014;25:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 65. | Yusuf SW, Iliescu C, Bathina JD, Daher IN, Durand JB. Antiplatelet therapy and percutaneous coronary intervention in patients with acute coronary syndrome and thrombocytopenia. Tex Heart Inst J. 2010;37:336-340. [PubMed] |