Published online Jan 21, 2022. doi: 10.12998/wjcc.v10.i3.1131
Peer-review started: July 25, 2021
First decision: October 22, 2021
Revised: November 4, 2021
Accepted: December 23, 2021
Article in press: December 23, 2021
Published online: January 21, 2022
Processing time: 174 Days and 6.2 Hours
Mycoplasma hominis (M. hominis), which causes central nervous system infections in adults, is very rare. It is also relatively difficult to culture mycoplasma and culturing requires special media, resulting in a high rate of clinical underdiagnosis. Therefore, clinicians often treat patients based on their own experience before obtaining pathogenic results and may ignore infections with atypical pathogens, thus delaying the diagnosis and treatment of patients and increasing the length of hospital stay and costs.
A 44-year-old man presented to the hospital complaining of recurrent dizziness for 1 year, which had worsened in the last week. After admission, brain magnetic resonance imaging (MRI) revealed a 7.0 cm × 6.0 cm × 6.1 cm lesion at the skull base, which was irregular in shape and had a midline shift to the left. Based on imaging findings, meningioma was our primary consideration. After lesion resection, the patient had persistent fever and a diagnosis of suppurative meningitis based on cerebrospinal fluid (CSF) examination. The patient was treated with the highest level of antibiotics (meropenem and linezolid), but the response was ineffective. Finally, M. hominis was detected by next-generation metagenomic sequencing (mNGS) in the CSF. Therefore, we changed the antibiotics to moxifloxacin 0.4 g daily combined with doxycycline 0.1 g twice a day for 2 wk, and the patient had a normal temperature the next day.
Mycoplasma meningitis after neurosurgery is rare. We can use mNGS to detect M. hominis in the CSF and then provide targeted treatment.
Core Tip: Mycoplasma meningitis after neurosurgery is relatively rare. Intracranial infections with atypical pathogens are difficult to identify. Because Mycoplasma hominis (M. hominis ) has no cell wall, it cannot be observed by Gram staining. Moreover, the difficulty of culturing M. hominis increases the challenge of clinical detection and often delays treatment. Next-generation metagenomic sequencing can be used to identify the pathogen in the early stage of the disease.
- Citation: Yang NL, Cai X, Que Q, Zhao H, Zhang KL, Lv S. Mycoplasma hominis meningitis after operative neurosurgery: A case report and review of literature. World J Clin Cases 2022; 10(3): 1131-1139
- URL: https://www.wjgnet.com/2307-8960/full/v10/i3/1131.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i3.1131
Mycoplasma hominis (M. hominis) is a common colonizer in the microflora of the genitourinary tract of many sexually active adolescent females. M. hominis can be found in the cervical or vaginal secretions of up to 50% of healthy women[1]. At present, it has been demonstrated that pathogenic M. hominis is mainly distributed in the oropharynx and urogenital tract[2]. M. hominis is associated with certain diseases of parturient women, their fetuses and newborns, but it is rare for M. hominis to cause central nervous system infections in adults. Because M. hominis has no cell wall, it cannot be observed by Gram staining. Moreover, the difficulty of culturing M. hominis increases the challenge of clinical detection and often delays treatment. Here, we report a case of M. hominis infection secondary to craniocerebral surgery detected by next-generation metagenomic sequencing (mNGS). We also reviewed relevant literature to analyze the clinical features, diagnosis and treatment methods of central nervous system infections caused by M. hominis to deepen the understanding of this type of infection among clinicians and improve the diagnosis and treatment options.
A 44-year-old man presented to our hospital complaining of worsening dizziness.
One year before admission, the patient suffered from repeated episodes of dizziness without blurred vision, nausea, vomiting or limb dysfunction. However, the symptom did not cause alarm. A week ago, his dizziness worsened, and he presented to the hospital.
Healthy, with no specific diseases.
Physical examination upon admission showed that the patient had no nystagmus, no neck rigidity, normal muscle strength and muscular tension of the limbs, and negative pathological signs.
On admission, the patient's examination results were completely normal, including leukocyte count, hypersensitive C-reactive protein, procalcitonin, electrolytes, liver and kidney function tests and coagulation function tests. On the third postoperative day, the leukocyte count was 14.8 × 109/L (reference range: 4-10 × 109/L), and the neutrophil count (NEUT%) was 89.5% (reference range: 40%-75%). The cerebrospinal fluid (CSF) examination showed 62.9 × 103 white blood cell (WBC)/μL, with a protein level of 8036 mg/L, glucose level of 3.8 mmol/L and chloride ion concentration of 139 mmol/L.
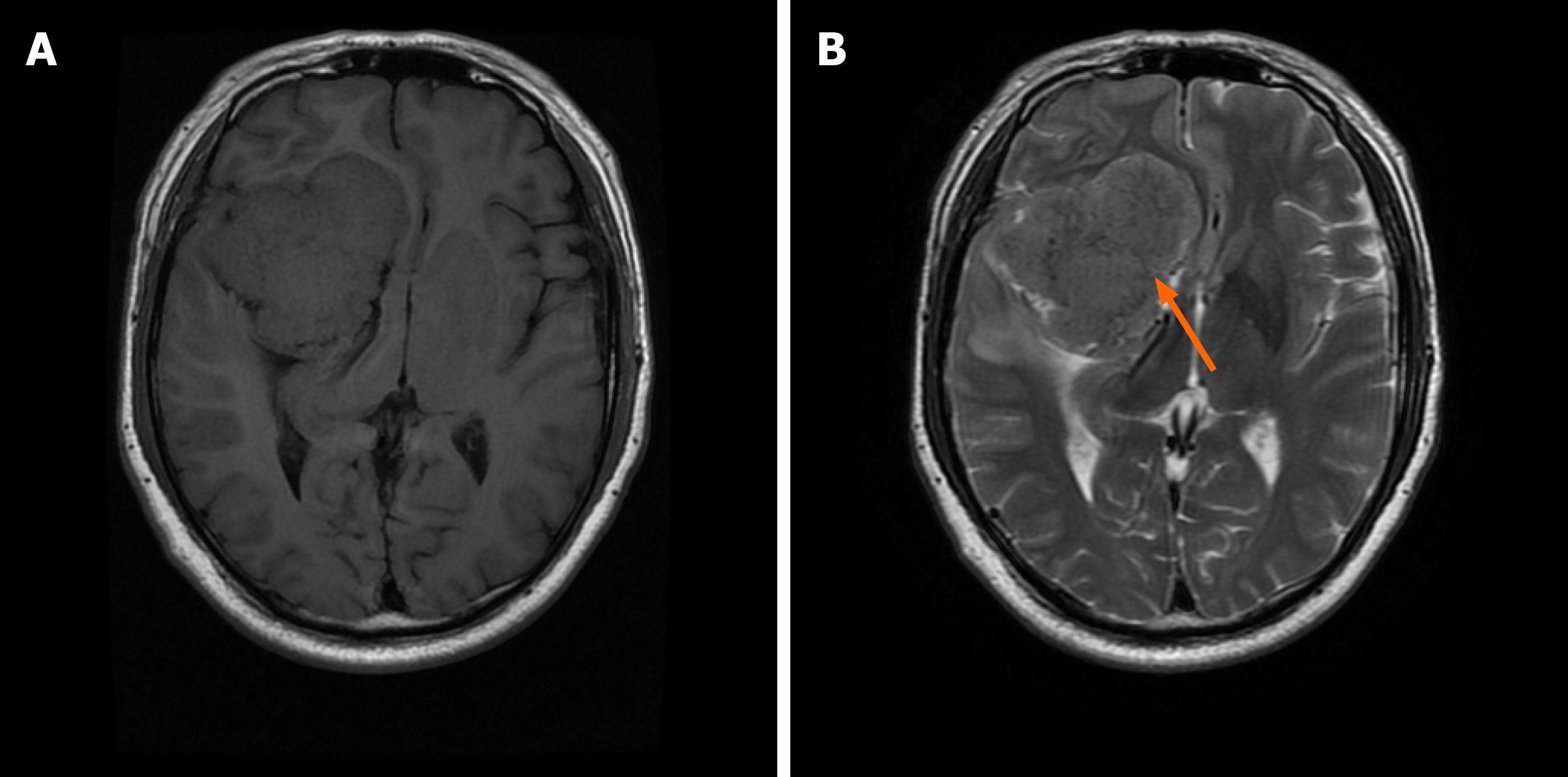
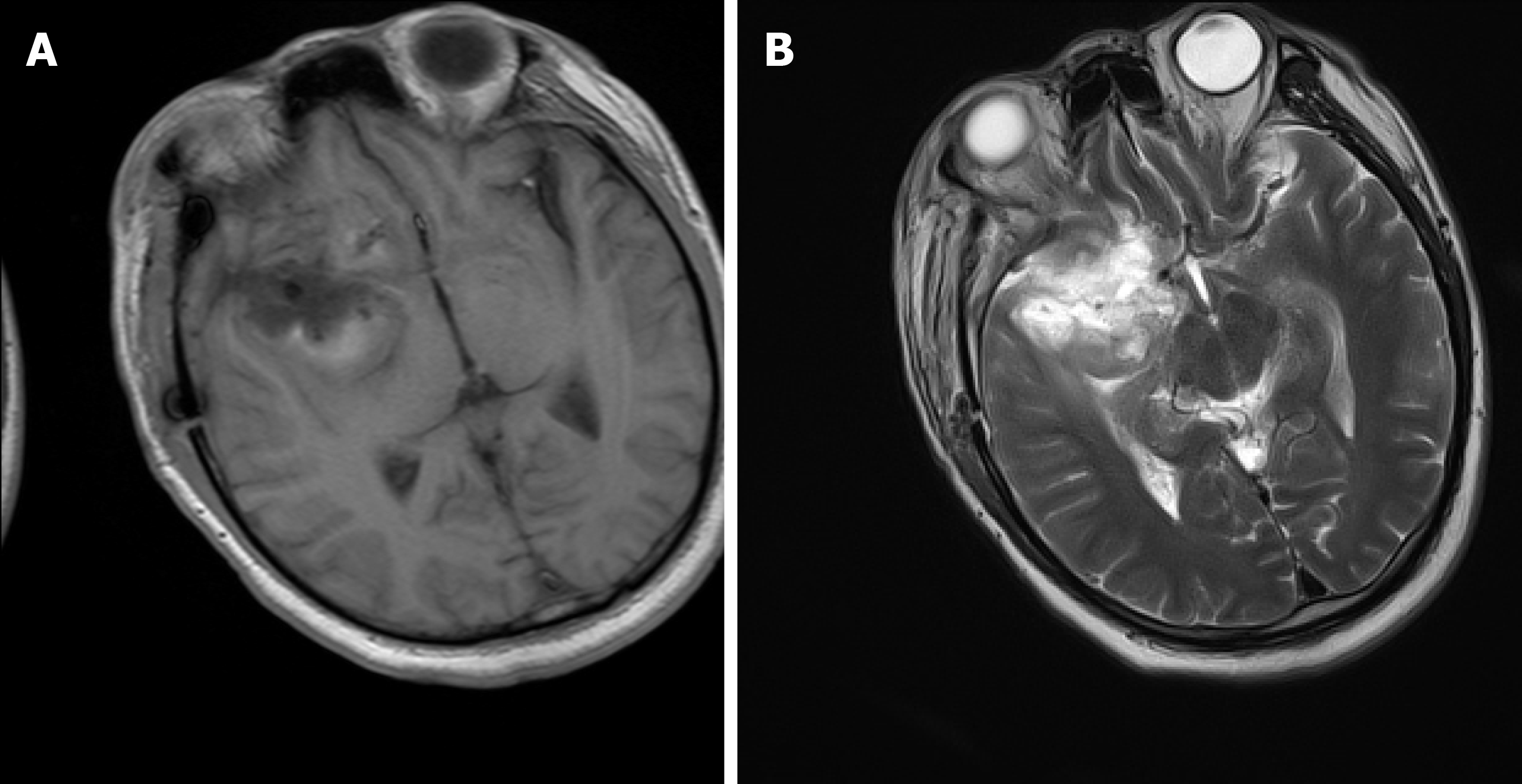
The brain magnetic resonance imaging (MRI) examination revealed a massive mass outside the right anterior and middle cranial base. The main body of the lesion was in the middle cranial base with an irregular shape and a size of approximately 7.0 cm × 6.0 cm × 6.1 cm. The right ventricle and cerebral peduncle were compressed, and the midline was shifted to the left (Figure 1). On postoperative day 10, we reviewed the brain MRI and excluded a brain abscess (Figure 2).
The initial diagnosis on admission was intracranial space-occupying meningioma. Meningioma, M. hominis meningitis and pulmonary infection were diagnosed postoperatively.
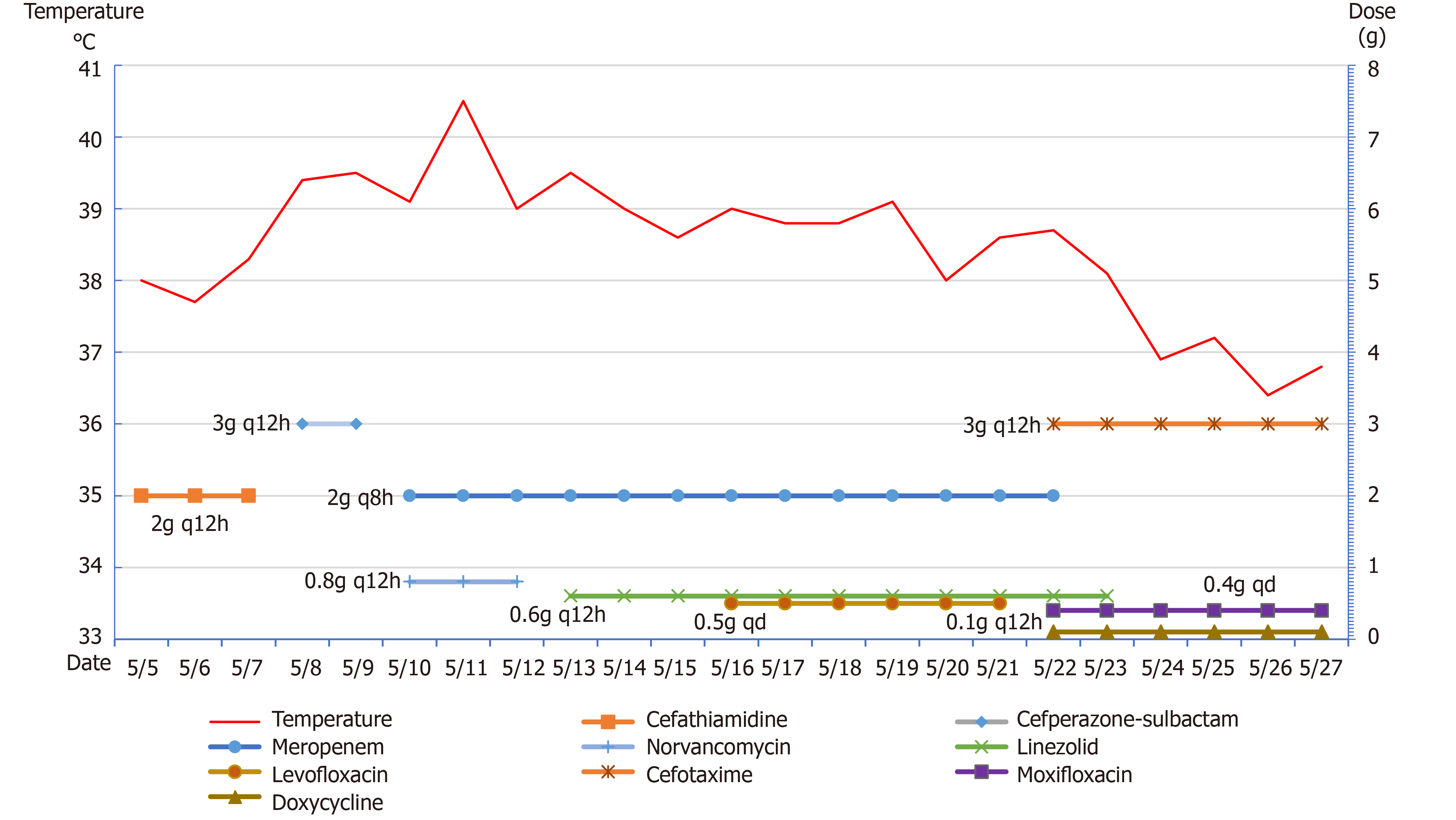
The patient was admitted to the hospital, and preoperative examinations were completed. The patient underwent intracranial tumor resection on May 4, 2020. The operation lasted approximately 9 h, and the intraoperative bleeding volume was 2000 mL. Preoperative and postoperative cefathiamidine was used to prevent infection. On the second day after surgery, the patient was conscious. The muscle strength of the left limb was approximately grade 3, whereas the muscle strength of the right limb was normal. The patient was extubated successfully on postoperative day 3. Also, on postoperative day 3, the patient developed fever with a temperature of 38.3°C. Laboratory studies revealed that the leukocyte count was 14.8 × 109/L (reference range: 4-10 × 109/L), and the NEUT% was 89.5% (reference range: 40%-75%). Then, we changed the antibiotic to cefoperazone-sulbactam. However, the patient's temperature continued to increase. At this time, we found that the patient had neck rigidity. Thus, we performed a lumbar puncture. The CSF examination showed a WBC level of 62.9 × 103 WBC/μL, protein level of 8036 mg/L, glucose level of 3.8 mmol/L and chloride ion concentration of 139 mmol/L. Blood cultures drawn on postoperative day 3 revealed Staphylococcus infection. The antibiotics were changed to meropenem and norvancomycin on postoperative day 6, and a brain abscess was excluded by brain MRI (Figure 2). M. hominis was detected in the CSF by mNGS on postoperative day 12. At that time, we believed that M. hominis meningitis was rare, the possibility of mycoplasma intracranial infection was low, and the possibility of contamination was high. Thus, we did not adjust the treatment plan. Afterwards, the patient was treated with linezolid and levofloxacin successively, but the body temperature still fluctuated between 38 °C and 39 °C. Just when we were at a loss, we discussed and developed a treatment plan with the neurosurgeons, infectious disease specialists, and hematologists and decided to use special media to culture the CSF for mycoplasma. We also reviewed the literature on M. hominis meningitis. A total of 19 studies published from inception to the end of June 2020 were retrieved, including 11 cases of M. hominis brain abscess, 6 cases of meningitis and 2 cases of spinal cord abscess (Table 1). Finally, M. hominis was cultured from the CSF, which confirmed the mNGS results. We finally changed the antibiotic to moxifloxacin combined with doxycycline on postoperative day 18. The patient's temperature returned to normal on the second day after adjustment of the treatment plan, and the patient was later discharged from the hospital (Figure 3).
| Ref. | Year published | Country | Sex | Age (yr) | History | Preoperative diagnosis | Risk factors | Clinical symptoms | DM | Specimens | IM | Antibiotics used after diagnosis | Outcomes |
| Paine et al[15] | 1950 | USA | M | 20 | No | Eyeball trauma | Head trauma | Fever, headache, neck stiffness | C | P | BA | St | Cure |
| Payan et al[16] | 1981 | USA | M | 29 | No | Subdural hematoma, brain contusion | Motor vehicle accident | Fever, disturbance of consciousness | C | P | BA | Te+Er | Cure |
| McMahon et al[17] | 1990 | USA | M | 76 | Hypertension | Subarachnoid hemorrhage | Urethral catheterization | Fever, disturbance of consciousness | C | CSF | Meningitis | - | Death |
| Kersten et al[18] | 1995 | USA | M | 20 | No | Eye contusion, hematoma of frontotemporal lobe | Motor vehicle accident, head trauma, hormone therapy | Fever, right eye swelling | C | P | Right eye abscess, BA | Dox + Cli | Cure |
| Zheng et al[19] | 1997 | USA | F | 22 | No | Right frontal lobe cerebral hemorrhage | Vaginal delivery | Fever, left-sided weakness | ELISA | P | BA | - | Cure |
| Cohen et al[20] | 1997 | USA | F | 18 | No | Subdural hemorrhage, ventricular hemorrhage | Motor vehicle accident | Fever | C | CSF | Meningitis | Dox + Cip + Ery | Cure |
| House et al[21] | 2003 | USA | F | 40 | No | Cavernous hemangioma of the right frontal lobe | Perineal ulcers | Fever, nausea, limb dysfunction | C + NGS | P | BA | Cip + Met | Cure |
| Kupila et al[7] | 2006 | Finland | M | 40 | No | Scalp laceration | Head trauma, cystoscopy, catheterization | Disturbance of consciousness | NGS | P | BA | Te | Cure |
| McCarthy et al[22] | 2008 | Australia | M | 48 | No | Intracranial colloid cyst | Surgical infection | Fever, disturbance of consciousness | NGS + C | Subdural empyema, bone flap | BA | Gat | Cure |
| Al Masalma et al[23] | 2011 | USA | F | 41 | No | Spontaneous abortion | Dilatation and curettage | Disturbance of consciousness | NGS | P | BA | Dox | Cure |
| Lee et al[24] | 2012 | Netherlands | F | 48 | No | Subarachnoid hemorrhage | Ventricular drainage tube | Fever | NGS + C | CSF | Meningitis | Mox | Cure |
| Sato et al[25] | 2012 | Japan | M | 26 | Hypogammaglobulinemia | Arthritis | Hypogammaglobulinemia | Joint swelling and pain, headache | C+NGS | CSF, joint effusion, blood | Meningitis | - | Death |
| Henao-Martínez et al[26] | 2012 | USA | M | 40 | No | Right subdural hematoma, subarachnoid hemorrhage, cerebral contusion | Head trauma | Fever | C+NGS | Brain debridement tissue | BA | Dox | Cure |
| Pailhoriès et al[27] | 2014 | France | M | 43 | Chronic alcoholism, TIA, epilepsy | Subdural hematoma, left frontal hematoma | Head trauma, urethral catheterization | Fever | MALDI-TOF MS、NGS | Electrodes, P | BA | Lev + Dox | Cure |
| Hos et al[28] | 2015 | Germany | F | 21 | No | Spinal abscess | Vaginal delivery, epidural blood tape therapy | Fever, neck pain, vomiting | NGS+C | CSF | SA | Mox | Cure |
| Zhou et al[29] | 2016 | China | M | 79 | Hypertension | Cerebral hemorrhage | Urethral catheterization | Fever, disturbance of consciousness, right-sided weakness | NGS | CSF | Meningitis | Azi + Dox + Min | Cure |
| Reissier et al[30] | 2016 | France | M | 39 | Hypertension, chronic alcoholism | Subarachnoid hemorrhage | Cystostomy | Disturbance of consciousness, fever | C, real-time PCR | CSF | Meningitis, hydrocephalus | Mox | Death |
| Parsonson et al[31] | 2016 | Australia | F | 30 | Polycystic ovary syndrome, depression, obesity | Lumbar disc herniation | Repeated operations | Festering wounds | NGS | P | SA | Mox | Cure |
| Bergin et al[32] | 2017 | Singapore | M | 57 | No | Arteriovenous malformation with hemorrhage | Urethral catheterization | Fever | NGS | Surgical specimens | BA | Dox | Cure |
At follow-up 1 year later, the muscle strength of the patient's left limb had returned to normal, and the patient could work normally.
Intracranial infection is a common complication after neurosurgery with a reported incidence of less than 10% and a high incidence at 3 to 7 d postoperatively. Infection is mainly caused by Gram-positive bacteria, which can manifest as subdural empyema, brain abscess, ventriculitis, or meningoencephalitis[3,4]. In recent years, the epidemiology of pathogenic bacteria causing intracranial infections after neurosurgery has changed. Gram-negative bacteria exhibit an obvious increasing trend, and multidrug-resistant or extensively drug-resistant Acinetobacter baumannii also exhibits a gradually increasing trend[5]. Intracranial infection with M. hominis is common in neonates but rare in adults after craniocerebral surgery. Current studies have found that cerebrospinal fluid leakage, ventricular drainage, multiple operations, surgical incision infection, and long operation time (greater than 4 h) are independent risk factors for intracranial infection after craniocerebral surgery[6]. There are three main sources of intracranial infection with mycoplasma: direct contamination during trauma, direct contamination during surgery, or bacteremia caused by urogenital tract manipulation secondary to brain site infection. Mycoplasma contains surface proteins that promote cell adhesion and can spread to other sites, leading to infection when the mucosa is damaged, such as with instrument manipulation, surgery, and trauma[1]. Although the results of urine culture were negative many times in this patient, the urinary catheter was continuously indwelling after surgery. Because the urinary tract is a common site of mycoplasma, the possibility of intracranial infection caused by the urinary tract could not be excluded in this patient. Earlier, Kupila et al[7] reported a case of brain abscess with M. hominis secondary to cystoscopy and an indwelling catheter. In this case, the risk of secondary intracranial infection after surgery was significantly increased due to the large tumor volume, long operation time, greater volume of intraoperative bleeding, and presence of a postoperative extradural drainage tube. The patient developed fever on postoperative day 3, and Staphylococcus was detected in blood cultures. Early empirical coverage of Gram-positive bacteria was performed, but the treatment was ineffective. During treatment, we reviewed the relevant domestic and international literature. There have been a few reports on M. hominis infection in adults after craniocerebral surgery. In addition, we lacked clinical experience, so the treatment for M. hominis was delayed. Fortunately, the patient was finally cured and discharged.
At present, mycoplasma culture is the main method for detection of mycoplasma in domestic medical institutions, and this process mainly uses liquid medium for direct culture with simultaneous drug sensitivity tests. Mycoplasma releases ammonia gas by decomposing arginine, resulting in pH changes in the liquid medium and thus a change in the color of the indicator to infer the culture result. Because cholesterol is an important component of the cell membrane of mycoplasma and mycoplasma itself does not have the ability to synthesize it, animal serum must be added to the culture medium in vitro to provide cholesterol components. Therefore, the liquid medium must contain arginine and cholesterol. If the solid culture method is adopted, the specimen is cultured in a CO2 environment for 24-48 h after inoculation and characteristic "fried egg-like" colonies can be observed under the microscope. Due to the uncertainty of the factors leading to pH changes in liquid media, false-positive results may occur. Therefore, the liquid culture method can be combined with the solid culture method in clinical practice to improve the mycoplasma detection rate. The possibility of mycoplasma infection was not considered during the culture of the CSF specimen of this patient, and no special medium was used. Thus, the results of repeated culture were negative. After the mNGS test results suggested M. hominis, we cultured the CSF again using special medium, and the results confirmed the intracranial infection caused by M. hominis. Most of the cases we reviewed were diagnosed by mNGS, which not only directly sequences the genomes of samples but also identifies a variety of unknown pathogens in the samples. Compared with traditional culture methods, mNGS requires less time and is more efficient[8]. Long et al[9] showed that, compared with blood cultures, mNGS had a higher sensitivity and pathogen detection rate (30.77% vs 12.82%). Currently, the conserved region of 16S rRNA is the main gene sequence used for the construction of primers. Studies have found that the application of 16S rRNA by real-time reverse transcription PCR (qRT-PCR) can further improve the positive rate of specimen detection and eliminate false-positives[10].
Because mycoplasmas lack a cell wall, they are resistant to β-lactam and glycopeptide antibiotics that act on the cell wall. Tetracyclines that interfere with protein synthesis are commonly used to treat mycoplasmas, which are also sensitive to quinolones that inhibit DNA replication. M. hominis is typically resistant to macrolides and aminoglycosides. In the cases reviewed, 9 patients were switched to tetracycline antibiotics after the pathogen was confirmed as M. hominis, and all the patients were cured. In patients with meningitis caused by M. hominis, if doxycycline treatment fails, clindamycin or fluoroquinolones may be used instead[11]. In the treatment of this patient, levofloxacin was used in the early stages, but the treatment effect was not ideal. After the combined application of moxifloxacin and doxycycline, the patient's body temperature and infection indices gradually improved. M. hominis was most sensitive to doxycycline and minocycline but more resistant to erythromycin, norfloxacin and clarithromycin[12]. Although some studies have shown that the drug resistance rate of levofloxacin to mycoplasma has exhibited a declining trend in recent years, the drug resistance rate of M. hominis is approximately 23.08%[13]. However, Zhang et al[14] used PCR to amplify drug-resistant genes and found that the drug resistance rate of M. hominis to levofloxacin reached 87.9% due to ParC S911 and ParC K144R gene variation. Therefore, doxycycline remains the drug of choice for the treatment of M. hominis.
M. hominis infection after craniocerebral surgery in adults is rare, but it can be clearly diagnosed by special culture or mNGS. The clinical prognosis is generally good when treated with targeted anti-infection therapy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Awad AK, Surani S S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Waites KB, Schelonka RL, Xiao L, Grigsby PL, Novy MJ. Congenital and opportunistic infections: Ureaplasma species and Mycoplasma hominis. Semin Fetal Neonatal Med. 2009;14:190-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17:697-728, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 926] [Cited by in RCA: 927] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 3. | Lin C, Zhao X, Sun H. Analysis on the risk factors of intracranial infection secondary to traumatic brain injury. Chin J Traumatol. 2015;18:81-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Sun JP, Wang F, Gu XY, Zhang GS, Wu JL, Han HE. Etiological characteristics and influencing factors for intracranial infections in patients after craniotomy. Zhonghua Yiyuan Ganran Zazhi. 2018;28:218-221. |
| 5. | Wu Y, Chen K, Zhao J, Wang Q, Zhou J. Intraventricular administration of tigecycline for the treatment of multidrug-resistant bacterial meningitis after craniotomy: a case report. J Chemother. 2018;30:49-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Sneh-Arbib O, Shiferstein A, Dagan N, Fein S, Telem L, Muchtar E, Eliakim-Raz N, Rubinovitch B, Rubin G, Rappaport ZH, Paul M. Surgical site infections following craniotomy focusing on possible post-operative acquisition of infection: prospective cohort study. Eur J Clin Microbiol Infect Dis. 2013;32:1511-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Kupila L, Rantakokko-Jalava K, Jalava J, Peltonen R, Marttila RJ, Kotilainen E, Kotilainen P. Brain abscess caused by Mycoplasma hominis: a clinically recognizable entity? Eur J Neurol. 2006;13:550-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 8. | Abril MK, Barnett AS, Wegermann K, Fountain E, Strand A, Heyman BM, Blough BA, Swaminathan AC, Sharma-Kuinkel B, Ruffin F, Alexander BD, McCall CM, Costa SF, Arcasoy MO, Hong DK, Blauwkamp TA, Kertesz M, Fowler VG Jr, Kraft BD. Diagnosis of Capnocytophaga canimorsus Sepsis by Whole-Genome Next-Generation Sequencing. Open Forum Infect Dis. 2016;3:ofw144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Long Y, Zhang Y, Gong Y, Sun R, Su L, Lin X, Shen A, Zhou J, Caiji Z, Wang X, Li D, Wu H, Tan H. Diagnosis of Sepsis with Cell-free DNA by Next-Generation Sequencing Technology in ICU Patients. Arch Med Res. 2016;47:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 10. | Dong Z, Li WB, Bai GC, Huang ZD, Yang B, Lin JH, Zhang WM. 16S rRNA Real-Time reverse transcription PCR in synovial fluid for diagnosis of periprosthetic joint infection. Zhonghua Guke Zazhi. 2016;36:1312-1318. [DOI] [Full Text] |
| 11. | Hata A, Honda Y, Asada K, Sasaki Y, Kenri T, Hata D. Mycoplasma hominis meningitis in a neonate: case report and review. J Infect. 2008;57:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Cheng L, Yan XH, Li YZ, Zhou WD, Wu RF, Tao P, Ye YY. Analysis of Mycoplasma infection in reproductive tract and drug sensitivity in infertile patients. Zhongguo Fuyou Baojian. 2020;35:1490-1492. [DOI] [Full Text] |
| 13. | Hu F, Zhan Y, Liu F, Liu YW. Analysis of distribution and drug resistance evolution of Ureaplasma urealyticum and Mycoplasma hominis isolated from genitourinary tract. Zhongguo Yiyuan Yaoxue Zazhi 2019; 39: 1791-1794. [DOI] [Full Text] |
| 14. | Zhang H, Zheng L, Zhao J, Ding S, Xia Y. Investigation of fluoroquinolone resistance mechanism in Mycoplasma hominis isolated from urogenital samples in a Chinese hospital. J Med Microbiol. 2019;68:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Paine TF, Murray R, Perlmutter I, Finland M. Brain abscess and meningitis associated with a pleuropneumonia-like organism: clinical and bacteriological observations in a case with recovery. Ann Intern Med. 1950;32:554-562. [DOI] [Full Text] |
| 16. | Payan DG, Seigal N, Madoff S. Infection of a brain abscess of Mycoplasma hominis. J Clin Microbiol. 1981;14:571-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | McMahon DK, Dummer JS, Pasculle AW, Cassell G. Extragenital Mycoplasma hominis infections in adults. Am J Med. 1990;89:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Kersten RC, Haglund L, Kulwin DR, Ma'luf R, DeConciliis C. Mycoplasma hominis orbital abscess. Arch Ophthalmol. 1995;113:1096-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Zheng X, Olson DA, Tully JG, Watson HL, Cassell GH, Gustafson DR, Svien KA, Smith TF. Isolation of Mycoplasma hominis from a brain abscess. J Clin Microbiol. 1997;35:992-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Cohen M, Kubak B. Mycoplasma hominis meningitis complicating head trauma: case report and review. Clin Infect Dis. 1997;24:272-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | House P, Dunn J, Carroll K, MacDonald J. Seeding of a cavernous angioma with Mycoplasma hominis: case report. Neurosurgery. 2003;53:749-52; discussion 752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | McCarthy KL, Looke DF. Successful treatment of post-neurosurgical intracranial Mycoplasma hominis infection using gatifloxacin. J Infect. 2008;57:344-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Al Masalma M, Drancourt M, Dufour H, Raoult D, Fournier PE. Mycoplasma hominis brain abscess following uterus curettage: a case report. J Med Case Rep. 2011;5:278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Lee EH, Winter HL, van Dijl JM, Metzemaekers JD, Arends JP. Diagnosis and antimicrobial therapy of Mycoplasma hominis meningitis in adults. Int J Med Microbiol. 2012;302:289-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Sato H, Iino N, Ohashi R, Saeki T, Ito T, Saito M, Tsubata Y, Yamamoto S, Murakami S, Kuroda T, Tanabe Y, Fujisawa J, Murai T, Nakano M, Narita I, Gejyo F. Hypogammaglobulinemic patient with polyarthritis mimicking rheumatoid arthritis finally diagnosed as septic arthritis caused by Mycoplasma hominis. Intern Med. 2012;51:425-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Henao-Martínez AF, Young H, Nardi-Korver JJ, Burman W. Mycoplasma hominis brain abscess presenting after a head trauma: a case report. J Med Case Rep. 2012;6:253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Pailhoriès H, Rabier V, Eveillard M, Mahaza C, Joly-Guillou ML, Chennebault JM, Kempf M, Lemarié C. A case report of Mycoplasma hominis brain abscess identified by MALDI-TOF mass spectrometry. Int J Infect Dis. 2014;29:166-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Hos NJ, Bauer C, Liebig T, Plum G, Seifert H, Hampl J. Autoinfection as a cause of postpartum subdural empyema due to Mycoplasma hominis. Infection. 2015;43:241-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Zhou M, Wang P, Chen S, Du B, Du J, Wang F, Xiao M, Kong F, Xu Y. Meningitis in a Chinese adult patient caused by Mycoplasma hominis: a rare infection and literature review. BMC Infect Dis. 2016;16:557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Reissier S, Masson R, Guérin F, Viquesnel G, Petitjean-Lecherbonnier J, Pereyre S, Cattoir V, Isnard C. Fatal nosocomial meningitis caused by Mycoplasma hominis in an adult patient: case report and review of the literature. Int J Infect Dis. 2016;48:81-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Parsonson F. Mycoplasma hominis infection following neurosurgical intervention in a patient with spinal cord compression. JMM Case Rep. 2016;3:e005023. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Bergin SM, Mendis SM, Young B, Binti Izharuddin E. Postoperative Mycoplasma hominis brain abscess: keep it in mind! BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |