Published online Jan 21, 2022. doi: 10.12998/wjcc.v10.i3.1016
Peer-review started: June 2, 2021
First decision: June 25, 2021
Revised: July 5, 2021
Accepted: December 22, 2021
Article in press: December 22, 2021
Published online: January 21, 2022
Processing time: 227 Days and 11.5 Hours
Sjogren’s syndrome (SS), which affect salivary gland function, is an autoimmune disease. SS may involve extraglandular organs. Approximately 10 to 20 percent of SS patients have clinically significant lung disease, but presentation of pulmonary amylodosis is extremly rare. The incidence of benign monoclonal gammopathy in SS patients is high, but multiple myeloma is rare. No case involving the simultaneous occurrence of two rare diseases, pulmonary amyloidosis and multiple myeloma, in the same patient with SS has been reported so far.
A 41-year-old male patient was referred to our hematology department due to incidentally detected gastric plasmacytoma. He had been diagnosed with SS four years earlier. Multiple miliary nodules, ground glass opacity in both lung fields, and enlargement of both inguinal lymph nodes was observed on chest and abdomen computer tomography. Based on the pathological findings of lung and lymph node biopsied specimens, the patient was diagnosed with pulmonary amyloidosis and multiple myeloma. Pulmonary amyloidosis and multiple myeloma associated with SS has rarely been reported.
This is an extremely rare case of simultaneous pulmonary amyloidosis and mul
Core Tip: Sjogren’s syndrome (SS) is known for its involvement in exocrine glands, and may also involve extraglandular organs. Interstitial lung disease is the most common pulmonary abnormality in primary SS, but pulmonary amyloidosis is rare. In addition, the incidence of benign monoclonal gammopathy in SS patients is relatively high, but multiple myeloma is very rare. Herein, we report the extremely rare case of simultaneous pulmonary amyloidosis and multiple myeloma in the same patient with SS.
- Citation: Kim J, Kim YS, Lee HJ, Park SG. Pulmonary amyloidosis and multiple myeloma mimicking lymphoma in a patient with Sjogren’s syndrome: A case report. World J Clin Cases 2022; 10(3): 1016-1023
- URL: https://www.wjgnet.com/2307-8960/full/v10/i3/1016.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i3.1016
Primary Sjogren’s syndrome (SS) is a chronic systemic autoimmune disease characterized by decreased organ function by lymphocyte infiltration, not only exocrine gland but also extraglandular organ[1]. Major B cell activation is the main pathogenesis of SS and continuous activation of B cells can lead to clonal proliferation and malignant lymphoma[2-4]. Clonal proliferation of B cells may lead to both monoclonal gammopathy of undetermined significance, a benign monoclonal gammopathy, and multiple myeloma (MM), a hematologic malignancy[5-9]. However, in practice, multiple myeloma is very rare[6,7,10]. Amyloidosis is a heterogeneous group of disorders characterized by deposits in the extracellular matrix of abnormal protein material. The incidence of amyloidosis is low and that of amyloidosis with involvement of only the respiratory system is extremely low[11,12]. In particular, amyloidosis is a rare cause of pulmonary infiltration in SS[13]. Simultaneous occurrence of pulmonary amyloidosis and multiple myeloma associated with SS is extremely rare.
A 46-year-old male patient was admitted for treatment of an incidental gastric plasmacytoma.
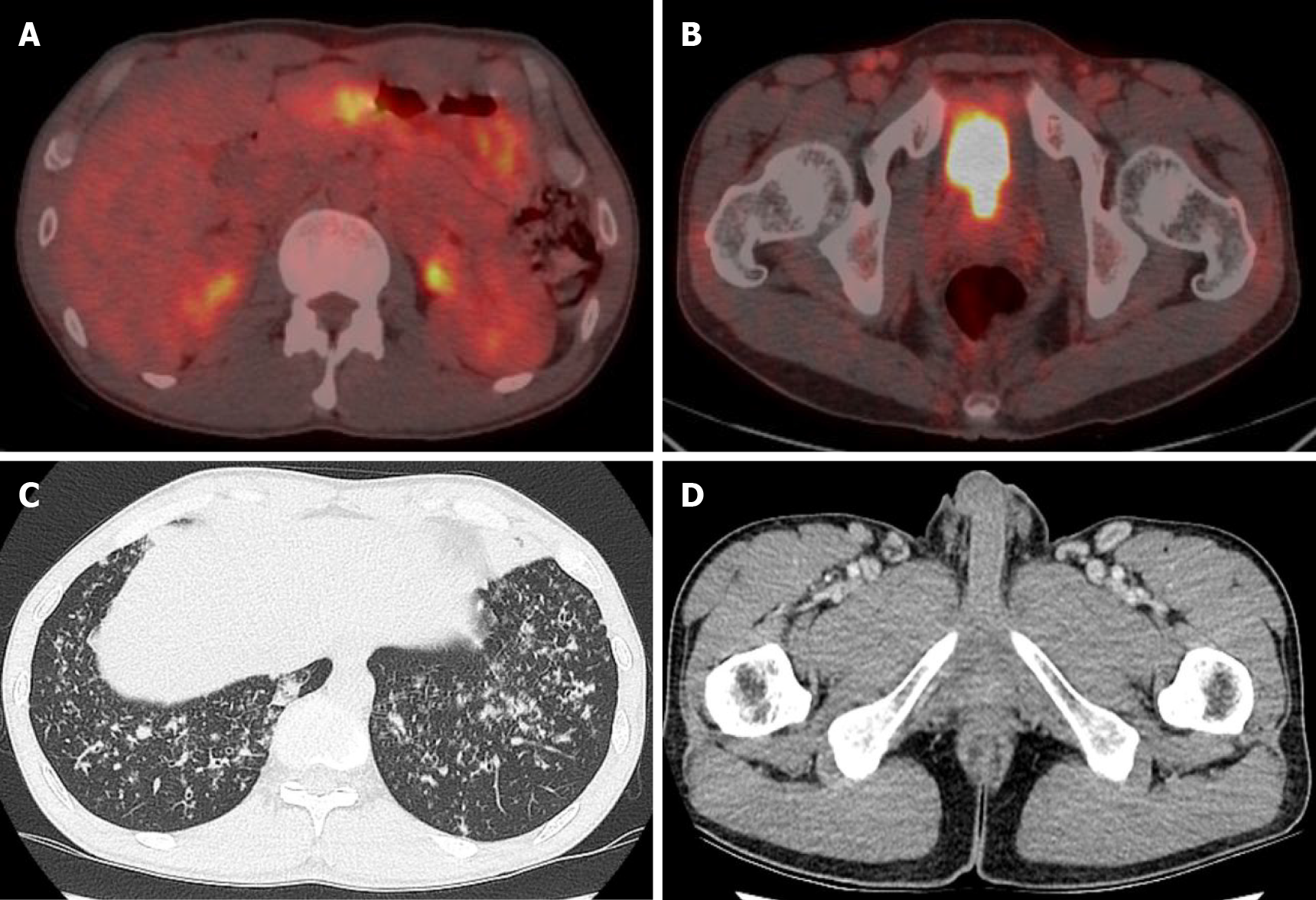
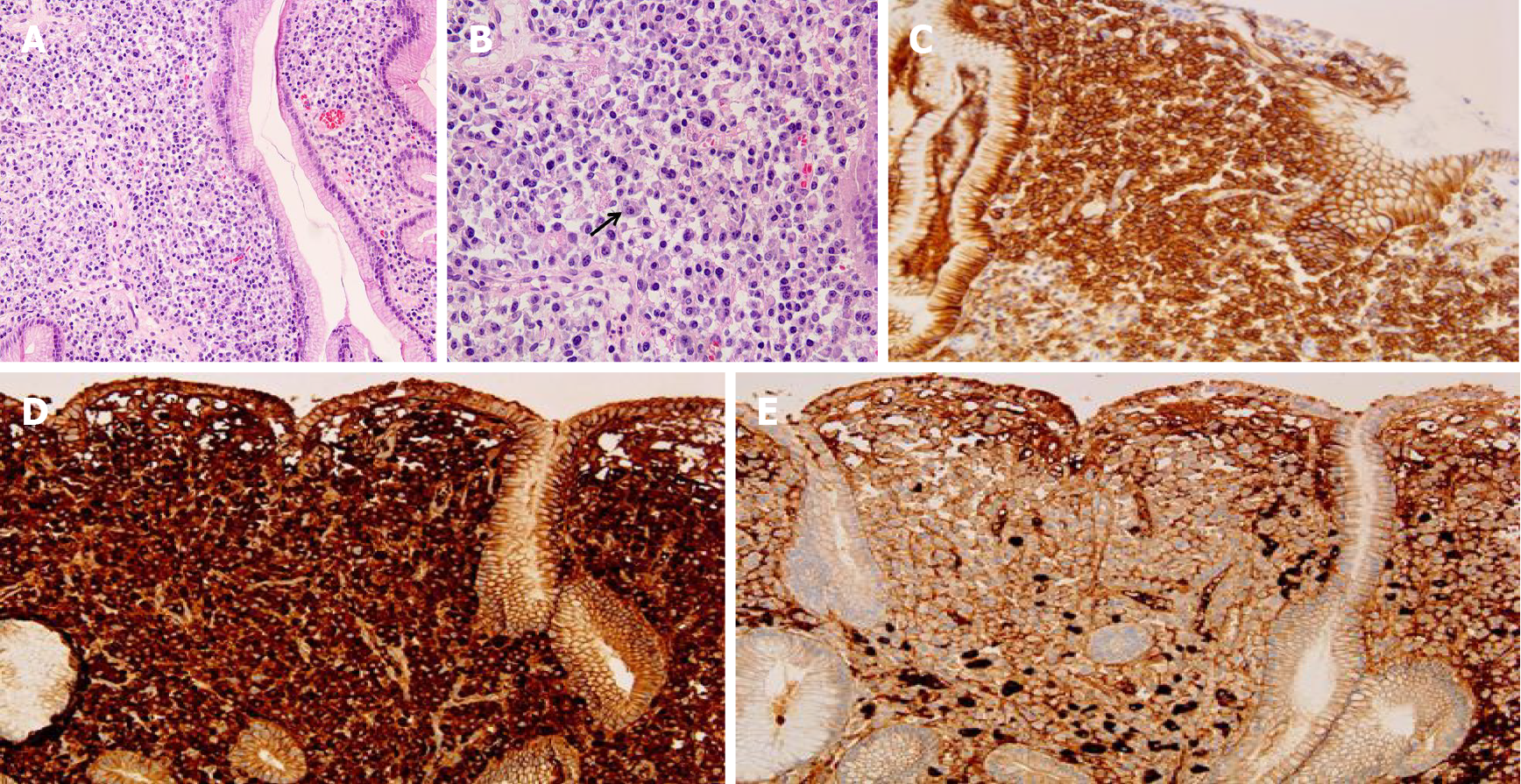
The patient was checked by a positron emission tomography (PET) scan due to enlargement of both inguinal lymph nodes more than two months ago. The PET scan revealed hypermetabolic activity in the gastric body, both inguinal lymph nodes (Figure 1A). A gastroscopic biopsy of the stomach lesion was performed and diagnosed with plasmacytoma (Figure 2).
The patient had not been diagnosed with any diseases.
The patient had been diagnosed with SS four years ago due to dry eyes and dry mouth, and his symptoms improved after treatment with methotrexate, hydroxychloroquine. However, prednisolone was added two months ago due to swelling of the parotid glands and enlargement of lymph nodes.
A physical examination revealed painless lymph nodes of variable size in both inguinal areas and both neck areas.
The complete blood count results, with normal ranges in parentheses, were as follows: white blood cells, 5160 × 103/μL (4.0–10.0 × 103/μL); hemoglobin, 13.3 g/dL (12–16 g/dL); platelets, 305 × 103/μL (150–400 × 103/μL). Blood biochemistry results were as follows: total protein 9.03 g/dL (5.3-7.4 g/dL); albumin 3.74g/dL (3.5-5.2 g/dL), A/G ratio 0.71 (1.0-2.0); C-reactive protein, 0.49 mg/dL (0–0.3 mg/dL). The results of serum protein electrophoresis showed an increase in the total protein amount, a decrease of albumin, and a slightly sharp increase of gamma-globulin. The results of serum immunofixation electrophoresis revealed oligoclonal gammopathy; multiple dense bands were observed in IgG, IgA, kappa, and lambda antisera. The results of Ig quantification showed IgG 2540 mg/dL (700-1600); IgM 18.3 mg/dL (40-230 mg/dL); IgA 1220 mg/dL (70-400 mg/dL). B2-microglobulin level was increased to 2.8 mg/L (0.0-2.4 mg/L). The results of a serum free light chain assay revealed increased kappa light chain (420.60 mg/L, normal 3.3-19.40 mg/L) and an increased kappa/lambda light chain ratio (34.9, normal 0.26-1.65). Alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, lactate dehydrogenase, calcium, and creatinine were all within normal limits.
The PET-computed tomography (CT) revealed hypermetabolic activity in the gastric body, a mild increase in metabolic activity in both inguinal areas, and ground glass opacities (GGO) and centrilobular nodules in both lung fields (Figure 1A). A chest CT showed patch GGO and multiple nodules in both lung fields (Figure 1C) and an abdominal CT showed multiple enlarged lymph nodes in the bilateral inguinal area (Figure 1D).
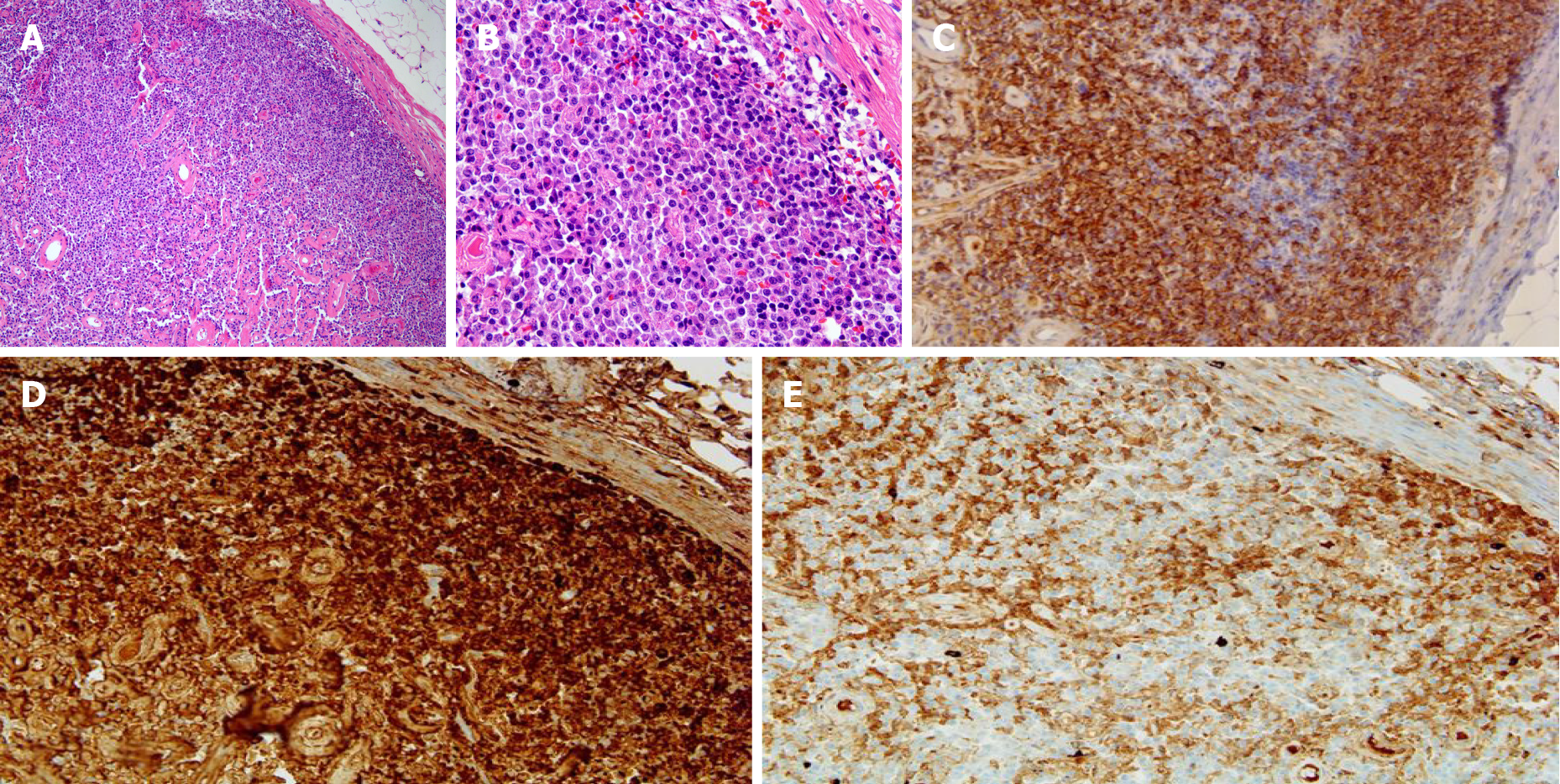
An excisional lymph node biopsy was performed for differential diagnosis of pulmonary tuberculosis with tuberculous lymphadenitis from lymphoma. Biopsy of the right inguinal lymph node revealed diffuse infiltration of plasma cells, which were positive for CD 138, Kappa, and lambda chain immunohistochemical staining (Figure 3). The results of a bone marrow biopsy showed that plasmas cells were increased to 34% and immunostain of IgG, IgA, kappa, and lambda was positive.
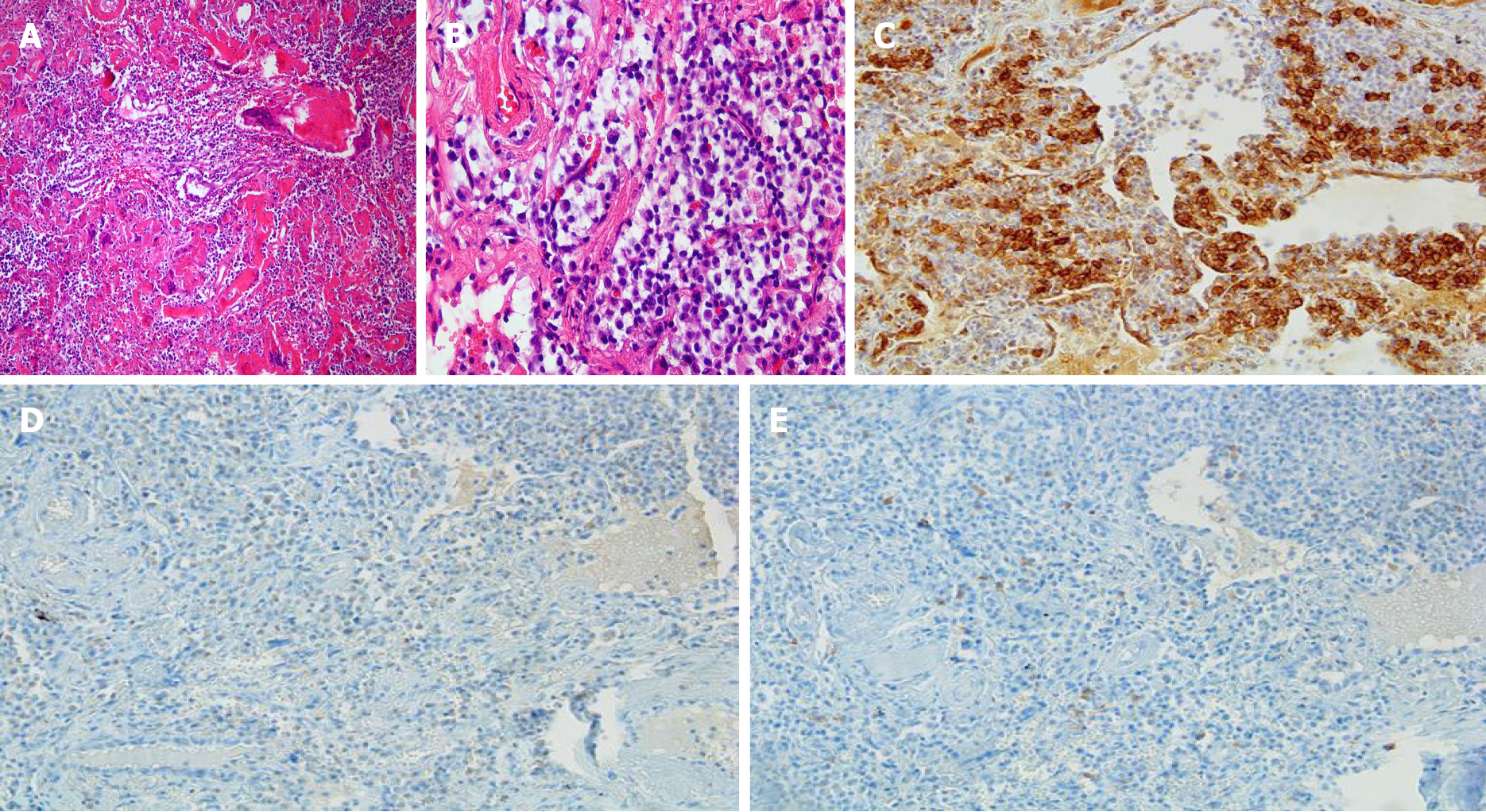
A lung biopsy was also performed to rule out pulmonary tuberculosis, interstitial lung disease, or lung involvement of multiple myeloma. Lung biopsy revealed chronic inflammation showing multinucleated giant cells and deposition of amorphous proteinaceous material (Figure 4A). Infiltration of lymphoplasmacytes was observed in the interstitium and perivascular area (Figure 4B). In addition, Congo-red stain for amorphous proteinaceous material revealed apple green birefringence under a polarized microscope. Mycobacterium tuberculosis nested polymerase chain reaction to exclude pulmonary tuberculosis was negative.
We diagnosed the patient with pulmonary amyloidosis and multiple myeloma involving extramedullary organs (stomach, lymph nodes) associated with SS.
Systemic chemotherapy was planned for the multiple myeloma, however the patient was transferred to a larger hospital.
The patient was transferred to another hospital and underwent chemotherapy. He is on regular follow up for SS to our rheumatology department and is on medication.
Although SS is typically known as an autoimmune disorder, it can also present as lymphoproliferative disease. The form of lymphoproliferative disease can present as polyclonal lymphocytic infiltration of the salivary glands, oligo- or monoclonal B cell proliferation resulting in clonally derived lymphoproliferative disorders such as monoclonal gammopathy, light-chain amyloidosis, and malignant lymphoma[3,4,6-9]. SS often involves interstitial lung disease, sometimes primary pulmonary lymphoma, pleuritis, but occurrence of pulmonary amyloidosis is very rare[14].
Amyloidosis is a disease group; certain proteins such as amyloid fibrils are deposited in extracellular tissue. Amyloidosis can be classified as primary and secondary. Primary amyloidosis is caused by clonal plasma cell proliferation which appears in monoclonal gammopathy or myeloma and the associated abnormal proteins consist of fragments of immunoglobulin light chain such as kappa and lambda (light chain amyloidosis)[15]. Secondary amyloidosis is the reactive systemic amyloidosis usually associated with chronic inflammatory diseases or neoplasms and the associated fibrils are composed of fragments of the acute phase reactant serum amyloid A (reactive amyloidosis)[15]. These two types are the most common form of amyloidosis. Amyloid deposition is rare in SS patients, but when it does occur, multiple organs are affected, such as skin, kidney, breast, tongue, and lymph nodes, as well as the lung[16-21]. However, only about 50 cases related to primary SS and pulmonary amyloidosis have been reported so far[22]. Amyloidosis of the lungs has three different clinicopathologic forms: diffuse alveolar-septal amyloidosis (diffuse parenchymal amyloidosis), nodular pulmonary amyloidosis (nodular parenchymal amyloidosis), and tracheobronchial amyloidosis[23]. Diffuse alveolar-septal amyloidosis is characterized by amyloid deposition in the alveolar septa and vessel walls, and transbronchial amyloidosis is characterized by amyloid deposition in various segments of the tracheobronchial tree[23]. Nodular pulmonary amyloidosis can be defined as one or more tumor-like amyloid deposits involving the lungs[23]. Pathologically, well circumscribed nodules with homogeneous and dense eosinophilic material, lymphocytes and plasma cells are generally found within or nearby nodules[23]. Other pathologic findings that may appear include foreign body giant cells, calcifications, and bony or cartilaginous areas[23]. Lung biopsy of our patient revealed chronic inflammatory nodules showing multinucleated giant cells and deposition of amorphous proteinaceous material (Figure 4A). Infiltration of lymphoplasmacytes was observed in the interstitium and perivascular area (Figure 4B). Congo-red stain for amorphous proteinaceous material revealed apple green birefringence under a polarized microscope.
In MM, monoclonal immunoglobulin is produced by plasma cell neoplastic proliferation of plasma cells. There are two categories of MM, according to diagnostic criteria for plasma cell proliferative disorders. The first is asymptomatic multiple myeloma (smoldering multiple myeloma), which is serum monoclonal protein more than 3 g/dL and/or clonal bone marrow plasma cells more than 10% but absence of end-organ damage[5]. Symptomatic multiple myeloma satisfies all of the following: more than 10% clonal bone marrow plasma cells and presence of serum and/or urinary monoclonal protein and evidence of end-organ damage[5].
This patient had increased serum protein IgG, IgA, and Kappa light chain, and plasma cells in the bone marrow were increased to 34%. Although there was no end-organ damage such as anemia, renal insufficiency, hypercalcemia, and bone lesions, plasmacytomas were observed in multiple lymph nodes and stomach as end-organ involvement. This patient was expressed as the type of extramedullary plasmacytoma in multiple myeloma.
Plasmacytomas, tumors composed of plasma cells, are histologically identical to those observed in MM[24]. Those that occur solely in the bone are designated solitary plasmacytoma of bone[25]. Those that arise outside bone in soft tissues are called solitary extramedullary plasmacytoma (EMP)[25]. EMP can arise anywhere in the body. The incidence of extramedullary disease with newly diagnosed MM is variable, ranging from 7%-13%[26]. EMP arise most commonly from direct extension of primary bone tumors, but rarely they may also result from hematogenous spread involving distant organs[26].
The incidence of benign monoclonal gammopathy in SS patients is relatively high and a prevalence of monoclonal gammopathy in primary SS patients of 7% to 22% was recently reported[6-8], however, the prevalence of MM is very rare[6,7,10]. IgG is the most common class associated with MM[7]. This report describes a patient with SS associated with IgG and IgA-kappa-type MM.
Amyloidosis and MM associated primary SS is rare but can occur respectively. However, no cases of simultaneous occurrence of MM and amyloidosis in patients with SS have been reported. Of course, there is a limitation which cannot rule out the occurrence of pulmonary amyloidosis caused by MM. However, abnormal proteins of pulmonary amyloidosis caused by MM consist of fragments of immunoglobulin light chains such as kappa and lambda. Pulmonary amyloidosis was diagnosed in our patient’s lung biopsy, but immunohistochemistry of kappa and lambda was negative. Therefore, we report a rare case diagnosed simultaneously with pulmonary amyloidosis and multiple myeloma associated with SS.
SS is a chronic inflammatory disease characterized by decreased organ function by lymphocyte infiltration, not only exocrine gland but also extraglandular organ. Interstitial lung disease is the most common pulmonary abnormality in primary SS, but pulmonary amyloidosis is rare. Monoclonal gammopathy can also occur, but progression to MM is rare in primary SS. To the best of our knowledge, simultaneous development of MM and pulmonary amyloidosis in primary SS patients has not been reported so far. Herein, we report the extremely rare case of pulmonary amyloidosis and multiple myeloma associated with SS.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Rheumatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Esch M, Le PH S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Fox RI. Sjögren's syndrome. Lancet. 2005;366:321-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1052] [Cited by in RCA: 1085] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 2. | Daridon C, Devauchelle V, Hutin P, Le Berre R, Martins-Carvalho C, Bendaoud B, Dueymes M, Saraux A, Youinou P, Pers JO. Aberrant expression of BAFF by B lymphocytes infiltrating the salivary glands of patients with primary Sjögren's syndrome. Arthritis Rheum. 2007;56:1134-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 154] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Theander E, Henriksson G, Ljungberg O, Mandl T, Manthorpe R, Jacobsson LT. Lymphoma and other malignancies in primary Sjögren's syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis. 2006;65:796-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 366] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 4. | Baimpa E, Dahabreh IJ, Voulgarelis M, Moutsopoulos HM. Hematologic manifestations and predictors of lymphoma development in primary Sjögren syndrome: clinical and pathophysiologic aspects. Medicine (Baltimore). 2009;88:284-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E, Richardson P, Landgren O, Paiva B, Dispenzieri A, Weiss B, LeLeu X, Zweegman S, Lonial S, Rosinol L, Zamagni E, Jagannath S, Sezer O, Kristinsson SY, Caers J, Usmani SZ, Lahuerta JJ, Johnsen HE, Beksac M, Cavo M, Goldschmidt H, Terpos E, Kyle RA, Anderson KC, Durie BG, Miguel JF. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538-e548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2320] [Cited by in RCA: 3141] [Article Influence: 285.5] [Reference Citation Analysis (0)] |
| 6. | Brito-Zerón P, Retamozo S, Gandía M, Akasbi M, Pérez-De-Lis M, Diaz-Lagares C, Bosch X, Bové A, Pérez-Alvarez R, Soto-Cárdenas MJ, Sisó A, Ramos-Casals M. Monoclonal gammopathy related to Sjögren syndrome: a key marker of disease prognosis and outcomes. J Autoimmun. 2012;39:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Tomi AL, Belkhir R, Nocturne G, Desmoulins F, Berge E, Pavy S, Miceli-Richard C, Mariette X, Seror R. Brief Report: Monoclonal Gammopathy and Risk of Lymphoma and Multiple Myeloma in Patients With Primary Sjögren's Syndrome. Arthritis Rheumatol. 2016;68:1245-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Brito-Zerón P, Kostov B, Fraile G, Caravia-Durán D, Maure B, Rascón FJ, Zamora M, Casanovas A, Lopez-Dupla M, Ripoll M, Pinilla B, Fonseca E, Akasbi M, de la Red G, Duarte-Millán MA, Fanlo P, Guisado-Vasco P, Pérez-Alvarez R, Chamorro AJ, Morcillo C, Jiménez-Heredia I, Sánchez-Berná I, López-Guillermo A, Ramos-Casals M; SS Study Group GEAS-SEMI. Characterization and risk estimate of cancer in patients with primary Sjögren syndrome. J Hematol Oncol. 2017;10:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 9. | Kang J, Kim H, Kim J, Choi S, Jung SY, Jang EJ, Cho SK, Sung YK. Risk of malignancy in Korean patients with primary Sjögren's syndrome. Int J Rheum Dis. 2020;23:1240-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Ota T, Wake A, Eto S, Kobayashi T. Sjögren's syndrome terminating with multiple myeloma. Scand J Rheumatol. 1995;24:316-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Baumgart JV, Stuhlmann-Laeisz C, Hegenbart U, Nattenmüller J, Schönland S, Krüger S, Behrens HM, Röcken C. Local vs. systemic pulmonary amyloidosis-impact on diagnostics and clinical management. Virchows Arch. 2018;473:627-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Lachmann HJ, Hawkins PN. Amyloidosis and the lung. Chron Respir Dis. 2006;3:203-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Strimlan CV. Pulmonary involvement in Sjögren's syndrome. Chest. 1986;89:901-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Stojan G, Baer AN, Danoff SK. Pulmonary manifestations of Sjögren's syndrome. Curr Allergy Asthma Rep. 2013;13:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Nomenclature of amyloid and amyloidosis. WHO-IUIS Nomenclature Sub-Committee. Bull World Health Organ. 1993;71:105-112. [PubMed] |
| 16. | Wey SJ, Chen YM, Lai PJ, Chen DY. Primary sjögren syndrome manifested as localized cutaneous nodular amyloidosis. J Clin Rheumatol. 2011;17:368-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Ooms V, Decupere M, Lerut E, Vanrenterghem Y, Kuypers DR. Secondary renal amyloidosis due to long-standing tubulointerstitial nephritis in a patient with Sjögren syndrome. Am J Kidney Dis. 2005;46:e75-e80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Kersemans P, Van Ongeval C, Van Steen A, Drijkoningen M. Amyloid deposition of the breast in primary Sjögren syndrome. JBR-BTR. 2006;89:313-314. [PubMed] |
| 19. | Haraguchi H, Ohashi K, Yamada M, Hasegawa M, Maeda S, Komatsuzaki A. Primary localized nodular tongue amyloidosis associated with Sjögren's syndrome. ORL J Otorhinolaryngol Relat Spec. 1997;59:60-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Serizawa I, Inubushi M, Kanegae K, Morita K, Inoue T, Shiga T, Itoh T, Fukae J, Koike T, Tamaki N. Lymphadenopathy due to amyloidosis secondary to Sjögren syndrome and systemic lupus erythematosus detected by F-18 FDG PET. Clin Nucl Med. 2007;32:881-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Rajagopala S, Singh N, Gupta K, Gupta D. Pulmonary amyloidosis in Sjogren's syndrome: a case report and systematic review of the literature. Respirology. 2010;15:860-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Ellender CM, McLean C, Williams TJ, Snell GI, Whitford HM. Autoimmune disease leading to pulmonary AL amyloidosis and pulmonary hypertension. Respirol Case Rep. 2015;3:78-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Khoor A, Colby TV. Amyloidosis of the Lung. Arch Pathol Lab Med. 2017;141:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 24. | Caers J, Paiva B, Zamagni E, Leleu X, Bladé J, Kristinsson SY, Touzeau C, Abildgaard N, Terpos E, Heusschen R, Ocio E, Delforge M, Sezer O, Beksac M, Ludwig H, Merlini G, Moreau P, Zweegman S, Engelhardt M, Rosiñol L. Diagnosis, treatment, and response assessment in solitary plasmacytoma: updated recommendations from a European Expert Panel. J Hematol Oncol. 2018;11:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 182] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 25. | Jiménez R, Rosinol L, Cibeira MT, Larrea CF, Blade E, Tovar N, Bladé J. Incidence and outcome of soft-tissue plasmacytomas in patients with multiple myeloma before and after the introduction of novel drugs. Blood. 2017;130:3140. [DOI] [Full Text] |
| 26. | Bladé J, Fernández de Larrea C, Rosiñol L, Cibeira MT, Jiménez R, Powles R. Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach. J Clin Oncol. 2011;29:3805-3812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 258] [Article Influence: 18.4] [Reference Citation Analysis (0)] |