Published online Aug 16, 2022. doi: 10.12998/wjcc.v10.i23.8063
Peer-review started: March 22, 2022
First decision: May 30, 2022
Revised: June 3, 2022
Accepted: July 18, 2022
Article in press: July 18, 2022
Published online: August 16, 2022
Processing time: 131 Days and 14.4 Hours
Rhythm control is the core part of the integrated management of atrial fibrillation (AF), especially in the early stages. Despite advances in catheter ablation (CA), the recurrence rate of AF after CA remains high. As a result, stratification and early management of AF recurrence after CA are critical. Currently, predictors of recurrence of AF after CA are mostly based on dysfunction caused by structural remodeling, apart from traditional risk factors. Atrial strain is a recently developed important parameter for detecting the deformability of atrial myocardium during the cardiac cycle prior to atrial remodeling. Although there is only preliminary evidence, atrial strain is still a promising parameter in predicting the recurrence of AF after CA at an early stage. This review focuses on the evaluation of atrial strain, the current applications of atrial strain in assessing atrial function, and predicting the recurrence of AF after CA. We summarize the contents related as follows: (1) CA for rhythm control in AF; (2) Evaluation methods of atrial strain; (3) Atrial strain in the remodeling and reverse remodeling of AF; and (4) Clinical applications of atrial strain in predicting the recurrence of AF after CA. Although there is accumulating evidence on the role of decreased atrial strain in the early prediction of AF recurrence, atrial strain is limited in clinical practice for lacking exact cut-off values and difficulty in distinguishing specific function phases of the atrium. More research is needed in the future to add strength to the early prediction value of atrial strain in AF recurrences.
Core Tip: Atrial fibrillation (AF) is the most common arrhythmia, and rhythm control, especially catheter ablation (CA) is the core part of the integrated management of AF. Despite protocol and devices advances in CA, the recurrence rate of AF after CA is still high. Atrial strain, the parameter of atrial deformation during the cardiac cycle, is closely related to atrial remodeling and atrial function. Furthermore, accumulating evidence showed the role of decreased atrial strain in the early prediction of AF recurrence. Further studies are needed to add strength to the early prediction value of atrial strain in AF recurrences.
- Citation: Yu ZX, Yang W, Yin WS, Peng KX, Pan YL, Chen WW, Du BB, He YQ, Yang P. Clinical utility of left atrial strain in predicting atrial fibrillation recurrence after catheter ablation: An up-to-date review. World J Clin Cases 2022; 10(23): 8063-8075
- URL: https://www.wjgnet.com/2307-8960/full/v10/i23/8063.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i23.8063
Atrial fibrillation (AF) is the most common chronic arrhythmia, with an estimated incidence of 2%–4% in adults worldwide[1]. According to the Global Burden of Disease Study 2017[2], there are 37.6 million cases globally, resulting in approximately 287200 deaths in 2017. With the population aging and prolonged survival of chronic disease, the burden of AF is still increasing rapidly. Besides, AF is associated with a higher incidence of ischemic stroke and cardiac mortality.
Rhythm control is the core part of the integrated management of AF[3], especially in the early stage. Catheter ablation (CA) targeting for the pulmonary vein–left atrium (PV-LA) conjunction area, known as PV isolation (PVI), is the most common way of restoring sinus rhythm. However, despite the enormous advances in the protocol and devices in PVI, the recurrence rate of AF after CA is still high[4].
Atrial remodeling is considered to be one potential mechanism for AF recurrence. Left atrial strain is related to structural remodeling in paroxysmal AF (PAF) and persistent AF (PeAF)[5]. Speckle tracking echocardiography (STE), commonly used in early left ventricular (LV) dysfunction assessment, has also been used in left atrial (LA) strain evaluation[6]. This article highlights the accumulating evidence on AF recurrence after CA and the role of LA strain assessment in the prediction of atrial remodeling and AF recurrence.
Whether rhythm control reduces cardiovascular risk in patients with AF has been debated for decades. Previous trials of rate control vs rhythm control in AF, such as the Atrial Fibrillation Rhythm Management Follow-up Study (AFFIRM) study[7], have not demonstrated the superiority of rhythm control in patients with AF. The recent EAST-AFNET 4 study[8] showed that compared to rate control, early rhythm control therapy (including antiarrhythmic drugs and/or CA) can significantly reduce the risk of major adverse cardiovascular outcomes (MACE) in patients with early-stage AF (diagnosis ≤ 1 year).
Since then, the treatment of AF has entered the era of rhythm control[9]. On the basis of adequate anticoagulation and management of cardiovascular risk factors and comorbid diseases, patients who are suitable for rhythm control should be converted and maintained in sinus rhythm to improve the prognosis of patients.
Antiarrhythmic drugs to maintain sinus rhythm often require long-term use, which increases the risk of side effects and limits the indications. CA targeting for PV-LA conjunction area, known as PVI, is the cornerstone way of restoring sinus rhythm. Compared to drug therapy, CA can lighten the AF burden, and improve quality of life[10,11].
Moreover, there have been enormous advances and optimizations in the protocol and devices in PVI, including cryoballoon ablation and additional atrial ablations etc.[3,9]. These advances have substantially improved the safety and efficacy of PVI. However, the high recurrence rate of AF after CA is still a concern in clinical practice[4]. As a result, stratification and early management of AF recurrence after CA are critical.
Technological advances have improved the success rate of CA for both PAF and PeAF. Still, recurrence of AF is a concern for both the patients and the cardiologists in clinical practice. Due to inconsistency in the definitions of procedural success and post-procedural recurrences, the estimation of CA success rate is challenging[3,12]. The commonly accepted AF recurrence as the occurrence of any symptomatic or asymptomatic atrial tachyarrhythmia lasting > 30 s 3 mo after the procedure[13]. Accordingly, the long-term success rate of CA for AF is between 50% and 80%[12,14].
The recurrence of AF after CA is the result of a complex interaction of many factors. However, aging, female gender, hypertension, PeAF, impairment of atrial function, etc.[15] are assumed to be related to AF recurrence after CA[16]. Apart from clinical risk factors, predictors of the recurrence of AF after CA are mostly based on the dysfunction caused by structural remodeling. Increasing attention has been attracted to the predictive role of atrial functional assessment and remodeling in the recurrence of AF after CA. Parameters for assessing left atrial (LA) remodeling and LA function[17,18], including biomarkers, electrocardiogram (ECG), and imaging parameters, are summarized in Table 1.
| Methods | Parameters | |
| Biomarkers | C-reactive protein | |
| Fibrinogen | ||
| B-type natriuretic peptide | ||
| Oxidative stress | ||
| Homocysteine and endothelin-1 | ||
| Renin-angiotensin-aldosterone system | ||
| ECG parameters | P-wave duration | |
| Intra-atrial conduction time | ||
| Dispersion of atrial fibrillation cycle lengths | ||
| Imaging parameters | Echocardiographic parameters | Left atrial diameter |
| Left atrial volume | ||
| Mitral inflow patterns | ||
| E/e’ index | ||
| Left atrial electromechanical conduction time | ||
| LA appendage ejectionfraction | ||
| Left atrial expansion index | ||
| Strain/strain rate | ||
| MRI and CT imaging | Pericardial fat | |
| Left atrial fibrosis | ||
| Ablation-related scarring | ||
| Pulmonary vein anatomy | ||
Patients with AF, especially those undergoing CA, are recommended to check transthoracic echocardiography (TTE) during routine follow-up. Apart from cardiac function, TTE can provide LA parameters that can represent the extent of LA remodeling and function. LA diameter, volume and ejection fraction are parameters that can be used in LA remodeling assessment and AF recurrence prediction[18]. In the meta-analysis by D’Ascenzo et al[19] that included 19 studies and 4357 AF patients, valve defect and LA diameter > 50 mm predicted AF recurrence after CA.
Owing to the low sensitivity, LA volume measurement to evaluate atrial function lacks accuracy and is limited in the prediction of recurrence of AF after CA, especially in the early stage. Parameters that can potently predict the recurrence of early AF are still needed. STE, commonly used in early LV dysfunction assessment, is also an important assessment method[20], in the quantification of LA myocardial deformation and remodeling[21].
Recently, LA strain has been proven to be superior to LA size as a predictor for AF recurrence after CA[22,23]. An increased LA strain, representing the decline in the deformability of LA, is related to a higher AF recurrence rate. Moreover, Sílvia et al[24] indicate LA strain is reliable in predicting the success of the CA procedure in AF, especially for the second CA.
Myocardial strain is a change in the distance between two points of the myocardium occurring in the cardiac cycle, expressed as a percentage, representing the fractional change in length of a myocardial segment. Strain is initially used to analyze ventricular function, and the resulting atrial strain provides a highly reproducible measurement of atrial myocardial deformation. Thus, subsequent research focuses on the LA strain.
The left atrium is a functional complex chamber, that plays an integral role in maintaining physiological hemodynamic and electrical stability of the heart. Apart from acting as a booster pump during late ventricular diastole to augment LV filling, it also serves as a reservoir to adapt the inflow volume from pulmonary veins during ventricular systole and isovolumic relaxation as well as serves as a passive conduit during early ventricular diastole[25]. In healthy people, the contribution of the LA reservoir, conduit, and booster pump to left ventricular filling is roughly 50%, 30% and 20% respectively[26]. The strains in the heart are longitudinal, circumferential and radial, but because of the fiber orientation and thinness of the atrial wall, only longitudinal strains are generally measured in the left atrium.
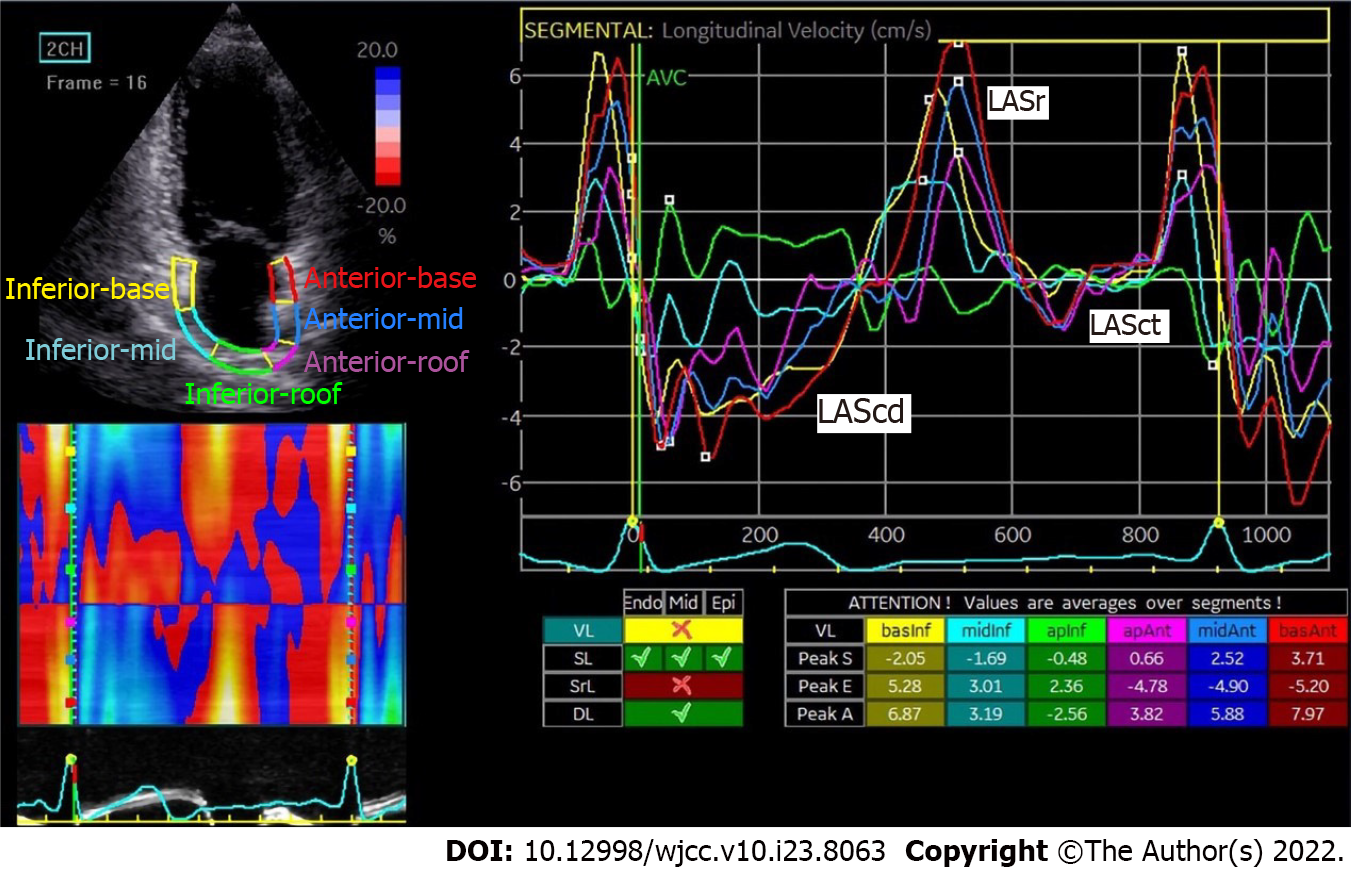
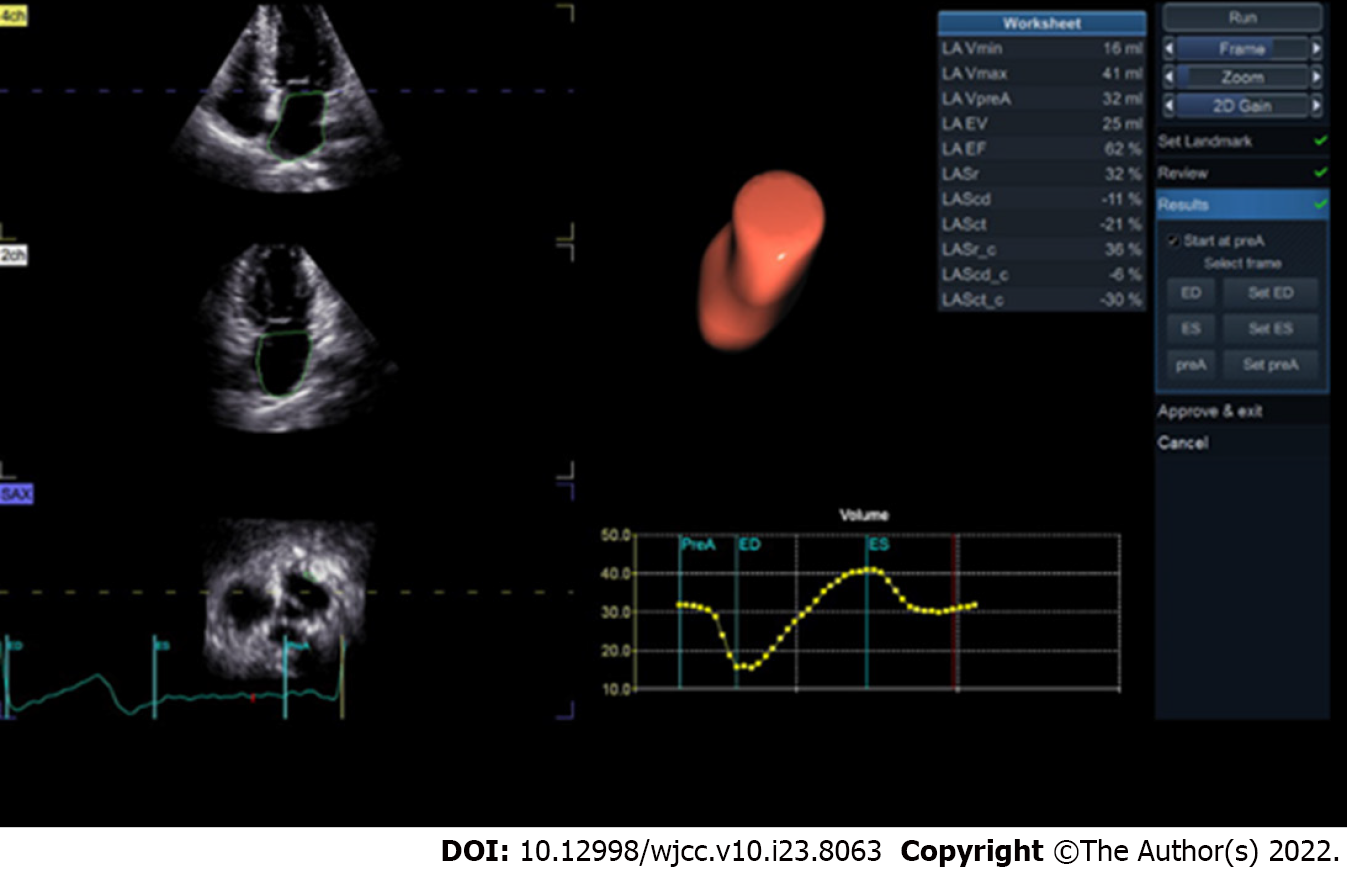
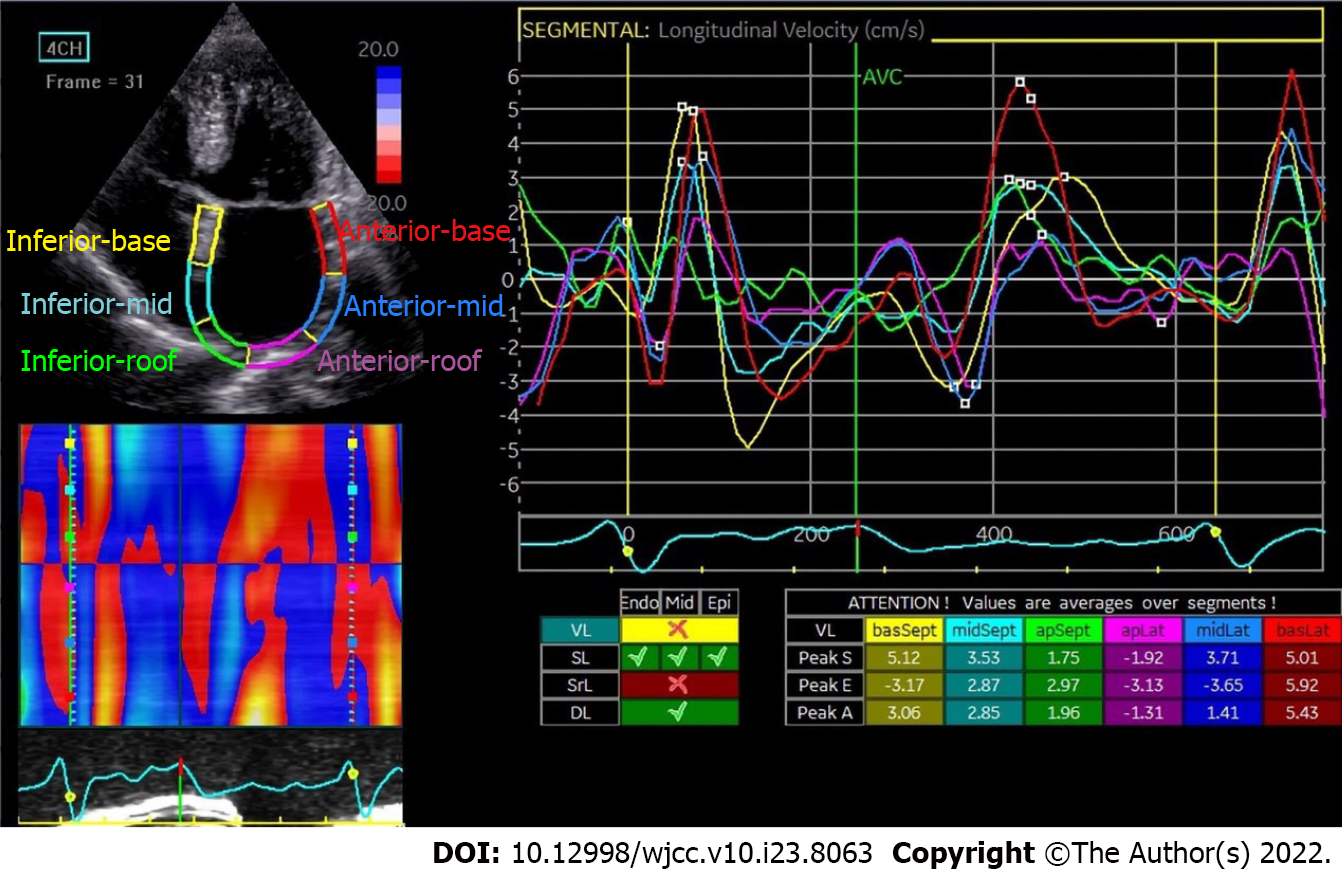
The first positive peak in the sinus rhythm strain curve corresponds to the phase before P wave in the ECG and represents LA reservoir strain (LASr) (Figure 1), reflecting the reserve function of the left atrium. The second negative peak (LASct) represents LA strain during contraction. The first negative peak and plateau reflect the LA strain when the atrium works as a conduit (LAScd). In a nutshell, LASr, LASct and LAScd respectively represent the reservoir, contractile and conduit functions of the left atrium. The specific measurements are as follows: (1) LASr: Strain change over reservoir phase in the cardiac cycle, expressed as the strain value at mitral valve opening minus the strain value at ventricular end diastole (positive value); (2) LAScd: Strain change over conduit phase in the cardiac cycle, expressed as the strain value at the beginning of atrial contraction minus the strain value at mitral valve opening (negative value); and (3) LASct: Only in patients with sinus rhythm is strain during the contraction phase measured as the difference between the strain value at ventricular end diastole and the onset of atrial contraction (negative value)[27].
Positive values are typically assigned to lengthening, thickening or clockwise rotation, whereas negative values are assigned to shortening, thinning or counterclockwise rotation. The baseline could be the electrocardiographic P wave or QRS complex, but patients with AF need measurements from the QRS complex.
Besides, the peak atrial longitudinal strain (PALS), which corresponds to the peak of the positive strain during left ventricular systole, is a crucial indicator of atrial compliance[27]. Furthermore, global atrial longitudinal strain (GALS) is a term that describes the average change in muscle length for visible segments[27,28].
With preliminary evidence of clinical applications of STE in atrial cardiomyopathy and valvular heart disease, the consensus on STE confirmed the ability of STE for assessing atrial function[29]. When feasible, atrial strain and three-dimensional (3D) atrial size and function assessment are used as part of the standard examination[30].
There has not been an accurate normal range for atrial strain parameters since the lack of standardization of measurement software and data processing software. Some preliminary research has led to the normal range of STE as a reference for evaluating atrial function. Pathan et al[31] performed a meta-analysis on 40 studies involving 2038 healthy subjects and tested the normal range of strain in the three functional states of the LA. The normal reference ranges were: LASr 39.4% (95% CI: 38.0%-40.8%), LAScd 23.0% (95% CI: 20.7%-25.2%), and LASct 17.4% (95% CI: 16.0%-19.0%). In 2019, Haji et al[32] described eight practical steps in measuring LA strain with TTE and strain software, and stressed the comprehensive clinical applications of LA strain in heart failure and AF.
Sun et al[33] noted that atrial strain significantly correlated with a few two-dimensional (2D) Doppler LV diastolic and LA function parameters. Peak strain and strain rate during LA contraction had a modest correlation with LA volumes and LV diastolic function.
There are distinct advantages and disadvantages to assessing LA strain after CA procedure in AF patients using 2D-STE and 3D-STE, cardiac computed tomography (CT) and cardiac magnetic resonance imaging (MRI) etc.
The use of cardiac CT is limited in the assessment of LA strain due to radiation exposure. For better visualization of the LA border, CT angiography has also been used, which can result in renal function impairment by using the iodine contrast agent. As a result of short data acquisition duration (PR interval 120-200 ms), it is difficult to distinguish LA volume changes over the functional phases[34]. In a small study of Szilveszter et al[35] cardiac CT slightly but consistently underestimated both LV and LA absolute global strain values.
Cardiac MRI is rarely used in clinical practice in LA strain assessment due to its high cost and lengthy examination time, making it unsuitable for heart disease patients, such as heart failure. Feature-tracking cardiac MRI is a novel and practical approach to assessing LA deformation that uses standard cine images and does not require additional tagging sequences[36]. Cardiac MRI can directly detect pathological features such as myocardial scars and fibrosis by late gadolinium enhancement imaging, thus accurately displaying the endocardial boundary, and providing details beyond structure and function[37]. Kuppahlly et al[5] have confirmed a negative correlation between myocardial fibrosis level and LA strain by cardiac MRI in AF patients. Moreover, PeAF patients have more myocardial fibrosis than PAF patients, implying a link between atrial remodeling, LA mechanical dysfunction, and AF prognosis.
Echocardiography is still the most commonly used examination to assess cardiac function because of its simplicity and convenience[21]. Tissue Doppler imaging (TDI) has traditionally been used in clinical practice. TDI evaluates the LA function using volume measurement, susceptible to angle dependence, noise interference, artifacts, and other factors[22].
STE is a new angle-independent quantitative technology that evaluates myocardial function by analyzing points on 2D gray-scale ultrasound images. In 2015, American/European Society of Echocardiography guidelines recommended the use of 2D-STE to analyze LA volume[38]. Among the techniques for evaluating atrial strain 2D-STE and 3D-STE have better predictive ability in AF rec
Hwang et al[41] reported that applying artificial intelligence algorithms to the STE radial strain of the left atrium can assess outcome status after AF ablation more accurately and sensitively. They developed a deep convolutional neural networks (CNN) model based on curved M-mode STE images, which may be a novel approach to evaluate the LA dysfunction. CNN may accurately classify the curved M-mode images of global strain in patients and provide detailed spatiotemporal information about the deformation sufficiently.
Although STE may not be possible to track accurately the LA deformation, because of the thinner atrial wall, being interrupted by the LA appendage and the four pulmonary vein openings, and shortening and extending uniformly[42], it is still a convenient and practical method in assessing LA strain compared to other assessment methods.
Atrial remodeling, including electrical and structural remodeling[43], is a common pathophysiological feature of AF, interacting with one another and ultimately causing dysfunction. Electrical remodeling includes changes in the properties of ion channels that affect atrial myocardium activation and conduction, resulting in longer atrial conduction times. Structural remodeling refers to microscopic structural changes such as myocardial hypertrophy, fibrosis, and muscle fiber arrangement disorder, leading to decreased atrial compliance and contractility. An electrophysiological study with detailed biatrial electroanatomic mapping has demonstrated that right atrial remodeling could accurately correlate with LA remodeling[44]. The imbalance between collagen synthesis and degradation and a fibrotic atrial substrate has consequences for LA electromechanical function, eventually leading to the occurrence of AF[45,46]. In contrast, atrial electrophysiological remodeling, cellular structure remodeling, myocardial lysis, interstitial fiber deposition, and extra-atrial matrix changes occur shortly after the onset of AF[47,48]. These changes cause a slow and gradual remodeling of the atrium and promote the recurrence and continuous occurrence of arrhythmia.
Extensive fibrosis is the cause of atrial arrhythmia, atrial stiffness increases, and atrial activities decrease, which is only seen in a small percentage of PeAF patients[49]. Atrial fibrosis occurs before changes in the macroscopic structure of the atrium[50,51], which is used to predict the outcome of CA. It takes a long time for the atrium to regain normal contractile function after cardioversion[52].
LA presents reservoir, conduit and contractile functions in sequence during the cardiac cycle. The compliance or stiffness of the atrial wall determines the deformability of the atrial muscle[53]. Kuppahally et al[5] proposed that LASr is a surrogate for fibrosis in patients with PeAF. In AF, increased atrial stiffness, weakened elasticity, decreased atrial compliance, and contractility result in lower strain and dysfunction of atrial reservoir function when compared to sinus rhythm[54]. Patients with AF have less strain, which reduces their atrial myocardial deformability. It is assumed that the deformability of the myocardium is further compromised during the development of AF.
Due to changes in atrial structure, the contractile function is almost lost in AF, and reservoir and conduit functions are both reduced. As a result, the strain curves in AF differ from those in sinus rhythm (Figure 3).
Reverse remodeling improves the response to medical or nonmedical intervention, whereas LA remodeling represents a state of maladaptive deterioration[55]. The reverse modeling increase, or anti-remodeling, is confirmed after restoring sinus rhythm through medical treatment and cardioversion[56]. The reduced baseline LA deformability assessed by STE has been shown to help identify patients at high risk of AF recurrence after CA in both PAF and PeAF[57]. While the CA is successful, the left atrium may undergo reverse remodeling, improving its function. Although the exact pathophysiology of LA reverse remodeling is unknown, it is critical in determining the prognosis of the left atrium, which may help to reduce the risk of AF recurrence. LV function can be improved by sinus rhythm recovery after CA, and LA strain and strain rate improve as LV systolic pressure strain improves. The LA strain may improve in the 3 mo following a successful CA procedure for AF[58]. Similarly, patients with AF had lower PLAS than healthy subjects, but it increased significantly at 3 and 12 mo after CA[59]. Furthermore, 63% of patients had reversal remodeling of AF after CA, accompanied by an improvement in LA strain. The baseline LA strain is a reliable predictor of LA reverse reconstruction[60].
Atrial strain is essential in detecting an increase in atrial stress early on, which other indexes can hardly accomplish. The strain is useful in determining the recurrence of AF following the CA procedure. Unfortunately, no clear strain value exists to predict the occurrence of a recurring event, and a large amount of data is still required to complete the evidence.
The clinical use of the LA strain has grown rapidly in recent years. The application of LA strain in AF patients not only includes assessing LA fibrosis and dysfunction, calculating thrombosis risk, etc., but also in predicting the presence/recurrence of AF. Table 2 shows the specifics of clinical application in atrial strain after CA.
| Ref. | Year (of data acquisition) | Population | Sample size (n) | Technology | Conclusion |
| Wen et al[77] (2021) | 2009-2011 | America | 144 | 2D-STE | Patients with recurrence had higher LASct 1-d than that in non-recurrence subjects LASct 1-d post-procedure predicts arrhythmia recurrence at long-term follow-up |
| Uziębło-Życzkowska et al[72] (2021) | 2019-2020 | Poland | 172 | 2D-STE | LASr and LASct were all associated with LA increased pressure in AF patients after CA |
| Pilichowska-Paszkiet et al[71] (2021) | July 2011 to January 2014 | Poland | 208 | 2D-STE | In patients with PAF, parameters reflecting LA compliance LASr and LAScd are independent and strong predictors of CA outcome |
| Koca et al[76] (2020) | follow-up 1 yr | Turkey | 190 | 2D-STE | LA strain in both 2 chambers and 4 chambers, and GALS were significantly lower in patients with AF recurrence. GALS should be included in routine evaluations to determine long-term AF recurrence preoperatively |
| Hanaki et al[74] (2020) | January 2013 to December 2016 | Japan | 100 | 2D-STE | In patients with long-standing PeAF, the inability to restore SR and lower LASr after AAD/ECV treatment independently and incrementally predicts the recurrence after CA |
| Csécs et al[65] (2020) | Follow-up 3 mo | America | 55 | CMR | Peak longitudinal atrial strain was significant predictor of arrhythmia recurrence and arrhythmia recurrence |
| Yan et al[64] (2019) | October 2016 to December 2017 | China | 32 | 2D-STE | The strain rates in the lateral wall base segment, interval middle segment, and middle segment of the lateral wall and GALS were significantly decreased in the patients with AF recurrence |
| Chen et al[6] (2019) | May 2015 to June 2016 | China | 40 | 2D-STE | The LA reservoir, conduit and contractile strain in septal segments significantly decreased in the PAF patients with low-voltage zone after CA. Besides, global strain tended to be an independent determinant of LA fibrosis |
| Bai et al[78] (2018) | 2013-2014 | China | 87 | 2D-STE | Peak right atrial longitudinal strain, peak LA longitudinal strain, and combined both are important factors associated with AF recurrence following CA in patients with chronic lung diseases |
| Parwani et al[62] (2017) | January 2010 to January 2013 | Germany | 102 | 2D-STE | Patients with recurrence of atrial arrhythmias after both the first and the second CA procedure had significantly lowered LA strain than those without recurrence |
| Mochizuki et al[39] (2017) | February 2013 to December 2014 | Japan | 42 | 3D-STE | In both the PAF and PeAF populations, patients with recurrence presented with significantly impaired GALS compared with patients without recurrence. LA strain determined by 3D-STE is a novel and better predictor of AF recurrence after CA than that determined by 2D-STE or other known predictors |
| Ma et al[57] (2017) | March 2013 to March 2015 | China | 115 | 2D-STE | Patients with recurrence presented with significantly impaired GALS compared with patients without recurrence. In both PAF and PeAF, decreased baseline LA deformation capabilities assessed by 2D-STE can help to identify patients at high risk of AF recurrence after catheter ablation |
| Habibi et al[66] (2016) | January 2011 to September 2013 | America | 121 | CMR | LA reservoir function was independently associated with recurrent AF/AT after PVI. Peak LA strain improved prediction of recurrent AT/AF compared to the baseline clinical model |
| Gucuk Ipek et al[69] (2016) | 2010-2013 | America | 119 | CMR | Baseline reservoir, conduit, and contractile function of the LA were significantly impaired in patients with incident LA flutter |
| Yasuda et al[63](2015) | July 2010 to March 2012 | Japan | 100 | 2D-STE | Patients with AF recurrence had significantly a lower LA global strain and lower LA lateral total strain than those who maintained sinus rhythm. LA global strain could predict AF recurrence after CA |
| Montserrat et al[24] (2015) | Follow-up 6 mo | Spain | 83 | 2D-STE | LASr and LASct were significantly lower in the second RFCA patients. LASr independent predictor of arrhythmia suppression after first RFCA and after a second RFCA |
| Motoki et al[75] (2014) | June 2008 to May 2010 | Australia | 319 | 2D-STE | Patients with LA total strain < 23.2% showed a higher incidence of AF recurrence. baseline LA total strain was associated with rhythm outcome after catheter ablation |
| La Meir et al[59] (2013) | 2007-2011 | Netherlands | 33 | 2D-STE | The peak systolic strain and the peak strain rate were lower in patients with atrial fibrillation than in the controls. It had increased significantly at 3 mo and 12 mo after surgery |
| Hammerstingl et al[23] (2012) | 2008-2010 | Germany | 103 | 2D-STE | The assessment of global LA strain with 2D-STE identifies patients with a high risk for AF recurrence after ablation procedures |
| Tops et al[60] (2011) | Follow-up 12 mo | Netherlands | 122 | 2D-STE | 63% of the patients exhibited LA reverse remodeling after CA for AF, with a concomitant improvement in LA strain. LA strain at baseline was an independent predictor of LA reverse remodeling |
| Mirza et al[79] (2011) | January 2005 to April 2009 | America | 63 | 2D-STE | global and regional systolic and diastolic strains and SR were reduced in patients with recurrent AF. Regional LA lateral wall LS is a preprocedural determinant of AFR in patients undergoing CA, independent of LA enlargement |
| Hwang et al[73] (2009) | Follow-up 9 mo | Korea | 40 | 2D- STE | The lower systolic strain of LA was strongly associated with recurrence after catheter ablation |
| Schneider et al)[61] (2008) | March 2003 to October 2006 | Germany | 118 | 2D-STE | Patients with higher atrial strain and SR after catheter ablation appear to have a greater likelihood of maintenance of sinus rhythm |
Decreased LA strain is generally accepted to be related to AF recurrence after cardioversion or CA. LA strain is superior in early detection of LA functional impairment than other structural change parameters by TTE. Patients with AF recurrence had significantly lower LA strain when compared to those who maintained sinus rhythm after CA[61-63]. Moreover, in patients with AF and atrial flutter, atrial function impairment, reduction in LA strain, and atrial compliance impairment all come before structural reconstruction and LA size changes[23].
PALS and GALS are two indexes used to represent LA strain changes over the cardiac cycle. Several studies have shown that GALS in the basal, midseptal, or midlateral walls of patients with AF recurrence after CA decreased significantly[39,57,64]. The baseline GALS is related to the rhythm maintenance after CA, and GALS < 23.2% shows a higher rate of AF recurrence[39]. The global and regional LA strains were both reduced in patients with AF recurrence[24] .
PALS is frequently an independent predictor of LA fibrosis[6] and arrhythmia recurrence[63,65,66]. PALS is significantly reduced in patients with recurrence of AF[63]. Nielsen et al[67] conducted research on a meta-analysis of 12 studies involving 1025 subjects, revealing that lower PALS is associated with an increased risk of AF recurrence following CA. They determined that 12.8% was the cut-off value for PALS to predict AF recurrence, with a weighted mean sensitivity of 80% (range 74%-86%) and specificity of 87% (range 71%-98%). Also, in another recent study, it has been confirmed that LA strain and strain rate are more independent than other parameters in predicting the possibility of AF recurrence after CA[68,69]. Similarly, Bajraktari et al[70] did the meta-analysis of 85 studies including 16126 patients and verified that LA diameter > 50 mm, volume > 150 mL, and strain < 19%, have a negative effect on maintaining sinus rhythm after CA.
Consistent with GALS and PALS changes, LA strain in the different phases, LASr, LAScd and LASct are all significantly reduced in PAF patients after CA[6].
When it comes to a specific phase, LASr is decreased in patients with AF recurrence after CA. Physiologically, LASr reduction is related to the increase in LA pressure, compromised LA compliance and high fibrosis status[24,71-76]. Decreased LAScd, has also been found related to AF recurrence after CA in limited evidence due to the difficulty in discrimination and vulnerability to be affected by atrial remodeling[71]. LASct is a more sensitive parameter that reflects the structure of the atrium. Decreased LASct is also related to AF recurrence after CA. Besides, it can predict the AF recurrence during long-term follow-up[77].
Still, the evaluation of LA strain is restricted in AF rhythm. Also, due to the limited evidence, the exact cut-off value of LA strain in a specific phase has not been proposed.
Compared to LA strain, right atrial strain is more commonly used in the atrial assessment of potential right heart disease. Peak right atrial longitudinal strain, peak LA longitudinal strain, and the combination of the two have also been reported can predict the recurrence of AF after CA in patients with chronic lung disease[78].
Rhythm control is the core part of the integrated management of AF. Despite protocol and devices advances in CA, the recurrence rate of AF after CA is still high. Atrial strain, the parameter of atrial deformation during the cardiac cycle, is closely related to atrial remodeling and atrial function. Furthermore, accumulating evidence shows the role of decreased atrial strain in the early prediction of AF recurrence. Further studies are needed to add strength to the early prediction value of atrial strain in AF recurrences.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: European Society of Cardiology, No. 871093.
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Conti S, Italy; Mizutani Y, Japan S-Editor: Wang DM L-Editor: Kerr C P-Editor: Wang DM
| 1. | Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. Epidemiology of Atrial Fibrillation in the 21st Century: Novel Methods and New Insights. Circ Res. 2020;127:4-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 911] [Article Influence: 182.2] [Reference Citation Analysis (0)] |
| 2. | GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789-1858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9354] [Cited by in RCA: 8390] [Article Influence: 1198.6] [Reference Citation Analysis (4)] |
| 3. | Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3176] [Cited by in RCA: 6466] [Article Influence: 1616.5] [Reference Citation Analysis (0)] |
| 4. | Yamaguchi T, Marrouche NF. Recurrence Post-Atrial Fibrillation Ablation: Think Outside the Pulmonary Veins. Circ Arrhythm Electrophysiol. 2018;11:e006379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovski EG, Vijayakumar S, Rao SN, Blauer J, Fish EN, Dibella EV, Macleod RS, McGann C, Litwin SE, Marrouche NF. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging. 2010;3:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 534] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 6. | Chen Y, Li Z, Shen X, Wang W, Kang Y, Qiao Z, Wang X, Pu J. Assessment of left atrial remodeling in paroxysmal atrial fibrillation with speckle tracking echocardiography: a study with an electrophysiological mapping system. Int J Cardiovasc Imaging. 2019;35:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Olshansky B, Rosenfeld LE, Warner AL, Solomon AJ, O'Neill G, Sharma A, Platia E, Feld GK, Akiyama T, Brodsky MA, Greene HL; AFFIRM Investigators. The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study: approaches to control rate in atrial fibrillation. J Am Coll Cardiol. 2004;43:1201-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 237] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, Hamann F, Heidbüchel H, Hindricks G, Kautzner J, Kuck KH, Mont L, Ng GA, Rekosz J, Schoen N, Schotten U, Suling A, Taggeselle J, Themistoclakis S, Vettorazzi E, Vardas P, Wegscheider K, Willems S, Crijns HJGM, Breithardt G; EAST-AFNET 4 Trial Investigators. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N Engl J Med. 2020;383:1305-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 1370] [Article Influence: 274.0] [Reference Citation Analysis (0)] |
| 9. | Parameswaran R, Al-Kaisey AM, Kalman JM. Catheter ablation for atrial fibrillation: current indications and evolving technologies. Nat Rev Cardiol. 2021;18:210-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 10. | Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, Daniels MR, Bahnson TD, Poole JE, Rosenberg Y, Lee KL, Packer DL; CABANA Investigators. Effect of Catheter Ablation vs Medical Therapy on Quality of Life Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA. 2019;321:1275-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 451] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 11. | Blomström-Lundqvist C, Gizurarson S, Schwieler J, Jensen SM, Bergfeldt L, Kennebäck G, Rubulis A, Malmborg H, Raatikainen P, Lönnerholm S, Höglund N, Mörtsell D. Effect of Catheter Ablation vs Antiarrhythmic Medication on Quality of Life in Patients With Atrial Fibrillation: The CAPTAF Randomized Clinical Trial. JAMA. 2019;321:1059-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 250] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 12. | Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, Chen PS, Chen SA, Chung MK, Cosedis Nielsen J, Curtis AB, Davies DW, Day JD, d'Avila A, Natasja de Groot NMS, Di Biase L, Duytschaever M, Edgerton JR, Ellenbogen KA, Ellinor PT, Ernst S, Fenelon G, Gerstenfeld EP, Haines DE, Haissaguerre M, Helm RH, Hylek E, Jackman WM, Jalife J, Kalman JM, Kautzner J, Kottkamp H, Kuck KH, Kumagai K, Lee R, Lewalter T, Lindsay BD, Macle L, Mansour M, Marchlinski FE, Michaud GF, Nakagawa H, Natale A, Nattel S, Okumura K, Packer D, Pokushalov E, Reynolds MR, Sanders P, Scanavacca M, Schilling R, Tondo C, Tsao HM, Verma A, Wilber DJ, Yamane T; Document Reviewers:. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20:e1-e160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 758] [Cited by in RCA: 796] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 13. | Mujović N, Marinković M, Lenarczyk R, Tilz R, Potpara TS. Catheter Ablation of Atrial Fibrillation: An Overview for Clinicians. Adv Ther. 2017;34:1897-1917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 14. | Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HS, Sullivan T, Roberts-Thomson KC, Sanders P. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2:e004549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 543] [Cited by in RCA: 578] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 15. | Kallistratos MS, Poulimenos LE, Manolis AJ. Atrial fibrillation and arterial hypertension. Pharmacol Res. 2018;128:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Mujović N, Marinković M, Mihajlović M, Mujović N, Potpara TS. Risk factor modification for the primary and secondary prevention of atrial fibrillation. Part 2. Kardiol Pol. 2020;78:192-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Vizzardi E, Curnis A, Latini MG, Salghetti F, Rocco E, Lupi L, Rovetta R, Quinzani F, Bonadei I, Bontempi L, D'Aloia A, Dei Cas L. Risk factors for atrial fibrillation recurrence: a literature review. J Cardiovasc Med (Hagerstown). 2014;15:235-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Leung M, Abou R, van Rosendael PJ, van der Bijl P, van Wijngaarden SE, Regeer MV, Podlesnikar T, Ajmone Marsan N, Leung DY, Delgado V, Bax JJ. Relation of Echocardiographic Markers of Left Atrial Fibrosis to Atrial Fibrillation Burden. Am J Cardiol. 2018;122:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | D'Ascenzo F, Corleto A, Biondi-Zoccai G, Anselmino M, Ferraris F, di Biase L, Natale A, Hunter RJ, Schilling RJ, Miyazaki S, Tada H, Aonuma K, Yenn-Jiang L, Tao H, Ma C, Packer D, Hammill S, Gaita F. Which are the most reliable predictors of recurrence of atrial fibrillation after transcatheter ablation? Int J Cardiol. 2013;167:1984-1989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 20. | Yuda S, Muranaka A, Miura T. Clinical implications of left atrial function assessed by speckle tracking echocardiography. J Echocardiogr. 2016;14:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Cameli M, Lisi M, Righini FM, Mondillo S. Novel echocardiographic techniques to assess left atrial size, anatomy and function. Cardiovasc Ultrasound. 2012;10:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Saraiva RM, Demirkol S, Buakhamsri A, Greenberg N, Popović ZB, Thomas JD, Klein AL. Left atrial strain measured by two-dimensional speckle tracking represents a new tool to evaluate left atrial function. J Am Soc Echocardiogr. 2010;23:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 264] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 23. | Hammerstingl C, Schwekendiek M, Momcilovic D, Schueler R, Sinning JM, Schrickel JW, Mittmann-Braun E, Nickenig G, Lickfett L. Left atrial deformation imaging with ultrasound based two-dimensional speckle-tracking predicts the rate of recurrence of paroxysmal and persistent atrial fibrillation after successful ablation procedures. J Cardiovasc Electrophysiol. 2012;23:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Montserrat S, Gabrielli L, Bijnens B, Borràs R, Berruezo A, Poyatos S, Brugada J, Mont L, Sitges M. Left atrial deformation predicts success of first and second percutaneous atrial fibrillation ablation. Heart Rhythm. 2015;12:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Gutman J, Wang YS, Wahr D, Schiller NB. Normal left atrial function determined by 2-dimensional echocardiography. Am J Cardiol. 1983;51:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Delgado V, Di Biase L, Leung M, Romero J, Tops LF, Casadei B, Marrouche N, Bax JJ. Structure and Function of the Left Atrium and Left Atrial Appendage: AF and Stroke Implications. J Am Coll Cardiol. 2017;70:3157-3172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 137] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 27. | Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, D'Hooge J, Donal E, Fraser AG, Marwick T, Mertens L, Popescu BA, Sengupta PP, Lancellotti P, Thomas JD, Voigt JU; Industry representatives; Reviewers: This document was reviewed by members of the 2016–2018 EACVI Scientific Documents Committee. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2018;19:591-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 1055] [Article Influence: 175.8] [Reference Citation Analysis (0)] |
| 28. | Kupczyńska K, Mandoli GE, Cameli M, Kasprzak JD. Left atrial strain - a current clinical perspective. Kardiol Pol. 2021;79:955-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, Horton K, Ogunyankin KO, Palma RA, Velazquez EJ. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32:1-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1366] [Cited by in RCA: 1474] [Article Influence: 245.7] [Reference Citation Analysis (0)] |
| 30. | Voigt JU, Mălăescu GG, Haugaa K, Badano L. How to do LA strain. Eur Heart J Cardiovasc Imaging. 2020;21:715-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | Pathan F, D'Elia N, Nolan MT, Marwick TH, Negishi K. Normal Ranges of Left Atrial Strain by Speckle-Tracking Echocardiography: A Systematic Review and Meta-Analysis. J Am Soc Echocardiogr. 2017;30:59-70.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 367] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 32. | Haji K, Wong C, Wright L, Ramkumar S, Marwick TH. Left Atrial Strain Performance and its Application in Clinical Practice. JACC Cardiovasc Imaging. 2019;12:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Sun JP, Yang Y, Guo R, Wang D, Lee AP, Wang XY, Lam YY, Fang F, Yang XS, Yu CM. Left atrial regional phasic strain, strain rate and velocity by speckle-tracking echocardiography: normal values and effects of aging in a large group of normal subjects. Int J Cardiol. 2013;168:3473-3479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Sheth PJ, Danton GH, Siegel Y, Kardon RE, Infante JC Jr, Ghersin E, Fishman JE. Cardiac Physiology for Radiologists: Review of Relevant Physiology for Interpretation of Cardiac MR Imaging and CT. Radiographics. 2015;35:1335-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Szilveszter B, Nagy AI, Vattay B, Apor A, Kolossváry M, Bartykowszki A, Simon J, Drobni ZD, Tóth A, Suhai FI, Merkely B, Maurovich-Horvat P. Left ventricular and atrial strain imaging with cardiac computed tomography: Validation against echocardiography. J Cardiovasc Comput Tomogr. 2020;14:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Kowallick JT, Morton G, Lamata P, Jogiya R, Kutty S, Hasenfuß G, Lotz J, Nagel E, Chiribiri A, Schuster A. Quantification of atrial dynamics using cardiovascular magnetic resonance: inter-study reproducibility. J Cardiovasc Magn Reson. 2015;17:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Siebermair J, Kholmovski EG, Marrouche N. Assessment of Left Atrial Fibrosis by Late Gadolinium Enhancement Magnetic Resonance Imaging: Methodology and Clinical Implications. JACC Clin Electrophysiol. 2017;3:791-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 38. | Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3897] [Cited by in RCA: 5492] [Article Influence: 549.2] [Reference Citation Analysis (0)] |
| 39. | Mochizuki A, Yuda S, Fujito T, Kawamukai M, Muranaka A, Nagahara D, Shimoshige S, Hashimoto A, Miura T. Left atrial strain assessed by three-dimensional speckle tracking echocardiography predicts atrial fibrillation recurrence after catheter ablation in patients with paroxysmal atrial fibrillation. J Echocardiogr. 2017;15:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Seo Y, Ishizu T, Atsumi A, Kawamura R, Aonuma K. Three-dimensional speckle tracking echocardiography. Circ J. 2014;78:1290-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Hwang YT, Lee HL, Lu CH, Chang PC, Wo HT, Liu HT, Wen MS, Lin FC, Chou CC. A Novel Approach for Predicting Atrial Fibrillation Recurrence After Ablation Using Deep Convolutional Neural Networks by Assessing Left Atrial Curved M-Mode Speckle-Tracking Images. Front Cardiovasc Med. 2020;7:605642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J. 2016;37:1196-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 557] [Cited by in RCA: 597] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 43. | Wijesurendra RS, Casadei B. Mechanisms of atrial fibrillation. Heart. 2019;105:1860-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 44. | Prabhu S, Voskoboinik A, McLellan AJA, Peck KY, Pathik B, Nalliah CJ, Wong GR, Azzopardi SM, Lee G, Mariani J, Ling LH, Taylor AJ, Kalman JM, Kistler PM. A comparison of the electrophysiologic and electroanatomic characteristics between the right and left atrium in persistent atrial fibrillation: Is the right atrium a window into the left? J Cardiovasc Electrophysiol. 2017;28:1109-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Jalife J, Kaur K. Atrial remodeling, fibrosis, and atrial fibrillation. Trends Cardiovasc Med. 2015;25:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 219] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 46. | Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 819] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 47. | Pathak R, Lau DH, Mahajan R, Sanders P. Structural and Functional Remodeling of the Left Atrium: Clinical and Therapeutic Implications for Atrial Fibrillation. J Atr Fibrillation. 2013;6:986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 48. | Lau DH, Linz D, Schotten U, Mahajan R, Sanders P, Kalman JM. Pathophysiology of Paroxysmal and Persistent Atrial Fibrillation: Rotors, Foci and Fibrosis. Heart Lung Circ. 2017;26:887-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 49. | Hirsh BJ, Copeland-Halperin RS, Halperin JL. Fibrotic atrial cardiomyopathy, atrial fibrillation, and thromboembolism: mechanistic links and clinical inferences. J Am Coll Cardiol. 2015;65:2239-2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 163] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 50. | Lacalzada-Almeida J, García-Niebla J. How to detect atrial fibrosis. J Geriatr Cardiol. 2017;14:185-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 51. | Valderrábano M, Shah DJ. Structure Predicts (Dys)Function: Atrial Fibrosis, Function, and Incident Atrial Fibrillation. JACC Cardiovasc Imaging. 2020;13:1701-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Kagawa Y, Fujii E, Fujita S, Ito M. Association between left atrial reverse remodeling and maintenance of sinus rhythm after catheter ablation of persistent atrial fibrillation. Heart Vessels. 2020;35:239-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 53. | Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014;63:493-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 738] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 54. | Abhayaratna WP, Fatema K, Barnes ME, Seward JB, Gersh BJ, Bailey KR, Casaclang-Verzosa G, Tsang TS. Left atrial reservoir function as a potent marker for first atrial fibrillation or flutter in persons > or = 65 years of age. Am J Cardiol. 2008;101:1626-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 55. | Thomas L, Abhayaratna WP. Left Atrial Reverse Remodeling: Mechanisms, Evaluation, and Clinical Significance. JACC Cardiovasc Imaging. 2017;10:65-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 275] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 56. | Casaclang-Verzosa G, Gersh BJ, Tsang TS. Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation. J Am Coll Cardiol. 2008;51:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 371] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 57. | Ma XX, Zhang YL, Hu B, Zhu MR, Jiang WJ, Wang M, Zheng DY, Xue XP. The usefulness of global left atrial strain for predicting atrial fibrillation recurrence after catheter ablation in patients with persistent and paroxysmal atrial fibrillation. Arch Cardiovasc Dis. 2017;110:447-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 58. | Marsan NA, Tops LF, Holman ER, Van de Veire NR, Zeppenfeld K, Boersma E, van der Wall EE, Schalij MJ, Bax JJ. Comparison of left atrial volumes and function by real-time three-dimensional echocardiography in patients having catheter ablation for atrial fibrillation with persistence of sinus rhythm versus recurrent atrial fibrillation three months later. Am J Cardiol. 2008;102:847-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | La Meir M, Gelsomino S, Lucà F, Pison L, Rao CM, Wellens F, Maessen JG. Improvement of left atrial function and left atrial reverse remodeling after minimally invasive radiofrequency ablation evaluated by 2-dimensional speckle tracking echocardiography. J Thorac Cardiovasc Surg. 2013;146:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Tops LF, Delgado V, Bertini M, Marsan NA, Den Uijl DW, Trines SA, Zeppenfeld K, Holman E, Schalij MJ, Bax JJ. Left atrial strain predicts reverse remodeling after catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2011;57:324-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 61. | Schneider C, Malisius R, Krause K, Lampe F, Bahlmann E, Boczor S, Antz M, Ernst S, Kuck KH. Strain rate imaging for functional quantification of the left atrium: atrial deformation predicts the maintenance of sinus rhythm after catheter ablation of atrial fibrillation. Eur Heart J. 2008;29:1397-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 62. | Parwani AS, Morris DA, Blaschke F, Huemer M, Pieske B, Haverkamp W, Boldt LH. Left atrial strain predicts recurrence of atrial arrhythmias after catheter ablation of persistent atrial fibrillation. Open Heart. 2017;4:e000572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 63. | Yasuda R, Murata M, Roberts R, Tokuda H, Minakata Y, Suzuki K, Tsuruta H, Kimura T, Nishiyama N, Fukumoto K, Aizawa Y, Tanimoto K, Takatsuki S, Abe T, Fukuda K. Left atrial strain is a powerful predictor of atrial fibrillation recurrence after catheter ablation: study of a heterogeneous population with sinus rhythm or atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2015;16:1008-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 64. | Yan Y, Li XL. [Evaluation of Left Atrial Structure and Function with Two-dimensional Speckle Tracking Imaging and Real-time Three-dimensional Imaging in Patients with Paroxysmal Atrial Fibrillation After Radiofrequency Catheter Ablation]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2019;50:390-395. [PubMed] |
| 65. | Csécs I, Yamaguchi T, Kheirkhahan M, Czimbalmos C, Fochler F, Kholmovski EG, Morris AK, Kaur G, Vago H, Merkely B, Chelu MG, Marrouche NF, Wilson BD. Left atrial functional and structural changes associated with ablation of atrial fibrillation - Cardiac magnetic resonance study. Int J Cardiol. 2020;305:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 66. | Habibi M, Lima JAC, Gucuk Ipek E, Zimmerman SL, Zipunnikov V, Spragg D, Ashikaga H, Rickard J, Marine JE, Berger RD, Calkins H, Nazarian S. The association of baseline left atrial structure and function measured with cardiac magnetic resonance and pulmonary vein isolation outcome in patients with drug-refractory atrial fibrillation. Heart Rhythm. 2016;13:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 67. | Nielsen AB, Skaarup KG, Lassen MCH, Djernæs K, Hansen ML, Svendsen JH, Johannessen A, Hansen J, Sørensen SK, Gislason G, Biering-Sørensen T. Usefulness of left atrial speckle tracking echocardiography in predicting recurrence of atrial fibrillation after radiofrequency ablation: a systematic review and meta-analysis. Int J Cardiovasc Imaging. 2020;36:1293-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 68. | Liżewska-Springer A, Dąbrowska-Kugacka A, Lewicka E, Drelich Ł, Królak T, Raczak G. Echocardiographic predictors of atrial fibrillation recurrence after catheter ablation: A literature review. Cardiol J. 2020;27:848-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 69. | Gucuk Ipek E, Marine JE, Habibi M, Chrispin J, Lima J, Rickard J, Spragg D, Zimmerman SL, Zipunnikov V, Berger R, Calkins H, Nazarian S. Association of left atrial function with incident atypical atrial flutter after atrial fibrillation ablation. Heart Rhythm. 2016;13:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Bajraktari G, Bytyçi I, Henein MY. Left atrial structure and function predictors of recurrent fibrillation after catheter ablation: a systematic review and meta-analysis. Clin Physiol Funct Imaging. 2020;40:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 71. | Pilichowska-Paszkiet E, Baran J, Kułakowski P, Zaborska B. Echocardiographic assessment of left atrial function for prediction of efficacy of catheter ablation for atrial fibrillation. Medicine (Baltimore). 2021;100:e27278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Uziębło-Życzkowska B, Krzesiński P, Jurek A, Krzyżanowski K, Kiliszek M. Correlations between left atrial strain and left atrial pressures values in patients undergoing atrial fibrillation ablation. Kardiol Pol. 2021;79:1223-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | Hwang HJ, Choi EY, Rhee SJ, Joung B, Lee BH, Lee SH, Kim J, Lee MH, Jang Y, Chung N, Kim SS. Left atrial strain as predictor of successful outcomes in catheter ablation for atrial fibrillation: a two-dimensional myocardial imaging study. J Interv Card Electrophysiol. 2009;26:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 74. | Hanaki Y, Machino-Ohtsuka T, Aonuma K, Komatsu Y, Machino T, Yamasaki H, Igarashi M, Sekiguchi Y, Nogami A, Ieda M. Preprocedural restoration of sinus rhythm and left atrial strain predict outcomes of catheter ablation for long-standing persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2020;31:1709-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 75. | Motoki H, Negishi K, Kusunose K, Popović ZB, Bhargava M, Wazni OM, Saliba WI, Chung MK, Marwick TH, Klein AL. Global left atrial strain in the prediction of sinus rhythm maintenance after catheter ablation for atrial fibrillation. J Am Soc Echocardiogr. 2014;27:1184-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 76. | Koca H, Demirtas AO, Kaypaklı O, Icen YK, Sahin DY, Koca F, Koseoglu Z, Baykan AO, Guler EC, Demirtas D, Koc M. Decreased left atrial global longitudinal strain predicts the risk of atrial fibrillation recurrence after cryoablation in paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2020;58:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 77. | Wen S, Indrabhinduwat M, Brady PA, Pislaru C, Miller FA, Ammash NM, Nkomo VT, Padang R, Pislaru SV, Lin G. Post Procedural Peak Left Atrial Contraction Strain Predicts Recurrence of Arrhythmia after Catheter Ablation of Atrial Fibrillation. Cardiovasc Ultrasound. 2021;19:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 78. | Bai Y, Zhao Y, Li J, Zhang Y, Bai R, Du X, Dong JZ, He YH, Ma CS. Association of peak atrial longitudinal strain with atrial fibrillation recurrence in patients with chronic lung diseases following radiofrequency ablation. Intern Med J. 2018;48:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 79. | Mirza M, Caracciolo G, Khan U, Mori N, Saha SK, Srivathsan K, Altemose G, Scott L, Sengupta P, Jahangir A. Left atrial reservoir function predicts atrial fibrillation recurrence after catheter ablation: a two-dimensional speckle strain study. J Interv Card Electrophysiol. 2011;31:197-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |