Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.7950
Peer-review started: December 13, 2021
First decision: April 8, 2022
Revised: April 29, 2022
Accepted: June 23, 2022
Article in press: June 23, 2022
Published online: August 6, 2022
Processing time: 221 Days and 3.1 Hours
Primary spinal cord (PSC) glioblastoma (GB) is an extremely rare but fatal primary tumor of the central nervous system and associated with a poor prognosis. While typical tumor imaging features are generally easy to recognize, glioblastoma multiforme can have a wide range of imaging findings. Atypical GB is often misdiagnosed, which usually delays the optimal time for treatment. In this article, we discuss a clinical case of pathologically confirmed PSC GB under the guise of benign tumor imaging findings, as well as the most recent literature pertaining to PSC GB.
A 70-year-old female complained of limb weakness lasting more than 20 d. Irregular masses were observed inside and outside the left foramina of the spinal canal at C7-T1 on medical imaging. Based on the imaging features, radiologists diagnosed the patient with schwannoma. Tumor resection was performed under general anesthesia. The final histopathological findings revealed a final diagnosis of PSC GB, world health organization Grade IV. The patient subsequently underwent a 4-wk course of radiotherapy (60 Gy in 20 fractions) combined with temozolomide chemotherapy. The patient was alive at the time of submission of this manuscript.
Atypical GB presented unusual imaging findings, which led to misdiagnosis. Therefore, a complete recognition of imaging signs may facilitate early accurate diagnosis.
Core Tip: This is the first literature review to summarize the imaging features of surgical and pathologically confirmed glioblastoma in the spinal cord. Lesions reported in previous cases were located in the spinal canal exclusively. Intramedullary glioma that extended beyond the spinal canal has not been reported before. In this case, the tumor grew across and beyond the spinal canal and appeared benign, which led to the misdiagnosis of neurogenic tumors. A definitive diagnosis requires histopathological confirmation. However, complete recognition of the imaging signs of the disease may facilitate early accurate diagnosis.
- Citation: Liang XY, Chen YP, Li Q, Zhou ZW. Atypical imaging features of the primary spinal cord glioblastoma: A case report. World J Clin Cases 2022; 10(22): 7950-7959
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/7950.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.7950
Glioblastoma (GB) is the most common malignancy of the nervous system. Primary spinal cord (PSC) GB is a fairly rare tumor, accounting for 1% to 5% of all GB and 1.5% of all spinal cord neoplasms[1]. Due to its rare occurrence, most reported studies are either case reports or focused on its treatment and prognosis. Apart from histopathological findings, a precise imaging diagnosis also plays an essential role in the prognosis of GB. Furthermore, as it presents a variety of imaging features, it is difficult to differentiate this tumor from ependymoma and astrocytoma as well as neurogenic neoplasms[2,3]. To the best of our knowledge, this is the first literature review to summarize the imaging findings of this rare disease. Due to a misdiagnosed case of pathologically confirmed PSC GB with atypical imaging features in our clinical practice (Figure 1), we reviewed and summarized the imaging and clinical features of PSC GB from 2011 to 2021. By aggregating these data, we intend to improve the diagnostic accuracy of PSC GB for radiologists and clinicians.
A 70-year-old female, with no history of previous illnesses, complained of limb weakness and an inability to walk lasting more than 20 d.
The patient reported feeling weak in her limbs, especially her left arm and leg. She described that, in the previous 2 wk, she had been unable to walk as before.
The patient had no chronic illnesses or history of surgery.
The patient did not have any significant family history.
Physical examination revealed no deformity in the spine and limbs, with a normal cranial nerve sensation detected. However, there was decreased needling reflection below the bilateral nipples and a muscle strength score of Grade 0 in both lower limbs. Additionally, bilateral abdominal reflexes, anal sphincter reflex, bilateral knee tendon, and Achilles tendon reflexes were absent.
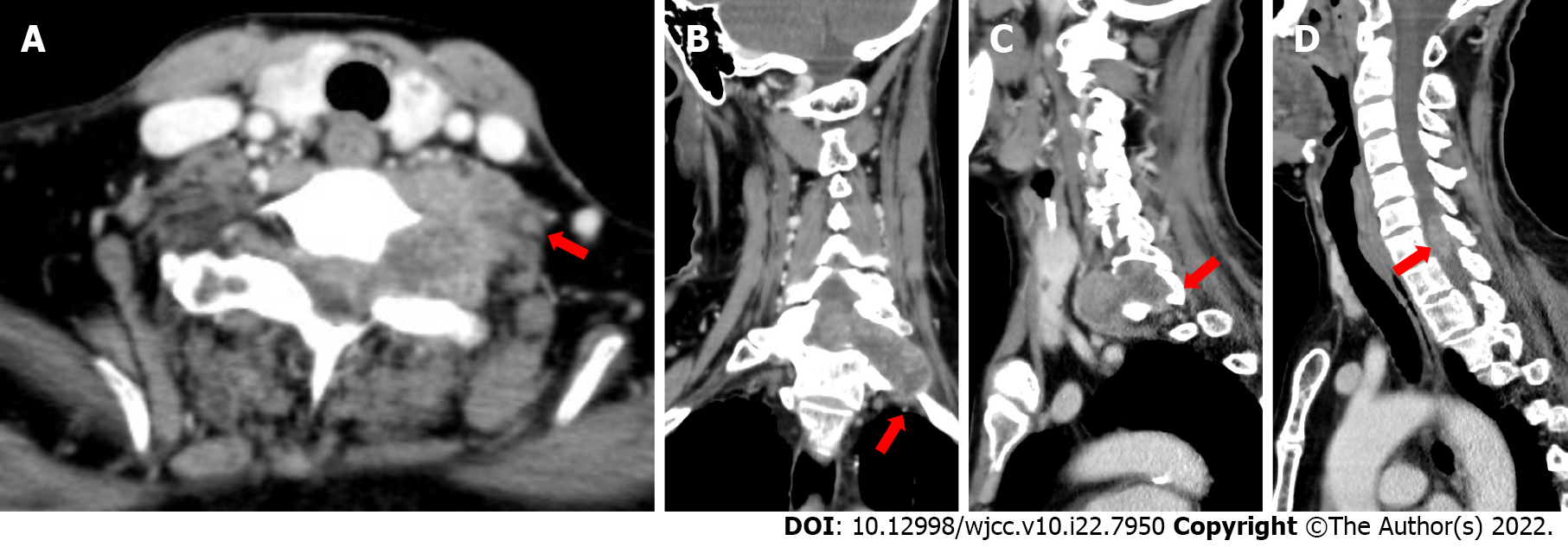
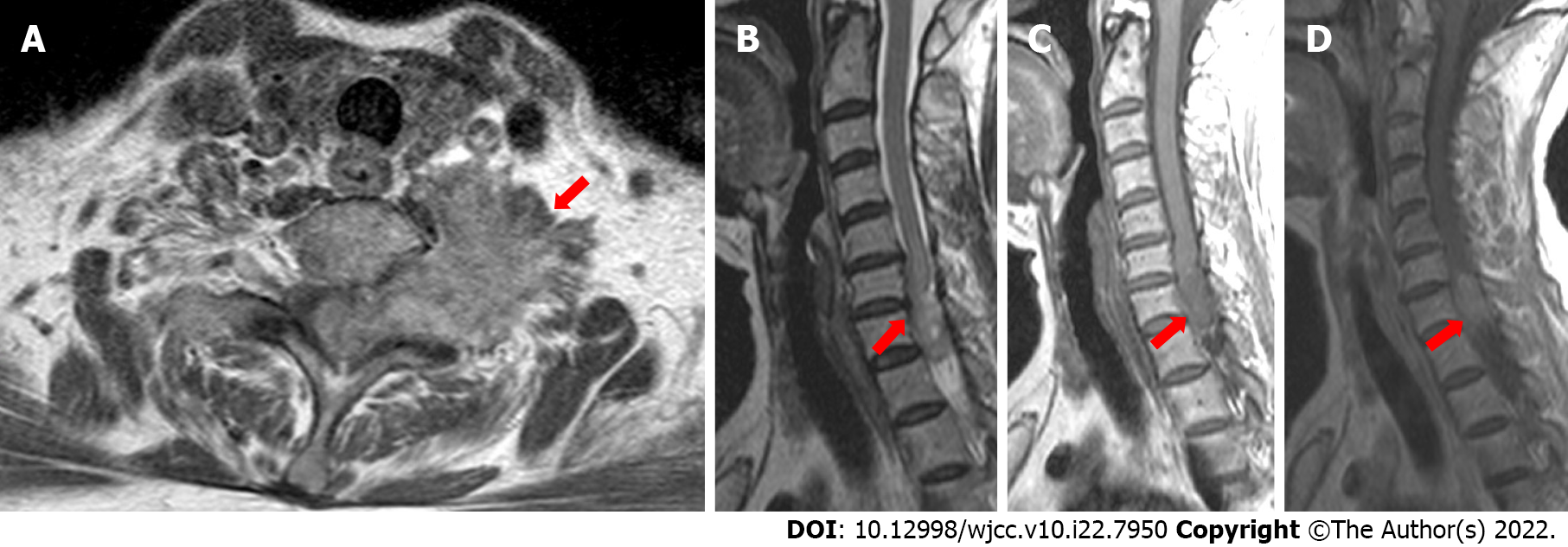
In the cervical computed tomography and magnetic resonance imaging (MRI) scans (Figures 2 and 3), irregular masses were observed inside and outside of the left foramina of the spinal canal, at C7-T1. The margins between the medial part and the spinal cord were not clear, whereas the lateral margin was rough, and the foramina was enlarged, with a significantly heterogeneous enhancement.
According to the results of physical examinations and imaging features, the physicians and radiologists made an initial diagnosis of a benign tumor, like schwannoma. A section surgery was recommended.
Based on the imaging features, radiologists, and multidisciplinary expert conclusion, the final diagnosis was schwannoma.
After the primary diagnosis, C7-T1 spinal canal tumor resection was performed under general anesthesia. During the procedure, the surgeons noticed edema from the C7-T1 spinal cord, with the residing tumor on the left side, which was obscuring the boundary of the spinal cord. The tumor crossed the foramina and was in proximity to the vertebral and internal carotid arteries and left lung tip. The C7 nerve root was observed passing through it, with a visible small amount of subarachnoid hemorrhage. After resection of the tumor (Figure 4A), histopathological findings revealed a primary spinal cord glioblastoma, World Health Organization (WHO) Grade IV (Figure 4B and C).
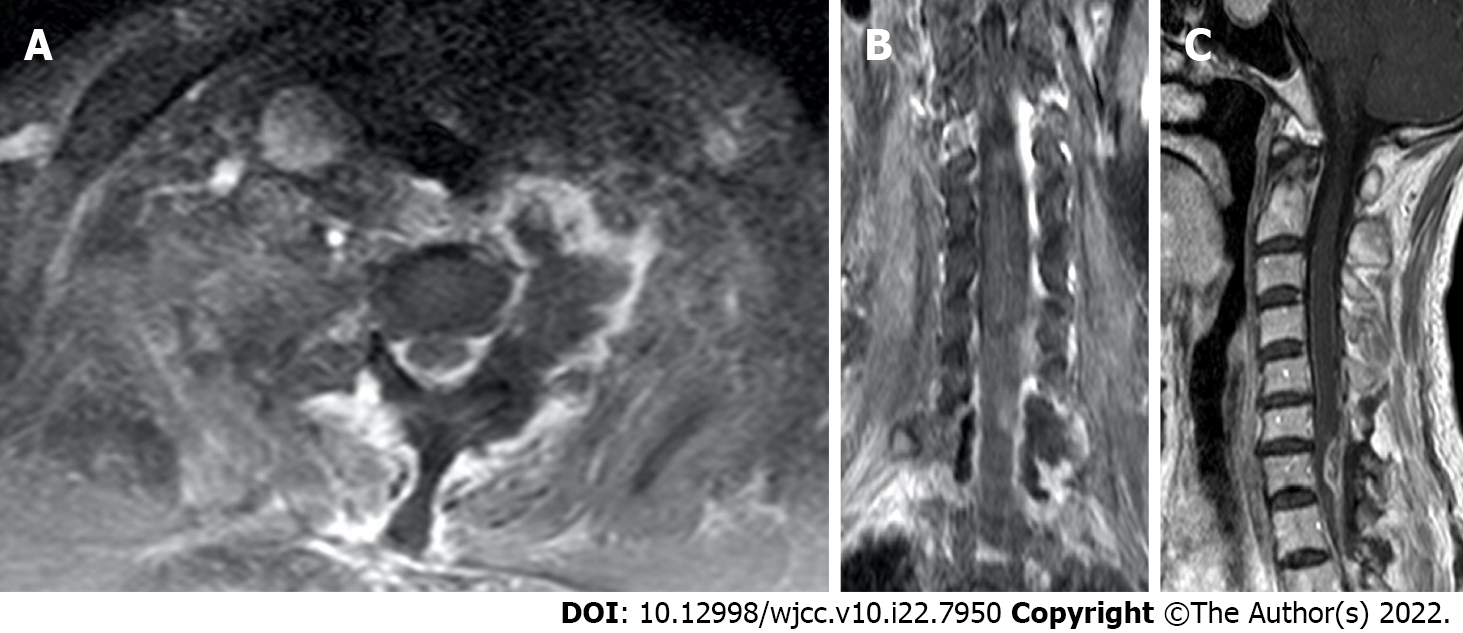
The patient underwent MRI re-examination 1 wk after surgery (Figure 5). She subsequently underwent a 4-wk course of radiotherapy (60 Gy in 20 fractions) combined with temozolomide chemotherapy.
For the next 6 mo, the patient’s upper limb muscle strength was Grade II, although lower limb muscle strength was Grade 0, with no relief in incontinence. However, the patient is still alive at this time (day of submission of this manuscript).
This is one of the rare cases of PSC GB that was characterized by tumor growth across and beyond the spinal canal, like a benign tumor, which led to the misdiagnosis of neurogenic tumors. To the best of our knowledge, an intramedullary glioma that extends beyond the spinal canal has not been reported before. Also, lesions reported previously were located in the spinal canal exclusively.
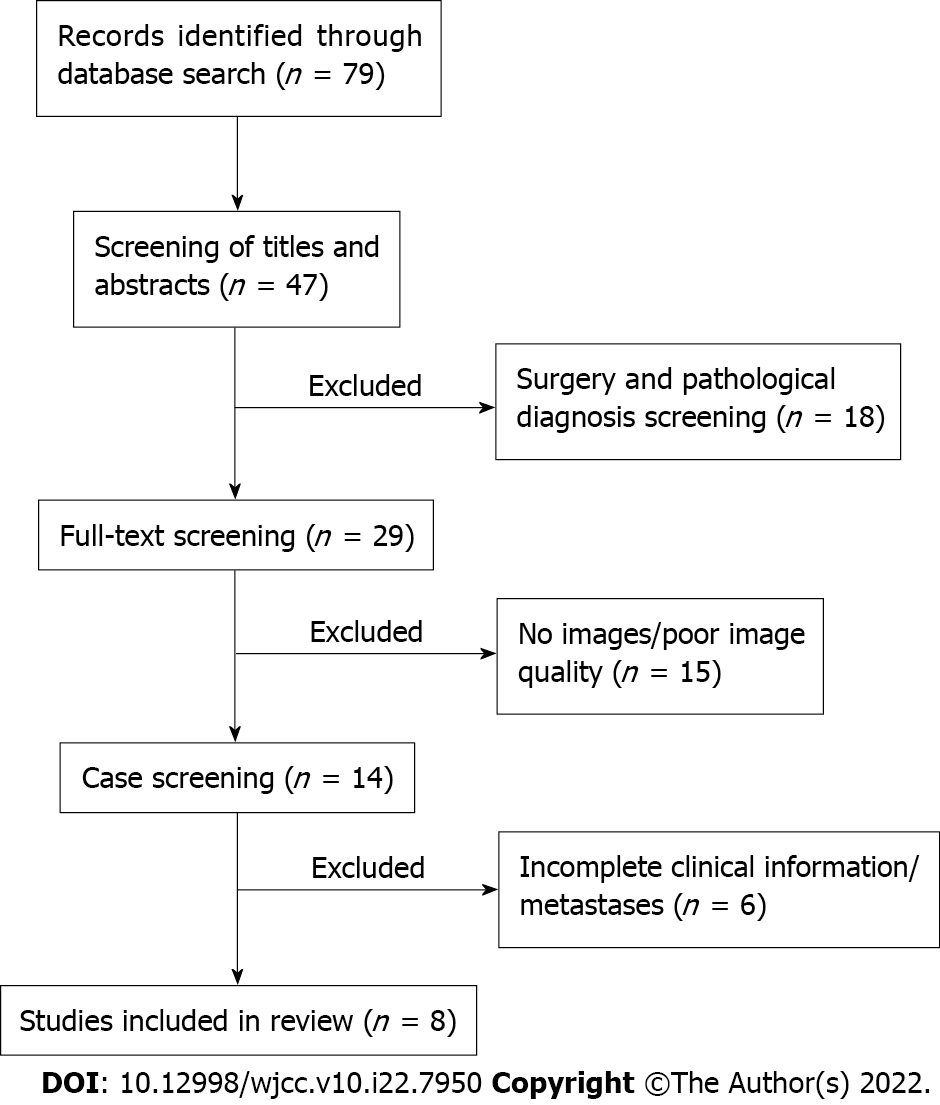
For conducting the extensive literature review, articles were selected in PubMed and Google Scholar for the most recent 10 years (2011-2021) by using search terms of “spinal cord glioblastoma,” “spinal cord malignant glioma,” and “spinal cord malignant neoplasm.” The search resulted in 79 publications, which were carefully screened by two authors (Liang XY and Zhou ZW) based on the following criteria: (1) Patients with surgically and pathologically confirmed PSC GB; (2) Patients with whole clinical information and high-quality preoperative computed tomography/MR images; and (3) Only literature available in English language. However, publications were excluded from the study if they reported: (1) PSC GB with other malignant or benign tumors; (2) Patients with secondary spinal cord GB who had previously undergone low-grade glioma resection; or (3) Patients who had been diagnosed with primary cerebral GB secondary metastases. The publications were also examined for patient clinical details and reported department to rule out duplicates. After the screening, 8 studies (involving 11 patients) fulfilled our requirements and were included in the analysis. The clinical and imaging characteristics of these 11 lesions are listed in Table 1. The detailed screening flow chart is shown in Figure 6.
| Case No. | Ref. | Age/sex | Symptoms | Lesion location | Intramedullary only | Imaging findings | Surgery/therapy | Survival in month |
| 1 | Ferrante et al[3], 2021 | 34 yr/F | MW, SD | Nearly entire cervical spinal cord and medulla oblongata | Yes | Isointense to hypointense on T1WI, isointense to hyperintense on T2WI, homogeneous enhancement | None | NR |
| 64 yr/M | MW, SD | Conus medullaris | Yes | Hyperintense on T2WI, peripheral enhancement, necrosis enhancing nodule | STR, radio/chemotherapy | 33 | ||
| 47 yr/M | Paresthesia, pain, MW, SD | Cervical and thoracic | Yes | Multifocal lesion (C5-C7) and (T3-T5), peripheral enhancement, necrotic center | Biopsy, radio/chemotherapy | 10 | ||
| 2 | Shen et al[4], 2020 | 47 yr/F | Paresthesia, paralysis | T12 | Yes | T12 nodular, isointense to hyperintense on T2WI, central significant enhancement | surgical resection, NR | NR |
| 3 | Delgado et al[5], 2019 | 21 yr/F | Pain, weakness, paresthesia | T10-T12, L1 | Yes | 60 mm expansile mass, T10-T12 down to L1, nodular significant enhancement | NTR, radio/chemotherapy | Recurrence in 4 mo |
| 4 | Chanchotisatien et al[6], 2019 | 27 yr/F | Dysuria, numbness, weakness, back Pain | T4-T12, T5-T12, respectively | No | Homogenous, hyperintense T2WI, intra-/extramedullary regions from T4-T12 and T5-T12, respectively, homogenous enhancement | Biopsy, radio/chemotherapy | NR |
| 5 | Caro-Osorio et al[7], 2018 | 48 yr/F | Numbness, pain | C1-C3 | Yes | Hypointense on T1WI, hyperintense on T2WI, heterogeneous enhancement | STR, radio/chemotherapy | 8 |
| 6 | Shen et al[1], 2017 | 15 yr/F | Numbness, weakness | C4-C7 | Yes | Hyperintense on T2WI, inhomogeneous enhancement | NTR, radio/chemotherapy | 13 |
| 7 | Nunn et al[12], 2017 | 31 yr/M | MW, SD | T9-T2 | Yes | Hyperintense on T2WI, spinal cord expanding, heterogeneous enhancement | Surgical resection, radio/chemotherapy | 14 |
| 8 | Prasad et al[13], 2012 | 15 yr/F | Paraparesis, dysesthesia, urinary incontinence, SD | C6-T7 | Yes | Enlargement of cord, heterogeneously enhancing | STR, radio/chemotherapy | 6 |
| 35 yr/M | Pain, paraplegia, urinary incontinence, SD | T11-L1 | Yes | Enlargement of cord, heterogeneously enhancing | NTR, radio/chemotherapy | 15 |
Generally, the typical imaging features of GB can be easily recognized by most radiologists. However, benign-looking masses, cystic lesions, multifocal/multicentric tumors, and spinal cord abnormalities may all represent features of GB[2]. Therefore, a precise preoperative neuroimaging diagnosis is much more difficult, owing to the high variability of PSC GB. MRI is widely used in early diagnosis and preoperative evaluation of PSC GBs as a non-destructive and high-qualified imaging modality[2,3]. Usually, PSC GBs appear as infiltrative and expansile intramedullary lesions, with T2 hyperintensity and T1 isointensity or hypointensity with different heterogeneous enhancement post-contrast on T1[2,3]. Both the location and shape of the tumor play significant roles in the radiological differential diagnosis of intramedullary lesions.
In our case, the tumor was detected as a dumbbell mass, spanning the intramedullary and extramedullary region through the foramina. This disguised appearance led us to an erroneous diagnosis of the neurogenic tumor. However, in the reviewed publications, the tumor had an appearance of nodular[3,4], elongated lesions, growing along the spinal cord and confined to the intramedullary area[1,5-7].
The distinction of PSC GB from pathologies like myelitis or other types of intramedullary tumors, such as astrocytomas or ependymomas, are most difficult due to its nonspecific and often very similar manifestations. The majority of ependymomas are often hyperintense on T2-weighted imaging with inhomogeneous enhancement after injection of gadolinium. Usually, ependymomas show high uptake of 18F-fluorodeoxyglucose, while myxopapillary ependymomas have low uptake of fluorodeoxyglucose[4]. Pilocytic astrocytomas often appear as a well-circumscribed mass with a highly enhanced T2 signal. Nonconventional MRI sequences, such as diffusion tensor images and dynamic susceptibility contrast perfusion weighted imaging, may provide more comprehensive information, which can be useful to differentiate and grade the lesion[8].
A normalized (referenced to brain region of interest) relative cerebral blood volume threshold of 1.75 has been used to predict GB progression. An increase in normalized relative cerebral blood volume (> 1.75) with a decrease in fractional anisotropy of the corresponding region of the tumor might suggest a high-grade glioma[8]. In our case, the tumor demonstrated atypical MRI and computed tomography appearances of PSC GB, although prior familiarity with these imaging features may help to differentiate from other intramedullary tumors.
PSC GB is extremely rare, reported in about 1.5% of all the spinal cord tumors[9]. Due to its rare occurrence, few studies focus on it. Most of the published literature on PSC GB are case reports. Therefore, due to the lack of properly related literature, the clinical features, optimal therapy, and prognosis of PSC GB remain controversial[10]. This tumor is more prone to grow in the cervical or cervicothoracic region and is associated with severe disability and poor prognosis in most patients[11]. The early symptoms of these patients are usually weakness of unilateral or bilateral limbs[1,3,5], sensorimotor disorders, and incontinence[6,12,13]. However, when accompanied by metastasis of other parts, the symptoms are usually different.
To the best of our knowledge, the largest sample study involving 190 PSC GB patients was reported by Moinuddin et al[14]. They found that PSC GB had a higher incidence in the younger age group (age < 18). In contrast, our patient’s age (70 years old) contributes to the rarity of the present case. They also reported a better overall survival in the 18-year-old to 65-year-old group (13.2 mo)[14]. Previous studies have also suggested that genetic mutations may be a contributing factor for the onset of the disease. It has been observed that patients with constitutional mismatch repair deficiency are more prone to develop high-grade gliomas in the central nervous system[15].
Due to its diverse and heterogeneous nature, PSC GB is difficult to diagnose merely by imaging techniques. Most PSC GB are confirmed by histopathological parameters, including nuclear atypia, mitotic activity, vascular proliferation, and necrosis[16,17], along with immunohistochemical parameters, such as glial fibrillary acidic protein and S-lfln protein 100 (S-100) positivity with a high Ki-67 index[16-18].
The tissue section of our patient revealed many pleomorphic astrocytic cells with marked nuclear atypia and brisk mitotic activity, which was accompanied by necrosis and microvascular proliferation. The diagnosis of WHO Grade IV GB is mainly based on the routine histopathological findings[16]. Immunohistochemically, the cells were slightly positive for glial fibrillary acidic protein, p53, and vimentin but were negative for S-100, isocitrate dehydrogenase, and histone H3 lysine 27 methylation (H3K27M). The proliferation index (measured by Ki67) was about 30% in the resected tumor. High-grade glioma with isocitrate dehydrogenase gene mutation and positive for H3K27M is reported to have a significantly better prognosis[17,19,20]. However, H3K27M-positive PSC GBs are also at risk of developing local hemorrhage[18]. Before the submission of the current manuscript, our patient was still alive after surgery and radio-/chemotherapy. The survival of our patient might be attributed to an H3K27M mutation.
Although not implying causation, there is a trend towards improved overall survival with the use of chemotherapy and surgical resection for patients suffering from GB[21]. Despite the advancement in surgical techniques and postoperative adjuvant therapies, PSC GB still has an overall poor prognosis[9,21]. The overall survival in patients with PSC GB is approximately 10-14 mo[9]. The disease often leads to severe neurological deficits and poor quality of life, even after treatment.
In our case, the patient underwent surgical resection with subsequent radiotherapy and chemotherapy. Unfortunately, neither treatment alleviated or improved her symptoms nor improved her quality of life. According to a single-center retrospective study by Cheng et al[22], the extent of surgical resection did not have any significance, but radiotherapy accompanied with postoperative chemotherapy was the major prognostic factor for longer survival in adult GB patients. However, the previous study reported that gross-total resection followed by radiotherapy had beneficial effects on the overall outcome of PSC GB in the pediatric age group[23,24]. More effective and targeted therapies need specific investigation in the future for helping patients with unique gene mutations achieve better prognosis[25,26]. Further, broadening treatment avenues would help improve the efficacy of therapies and help in recovery for these patients.
Due to the large mass in C7-T1, the conventional cervical MR field of view was not able to cover the whole lesion.
A definitive PSC GB diagnosis requires histopathological confirmation. However, complete recognition of the imaging signs of the disease may facilitate early accurate diagnosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Burger MC, Germany; Kołat D, Poland S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Shen CX, Wu JF, Zhao W, Cai ZW, Cai RZ, Chen CM. Primary spinal glioblastoma multiforme: A case report and review of the literature. Medicine (Baltimore). 2017;96:e6634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Khandwala K, Mubarak F, Minhas K. The many faces of glioblastoma: Pictorial review of atypical imaging features. Neuroradiol J. 2021;34:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Ferrante P, Mora JA, Salazar L, Sáez EM, Auger C, Rovira À. MR imaging findings in primary spinal cord glioblastoma. Radiol Case Rep. 2021;16:72-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Shen G, Ma H, Pan L, Su M, Kuang A. FDG PET/CT and MRI in Primary Spinal Cord Glioblastoma. Clin Nucl Med. 2020;45:e144-e145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Delgado BJ, Moosavi L, Rangel E, Stull W, Polineni RD, Chen J, Cobos E. An Unusual Presentation of Spinal Giant Cell Glioblastoma in a 21-Year-Old Female. J Investig Med High Impact Case Rep. 2019;7:2324709619868255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Chanchotisatien A, Xiong J, Yu J, Chu S. Exophytic Primary Intramedullary Spinal Cord Glioblastoma: Case Report and Critical Review of Literature. World Neurosurg. 2019;122:573-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Caro-Osorio E, Herrera-Castro JC, Barbosa-Quintana A, Benvenutti-Regato M. Primary Spinal Cord Small-Cell Glioblastoma: Case Report and Literature Review. World Neurosurg. 2018;118:69-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Liu X, Germin BI, Ekholm S. A case of cervical spinal cord glioblastoma diagnosed with MR diffusion tensor and perfusion imaging. J Neuroimaging. 2011;21:292-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Alharbi B, Alammar H, Alkhaibary A, Alharbi A, Khairy S, Alassiri AH, AlSufiani F, Aloraidi A, Alkhani A. Primary spinal cord glioblastoma: A rare cause of paraplegia. Surg Neurol Int. 2022;13:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Cheng L, Yao Q, Ma L, Duan W, Guan J, Zhang C, Wang K, Liu Z, Jian F, Wu H, Chen Z, Wang X, Wang Z. Predictors of mortality in patients with primary spinal cord glioblastoma. Eur Spine J. 2020;29:3203-3213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Lakhdar F, Benzagmout M, Chakour K, Chaoui FM. Primary bulbo-medullary glioblastoma in a child: case report. Childs Nerv Syst. 2019;35:2417-2421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Nunn A, Polyzoidis S, Piechowski-Jozwiak B, Brazil L, Ashkan K. Primary glioblastoma multiforme of the conus medullaris with leptomeningeal metastasis. J Neurol Sci. 2017;381:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Prasad GL, Borkar SA, Subbarao KC, Suri V, Mahapatra AK. Primary spinal cord glioblastoma multiforme: a report of two cases. Neurol India. 2012;60:333-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Moinuddin FM, Alvi MA, Kerezoudis P, Wahood W, Meyer J, Lachance DH, Bydon M. Variation in management of spinal gliobastoma multiforme: results from a national cancer registry. J Neurooncol. 2019;141:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Jarrar SM, Daoud SS, Jbarah OF, Albustami IS, Daise MA. Primary cervical glioblastoma multiforme as a presentation of constitutional mismatch repair deficiency: Case report and literature review. Ann Med Surg (Lond). 2021;64:102263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Song D, Xu D, Gao Q, Hu P, Guo F. Intracranial Metastases Originating From Pediatric Primary Spinal Cord Glioblastoma Multiforme: A Case Report and Literature Review. Front Oncol. 2020;10:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Yi S, Choi S, Shin DA, Kim DS, Choi J, Ha Y, Kim KN, Suh CO, Chang JH, Kim SH, Yoon DH. Impact of H3.3 K27M Mutation on Prognosis and Survival of Grade IV Spinal Cord Glioma on the Basis of New 2016 World Health Organization Classification of the Central Nervous System. Neurosurgery. 2019;84:1072-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 18. | Akinduro OO, Garcia DP, Higgins DMO, Vivas-Buitrago T, Jentoft M, Solomon DA, Daniels DJ, Pennington Z, Sherman WJ, Delgardo M, Bydon M, Kalani MA, Zanazzi G, Tsankova N, Bendok BR, McCormick PC, Sciubba DM, Lo SL, Clarke JL, Abode-Iyamah K, Quiñones-Hinojosa A. A multicenter analysis of the prognostic value of histone H3 K27M mutation in adult high-grade spinal glioma. J Neurosurg Spine. 2021;35:834-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4838] [Cited by in RCA: 4496] [Article Influence: 281.0] [Reference Citation Analysis (0)] |
| 20. | Lu VM, Alvi MA, McDonald KL, Daniels DJ. Impact of the H3K27M mutation on survival in pediatric high-grade glioma: a systematic review and meta-analysis. J Neurosurg Pediatr. 2018;23:308-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 21. | Haque W, Verma V, Barber S, Tremont IW, Brian Butler E, Teh BS. Management, outcomes, and prognostic factors of adult primary spinal cord gliomas. J Clin Neurosci. 2021;84:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Darbari S, Manjunath N, Doddamani RS, Meena R, Nambirajan A, Sawarkar D, Singh PK, Garg K, Chandra PS, Kale SS. Primary spinal cord glioblastoma multiforme: a single-center experience. Br J Neurosurg. 2022;1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Konar SK, Bir SC, Maiti TK, Nanda A. A systematic review of overall survival in pediatric primary glioblastoma multiforme of the spinal cord. J Neurosurg Pediatr. 2017;19:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Yang R, Isaacs AM, Cadieux M, Hirmer TJ, CreveCoeur TS, Lapointe AP, Opoku-Darko M, Premji Z, Riva-Cambrin J, Gallagher CN. Primary and metastatic glioblastoma of the spine in the pediatric population: a systematic review. Childs Nerv Syst. 2021;37:1849-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Grady C, Melnick K, Porche K, Dastmalchi F, Hoh DJ, Rahman M, Ghiaseddin A. Glioma Immunotherapy: Advances and Challenges for Spinal Cord Gliomas. Neurospine. 2022;19:13-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | He JH, Wang J, Yang YZ, Chen QX, Liu LL, Sun L, Hu WM, Zeng J. SSTR2 is a prognostic factor and a promising therapeutic target in glioma. Am J Transl Res. 2021;13:11223-11234. [PubMed] |