Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7592
Peer-review started: February 23, 2022
First decision: March 25, 2022
Revised: March 29, 2022
Accepted: June 18, 2022
Article in press: June 18, 2022
Published online: July 26, 2022
Processing time: 137 Days and 20.4 Hours
Tension pneumothorax of the contralateral lung during single-lung ventilation (SLV) combined with artificial pneumothorax can cause cardiac arrest due to bilateral pneumothorax. If not rapidly diagnosed and managed, this condition can lead to sudden death. We describe the emergency handling procedures and rapid diagnostic methods for this critical emergency situation.
We report a case of bilateral pneumothorax in a neonatal patient who underwent thoracoscopic esophageal atresia and tracheoesophageal fistula repair under the combined application of SLV and artificial pneumothorax. The patient suffered sudden cardiac arrest and received emergency treatment to revive her. The recognition of dangerous vital sign parameters, rapid evacuation of the artificial pneumothorax, and initiation of lateral position cardiopulmonary resuscitation while simultaneously removing the endotracheal tube to the main airway are critically important. Moreover, even though the sinus rhythm was restored, the patient’s continued tachycardia, reduced pulse pressure, and depressed pulse oximeter waveform were worrisome. We should highly suspect the possibility of pneumothorax and use rapid diagnostic methods to make judgment calls. Sometimes thoracoscopy can be used for rapid examination; if the mediastinum is observed to be shifted to the right, it may indicate tension pneumothorax. This condition can be immediately relieved by needle thoracentesis, ultimately allowing the safe completion of the surgical procedure.
Bilateral pneumothorax during SLV combined with artificial pneumothorax is rare but can occur at any time in neonatal thoracoscopic surgery. Therefore, anesthesiologists should consider this possibility, be alert, and address this rare but critical complication in a timely manner.
Core Tip: Tension pneumothorax on the contralateral lung during single-lung ventilation (SLV) combined with artificial pneumothorax can lead to cardiac arrest due to bilateral pneumothorax. If not rapidly diagnosed and managed, it can lead to sudden death. Bilateral pneumothorax during SLV combined with artificial pneumothorax is rare but can occur at any time in neonatal thoracoscopic surgery. We describe the emergency handling procedures and rapid diagnostic methods for this critical emergency situation.
- Citation: Zhang X, Song HC, Wang KL, Ren YY. Considerations of single-lung ventilation in neonatal thoracoscopic surgery with cardiac arrest caused by bilateral pneumothorax: A case report. World J Clin Cases 2022; 10(21): 7592-7598
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7592.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7592
The thoracoscopic repair of esophageal atresia (EA) and tracheoesophageal fistula (TEF) has gained increased acceptance, but it is a highly technical and challenging procedure[1]. To expand the surgical space, the nondependent lung is intentionally collapsed using surgically guided intrathoracic carbon dioxide (CO2) insufflation (artificial pneumothorax, AP) combined with single-lung ventilation (SLV)[2]. However, for neonates, the combination of these two approaches can potentially pose serious pathophysiological changes. The most common complications of thoracoscopic repair include the development of high airway pressure, hypoxemia and hypercapnia[3]. The present case report details a neonatal patient who underwent thoracoscopic EA/TEF repair using the combination of AP and SLV, which was complicated by the development of bilateral pneumothorax and cardiac arrest.
A preterm female born at 36 wk gestation received nCPAP-assisted ventilation due to dyspnea, during which it was difficult to place an indwelling nasogastric tube.
The neonatal patient was delivered by cesarean section at 36 wk due to intrauterine distress and reduced flow in the umbilical artery. The patient gradually developed dyspnea for 10 min after birth, which was characterized by shortness of breath and cyanosis, and the neonate was then transferred to the Neonatal Intensive Care Unit (NICU). She was given nCPAP-assisted ventilation, during which it was difficult to place an indwelling nasogastric tube. Due to the history of amniotic fluid, EA was not excluded.
The patient had no history of other illnesses.
The patient had no family history of illness.
The physical examination revealed a premature infant's appearance and poor physical development (height, 40 cm; weight, 1.93 kg), a blood pressure of 75/45 mmHg, a pulse rate of 140 beats per min, and a respiratory rate (RR) of 46 breaths/min. Her oxygen saturation on a pulse oximeter was 93% in room air.
Blood tests showed an Hb level of 14.5 g/dL, hematocrit (Hct) of 75%, platelet count of 187 × 109 cells/L, leukocyte count of 16.83 × 109/L, and C-reactive protein of 5.68 mg/L. Other blood tests showed no significant abnormalities.
Upper gastrointestinal radiography (a small amount of contrast agent was injected through the orogastric tube) showed that the middle and upper esophagus was markedly dilated, the proximal blind end of the esophagus was located at the level of the third thoracic vertebra, and there was significantly less physiological gas accumulation in the abdomen (Figure 1). Chest computed tomography suggested neonatal pneumonia and no pleural effusion. Echocardiography showed no obvious abnormalities.
She was initially diagnosed with type III EA, which is the most common proximal EA, with a distal TEF.
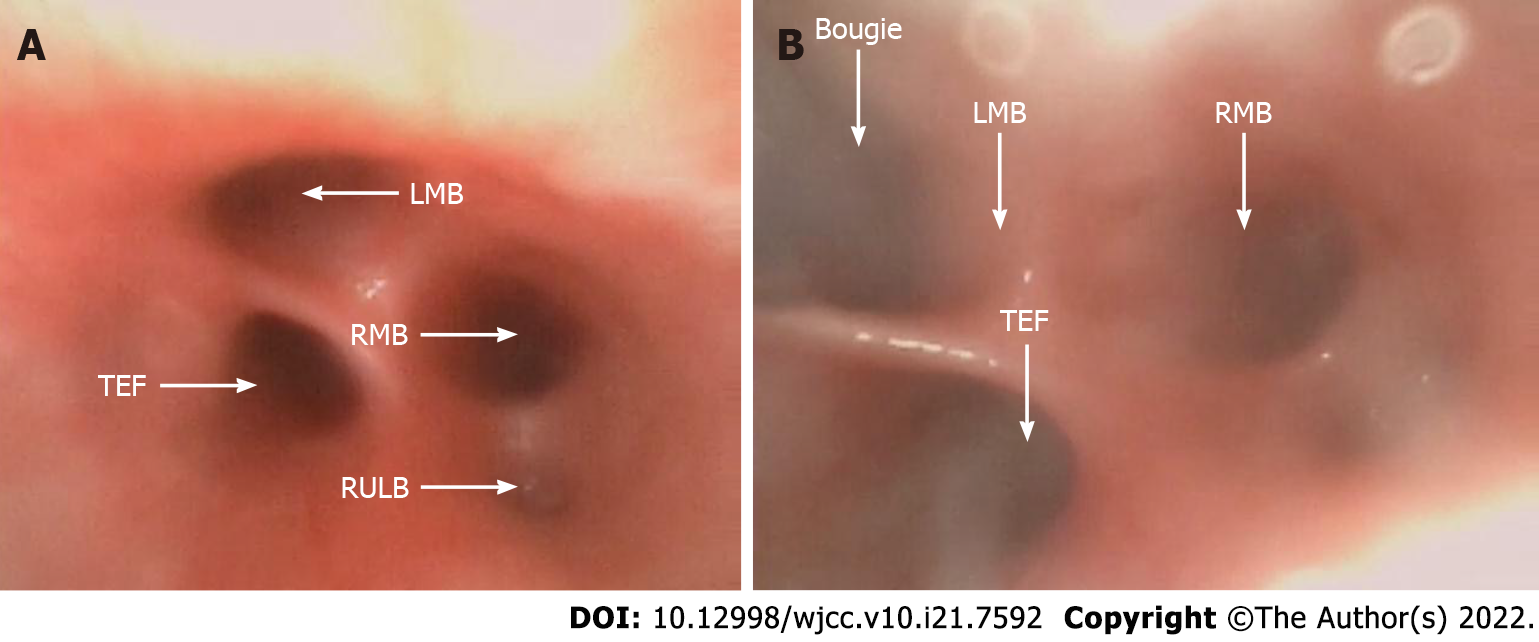
The patient underwent surgery on the third day after her birth. In preparation for thoracoscopic repair, the patient first was allowed to continue to spontaneously breathe during general anesthesia with 6% sevoflurane carried by 100% oxygen through a face mask. A 2.8-mm fiberoptic bronchoscope (FOB) was inserted into the airway orally through a swivel adaptor connected to the mask. The fistula was located proximal to the carina and trifurcation (Figure 2A). Then, a bougie was introduced into the left mainstem bronchus under the FOB (Figure 2B), and a 3.0 mm cuffed endotracheal tube (ETT) was positioned into the left mainstem bronchus using the bougie as a guide. We confirmed the SLV by chest auscultation, as the 2.8-mm FOB could not pass through the ETT. One milligram of rocuronium was then administered intravenously, left radial artery cannulation and right internal jugular vein catheterization were performed, and she was placed in the left lateral decubitus position. AP was established by insufflation of CO2 into the right thoracic cavity at a pressure of 4 mmHg. Mechanical ventilation was conducted with a pressure control of 24 cmH2O, a RR of 28 breaths/min, and a positive end expiratory pressure 3 cmH2O.
The end-tidal CO2 (ETCO2) level continuously rose during surgery. When the operative procedure started at 1 h, the arterial partial pressure of CO2 (PaCO2) was 62 mmHg. The control pressure was gradually increased to 30 mmHg, and the AP pressure was reduced to 2 mmHg to meet the tidal volume requirements. Two hours into the operation, a further increase in the PaCO2 to 85 mmHg prompted communication with the surgeon. At this time, the fistula was ligated, and the surgeon performed an end-to-end anastomosis during the operation.
The surgeon thought that the distance between the two ends of the esophagus was small, so the success rate of the operation was very high. To increase the tidal volume and RR, manual ventilation with 100% oxygen was instituted, which also washed out the retained CO2 after two minutes of using recruitment maneuvering techniques. During the establishment of AP and CO2 insufflation, the patient suffered sudden cardiac arrest, with her heart rate, arterial blood pressure and pulse oximeter suddenly flattening on the monitor.
The CO2 in the right thoracic cavity was immediately evacuated by opening the trocar, and the surgeon initiated lateral cardiac compressions, with one palm against the child's back and by using two-finger anterior chest compressions. With an intravenous injection of 10 μg of epinephrine, a normal sinus heart rhythm was restored. However, the monitor showed an HR of 180 beats/min, an ABP of 53/47 mmHg with reduced pulse pressure, and a poor pulse oximetry waveform. Thoracoscopy showed that the mediastinal structures were shifted to the right, strongly indicating the presence of a left tension pneumothorax.
We examined the ETT and anesthesia breathing circuit and then removed the ETT from the main airway. Auscultation of bilateral lung breath sounds showed that the breath sounds on the left were weak, while the breath sounds on the right were strong. The right thoracoscopic incision was covered with gauze, and the patient was moved to the supine position. A needle thoracocentesis was performed with a 22 G intravenous indwelling needle in the second left interspace midclavicular line, which caused immediate improvement in the ABP and pulse oximeter waveforms. After approximately 50 mL of gas had been extracted, the indwelling needle was replaced with a standard chest drain. The patient's vital signs then returned to normal, with an ABP of 58/39 mmHg, a HR of 165 beats/min, and a pulse oximeter reading of 93%. With the restoration of stable hemodynamics, the thoracoscopy was converted to thoracotomy and was successfully completed.
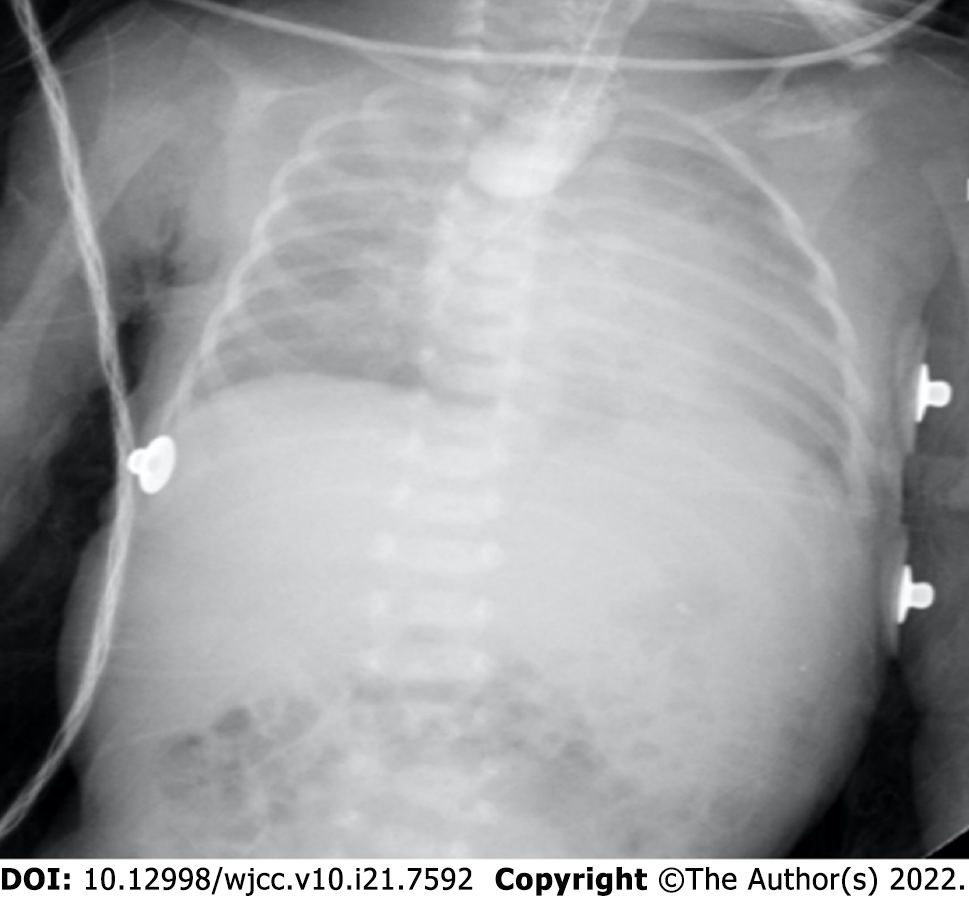
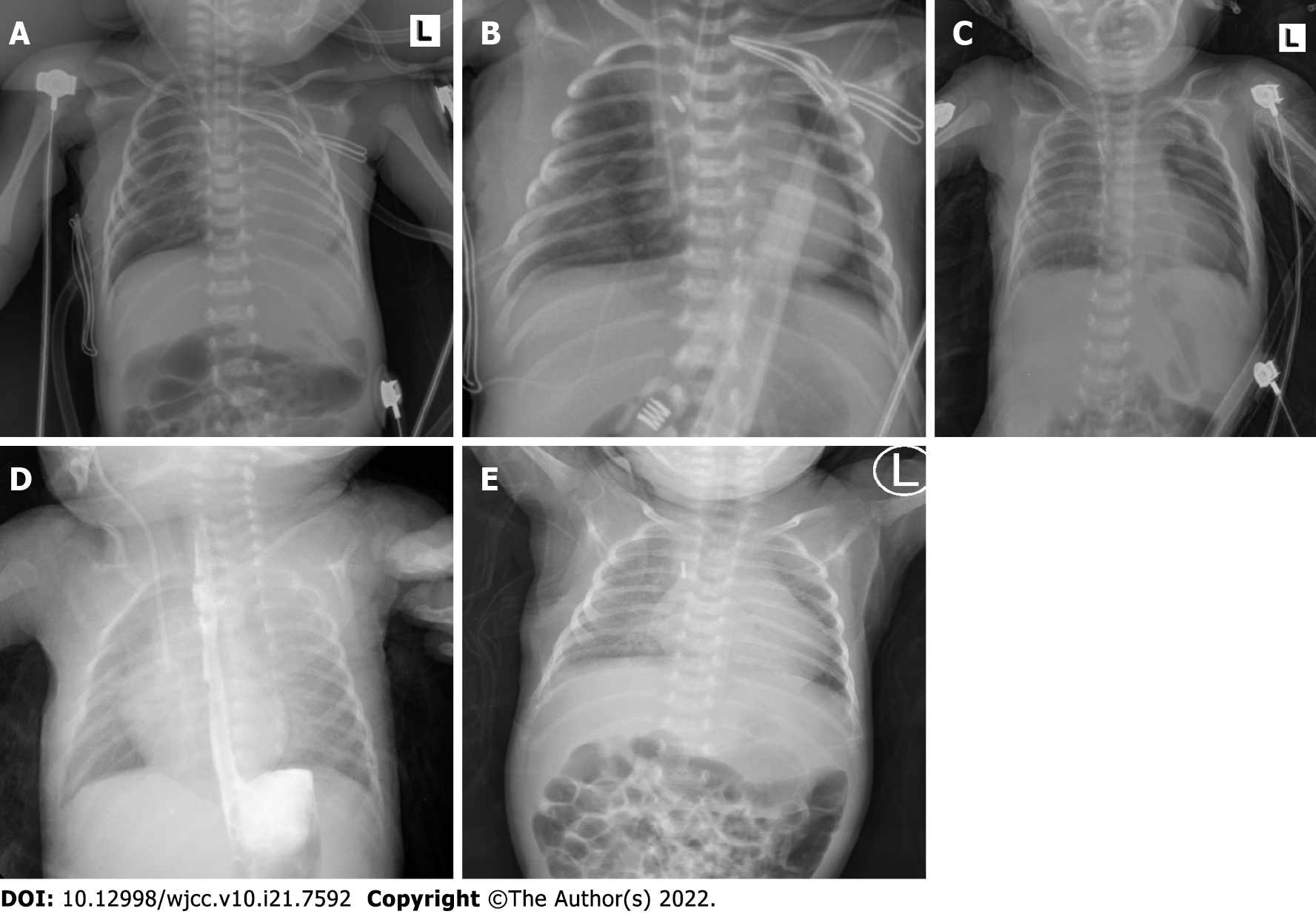
After surgery, the patient was immediately transferred to the cardiac intensive care unit under endotracheal intubation. The chest X-ray revealed partial atelectasis of the left lung after the operation (Figure 3A). The patient was treated with positive pressure ventilation. The chest X-ray taken on the first postoperative day revealed good re-expansion of the left lung (Figure 3B). Bedside ultrasonography was performed, and no pleural effusion was found. The ETT was removed on the fifth postoperative day. Because the patient developed recurrent pneumothorax and pulmonary exudation, the air leakage persisted, and the patient's right chest tube was removed on the 12th day after the operation. The chest X-ray taken on the 13th postoperative day showed that a pneumothorax had developed again on the left side (Figure 3C); therefore, the left chest tube was not removed until the 15th postoperative day. Then, she was transferred out of the NICU. Postoperative upper gastrointestinal radiography showed that the surgical effect was good (Figure 3D). The patient had a repeat chest X-ray, which showed marked improvement, and she was subsequently discharged (Figure 3E).
Neonatal pneumothorax is an uncommon event during the perioperative period. There are many risk factors for pneumothorax in neonates, such as prematurity, neonatal respiratory distress syndrome, pneumonia and positive pressure ventilation[4]. In the present case, the patient care was complicated by congenital pneumonia, and she received CPAP treatment before surgery. To expand the surgical space, we used a combination of AP and SLV. The methods available for neonatal SLV are limited[5]. The smallest FOB (2.8 mm) available in our hospital was too thick to use the ETT. We chose a compromise method, by using the fiberoptic bronchoscope to guide the bougie into the left main bronchus and then through the bougie into the ETT. It can be more accurate to place the ETT into the left main bronchus in order to avoid the fistula. At the start of surgery, the patient was placed in the left lateral decubitus position with AP in the right lung and left SLV. Unexpectedly, she developed bilateral pneumothorax, which resulted in cardiac arrest. If right-sided AP was not used, the tension pneumothorax on the left may not have led to sudden cardiac arrest. A possible cause of the left pneumothorax was the higher-pressure ventilation from the single bronchus intubation and SLV. Niwas et al[6] reported that neonatal pneumothorax cases mainly occurred on the right side (72.5%), and 67% of these cases were related to endotracheal intubation of the single bronchus. Another potential cause was the use of intraoperative manual ventilation and continuous strong positive pressure ventilation with the consequent rupture of the left pulmonary alveoli, leading to a tension pneumothorax. In previous case reports, many cases of tension pneumothorax during SLV in adults have been associated with barotrauma[7]. During SLV, the use of recruitment maneuvering techniques at high pressures can lead to barotraumatic and even contralateral tension pneumothorax[8]. In addition, another possible cause was the use of an intubation bougie to guide single bronchus intubation. Literature reports have indicated that the use of intubation bougies in neonates may cause a pneumothorax. Kumar and Walker reported a case of pneumothorax as a result of airway perforation by using a bougie[9]. Two other cases of neonatal pneumothorax reported by Parekh et al[10] were suspected to be caused by trauma when a bougie is used to guide endotracheal intubation.
Normally, the clinical manifestations of tension pneumothorax in neonates receiving mechanical ventilation include immediate increased airway pressure, progressively decreased pulse oximeter readings, decreased heart rate, sometimes decreased or disappearing end tidal CO2 concentrations, and loss of breath sounds of the affected side[11]. However, some findings are generally considered to be reasonable in neonatal thoracoscopy, such as decreased HR, ABP, pulse oximeter readings, and a narrowing of pulse pressure. In this case, the patient developed tension pneumothorax and immediately experienced cardiac arrest. If the situation permits, in addition to auscultation, chest transillumination and lung ultrasound are also fast and effective methods for the diagnosis of pneumothorax[12,13].
In this case, the blood gas analysis of the patient showed that her PaCO2 exceeded 85 mmHg and that she developed hypercapnia. By suspending the surgery, we increased the RR and tidal volume to quickly expel CO2 from the alveolar spaces. Severe acidosis, mainly due to hypercapnia caused by the application of CO2 to develop an AP, is a common feature and regarded as a significant drawback of neonatal thoracoscopy[14,15]. However, several studies have shown that the decrease in SaO2 and pH and the increase in PaCO2 are reversible during neonatal thoracoscopy and AP[14,15,16]. The regional cerebral oxygen saturation (rSO2) remained stable, suggesting no hampering of cerebral oxygenation by thoracoscopic surgery. In one study, further follow-up showed that neurodevelopmental outcomes of these neonates were favorable in the first 24 months[14]. In general, no matter what happens to the other blood gas parameters, maintaining a normal rSO2 is very important to prevent brain injury.
The cause of cardiac arrest in this patient was mediastinal displacement and compression of the heart and large blood vessels due to bilateral tension pneumothorax. We immediately started chest compressions with the patient in the lateral position and gave the patient an intravenous injection of epinephrine. At the same time, rapid evacuation of CO2 and removal of the ETT in the main airway were also critically important. Moreover, even though a normal sinus rhythm was restored, the patient’s continued tachycardia, reduced pulse pressure, and depressed pulse oximeter waveform were still worrisome. In these types of cases should highly suspect the possibility of pneumothorax and use rapid diagnostic methods to make judgment calls. Sometimes thoracoscopy can be used for rapid examination; if the mediastinum is observed to have shifted to the right, tension pneumothorax may be present. This condition is immediately relieved by needle thoracentesis, which ultimately allows for safe completion of the surgical procedure.
Pneumothorax is rare but can occur at any time during neonatal thoracoscopic surgery. In addition to the common risk factors for neonatal pneumothorax, AP and SLV are two special risk factors that can lead to the possibility of bilateral pneumothorax. Therefore, anesthesiologists should consider and be alert to the occurrence of bilateral pneumothorax in neonatal thoracoscopic surgery. If not found and managed adequately in a rapid manner, the conditions can lead to cardiac arrest and sudden death.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lee S, South Korea; Tajiri K, Japan S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Wu Y, Kuang H, Lv T, Wu C. Comparison of clinical outcomes between open and thoracoscopic repair for esophageal atresia with tracheoesophageal fistula: a systematic review and meta-analysis. Pediatr Surg Int. 2017;33:1147-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Kashyap L, Nisa N, Chowdhury AR, Khanna P. Safety issues of endobronchial intubation for one-lung ventilation in video-assisted thoracoscopic surgery in neonates: Can we extubate on the table? Saudi J Anaesth. 2017;11:254-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Zani A, Lamas-Pinheiro R, Paraboschi I, King SK, Wolinska J, Zani-Ruttenstock E, Eaton S, Pierro A. Intraoperative acidosis and hypercapnia during thoracoscopic repair of congenital diaphragmatic hernia and esophageal atresia/tracheoesophageal fistula. Paediatr Anaesth. 2017;27:841-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Vibede L, Vibede E, Bendtsen M, Pedersen L, Ebbesen F. Neonatal Pneumothorax: A Descriptive Regional Danish Study. Neonatology. 2017;111:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Schmidt C, Rellensmann G, Van Aken H, Semik M, Bruessel T, Enk D. Single-lung ventilation for pulmonary lobe resection in a newborn. Anesth Analg. 2005;101:362-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Niwas R, Nadroo AM, Sutija VG, Gudavalli M, Narula P. Malposition of endotracheal tube: association with pneumothorax in ventilated neonates. Arch Dis Child Fetal Neonatal Ed. 2007;92:F233-F234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Lee SK, Seo KH, Kim YJ, Youn EJ, Lee JS, Park J, Moon HS. Cardiac arrest caused by contralateral tension pneumothorax during one-lung ventilation: - A case report. Anesth Pain Med (Seoul). 2020;15:78-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Hoechter DJ, Speck E, Siegl D, Laven H, Zwissler B, Kammerer T. Tension Pneumothorax During One-Lung Ventilation - An Underestimated Complication? J Cardiothorac Vasc Anesth. 2018;32:1398-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Sakhuja P, Ferenc BA, Fierens I, Garg A, Ratnavel N. Association of pneumothorax with use of a bougie for endotracheal intubation in a neonate. J Paediatr Child Health. 2019;55:376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Parekh UR, Maguire AM, Emery J, Martin PH. Pneumothorax in neonates: Complication during endotracheal intubation, diagnosis, and management. J Anaesthesiol Clin Pharmacol. 2016;32:397-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Huang TJ, Ahmed A, D'Souza D, Awad H. Delayed diagnosis of contralateral tension pneumothorax during robotic lung wedge resection. J Clin Anesth. 2018;45:30-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Sharma D, Murki S, Pratap T. Point of care in nursery to diagnose pneumothorax in neonates by new use of LED torch. Med Dr. D.Y. Patil Univers. 2016;9:124-126. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Fei Q, Lin Y, Yuan TM. Lung Ultrasound, a Better Choice for Neonatal Pneumothorax: A Systematic Review and Meta-analysis. Ultrasound Med Biol. 2021;47:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Bishay M, Giacomello L, Retrosi G, Thyoka M, Garriboli M, Brierley J, Harding L, Scuplak S, Cross KM, Curry JI, Kiely EM, De Coppi P, Eaton S, Pierro A. Hypercapnia and acidosis during open and thoracoscopic repair of congenital diaphragmatic hernia and esophageal atresia: results of a pilot randomized controlled trial. Ann Surg. 2013;258:895-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 15. | Costerus S, Vlot J, van Rosmalen J, Wijnen R, Weber F. Effects of Neonatal Thoracoscopic Surgery on Tissue Oxygenation: A Pilot Study on (Neuro-) Monitoring and Outcomes. Eur J Pediatr Surg. 2019;29:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Tytgat SH, van Herwaarden MY, Stolwijk LJ, Keunen K, Benders MJ, de Graaff JC, Milstein DM, van der Zee DC, Lemmers PM. Neonatal brain oxygenation during thoracoscopic correction of esophageal atresia. Surg Endosc. 2016;30:2811-2817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |