Published online Jun 16, 2022. doi: 10.12998/wjcc.v10.i17.5899
Peer-review started: January 22, 2022
First decision: February 24, 2022
Revised: March 3, 2022
Accepted: April 4, 2022
Article in press: April 4, 2022
Published online: June 16, 2022
Liver metastasis of duodenal gastrointestinal stromal tumor (GIST) is rare. Most reports mainly focus on its treatment and approaches to surgical resection, while details on its contrast-enhanced ultrasound (CEUS) findings are lacking. The diagnosis and imaging modalities for this condition remain challenging.
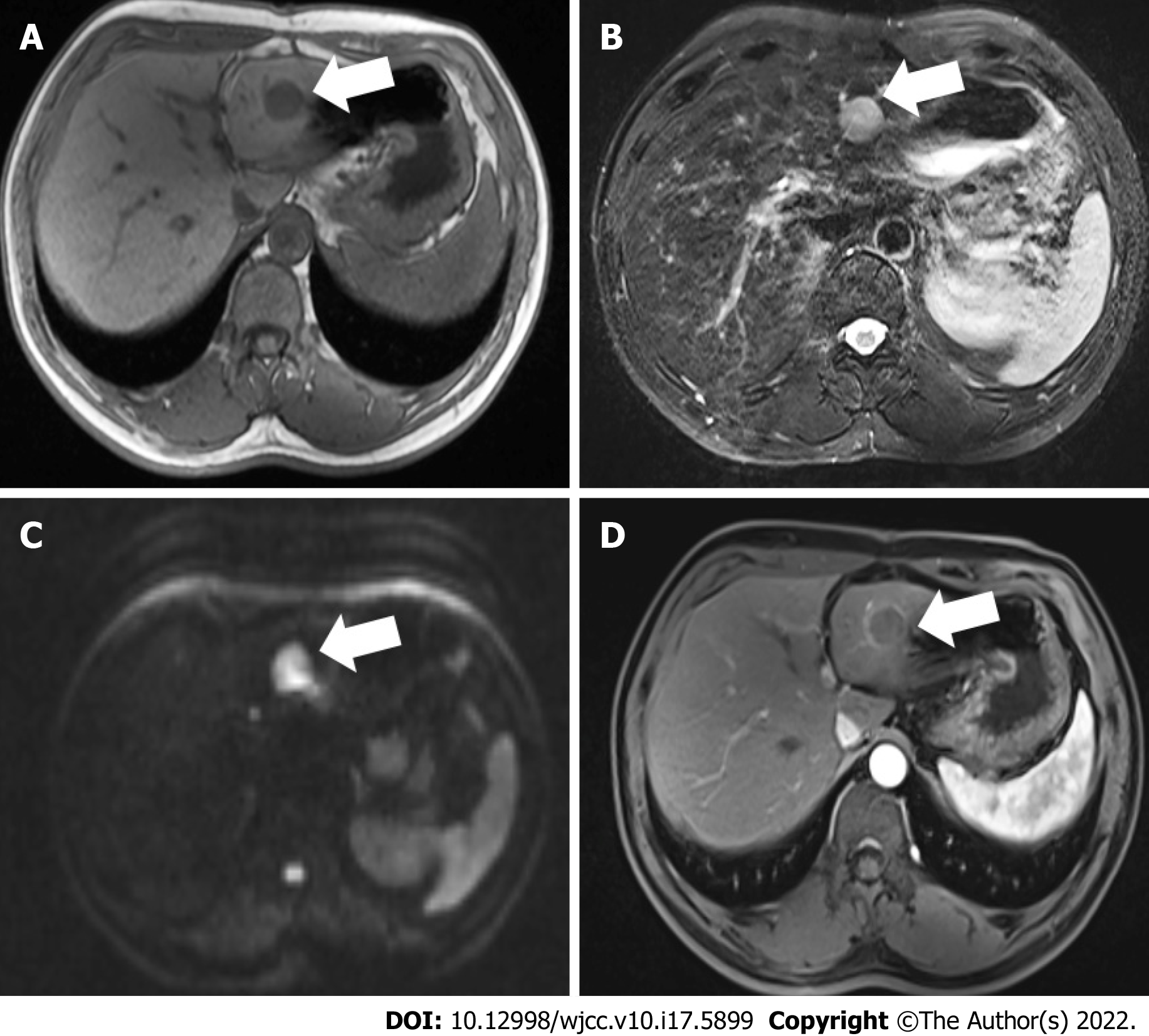
A 53-year-old Chinese man presented with mild signs and symptoms of the digestive tract. He underwent routine examinations after GIST surgery. Magnetic resonance imaging showed a 2.3 cm hepatic space-occupying lesion. All the laboratory test results were within normal limits. For further diagnostic con
H-CEUS is useful for detecting microcirculation differences, wash-in patterns, and vascular morphogenesis and diagnosing liver metastasis of duodenal GIST.
Core Tip: Gastrointestinal stromal tumors (GISTs) are the most common types of gastrointestinal mesenchymal tumors. The liver is considered the most common organ target of metastasis; however, liver metastasis of duodenal GIST is extremely rare. We describe a new imaging modality, high frame rate contrast-enhanced ultrasound, for detecting microcirculation differences in the lesion, wash-in patterns during the early arterial phase, and vascular morphogenesis due to its high frame rate, during which liver metastasis of duodenal GIST can be diagnosed accurately. No complications were observed in our patient. We recommend this new technology for the diagnosis of liver metastasis of duodenal GIST.
- Citation: Chen JH, Huang Y. High-frame-rate contrast-enhanced ultrasound findings of liver metastasis of duodenal gastrointestinal stromal tumor: A case report and literature review. World J Clin Cases 2022; 10(17): 5899-5909
- URL: https://www.wjgnet.com/2307-8960/full/v10/i17/5899.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i17.5899
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors originating from the digestive tract, and they account for 1%-3% of gastrointestinal tumors. Their clinical manifestations have not been established, and no specific tumor markers have been reported; approximately 15%-50% of GISTs are found due to liver metastasis[1]. Duodenal GISTs are rare, accounting for 12%-18% of small intestine GISTs and 1%-4% of all GISTs, and only 6 cases of liver metastasis of duodenal GISTs have been reported[2-7]. Usually, computed tomography (CT) scan, ultrasound endoscopy, and digestive tract contrast can help in the evaluation of the size, local immersion, and metastasis and location of the GIST[8]. Herein, we report a case of metastatic duodenal GIST that was initially accurately diagnosed by high frame rate contrast-enhanced ultrasound (H-CEUS). This report is intended to introduce a new imaging technology, which could not only provide important information in diagnosing rare disease liver metastasis of duodenal GIST but may also contribute to the diagnosis of many other hepatic lesions.
A 53-year-old Chinese man was admitted to our hospital with a progressively enlarged liver lesion for 2 years.
The lesion in the liver had been found during a routine re-examination after surgery two years earlier, and no significant progression was observed during subsequent annual examinations until three months earlier. The maximum diameter of the lesion had grown from 1.3 cm to 3.5 cm within a year, accompanied by mild abdominal discomfort. The patient reported to our hospital for further diagnosis.
The patient had a 30-year history of fatty liver but no history of hepatitis and liver cirrhosis and had undergone duodenal stromal tumor resection twice in our hospital in March 2012 and September 2016. He had no significant symptoms but slightly abdominal discomfort occasionally. His dietary was regular, but the sleep was not that well.
The patient had a 30-year history of alcohol consumption. His father had a gastrointestinal-related disease, but the details are unknown.
The entire abdomen was soft, and there was no pressure pain, rebound pain, and muscle tension. A vertical surgery scar of approximately 15 cm was found. All the other vital signs were stable, and no positive signs were revealed.
The results of the laboratory tests were negative, except an ALT level of 75 U/L. The tumor markers, AFP, CEA, CA19-9, had normal concentrations. The blood tests and fecal, coagulation function, and Helicobacter pylori antibody examinations showed normal results.
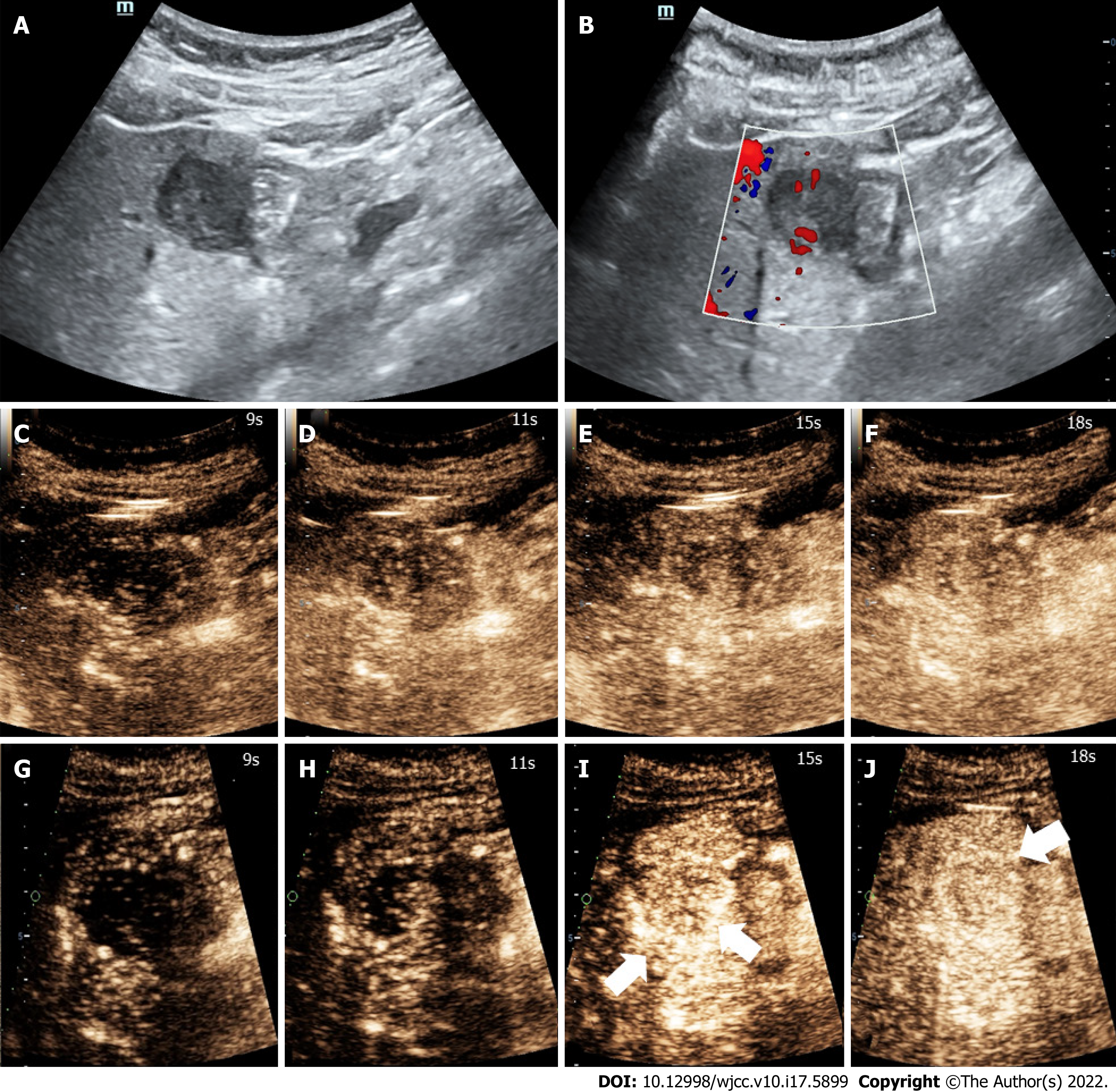
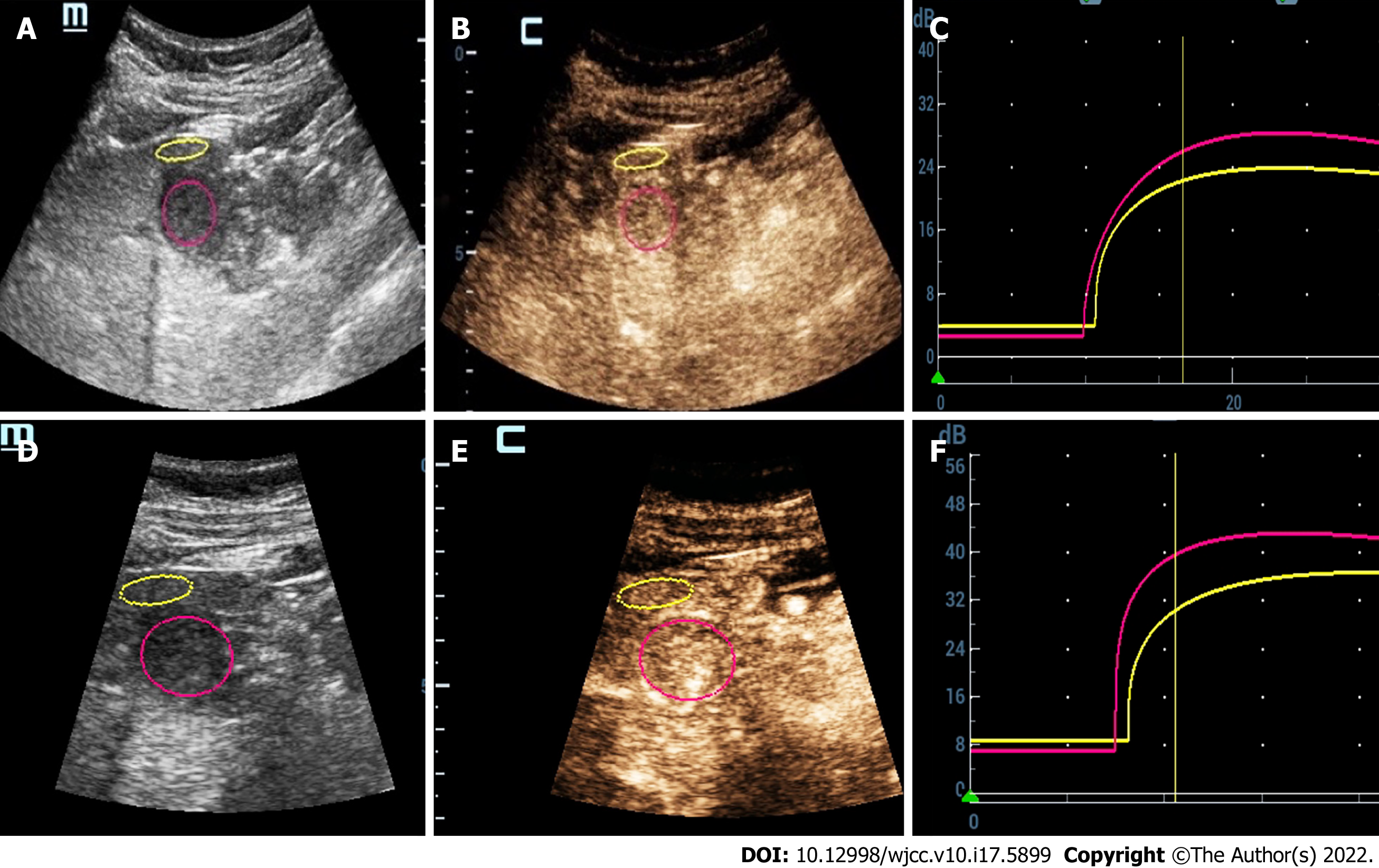
Three months earlier, magnetic resonance imaging (MRI) showed a 2.3 cm occupying lesion in the left external liver lobe (Figure 1). The patient underwent an abdominal ultrasound examination using the Resona9 ultrasound system (Mindray Medical International, China) equipped with an SC6-1U (1-6 MHz) transducer. Conventional ultrasound (US) showed an uneven hypo-echo lesion with a peripheral hypoechoic halo located in the left lobe of the liver. The lesion had an approximate size of 3.5 × 2.2 × 2.4 cm3, a round shape, and slightly clear margins. Color Doppler flow imaging (CDFI) showed the dot-linear blood flow signal within the lesion (Figure 2A and B). Given the history of duodenal GIST, the patient’s doctor suggested further CEUS diagnosis and obtained patient's consent. The depth, gain, and focus were thoroughly adjusted for optimal display according to the operator's habits. After a bolus injection of 1.5 mL of Sonovue (Bracco, Italy) suspension, an ultrasound contrast agent, with 5 mL physiological saline (Italy, Bracco), the timer was activated. The target lesion and surrounding liver parenchyma were continuously observed for 5 min. Based on the accepted guidelines, the arterial, portal, and late phases were defined after 10-30 s, 30-120 s, and 121-360 s of the contrast agent injection, respectively. On CEUS, the solid nodule appeared heterogeneous, and there was hyper-enhancement during the arterial phase without a significant concentric perfusion and rim-like enhancement (Figure 2C-F). Quantitative analysis showed a peak intensity difference between the lesion and liver parenchyma (ΔPI) of 3.58 dB (Figure 3A-C). The enhancement of the lesion was washed out rapidly and gradually; a heterogeneous enhancement and hypo-enhancement were observed during the portal and late phases. During the late phase, the contrast agent was barely perfused. These features were suggestive of malignancy. To observe the process and direction of the contrast agent more precisely and better assess the liver lesion, we carried out a second H-CEUS after the patient rested for 1 h. During the arterial phase, a solid lesion that was enhanced from the periphery to the center was observed; a heterogeneous hyper-enhancement at peak was also observed. We also found two irregular branch-like vascular columns around the lesion. Rim enhancement was more significant this time (Figure 3G-J). The video showing the H-CEUS in arterial phase is displayed (Video 1). Quantitative analysis showed a peak intensity difference between the lesion and liver parenchyma (ΔPI) of 6.63 dB (Figure 3D-F). Similar to the CEUS findings, the contrast agent was washed out rapidly during the portal and late phases, and, finally, no enhancement was observed. The arterial phase of the lesion was suggestive of a malignancy, and metastasis was suspected based on the history.
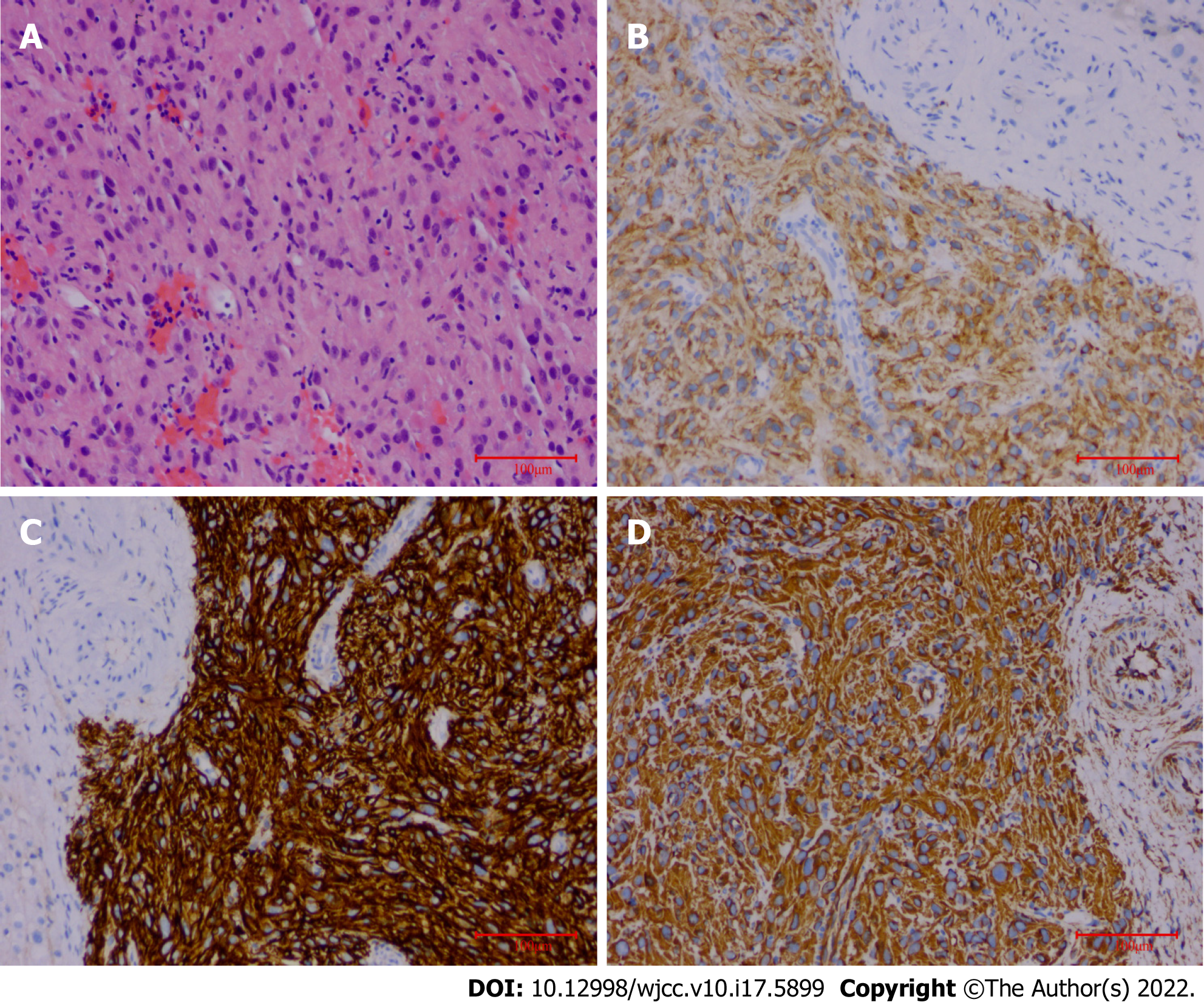
The final pathological findings were as follows: (1) Microscopic: the short and spindle cells were patchy; and (2) Immunohistochemical results: CD117, Dog-1, and Vimentin were positive, and Ki-67 was > 5% (Figure 4).
Molecular testing revealed c-Kit 11 (c.1655_1699del 15 type), c-Kit 9, c-Kit 13, c-Kit 17, PDGFRα12, and PDGFRα18 wild type mutations, which were associated with poor prognosis (Figure 5).
The final diagnosis of the presented case was liver metastasis of duodenal GIST.
The patient currently takes 400 mg imatine per day and has no other symptoms. Although the patient had not quit drinking, he had reduced the frequency and amount of alcohol consumption to a large extent.
The patient remained on imatine therapy, and was followed up.
Duodenal GIST is rare, accounting for 12%-18% of small intestine GISTs and 1%-4% of all GISTs[9-11]. The clinical features of duodenal GIST include mild gastrointestinal bleeding, abdominal pain, and an abdominal mass[12]. The diagnosis of duodenal GIST is usually based on histopathological and imaging findings. Usually, ultrasound endoscopy, CT scan, MRI, and digestive tract contrast can help in evaluating the size, local immersion, metastasis, and location of the GIST[13-15].
Metastatic liver cancer (MLC) is one of the most common metastatic tumors, and is usually associated with a lower survival rate[16,17]. The most common type of liver metastasis derives from colorectal cancer. GISTs only account for 1%-3% of gastrointestinal tumors. Due to the higher malignancy and poor prognosis of MLCs, early detection and therapy are of great significance.
CT and MRI are the preferred imaging modalities for diagnosing MLC. Liver metastasis often shows heterogeneous hypodense lesions with progressive concentric enhancement in CT scans, with a sensitivity of up to 97%[18], while the MRI technique can detect smaller lesions[18,19]. These two imaging modalities are not used in real-time evaluation, and their scanning durations are longer. Their radiation and high cost should be taken into consideration for neonates and pregnant women. CEUS has been widely used to detect focal liver lesions (FLLs) in recent years[20,21], but there have only been 10 reports of liver metastatic lesions so far (Table 1). Herein, we collected information regarding the age, gender, clinical manifestation, MLC’s originated organs, treatment, and follow-up from four cases and six clinical research studies. As shown in Table 1, MLCs mainly originated from gastrointestinal organs, except for three patients in whom the primary malignant tumors were medullary thyroid cancer[22], choroidal melanoma[23] and breast cancer[24]. MLCs usually have no specific symptoms. The US and CEUS features are depicted in Table 1. We found that MLCs often present as oval or round with irregular margins, and their echo characteristics are not specific. For MLCs from colorectal and ileal lesions, there is a central part with no echo[25,26]. They may consist of a hemorrhage and a necrotic area. It was reported that approximately 50% of all GISTs show cystic or necrotic areas[27]. CEUS is more sensitive in detecting avascular areas than US when part of the isoechoic or hypoechoic area was necrotic. Regarding the CEUS findings, the majority of these cases presented with homogeneous or heterogeneous hyper-enhancement during the arterial phase, mostly earlier than liver parenchyma. The contrast agents washed out very rapidly during the portal phase and presented hypo-enhancement until the end of the late phase. Wu et al[28] showed that metastatic lesion presented non-enhancement during the late phase. Zhang et al[29] found that lung primary lesion’s MLC showed no significant enhancement in CEUS, which was contrary to other findings. We deduced that it was probably associated with the pathological type, but more supportive literature was required to make further conclusions. For this present case, CEUS showed a solid nodule that appeared heterogeneous and hyper-enhanced during the arterial phase; these were consistent with malignant liver lesion perfusion patterns previously reported[20,30]. As we all know, hyper-enhancement of atypical hemangiomas during the arterial phase or non-enhancement during the portal and late phases can lead to a misleading diagnosis. Intrahepatic cholangiocellular carcinomas behave like metastases, washing out rapidly and appearing as defects during the late phase[31]. Consequently, further imaging characteristics are needed for a precise diagnosis.
| Ref. | Country | Age/gender | Clinical manifestation | Organs originated | US features | CEUS features | Contrast agent/dosage | Treatment | Follow-up |
| Zhou J et al[22], 2017 | China | 33/F | Increase in calcitonin and CEA | MTC | Hyper-and net-like; echogenicity, clear margin, well-defined shape | Hyper-enhancement during the arterial phase and hypo-enhancement in portal and parenchyma phase | Sonovue 1.2 mL | Surgery | Calcitonin and CEA remained normal |
| Corvino et al[42], 2015 | Italy | 41/M | No specific symptoms | Rectal melanoma | Solitary hypo-anechoic complex cystic lesion with a thin internal septum | Hyper-enhancement of the cystic wall and intra-cystic septation during the arterial phase, rapid wash-out, and hypo-enhancement during the portal and late phases | Sonovue 2.4 mL | Surgery | N/A |
| Toni et al[23], 2011 | Germany | 36/M | No specific symptoms | Choroidal melanoma | Iso-echogenicity | Homogeneous hyper-enhancement in arterial phase; hypo-enhancement in portal phase and punched-out enhancement defect; in late phase | Sonovue 2.0 mL | Surgery | N/A |
| Paulatto et al[25], 2020 | France | 74/M | N/A | NOS of colon | N/A | Central part remains hypoechoic | N/A | Surgery | N/A |
| Ishikawa et al[43], 2021 | Japan | N/A | N/A | Pancreas | Round with irregular margin | Strong peripheral enhancement in the arterial phase, early washout, hypo-enhancement in the portal, and post-vascular phases | Sonazoid 0.015 mL/kg | N/A | N/A |
| Michima et al[24], 2016 | Japan | N/A | N/A | Breast | Clearly round, oval, or lobulated solid focal lesions, irregular margin. | Hypoechoic defects in enhancing parenchyma in the portal venous or postvascular phase | Sonazoid 0.015 mL/kg | N/A | N/A |
| Yang DP et al[26], 2020 | China | N/A | N/A | Colorectum | Hypo- or mix- echogenicity and anechoic area | Capsule enhancement, starting time of washout of > 40 s, un-enhancement area, and proportion of non-enhancement area > 50% | Sonovue 2.4 mL | N/A | N/A |
| Wu et al[28], 2020 | China | N/A | N/A | Colorectum | Not mentioned | Peripheral nodular enhancement, heterogeneous hyper-enhancement, or rim-like enhancement during the arterial phase and a non-enhancement area during the late phase | Sonovue 2.0 mL | N/A | N/A |
| Schwarze et al[44], 2019 | Germany | N/A | N/A | NET of ileum | Anechoic oval-shaped | Earlier wash-in, hyperenhancement during the arterial phase, and hypo-enhancement in the portal phase | Not mentioned | N/A | N/A |
| Schwarze et al[44], 2019 | Germany | N/A | N/A | Pancreas | N/A | Earlier wash-in, hyperenhancement during the arterial phase, and hypo-enhancement during the portal phase | Not mentioned | N/A | N/A |
| Zhang GD et al[29], 2013 | China | N/A | N/A | Stomach | Round with regular margin, iso-echo | Earlier rim-like enhancement and washed out in portal phase, non-enhancement in late phase | Sonovue 2.4 mL | N/A | N/A |
| Zhang GD et al[29], 2013 | China | N/A | N/A | Lung | Oval and hypo-echo | No significant enhancement, hypo-perfusion compared with liver parenchyma | Sonovue 2.4 mL | N/A | N/A |
The contrast agent was a pure-blood pool tracer for showing microcirculation perfusion of solid organs[21,32]. It can detect smaller (> 40 μm) blood vessels better than CDFI (> 100 μm) and is widely used in FLLs[33,34]. While it is partly affected by the frame rate, the frame frequency of CEUS is within 9-15 Hz, which is probably not adequate for detecting quick wash-in progression and the vascular architecture during the early arterial phase. H-CEUS provides a frame rate of tens of thousands of images per second to compensate for the reduced focusing of the acoustic beam and enhance the signal-to-noise ratio. H-CEUS can show microcirculation differences and wash-in patterns during the early arterial phase and vascular morphogenesis. In this case of H-CEUS technology, the enhancement appeared at the peripheral part of the lesion, and there was concentric perfusion. This may be mainly due to the increase in frame rate, which facilitated a better display of the first enhancement area, and the site where the change in enhancement over time demonstrated the direction of contrast perfusion. Nevertheless, when the image frame rate is low, it is impossible to accurately display the contrast enhancement area, as the enhancement appears almost simultaneously in various parts of the lesion. Instead, it is presented as an entire perfusion on CEUS. Besides, vascular morphology is one of the important features for identifying and diagnosing the nature of FLL, and irregular vascular morphology is usually the main manifestation of malignant FLL[35,36]. The vascular morphology can be demonstrated by recording the path of contrast agent microbubbles, as the bubbles cannot enter the tissue gap through the vessel wall, and can only continuously move in the vessel[37-40]. The arterial phase is important for observing the vascular morphology, and rapid flow of arterial blood leads to the rapid movement of contrast agents. Therefore, only when the frame rate of contrast imaging reaches a certain threshold. In our case, we observed irregular branch-like vascular columns, which suggested the presence of malignancy; resulting in the final diagnosis of “liver metastasis of duodenal GIST.” Compared with the CEUS technology, H-CEUS is more suitable for accurately detecting the movement of contrast agents to ascertain the vascular morphology of liver lesions.
The prognosis and treatment of liver metastasis of duodenal GIST have not been established due to the limited number of reported cases[2,3]. Based on our literature review, we found that distant metastases can occur years after the surgical excision of the primary duodenal lesion. Both patients reported in the two cases experienced bleeding after rupture of the liver metastatic lesion[2,3,6,41]. Given that liver metastases of duodenal GISTs are negatively correlated with disease prognosis, early detection is important. Consequently, our report is useful, as it is the first to highlight the value and utility of H-CEUS for diagnosis of patients with liver metastases of duodenal GISTs. This case reminds clinicians that the H-CEUS technology can facilitate the diagnosis of liver metastatic cancers, resulting in improved diagnosis, treatment, and outcomes.
Duodenal GISTs are relatively rare, but they usually have a poor prognosis and are associated with high incidences of metastases. The H-CEUS findings were as follows: a solid lesion enhanced from the periphery to the center with two irregular branch-like vessel columns during the early arterial phase with peak heterogeneous hyper-enhancement and rim enhancement. The contrast agent was washed out rapidly during the portal phase and showed no enhancement during the late phase. To the best of our knowledge, this report is the first to describe the H-CEUS patterns of this disease. It also highlights the challenges associated with the CEUS findings of metastatic hepatic cancers.
We thank Dr. Jin Y, Department of Gastroenterology, Shengjing Hospital of China Medical University for her pathological diagnosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kim YJ, Malaysia; Madian A, Egypt S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Gaitanidis A, Alevizakos M, Tsaroucha A, Simopoulos C, Pitiakoudis M. Incidence and predictors of synchronous liver metastases in patients with gastrointestinal stromal tumors (GISTs). Am J Surg. 2018;216:492-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Mori T, Kataoka M, Kaiho T, Yanagisawa S, Nishimura M, Kobayashi S, Okaniwa A, Ashizawa Y, Oka Y, Ohya M. [A Case of Liver Metastasis 28 Years after Resection for Gastrointestinal Stromal Tumor of Duodenum]. Gan To Kagaku Ryoho. 2020;47:1810-1812. [PubMed] [Cited in This Article: ] |
| 3. | Koike K, Kubota H, Takayanagi Y, Ikeda T, Ito T. [A case of gastrointestinal stromal tumor of the duodenum with ruptured liver metastasis during administration of imatinib]. Nihon Shokakibyo Gakkai Zasshi. 2020;117:914-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 4. | Kishi Y, Takaki S, Fukuhara T, Mori N, Okanobu H, Tsuji K, Maeda T, Fujiwara M, Nagai K, Furukawa Y. [A case of liver metastasis 11 years after resection of a gastrointestinal stromal tumor of the duodenum]. Nihon Shokakibyo Gakkai Zasshi. 2019;116:1030-1038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 5. | Stratopoulos C, Soonawalla Z, Piris J, Friend PJ. Hepatopancreatoduodenectomy for metastatic duodenal gastrointestinal stromal tumor. Hepatobiliary Pancreat Dis Int. 2006;5:147-150. [PubMed] [Cited in This Article: ] |
| 6. | Sakata M, Kaneyoshi T, Fushimi T, Watanabe J. Rare cause of cystic liver lesions: Liver metastasis of gastrointestinal stromal tumors. JGH Open. 2021;5:408-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Inoue M, Shishida M, Watanabe A, Kajikawa R, Kajiwara R, Sawada H, Ohmori I, Miyamoto K, Ikeda M, Toyota K, Sadamoto S, Takahashi T. A liver metastasis 7 years after resection of a low-risk duodenal gastrointestinal stromal tumor. Clin J Gastroenterol. 2021;14:1464-1469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | McGuirk M, Gachabayov M, Gogna S, Da Dong X. Robotic duodenal (D3) resection with Roux-en-Y duodenojejunostomy reconstruction for large GIST tumor: Step by step with video. Surg Oncol. 2021;36:130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Cassier PA, Ducimetière F, Lurkin A, Ranchère-Vince D, Scoazec JY, Bringuier PP, Decouvelaere AV, Méeus P, Cellier D, Blay JY, Ray-Coquard I. A prospective epidemiological study of new incident GISTs during two consecutive years in Rhône Alpes region: incidence and molecular distribution of GIST in a European region. Br J Cancer. 2010;103:165-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731-1741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 425] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 11. | Sandvik OM, Søreide K, Kvaløy JT, Gudlaugsson E, Søreide JA. Epidemiology of gastrointestinal stromal tumours: single-institution experience and clinical presentation over three decades. Cancer Epidemiol. 2011;35:515-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Qiu H, Zhang P, Feng X, Chen T, Sun X, Yu J, Chen Z, Li Y, Tao K, Li G, Zhou Z. Changes of diagnosis and treatment for gastrointestinal stromal tumors during a 18-year period in four medical centers of China. Zhonghua Weichang Waike Zazhi. 2016;19:1265-1270. [PubMed] [Cited in This Article: ] |
| 13. | Karaca C, Turner BG, Cizginer S, Forcione D, Brugge W. Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc. 2010;71:722-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Park CH, Kim EH, Jung DH, Chung H, Park JC, Shin SK, Lee YC, Kim H, Lee SK. Impact of periodic endoscopy on incidentally diagnosed gastric gastrointestinal stromal tumors: findings in surgically resected and confirmed lesions. Ann Surg Oncol. 2015;22:2933-2939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Zhou Z, Lu J, Morelli JN, Hu D, Li Z, Xiao P, Hu X, Shen Y. Utility of noncontrast MRI in the detection and risk grading of gastrointestinal stromal tumor: a comparison with contrast-enhanced CT. Quant Imaging Med Surg. 2021;11:2453-2464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Obayashi J, Kawaguchi K, Manabe S, Nagae H, Wakisaka M, Koike J, Takagi M, Kitagawa H. Prognostic factors indicating survival with native liver after Kasai procedure for biliary atresia. Pediatr Surg Int. 2017;33:1047-1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Hara Y, Ogata Y, Shirouzu K. Early tumor growth in metastatic organs influenced by the microenvironment is an important factor which provides organ specificity of colon cancer metastasis. J Exp Clin Cancer Res. 2000;19:497-504. [PubMed] [Cited in This Article: ] |
| 18. | Tsili AC, Alexiou G, Naka C, Argyropoulou MI. Imaging of colorectal cancer liver metastases using contrast-enhanced US, multidetector CT, MRI, and FDG PET/CT: a meta-analysis. Acta Radiol. 2021;62:302-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology. 2010;257:674-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 420] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 20. | Wilson SR, Feinstein SB. Introduction: 4th Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (CEUS) in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM and FLAUS. Ultrasound Med Biol. 2020;46:3483-3484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC, Chaubal NG, Chen MH, Clevert DA, Correas JM, Ding H, Forsberg F, Fowlkes JB, Gibson RN, Goldberg BB, Lassau N, Leen EL, Mattrey RF, Moriyasu F, Solbiati L, Weskott HP, Xu HX; World Federation for Ultrasound in Medicine; European Federation of Societies for Ultrasound. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 473] [Cited by in F6Publishing: 479] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 22. | Zhou J, Luo Y, Ma BY, Ling WW, Zhu XL. Contrast-enhanced ultrasound diagnosis of hepatic metastasis of concurrent medullary-papillary thyroid carcinoma: A case report. Medicine (Baltimore). 2017;96:e9065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | De Toni EN, Gallmeier E, Auernhammer CJ, Clevert DA. Contrast-enhanced ultrasound for surveillance of choroidal carcinoma patients: features of liver metastasis arising several years after treatment of the primary tumor. Case Rep Oncol. 2011;4:336-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Mishima M, Toh U, Iwakuma N, Takenaka M, Furukawa M, Akagi Y. Evaluation of contrast Sonazoid-enhanced ultrasonography for the detection of hepatic metastases in breast cancer. Breast Cancer. 2016;23:231-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Paulatto L, Dioguardi Burgio M, Sartoris R, Beaufrère A, Cauchy F, Paradis V, Vilgrain V, Ronot M. Colorectal liver metastases: radiopathological correlation. Insights Imaging. 2020;11:99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Yang D, Zhuang B, Wang W, Xie X. Differential diagnosis of liver metastases of gastrointestinal stromal tumors from colorectal cancer based on combined tumor biomarker with features of conventional ultrasound and contrast-enhanced ultrasound. Abdom Radiol (NY). 2020;45:2717-2725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Baheti AD, Shinagare AB, O'Neill AC, Krajewski KM, Hornick JL, George S, Ramaiya NH, Tirumani SH. MDCT and clinicopathological features of small bowel gastrointestinal stromal tumours in 102 patients: a single institute experience. Br J Radiol. 2015;88:20150085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Wu XF, Bai XM, Yang W, Sun Y, Wang H, Wu W, Chen MH, Yan K. Differentiation of atypical hepatic hemangioma from liver metastases: Diagnostic performance of a novel type of color contrast enhanced ultrasound. World J Gastroenterol. 2020;26:960-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 15] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Zhang GD, Zou SD, Wang H, He J, Huang D. [Contrast-enhanced ultrasound in diagnosing 55 cases of various types of liver metastatic carcinoma]. Shijie Huaren Xiaohua Zazhi. 2013;21:2849-2850. [DOI] [Cited in This Article: ] [Cited by in CrossRef: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Chammas MC, Bordini AL. Contrast-enhanced ultrasonography for the evaluation of malignant focal liver lesions. Ultrasonography. 2022;41:4-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Xu HX, Lu MD, Liu GJ, Xie XY, Xu ZF, Zheng YL, Liang JY. Imaging of peripheral cholangiocarcinoma with low-mechanical index contrast-enhanced sonography and SonoVue: initial experience. J Ultrasound Med. 2006;25:23-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Sidhu PS, Cantisani V, Dietrich CF, Gilja OH, Saftoiu A, Bartels E, Bertolotto M, Calliada F, Clevert DA, Cosgrove D, Deganello A, D'Onofrio M, Drudi FM, Freeman S, Harvey C, Jenssen C, Jung EM, Klauser AS, Lassau N, Meloni MF, Leen E, Nicolau C, Nolsoe C, Piscaglia F, Prada F, Prosch H, Radzina M, Savelli L, Weskott HP, Wijkstra H. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. 2018;39:e2-e44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 398] [Cited by in F6Publishing: 477] [Article Influence: 79.5] [Reference Citation Analysis (1)] |
| 33. | Jiang Y, Zhang M, Zhu Y, Zhu D. Diagnostic role of contrast-enhanced ultrasonography vs conventional B-mode ultrasonography in cirrhotic patients with early hepatocellular carcinoma: a retrospective study. J Gastrointest Oncol. 2021;12:2403-2411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Guo HL, Lu XZ, Hu HT, Ruan SM, Zheng X, Xie XY, Lu MD, Kuang M, Shen SL, Chen LD, Wang W. Contrast-Enhanced Ultrasound-Based Nomogram: A Potential Predictor of Individually Postoperative Early Recurrence for Patients With Combined Hepatocellular-Cholangiocarcinoma. J Ultrasound Med. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Ferraioli G, Meloni MF. Contrast-enhanced ultrasonography of the liver using SonoVue. Ultrasonography. 2018;37:25-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Zarzour JG, Porter KK, Tchelepi H, Robbin ML. Contrast-enhanced ultrasound of benign liver lesions. Abdom Radiol (NY). 2018;43:848-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Kang XN, Zhang XY, Bai J, Wang ZY, Yin WJ, Li L. Analysis of B-ultrasound and contrast-enhanced ultrasound characteristics of different hepatic neuroendocrine neoplasm. World J Gastrointest Oncol. 2019;11:436-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 5] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Jang HJ, Kim TK, Burns PN, Wilson SR. CEUS: An essential component in a multimodality approach to small nodules in patients at high-risk for hepatocellular carcinoma. Eur J Radiol. 2015;84:1623-1635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Kitao A, Zen Y, Matsui O, Gabata T, Nakanuma Y. Hepatocarcinogenesis: multistep changes of drainage vessels at CT during arterial portography and hepatic arteriography--radiologic-pathologic correlation. Radiology. 2009;252:605-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 40. | Fukukura Y, Nakashima O, Kusaba A, Kage M, Kojiro M. Angioarchitecture and blood circulation in focal nodular hyperplasia of the liver. J Hepatol. 1998;29:470-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 87] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Sakakura C, Kumano T, Mizuta Y, Yamaoka N, Sagara Y, Hagiwara A, Otsuji E. Successful treatment of huge peritoneal metastasis from duodenal gastrointestinal stromal tumor resistant for imatinib mesylate. Gan To Kagaku Ryoho. 2007;34:2144-2146. [PubMed] [Cited in This Article: ] |
| 42. | Corvino A, Catalano O, Corvino F, Petrillo A. Rectal melanoma presenting as a solitary complex cystic liver lesion: role of contrast-specific low-MI real-time ultrasound imaging. J Ultrasound. 2016;19:135-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Ishikawa T, Ohno E, Mizutani Y, Iida T, Koya T, Sasaki Y, Ogawa H, Kinoshita F, Hirooka Y, Kawashima H. Comparison of contrast-enhanced transabdominal ultrasonography following endoscopic ultrasonography with GD-EOB-DTPA-enhanced MRI for the sequential diagnosis of liver metastasis in patients with pancreatic cancer. J Hepatobiliary Pancreat Sci. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Schwarze V, Marschner C, Grosu S, Rübenthaler J, Knösel T, Clevert DA. [Modern sonographic imaging of abdominal neuroendocrine tumors]. Radiologe. 2019;59:1002-1009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |