Published online Jun 16, 2022. doi: 10.12998/wjcc.v10.i17.5655
Peer-review started: November 13, 2021
First decision: December 27, 2021
Revised: March 25, 2022
Accepted: April 4, 2022
Article in press: April 4, 2022
Published online: June 16, 2022
Processing time: 208 Days and 3.4 Hours
Peroral endoscopic myotomy (POEM) is a safe and effective endoscopic treatment for achalasia. However, postoperative pain management for these patients is often neglected by anesthesiologists because of the short operative time, short hospital stay and the minimally invasive nature of the procedure.
To assess the pain and sleep quality of achalasia patients receiving the POEM procedure and investigate factors that affect postoperative pain.
This observational study included patients with achalasia who underwent POEM at Zhongshan Hospital from December 2017 to March 2018. General anesthesia was performed with endotracheal intubation. The postoperative visual analog scale (VAS), postoperative sleep quality, basic patient information, and surgical parameters were collected. Depending on whether the 12-h post-POEM VAS score was less than 4, patients were divided into two groups, a well-controlled pain group and a poorly controlled pain group. Univariate, multivariate, and stepwise logistic regression analyses were used to investigate risk factors for poor pain control. A prediction model of post-POEM pain risk was established in the form of a nomogram. The calibration curve and receiver operating characteristic curve were used to evaluate the clinical usage of the prediction model. Repeated measures analysis of variance and simple effect analysis were used to verify whether differences in the VAS and sleep scores of the high- and low-risk groups, divided by the model from the raw data, were statistically significant.
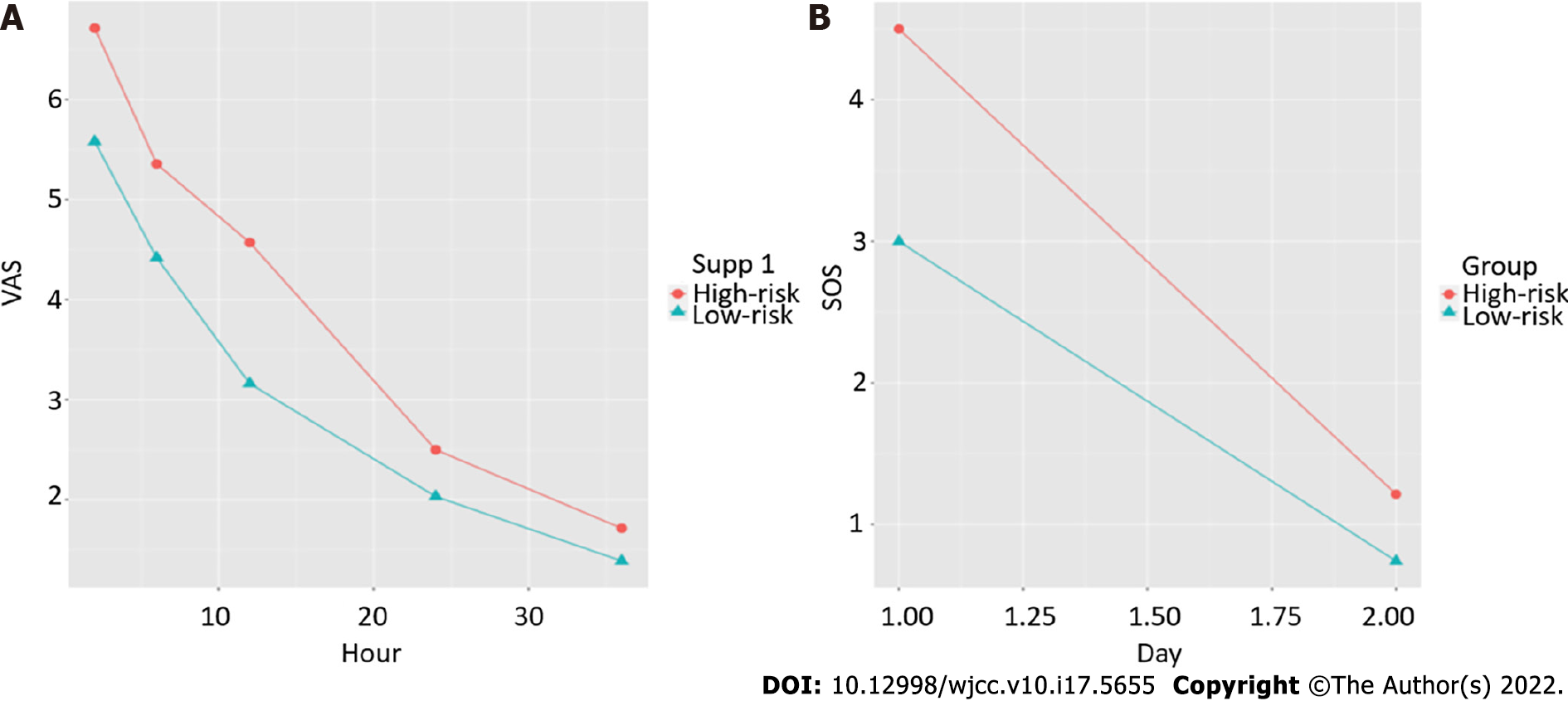
A total of 45 eligible patients were included. Multivariate logistic regression and further stepwise logistic regression analysis found that the preoperative Eckardt score [odds ratio (OR): 1.82, 95% confidence interval (CI): 1.17-2.84, P < 0.001], previous treatment (OR: 7.59, 95%CI: 1.12-51.23, P = 0.037) and the distance between the end of the muscle incision and the cardia (OR: 1.52, 95%CI: 0.79-293.93, P = 0.072) were risk factors for post-POEM pain. Repeated measures analysis of variance demonstrated that VAS (P = 0.0097) and sleep scores (P = 0.043) were higher in the high-risk group, and the interactions between the two main effects were obvious (VAS score: P = 0.019, sleep score: P = 0.035). Further simple effect analysis found that VAS scores were higher in the high-risk group at 2 h, 6 h and 12 h (P = 0.005, P = 0.019, P < 0.001), and sleep scores were higher in the high-risk group at day 1 (P = 0.006).
Achalasia patients who underwent POEM experienced serious postoperative pain, which may affect sleep quality. A higher Eckardt score, previous treatment, and a longer distance between the muscle incision ending and the cardia were risk factors for poor post-POEM pain control.
Core Tip: Post-peroral endoscopic myotomy (POEM) pain management has been neglected by anesthesiologists and endoscopists. In the present study, we found that achalasia patients experienced moderate to severe postoperative pain after the POEM procedure. The preoperative Eckardt score, previous treatment, and a longer distance between the muscle incision ending and the cardia were high risk factors for postoperative pain.
- Citation: Chen WN, Xu YL, Zhang XG. High Eckardt score and previous treatment were associated with poor postperoral endoscopic myotomy pain control: A retrospective study. World J Clin Cases 2022; 10(17): 5655-5666
- URL: https://www.wjgnet.com/2307-8960/full/v10/i17/5655.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i17.5655
Achalasia is a decrease in esophageal tension and esophageal dilation caused by a reduction in esophageal ganglion cells, disappearance of the thoracic esophagus, and diastolic dysfunction of the lower esophageal sphincter (LES). Clinical symptoms include dysphagia, gastroesophageal reflux, chest pain, weight loss, and anemia[1]. The incidence of achalasia is approximately 1.6/100000, regardless of sex and ethnicity, and is higher in younger patients[2-4].
Noninvasive treatments for achalasia include endoscopic botulinum toxin injection, esophageal stenting, and pneumatic balloon dilation. Invasive treatment can be performed with cardiomyotomy (also known as Heller surgery), which involves permanent surgical cutting of the LES[5,6]. In 2010, peroral endoscopic myotomy (POEM) was introduced as a clinical application by Inoue et al[7], and its use has rapidly spread worldwide. POEM surgery is a safe and effective treatment for achalasia, as verified by a large number of clinical studies, and has become a first-line treatment for achalasia[8], replacing Heller surgery and other noninvasive treatments.
Several published studies mainly focused on comparing the postoperative pain of achalasia patients receiving either the POEM procedure or laparoscopic Heller’s myelotomy (LMH), but their conclusions were inconsistent[9-13]. Meanwhile, postoperative pain management differs among studies, varying from oral opioids and intravenous nonsteroidal anti-inflammatory drugs to intraoperative nerve block[10-12,14]. There was little general agreement on the postoperative pain management of achalasia patients. Thus, the authors conducted this retrospective study to examine the postoperative pain intensity of achalasia patients receiving the POEM procedure and to investigate possible risk factors for postoperative pain.
This study was approved by the Ethics Committee of Zhongshan Hospital at Fudan University, No. B2018-004R. This was a preliminary study for a prospective study conducted at the Department of Anesthesiology within Zhongshan Hospital at Fudan University.
Inclusion criteria: (1) Male or female. Age between 18 and 75 years; (2) With diagnosis of achalasia; (3) Received POEM surgery in Zhongshan Hospital Fudan University from November 2017 to April 2018; (4) Complete anesthesia record and operative report; and (5) Signed informed consent.
Exclusion criteria: (1) Past experience with Heller surgery or POEM surgery; (2) Preoperative chronic pain management; and (3) Cognitive dysfunction or an inability to attend follow-up.
The diagnosis of achalasia was established based on the following: (1) Clinical symptoms; (2) X-ray angiography of the esophagus and sputum; (3) Esophagogastroduodenoscopy; (4) Esophageal manometry; and (5) Chest computed tomography (CT) scan[15].
The main results collected were the visual analog scale (VAS) scores at 2 h, 6 h, 12 h, 24 h, and 36 h after surgery. The secondary results collected were sleep quality on the night of surgery and on the night of the first day after surgery. A self-reported sleep questionnaire was used to investigate postoperative sleep quality, which included 6 yes-or-no questions. A score of 1 indicated the best quality sleep, and the highest score of 6 indicated the worst sleep quality. A correlation analysis of postoperative pain scores at each follow-up time with postoperative sleep quality and pain scores with related factors was performed. Related factors included the following: (1) Patient demographic information (sex, age, illness duration, disease grade and previous treatment); and (2) Surgical parameters (surgical time, anesthesia time, tunnel length, length of muscle incision, length between the muscle incision ending and the cardia, and perioperative opioid dose).
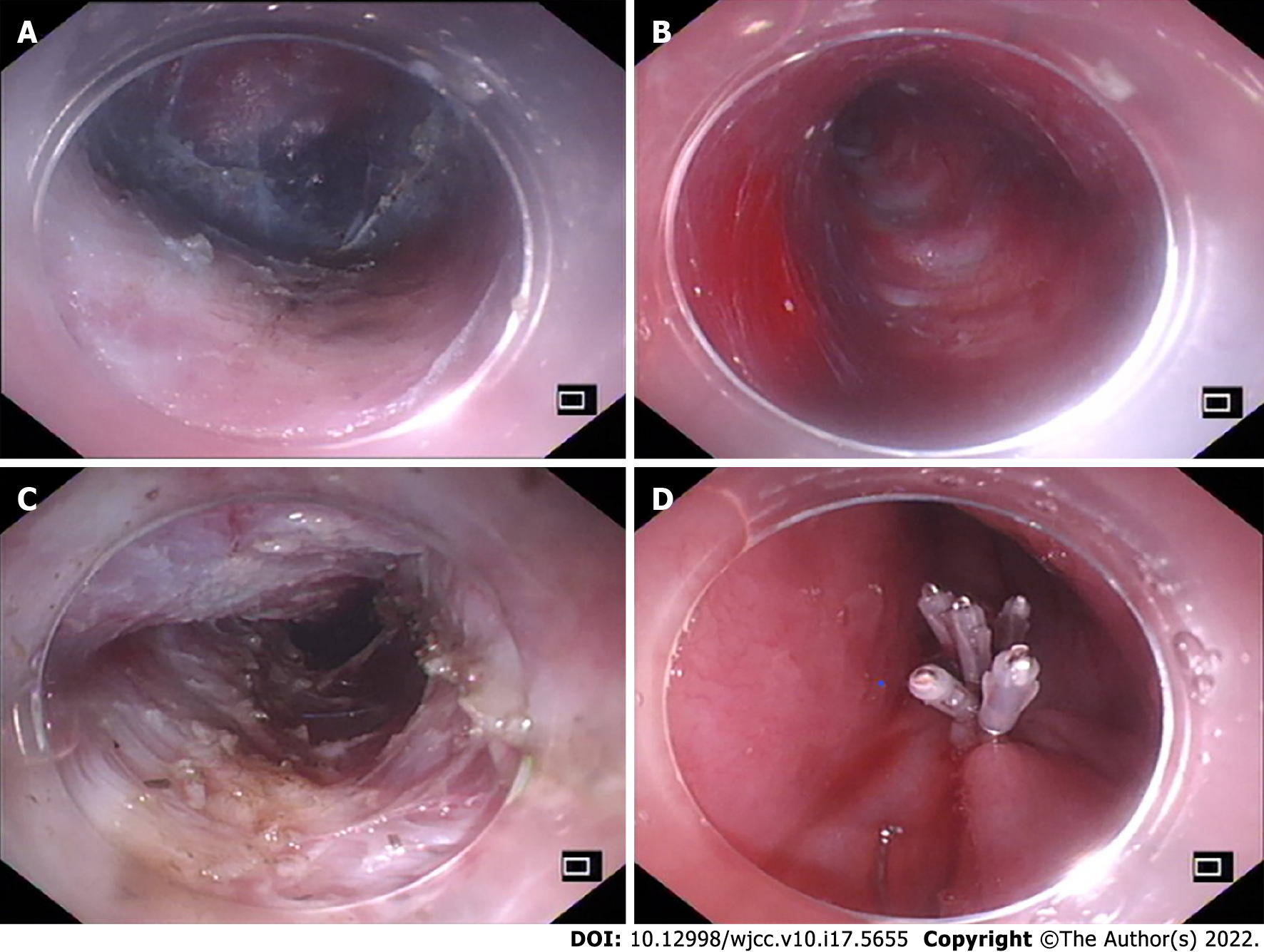
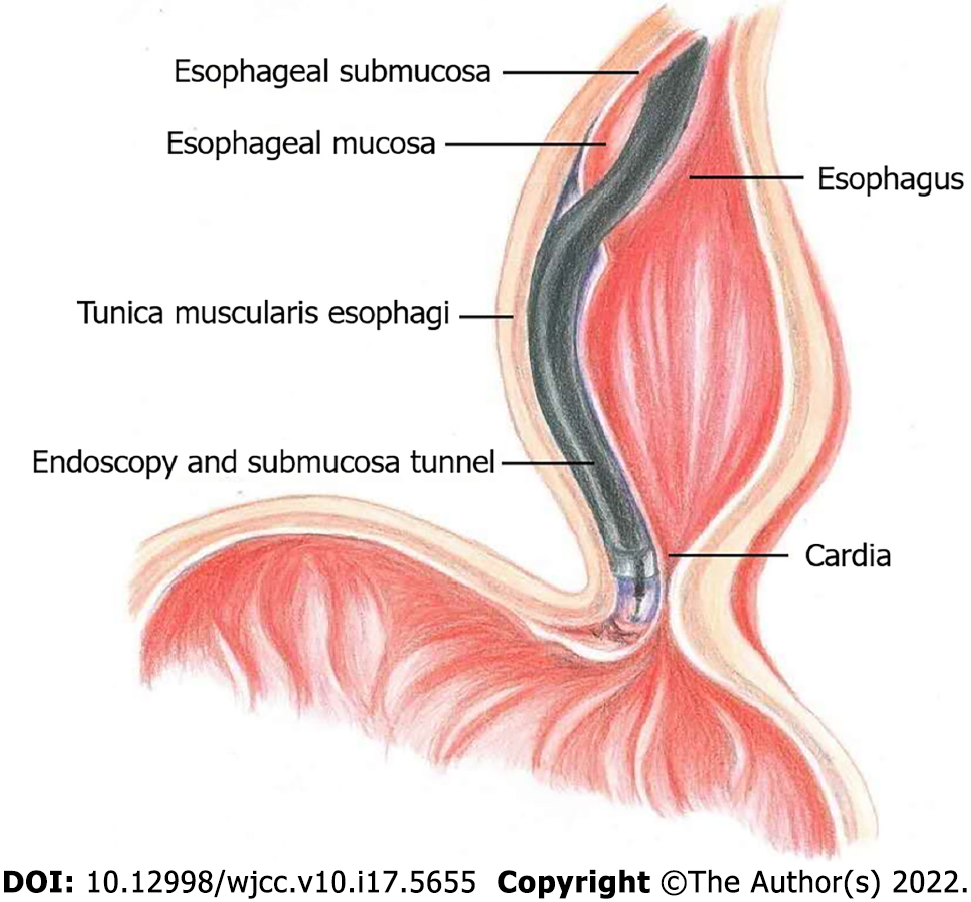
POEM surgery was performed at the endoscopy center of the hospital by endoscopic specialists who had received specialized POEM surgery training. Saline was submucosally injected 10-12 cm above the gastroesophageal junction (GEJ), and the mucosal layer was longitudinally cut to form a mucosal opening to expose the stratum submucosa (Figure 1A). Submucosal injection was performed while the mucosa was separated to form a submucosal tunnel (3-4 cm) to the distal end of the GEJ (Figure 1B). The myometrial incision was performed from the distal end of the mucosal tunnel opening at 2 cm to at least 2 cm to the distal end of the GEJ (Figure 1C). After proper hemostasis, the mucosal opening was closed with a hemostatic clip (Figure 1D). To avoid the occurrence of air-related complications, carbon dioxide inflation was continued throughout the procedure (Figure 2).
The procedures were performed on patients under general anesthesia with endotracheal intubation. Patients were maintained on carbohydrate drinks the day before the procedure, and oral intake was withheld for 12 h before the surgery. Esophagoscopy was performed while the patients were awake to clear the esophagus. Rapid sequence induction was employed with propofol (2-2.5 mg/kg), succinylcholine (2 mg/kg) and remifentanil (1 μg/kg). Anesthesia was maintained with desflurane (0.8 MAC), fentanyl 2-4 μg/kg, remifentanil 0.05-0.1 μg/kg per min. Cis-atracurium (0.03 mg/kg per hour) was used to assist intraoperative mechanical ventilation. The ventilator was set up as follows: Tidal volume 8 mL/kg, respiratory rate 10-12/min, and etCO2 maintained between 35-45 mmHg. A protective ventilation strategy was adopted to maintain the peak airway pressure below 30 mmHg and the plateau airway pressure below 25 mmHg. A certain degree of hypercapnia was considered as acceptable (etCO2 lower than 60 mmHg). Two grams of acetaminophen and 4 mg of ondansetron were given before the end of the operation. After the operation, the awakened patients were sent to the postanesthesia care unit (PACU) with a 25 μg titration of fentanyl to maintain a VAS score < 4 and were sent to the ward after a one-hour observation period. To control postoperative pain, 3 mg of dizocine was given intravenously at 8 pm on the day of the surgery and at 8 am on the first day after surgery.
Although these were quite rare, POEM-related adverse events included postoperative transfer to the intensive care unit, unstable vital signs, readmission, change to open surgery, postoperative invasive operation, delayed mucosal barrier failure, delayed bleeding and transfusion of blood, hydrothorax, pneumothorax, and extended hospital stay (> 5 d) due to functional impairment[16]. Anesthesia-related adverse events included reflux aspiration, delayed awakening time for more than 30 min, reintubation, postoperative nausea and vomiting.
The data were expressed as numerical values and percentages, and continuous data were expressed using the mean ± SD. All patients were divided into groups according to the 12-h postoperative VAS score; the poorly controlled pain group had a VAS score greater than or equal to 4, and the well-controlled pain group had a VAS score less than 4. Student's t test or the Wilcoxon rank sum test was used to compare quantitative variables, and the chi-square test or Fisher's exact test was used for qualitative variables. Univariate, multivariate, and stepwise logistic regression analyses were used to determine risk factors for poor pain control. The nomogram was formed by the optimal model fitted by logistic regression analysis. The area under the receiver operating characteristic curve (AUC) of the model was evaluated. Repeated measures analysis and of variance and simple effect analysis were used to verify whether the differences in the VAS and sleep scores between the high- and low-risk groups, divided by the model from the raw data, were statistically significant. P values over 0.05 were considered as statistically significant. The test was two-sided. All analyses were performed using RStudio software version 1.3.1093.
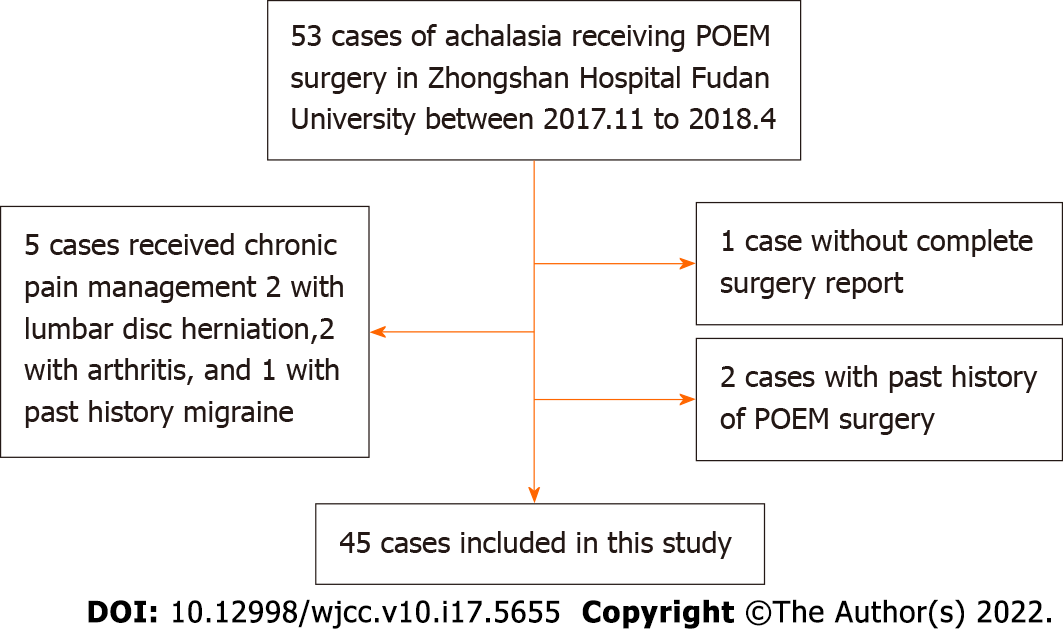
The flowchart of patient selection is summarized in Figure 3. Forty-five patients were enrolled with a mean age of 43.2 ± 15.7 years, including 24 males and 21 females. The average duration of symptoms was 6.0 ± 6.3 years, with an average preoperative Eckardt score of 7.6 ± 2.1 and esophageal dilation of 42.9 ± 10.7 cm. Ten (22.2%) patients had a past history of treatment (Table 1).
| Total | VAS < 4 | VAS ≥ 4 | P value | |
| Sex (%) | ||||
| Female | 21 (46.7) | 8 (38.1) | 13 (54.2) | 0.44 |
| Male | 24 (53.3) | 13 (61.9) | 11 (45.8) | |
| Age | 43.2 ± 15.7 | 45.0 ± 18.0 | 41.5 ± 13.6 | 0.60 |
| Symptom duration (yr) | 6.0 ± 6.3 | 4.1 ± 4.9 | 7.5 ± 7.0 | 0.019 |
| Eckardt score | 7.6 ± 2.1 | 6.7 ± 2.2 | 8.4 ± 1.6 | 0.013 |
| Esophageal diameter (mm) | 42.9 ± 10.7 | 39.0 ± 10.0 | 46.3 ± 10.4 | 0.022 |
| Esophageal dilatation classification (%) | ||||
| Ⅰ | 20 (44.4) | 13 (61.9) | 7 (29.2) | 0.088 |
| Ⅱ | 21 (46.7) | 7 (33.3) | 14 (58.3) | |
| Ⅲ | 4 (8.9) | 1 (4.8) | 3 (12.5) | |
| Previous treatment (%) | ||||
| No | 35 (77.8) | 19 (90.5) | 16 (66.7) | 0.078 |
| Yes | 10 (22.2) | 2 (9.5) | 8 (33.3) | |
| Operative time (min) | 61.3 ± 18.8 | 58.8 ± 16.9 | 63.5 ± 20.4 | 0.46 |
| Anesthesia time (min) | 74.1 ± 21.0 | 72.1 ± 17.5 | 75.8 ± 23.9 | 0.73 |
| Tunnel length (cm) | 12.7 ± 1.2 | 12.6 ± 0.6 | 12.8 ± 1.5 | 0.56 |
| Myotomy length (cm) | 10.7 ± 1.1 | 10.6 ± 0.7 | 10.8 ± 1.4 | 0.35 |
| Distance from muscle incision ending to cardia (cm) | 2.1 ± 0.3 | 2.0 ± 0.2 | 2.2 ± 0.4 | 0.22 |
| Fentanyl dosage (ug) (%) | ||||
| 100 | 37 (82.2) | 16 (76.2) | 21 (87.5) | 0.44 |
| 150 | 8 (17.8) | 5 (23.8) | 3 (12.5) | |
| Remifentanil dosage (ug) | 265.5 ± 90.8 | 264.8 ± 85.3 | 266.1 ± 97.2 | 1.0 |
With regard to the surgical procedure, the mean surgery and anesthesia times were 61.3 ± 18.8 min and 74.1 ± 21.0 min, respectively. The average submucosal tunnel length, myotomy length, and distance between the muscle incision ending and the cardia were 12.7 ± 1.2 cm, 10.7 ± 1.1 cm, and 2.1 ± 0.3 cm, respectively. Thirty-seven patients (82.2%) were administered 100 μg fentanyl during the surgery, and 8 patients (17.8%) were given 150 μg fentanyl. The total intraoperative remifentanil dose was 265.5 ± 90.8 μg. No patient received fentanyl in the PACU (Table 1).
A 12-h postoperative VAS score ≥ 4 was defined as poor post-POEM pain control. As shown in Table 1, patients with poorly controlled postoperative pain had prolonged symptom duration (VAS ≥ 4 vs VAS < 4, 7.5 years vs 4.1 years, P = 0.019), a higher preoperative Eckardt score (VAS ≥ 4 vs VAS < 4, 8.4 vs 6.7, P = 0.013), and a larger esophageal diameter (VAS ≥ 4 vs VAS < 4, 46.3 mm vs 39 mm, P = 0.022). Further univariate logistic regression analyses demonstrated that the preoperative Eckardt score (P = 0.011) and preoperative esophageal dilatation (P = 0.028) were potential risk factors for poor pain control. Multivariate logistic regression analysis and further stepwise logistic regression were used to exclude the internal effects of the participants, and the optimal fitted model for the risk of poor pain control was constructed. We found that the preoperative Eckardt score (OR: 1.82, 95%CI: 1.17-2.84, P < 0.001), previous treatment (OR: 7.59, 95%CI: 1.12-51.23, P = 0.037) and the distance between the muscle incision ending and the cardia (OR: 1.52, 95%CI: 0.79-293.93, P = 0.072) were risk factors and were included in the model for predicting postoperative pain (Table 2).
| Parameter | Univariate | Multivariate | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Sex | 0.52 (0.16-1.71) | 0.283 | ||
| Age | 0.99 (0.95-1.02) | 0.45 | ||
| Duration | 1.12 (0.98-1.27) | 0.091 | ||
| Eckardt Score | 1.64 (1.12-2.40) | 0.011 | 1.82 (1.17-2.84) | < 0.001 |
| Esophageal diameter | 1.07 (1.01-1.14) | 0.028 | ||
| Esophageal dilatation classification | 3.71 (1.02-13.51) | 0.046 | ||
| 5.57 (0.48-64.09) | 0.17 | |||
| Previous treatment | 4.75 (0.88-25.65) | 0.07 | 7.59 (1.12-51.23) | 0.037 |
| Operative time | 1.01 (0.98-1.05) | 0.40 | ||
| Anesthesia time | 1.01 (0.98-1.04) | 0.55 | ||
| Tunnel length | 1.18 (0.69-2.01) | 0.54 | ||
| Myotomy length | 1.20 (0.69-2.09) | 0.52 | ||
| Distance from muscle incision ending to cardia | 4.00 (0.41-39.00) | 0.23 | 1.52 (0.79-293.93) | 0.072 |
| Fentanyl dosage | 0.46 (0.095-2.20) | 0.33 | ||
| Remifentanil dosage | 1.00 (0.99-1.01) | 0.96 | ||
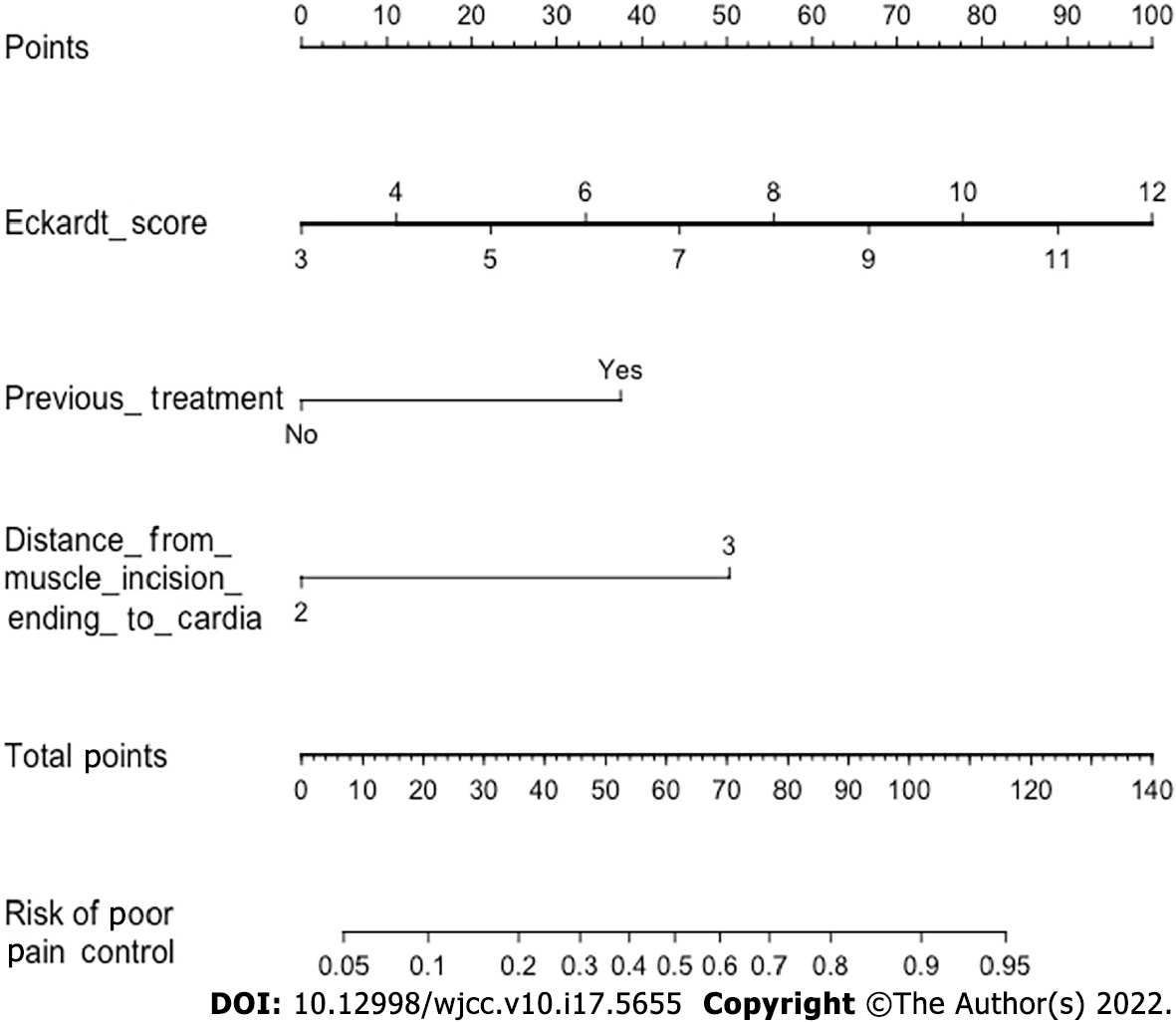
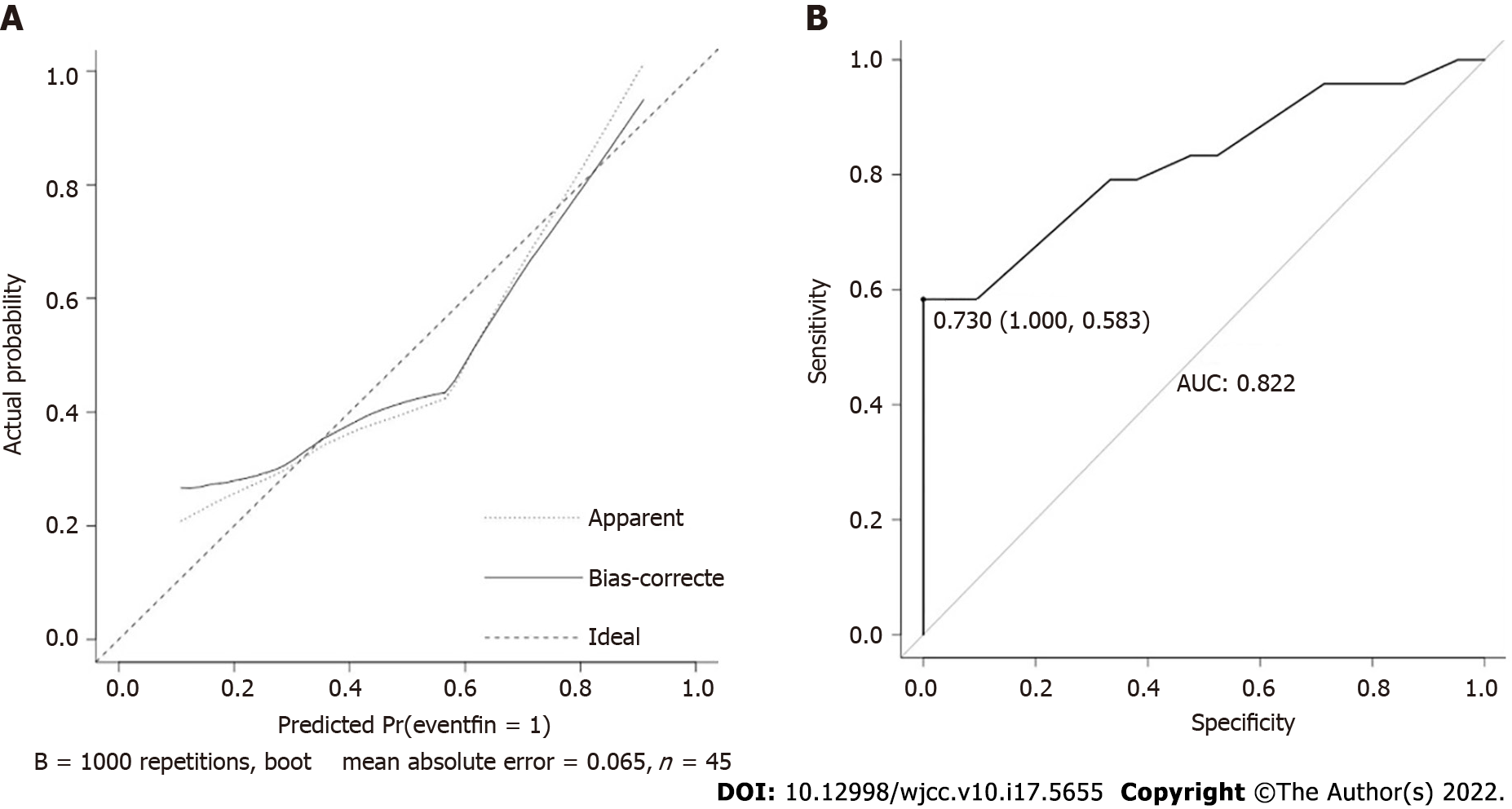
We further built a nomogram, as shown in Figure 4, to illustrate and visualize the relationship between the potential risk factors and postoperative pain. To calculate the risk for postoperative pain, each parameter was assigned a point vertically corresponding to the "points" axis, and the sum of the points was plotted on the “total points” axis. The risk of poor post-POEM pain control is the value on the “Risk of poor pain control” axis at the vertical position from the corresponding point on the “Total Points” axis. To evaluate the effect of the nomogram, we used the raw data for internal verification to obtain the calibration and receiver operating characteristic curve (ROC) curves. The calibration curve demonstrated good agreement between the predicted value and the actual value (Figure 5A). The ROC curve reflected a good classification ability, with an AUC value of 0.822 and an optimal cutoff of 0.730 (Figure 5B).
Patients were then divided into high- and low-risk groups according to the calculated cutoff from the ROC curve. To investigate whether postoperative VAS and sleep scores differed between the groups, a repeated measures analysis of variance was conducted, and we found that patients in the high-risk group had higher VAS (P = 0.0097) and sleep scores (P = 0.043) (Table 3). A further simple effect analysis found that VAS scores were higher in the high-risk group at 2 h, 6 h and 12 h (P = 0.005, P = 0.019, P < 0.001), and sleep scores were higher in the high-risk group at day 1 (P = 0.006) (Table 4). Line charts of the VAS and sleep scores of the two groups are presented to visually represent the trend in their variation (Figure 6).
| F value | P value | ||
| VAS | Groups | 7.33 | 0.0097 |
| Time | 226.47 | < 0.001 | |
| Groups: Time | 3.03 | 0.019 | |
| Sleep score | Groups | 4.35 | 0.043 |
| Time | 138.57 | < 0.001 | |
| Groups: Time | 4.72 | 0.035 |
| F value | P value | ||
| VAS | Risk (2 h) | 8.34 | 0.005 |
| Risk (6 h) | 5.71 | 0.019 | |
| Risk (12 h) | 12.91 | < 0.001 | |
| Risk (24 h) | 1.42 | 0.24 | |
| Risk (36 h) | 0.69 | 0.41 | |
| Sleep score | Risk (day 1) | 8.05 | 0.006 |
| Risk (day 2) | 0.80 | 0.375 |
Postoperative pain management of achalasia patients who undergo the POEM procedure is often neglected by anesthesiologists due to the short operative time and minimally invasive nature of the POEM procedure. In this retrospective study, we included 45 achalasia patients who received POEM at our medical center, evaluated their postoperative pain intensity and sleep quality, and explored factors associated with post-POEM pain. Our results demonstrated that patients with postoperative VAS scores (12 h after surgery) greater than or equal to 4 had longer symptom durations, higher preoperative Eckardt scores, and larger esophageal diameters. The Eckardt score, previous treatment for achalasia, and the distance between the muscle incision ending and the cardia were associated with poor postoperative pain control and were further included in the model for predicting postoperative pain. Patients in the high-risk group had higher VAS and sleep scores.
POEM has been proven to be a safe and effective endoscopic procedure for treating achalasia[16,17]. Previous studies on anesthesia management in POEM procedures have mostly focused on intraoperative anesthesia, reflux aspiration, and anesthesia-related complications[18-20]. Tanaka et al[18] administered remifentanil during surgery, and patients complained of postoperative upper abdominal pain. Twenty of the twenty-eight enrolled patients received diclofenac sodium turunda within 24 h after surgery, among whom 3 patients were given additional pentazocine. Nishihara et al[20] also delivered remifentanil during surgery but did not report any information about postoperative pain. Another retrospective study of 12 patients performed by Misra et al[21] revealed that patients had mild to moderate pain after POEM. These patients received fentanyl, hydromorphone, morphine, acetaminophen, oxycodone, and ketorolac as postoperative analgesia; however, there were no records on opioid use during surgery. Thus, the present study is the first retrospective study incorporating complete anesthesia management data and medical records to evaluate postoperative pain intensity and investigate potential risk factors among achalasia patients who underwent POEM.
No patient complained of pain during the telephone follow-up on the 28th day after surgery, suggesting that POEM may not lead to chronic pain. This result was consistent with previous research conducted by Misra et al[21], which revealed that postoperative pain associated with the POEM procedure seldom lasts for months.
A comparison of the baseline characteristics between groups demonstrated that patients who suffered from postoperative pain had longer symptom durations, higher Eckardt scores and larger esophageal diameters, i.e., more severe cases. Further stepwise logistic regression analyses showed that a higher Eckardt score and previous treatment for achalasia were associated with poor postoperative pain control (VAS ≥ 4 at 12 h after surgery). Patients with higher Eckardt scores had worse symptoms of achalasia and were also more likely to have relapsed or intractable achalasia, resulting in increased surgical difficulty and greater surgical wounds, which may contribute to severe postoperative pain. Interestingly, we also found that the 12-h postoperative VAS score was associated with the distance between the muscle incision ending and the cardia; the longer the distance, the more severe the pain. However, there was no significant difference between the VAS < 4 and VAS ≥ 4 groups in terms of surgical parameters. The suggestion for the range of submucosal tunnels was from 10-12 cm above the GEJ to 3-4 cm below the GEJ[7]. Previous studies showed that patients with shorter tunnels (10.7 ± 1.4 cm) had fewer complications than those with longer tunnels (13.1 ± 0.8 cm)[22]. Meanwhile, the range of myotomy is still controversial. It was suggested that excess myotomy was unnecessary, while insufficient myotomy may lead to recurrence. A muscle incision ending within 3 cm of the cardia was recommended. However, commonly used endoscopic markers of the gastric side are inaccurate, particularly in patients who have received previous treatment, such as balloon dilation or Botox injection of the LES[23]. Thus, anesthesiologists and endoscopists should pay attention to not only the surgical procedure itself but also the severity of achalasia.
With the small number of patients enrolled in this study, meaningful conclusions are still limited to some degree. This was an observational study performed during the early stage of an randomized controlled trial (RCT), and we will further explore the optimal approach to postoperative analgesia in patients who underwent POEM surgery in the RCT study and will cooperate with endoscopists to explore the effect of the POEM modus operandi (intrapleural tunnel length and myotomy position) on postoperative pain.
In summary, achalasia patients who underwent the POEM procedure experienced moderate to severe postoperative pain that required pain management. Patients with higher preoperative Eckardt scores, previous treatments, and longer distances between the muscle incision ending and the cardia may have a higher risk for postoperative pain.
Achalasia patients receiving POEM surgery experienced moderate to severe postoperative pain, which affected their sleep quality. Patients with higher preoperative Eckardt scores, previous treatments, and longer distances between the muscle incision ending and the cardia have a higher risk for poor postoperative pain control. Anesthetists and endoscopists should pay more attention to the severity of achalasia than to the POEM procedure itself when evaluating the risk for post-POEM pain.
Postoperative pain management for peroral endoscopic myotomy (POEM) is often neglected by anesthesiologists because of the short operative time, short hospital stay and the minimally invasive nature of the procedure.
The authors conducted this retrospective study to examine the postoperative pain intensity of achalasia patients receiving the POEM procedure and to investigate possible risk factors for postoperative pain.
To achieve better postoperative pain management.
We included patients with achalasia who underwent POEM at Zhongshan Hospital from December 2017 to March 2018. The postoperative visual analog scale, postoperative sleep quality, basic patient information, and surgical parameters were collected.
The preoperative Eckardt score [odds ratio (OR): 1.82, 95% confidence interval (CI): 1.17-2.84, P < 0.001], previous treatment (OR: 7.59, 95%CI: 1.12-51.23, P = 0.037) and the distance between the end of the muscle incision and the cardia (OR: 1.52, 95%CI: 0.79-293.93, P = 0.072) were risk factors for post-POEM pain.
Achalasia patients who underwent POEM experienced serious postoperative pain, which may affect sleep quality. A higher Eckardt score, previous treatment, and a longer distance between the muscle incision ending and the cardia were risk factors for poor post-POEM pain control.
We will further explore the optimal approach to postoperative analgesia in patients who underwent POEM surgery in the randomized controlled trial study and will cooperate with endoscopists to explore the effect of the POEM modus operandi (intrapleural tunnel length and myotomy position) on postoperative pain.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cascella M, Italy; Pallav K, United States S-Editor: Fan JR L-Editor: A P-Editor: Yu HG
| 1. | Winter H, Shukla R, Elshaer M, Riaz AA. Current management of achalasia—a review. Br J Med Pract. 2015;8:a810. |
| 2. | O'Neill OM, Johnston BT, Coleman HG. Achalasia: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2013;19:5806-5812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 159] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Sadowski DC, Ackah F, Jiang B, Svenson LW. Achalasia: incidence, prevalence and survival. A population-based study. Neurogastroenterol Motil. 2010;22:e256-e261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 4. | Francis DL, Katzka DA. Achalasia: update on the disease and its treatment. Gastroenterology. 2010;139:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Campos GM, Vittinghoff E, Rabl C, Takata M, Gadenstätter M, Lin F, Ciovica R. Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg. 2009;249:45-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 459] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 6. | Boeckxstaens GE, Annese V, des Varannes SB, Chaussade S, Costantini M, Cuttitta A, Elizalde JI, Fumagalli U, Gaudric M, Rohof WO, Smout AJ, Tack J, Zwinderman AH, Zaninotto G, Busch OR; European Achalasia Trial Investigators. Pneumatic dilation versus laparoscopic Heller's myotomy for idiopathic achalasia. N Engl J Med. 2011;364:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 580] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 7. | Inoue H, Minami H, Kobayashi Y, Sato Y, Kaga M, Suzuki M, Satodate H, Odaka N, Itoh H, Kudo S. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1233] [Article Influence: 82.2] [Reference Citation Analysis (1)] |
| 8. | Pasricha PJ, Hawari R, Ahmed I, Chen J, Cotton PB, Hawes RH, Kalloo AN, Kantsevoy SV, Gostout CJ. Submucosal endoscopic esophageal myotomy: a novel experimental approach for the treatment of achalasia. Endoscopy. 2007;39:761-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 360] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 9. | Murata H, Ichinomiya T, Hara T. Anesthesia for peroral endoscopic myotomy in Japan. Curr Opin Anaesthesiol. 2019;32:511-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Hungness ES, Teitelbaum EN, Santos BF, Arafat FO, Pandolfino JE, Kahrilas PJ, Soper NJ. Comparison of perioperative outcomes between peroral esophageal myotomy (POEM) and laparoscopic Heller myotomy. J Gastrointest Surg. 2013;17:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 11. | Docimo S Jr, Mathew A, Shope AJ, Winder JS, Haluck RS, Pauli EM. Reduced postoperative pain scores and narcotic use favor per-oral endoscopic myotomy over laparoscopic Heller myotomy. Surg Endosc. 2017;31:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Ujiki MB, Yetasook AK, Zapf M, Linn JG, Carbray JM, Denham W. Peroral endoscopic myotomy: A short-term comparison with the standard laparoscopic approach. Surgery. 2013;154:893-7; discussion 897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | Talukdar R, Inoue H, Nageshwar Reddy D. Efficacy of peroral endoscopic myotomy (POEM) in the treatment of achalasia: a systematic review and meta-analysis. Surg Endosc. 2015;29:3030-3046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 14. | Elmunzer BJ, Lewis BR, Miller KF, Wolf BJ, Zeiler L, Gutman DA, Elias P, Tansel A, Moran RA, Bolin ED. Paravertebral anesthetic nerve block for pain control after peroral endoscopic myotomy. Tech Innov Gastrointest Endosc. 2021;23:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol. 2013;108:1238-49; quiz 1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 356] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 16. | Zhang XC, Li QL, Xu MD, Chen SY, Zhong YS, Zhang YQ, Chen WF, Ma LL, Qin WZ, Hu JW, Cai MY, Yao LQ, Zhou PH. Major perioperative adverse events of peroral endoscopic myotomy: a systematic 5-year analysis. Endoscopy. 2016;48:967-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 17. | Li QL, Wu QN, Zhang XC, Xu MD, Zhang W, Chen SY, Zhong YS, Zhang YQ, Chen WF, Qin WZ, Hu JW, Cai MY, Yao LQ, Zhou PH. Outcomes of per-oral endoscopic myotomy for treatment of esophageal achalasia with a median follow-up of 49 months. Gastrointest Endosc. 2018;87:1405-1412.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 18. | Tanaka E, Murata H, Minami H, Sumikawa K. Anesthetic management of peroral endoscopic myotomy for esophageal achalasia: a retrospective case series. J Anesth. 2014;28:456-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Löser B, Werner YB, Punke MA, Saugel B, Haas S, Reuter DA, Mann O, Duprée A, Schachschal G, Rösch T, Petzoldt M. Anesthetic considerations for patients with esophageal achalasia undergoing peroral endoscopic myotomy: a retrospective case series review. Can J Anaesth. 2017;64:480-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Nishihara Y, Yoshida T, Ooi M, Obata N, Izuta S, Mizobuchi S. Anesthetic management and associated complications of peroral endoscopic myotomy: A case series. World J Gastrointest Endosc. 2018;10:193-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Misra L, Fukami N, Nikolic K, Trentman TL. Peroral endoscopic myotomy: procedural complications and pain management for the perioperative clinician. Med Devices (Auckl). 2017;10:53-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Ma XB, Linghu EQ, Wang NJ, Wang XD, Du H, Meng JY, Wang HB, Zhu J, Tang P, Huang QY, Zhao XW, Chai GQ, Kong JY, Qiu XY. Application of peroral endoscopic myotomy with short tunnel for Ling IIc type achalasia. Chin J Laparoscopic Sur. 2014;7:271-274. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Grimes KL, Inoue H, Onimaru M, Ikeda H, Tansawet A, Bechara R, Tanaka S. Double-scope per oral endoscopic myotomy (POEM): a prospective randomized controlled trial. Surg Endosc. 2016;30:1344-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |