Published online Jan 7, 2022. doi: 10.12998/wjcc.v10.i1.143
Peer-review started: August 22, 2021
First decision: October 22, 2021
Revised: November 4, 2021
Accepted: November 25, 2021
Article in press: November 25, 2021
Published online: January 7, 2022
Processing time: 130 Days and 6.5 Hours
Cardiogenic embolism caused by patent foramen ovale (PFO) is a common etiology of cryptogenic stroke (CS), particularly in young and middle-aged patients. Studies about right-to-left shunt (RLS) detection using contrast-enhanced transcranial Doppler (c-TCD) are numerous. According to the time phase and number of microbubbles detected on c-TCD, RLS can be classified and graded. We hypothesized that the characteristics of an infarction lesion on diffusion-weighted imaging differs when combining the type and grade of RLS on c-TCD in patients with PFO-related CS.
To explore the characteristics of infarction lesions on diffusion-weighted imaging when combining the RLS type and grade determined by c-TCD.
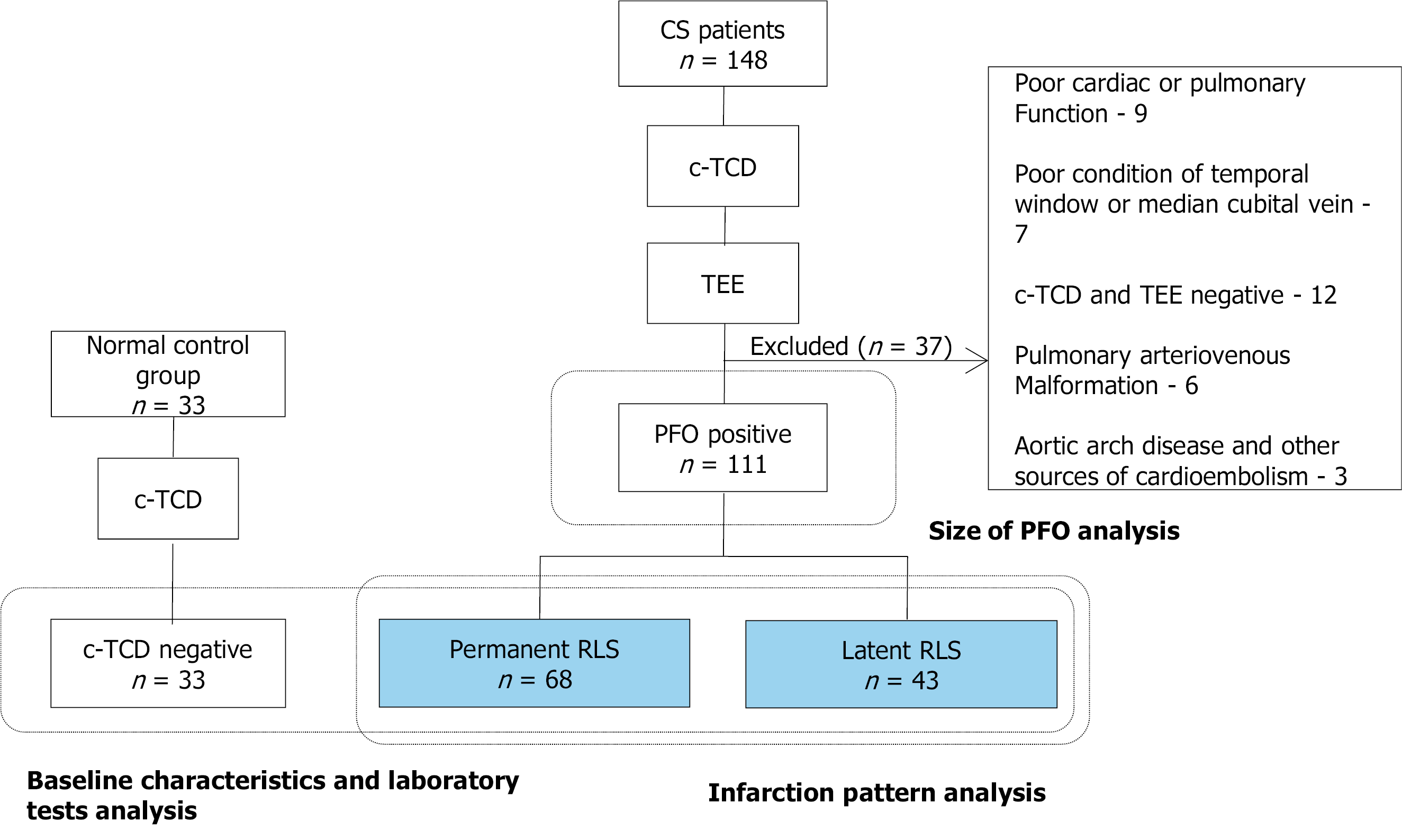
We retrospectively evaluated CS patients from August 2015 to December 2019 at a tertiary hospital. In total, 111 PFO-related CS patients were divided according to whether RLS was permanent (microbubbles detected both at resting state and after the Valsalva maneuver) or latent (microbubbles detected only after the Valsalva maneuver) on c-TCD. Each group was subdivided into small, mild and large RLS according to the grade of shunt on c-TCD. A normal control group was composed of 33 patients who suffered from simple dizziness. Intragroup and intergroup differences were analyzed in terms of clinical, laboratory and diffusion-weighted imaging lesion characteristics. The correlation between RLS grade evaluated by c-TCD and size of PFO determined by transesophageal echocardiography were also analyzed.
In 111 patients with PFO-related CS, 68 had permanent RLS and 43 had latent RLS. Clinical characteristics and laboratory tests were not significantly different among the permanent RLS, latent RLS and normal control groups. The proportion of patients with multiple territory lesions in the permanent RLS group (50%) was larger than that in the latent RLS group (27.91%; P = 0.021). Posterior circulation was more likely to be affected in the latent RLS group than in the permanent RLS group (30.23% vs 8.82%, P = 0.004). Permanent-large and latent-large RLS were both more likely to be related to multiple (Ptrend = 0.017 and 0.009, respectively), small (Ptrend = 0.035 and 0.006, respectively) and cortical (Ptrend = 0.031 and 0.033, respectively) lesions. The grade of RLS evaluated by c-TCD was correlated to the size of PFO determined by transesophageal echocardiography (r = 0.758, P < 0.001).
Distribution of the infarct suggested the possible type of RLS. Multiple, small and cortical infarcts suggest large RLS induced by a large PFO.
Core Tip: This retrospective study analyzed the relationship between right-to-left shunt (RLS) detected on contrast-enhanced transcranial Doppler (c-TCD) and infarction pattern detected on diffusion-weighted imaging in patients with patent foramen ovale (PFO)-related cryptogenic stroke, when combining the type and grade of RLS. Permanent RLS induced by PFO was more likely to involve multiple territories, while latent RLS tended to affect the posterior circulation. RLS grade evaluated by c-TCD was correlated to size of PFO determined by transesophageal echocardiography. Multiple, small and cortical infarcts suggested large RLS induced by a large PFO.
- Citation: Xiao L, Yan YH, Ding YF, Liu M, Kong LJ, Hu CH, Hui PJ. Evaluation of right-to-left shunt on contrast-enhanced transcranial Doppler in patent foramen ovale-related cryptogenic stroke: Research based on imaging. World J Clin Cases 2022; 10(1): 143-154
- URL: https://www.wjgnet.com/2307-8960/full/v10/i1/143.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i1.143
Ischemic stroke is more common than hemorrhagic stroke[1]. Cryptogenic stroke (CS), a subtype of ischemic stroke with no determined etiology after an extensive investigation, accounts for 40% of all types of ischemic stroke[2,3]. Seeking the underlying causes of CS can guide treatment and reduce or prevent the reoccurrence of stroke. Previous studies have indicated that patent foramen ovale (PFO) occurs in approximately half of CS patients, particularly among those who are of young age[4-6]; moreover, this percentage is considerably higher than in the normal healthy population. Paradoxical embolism caused by right-to-left shunt (RLS) of a PFO is considered an important pathogenesis for CS[7,8].
CS patients with PFO show characteristic infarction patterns on diffusion-weighted imaging (DWI), as compared to CS patients without PFO. This characteristic becomes more obvious as the grade of shunt increases on contrast-enhanced transcranial Doppler (c-TCD)[9-11]. In latent RLS, microbubbles are detected on c-TCD only after the valsalva maneuver (VM), whereas in permanent RLS, microbubbles are also detected at rest[12]. In this study, we sought to determine the relationship between RLS on c-TCD and the infarction pattern on DWI in PFO-related CS patients when combining the type and grade of RLS. The RLS grade evaluated by c-TCD was compared to the size of PFO determined by transesophageal echocardiography (TEE).
We retrospectively evaluated CS patients who attended the stroke center at the First Affiliated Hospital of Soochow University within 72 h from onset of symptoms. The study was carried out from August 2015 to December 2019. According to the TOAST criteria, CS is defined upon determination of no specific cause after an extensive evaluation[2]. The data for study included findings from clinical examinations, standard laboratory tests, brain imaging (computed tomography and magnetic resonance imaging), vascular imaging (color-coded duplex sonography, magnetic resonance or conventional angiography), 12-lead and 24 h electrocardiographic analyses, and echocardiographic analysis.
During the study period, 238 of the ischemic stroke patients admitted to our center had been diagnosed with CS. Of these, 148 underwent examination by c-TCD and TEE; the 16 patients among them who did not complete c-TCD or TEE detection due to poor cardiac or pulmonary function and a poor condition of temporal window or median cubital vein were excluded. Additionally, 12 patients with a negative result for both c-TCD and TEE, 6 patients with pulmonary arteriovenous malformation (detected via computed tomography pulmonary angiography[13]) and 3 patients with aortic arch disease and other sources of cardioembolism (detected by transthoracic echocardiography[14]) were excluded. Finally, a total of 111 CS patients with PFO were included in the current study (Figure 1).
For all included patients, findings from DWI of brain magnetic resonance imaging and TEE detection were assessed. A normal control group of 33 patients with simple dizziness who were age-matched to the included CS patients and admitted to hospital in the corresponding period were chosen. The normal control group had no neurological signs, no cerebral infarction, and negative c-TCD. The baseline characteristics and laboratory tests were evaluated to determine differences between PFO-related CS patients and the normal control group.
All examinations performed by TCD detector (EMS-9PB; Shenzhen Delica Medical Equipment, Shenzhen, China) were conducted by an experienced neuroscientist (> 300 case experience). In practice, with the patient lying in the supine position, a handheld 1.6 MHz probe was placed at the left temporal window to monitor the middle cerebral artery. The single-channel dual-depth mode was selected, with two depths (48-52 mm and 60-64 mm) having a depth difference of 12 mm. The detector was set to a sample volume of 10 mm (of note, the depth difference should be greater than the sample volume) and a low gain for embolus to distinguish from the background spectrum.
Before the test, an 18-gauge needle was aseptically inserted into the left median cubital vein of the patient. The contrast agent was prepared in a saline bag using a mixture of 9 mL of saline, 1 mL of sterile air, and a single drop of blood from the patient. Microbubbles were then created by rapid mechanical mixing (20 times) by means of two 10 mL syringes connected by a three-way stopcock. Immediately after, the contrast agent was injected into the prepared median cubital vein as a bolus. The procedure was performed during: (1) resting state; (2) conventional VM, for which the patients had been trained prior to inhale deeply and hold their breath; and (3) quantitative VM, for which the patients had been trained prior to inhale deeply and blow into the connecting tube of a VM quantitative device. A middle cerebral artery flow velocity with a 25% decrease was used to indicate that the conventional VM was effective[15]. For the quantitative VM specifically, the participants had been trained to blow into the connecting tube so that the pressure gauge reached 40 mmHg[16]. The conventional VM and quantitative VM were monitored over a period of 10 s, starting 5 s after the time the contrast agent injection had been initiated. Each maneuver had an interval of at least 2 min from the last observed microbubble signal (MBs) to ensure that the microbubbles were completely metabolized. The MBs was detected as a series of high-intensity transient signal, defined by the typical chirping sound within the doppler flow spectrum. The spike-like appearance on the spectrogram produced by reprocessing analysis was distinguishable from the noise.
The inspection result in each state was recorded using a five-level categorization system[6] based on the number of MBs observed, as follows: Grade 0, negative; Grade I, 1 ≤ MBs ≤ 10; Grade II, 10 < MBs ≤ 25; Grade III, > 25 MBs and no curtain; and Grade IV, curtain, a single MBs could not be identified. To improve the analysis, we further categorized the CS patients based on the level of the shunt, as follows: small RLS, Grade I; mild RLS: Grade II; large RLS, Grade III and IV.
TEE was performed using a cardiac ultrasound machine (Vivid E9; GE Healthcare, Chicago, IL, United States) with a 6VT-D transesophageal probe at a frequency range of 3.0-8.0 MHz. All patients were examined in a fasting state. For each, after local pharyngeal anesthesia was applied (10% topical lidocaine spray), the probe was quickly inserted into the esophagus through the mouth until it reached the pharynx. The width of the fissure between the primary septum and secondary septum was measured as the size of PFO in all confirmed PFO patients.
Brain magnetic resonance imaging was conducted within 24 h of admission for all patients by use of a MAGNETOM Skyra 3T system (Siemens, Munich, Germany). The obtained DWI images were reviewed by experienced neuroradiologists (including Chief Physician) who were blinded to the study groups.
The ischemic lesions were classified based on the amount (single or multiple) and size (small: < 1 cm diameter; large: > 1 cm diameter)[11]. According to the vascular territory involved, the lesions were divided into anterior circulation, posterior circulation and multiple territories[11]. The lesions were also classified based on cortical involvement (only-cortical, cortical–subcortical, and only-subcortical). We further synthesized and divided the infarction patterns into single territory single lesion, single territory multiple lesions, multiple territories small scattered lesions, and multiple territories large and small lesions, in accordance with a previous study[17].
Data were statistically analyzed using SPSS statistical software, version 25.0 (IBM Corp., Armonk, NY, United States). For continuous variables, one-way analysis of variance or the Kruskal–Wallis test were used to compare between-group differences. The least significant difference test or the adjusted Mann–Whitney U rank sum test were chosen as the method for intragroup multiple comparisons. For nominal variables, intragroup and intergroup differences were analyzed using the Pearson χ2 test, χ2 test for trends, Fisher’s exact test or partitioning χ2 test. Spearman rank correlation was used to analyze the correlation between the grade of RLS evaluated by c-TCD and the size of PFO determined by TEE. The level of significance was set at P < 0.05.
A total of 68 patients (61.26%) were classified as having permanent RLS and 43 patients (38.74%) as having latent RLS based on c-TCD. The differences in age, sex, blood pressure, smoking, alcohol intake, body mass index and history of stroke did not reach statistical significance in comparison between the CS patients with permanent or latent RLS and the normal control group. We then analyzed the findings from laboratory tests and observed no statistically significant differences among the three groups for blood lipid parameters, blood glucose, blood cell count, coagulation indicators, inflammatory biomarkers, and blood uric acid (Table 1).
| Permanent RLS (n = 68) | Latent RLS (n = 43) | Normal control group (n = 33) | P value | |
| Age (SD) | 46.25 (11.54) | 42.26 (11.95) | 45.39 (13.57) | 0.161 |
| Male (%) | 37 (54.41) | 28 (65.12) | 22 (66.67) | 0.375 |
| Blood pressure | ||||
| Systolic pressure (SD) | 129.68 (16.23) | 130.67 (19.25) | 128.97 (15.05) | 0.988 |
| Diastolic pressure (SD) | 81.12 (11.46) | 82.42 (14.31) | 77.73 (10.49) | 0.386 |
| Smoking (%) | 11 (16.18) | 13 (30.23) | 12 (36.36) | 0.057 |
| Alcohol intake (%) | 5 (7.35) | 7 (16.28) | 7 (21.21) | 0.120 |
| BMI (SD) | 24.40 (1.25) | 24.55 (1.29) | 24.84 (1.25) | 0.180 |
| Previous stroke history (%) | 10 (14.71) | 7 (16.28) | 0 (0) | 0.055 |
| Total cholesterol (SD) | 4.24 (0.85) | 4.25 (1.07) | 4.50 (0.92) | 0.390 |
| High-density cholesterol(SD) | 1.21 (0.29) | 1.11 (0.24) | 1.27 (0.32) | 0.082 |
| Low-density cholesterol(SD) | 2.44 (0.78) | 2.56 (0.93) | 2.65 (0.82) | 0.418 |
| Triglyceride (SD) | 1.35 (0.64) | 1.37 (0.66) | 1.43 (0.51) | 0.502 |
| Blood glucose (SD) | 5.08 (1.27) | 4.94 (0.83) | 5.22 (1.20) | 0.728 |
| RBC (SD) | 4.63 (0.46) | 4.70 (0.58) | 4.64 (0.48) | 0.795 |
| Platelet (SD) | 220.29 (53.28) | 216.02 (78.30) | 237.0 (55.83) | 0.061 |
| Fibrinogen (SD) | 2.72 (0.69) | 2.99 (1.46) | 2.54 (0.58) | 0.524 |
| D-dimer (SD) | 0.48 (0.35) | 0.47 (0.32) | 0.41 (0.32) | 0.428 |
| APTT (SD) | 26.61 (4.47) | 26.79 (5.72) | 25.85 (3.71) | 0.621 |
| WBC (SD) | 7.12 (2.24) | 6.67 (1.86) | 6.99 (1.55) | 0.540 |
| hs-CRP (SD) | 2.63 (3.98) | 2.65 (3.50) | 1.38 (1.24) | 0.619 |
| ESR (SD) | 10.54 (8.70) | 9.47 (6.95) | 8.48 (4.49) | 0.608 |
| BUA (SD) | 300.20 (99.11) | 319.22 (66.32) | 311.93 (58.76) | 0.068 |
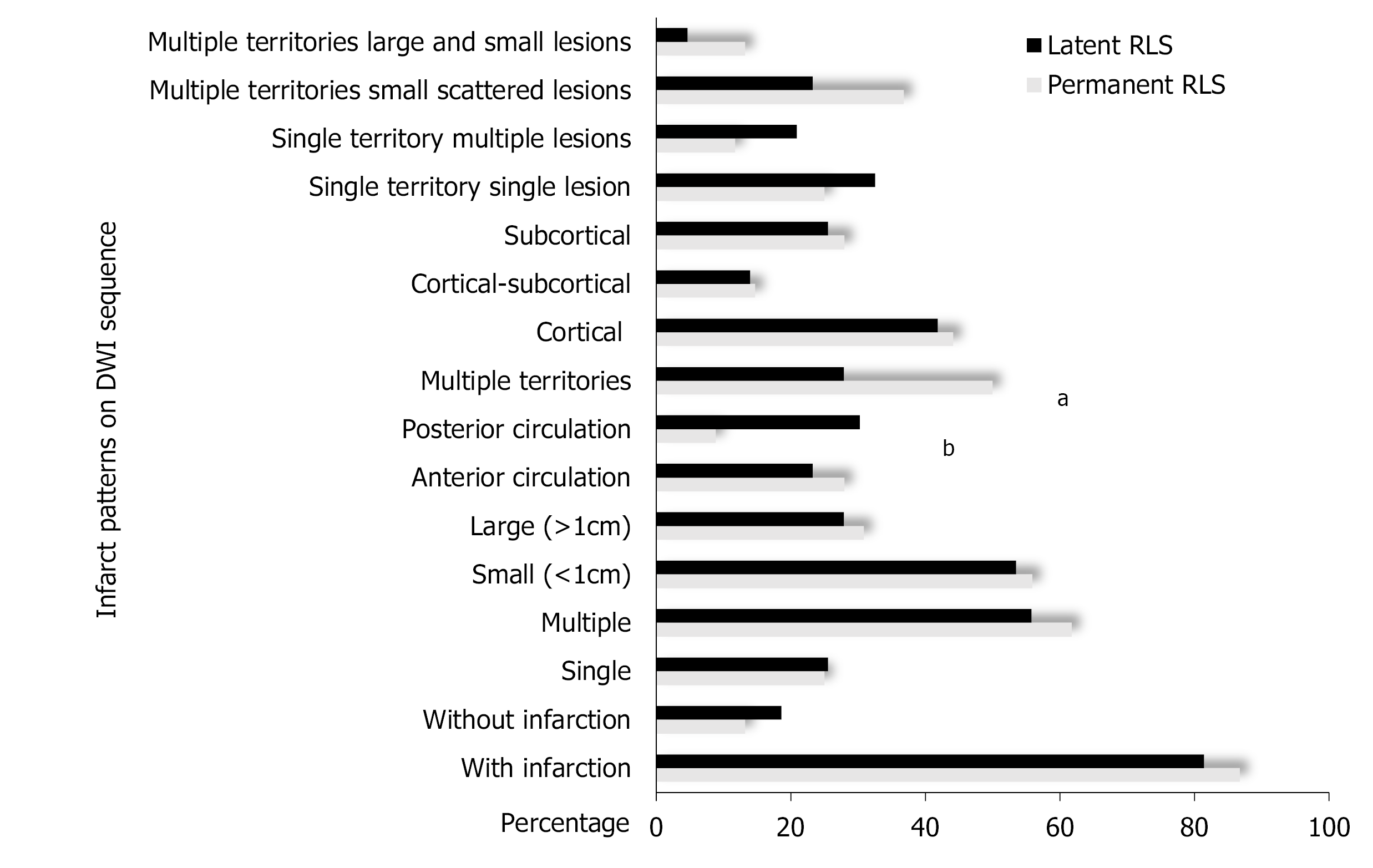
The DWI lesions of CS patients with different types and grades of RLS were compared and analyzed. First, the DWI lesions were compared between the permanent RLS group and the latent RLS group. In the permanent RLS group, 86.76% of the patients showed DWI lesions; in the latent RLS group, 81.40% of the patients showed DWI lesions. These rates were not significantly different between the two groups. We also found no significant differences in the number, size and cortical involvement of DWI lesions between the permanent RLS group and the latent RLS group. The proportion of patients with multiple territory lesions in the permanent RLS group (50.00%) was significantly greater than that in the latent RLS group (27.91%; P = 0.021). However, the latent RLS group was more likely to show an effect on posterior circulation (P = 0.004) (Figures 2 and 3).
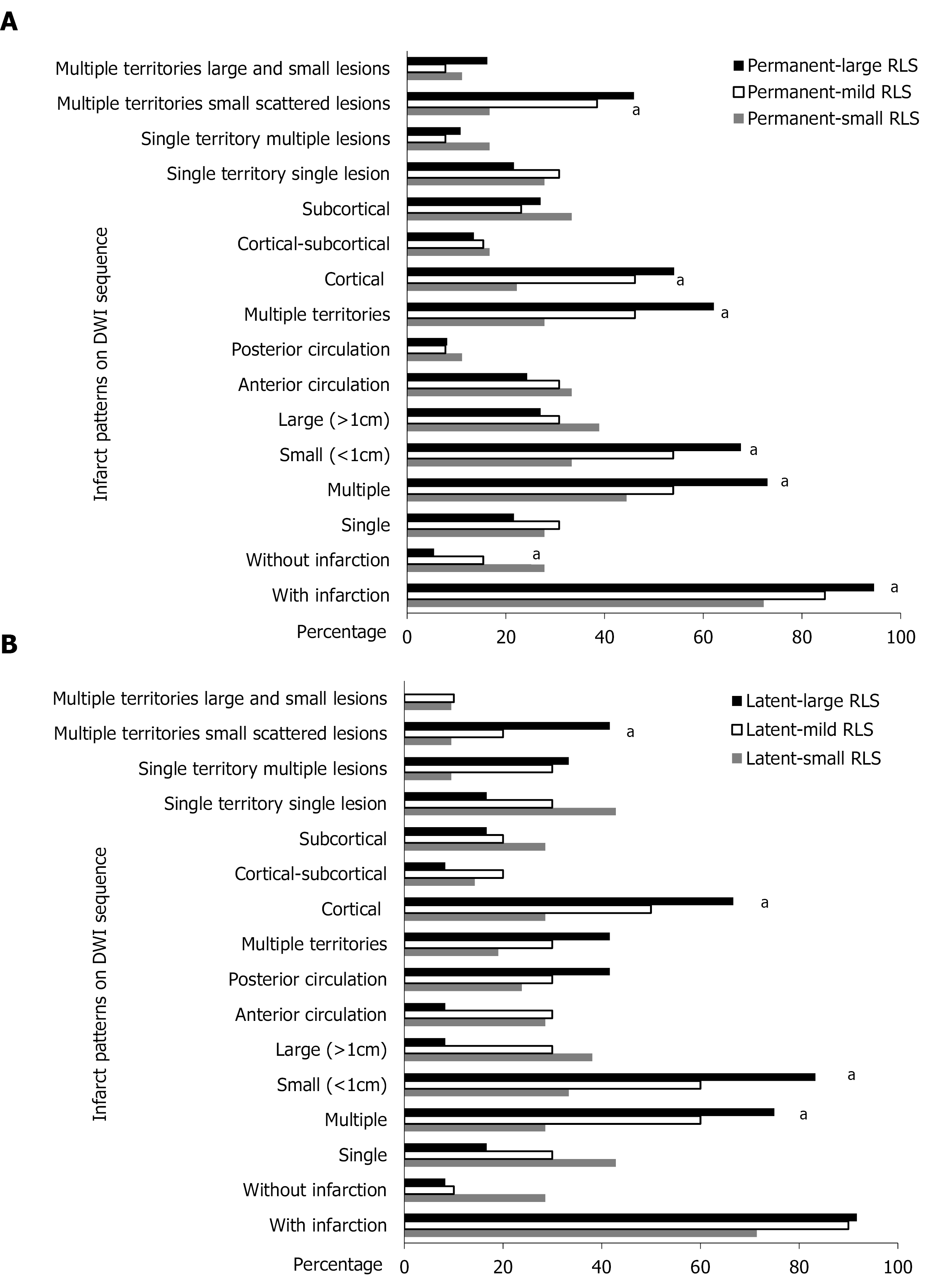
We then subcategorized the permanent RLS and latent RLS groups into small RLS, mild RLS and large RLS according to the grade of shunt on c-TCD. In the permanent RLS group, 18 patients had small RLS, 13 patients had mild RLS, and 37 patients had large RLS. The proportion of patients with DWI lesions was 72.22% in the small RLS subgroup, 84.61% in the mild RLS subgroup, and 94.59% in the large RLS subgroup (Ptrend = 0.022). The proportion of multiple lesions was 44.44% in the small RLS subgroup, 53.85% in the mild RLS subgroup, and 72.97% in the large RLS subgroup (Ptrend = 0.035). The proportion of small lesions was 33.33% in the small RLS subgroup, 53.85% in the mild RLS subgroup, and 67.57% in the large RLS subgroup (Ptrend = 0.017). The proportion of multiple territory lesions was 27.78% in the small RLS subgroup, 46.15% in the mild RLS subgroup, and 62.16% in the large RLS subgroup (Ptrend = 0.017). Cortical involvement varied from 22.22% in the small RLS subgroup to 46.15% in the mild RLS subgroup and 54.05% in the large RLS subgroup (Ptrend = 0.031). The grade of RLS also showed a positive tendency with multiple territories and small scattered lesions, varying from 16.67% in the small RLS subgroup to 38.46% in the mild RLS subgroup and 45.95% in the large RLS subgroup (Ptrend = 0.041) (Figure 4A).
In the latent RLS group, 21 patients had small RLS, 10 patients had mild RLS, and 12 patients had large RLS. No statistically significant difference was found for the proportion of patients with DWI lesions and the distribution of lesions according to the different grades of RLS. The proportion of multiple lesions varied from 28.57% in the small RLS subgroup to 60.00% in the mild RLS subgroup and 75.00% in the large RLS subgroup (Ptrend = 0.009). The proportion of small lesions varied from 33.33% in the small RLS subgroup to 60% in the mild RLS subgroup and 83.33% in the large RLS subgroup (Ptrend = 0.006). The proportion of cortical involvement varied from 28.57% in the small RLS subgroup to 50.00% in the mild RLS subgroup and 66.67% in the large RLS subgroup (Ptrend = 0.033). Multiple territory and small scattered lesions varied from 9.52% in the small RLS subgroup to 20.00% in the mild RLS subgroup and 41.67% in the large RLS subgroup (Ptrend = 0.033) (Figure 4B).
Finally, the DWI lesions of CS patients with an equivalent grade of RLS between the permanent RLS group and the latent RLS group were compared. A statistically significant difference was found only in the proportions of posterior circulation lesions of the large RLS subgroup for the permanent RLS group (8.11%) and the latent RLS group (41.67%; P = 0.007). No statistically significant difference was found in mild or small RLS between the permanent RLS group and the latent RLS group.
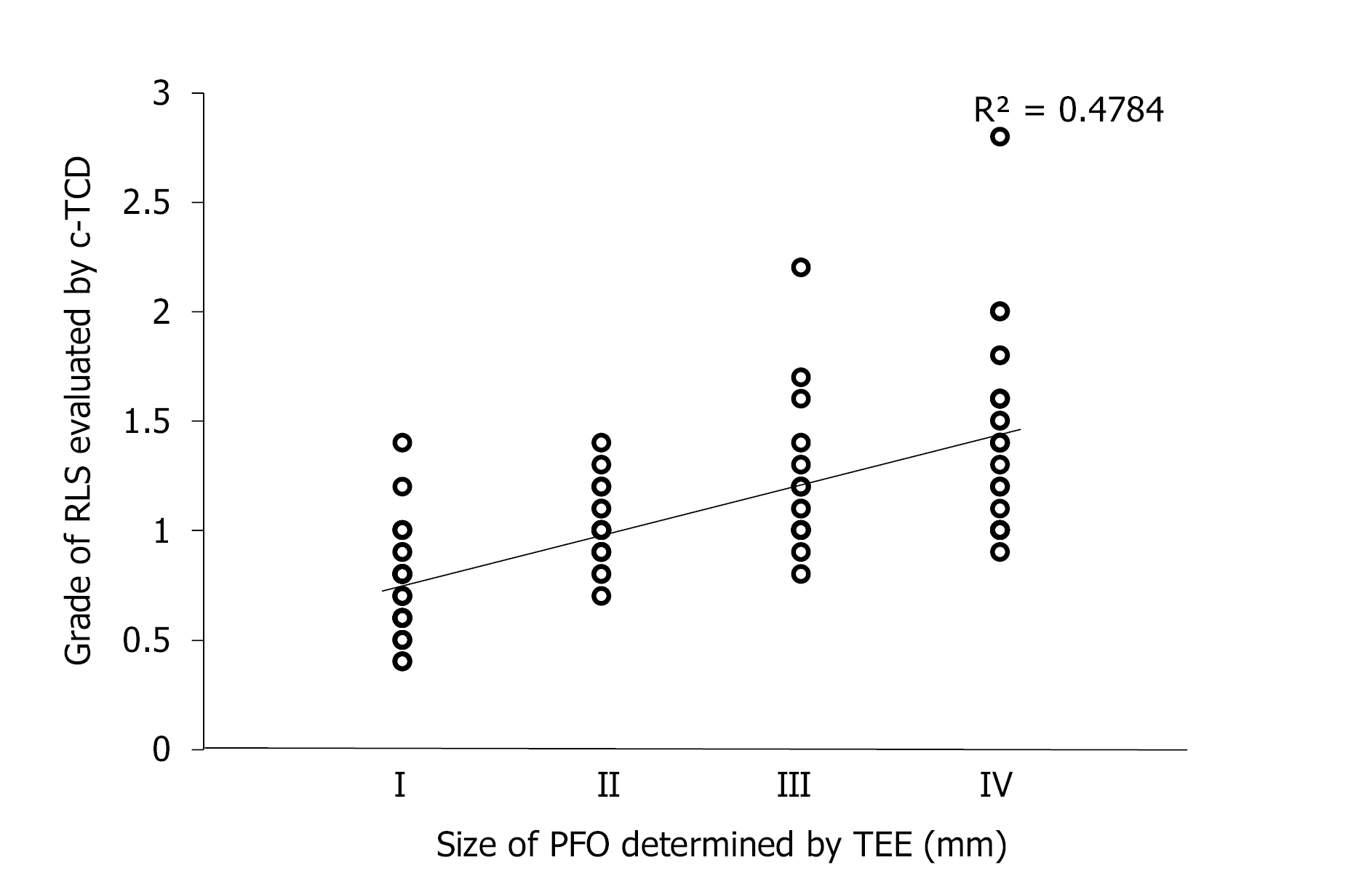
The width of fissure between the primary septum and secondary septum was measured as the size of PFO in all PFO-related CS patients. The grade of RLS evaluated by c-TCD was found to correlate statistically with the size of PFO determined by TEE (r = 0.758, P < 0.001) (Figure 5).
As previously reported, RLS is regarded as a common cause of CS in young and middle-aged adults[18,19]. In the current study, we observed that the average age of CS patients was < 55 years. Krassen et al[20] indicated that PFO was more prevalent in men with CS than in women with CS. However, the same was not observed in the current study. Furthermore, the vascular risk factors of CS patients were evaluated. We found no significant difference between CS patients with permanent RLS and latent RLS and the normal control group. The results indicated that other mechanisms of stroke, such as paradoxical embolism, may exist in these patients, which was similar to findings of previous studies[11,21].
In the present study, we demonstrated characteristic infarction patterns on DWI depending on the different types and grades of RLS in PFO-related CS patients. Patients with permanent RLS were more likely to involve multiple territories, and patients with latent RLS were more likely to involve posterior circulation. We also observed this tendency in latent-large RLS patients. The high prevalence of posterior circulatory infarction in latent RLS patients can be attributed to the reduced innervation of the sympathetic nervous system and the increased blood flow to the posterior circulatory area after the provocation maneuver[22,23]. Meanwhile, infarction in permanent RLS patients exhibited the tendency to involve multiple vascular territories because of the existence of microemboli, both at a resting state and after the provocation maneuver[13].
We also found that the DWI lesions exhibited certain characteristics as the grade of shunt increased, for both the permanent RLS group and the latent RLS group. These lesions were more likely to be multiple, small and cortical lesions. This infarction pattern was consistent with the main characteristic of PFO-related stroke reported in previous studies[24,25]. PFO is an imbricate structure and a potential fissure, which usually only allows for passage of smaller microemboli[26]. If the grade of RLS increases, the number of microemboli that can pass through the PFO will increase relative to the blood flow to the peripheral vessels of the brain. This provides an anatomical basis for multiple, small and cortical lesion patterns.
In accordance with the five-level categorization system based on the number of MBs[6], we conducted semiquantitative grading by c-TCD. Although the semiquantitative grading could not diagnose the presence of PFO in our study, we discovered that once PFO was confirmed by TEE, the grade of RLS evaluated by c-TCD was related to the size of PFO as determined by TEE. However, the specific relationship was not very clear and may be explained by several reasons. One is that the number of MBs appearing in the left atrium detected by contrast TEE is not uniformly evaluated in the literature. Thus, the grade of RLS could not be compared with the size of PFO in the same inspection. Another reason is that the size of PFO measured by TEE could be larger than the real value because of the increased pressure in the right atrium[27]. Moreover, a previous study demonstrated that the size of PFO increases with age[28].
In the current study, 38% of the PFO-CS patients had latent RLS. We found that several patients with latent RLS (detected by c-TCD) were initially negative on TEE. Once we increased the intrathoracic pressure in these patients by appropriately pressurizing their abdomen, the RLS fascicles at the atrial level were observed. This problem could be attributed to difficulty in performing the VM effectively during TEE probe intubation or after sedation; consequently, an inadequate pressure gradient occurs between the right and left atria[29]. This may have led to underestimation or even misdiagnosis of latent RLS induced by PFO on TEE.
TEE is currently regarded as the gold standard for diagnosis of PFO. However, TEE is a semi-invasive detection method, and some patients refuse the procedure due to intolerance. Also, because PFO represents a potential fissure, TEE may miss small PFOs, suggesting that use of TEE may not provide the highest sensitivity for RLS detection[30]. At present, c-TCD is the most commonly used method for detecting RLS clinically, including intracardiac and extracardiac RLS[14]. According to the time when microbubbles appear and whether the signal changed after VM, we could identify intracardiac and extracardiac RLS[31]. The sensitivity and specificity of c-TCD for diagnosis of PFO were reported to be 96.1% and 92.4% respectively[32]. In our study, patients were trained to blow into the connecting tube of a VM quantitative device so that the pressure gauge reached 40 mmHg. Previous studies have reported that the use of quantitative VM significantly improved the positive rate of c-TCD detection of RLS[15]. Indeed, as a noninvasive, effective and repeatable technique, c-TCD has been used to screen PFO through recognition of RLS.
There are two major strengths of this study. The first is the application of quantitative VM in c-TCD ensured effectiveness of the detection. The second is the analysis of DWI lesions of PFO-related CS patients by combining different types and grades of RLS detected by c-TCD and the comparison of the multiple features of the infarct. However, our study also included several limitations. First, the study was retrospective and conducted in a single center; Second, to ensure the integrity of data, all cases selected were inpatients, hence presenting a potential for selection bias; and third, the sample size of this study was not adequately large. Therefore, expanding the sample size and sample selection range as well as conducting in-depth and comprehensive studies are necessary to fully elucidate the mechanism of stroke in PFO-related CS patients.
CS patients with multiple, small and cortical infarcts should be advised to receive c-TCD to determine RLS induced by PFO. Multiple territory infarcts indicate permanent RLS, while posterior circulation infarcts may indicate latent RLS. This infarction pattern suggests that distribution of the infarct may provide clues for c-TCD screening of PFO.
For future research, we will continue to study the mechanism of stroke in patent foramen ovale (PFO)-related cryptogenic stroke (CS) patients, and we plan to cooperate with other stroke centers to expand the range of sample selection and sample size. This will allow more accurate and clinically consistent research results.
In this study, we propose that multiple territory infarctions may indicate permanent right-to-left shunt (RLS), while posterior circulation infarction may indicate latent RLS, which suggests that the distribution of infarct may provide clues for contrast-enhanced transcranial Doppler (c-TCD) screening for PFO. This study proposed a new method of analyzing the characteristics of infarction lesions by combining the type and grade of RLS detected on c-TCD.
The distribution of infarcts indicated a trend for the type of RLS. This observation could contribute to c-TCD screening for PFO. However, this study did have a potential selection bias, and the sample size was not large enough.
This was a retrospective study. The normal group (non-CS patients) was selected for control study, and the CS patient group was divided into specific groups for intragroup and intergroup analyses. Patients in the CS patient group received both c-TCD and transesophageal echocardiography, which allowed us to observe patterns in the infarct lesions.
To find the association between the characteristics of infarction lesions and the type and grade of RLS in PFO-related CS patients. This information will provide a basis for future mechanism research of stroke in PFO-related CS patients.
We aimed to find an association between the characteristics of infarction lesions and the type and grade of RLS in PFO-related CS patients. We hypothesized that we could predict the type and grade of shunt detected during c-TCD examination by the characteristics of the infarction lesions, which would guide the effectiveness of c-TCD.
CS is relatively common in young people. RLS caused by PFO is an important risk factor for CS. Determining the characteristics of infarction lesions in PFO-related CS patients and the type and grade of RLS is of great value in the search for the etiology of CS.
The authors would like to extend their gratitude to the patients who consented to the use of their clinical and image data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neuroimaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gokce E S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson DJ, Schwamm LH, Wilson JA. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1065] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 2. | Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7474] [Cited by in RCA: 8875] [Article Influence: 277.3] [Reference Citation Analysis (0)] |
| 3. | Saver JL. CLINICAL PRACTICE. Cryptogenic Stroke. N Engl J Med. 2016;374:2065-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 203] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 4. | Hara H, Virmani R, Ladich E, Mackey-Bojack S, Titus J, Reisman M, Gray W, Nakamura M, Mooney M, Poulose A, Schwartz RS. Patent foramen ovale: current pathology, pathophysiology, and clinical status. J Am Coll Cardiol. 2005;46:1768-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 212] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 5. | Windecker S, Stortecky S, Meier B. Paradoxical embolism. J Am Coll Cardiol. 2014;64:403-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 6. | Webster MW, Chancellor AM, Smith HJ, Swift DL, Sharpe DN, Bass NM, Glasgow GL. Patent foramen ovale in young stroke patients. Lancet. 1988;2:11-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 550] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 7. | Fonseca AC, Ferro JM. Cryptogenic stroke. Eur J Neurol. 2015;22:618-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Elmariah S, Furlan AJ, Reisman M, Burke D, Vardi M, Wimmer NJ, Ling S, Chen X, Kent DM, Massaro J, Mauri L; CLOSURE I Investigators. Predictors of recurrent events in patients with cryptogenic stroke and patent foramen ovale within the CLOSURE I (Evaluation of the STARFlex Septal Closure System in Patients With a Stroke and/or Transient Ischemic Attack Due to Presumed Paradoxical Embolism Through a Patent Foramen Ovale) trial. JACC Cardiovasc Interv. 2014;7:913-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Stecco A, Quagliozzi M, Soligo E, Naldi A, Cassarà A, Coppo L, Rosso R, Bongo AS, Amatuzzo P, Buemi F, Guenzi E, Carriero A. Can neuroimaging differentiate PFO and AF-related cardioembolic stroke from the other embolic sources? Radiol Med. 2017;122:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | He D, Shi Q, Xu G, Hu Z, Li X, Li Q, Guo Y, Xu S, Lin Y, Yu Z, Wang W, Luo X. Clinical and infarction patterns of PFO-related cryptogenic strokes and a prediction model. Ann Clin Transl Neurol. 2018;5:1323-1337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Nam KW, Guk HS, Kwon HM, Lee YS. Diffusion-Weighted Imaging Patterns According to the Right-to-Left Shunt Amount in Cryptogenic Stroke. Cerebrovasc Dis. 2019;48:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | He Y, Deng J, Tu J, Zhang H, Guo Y. Is inferior vena cava compression an alternative for valsalva maneuver in contrast-enhanced transcranial doppler? Echocardiography. 2020;37:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Kucukoglu MS, Sinan UY, Kilickesmez KO, Saltık IL, Dursun M, Gurmen T. Pulmonary arteriovenous fistula coexisting with secundum atrial septal defect. An unusual cause of stroke in a young woman. Herz. 2012;37:567-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, Tirschwell DL; RESPECT Investigators. Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. N Engl J Med. 2017;377:1022-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 714] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 15. | Guo YZ, Gao YS, Guo ZN, Niu PP, Yang Y, Xing YQ. Comparison of Different Methods of Valsalva Maneuver for Right-to-left Shunt Detection by Contrast-Enhanced Transcranial Doppler. Ultrasound Med Biol. 2016;42:1124-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Van H, Poommipanit P, Shalaby M, Gevorgyan R, Tseng CH, Tobis J. Sensitivity of transcranial Doppler versus intracardiac echocardiography in the detection of right-to-left shunt. JACC Cardiovasc Imaging. 2010;3:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Bang OY, Seok JM, Kim SG, Hong JM, Kim HY, Lee J, Chung PW, Park KY, Kim GM, Chung CS, Lee KH. Ischemic stroke and cancer: stroke severely impacts cancer patients, while cancer increases the number of strokes. J Clin Neurol. 2011;7:53-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Piechowski-Jozwiak B, Bogousslavsky J. Stroke and patent foramen ovale in young individuals. Eur Neurol. 2013;69:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Ryoo S, Chung JW, Lee MJ, Kim SJ, Lee JS, Kim GM, Chung CS, Lee KH, Hong JM, Bang OY. An Approach to Working Up Cases of Embolic Stroke of Undetermined Source. J Am Heart Assoc. 2016;5:e002975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Nedeltchev K, Wiedmer S, Schwerzmann M, Windecker S, Haefeli T, Meier B, Mattle HP, Arnold M. Sex differences in cryptogenic stroke with patent foramen ovale. Am Heart J. 2008;156:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Miranda B, Fonseca AC, Ferro JM. Patent foramen ovale and stroke. J Neurol. 2018;265:1943-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 22. | Hayashida K, Fukuchi K, Inubushi M, Fukushima K, Imakita S, Kimura K. Embolic distribution through patent foramen ovale demonstrated by (99m)Tc-MAA brain SPECT after Valsalva radionuclide venography. J Nucl Med. 2001;42:859-863. [PubMed] |
| 23. | Kim BJ, Kim NY, Kang DW, Kim JS, Kwon SU. Provoked right-to-left shunt in patent foramen ovale associates with ischemic stroke in posterior circulation. Stroke. 2014;45:3707-3710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Xu WH, Xing YQ, Yan ZR, Jiang JD, Gao S. Cardiac right-to-left shunt subtypes in Chinese patients with cryptogenic strokes: a multicenter case-control study. Eur J Neurol. 2014;21:525-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Gempp E, Blatteau JE, Stephant E, Louge P. Relation between right-to-left shunts and spinal cord decompression sickness in divers. Int J Sports Med. 2009;30:150-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Kim JW, Kim SJ, Yoon CW, Park CH, Kang KW, Kim SK, Kim YH, Bang OY. Association between the amount of right-to-left shunt and infarct patterns in patients with cryptogenic embolic stroke: a transcranial Doppler study. Int J Stroke. 2013;8:657-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Chen J, Chen L, Hu W, Ni X, Zhang Z, Feng X, Fan Z, Chen C, Qiu F, Shao B. A comparison of contrast transthoracic echocardiography and contrast transcranial Doppler in cryptogenic stroke patients with patent foramen ovale. Brain Behav. 2019;9:e01283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59:17-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1669] [Cited by in RCA: 1577] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 29. | Rodrigues AC, Picard MH, Carbone A, Arruda AL, Flores T, Klohn J, Furtado M, Lira-Filho EB, Cerri GG, Andrade JL. Importance of adequately performed Valsalva maneuver to detect patent foramen ovale during transesophageal echocardiography. J Am Soc Echocardiogr. 2013;26:1337-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Wessler BS, Kent DM, Thaler DE, Ruthazer R, Lutz JS, Serena J. The RoPE Score and Right-to-Left Shunt Severity by Transcranial Doppler in the CODICIA Study. Cerebrovasc Dis. 2015;40:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Komar M, Olszowska M, Przewłocki T, Podolec J, Stępniewski J, Sobień B, Badacz R, Kabłak-Ziembicka A, Tomkiewicz-Pająk L, Podolec P. Transcranial Doppler ultrasonography should it be the first choice for persistent foramen ovale screening? Cardiovasc Ultrasound. 2014;12:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Katsanos AH, Psaltopoulou T, Sergentanis TN, Frogoudaki A, Vrettou AR, Ikonomidis I, Paraskevaidis I, Parissis J, Bogiatzi C, Zompola C, Ellul J, Triantafyllou N, Voumvourakis K, Kyritsis AP, Giannopoulos S, Alexandrov AW, Alexandrov AV, Tsivgoulis G. Transcranial Doppler versus transthoracic echocardiography for the detection of patent foramen ovale in patients with cryptogenic cerebral ischemia: A systematic review and diagnostic test accuracy meta-analysis. Ann Neurol. 2016;79:625-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |