Published online Mar 22, 2016. doi: 10.5498/wjp.v6.i1.177
Peer-review started: July 11, 2015
First decision: October 30, 2015
Revised: November 19, 2015
Accepted: December 29, 2015
Article in press: January 4, 2016
Published online: March 22, 2016
Processing time: 253 Days and 9 Hours
AIM: To identify findings concerning white matter (WM) fibre microstructural alterations in anorexia nervosa (AN).
METHODS: A systematic electronic search was undertaken in several databases up to April 2015. The search strategy aimed to locate all studies published in English or Spanish that included participants with AN and which investigated WM using diffusion tensor imaging (DTI). Trials were assessed for quality assessment according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses checklist and a published quality index guideline.
RESULTS: A total of 6 studies met the inclusion criteria, four of people in the acute state of the illness, one included both recovered and unwell participants, and one included people who had recovered. Participants were female with ages ranging from 14 to 29 years. All studies but one measured a range of psychopathological features. Fractional anisotropy and mean diffusivity were the main DTI correlates reported. Alterations were reported in a range of WM structures of the limbic system, most often of the fornix and cingulum as well as the fronto-occipital fibre tracts, i.e., regions associated with anxiety, body image and cognitive function. Subtle abnormalities also appeared to persist after recovery.
CONCLUSION: This diversity likely reflects the symptom complexity of AN. However, there were few studies, they applied different methodologies, and all were cross-sectional.
Core tip: The present systematic review identifies the latest research on white matter (WM) brain alterations in anorexia nervosa (AN). The WM architecture has been poorly understood due to its structure forming deep parts of the brain. It transmits information between cortical and subcortical structures. New advances in imaging methods with diffusion tensor imaging, allow its characterization and integrity analysis. Alterations in areas of fornix, cingulum, corpus callosum, cerebellum, superior longitudinal fasciculus and thalamus have been found in AN. They could be related to symptoms like anxiety, body image perception, reward processing and cognitive abilities.
- Citation: Martin Monzon B, Hay P, Foroughi N, Touyz S. White matter alterations in anorexia nervosa: A systematic review of diffusion tensor imaging studies. World J Psychiatr 2016; 6(1): 177-186
- URL: https://www.wjgnet.com/2220-3206/full/v6/i1/177.htm
- DOI: https://dx.doi.org/10.5498/wjp.v6.i1.177
Anorexia nervosa (AN) is a serious illness and has the highest standardized mortality ratio of all psychiatric disorders[1]. According to the DSM-5 criteria, a person in the sick state is underweight for their for age and height, fears gaining weight or becoming fat, and persists in behaviours to avoid weight gain, and weight/shape overvaluation or body image disturbance[2]. In young people it is the third commonest chronic illness and has a mortality rate 12 times higher than the death rate from all causes in young women[3]. In addition to acute effects of self-starvation there may be severe long term medical and psychological sequalae[4].
AN is multidetermined, and as well as psychological and environmental aetiologies, there is increasing evidence for biological underpinnings of its pathophysiology[5]. These include genetic, neuroendocrinologic and metabolic pathways. Furthermore, studies of the brain structure in this mental disorder have identified structural abnormalities. Severely underweight individuals with AN have altered brain function that is considered more profound than found in any other psychiatric disorder[6]. First computed tomography and then functional magnetic resonance imaging and other neuroimaging techniques have been used to study brain alterations in AN[7]. (FMRI measures the brain activity by detecting associated changes in blood flow, on the premise that cerebral blood flow reflects neuronal activation[8]).
The mechanisms addressing brain alterations remains unclear, but changes in brain grey matter (GM) and white matter (WM) might underlie altered brain function and behaviour[9]. Both fibres are part of the central nervous system (CNS) which co-ordinates basic activities of the human body. The GM is related to processing and cognition whilst the WM co-ordinates different brain regions. The first studies found differences in the GM of AN compared to healthy people and other eating disorders. However, the peculiar architecture of WM and the limited availability of imaging methods to study the WM delayed its investigation. Now, new advances in imaging procedures such as diffusion tensor imaging (DTI) allow the study of the structure of WM and its implication in the pathology of AN.
Volumetric brain imaging studies have been used to study GM and WM volume abnormalities in underweight adolescent and adult people with AN[10]. Several have reported loss of global and regional GM and often WM volume. After 2 or 3 d of dehydration these volumes are significantly lower, whereas hyperhydration is associated with higher GM and WM volumes[11].
Functionally connected brain regions utilising WM axons are considered to modulate higher order human behaviours, cognitions and emotions[12]. Investigating WM functionality thus may elucidate the cortical networks and circuitries that underpin the clinical features of AN. Kazlouski et al[13] (2011), were the first to use DTI to identify microstructural WM alterations in adults with AN. They found decreased fractional anisotropy (FA) in the cingulum, fronto-occipital and fimbria-fornix WM tracts.
The normal maturation and reorganization of WM during adolescence and young adult years is thought likely to be disturbed and explain in part the onset of AN[14]. WM fibres such injured WM fibres may not recover in those with enduring illness and years of starvation[15]. Simultaneously, other studies associate nutritional status with quickly occurring GM and WM changes[11].
A variety of studies have previously used the DTI technology showing reduced FA in a multitude of regions across mood, psychotic, anxiety and substance use psychiatric disorders[16]. In AN, the limited research has just began to identify functionally related brain areas structures[13]. Therefore, added to the difficulty to identify AN prior to illness onset, it remains unclear if the structural brain abnormalities are a prerequisite or consequence of the development of AN[5].
Findings are very heterogeneous with respect to WM abnormalities regarding to brain region and behaviour-relationship. Hence, the aim of the current systematic review is to critically examine and synthesise the existing research that has investigated WM microstructural alterations in individuals with AN using DTI. Specifically, the functions of implicated brain areas and relationships with psychopathological measures, and implications for understanding the pathophysiology of AN will be described.
The search strategy was designed to identify all studies of subjects with eating disorders in which microstructure of brain WM brain fibres was analysed through DTI. The literature search was conducted in November 17th 2014 in SCOPUS and Pubmed electronic databases. The following search terms were used: “DTI” AND (“AN” OR “eating disorders”). All fields were included in order to expand the quest. Trials were incorporated if they were published in English and peer-reviewed.
Secondly, we included an additional search in the Spanish language. In December 17th 2014, we introduced the following terms on Lilacs and CSIC electronic databases: “imagen de tensor de difusión” Y (“anorexia nerviosa” O “trastornos de alimentación”). The findings were non-existent in in both of these databases.
In order to update the search, on April 28th 2015 we conducted a third data search on SCOPUS, PubMed, Lilacs and CSIC electronic databases. We used the terms previously described.
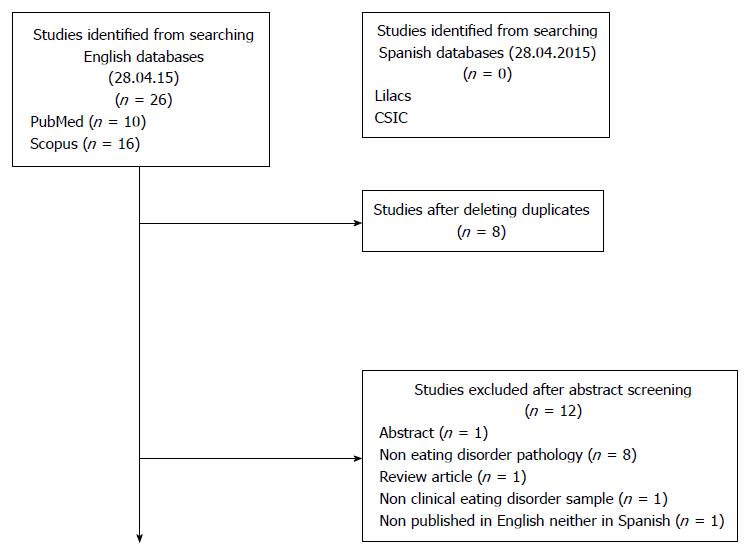
A total of 26 papers were retrieved, from which 8 duplicates were removed. The studies were screened to determine the suitability according to this review by two authors independently. Data extraction and quality assessment were done by Beatriz Martin Monzon, checked for agreement by Nasim Foroughi and disagreements were reviewed by Phillipa Hay. According to the inclusion criteria, 7 papers were excluded based on the abstract, leaving a total of 6 articles for full review. A detailed map of the search strategy stage is shown in Figure 1.
In order to prevent selection bias, the following inclusion criteria were decided on prior to the review. The studies should be: (1) published in a peer-reviewed journal; (2) available either in English or Spanish; (3) participants were over 14 years of age and under 65 years of age; (4) the experimental group should be composed of participants who met the diagnostic criteria for current or lifetime AN according to DSM-5 or DSM-IV criteria; (5) the control group should be composed of healthy participants; and (6) correlates of brain WM fibre were assessed using DTI measures.
Papers were excluded if they were dissertations, review articles or abstracts. No restrictions were placed on year of publication, gender of participants and body mass index (BMI).
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines[17].
The final retrieved articles were assessed using the Quality Index from Downs and Black[18]. The “checklist for measuring study quality” is a tool for assessing the quality of randomised and non-randomised trials, which provides a profile of their methodological strengths and weaknesses. It has reported high internal (KR-20: 0.89) and external consistency (KR-20: 0.54), tested using the Kuder-Richardson formula 20[19].
The 6 studies selected are indicative of the advances in the DTI technique for investigating WM fibre in AN patients. Table 1 presents the main characteristics of the included studies and Table 2 the characteristics of their participant samples. Three of the studies were nested in United States, one in Japan, one in Germany and another in Spain.
| Ref. | AN subtype | ED subtype | Study purpose | Method DTI | Assessment |
| Kazlouski et al[13] | Current AN restricting and purging subtype | AN purging and restricting subtype | Test of the brain WM integrity alteration and its relation to heightened anxiety in AN | GE 3 Tesla whole-body MRI scanner, maximum gradient amplitude of 40 Mt/M and maximum slew rate of 150 T/m per second, 8-channel phased-array head coil | SCID-CV |
| EDI-3 | |||||
| TCI | |||||
| STAI | |||||
| BDI | |||||
| Frank et al[49] | Current AN restricting and purging subtype | AN | Analysis of GM and WM in a sample of adolescents in comparison to adults | Signa 3T Scanner, axial, 3-dimensional, T-1 weighted magnetization-prepared rapid acquisition gradient echo | C-DISC |
| EDI-3 | |||||
| TCI | |||||
| STAI | |||||
| CDI | |||||
| SPSRQ | |||||
| 9 point Likert scale sweetness-pleasantness of 1 molar sucrose | |||||
| Nagahara et al[7] | Current AN, NS subtype | AN | Exploration of WM abnormalities in AN patients compared to HC | Whole body 3 Tesla MR system with an eight-channel phased-array head coil | SCID-CV |
| EDI-2 | |||||
| BDI-II | |||||
| Via et al[53] | Current AN restricting subtype | AN | Assessment of WM microstructure in a clinical sample of AN patients | GE Signa Excite scanner at 1.5 T equipped with an 8-channel phased-array head coil | SCID-CV |
| SCID-I/NP | |||||
| GHQ-28 | |||||
| EDI-2 | |||||
| HAM-D | |||||
| HAM-A | |||||
| Frieling et al[5] | Current AN with NS subtype and recovered women from AN | AN and recovered women from AN | Assessment of the microstructural integrity of WM pathways through DTI in women with AN | 3 T Siemens-scanner, with a gradient field strength up to 45 MT/m | None |
| Yau et al[39] | Recovered women from restricting type AN | Recovered women from restricting type AN | Examination of the microstructural alterations of WM integrity in recovered AN women | 3.0 Tesla Discovery MR750 scanner with an 8-channel phase-array head coil | SCID-CV |
| MINI | |||||
| TCI | |||||
| STAI | |||||
| MPS |
| Ref. | AN subtype | Total size | Sample characteristics | ||
| n | |||||
| Gender | |||||
| Mean ± SD: | |||||
| Age/years | |||||
| BMI | |||||
| Duration of illness/years | |||||
| ED group (AN) | Recovered AN | Healthy group | |||
| Kazlouski et al[13] | Current AN restricting and purging subtype | n = 33 | n = 16 (10 resAN, 6 purgAN) | - | n = 17 |
| Female | Female | ||||
| 23.9 ± 7 SD | 25.1 ±4 SD | ||||
| 16.5 ± 1 SD | 21.5 ±1 SD | ||||
| 7.5 ± 8 SD | NA | ||||
| Frank et al[49] | Current AN restricting and purging subtype | n = 41 | n = 19 (17 resAN, 2 purgAN) | - | n =22 |
| NS | Female | ||||
| 15.4 ± 1.4 SD | 14.8 ± 1.8 SD | ||||
| 16.2 ± 1.1 SD | 21.3 ± 1.9 SD | ||||
| NS | NA | ||||
| Nagahara et al[7] | Current AN, NS subtype | n = 35 | n = 17 | - | n = 18 |
| Female | Female | ||||
| 23.8 ± 6.68 SD | 26.2 ± 5.6 SD | ||||
| 13.6 ± 1.3 SD | 19.9 ± 2.0 SD | ||||
| 4.93 ± 4.9 SD | NA | ||||
| Via et al[53] | Current AN restricting subtype | n = 38 | n = 19 | - | n = 19 |
| Female | Female | ||||
| 28.37 ± 9.55 SD | 28.63 ± 8.58 SD | ||||
| 17.03 ± 1.09 SD | 21.09 ± 1.80 SD | ||||
| 6.52 ± 6.03 SD | NA | ||||
| Frieling et al[5] | Current AN with NS subtype and recovered women from AN | n = 41 | n = 12 | n = 9 | n = 20 |
| Female | Female | Female | |||
| 26.8 ± 6.94 SD | 27.44 ± 5.3 SD | 24.80 ± 2.6 SD | |||
| 15.18 ± 1.39 SD | 19.31 ± 1.39 SD | 19.60 ± 0.94 SD | |||
| NS | NS | NA | |||
| Yau et al[39] | Recovered women from restricting type AN | n = 22 | - | n = 12 | n = 10 |
| Female | Female | ||||
| 28.7 ± 7.9 SD | 26.7 ± 5.4 SD | ||||
| 21.2 ± 1.5 SD | 22.0 ± 1.1. SD | ||||
| 5.6 ± 5.2 | NA | ||||
All the studies included a group of AN people and a control or healthy group. From this total, 2 studies also included recovered AN women as one of the groups of participants investigated. As inclusion criteria, it was mandatory to be diagnosed as AN according to the DSM-IV criteria and that the BMI was measured. All of them were comprised of women with mean ages ranging from 14 to 29 years old.
All 6 papers used a cross-sectional design. Three studies used a Tesla scanner, 2 a Signa scanner and one a Siemens model scanner for the diffusion tensor templates. Five papers used self-report questionnaires to assess psychopathological characteristics and one did not report use of assessment instruments.
Table 3 presents the main outcomes of each study and author’s interpretation of the findings. Following the DTI procedures, the brain areas selected by the authors as possibly shown abnormalities are the fornix (and its connection to the fimbria tract), the cingulum, the thalamus (several nuclei), the superior longitudinal fasciculus, the corona radiata, the cerebellum and the fronto-occipital fibre tracts. Their main functions are emotion processing, body perception, reward processing, anxiety and cognitive style.
| Ref. | AN subtype | Outcomes: DTI measures and areas in AN | Author interpretation |
| Kazlouski et al[13] | Current AN restricting and purging subtype | FA: Lower in the bilateral fimbria-fornix, fronto-occipital and cingulum fiber tracts | Anxiety is predicted by the fimbria-fornix FA value. Thus, reduced WM integrity could provide a mechanism for heightened anxiety |
| Frank et al[49] | Current AN restricting and purging subtype | FA: Lower in fornix, cingulum and corpus callosum (corona radiata and forceps mayor) | Abnormal fornix integrity could lead to altered feedback between limbic and higher-order brain structures. The corpus callosum could be implicated in taste processing |
| Nagahara et al[7] | Current AN, NS subtype | FA: Lower value in the left cerebellum | WM abnormalities in the fornix and the cerebellum may be neural substrates of the pathophysiology of AN. The fornix is one of the important components of the Papez circuit, which links the limbic system with other brain structures. The correlation of WM alteration with physical severity, including BMI and duration of illness may indicate that WM alteration is more relevant with regard to physical severity rather than psychological severity |
| MD: Higher value in the fornix | |||
| Via et al[53] | Current AN restricting subtype | FA: Lower in the parietal part of the left SLF and the fornix. | The left SLF seem relevant to body image distortion as well as other cognitive processes like the called weak central coherence. The fornix is a key structure involved in the regulation of body-energy balance and processing of reward responses |
| MD: Higher in the SLF and the fornix. They also reported significantly increased MD in the fornix, accompanied by decreased FA and increased RD and AD | |||
| Frieling et al[5] | Current AN with NS subtype and recovered women from AN | FA (AN and recAN): Lower in the posterior thalamic radiation bilaterally (which includes the of optic radiation) and the left mediodorsal thalamus | The posterior thalamic radiation fibres project to areas involved in the processing of the body image, whose alteration could explain the AN body image distortion. The left mediodorsal thalamic nucleus is connected to regions contributing to impairments in cognitive domains, especially set/shifting ability, executive control, habit learning and reward processing |
| Yau et al[39] | Recovered women from restricting type AN | FA (recAN): Insignificant alteration | Lower MD was associated with harm avoidance, suggesting a possible underlying trait associated with AN. Localization of disturbances in frontal-parietal and cingulum WM suggests that these pathways, which are important for cognitive control, may be susceptible to core AN pathology. Malnutrition seems to have potentially lasting effects on WM integrity and degree of recovery |
| MD: Lower in frontal, parietal and cingulum |
Overall, the studies met most of the quality criteria considered for this review (Table 4). A maximum score of 8 points based in a yes/no/partially scale response was developed to grade quality. The score range was from 4 to 7 points, with mean of 5.75 and SD of 1.57.
| Ref. | Q1 | Q2 | Q3 | Q5 | Q6 | Q15 | Q18 | Q25 |
| Hypothesis clearly described | Main outcomes clearly described in the introduction or methods section | Participants characteristics clearly described | Distributions of confounders in each group of subjects clearly described | Main study findings clearly described | Study measurement blind | Appropriateness of the statistical tests | Adjustment for confounding | |
| Kazlouski et al[13] | Yes | Yes | Yes | No | Yes | U | U | No |
| Frank et al[49] | Yes | Yes | Yes | Partially | Yes | U | Yes | Yes |
| Nagahara et al[7] | Yes | Yes | Yes | No | Yes | U | Yes | No |
| Via et al[53] | Yes | Yes | Yes | Yes | Yes | U | Yes | Yes |
| Frieling et al[5] | Yes | Yes | Yes | Yes | Yes | U | Yes | Yes |
| Yau et al[39] | Yes | Yes | Yes | No | Yes | U | Yes | No |
Specifically, all the papers described the hypothesis or main study objective. The main outcomes to be investigated were clearly described in the Introduction or Method section. The participant characteristics were clearly described, often being displayed in a chart or table. The intervention of interest wasn’t applicable to the present systematic review, since none of the retrieved studies developed an interventional design. The main weakness of the studies was the description of the confounders’ distribution. This was clearly described in one, partially in two and not provided in three studies. Adjustment for confounding was reported in three studies only. Also, most studies did not include another mental health disorder such as depression in participants in a control group. This was clearly described in one study, partially in two studies and not provided in three studies. The main study findings were clearly described in all the papers. All but one study, which didn’t provide enough information, selected appropriate statistical tests for each research study measurement.
DTI measures reported in the six included papers: Water diffusion direction along the WM tracts was measured with FA and MD was used as an indicator of white matter integrity, a scalar measure reflecting the magnitude of diffusion. Mean diffusivity (MD) or in other term apparent diffusion coefficient provides information about the average diffusion-freedom water molecules have in the brain tissue, and correlates with local cell breakdown[20-22].
Psychopathological measures: To confirm the diagnoses of AN, five of the 6 collected studies used the Structured Clinical Interview for DSM-IV Axis I Disorders. Specifically, four studies used the clinician version[23] and one of them included the non-patient edition for the sample group[24] and the General Health Questionnaire[25]. One of the studies chose the Computerized Diagnostic Interview for Children[26] and one The Mini-International Neuropsychiatric Interview[27].
In general, the majority of the studies used the same assessment instruments for general eating disorders symptoms, depression as well as anxiety. The presence of symptoms and psychological features typically presented in AN was assessed through the eating disorders inventory-3[28] in two studies whilst its second version[29] was used in other 2. Three studies complemented it with the temperament and character inventory[30]. Mood was assessed with the beck depression inventory first edition (BDI)[31] and its second edition (BDI-II)[32] in two studies. Three studies used the state-trait anxiety inventory[33] to measure anxiety levels. Other questionnaires applied the hamilton rating scale for depression[34] and the hamilton rating scale for anxiety[35,36], the children’s depression inventory[36], the revised sensitivity to reward and punishment questionnaire[37] and the multidimensional perfectionism scale[38]. In addition, in one study a molar sucrose and a control solution for sweetness and pleasantness were rated on a 9-point Likert scale.
The neurobiology of AN is still poorly understood. This systematic review reports research that investigated WM fibre tracts using advances in DTI procedures. Sample selection criteria, type of scanner and psychopathological measures used, as well as DTI correlates with brain functions connections were documented. Six studies were included and WM fibre changes were found in tracts relevant to emotional brain circuits as well as to neurocognitive brain structures.
Four of the six included studies performed the WM fibre measurement in patients at the sick state, one included recovered AN patients and another one studied the possible WM alterations in recovered and as well AN acute patients. As a control measure, some studies included the indicator of low BMI in the healthy sample to make the groups comparable. The criteria for recovery differed in the two studies that included such participants. Specifically, in the study of Frieling et al[5], the participants didn’t meet criteria for a diagnosis of AN at the time of inclusion, and had a BMI above 18 km/m2. In contrast, Yau et al[39] defined recovery based on symptoms of no food restriction, no compulsive exercise and not engaging in any other ED behaviours as well as sustaining a stable weight (± 3 kg and 90% to 120% of mean body weight) with regular menstrual cycles.
Four of the studies found either a low FA value or a high MD correlate in the fornix. This formation is an important WM structure that originates from the hippocampus as the fimbria-fornix tract and projects superior-anteriorly toward the midline, forming the body of the fornix[40]. It is a part of the Papez Circuit, an element of the limbic system involved in the regulation of emotions by higher order frontal cortical brain regions. It is linked to the hypothalamus, the amygdala as well as to prefrontal areas like the ventral striatum, the orbitofrontal cortex and the cingulate cortex[41]. The fornix is a key connection involved in the regulation of body-energy balance and processing of reward responses. It has been associated with trait anxiety and harm avoidance and responds to leptin (feeding inhibiting hormone)[42]. The last studies suggest that fornix alterations in AN could be involved in the characteristic of food restriction, disrupted meal patterns, altered food reward processing and difficulties making behaviour changes[43]. Previous studies in rodents have identified those altered patterns[44].
Another well reported brain structure in three of the retrieved studies is the cingulum. It is composed of a WM association fibres bound from the cingulate gyrus to the entorhinal cortex in the brain. It is part of the limbic system which connects frontal lobe regions with posterior structures as the temporal lobe and hippocampus. They comprise a network that integrates the necessary behaviours for executive function and emotion recognition[45]. Alteration in this structure could be related to altered emotion identification and processing in AN, as well as disturbances in cognitive control[46].
At the same time, two studies found reduced FA in fronto-occipital association fibres. These tracts connect frontal with occipital and posterior parietal and temporal lobes. They integrate auditory and visual association cortices and may have a role in the spatial awareness and neglect, as well as emotion recognition and expression[47]. The fronto-occipital WM abnormality could be related to altered body perception shown in AN. It has been associated with abnormal recognition of emotions in others and emotion regulation within themselves[48].
Frieling et al[5] (2012) identified bilateral reductions of FA maps in the posterior thalamic radiation and the left mediodorsal thalamus. Alterations of the posterior thalamic radiations are of special interest because they are connected with the occipital, temporal and parietal lobes which are all involved with the cortical processing of body image perception. In contrast, the left mediodorsal thalamus is linked to brain structures implied in cognitive domains related to the shifting ability, executive control, habit learning and reward processing.
Alterations of WM fibre were seen as well in adolescent AN samples. Frank et al[49] (2013) registered a low FA in the corpus callosum. This area facilitates communication between left and right sided brain structures and has been implicated in taste processing. Parent et al[50] (1996) found an alteration in the corona radiate and forceps major, a group of bundles that connect the cerebral cortex with the basal ganglia and spinal cord. Both structures are related to taste, whose significance could be related to altered taste and processing in AN[51].
There are several brain structures that integrate a functional network connecting the prefrontal and parietal areas which may be implicated in the body image distortion found in AN. The superior longitudinal fasciculus is the major association WM tract connecting these regions[52]. Accordingly, Via et al[53] (2014) reported significant FA decreases in the parietal areas of the superior longitudinal fasciculus, which sets the basis for the representation of the body self-image[54]. Proprioception, size and spatial judgment, visual imagery and the integration of visual information are properties of body image. Apart from that, the authors suggested the possible implication of the superior longitudinal fasciculus in other cognitive processes such as the “weak central coherence” reported in people with AN. This cognitive style is characterized by an enhanced attention to local details at the expense of global processing and is found in several disorders[55]. These abnormalities are thought to depend on alterations of long-range connections between prefrontal and parieto-occipital areas, which could be also implicated in AN[56].
One of the studies[39], reported altered WM patterns in parietal areas of AN subjects. The authors found reduced diffusivity in fronto-parietal areas that was associated with cognitive control to be related to increased post-error slowing during response inhibition[57]. These results are consistent with increased cognitive control and error perseveration[58]. Thus, a low FA value may be associated with typical AN traits such as enhanced cognitive control and clinical perfectionism[6].
Moreover, it has been seen that abnormalities in the left cerebellum exist in AN patients. Nagahara et al[7] (2014) found a low FA value in the lateral zone of this structure. It projects to the dentate nucleus which has connections with the ventral anterior nucleus and ventral lateral nucleus of the thalamus. Added to the functions that we have specified previously, the thalamus facilitates coordinated movements and has a direct bidirectional connection with regions involved in the regulation of food intake. Specifically, the connections with several hypothalamic nuclei (the lateral hypothalamic areas, the dorsomedial hypothalamic nucleus and the ventromedial hypothalamic nucleus) could prompt abnormal food intake behaviour in AN[59]. Furthermore, the cerebellum also relates social, empathic and emotional function, including fear response[60]. Altogether, the alterations of these functions may underline the pathophysiology of AN[61].
In sum, the pathophysiology of AN is still unknown but the limbic system is undoubtedly implicated as many of the DTI studies found alterations in this network. The connections with the prefrontal areas might explain the dysfunction which appears in the cognitive style and emotion procession. Two main theories are considered in understanding these neurocognitive mechanisms in AN. The alterations in WM fibre could reflect predisposing personality traits and neurocognitive style. On the other hand, the effects of undernutrition could cause these abnormalities in WM.
Assessment instruments varied between the studies based on the analysis method. Most of the studies selected a combined method of assessment including cognitive assessment via questionnaires and MRI scanning for analysis of the WM fibre. Although some of the questionnaires were commonly used in most of the studies, the diverse range of used questionaries is problematic for comparing between studies. There was also diversity in the type of scanner used for imaging, the patient’s position in the scanner, and the selected regions of interest.
Previous studies have analysed specified DTI measures, including FA and MD. The axial diffusion coefficient has also been used in some of the previous studies. Future studies should consider investigating the relationship between the DTI measures and indirect measures. The exposed FA and MD are broad measures that could be driven by a number of factors (e.g., axonal ordering, density, degree of myelination). For this reason, axial diffusivity and radial diffusivity (RD) are thought to index more specific aspects of WM pathology, being more sensitive to changes in integrity and myelination, respectively. The combined quantification of direct and indirect measurements is recommended to better characterize any putative changes in WM structure.
All the studies used a cross-sectional design and there is a need for longitudinal studies to examine the WM alterations over time to determine if changes found in the recovered state are a persistent “scare” of illness effects such as starvation, or are innate to the disorder. Furthermore, future research should consider more comprehensive examination of myelination changes in people with AN. Changes in RD have been observed after different periods of cognitive treatment or meditation. This may reflect a brain plastic response that could provide new insights for understanding recovery in AN.
Finally, this review was limited in that only four databases were used and two of which were English language databases. However these two were the most relevant databases to the topic, and one, Scopus, is a composite of papers from many sources including several other databases such as MEDLINE and EMBASE. Insufficient funding for translations also precluded sourcing literature published in languages excepting those which the authors were fluent in.
In conclusion, the results of our review suggest that WM changes exist in brain fibres of people with AN. The research also showed that the changes appear in the brain during the sickness stage and the recovery stage in patients with AN. The main brain alterations seem to involve tracts of the limbic system, as the fornix. A prompt treatment plan for the sickness and recovery stages seems crucial for minimising the progression of brain impairments. However, there were few studies and problems of inconsistent assessment methodologies and a need for replication of findings and longitudinal study design.
There is evidence for biological underpinnings in anorexia nervosa (AN) that argue in favour of structural brain abnormalities. Previous research performed with Voxel Based Morphometry has found reduced grey matter (GM) volumes in people with AN compared to healthy controls. White matter (WM) is the second major component of the central nervous system (CNS) and it coordinates communication between different brain regions.
Very little research has been performed on WM in AN participants due to limited technologies able to investigate its particular structure. New advances with diffusion tensor imaging (DTI) however have identified alterations in several areas of the brain WM providing new insights into the structure and related function of WM in people with AN.
This review synthesises all the studies to date (April 2015) using DTI to assess the WM integrity in AN. Alterations in a range of WM structures of the limbic system were identified in regions associated with anxiety, body image and cognitive function. Subtle abnormalities also appeared to persist after recovery but no studies were prospective.
This systematic review synthetises high quality research evidence related to WM brain alterations in AN. It provides researchers with the latest knowledge available and directions for future research.
WM: Component of the CNS that distributes information between different brain areas; GM: Component of the CNS related to processing and cognition activities; DTI: Magnetic resonance imaging method that allows the mapping of the diffusion of molecules in the brain, in vivo and non-invasively. It is able to delineate fibre tracts within the WM; Fractional anisotropy (FA): A value that describes the degree of anisotropy of a diffusion process. It is thought to reflect myelination of WM; Mean diffusivity: An opposed value to FA which reflects the isotropy of the diffusion.
The present systematic review aims to identify findings concerning WM fibre microstructural alterations in AN. The authors find that WM fibre is altered in diverse areas of brain, the diversity possibly reflecting the symptom complexity of the AN.
P- Reviewer: Shi YY S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Papadopoulos FC, Ekbom A, Brandt L, Ekselius L. Excess mortality, causes of death and prognostic factors in anorexia nervosa. Br J Psychiatry. 2009;194:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 288] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 2. | American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington D.C, 2013. . |
| 3. | Sullivan PF. Mortality in anorexia nervosa. Am J Psychiatry. 1995;152:1073-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 438] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 4. | Steinhausen HC. The outcome of anorexia nervosa in the 20th century. Am J Psychiatry. 2002;159:1284-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 999] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 5. | Frieling H, Fischer J, Wilhelm J, Engelhorn T, Bleich S, Hillemacher T, Dörfler A, Kornhuber J, de Zwaan M, Peschel T. Microstructural abnormalities of the posterior thalamic radiation and the mediodorsal thalamic nuclei in females with anorexia nervosa--a voxel based diffusion tensor imaging (DTI) study. J Psychiatr Res. 2012;46:1237-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10:573-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 583] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 7. | Nagahara Y, Nakamae T, Nishizawa S, Mizuhara Y, Moritoki Y, Wada Y, Sakai Y, Yamashita T, Narumoto J, Miyata J. A tract-based spatial statistics study in anorexia nervosa: abnormality in the fornix and the cerebellum. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Huettel S, Song A, McCarthy G. Functional Magnetic Resonance Imaging. 3rd ed. Hardcover, 2014. . |
| 9. | Hao X, Xu D, Bansal R, Dong Z, Liu J, Wang Z, Kangarlu A, Liu F, Duan Y, Shova S. Multimodal magnetic resonance imaging: The coordinated use of multiple, mutually informative probes to understand brain structure and function. Hum Brain Mapp. 2013;34:253-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Van den Eynde F, Suda M, Broadbent H, Guillaume S, Van den Eynde M, Steiger H, Israel M, Berlim M, Giampietro V, Simmons A. Structural magnetic resonance imaging in eating disorders: a systematic review of voxel-based morphometry studies. Eur Eat Disord Rev. 2012;20:94-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Streitbürger DP, Möller HE, Tittgemeyer M, Hund-Georgiadis M, Schroeter ML, Mueller K. Investigating structural brain changes of dehydration using voxel-based morphometry. PLoS One. 2012;7:e44195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Dehaene S, Changeux JP. Reward-dependent learning in neuronal networks for planning and decision making. Prog Brain Res. 2000;126:217-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Kazlouski D, Rollin MD, Tregellas J, Shott ME, Jappe LM, Hagman JO, Pryor T, Yang TT, Frank GK. Altered fimbria-fornix white matter integrity in anorexia nervosa predicts harm avoidance. Psychiatry Res. 2011;192:109-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Connan F, Campbell IC, Katzman M, Lightman SL, Treasure J. A neurodevelopmental model for anorexia nervosa. Physiol Behav. 2003;79:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 154] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Asato MR, Terwilliger R, Woo J, Luna B. White matter development in adolescence: a DTI study. Cereb Cortex. 2010;20:2122-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 377] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 16. | White T, Nelson M, Lim KO. Diffusion tensor imaging in psychiatric disorders. Top Magn Reson Imaging. 2008;19:97-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9247] [Cited by in RCA: 8877] [Article Influence: 554.8] [Reference Citation Analysis (0)] |
| 18. | Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5177] [Cited by in RCA: 5937] [Article Influence: 219.9] [Reference Citation Analysis (0)] |
| 19. | Kuder G, Richardson M. The theory of the estimation of test reliability. Psychometrika. 1937;2:151-160. [DOI] [Full Text] |
| 20. | Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 835] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 21. | Cohen MX, Schoene-Bake JC, Elger CE, Weber B. Connectivity-based segregation of the human striatum predicts personality characteristics. Nat Neurosci. 2009;12:32-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 214] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 22. | Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. 1996. J Magn Reson. 2011;213:560-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 304] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 23. | First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders. Clinician Version (SCID-CV) ed. Washington, D.C. : American Psychiatric Press, Inc 1996; . |
| 24. | First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV). Clinician version ed. Washington: D.C. American Psychiatric Press 2002; . |
| 25. | Goldberg D. Manual of the General Health Questionnaire. UK: Windsor (UK) 1978; . |
| 26. | Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28-38. [PubMed] |
| 27. | Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22-33; quiz 34-57. [PubMed] |
| 28. | Garner D. Eating Disorder Inventory-3. Professional Manual. 3rd ed. Lutz, FL: Psychological Assessment Resources, Inc 2004; . |
| 29. | Garner D. EDI-2. Eating disorder inventory-2. Professional manual. 2nd ed. Odessa, FL: Psychological Assessment Resources 1991; . |
| 30. | Cloninger R. The temperament and character inventory (TCI): A guide to its development and use. 1st ed. St. Louis, MO: Center for Psychobiology of Personality, Washington University 1994; . |
| 31. | Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561-571. [PubMed] |
| 32. | Kojima M, Furukawa TA, Takahashi H, Kawai M, Nagaya T, Tokudome S. Cross-cultural validation of the Beck Depression Inventory-II in Japan. Psychiatry Res. 2002;110:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 340] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 33. | Spielberger C, Gorssuch R, Lushene P, Vagg P, Jacobs G. Manual for the State-Trate Anxiety Inventory. 1st ed. Palo Alto, CA: Consulting Psychologists Press, Inc 1983; . |
| 34. | Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62. [PubMed] |
| 35. | Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6169] [Cited by in RCA: 6789] [Article Influence: 102.9] [Reference Citation Analysis (0)] |
| 36. | Kovacs M. Children’s Depression Inventory. 1st ed. North Tonawanda: NY Multi-Health Systems, Inc 1992; . |
| 37. | Torrubia R, Ávila C, Moltó J, Caseras X. The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Pers Individ Dif. 2001;31 Suppl 6:837-862. [RCA] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 828] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 38. | Frost RO, Marten P, Lahart C, Rosenblate R. The Dimensions of Perfectionism. Cognitive Ther Res. 1990;14 Suppl 5:449-468. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2175] [Cited by in RCA: 1851] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 39. | Yau WY, Bischoff-Grethe A, Theilmann RJ, Torres L, Wagner A, Kaye WH, Fennema-Notestine C. Alterations in white matter microstructure in women recovered from anorexia nervosa. Int J Eat Disord. 2013;46:701-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Saunders RC, Aggleton JP. Origin and topography of fibers contributing to the fornix in macaque monkeys. Hippocampus. 2007;17:396-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 41. | Mettler LN, Shott ME, Pryor T, Yang TT, Frank GK. White matter integrity is reduced in bulimia nervosa. Int J Eat Disord. 2013;46:264-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 309] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 43. | Keating C, Tilbrook AJ, Rossell SL, Enticott PG, Fitzgerald PB. Reward processing in anorexia nervosa. Neuropsychologia. 2012;50:567-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 44. | Osborne B, Silverhart T, Markgraf C, Seggie J. Effects of fornix transection and pituitary-adrenal modulation on extinction behavior. Behav Neurosci. 1987;101:504-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Hutchins T, Herrod H, Quigley E, Anderson J, Salzman K. Dissecting the White Matter Tracts: Interactive Diffusion Tensor. Imaging Teaching Atlas. University of Utah Department of Neuroradiology; Online Atlas. Available from: http://www.asnr2.org/neurographics/7/1/26/White Matter Tract Anatomy/DTI tutorial 1.html05/19. |
| 46. | Bunge SA, Wright SB. Neurodevelopmental changes in working memory and cognitive control. Curr Opin Neurobiol. 2007;17:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 251] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 47. | Philippi CL, Mehta S, Grabowski T, Adolphs R, Rudrauf D. Damage to association fiber tracts impairs recognition of the facial expression of emotion. J Neurosci. 2009;29:15089-15099. [PubMed] |
| 48. | Harrison A, Tchanturia K, Treasure J. Attentional bias, emotion recognition, and emotion regulation in anorexia: state or trait? Biol Psychiatry. 2010;68:755-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 49. | Frank GK, Shott ME, Hagman JO, Yang TT. Localized brain volume and white matter integrity alterations in adolescent anorexia nervosa. J Am Acad Child Adolesc Psychiatry. 2013;52:1066-1075.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 50. | Parent A, Carpenter M. Carpenter’s human neuroanatomy. 9th ed. André Parent ed. United States: Baltimore, Williams & Wilkins 1996; . |
| 51. | Fabri M, Polonara G, Mascioli G, Salvolini U, Manzoni T. Topographical organization of human corpus callosum: an fMRI mapping study. Brain Res. 2011;1370:99-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 52. | Devue C, Brédart S. The neural correlates of visual self-recognition. Conscious Cogn. 2011;20:40-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 53. | Via E, Zalesky A, Sánchez I, Forcano L, Harrison BJ, Pujol J, Fernández-Aranda F, Menchón JM, Soriano-Mas C, Cardoner N. Disruption of brain white matter microstructure in women with anorexia nervosa. J Psychiatry Neurosci. 2014;39:367-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 54. | Gaudio S, Quattrocchi CC. Neural basis of a multidimensional model of body image distortion in anorexia nervosa. Neurosci Biobehav Rev. 2012;36:1839-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 55. | Baron-Cohen S, Belmonte MK. Autism: a window onto the development of the social and the analytic brain. Annu Rev Neurosci. 2005;28:109-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 203] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 56. | Fonville L, Lao-Kaim NP, Giampietro V, Van den Eynde F, Davies H, Lounes N, Andrew C, Dalton J, Simmons A, Williams SC. Evaluation of enhanced attention to local detail in anorexia nervosa using the embedded figures test; an FMRI study. PLoS One. 2013;8:e63964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Fjell AM, Westlye LT, Amlien IK, Walhovd KB. A multi-modal investigation of behavioral adjustment: post-error slowing is associated with white matter characteristics. Neuroimage. 2012;61:195-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Danielmeier C, Ullsperger M. Post-error adjustments. Front Psychol. 2011;2:233. [PubMed] |
| 59. | Zhu JN, Wang JJ. The cerebellum in feeding control: possible function and mechanism. Cell Mol Neurobiol. 2008;28:469-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 60. | Villanueva R. The cerebellum and neuropsychiatric disorders. Psychiatry Res. 2012;198:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 61. | Oldershaw A, Hambrook D, Stahl D, Tchanturia K, Treasure J, Schmidt U. The socio-emotional processing stream in Anorexia Nervosa. Neurosci Biobehav Rev. 2011;35:970-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |