Copyright
©The Author(s) 2025.
World J Crit Care Med. Sep 9, 2025; 14(3): 101462
Published online Sep 9, 2025. doi: 10.5492/wjccm.v14.i3.101462
Published online Sep 9, 2025. doi: 10.5492/wjccm.v14.i3.101462
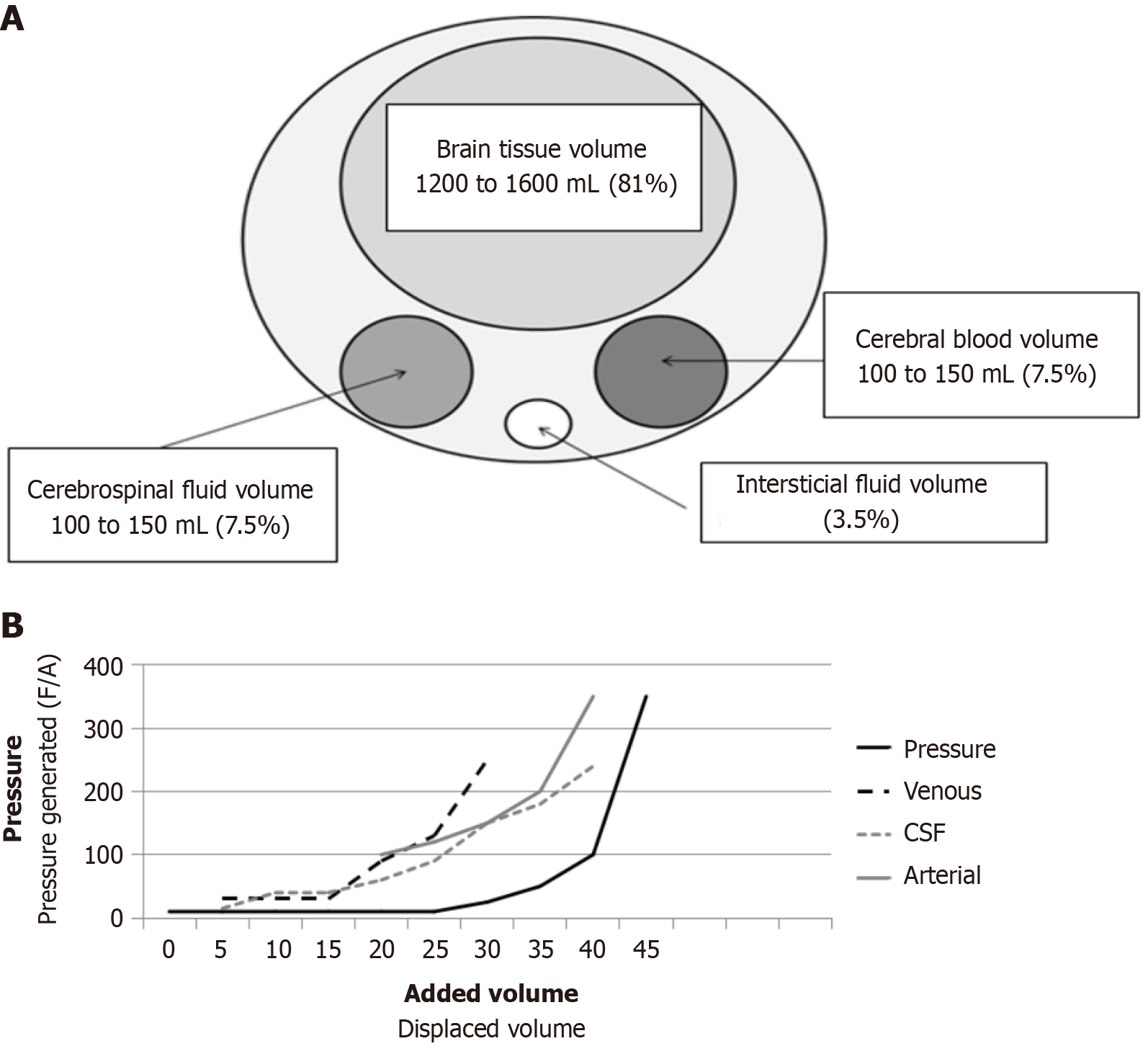
Figure 1 Maintain the intracranial pressure.
A: Monro-Kellie law. Brain compartments with their respective volumes and percentage of total volume; B: Langfitt’s pressure-volume curve showing the delay in pressure increase relative to volume increase, which suggests the existence of a compensatory mechanism. The generated pressure, calculated as force/area, is a consequence of the displaced volume. Firstly, the first capacitance system, the venous volume, is displaced, followed by the cerebrospinal fluid, and finally the arterial volume. F/A: Force/area; CSF: Cerebrospinal fluid.
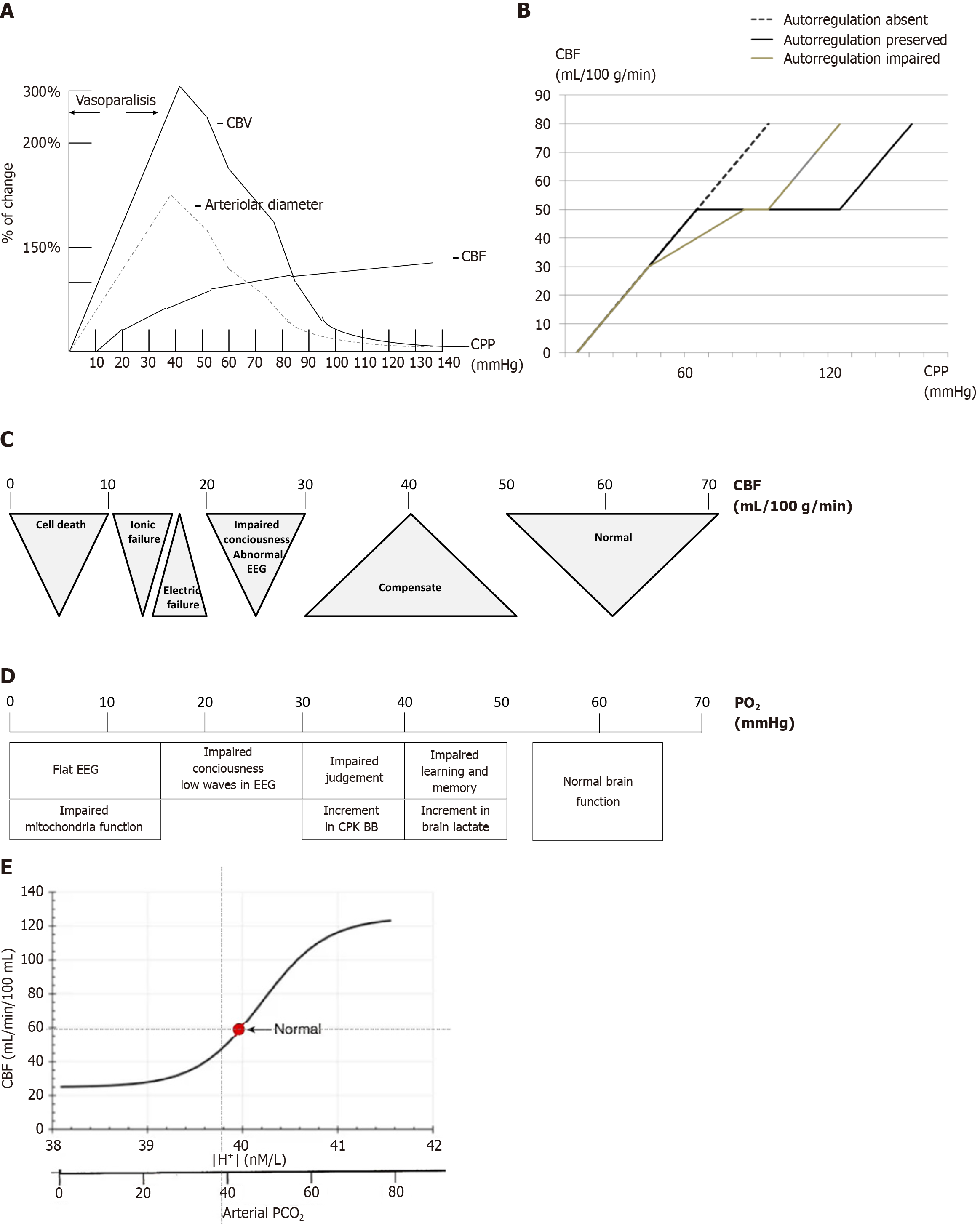
Figure 2 Relationship between cerebral blood flow and cerebral perfusion pressures.
A: Solid line represents preserved autoregulation, dashed line represents impaired autoregulation, and dotted line represents abolished autoregulation. Note that both altered and abolished autoregulation require higher cerebral perfusion pressures to maintain adequate cerebral blood flow; B: Relative changes in cerebral blood volume, vascular diameter, and cerebral blood flow in response to changes in cerebral perfusion pressures; C: Cerebral blood flow thresholds. The critical threshold for cellular death is below 10 mL/100 g/minute; D: Hypoxic thresholds. Clinical neurological signs emerge only at significantly low partial oxygen levels (below 40 mmHg); E: A typical graph illustrating this relationship places cerebral blood flow on the y-axis and partial carbon dioxide pressure and pH on the x-axis. As partial carbon dioxide pressure and H+ concentrations increase, cerebral blood flow (CBF) rises sharply until it reaches a plateau, beyond which further increases have a diminishing impact on CBF. Conversely, as partial carbon dioxide pressure and H+ concentrations decrease, CBF declines until it reaches a lower plateau, where additional decreases have minimal effect on CBF. CBV: Cerebral blood volume; CBF: Cerebral blood flow; CPP: Cerebral perfusion pressures; PO2: Partial oxygen; PCO2: Partial carbon dioxide pressure; EEG: Electroencephalography; CPK BB: Brain creatin phosphokinase.
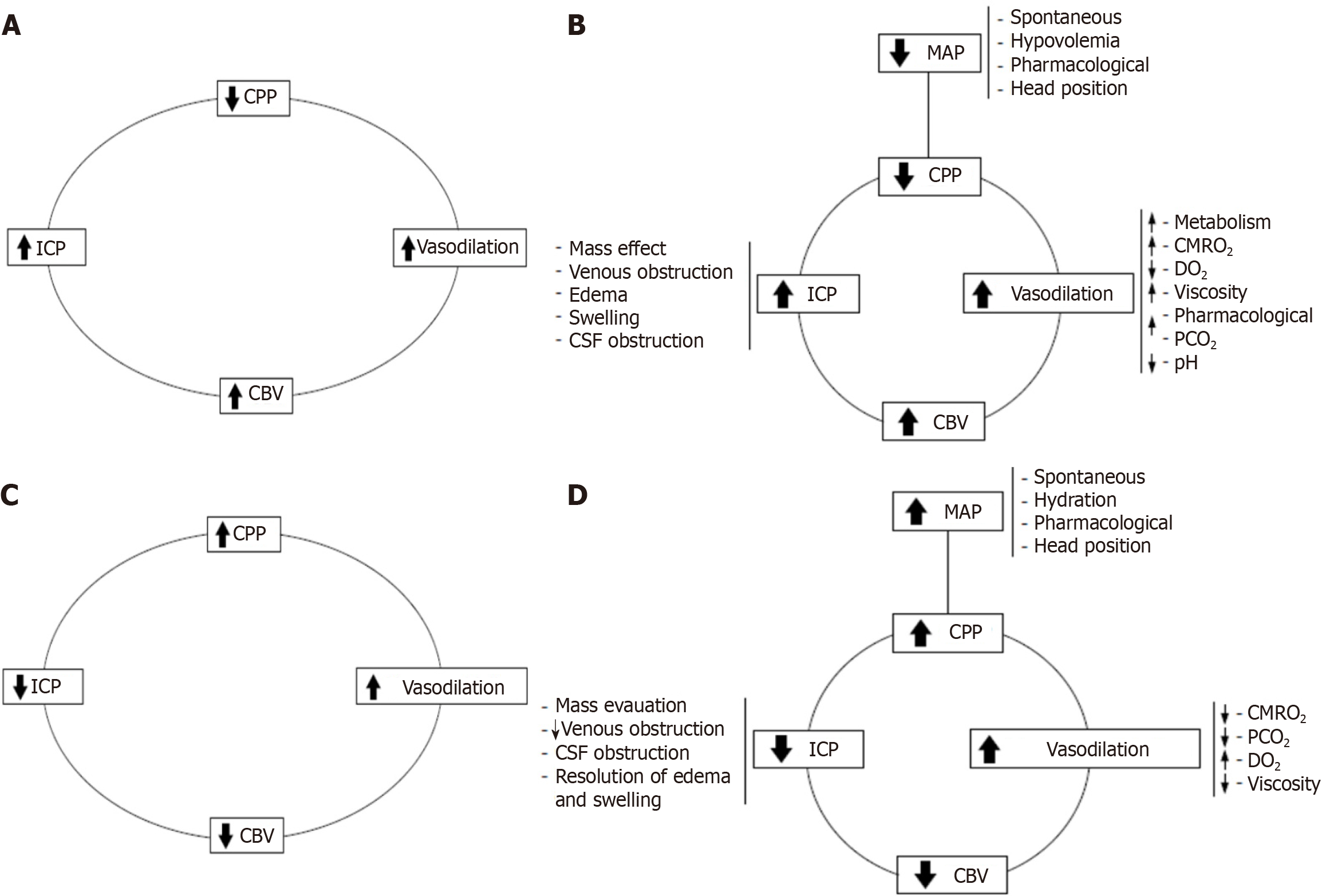
Figure 3 The vasodilator and vasoconstrictor cascades.
A: Vasodilator cascade. A decrease in cerebral perfusion pressures (CPP) triggers vasodilation as a compensatory mechanism to maintain cerebral blood flow. It is evident that vasodilation increases cerebral blood volume, which, according to the Monro-Kellie doctrine, raises intracranial pressure (ICP). This rise in ICP further reduces CPP, creating a self-perpetuating vicious cycle; B: Complex vasodilator cascade. This framework helps clarify many seemingly contradictory events and illustrates how the cycle can be initiated by any of its primary components; C: Vasoconstrictor cascade. With constant levels of partial carbon dioxide pressure, cerebral metabolic rate of oxygen consumption, and related variables, an increase in CPP induces vasoconstriction, which reduces cerebral blood volume and ICP, thereby enhancing CPP. This mechanism is effective only if the patient is within or near their autoregulatory range; D: Complex vasoconstrictor cascade. Management strategies can target any single component of the cascade or employ a combination of multiple components for optimal effect. CPP: Cerebral perfusion pressures; ICP: Intracranial pressure; CBV: Cerebral blood volume; MAP: Mean arterial pressure; CSF: Cerebrospinal fluid; CMRO2: Cerebral metabolic rate of oxygen; PCO2: Partial carbon dioxide pressure; DO2: Oxygen delivery.
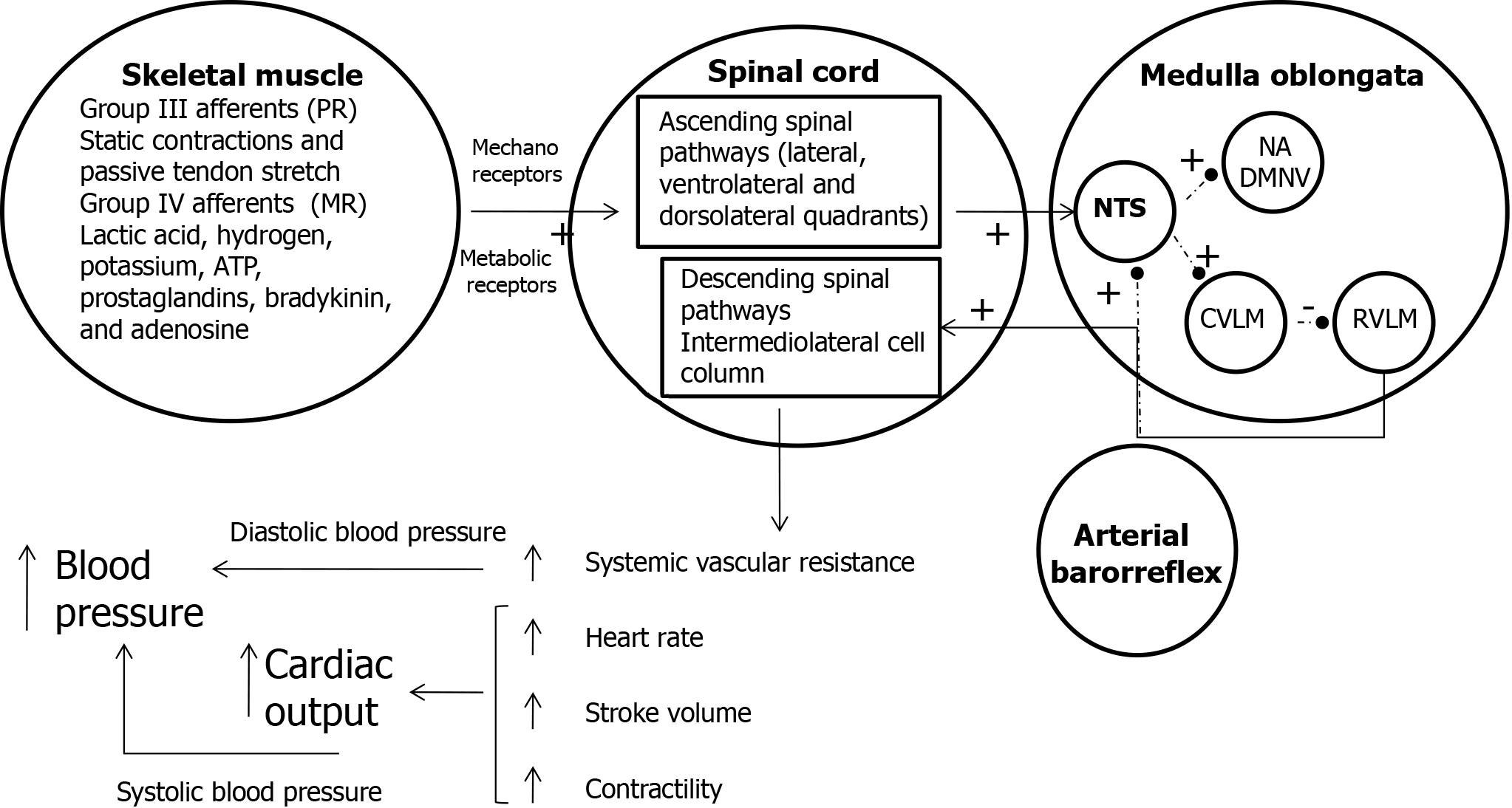
Figure 4 The combined action of the muscle metaboreflex, central command, and baroreceptors in cardiovascular regulation during exercise.
Upon the initiation of exercise, type III afferent fibers are stimulated by static muscle contraction and passive tendon stretch through mechanoreceptors (pressure response), while type IV afferent fibers are activated by the accumulation of metabolic byproducts. Ascending spinal pathways, mediated by various neurotransmitters, then stimulate the nucleus tractus solitarius (NTS) in the brainstem, generating an inhibitory response. Under normal conditions (indicated by dotted lines), the NTS regulates parasympathetic activity via the nucleus ambiguous and dorsal motor nucleus of the vagus, as well as sympathetic activity via the caudal ventrolateral medulla and rostral ventrolateral medulla (RVLM), in response to arterial baroreflex stimulation. When the NTS is inhibited, this leads to stimulation of the RVLM and inhibition of the nucleus ambiguus, dorsal motor nucleus of the vagus, and caudal ventrolateral medulla. Stimulation of the RVLM activates the intermediolateral cell column, which results in an increase in systemic vascular resistance, along with enhanced stroke volume, heart rate, and myocardial contractility. Together, these changes increase cardiac output. The rise in systemic vascular resistance contributes to an increase in diastolic pressure, while the augmentation of cardiac output raises systolic blood pressure. This integrated response ensures the maintenance of hemodynamic stability during exercise. PR: Pressure reflex; MR: Metabolic reflex; NTS: Nucleus tractus solitarius; NA: Nucleus ambiguous; DMNV: Dorsal motor nucleus of the vagus; CVLM: Caudal ventrolateral medulla; RVLM: Rostral ventrolateral medulla.
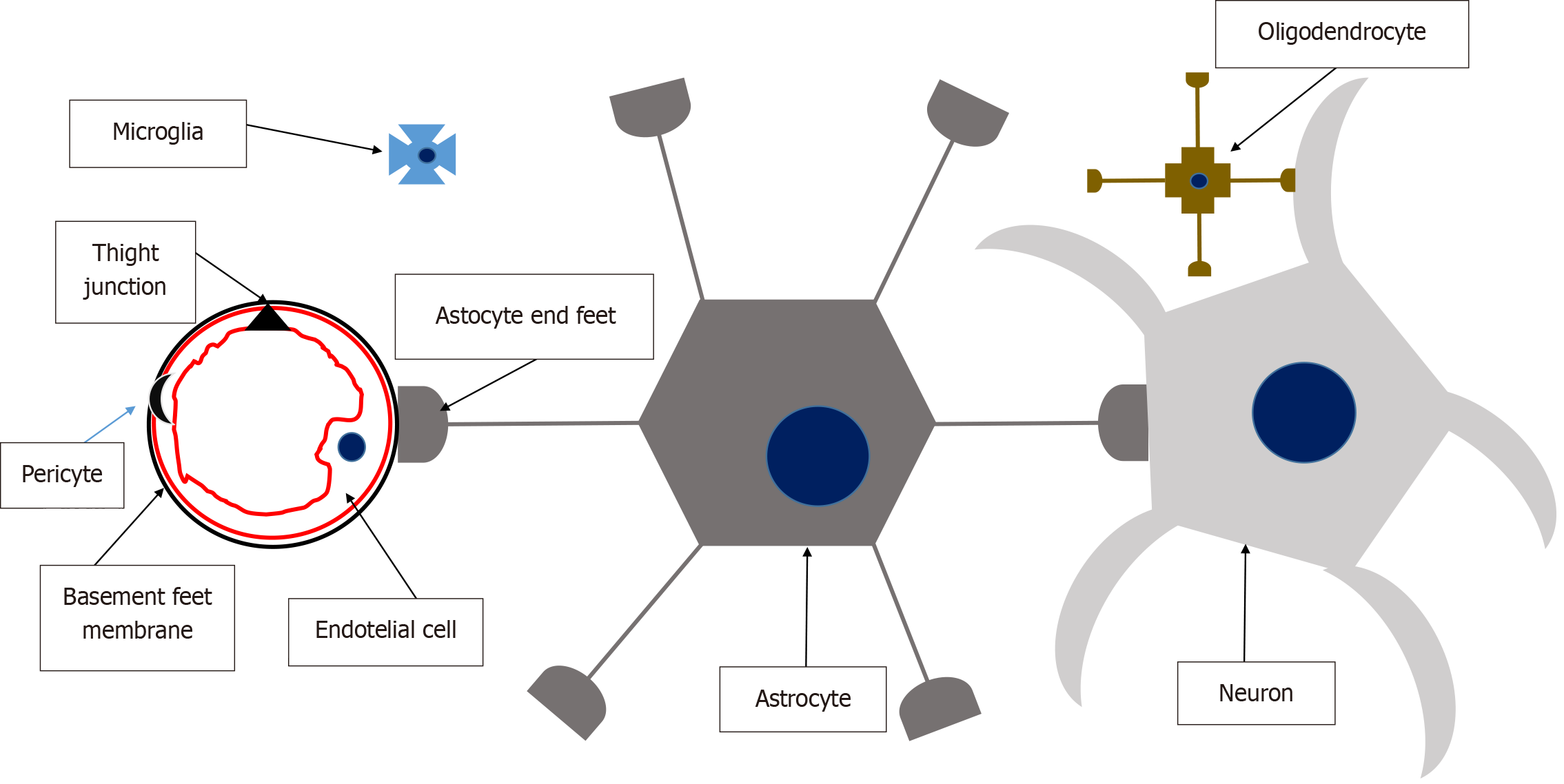
Figure 5 Neurovascular unit.
Cerebral endothelial cells form tight junctions that seal the paracellular aqueous diffusion pathways between cells, maintaining the integrity of the blood-brain barrier. Pericytes are discontinuously distributed along capillaries, positioned between the endothelium and the basement membrane, which forms a specialized extracellular matrix distinct from the astrocytic foot processes that encase it. Astrocytes play a pivotal role as a bridge between neurons and the endothelial complex, facilitating the passage of vasoactive peptides that regulate vascular dilation or constriction. Microglia serve as the immunocompetent cells of the neurovascular unit, providing immune surveillance. Solutes cross the blood-brain barrier through passive diffusion, driven by concentration gradients and lipophilicity, or via facilitated transport mechanisms involving active or passive transport through endothelial cells.
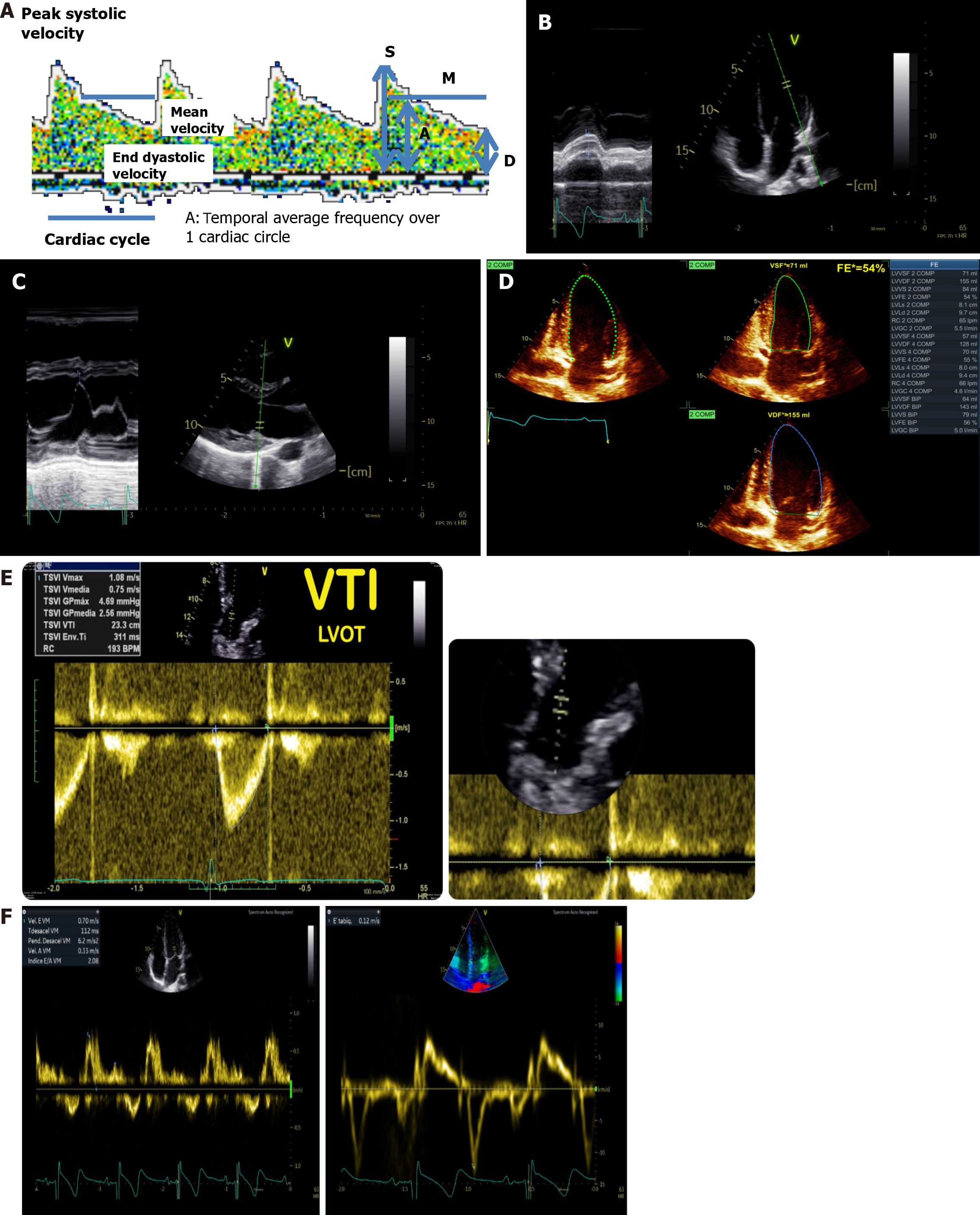
Figure 6 Assessment of cardio-cerebral coupling through ultrasound.
A: Transcranial Doppler spectrum real measurements; B: Mitral annulus plane systolic excursion measurements; C: E point septal separation; D: Ejection fraction by biplane Simpson’s method; E: Left ventricular outflow tract velocity time integral measurement; F: Transmitral Doppler. Left: Normal biphasic morphology, showing distinct E and A waves. Right: Normal triphasic morphology, with the addition of an L wave observed between the E and A waves. LVOT VTI: Left ventricular outflow tract velocity time.
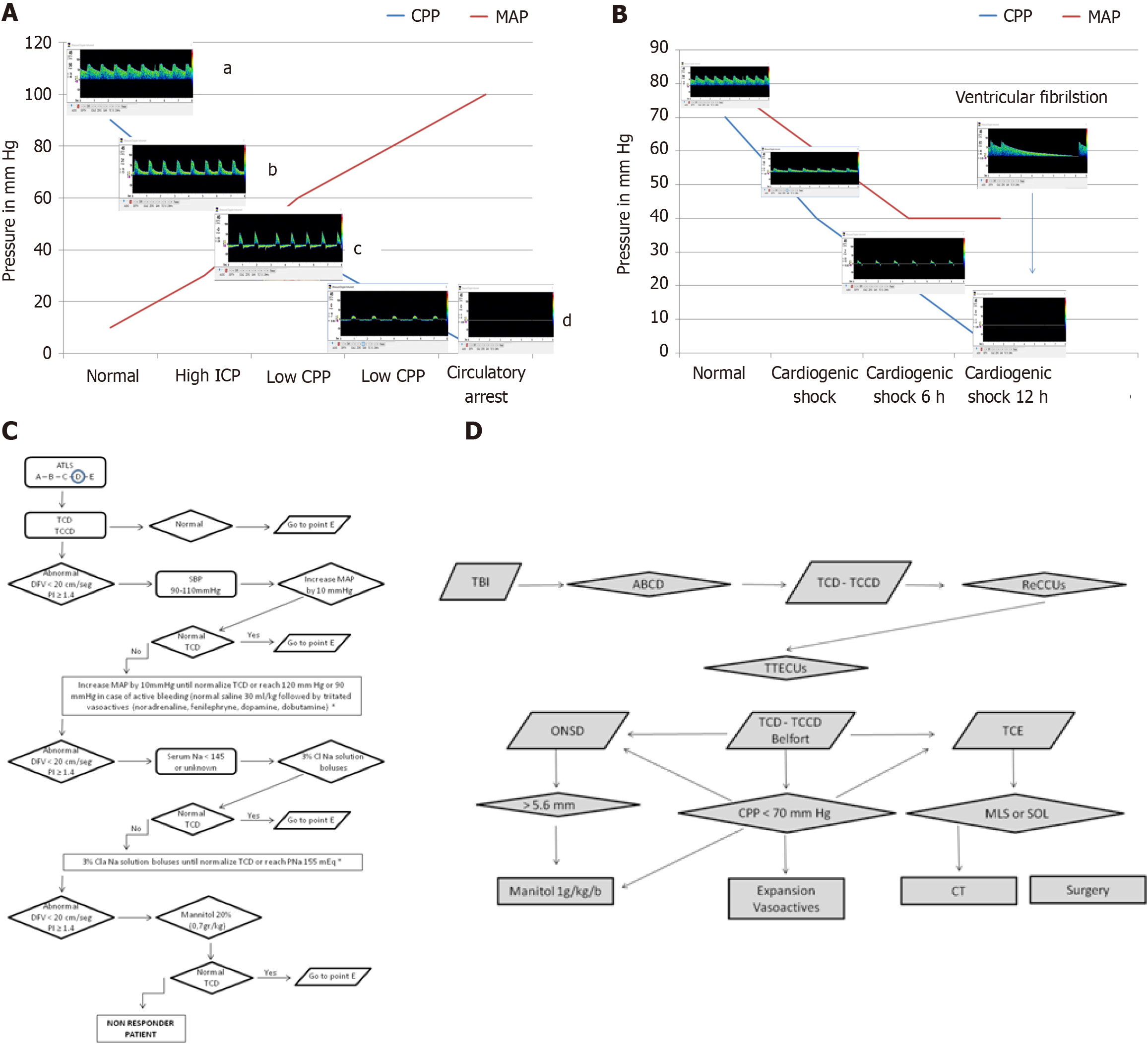
Figure 7 Cardio-cerebral coupling in health and disease.
A: Changes in flow velocities as cerebral perfusion pressures (CPP) decreases due to increasing intracranial pressure (ICP). aNormal flow: CPP at 90 mmHg and ICP at 10 mmHg ensures adequate cerebral perfusion with normal diastolic flow velocity (DFV) and pulsatility index. bHigh ICP: An increase in cerebral vascular resistance due to elevated ICP leads to compromised DFV and elevated pulsatility index. cLow CPP: Further increases in cerebral vascular resistance, coupled with low CPP, progress to reverberant flow. If sustained, this can evolve into small systolic spikes or complete flow absence. dCerebral circulatory arrest (CCA): The presence of reverberant flow or systolic spikes for more than 30 minutes is indicative of CCA, as defined by most brain death criteria; B: Changes in flow velocities as cardiogenic shock progresses. Early phase: Cardiogenic shock leads to a gradual decrease in all flow velocities, with a more pronounced reduction in DFV. Reverberant flow: After 6 hours, the typical pattern of reverberant flow emerges, characterized by oscillating flow without effective forward perfusion. CCA: Sustained reverberant flow or the appearance of complete flow absence (total CCA) is observed. Prolonged ventricular fibrillation can also result in this pattern. The presence of reverberant flow for more than 30 minutes is a reliable indicator of CCA, as defined by most brain death legislations; C: The ultrasound-guided cardio-cerebral resuscitation protocol. The Advanced Trauma Life Support® systematic approach includes airway, breathing, circulation, disability, and exposure; D: The ultrasound-guided brain injury treatment protocol. Following initial resuscitation using the ultrasound-guided brain injury treatment protocol, CPP is assessed using Belfort’s formula, ICP is estimated via optic nerve sheath diameter, and brain morphology is evaluated using B-mode transcranial ultrasound. If CPP < 70 mmHg, fluid expansion and vasoactive agents are immediately initiated. Simultaneously, optic nerve sheath diameter is measured. If ICP is elevated, a mannitol bolus is administered. If transcranial ultrasound reveals a midline shift or space-occupying lesion, the patient is immediately transferred to the computed tomography suite for surgical evaluation. This protocol ensures a systematic, ultrasound-guided approach to managing traumatic brain injury and optimizing cerebral perfusion. CPP: Cerebral perfusion pressures; ICP: Intracranial pressure; ATLS: Advanced Trauma Life Support®; TCD: Transcranial Doppler; TCCD: Transcranial color-coded Doppler; DFV: End-diastolic flow velocity; PI: Pulsatility index; SBP: Systolic blood pressure; MAP: Mean arterial pressure; TBI: Traumatic brain injury; ReCCUs: Ultrasound-guided cardio-cerebral resuscitation protocol; TTECUs: Ultrasound-guided brain injury treatment protocol; ONSD: Optic nerve sheath diameter; TCE: B-mode transcranial ultrasound; MLS: Midline shift; SOL: Space-occupying lesion; CT: Computed tomography.
- Citation: Previgliano IJ, Aboumarie HS, Tamagnone FM, Merlo PM, Sosa FA, Feijoo J, Carruega MC. Point of care ultrasound evaluation of cardio-cerebral coupling. World J Crit Care Med 2025; 14(3): 101462
- URL: https://www.wjgnet.com/2220-3141/full/v14/i3/101462.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v14.i3.101462