Published online Sep 9, 2025. doi: 10.5409/wjcp.v14.i3.103732
Revised: March 1, 2025
Accepted: March 12, 2025
Published online: September 9, 2025
Processing time: 199 Days and 8 Hours
Intra-arterial chemotherapy (IAC) has become a first-line standard treatment for retinoblastoma (RB). However, studies describing its adverse events are sparse, especially from the developing world. Our study described the outcomes and adverse events from a single center in South India.
To describe the challenges, treatment outcomes, and complications of selective IAC for RB in Indian eyes.
This study was a single center, retrospective study that included 17 patients with RB who underwent IAC using melphalan (5/7.5 mg) and topotecan (1/2 mg) (n = 12) or melphalan (5 mg) alone (n = 3) or triple therapy that included carboplatin (30 mg) along with these drugs (n = 2) between January 2018 and December 2023. In all, 17 IAC procedures were performed using selective ophthalmic artery cannulation. Treatment outcomes were evaluated in terms of tumor control, vitreous and subretinal seed control, complications, and globe salvage rates.
Out of the 17 patients, 11 were diagnosed with unilateral RB and 6 were diag
IAC is an effective way to control RB and globe preservation. In the Indian context we encountered many cha
Core Tip: Intra-arterial chemotherapy has become a first-line standard treatment for retinoblastoma. However, studies describing its adverse events are sparse, especially from the developing world. Our study described the challenges, treatment outcomes, and complications of selective intra-arterial chemotherapy for retinoblastoma in an Indian cohort. Our retrospective study showed that intra-arterial chemotherapy was effective in low resource settings with minimal adverse events.
- Citation: Das A, Saiteja K, Shah PK, Prema S, Narendran V. Outcomes and adverse events following intra-arterial chemotherapy for retinoblastoma: A single center study in South India. World J Clin Pediatr 2025; 14(3): 103732
- URL: https://www.wjgnet.com/2219-2808/full/v14/i3/103732.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i3.103732
Intra-arterial chemotherapy (IAC) has become a first-line treatment as well as a rescue therapy for retinoblastoma (RB). It was first described by Reese et al[1] in 1958, and they reported a 90% cure with a combination treatment employing triethylene melamine intramuscularly with X-ray. Subsequently, the procedure was refined by Kiribuchi[2] and Inomata and Kaneko[3]. Later, Abramson et al[4] performed IAC involving selective catheterization of the ophthalmic artery under fluoroscopic guidance for targeted chemotherapeutic drug delivery to the affected eye. Among the first nine cases treated with this technique, seven eyes destined for enucleation were salvaged with minimal complications.
It is well established that IAC is effective in unilateral group B, group C, group D, and to some extent group E according to the international classification of RB (ICRB). It has improved globe salvage rates and replaced enucleation in most cases[5-9]. Shields et al[10] concluded that using IAC plus additional intravitreal chemotherapy (IvitC) for vitreous seeding has improved globe salvage in eyes with advanced RB. Some authors have also explored bilateral sequential IAC for bilateral RB[11]. In a meta-analysis by Chen et al[12] comparing intravenous chemotherapy (IVC) vs IAC for RB, IAC was found to be superior to IVC with a higher overall success rate and better globe salvage in group D or group IV and group V eyes.
Very few studies have reported adverse events due to IAC, and this is particularly true for studies from developing countries. Implementing IAC for RB in resource-limited settings like India faces several challenges. The procedure requires advanced interventional radiology facilities and trained interventionists, which are available only in selected tertiary care centers. Additionally, high treatment costs, including procedural expenses and hospital stays, make it unaffordable for many patients. This study presented our 5-year experience with IAC, its outcomes, and adverse events in the era of systemic chemotherapy.
This retrospective study was conducted at a tertiary care referral eye hospital in South India. It received approval from the Institutional Ethics Committee and Institutional Review Board, adhering to the principles outlined in the Declaration of Helsinki. The study included patients diagnosed with RB who were treated with IAC at our Department of Oncology between January 2018 and December 2023.
Comprehensive data were collected for each patient, including demographic information, age at diagnosis, family history, tumor laterality, travel distance, clinical features at presentation, treatment details, complications, and outcomes. Additionally, any history of ocular and systemic treatment was documented. Ocular examination was conducted under general anesthesia, assessing tumor size, growth pattern, and the number of tumors per eye. Retinal imaging was performed using Retcam (Retcam III, Clarity Medical Systems, CA, United States). Tumors were classified using the ICRB and TNM staging system. Detailed measurements were taken to determine tumor size, proximity to optic disc and fovea, and degree of obscuration of the optic disc. The examination also evaluated the presence of retinal detachment as well as vitreous and subretinal seeds.
Patients underwent magnetic resonance imaging scans at the beginning of treatment and once a year in the case of bilateral tumors. Hematologic tests, including a complete blood analysis and basic coagulation profile, were performed at baseline and repeated after each IAC session to monitor for treatment-related changes. Written informed consent detailing potential risks and benefits was obtained from all patients.
The catheterization procedure for IAC was performed by an interventional radiologist in a catheterization laboratory. Under general anesthesia and strict aseptic precautions, the right or left common femoral artery was accessed using a 4-French pediatric short sheath. Using a 4-French Bernstein catheter, an ICA angiogram was performed to identify the ophthalmic artery. Anticoagulation was achieved with an intravenous injection of heparin (75 IU/kg). The ophthalmic artery was selectively cannulated using a Headway 17 microcatheter, and drug infusion was performed. A choroidal blush was observed. After confirming antegrade flow in the ophthalmic artery via angiogram, chemotherapeutic drugs were administered over 30 min in a pulsatile infusion.
Post-procedure, angiography showed normal opacification of the ophthalmic and central retinal arteries. The sheath was removed, and manual compression was applied to the puncture site. The chemotherapeutic drugs used included melphalan (5/7.5 mg) and topotecan (1/2 mg) for bilateral therapy, with carboplatin (30 mg) added for triple therapy. Patients were discharged on the same day after 4 h of observation. Postoperative care included oral analgesics and topical steroids for 1 week.
Patients were followed up at monthly intervals, with IAC repeated as needed based on tumor regression patterns. Fundus examination was performed under general anesthesia to assess tumor regression, complications, and globe salvage rates. After completion of primary or secondary IAC, amblyopia therapy was initiated with an age-adapted patching regimen. Compliance was stressed to parents.
Statistical analysis was performed using Microsoft Excel 2013 (Microsoft Corporation, Redmond, WA, United States). Categorical variables were analyzed using the χ² test or Fisher’s exact test, while continuous variables were analyzed using the Mann-Whitney U test. Given the small sample size, P values were supported with effect size calculations (Cohen’s d for continuous data and Cramer’s V for categorical data), and 95% confidence intervals were calculated using bootstrapping.
The indication for IAC was classified as either primary (treatment-naïve tumor) or secondary (progressive, persistent, or recurrent tumor). Before the IAC procedure, patients underwent a complete hematological and coagulation profile, which was repeated two weeks post-procedure.
Seventeen eyes from 17 patients were treated with IAC in the IvitC era (2018–2023) at a tertiary care center in South India. The patients were divided into those treated with primary IAC alone (n = 9) and those treated with primary IAC plus adjuvant IvitC or systemic chemotherapy (n = 8). The mean age at presentation was 21 months (median: 14 months, range: 2–48 months). The mean interval between the first symptom and presentation was 6.5 months.
Each eye received an average of 1.5 IAC sessions (median: 1 session; range: 1-3 sessions). There were 6 bilateral cases and 11 unilateral cases. The most common presenting symptom was leukocoria (n = 9, 53%), followed by strabismus (n = 3, 18%), leukocoria with strabismus (n = 3, 18%), and leukocoria with red eye (n = 2, 11%). Genetic testing was performed in all 17 patients, revealing 6 cases of familial RB and 11 sporadic cases.
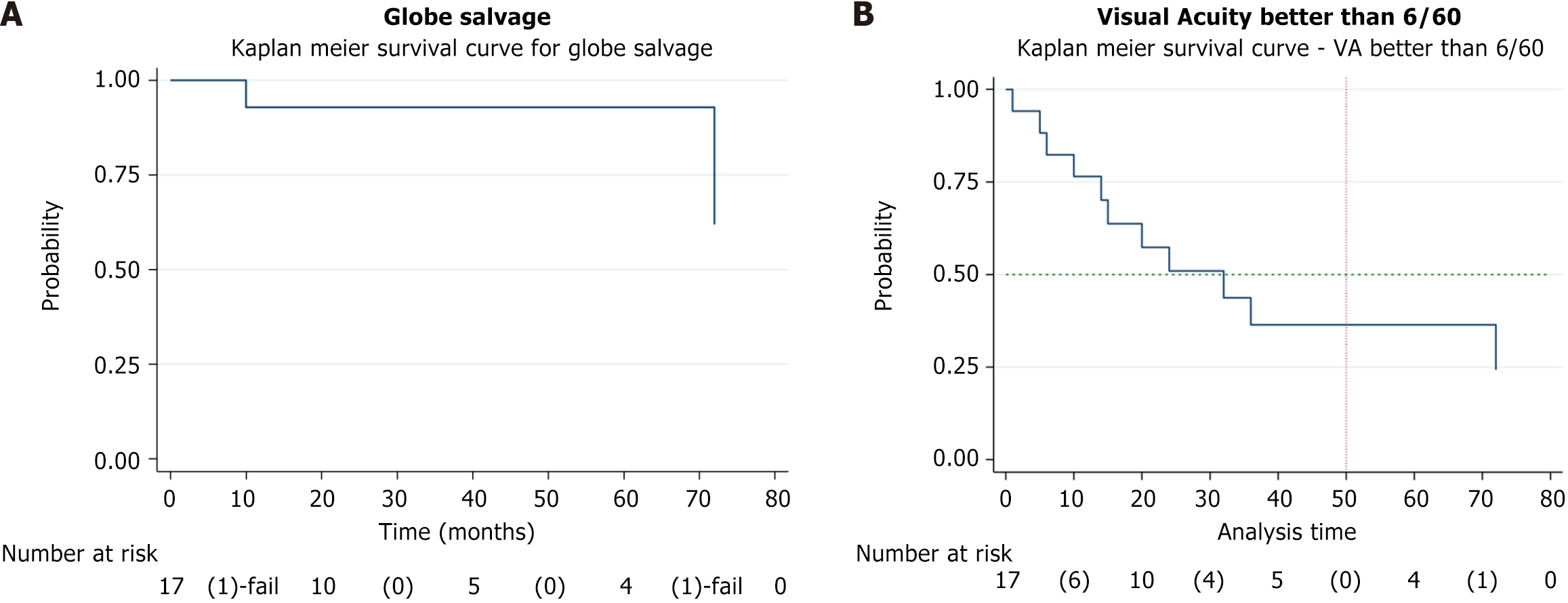
The study analyzed treatment outcomes, adverse effects, and tumor regression patterns in primary vs secondary IAC groups. Globe salvage was achieved in 15 eyes (88%). Visual acuity was > 6/60 in 4 eyes and < 6/60 in 11 eyes. The Kaplan-Meier analysis showed an event-free globe salvage rate of 93% at 5 years. Patient demographics along with disease laterality and inheritance details are provided in Table 1.
| Primary IAC (n = 9) | Secondary IAC with intravitreal chemotherapy (n = 8) | P value (effect size) | 95%CI | |
| Median age at presentation months | 14 (6, 48) | 18 (2, 36) | 0.73 (0.09) | 0.13-1.30 |
| Sex | ||||
| Male | 2 (22) | 3 (38) | 0.49 (0.17) | -2.80 to 3.80 |
| Female | 7 (78) | 5 (62) | ||
| Study eye | ||||
| Oculus dexter | 3 (33) | 5 (62) | 0.23 (0.29) | -3.90 to 6.80 |
| Oculus sinister | 6 (67) | 3 (38) | ||
| Laterality | ||||
| Unilateral | 8 (89) | 3 (38) | 0.03 (0.54) | -2.80 to 12.60 |
| Bilateral | 1 (11) | 5 (62) | ||
| Inheritance | ||||
| Hereditary | 1 (11) | 5 (63) | 0.03 (0.54) | -1.80 to 11.60 |
| Sporadic | 8 (89) | 3 (37) |
Average duration of symptoms at presentation was 4.3 months (median: 1 week, range: 1-48 weeks). Eyes with RB were classified according to the ICRB as group B (n = 5, 29%), group C (n = 1, 6%), group D (n = 4, 24%), and group E (n = 7, 41%). According to the TNM classification, the cT1 group had 5 eyes (30%) and the cT2 group had 12 eyes (70%). No signs of regional lymph node involvement were noted (N0), and there were no signs of intracranial or distant metastasis (cM0) in any of the patients. We observed that 4 patients traveled less than 100 km to our center, 10 traveled between 101-250 km, 2 traveled between 251-400 km, and 1 traveled more than 400 km. The mean tumors per eye was 1.1 in patients who underwent primary IAC and 1.5 in the secondary IAC group. Average tumor distance from the optic nerve was 2.25 mm in the primary IAC group and 1.8 mm in the secondary IAC group. The average tumor distance from the fovea was 3.5 mm in the primary IAC group vs 1.83 mm in the secondary IAC group. Out of 17 eyes, 4 patients (n = 4 eyes) had a total exudative retinal detachment at the initial presentation, and these eyes underwent primary IAC. Only one eye had seeds in the anterior chamber. All the clinical features of the 17 eyes in the primary and secondary IAC group are demonstrated in Table 2.
| International classification of retinoblastoma | IAC | Total | P value (Effect size) | 95%CI | |
| Primary IAC (n = 9) | Secondary (n = 8) | ||||
| Group A | 0 | 0 | 0 | 0.51 (0.38) | -0.90 to 1.10 |
| Group B | 2 | 3 | 5 | ||
| Group C | 0 | 1 | 1 | ||
| Group D | 2 | 2 | 4 | ||
| Group E | 5 | 2 | 7 | ||
| Total | 9 | 8 | 17 | ||
| Mean tumors per eye | 1.1 | 1.5 | |||
| Optic nerve distance (average) | 2.25 | 1.80 | |||
| Foveal distance (average) | 3.50 | 1.83 | |||
| Vitreous seeds quadrant | |||||
| None | 3 | 2 | 5 | 1.00 (0.30) | 0.37-1.63 |
| 1 quadrant | 2 | 1 | 3 | ||
| 2 quadrants | 1 | 2 | 3 | ||
| 3 quadrants | 2 | 1 | 3 | ||
| 4 quadrants | 1 | 2 | 3 | ||
| Subretinal seeds quadrant | |||||
| None | 6 | 5 | 11 | 1.00 (0.45) | 0.41-1.59 |
| 1 quadrant | 1 | 0 | 1 | ||
| 2 quadrants | 0 | 1 | 1 | ||
| 3 quadrants | 1 | 0 | 1 | ||
| 4 quadrants | 1 | 2 | 3 | ||
| Retinal detachment | |||||
| None | 5 | 8 | 13 | 0.47 (0.34) | -0.14 to 1.08 |
| 2 quadrants | 4 | 0 | 4 | ||
| Vitreous hemorrhage | |||||
| Yes | 1 | 0 | 1 | 1.00 (0.23) | 0.36-1.63 |
| No | 8 | 8 | 16 | ||
| Anterior chamber seeds | |||||
| Yes | 0 | 1 | 1 | 0.47 (0.26) | -0.20 to 1.14 |
| No | 9 | 7 | 16 | ||
| Neovascularization/neovascular glaucoma | |||||
| Yes | - | - | - | - | - |
| No | 9 | 8 | 17 | ||
Chemotherapeutic drugs used in IAC were melphalan (5/7.5 mg) and topotecan (1/2 mg) for bilateral therapy, with carboplatin (30 mg) added in cases of triple therapy. Triple therapy was given in 2 eyes only. The median number of cycles was 1 (1-3) in the primary IAC group vs 2 (1-3) in the secondary IAC group. Currently at our center we initiate with two drug regimens (melphalan + topotecan) for primary IAC. In the secondary IAC group, the mean number of IvitC was 3 (1-5), and the mean number of systemic chemotherapy (VEC regimen) was 8 (6-11).
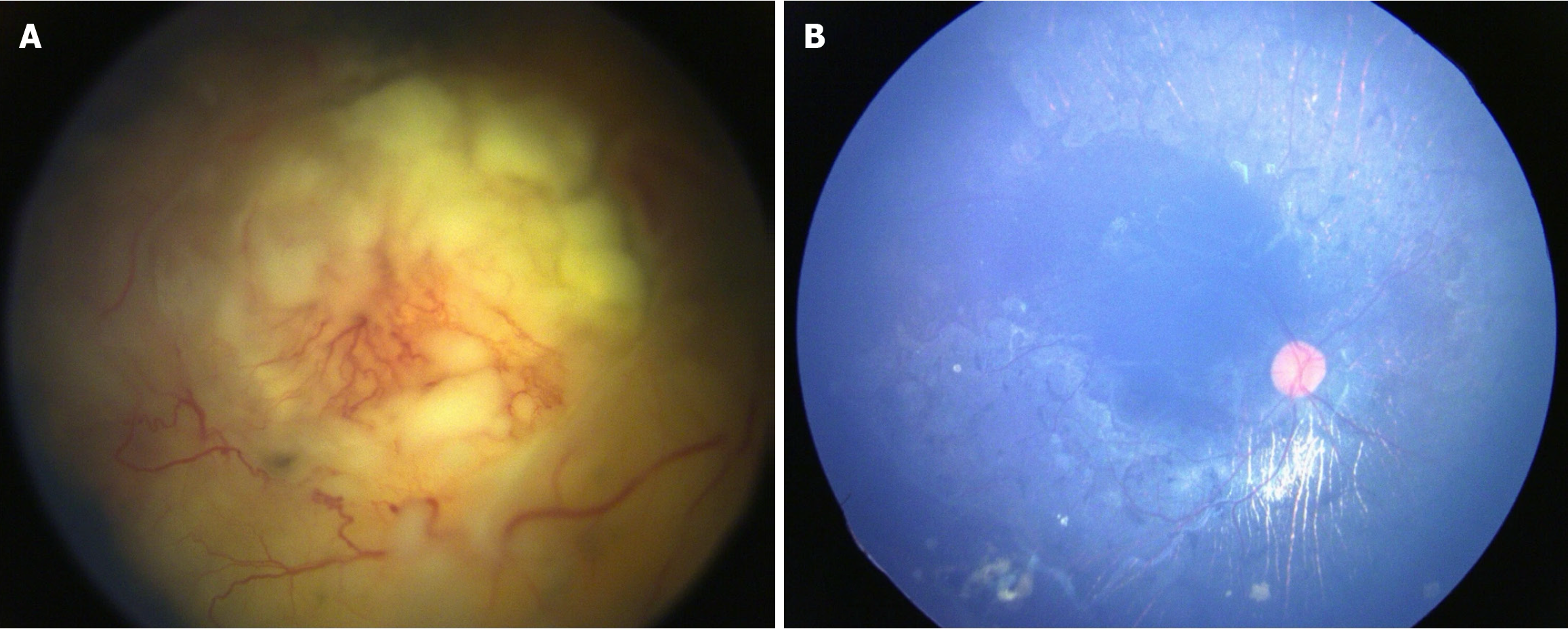
The median follow-up was 20 months (1-72) in the primary IAC group vs 28 months (10-72) in the secondary IAC group. There was no statistical significance between these two groups in terms of treatment outcomes. Treatment features and outcomes of primary vs secondary IAC are demonstrated in Table 3. Complete regression of the tumor was noted in 8 eyes (89%) in the primary group and 7 eyes (88%) in the secondary group. Type I regression was noted in 5 eyes (29%), type II regression in 2 eyes (13%), type III regression in 5 eyes (29%), and type IV regression in 5 eyes (29%) (Figure 1). Globe salvage was achieved in 15 eyes (88%). In terms of visual acuity of salvaged eyes, 4 eyes had more than 6/60 and 11 eyes had less than 6/60.
| IAC features | Primary IAC (n = 9) | Secondary IAC with intravitreal chemotherapy (n = 8) | P value | Total | |
| Median number of cycles | 1 (1-3) | 2 (1-3) | 0.403 | 1.7 | Mann Whitney U test |
| Melphalan only | 2 | 1 | 1.000 | 3 | |
| Melphalan + topotecan | 6 | 6 | 12 | Fisher’s exact test | |
| Melphalan + topotecan + carboplatin | 1 | 1 | 2 | ||
| Melphalan [median (range)] | 5 (3-12.5) | 5 (5-12.5) | 0.33 | 5 (3-12.5) | |
| Topotecan [median (range)] | 1 (1-3) | 1 (1-3) | 1 | 1 (1-3) | |
| Carboplatin [median (range)] | 30 (-) | 30 (-) | - | 30 (-) | |
| Intravitreal chemotherapy features | |||||
| Mean number of injections [median (range)] | - | 3 (1-5) | - | 3 (1-5) | |
| Melphalan only [median (range)] | - | 1 (16) | 1 (16) | ||
| Topotecan only [median (range)] | - | 2 (34) | 2 (34) | ||
| Melphalan + topotecan [median (range)] | - | 3 (50) | 3 (50) | ||
| Systemic chemotherapy | |||||
| Mean number of cycles [median (range)] | - | 8 (6-11) | - | 8 (6-11) | |
| Brachytherapy [median (range)] | - | 1 | 1 | ||
| Outcomes | |||||
| Follow-up months [median (range)] | 20 (1-72) | 28 (10-72) | 0.385 | 30.17 (1-72) | Mann Whitney U test |
| Tumor control | 8 (88) | 7 (88) | 1.00 | 15 (88) | Fisher’s exact test |
| Tumor control per ICRB | |||||
| Group B | 2 | 3 | 0.72 | 5 | Fisher’s exact |
| Group C | 0 | 1 | 1 | ||
| Group D | 2 | 1 | 3 | ||
| Group E | 4 | 2 | 6 | ||
| Enucleation per ICRB group | |||||
| Group B | 0 | 0 | - | 0 | |
| Group C | 0 | 0 | 0 | ||
| Group D | 0 | 1 | 1 | ||
| Group E | 1 | 0 | 1 | ||
| Visual acuity of salvaged eyes | n = 8 | n = 7 | 15 | ||
| > 6/60 [median (range)] | 2 (25) | 2 (28.6) | 1 | 4 | Fisher’s exact |
| < 6/60 [median (range)] | 6 (75) | 5 (71.4) | 11 |
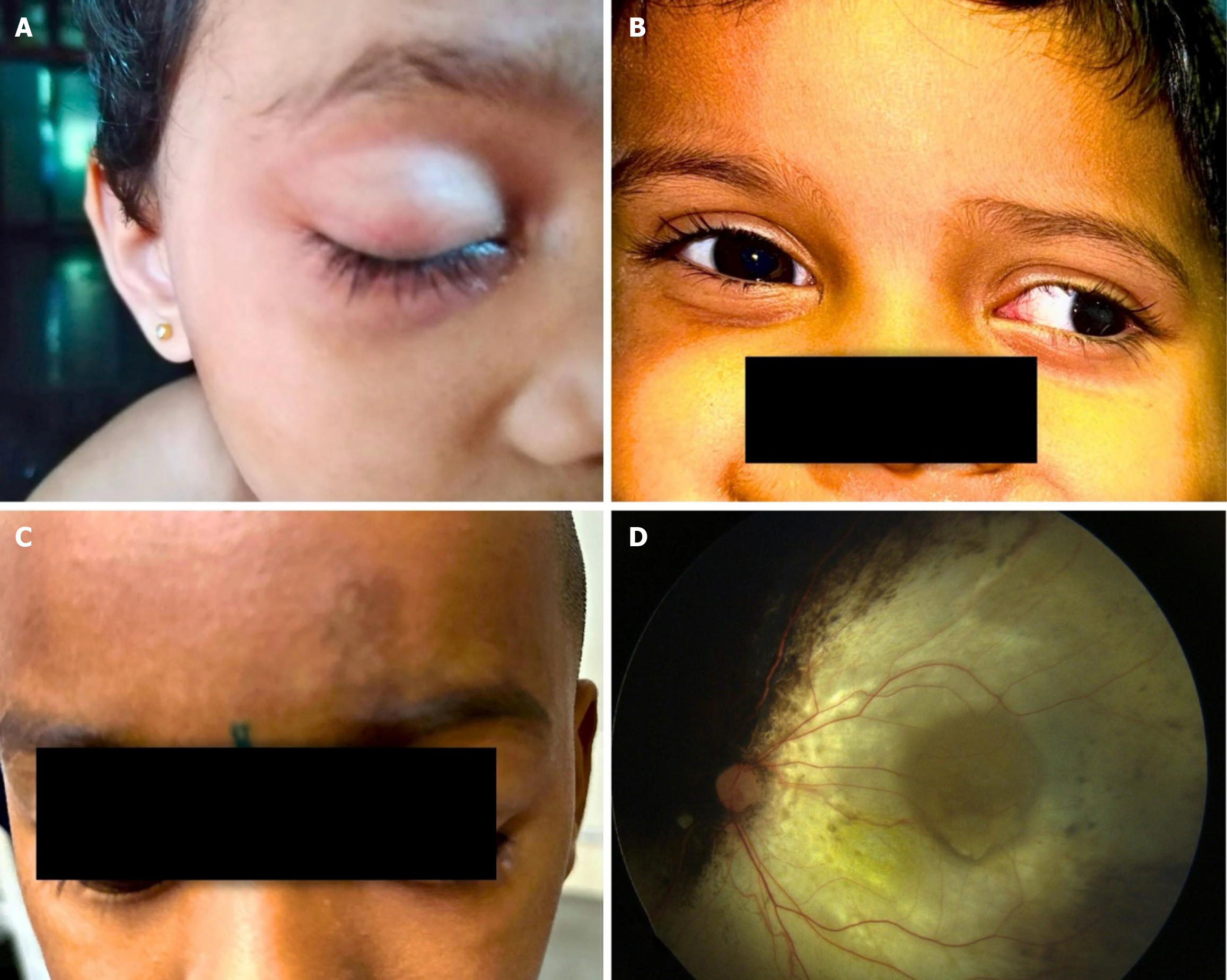
We noted vitreous hemorrhage (VH) (n = 3), rhegmatogenous RD (n = 2), choroidal ischemia (n = 1), isolated subretinal hemorrhage (n = 2), and extensive retinal pigment epithelium degeneration (n = 2) for adverse events. We also observed forehead pigmentation in 1 patient, third nerve palsy with complete ptosis in 1 patient, and 30-degree exotropia in 1 patient (Figure 2). The ptosis resolved in 3 months, and exotropia resolved in 5 months. There was no case of stroke, hemiplegia, metastasis, or death. The histopathological report of the two enucleated eyes had low risk features. Adverse effects along with details of the drug and dosage and fellow eye status have been illustrated in Table 4. The Kaplan-Meier analysis showed an event-free globe salvage rate of 93% at 5 years (Figure 3A) and following IAC showed 50% visual acuity presentation better than 6/60 (Figure 3B).
| Number | Intra-arterial chemotherapy cycles | Drug used with dose (mg) | Side effects | Fellow eye status | Best corrected visual acuity at last follow-up visit |
| 1 | 1 | M + T (5 + 1) | Nil | Normal | Fixing n following |
| 2 | 2 | M + T (5 + 1) | Nil | Normal | 6/6p |
| 3 | 1 | M + T (5 + 1) | Rhegamatogenous retinal detachment, vitreous hemorrhage | Normal | N/A |
| 4 | 2 | M + T (5 + 1) | Subretinal hemorrhage | Normal | Hand movements |
| 5 | 1 | M + T (5 + 1) | Forehead pigmentation | Normal | 6/15 |
| 6 | 3 | M + T + C (5/7.5 + 1/2 + 30) | Exotropia, vitreous hemorrhage | Normal | Fixing n following |
| 7 | 3 | M + T + C (5/7.5 + 1/2 + 30) | Retinal pigment epithelium degeneration | Enucleated | Fixing n following |
| 8 | 3 | M (5) | Lid edema, vitreous hemorrhage, rhegmatogenous retinal detachment, choroidal ischemia | Normal | N/A |
| 9 | 2 | M (4) | Nil | Normal | 6/60 |
| 10 | 1 | M + T (5 + 1) | Nil | Normal | Fixing n following |
| 11 | 1 | M + T (5 + 1) | Third nerve palsy with ptosis | Group E, regressed | Not seeing first card (Cardiff) |
| 12 | 1 | M + T (5 + 1) | Nil | Normal | Not seeing first card (Cardiff) |
| 13 | 1 | M + T (5 + 1) | Subretinal bleed | Normal | Fixing n following |
| 14 | 1 | M + T (5 + 1) | Nil | Group D, regressed | Not fixing n following |
| 15 | 2 | M + T (5 + 1) | Nil | Group B, regressed | Fixing n following |
| 16 | 2 | M + T (5 + 1) | Nil | Group C, regressed | Fixing n following |
| 17 | 2 | M (3) | Retinal pigment epithelium degeneration, melphalan toxicity | Group A, regressed | No perception of light |
There has been a paradigm shift in terms of management of RB from IVC to IAC along with IvitC for managing advanced RB with vitreous seeds[5,6,13-15]. Shields et al[16] showed the efficacy of melphalan and observed that intravitreal melphalan injection for persistent or recurrent vitreous RB seeding can provide tumor control with minimal toxicity and complications. Several authors also concluded that a combination of melphalan and/or topotecan gives a very good outcome in recurrent seed control and globe salvage as well as tumor control for several years[16-19]. These led to a superior method of tumor control and globe salvage thus reducing the number of enucleation and external beam radiation especially in advanced cases. Though it was a major breakthrough in the developed countries, it is still a challenge in developing countries like India. In the current scenario, IAC is available in very few centers in India as it requires a specialized interventional radiologist and adequate skills in performing the procedure. It is also an expensive procedure as it requires advanced equipment like microcatheters and imaging modalities and is not covered by the majority of the insurances available.
On the other hand, it is well justified that systemic chemotherapy has been the first-line treatment for RB for decades especially in developing and lower-income countries as the drugs are readily available[20]. But the systemic toxicity and multiple cycles associated with IVC cannot be ignored[21,22].
In this report, we described our 5-year experience with IAC and its outcomes and adverse events following the procedure and compared tumor control and globe salvage in eyes treated with IAC alone vs those who required additional IVC or IvitC along with IAC from a single center in India. There are very limited studies on IAC in RB from India. In this study, globe salvage was achieved in all group B, group C, and group D eyes without the need for IvitC except for 2 patients who underwent enucleation as they developed multiple relapses, VH, and rhegmatogenous retinal detachment (RRD). We compared primary IAC and secondary IAC, and we found no statistical difference between the varied management given to the patients.
No intervention is without risk and complications. In our study we observed a varied group of complications where few were procedure-related, and few were drug-related complications. Amongst adverse events after IAC, we observed that the most common event was VH (n = 3 eyes) out of which 2 eyes had concurrent RRD and were finally enucleated because of persistent relapse of the tumor along with RRD. Although VH itself is not a serious complication and usually resolves over time, it is significant in patients with RB as monitoring the tumor regression and possibility of relapse becomes difficult. Many authors have reported VH after the IAC procedure with rates up to 41.2%[23,24]. The rapid tumor shrinkage can disrupt the vascular supply causing vessel leakage and hemorrhage. RRD is also reported by many authors as an adverse effect post IAC. The mechanism of RRD post IAC may be due to tractional forces in response to the reduced volume of the tumor during IAC treatment. Munier et al[24] observed retinal detachment development in 9 out of 25 eyes in patients who received IAC with 5 total, 3 partial, and 1 peritumoral detachment. Shields et al[25] reported in their case series that RRD after IAC occurred with no adjacent local consolidation therapy such as cryotherapy, transpupillary thermotherapy, or argon laser photocoagulation, and they postulated that RRD occurs in 6% of patients, mostly in advanced eyes with extensive endophytic tumor and generally from an atrophic retinal hole after rapid tumor regression.
One of our patients presented with 30-degree exotropia with 3+ adduction limitation, which resolved over a period of a few months. Although ocular dysmotility after IAC has been previously reported, the etiology is variable between cases. They reported dysmotility was mainly due to extraocular muscle inflammation, and it resolved within a few months[26]. This is mainly observed with melphalan as it is a strong alkylating agent and can diffuse out into the orbital compartment causing an inflammatory reaction and edema. Lambert et al[27] reported ocular dysmotility because of medial rectus infarction and ischemia. They concluded that because the medial rectus receives its blood supply from the inferomedial muscular trunk, which is located downstream of the catheter tip, if is left vulnerable to adverse effects from IAC.
We also observed two other complications that are potential vision-threatening are choroidal ischemia/choroidal occlusive vasculopathy and extensive retinal pigment epithelium degeneration involving macula. We encountered 1 patient with choroidal ischemia and 2 patients with extensive retinal pigment epithelium degeneration. We assumed that higher levels of melanin in the retinal pigment epithelium in the Indian population may contribute to more toxicity due to prolonged drug retention or from direct cytotoxicity and vascular effects on retinal and choroidal circulation.
Muen et al[28] reported that 47% of the eyes had significant retinal pigment epithelium changes and were treated by intra-arterial melphalan (5 mg). They concluded that the dosage of melphalan (5 mg), which was uniform in all their patients, was responsible for these side effects. Munier et al[29] reported sectoral choroidal occlusive vasculopathy in two eyes (15%) 3-6 weeks after they started superselective ophthalmic artery chemotherapy (3 injections). They concluded that vasculopathy could be linked to the ophthalmic artery catheterism or from direct melphalan toxicity to the choroidal vascular bed, and/or to the retinal pigment epithelium and was not dose dependent.
Another retrospective study by Stathopoulos et al[30] from the same institute observed acute choroid ischemia developing in 35 eyes after a mean of two injections. They suggested that catheterization of the ophthalmic artery should be attempted from an ostial position or an external carotid approach to minimize the risk of potentially vision-threatening choroidal complications. We used a standard dose of 5 mg melphalan along with topotecan in 1 patient and 3 mg melphalan alone in another patient, and it is possible that this may have been a high dose for them.
Limitations of this study included its retrospective nature, potential selection bias, lack of long-term follow-up, and small number of patients as its accessibility was limited and an expensive affair in a developing nation. Even if it was accessible, IAC will only be available in selected tier 1 cities. The cost of one cycle of IAC ranges between 500 US dollars (USD) to 1300 USD. This cost goes even higher if a patient undergoes more than one cycle of IAC compared to standard systemic chemotherapy (VEC regimen), which is approximately 180 USD per cycle. But IAC offers several advantages over IVC such as reduced systemic adverse effects and fewer cycles of chemotherapy, and it gives a targeted drug delivery minimizing systemic exposure. We have recognized that primary IAC worked very well in unilateral RB without any adjuvant local therapy like thermotherapy or cryotherapy.
This study highlighted the effectiveness and challenges of IAC in managing RB in a resource-limited setting. While IAC has shown significant success in globe salvage, accessibility and cost remain key limitations. IvitC also plays an important role with active vitreous seeds along with the tumor. Hence, the combination of IAC and IvitC is very useful for both tumor and seed regression. Further studies with larger cohorts and long-term follow-up are needed from developing nations to optimize treatment protocols, measure the safety profile, address complications, and reduce the cost of IAC.
We would like to acknowledge Mrs. Padmavati Sivakumar for the statistical analysis and Dr. Devu T for help with the images.
| 1. | REESE AB, HYMAN GA, TAPLEY ND, FORREST AW. The treatment of retinoblastoma by x-ray and triethylene melamine. AMA Arch Ophthalmol. 1958;60:897-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Kiribuchi M. [Retrograde infusion of anti-cancer drugs to ophthalmic artery for intraocular malignant tumors]. Nippon Ganka Gakkai Zasshi. 1966;70:1829-1833. [PubMed] |
| 3. | Inomata M, Kaneko A. Chemosensitivity profiles of primary and cultured human retinoblastoma cells in a human tumor clonogenic assay. Jpn J Cancer Res. 1987;78:858-868. [PubMed] |
| 4. | Abramson DH, Dunkel IJ, Brodie SE, Kim JW, Gobin YP. A phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. Ophthalmology. 2008;115:1398-1404, 1404.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 326] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 5. | Shields CL, Lally SE, Leahey AM, Jabbour PM, Caywood EH, Schwendeman R, Shields JA. Targeted retinoblastoma management: when to use intravenous, intra-arterial, periocular, and intravitreal chemotherapy. Curr Opin Ophthalmol. 2014;25:374-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Shields CL, Manjandavida FP, Lally SE, Pieretti G, Arepalli SA, Caywood EH, Jabbour P, Shields JA. Intra-arterial chemotherapy for retinoblastoma in 70 eyes: outcomes based on the international classification of retinoblastoma. Ophthalmology. 2014;121:1453-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 196] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 7. | Shields CL, Bianciotto CG, Jabbour P, Ramasubramanian A, Lally SE, Griffin GC, Rosenwasser R, Shields JA. Intra-arterial chemotherapy for retinoblastoma: report No. 1, control of retinal tumors, subretinal seeds, and vitreous seeds. Arch Ophthalmol. 2011;129:1399-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 8. | Gobin YP, Dunkel IJ, Marr BP, Brodie SE, Abramson DH. Intra-arterial chemotherapy for the management of retinoblastoma: four-year experience. Arch Ophthalmol. 2011;129:732-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 315] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 9. | Abramson DH, Fabius AW, Issa R, Francis JH, Marr BP, Dunkel IJ, Gobin YP. Advanced Unilateral Retinoblastoma: The Impact of Ophthalmic Artery Chemosurgery on Enucleation Rate and Patient Survival at MSKCC. PLoS One. 2015;10:e0145436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Shields CL, Alset AE, Say EA, Caywood E, Jabbour P, Shields JA. Retinoblastoma Control With Primary Intra-arterial Chemotherapy: Outcomes Before and During the Intravitreal Chemotherapy Era. J Pediatr Ophthalmol Strabismus. 2016;53:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Abramson DH, Dunkel IJ, Brodie SE, Marr B, Gobin YP. Bilateral superselective ophthalmic artery chemotherapy for bilateral retinoblastoma: tandem therapy. Arch Ophthalmol. 2010;128:370-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Chen Q, Zhang B, Dong Y, Mo X, Zhang L, Huang W, Jiang H, Xia J, Zhang S. Comparison between intravenous chemotherapy and intra-arterial chemotherapy for retinoblastoma: a meta-analysis. BMC Cancer. 2018;18:486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Shields CL, Fulco EM, Arias JD, Alarcon C, Pellegrini M, Rishi P, Kaliki S, Bianciotto CG, Shields JA. Retinoblastoma frontiers with intravenous, intra-arterial, periocular, and intravitreal chemotherapy. Eye (Lond). 2013;27:253-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 14. | Francis JH, Abramson DH, Gaillard MC, Marr BP, Beck-Popovic M, Munier FL. The classification of vitreous seeds in retinoblastoma and response to intravitreal melphalan. Ophthalmology. 2015;122:1173-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Francis JH, Marr BP, Abramson DH. Classification of Vitreous Seeds in Retinoblastoma: Correlations with Patient, Tumor, and Treatment Characteristics. Ophthalmology. 2016;123:1601-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Shields CL, Manjandavida FP, Arepalli S, Kaliki S, Lally SE, Shields JA. Intravitreal melphalan for persistent or recurrent retinoblastoma vitreous seeds: preliminary results. JAMA Ophthalmol. 2014;132:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Berry JL, Shah S, Bechtold M, Zolfaghari E, Jubran R, Kim JW. Long-term outcomes of Group D retinoblastoma eyes during the intravitreal melphalan era. Pediatr Blood Cancer. 2017;64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Ghassemi F, Shields CL, Ghadimi H, Khodabandeh A, Roohipoor R. Combined intravitreal melphalan and topotecan for refractory or recurrent vitreous seeding from retinoblastoma. JAMA Ophthalmol. 2014;132:936-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Shields CL, Douglass AM, Beggache M, Say EA, Shields JA. Intravitreous Chemotherapy For Active Vitreous Seeding From Retinoblastoma: Outcomes After 192 Consecutive Injections. The 2015 Howard Naquin Lecture. Retina. 2016;36:1184-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Global Retinoblastoma Study Group. The Global Retinoblastoma Outcome Study: a prospective, cluster-based analysis of 4064 patients from 149 countries. Lancet Glob Health. 2022;10:e1128-e1140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 21. | Murphree AL, Villablanca JG, Deegan WF 3rd, Sato JK, Malogolowkin M, Fisher A, Parker R, Reed E, Gomer CJ. Chemotherapy plus local treatment in the management of intraocular retinoblastoma. Arch Ophthalmol. 1996;114:1348-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 276] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 22. | Friedman DL, Krailo M, Villaluna D, Gombos D, Langholz B, Jubran R, Shields C, Murphree L, O'Brien J, Kessel S, Rodriguez-Galindo C, Chintagumpala M, Meadows AT. Systemic neoadjuvant chemotherapy for Group B intraocular retinoblastoma (ARET0331): A report from the Children's Oncology Group. Pediatr Blood Cancer. 2017;64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Ong SJ, Chao AN, Wong HF, Liou KL, Kao LY. Selective ophthalmic arterial injection of melphalan for intraocular retinoblastoma: a 4-year review. Jpn J Ophthalmol. 2015;59:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Munier FL, Mosimann P, Puccinelli F, Gaillard MC, Stathopoulos C, Houghton S, Bergin C, Beck-Popovic M. First-line intra-arterial versus intravenous chemotherapy in unilateral sporadic group D retinoblastoma: evidence of better visual outcomes, ocular survival and shorter time to success with intra-arterial delivery from retrospective review of 20 years of treatment. Br J Ophthalmol. 2017;101:1086-1093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | Shields CL, Say EAT, Pefkianaki M, Regillo CD, Caywood EH, Jabbour PM, Shields JA. Rhegmatogenous Retinal Detachment After Intraarterial Chemotherapy For Retinoblastoma: The 2016 Founders Award Lecture. Retina. 2017;37:1441-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Shields CL, Bianciotto CG, Jabbour P, Griffin GC, Ramasubramanian A, Rosenwasser R, Shields JA. Intra-arterial chemotherapy for retinoblastoma: report No. 2, treatment complications. Arch Ophthalmol. 2011;129:1407-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Lambert NG, Winegar BA, Feola GP, Ramasubramanian A. Ocular dysmotility after intra-arterial chemotherapy for retinoblastoma. J AAPOS. 2015;19:574-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Muen WJ, Kingston JE, Robertson F, Brew S, Sagoo MS, Reddy MA. Efficacy and complications of super-selective intra-ophthalmic artery melphalan for the treatment of refractory retinoblastoma. Ophthalmology. 2012;119:611-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | Munier FL, Beck-Popovic M, Balmer A, Gaillard MC, Bovey E, Binaghi S. Occurrence of sectoral choroidal occlusive vasculopathy and retinal arteriolar embolization after superselective ophthalmic artery chemotherapy for advanced intraocular retinoblastoma. Retina. 2011;31:566-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Stathopoulos C, Bartolini B, Marie G, Beck-Popovic M, Saliou G, Munier FL. Risk Factors for Acute Choroidal Ischemia after Intra-arterial Melphalan for Retinoblastoma: The Role of the Catheterization Approach. Ophthalmology. 2021;128:754-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |