Published online Oct 18, 2016. doi: 10.5312/wjo.v7.i10.638
Peer-review started: February 26, 2016
First decision: May 13, 2016
Revised: June 22, 2016
Accepted: August 11, 2016
Article in press: August 15, 2016
Published online: October 18, 2016
Processing time: 229 Days and 6.3 Hours
Anterior cruciate ligament (ACL) reconstruction is one of the most common orthopedic procedures performed worldwide. In this regard, magnetic resonance imaging (MRI) represents a useful pre-operative tool to confirm a disruption of the ACL and to assess for potential associated injuries. However, MRI is also valuable post-operatively, as it is able to identify, in a non-invasive way, a number of aspects and situations that could suggest potential problems to clinicians. Graft signal and integrity, correct tunnel placement, tunnel widening, and problems with fixation devices or the donor site could all compromise the surgical outcomes and potentially predict the failure of the ACL reconstruction. Furthermore, several anatomical features of the knee could be associated to worst outcomes or higher risk of failure. This review provides a practical guide for the clinician to evaluate the post-surgical ACL through MRI, and to analyze all the parameters and features directly or indirectly related to ACL reconstruction, in order to assess for normal or pathologic conditions.
Core tip: There are several original studies and reviews in the literature that discuss magnetic resonance imaging (MRI) evaluation after anterior cruciate ligament (ACL) reconstruction. However, these are mostly focused on a single aspect such as graft signal intensity, tunnel placement, joint anatomy, or complications. This is a first known review to summarize all the aspects that should be evaluated through MRI after an ACL reconstruction, in order to perform a complete and global assessment of the post-operative status. The iconographic sections with practical and detailed explanation of measurements will serve as a useful reference for the MRI evaluation after ACL reconstruction in daily clinical practice.
- Citation: Grassi A, Bailey JR, Signorelli C, Carbone G, Wakam AT, Lucidi GA, Zaffagnini S. Magnetic resonance imaging after anterior cruciate ligament reconstruction: A practical guide. World J Orthop 2016; 7(10): 638-649
- URL: https://www.wjgnet.com/2218-5836/full/v7/i10/638.htm
- DOI: https://dx.doi.org/10.5312/wjo.v7.i10.638
Anterior cruciate ligament (ACL) reconstruction is one of the most common orthopedic procedures performed worldwide. It is considered the standard of care for young active patients who wish to return to sport practice after ACL injury[1]. Despite the lack of clear evidence of its ability to reduce the onset and progression of knee osteoarthritis (OA), ACL reconstruction is expected to prevent further meniscal and cartilage lesions that could occur in the ACL-deficient knee[2,3]. Usually, ACL reconstruction is performed arthroscopically (occasionally combined with extra-articular plasty/augmentation) using autologous graft such as Gracilis and Semitendinosus tendons (HS), bone-patellar tendon bone (BPTB), and Quadriceps Tendon (QT), or allogenic grafts such as BPTB, Achilles Tendon, and Posterior or Anterior Tibialis tendons. Graft fixation is obtained through a wide range of fixation devices, different in function, shape, size, material, biomechanical proprieties and positioning. The outcomes of ACL reconstruction are generally good; however, graft rupture or clinical failure can occur in 6%-12% of the cases[4].
In the field of ACL injury and reconstruction, magnetic resonance imaging (MRI) represents a useful pre-operative tool to confirm a disruption of the ACL and to assess for potential associated injuries. However, MRI is also valuable post-operatively to assess graft healing and maturation, to determine its position, and to evaluate potential complications or re-injury[5-7]. For example, a survey among expert surgeons of the German Arthroscopy Association (AGA) showed that MRI represents one of the decision-making criteria for return to sport activity in only 4% of those interviewed[8].
The purpose of this review is to provide a practical guide for the clinician to evaluate the post-surgical ACL through MRI, and to analyze all the parameters and features directly or indirectly related to ACL reconstruction, in order to assess normal or pathologic conditions.
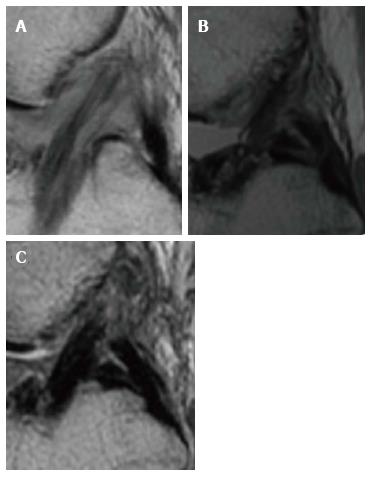
The signal evaluation of the neo-ligament, whose intensity is generally evaluated with a combined score (Table 1) or with software (Figure 1), represents a dynamic field of research.
| Item | Points |
| Integration: Synovial fluid at tunnel-graft interface | |
| Positive | 1 |
| Negative | 2 |
| Ligamentization: Graft signal pattern (> 50%) | |
| Hypointense | 3 |
| Isointense | 2 |
| Hyperintense | 1 |
| Characterization of graft | |
| Poor | 2 |
| Adequate | 3-5 |
From the biological point of view, the intra-articular graft undergoes a maturation and remodeling process lasting even beyond 24 mo, and consists of 4 steps: The initial avascular necrosis, the revascularization, cellular proliferation, and final remodeling[9]. It is generally agreed that initially the graft undergoes necrosis, showing hypocellularity especially in its central part. Cytokines are released as a consequence of necrosis, which then trigger growth factors for cell migration, proliferation, extracellular matrix (ECM) synthesis, and revascularization[10]. Maximum cellularity is observed during the proliferation phase as the cell number surpasses that of the intact ACL in numerous animal models[11]. Cell numbers then regress towards the intact ACL cellularity at the end of the proliferation phase. The tissue remodeling phase is started with cell-mediated restructuring of the extracellular matrix as an adaptive response to mechanical loading on the tendon graft. This whole process from tendon graft toward the acquisition of histologic and biomechanical properties similar to the native ACL is known as “ligamentization”.
This process could be indirectly monitored through MRI, as it has been proved that poor biomechanical properties and an incomplete graft maturation are related to a hyperintense graft signal on MRI[5,7]. Weiler et al[12] demonstrated in an animal model that a significantly elevated graft signal was present between the 6th and 12th weeks, and this condition was correlated to the lowest tensile stress of the graft (estimated around the 7%-16% of the initial values). However, from the 6th to the 24th month, the signal did not differ from that of the native ACL and the tensile stress increased, reaching around the 60% of the time-zero values. Furthermore, contrast-enhanced MRI with gadolinium showed the return to a graft signal similar to the native ACL by the 24th month, thus suggesting a late remodeling period. From the histological point of view, hyperintense signal was correlated to the presence of new hypervascular and hypercellular reparative tissue.
Despite the normal maturation process, it is important to know that an increase of signal intensity of the new-ACL, especially on the distal two-thirds, may also be due to graft impingement. This complication occurs when the grafts contacts the intercondylar notch during the extension of the knee. This has been implicated in the pathogenesis of the so-called “Cyclops lesion”, which consist of a fibrous injury to the anterior side of the graft, close to the site of greatest friction within the notch[5,13].
The structural composition of the graft and the presence or absence of the bone plugs has been shown to present different maturation behaviors and presentations with MRI. When BPTB autograft tendons are used, in the first month the graft usually presents a low-intensity signal in T1 and T2 sequences, similar to the original patellar tendon, mirroring the relatively avascular nature of the donor structure. Subsequently, during the remodeling phase, the graft is wrapped by synovial tissue and vascularized (Figure 2), with the consequent increase of MRI signal up to 16-18 mo. After this period, the graft will shortly reach a signal very similar to original ACL[5-7].
When Gracilis and Semitendinosus autograft tendons are used, in the 1st month it is possible to not necessarily observe a hypointense signal as with the BPTB graft, because of the multiple layer configuration of the graft that could cause an accumulation of a thin liquid stratum (hyperintense at the MRI) between the individual layers. This finding, sometimes combined with small liquid deposits in the tunnel-graft interface, can remain in the first post-operative year. After that, the maturation process continues similarly to that of the patellar tendon[5-7]. However, in a recent study on 26 patients undergoing single-bundle ACL reconstruction, it has been shown that the Gracilis and Semitendinosus autograft demonstrated slower maturation at 6 mo compared to an autograft quadriceps tendon with bone block, when measured with the signal/noise quotient[14]. The authors proposed the possibility of needing to modify rehabilitation according to the extent of graft maturation to prevent re-injury and maximize patient function.
Even allografts exhibit a similar behavior; however, it has been demonstrated that they have a much longer maturating process[15-17]. This has been confirmed by the persistence of a higher signal intensity compared to autografts for up to 2 years following ACL reconstruction[18].
In summary, the MRI evaluation of the neo-ligament signal indirectly allows us to obtain valuable information of the state of maturation, giving the clinician precious insight that can help guide rehabilitation and physical activity.
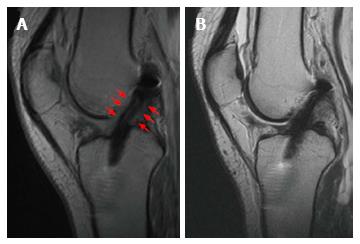
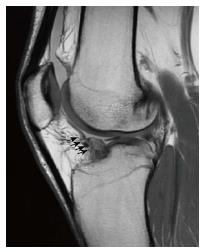
Apart from incorrect tunnel placement, one of the most common causes of ACL reconstruction failure is a new injury. A new disruption of the reconstructed ligament appears on MRI as increased signal intensity in the T2 sequence within the graft body[7,19] (Figure 3). However, this should be combined with a concordant clinical examination and a clear medical history of a new trauma. In fact, the mere MRI evaluation could sometimes be misleading, because of the discordance between clinical examination and MRI evaluation. In a series of 50 revision ACL reconstructions with a graft lesion confirmed by arthroscopy and clinical examination, the graft was read intact on MRI evaluation in 24% of cases. The discordance between MRI and clinical evaluation, and between MRI and arthroscopic evaluation was 52% and 44% respectively, especially in the cases of an insidious-onset mechanism of injury[19]. With arthroscopic evaluation as the diagnostic standard, the sensitivity of MRI to diagnose an ACL graft tear was 60%, and specificity 87%.
Therefore, it is possible to see an apparently normal graft on MRI but clinically or arthroscopically injured or elongated. To avoid this discrepancy and to improve the diagnostic power, some authors suggest the use of MR-arthrography, which can increase the sensitivity and specificity toward values near 100%[5]. Nevertheless the MRI should be considered an additional and not exclusive tool for the assessment of the post-operative ACL.
The correct positioning of the femoral and tibia tunnel represents a key technical step for the success of ACL reconstruction surgery. The MRI provides important information about those aspects, especially regarding the graft inclination.
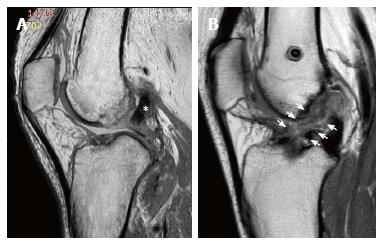
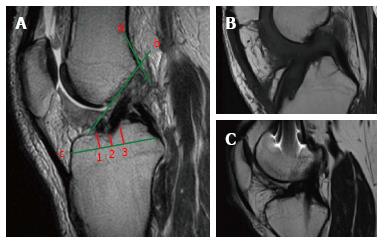
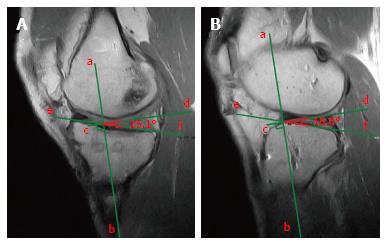
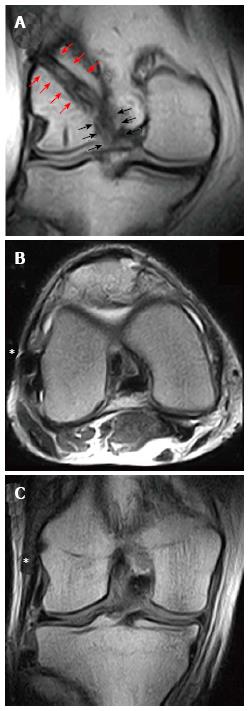
On the sagittal plane of the MRI the front border of the tibial tunnel should be localized behind a line that is tangential to the Blumensaat line (which is the line tangential to the intercondylar roof), without going beyond the midpoint of the proximal tibia with the knee in full extension (Figure 4A). The tibial tunnel center should ideally be located around the 42% mark of the entire sagittal distance of the tibial plateau as measured from the anterior edge of the tibia[20] (Figure 4A). It has been demonstrated that the native ACL is located between the 28% and 63% mark of the tibial plateau’s antero-posterior diameter, with the center at 46%[21]. If the tunnel is too forward, the risk of impingement of the graft with the intercondylar notch increases, possibly causing extension deficit or a Cyclops lesion (Figure 4B), while a tunnel too posterior could lead to a vertical graft (Figure 4) responsible of an incomplete control of knee antero-posterior and rotatory stability[22]. A controversial matter of debate is the correct positioning of the femoral tunnel. Despite the fact that the most accurate evaluation is obtained through arthroscopy, MRI could be helpful to identify gross malpositioning. It is generally accepted that the femoral tunnel should be located at the intersection of the posterior femoral cortex and the lateral wall of the intercondylar notch[23] (Figure 4A).
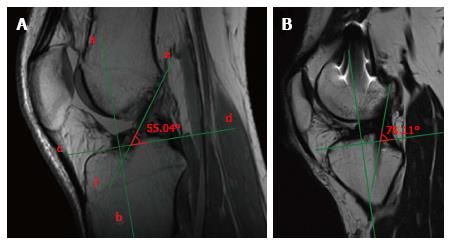
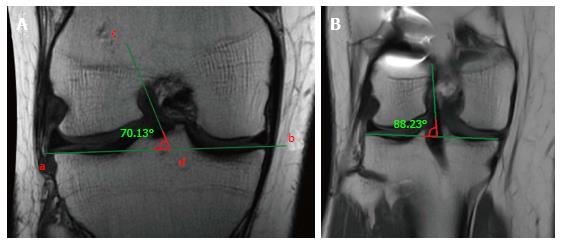
Regarding the bi-dimensional sagittal inclination, considering that the native ACL sagittal inclination ranges between 50°-60°[24], the graft inclination after ACL reconstruction should not exceed 60° (Figure 5A). A greater laxity could be linked to ACL reconstructions with sagittal graft inclination > 60° (Figure 5B).
Similarly, on the coronal plane, the graft inclination should be less than 75° (Figure 6A), as an excessively vertical graft sub-optimally controls rotatory laxity compared with a more horizontally placed graft[25] (Figure 6B). Usually, the coronal position of the tibial tunnel entrance does not represent an issue in the MRI evaluation after ACL reconstruction, as it is generally in the correct position under the femoral notch in the vast majority of the cases thanks to intra-operative anatomical landmarks; such as the anterior horn of lateral meniscus and the medial tibial eminence.
Tunnel inclination could therefore be correlated with success or failure of ACL reconstruction. Hosseini et al[26], reported a mean sagittal graft inclination of 69° in patients with failed ACL reconstruction scheduled for a revision procedure. Similarly, Mall et al[24] reported greater laxity in knees with sagittal graft inclination > 60° (Figure 5B); however, this did not affect general outcomes and the ability of these National Football League (NFL) athletes to return to high level sports practice. Furthermore, Fujimoto et al[27] found a mean coronal graft inclination of 79.5° in patients with grade 3 laxity after ACL reconstruction. Several factors could influence graft inclination, such as surgical experience, knee anatomy, and surgical technique. It has been demonstrated that trans-tibial (TT) femoral tunnel drilling tended to result in more vertical grafts in the sagittal plane compared to anteromedial portal (AMP) drilling (72°vs 53°)[28] or other methods of independent femoral tunnel drilling.
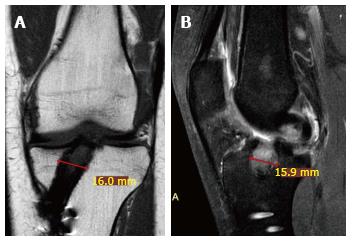
Tunnel morphology and possible enlargement should be evaluated appropriately using radiographs, computed tomography (CT), or MRI[29]. It has been demonstrated that intra-observer kappa scores for CT, radiographic and MRI evaluation of tunnel enlargement were 0.66, 0.50 and 0.37 respectively, and inter-observer kappa scores were 0.65, 0.39 and 0.32 respectively. Thus, MRI is considered a sub-optimal and not reliable tool to evaluate the progression of tunnel enlargement, while the CT represents the most reliable one. A precise tunnel measurement is in fact mandatory, as it could represent an indication for a staged revision procedure (usually with a tunnel diameter > 15 mm). However, through MRI it is possible to identify liquid collection and cyst formation inside the tibial tunnel, responsible of potential tunnel widening (Figure 7). Usually in Gracilis and Semitendinosus ACL reconstruction, such situations could occur within the first post-operative year. A hyperintense signal due to liquid collection in the tendon-tunnel interface could be present; however, with the tendency for spontaneous resolution[5-7]. Usually, tunnel enlargement occurs within the first 3 month and tends to remain stable up to 2 years if no tunnel malpositioning or graft lack of healing is present.
The exact etiology of tunnel widening is unknown[30], despite the fact that it has been related to several factors such as mechanical or biological mechanisms. Size mismatch between tunnel and graft dimension could allow sagittal micromotion at the graft-tunnel interface: This phenomenon, known as the “windshield wiper effect”, has been indicated as a potential cause of tunnel enlargement especially in the tibia, proximal to the fixation site. Similarly, longitudinal elongation, known as the “bungee cord effect” could also play a role. The use of a Gracilis and Semitendinosus graft coupled with cortical devices, that produce a low stiffness construct with a long tunnel length, have been demonstrated to be subjected to this phenomenon, leading to tunnel enlargement in the femoral side[31]. Furthermore, tunnel malposition could generate abnormal stresses and motion of the graft, leading to tunnel lysis. As tunnel enlargement has been noted to stabilize after the first 3 month post-operative, it is believed that the bone-to-bone or the tendon-to-bone healing within the tunnel could also influence the mechanical behavior of the graft. For the aforementioned reasons, early and aggressive rehabilitation, could potentially contribute to this phenomenon as well.
Regarding biological factors, it has been proposed that tunnel enlargement has been associated with allograft use secondary to a subclinical immunologic reaction[32], and to the presence of inflammatory cytokines within synovial fluid between the bone-graft tunnel interfaces, because of their osteolytic activity. Finally, a 12% increase size of the graft due to swelling has been reported through MRI after ACL reconstruction, and the consequent increased pressure within the tunnel could be responsible for necrosis and further cytokine release.
Despite the fact that tunnel enlargement does not appear to adversely affect clinical outcomes in the short term, the long-term relationship with potential knee laxity or increased traumatic failure is unknown[30]. Moreover, large tunnels could seriously complicate the revision procedure, especially regarding graft placement and fixation, sometimes requiring a staged procedure.
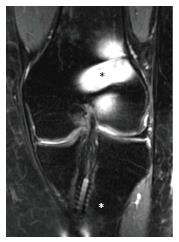
Fixation devices can be an issue in ACL post-operative MRI evaluation, because metallic devices could be responsible for disturbing artifacts (Figure 8). However, artifacts derived from metallic devices such as interference screws can be managed with software techniques to reduce metallic-hardware artifacts, reduction of slice thickness, and use of interecho spacing or short tau inversion recovery (STIR) techniques[33]. The use of bioabsorbable devices reduce the problems related to artifacts. However, despite possible artifacts due to fixation devices, their macroscopic mobilization, migration or rupture could be easily evidenced on MRI, and represent the cause of a potential reconstruction failure. Interference screws can migrate inside the joint damaging articular cartilage; suspensory extra-cortical systems can move inside the tunnel causing reduction of graft tensioning; cross pin fixation can migrate inside the soft tissues irritating muscles or tendons; while bioabsorbable materials can generate foreign-body inflammatory reaction. The latter circumstance could represent a severe event, due to an immune-mediate response. The production of inflammatory cytokine and the creation of a granuloma inside the bone around the screw or even in the surrounding soft-tissues, could possibly compromise the trabecular architecture weakening the bone[34]. Normally, bioabsorbable screws made in Poly-L-lactide (PLLA) or hydroxyapatite-PLLA have been reported to reabsorb slowly, necessitating up to 4 years to degrade[35]. Poly-D-L-lactide (PDLLA) screws have been reported clearly visible with MRI at 6-8 mo, show fragmentation and connective tissue ingrowth at 12-16 mo, and can be fully reabsorbed after 22 mo[36]. Differently, Polyglycolic Acid (PGA) screws have been shown to be completely reabsorbed even after 6-12 mo[37].
The MRI could be considered a precious tool also to evaluate aspects not directly relating to the neo-ACL, but closely related to reconstruction surgery, as those deriving from graft harvesting.
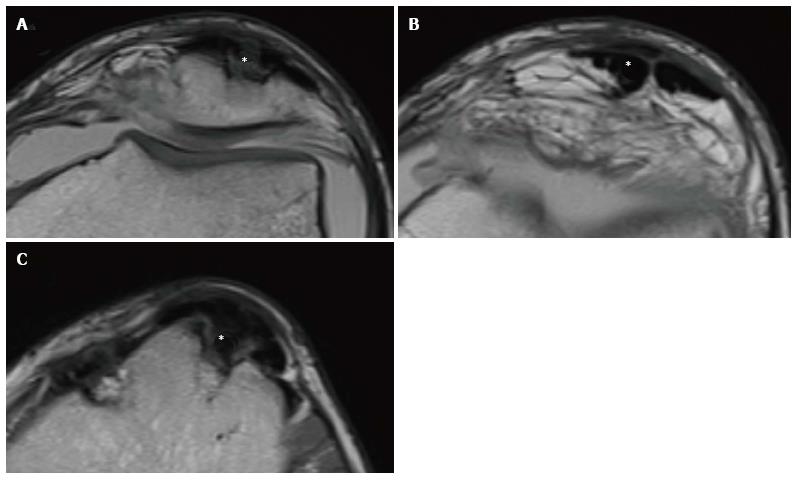
For example, the patellar tendon after the harvesting of its middle third, could appear thickened with enhanced signal in T1 and T2 sequences[38] (Figure 9). A gap between the medial and lateral third of the patellar tendon could be present, despite the fact it tends to disappear with time (Figure 10). In this regard, MRI studies have revealed the permanence of the tendon gap even after 10 years from reharvesting, and an increase of tendon thickness from 2 to 10 years, significantly higher compared to normal tendon[39]. Although rare, a patellar fracture, patellar tendon tears, bursitis or hematoma, especially after BPTB graft reharvesting, can be observed.
Sometimes, the Hoffa fat-pad could present an inflammatory reaction, highlighted by a hyperintense T2 signal at MRI fat-suppression sequences, due to hypertrophy and edema.
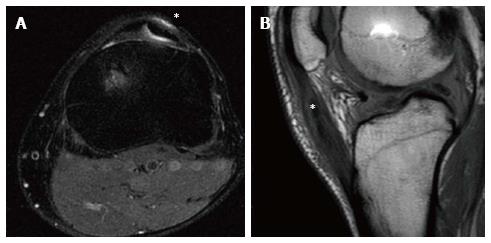
Another relatively frequent complication that can occur in the anterior knee compartment often related to graft harvesting is arthrofibrosis (Figure 11). Defined as a generic condition of post-operative stiffness (either extension or flexion), arthrofibrosis is believed to occur from ACL reconstruction being performed before post-traumatic inflammation has subsided or secondary to prolonged immobilization after ACL reconstruction. The arthrofibrosis can be diffuse or focal, and presents as an area of low-intermediate signal intensity extending anterior to the distal ACL[6] (Table 1).
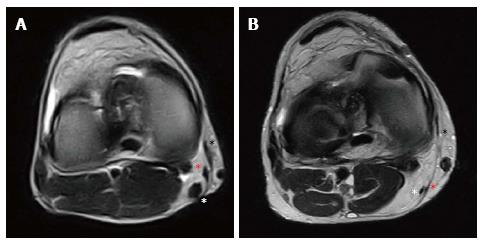
Regarding the “pes-anserinus”, it occasionally is subjected to donor-site pathology. A light fluid collection along the donor site can be present in the first post-operative month, until its complete disappearance around 12-18 mo. Tendon regeneration has been documented to occur through the so-called “lizard’s tail effect”, even if the quality for reharvesting could be questioned[40]. A 2014 systematic review of 18 publications demonstrated a mean regeneration rate for the semitendinosus and gracilis tendons of 70% or higher[41]. However, more recent literature supports a complete regeneration of the Semitendinosus during the 3rd-6th month in 60% of the cases, while Gracilis regeneration has been reported to be present after its harvesting in 30% of the cases (Figure 12). Complete regeneration of the whole “pes anserinus” was only noted in 10% of the cases, often with an ectopic re-insertion 1-2 cm below the joint line at the level of Sartorius fascia or medial head of Gastrocnemius. In 15% of the cases, complete absence of both tendons was noted. An initial hypertrophy, followed by a progressive volume reduction was described along the first 2 post-operative years, accompanied by muscular atrophy, retraction and fatty infiltration in up to 90% of the cases[42].
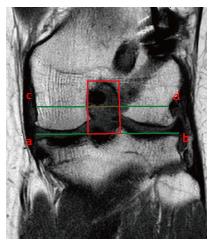
Christensen at al[43] evaluated the MRI of 35 patients with early ACL failure and 35 with no evidence of ACL graft failure, particularly medial (Figure 13A) and lateral (Figure 13B) tibial plateau sagittal slope. They found a higher lateral tibial plateau slope in patients that experienced graft failure (8.4°vs 6.5°). They estimated an odd ratio for graft failure of 1.6, 2.4 and 3.8 with a slope increase of 2°, 4° and 6° respectively. These findings were more evident in females. A similar conclusion was presented by Webb et al[44] using lateral radiographs. They described an incidence near 60% of graft re-rupture or contralateral ACL injury in patients with tibial slope > 12°. An increased tibial slope, especially the posterior slope of the lateral tibial plateau, is in fact a recognized risk factor for non-contact ACL injury[45]. However, there are some controversies in the literature, as some studies describing increased medial tibial plateau being the more important risk factor to ACL injury. Others believe the meniscal slope is a more accurate measure rather than the bony tibial plateau slope. Furthermore, different landmarks for measuremet, knee or long-leg X-ray, use of MRI, and other features, increase the variability of the measurement and the absence of complete agreement regarding this issue. However, despite the controversy in the literature, it is well accepted that increasing the posteriorslope overall increases risk of ACL injury or graft failure.
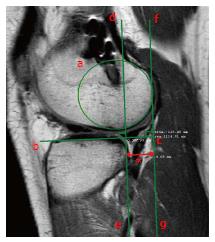
Notch shape is also considered a risk factor for non-contact ACL injury. A narrow notch has been measured in ACL-deficient patients, probably accounting for a smaller and weaker ACL compared to healthy patients with a wider notch[46]. Furthermore, in the setting of ACL reconstruction, Fujii et al[13] found a smaller notch cross-sectional area (Figure 14) in patients that developed the “cyclops lesion” due to notch impingement compared to complication-free patients (251.7 mm2vs 335.6 mm2).
Tanaka et al[47] reported an abnormal tibiofemoral relationship at MRI in patients with failed ACL reconstruction. An average of 5.7 mm of anterior tibial subluxation within the lateral compartment was reported, with a value greater than 15 mm in 12.5% of cases (Figure 15). The magnitude of anterior subluxation was 3.9 mm and 3.1 mm greater than the values of normal knees and knees with acute ACL tears, respectively. No noteworthy findings were reported for the medial compartment. This association between anterior displacement and failed ACL reconstruction may provide a mechanical explanation of suboptimal clinical results of ACL revision reconstruction.
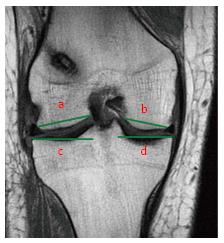
In the ACL-deficient condition, Musahl et al[48] noted a narrower lateral tibial plateau in patients with grade II pivot-shift compared to patients with grade I pivot-shift, (35.5 mm vs 30.3 mm) (Figure 16). However, this finding was significant only in female patients. No other anatomical parameters seemed to affect the pre-operative laxity. The authors suggested that bony anatomy contributes to the magnitude of knee laxity in the ACL-deficient knee; therefore, it could be argued that patients with specific anatomical features could represent patients with a higher risk of sub-optimal results of ACL reconstruction. In these cases of higher pre-operative laxity, the association of a lateral extra-articular plasty could be indicated[49] (Figure 17).
MRI represents an important tool for the post ACL reconstruction evaluation, due to its abilities to identify, in a non-invasive manner, a number of aspects and situations that could suggest potential problems to clinicians. Graft signal and integrity, correct tunnel placement or widening, and problems with fixation devices or donor site could all compromise the surgical outcomes and potentially determine the failure of the ACL reconstruction. However, this tool must not be used in isolation when assessing the post ACL reconstruction status. It should always be integrated with a careful clinical and medical history evaluation, as only an integrated approach to graft status and functionality is most effective in reducing potential diagnostic mistakes.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fanter NJ, Seijas R S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Irarrzaval S, Kurosaka M, Cohen M, Fu FH. Anterior cruciate ligament reconstruction. ISAKOS. 2016;1:38-52. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Fabricant PD, Lakomkin N, Cruz A, Spitzer E, Lawrence JTR, Marx RG. Early ACL reconstruction in children leads to less meniscal and articular cartilage damage when compared with conservative or delayed treatment. ISAKOS. 2016;1:10-15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Fabricant PD, Lakomkin N, Cruz A, Spitzer E, Marx RG. ACL reconstruction in youth athletes results in an improved rate of return to athletic activity when compared with non-operative treatment: a systematic review of the literature. ISAKOS. 2016;1:2059-7762. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Crawford SN, Waterman BR, Lubowitz JH. Long-term failure of anterior cruciate ligament reconstruction. Arthroscopy. 2013;29:1566-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 5. | Gnannt R, Chhabra A, Theodoropoulos JS, Hodler J, Andreisek G. MR imaging of the postoperative knee. J Magn Reson Imaging. 2011;34:1007-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Kulczycka P, Larbi A, Malghem J, Thienpont E, Vande Berg B, Lecouvet F. Imaging ACL reconstructions and their complications. Diagn Interv Imaging. 2015;96:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Naraghi A, White L. MRI evaluation of the postoperative knee: special considerations and pitfalls. Clin Sports Med. 2006;25:703-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 8. | Petersen W, Zantop T. Return to play following ACL reconstruction: survey among experienced arthroscopic surgeons (AGA instructors). Arch Orthop Trauma Surg. 2013;133:969-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Janssen RP, Scheffler SU. Intra-articular remodelling of hamstring tendon grafts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2014;22:2102-2108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 10. | Lui PP, Lee YW, Mok TY, Cheuk YC, Chan KM. Alendronate reduced peri-tunnel bone loss and enhanced tendon graft to bone tunnel healing in anterior cruciate ligament reconstruction. Eur Cell Mater. 2013;25:78-96. [PubMed] |
| 11. | Unterhauser FN, Bail HJ, Höher J, Haas NP, Weiler A. Endoligamentous revascularization of an anterior cruciate ligament graft. Clin Orthop Relat Res. 2003;276-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Weiler A, Peters G, Mäurer J, Unterhauser FN, Südkamp NP. Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging. A two-year study in sheep. Am J Sports Med. 2001;29:751-761. [PubMed] |
| 13. | Fujii M, Furumatsu T, Miyazawa S, Okada Y, Tanaka T, Ozaki T, Abe N. Intercondylar notch size influences cyclops formation after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2015;23:1092-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Ma Y, Murawski CD, Rahnemai-Azar AA, Maldjian C, Lynch AD, Fu FH. Graft maturity of the reconstructed anterior cruciate ligament 6 months postoperatively: a magnetic resonance imaging evaluation of quadriceps tendon with bone block and hamstring tendon autografts. Knee Surg Sports Traumatol Arthrosc. 2015;23:661-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Amendola A, Stolley MP. What do we really know about allografts? Clin Sports Med. 2009;28:215-222, vii-viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Ekdahl M, Wang JH, Ronga M, Fu FH. Graft healing in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16:935-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Gulotta LV, Rodeo SA. Biology of autograft and allograft healing in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26:509-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Li H, Tao H, Cho S, Chen S, Yao Z, Chen S. Difference in graft maturity of the reconstructed anterior cruciate ligament 2 years postoperatively: a comparison between autografts and allografts in young men using clinical and 3.0-T magnetic resonance imaging evaluation. Am J Sports Med. 2012;40:1519-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Waltz RA, Solomon DJ, Provencher MT. A Radiographic Assessment of Failed Anterior Cruciate Ligament Reconstruction: Can Magnetic Resonance Imaging Predict Graft Integrity? Am J Sports Med. 2014;42:1652-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Howell SM, Berns GS, Farley TE. Unimpinged and impinged anterior cruciate ligament grafts: MR signal intensity measurements. Radiology. 1991;179:639-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 109] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Frank RM, Seroyer ST, Lewis PB, Bach BR, Verma NN. MRI analysis of tibial position of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2010;18:1607-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Lee S, Kim H, Jang J, Seong SC, Lee MC. Intraoperative correlation analysis between tunnel position and translational and rotational stability in single- and double-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2012;28:1424-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Tomczak RJ, Hehl G, Mergo PJ, Merkle E, Rieber A, Brambs HJ. Tunnel placement in anterior cruciate ligament reconstruction: MRI analysis as an important factor in the radiological report. Skeletal Radiol. 1997;26:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Mall NA, Matava MJ, Wright RW, Brophy RH. Relation between anterior cruciate ligament graft obliquity and knee laxity in elite athletes at the National Football League combine. Arthroscopy. 2012;28:1104-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Saupe N, White LM, Chiavaras MM, Essue J, Weller I, Kunz M, Hurtig M, Marks P. Anterior cruciate ligament reconstruction grafts: MR imaging features at long-term follow-up--correlation with functional and clinical evaluation. Radiology. 2008;249:581-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Hosseini A, Lodhia P, Van de Velde SK, Asnis PD, Zarins B, Gill TJ, Li G. Tunnel position and graft orientation in failed anterior cruciate ligament reconstruction: a clinical and imaging analysis. Int Orthop. 2012;36:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Fujimoto E, Sumen Y, Deie M, Yasumoto M, Kobayashi K, Ochi M. Anterior cruciate ligament graft impingement against the posterior cruciate ligament: diagnosis using MRI plus three-dimensional reconstruction software. Magn Reson Imaging. 2004;22:1125-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Hantes ME, Zachos VC, Liantsis A, Venouziou A, Karantanas AH, Malizos KN. Differences in graft orientation using the transtibial and anteromedial portal technique in anterior cruciate ligament reconstruction: a magnetic resonance imaging study. Knee Surg Sports Traumatol Arthrosc. 2009;17:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Marchant MH, Willimon SC, Vinson E, Pietrobon R, Garrett WE, Higgins LD. Comparison of plain radiography, computed tomography, and magnetic resonance imaging in the evaluation of bone tunnel widening after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2010;18:1059-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Wilson TC, Kantaras A, Atay A, Johnson DL. Tunnel enlargement after anterior cruciate ligament surgery. Am J Sports Med. 2004;32:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 205] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 31. | Clatworthy MG, Annear P, Bulow JU, Bartlett RJ. Tunnel widening in anterior cruciate ligament reconstruction: a prospective evaluation of hamstring and patella tendon grafts. Knee Surg Sports Traumatol Arthrosc. 1999;7:138-145. [PubMed] |
| 32. | Fahey M, Indelicato PA. Bone tunnel enlargement after anterior cruciate ligament replacement. Am J Sports Med. 1994;22:410-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 204] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | White LM, Buckwalter KA. Technical considerations: CT and MR imaging in the postoperative orthopedic patient. Semin Musculoskelet Radiol. 2002;6:5-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Konan S, Haddad FS. Femoral fracture following knee ligament reconstruction surgery due to an unpredictable complication of bioabsorbable screw fixation: a case report and review of literature. J Orthop Traumatol. 2010;11:51-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Ma CB, Francis K, Towers J, Irrgang J, Fu FH, Harner CH. Hamstring anterior cruciate ligament reconstruction: a comparison of bioabsorbable interference screw and endobutton-post fixation. Arthroscopy. 2004;20:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 36. | Achtnich A, Forkel P, Metzlaff S, Zantop T, Petersen W. Degradation of poly-D-L-lactide (PDLLA) interference screws (Megafix ®). Arch Orthop Trauma Surg. 2014;134:1147-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Fink C, Benedetto KP, Hackl W, Hoser C, Freund MC, Rieger M. Bioabsorbable polyglyconate interference screw fixation in anterior cruciate ligament reconstruction: a prospective computed tomography-controlled study. Arthroscopy. 2000;16:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Peterson RK, Shelton WR, Bomboy AL. Allograft versus autograft patellar tendon anterior cruciate ligament reconstruction: A 5-year follow-up. Arthroscopy. 2001;17:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 39. | Lidén M, Ejerhed L, Sernert N, Bovaller A, Karlsson J, Kartus J. The course of the patellar tendon after reharvesting its central third for ACL revision surgery: a long-term clinical and radiographic study. Knee Surg Sports Traumatol Arthrosc. 2006;14:1130-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Yoshiya S, Matsui N, Matsumoto A, Kuroda R, Lee S, Kurosaka M. Revision anterior cruciate ligament reconstruction using the regenerated semitendinosus tendon: analysis of ultrastructure of the regenerated tendon. Arthroscopy. 2004;20:532-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Suijkerbuijk MA, Reijman M, Lodewijks SJ, Punt J, Meuffels DE. Hamstring Tendon Regeneration After Harvesting: A Systematic Review. Am J Sports Med. 2015;43:2591-2598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Tsifountoudis I, Bisbinas I, Kalaitzoglou I, Markopoulos G, Haritandi A, Dimitriadis A, Papastergiou S. The natural history of donor hamstrings unit after anterior cruciate ligament reconstruction: a prospective MRI scan assessment. Knee Surg Sports Traumatol Arthrosc. 2015; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Christensen JJ, Krych AJ, Engasser WM, Vanhees MK, Collins MS, Dahm DL. Lateral Tibial Posterior Slope Is Increased in Patients With Early Graft Failure After Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2015;43:2510-2514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 44. | Webb JM, Salmon LJ, Leclerc E, Pinczewski LA, Roe JP. Posterior tibial slope and further anterior cruciate ligament injuries in the anterior cruciate ligament-reconstructed patient. Am J Sports Med. 2013;41:2800-2804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 276] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 45. | Bisson LJ, Gurske-DePerio J. Axial and sagittal knee geometry as a risk factor for noncontact anterior cruciate ligament tear: a case-control study. Arthroscopy. 2010;26:901-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Sonnery-Cottet B, Archbold P, Cucurulo T, Fayard JM, Bortolletto J, Thaunat M, Prost T, Chambat P. The influence of the tibial slope and the size of the intercondylar notch on rupture of the anterior cruciate ligament. J Bone Joint Surg Br. 2011;93:1475-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 47. | Tanaka MJ, Jones KJ, Gargiulo AM, Delos D, Wickiewicz TL, Potter HG, Pearle AD. Passive anterior tibial subluxation in anterior cruciate ligament-deficient knees. Am J Sports Med. 2013;41:2347-2352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 48. | Musahl V, Ayeni OR, Citak M, Irrgang JJ, Pearle AD, Wickiewicz TL. The influence of bony morphology on the magnitude of the pivot shift. Knee Surg Sports Traumatol Arthrosc. 2010;18:1232-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Marcacci M, Zaffagnini S, Marcheggiani Muccioli GM, Neri MP, Bondi A, Nitri M, Bonanzinga T, Grassi A. Arthroscopic intra- and extra-articular anterior cruciate ligament reconstruction with gracilis and semitendinosus tendons: a review. Curr Rev Musculoskelet Med. 2011;4:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |