Published online May 28, 2014. doi: 10.4329/wjr.v6.i5.203
Revised: January 27, 2014
Accepted: April 17, 2014
Published online: May 28, 2014
Processing time: 191 Days and 23.4 Hours
AIM: To assess agreement between different forms of T2 weighted imaging (T2WI), and post-contrast T1WI in the depiction of fistula tracts, inflammation, and internal openings with that of a reference test.
METHODS: Thirty-nine consecutive prospective cases were enrolled. The following sequences were used for T2WI: 2D turbo-spin-echo (2D T2 TSE); 3D T2 TSE; short tau inversion recovery (STIR); 2D T2 TSE with fat saturation performed in all patients. T1WI were either a 3D T1-weighted prepared gradient echo sequence with fat saturation or a 2D T1 fat saturation [Spectral presaturation with inversion (SPIR)]. Agreement for each sequence for determination of fistula extension, internal openings, and the presence of active inflammation was assessed separately and blindly against a reference test comprised of follow-up, surgery, endoscopic ultrasound, and assessment by an independent experienced radiologist with access to all images.
RESULTS: Fifty-six fistula tracts were found: 2 inter-sphincteric, 13 trans-sphincteric, and 24 with additional tracts. The best T2 weighted sequence for depiction of fistula tracts was 2D T2 TSE (Cohen’s kappa = 1.0), followed by 3D T2 TSE (0.88), T2 with fat saturation (0.54), and STIR (0.19). Internal openings were best seen on 2D T2 TSE (Cohen’s kappa = 0.88), followed by 3D T2 TSE (0.70), T2 with fat saturation (0.54), and STIR (0.31). Detection of inflammation showed Cohen’s kappa of 0.88 with 2D T2 TSE, 0.62 with 3D T2 TSE, 0.63 with STIR, and 0.54 with T2 with fat saturation. STIR, 3D T2 TSE, and T2 with fat saturation did not make any contributions compared to 2D T2 TSE. Post-contrast 3D T1 weighted prepared gradient echo sequence with fat saturation showed better agreement in the depiction of fistulae (Cohen’s kappa = 0.94), finding internal openings (Cohen’s kappa = 0.97), and evaluating inflammation (Cohen’s kappa = 0.94) compared to post-contrast 2D T1 fat saturation or SPIR where the corresponding figures were 0.71, 0.66, and 0.87, respectively. Comparing the best T1 and T2 sequences showed that, for best results, both sequences were necessary.
CONCLUSION: 3D T1 weighted sequences were best for the depiction of internal openings and active inflammatory components, while 2D T2 TSE provided the best assessment of fistula extension.
Core tip: Both T1 (post-contrast) and T2 weighted sequences are needed for the best assessment of fistula-in-ano; T2 weighted imaging: 2D turbo-spin-echo (2D T2 TSE) is most useful for depiction in relation to sphincter muscles. 3D T2 TSE cannot replace 2D T2 TSE. Post-contrast 3D T1 weighted prepared gradient echo sequence with fat saturation is necessary for the depiction of internal openings and inflammation activity; short tau inversion recovery and fat saturated T2 sequences should be omitted from protocols.
- Citation: Torkzad MR, Ahlström H, Karlbom U. Comparison of different magnetic resonance imaging sequences for assessment of fistula-in-ano. World J Radiol 2014; 6(5): 203-209
- URL: https://www.wjgnet.com/1949-8470/full/v6/i5/203.htm
- DOI: https://dx.doi.org/10.4329/wjr.v6.i5.203
Treatment of fistula-in-ano requires adequate knowledge of its extensions[1-5]. Magnetic resonance imaging (MRI) is the single best imaging modality for this purpose, although sometimes the addition of other tests such as endoanal ultrasound (EUS) is required. During recent years, the focus of MRI research on fistula-in-ano has been two-fold; most previous research focused on comparing MRI with EUS or examination under anesthesia (EUA)[6-8], but during more recent years, as efficacy of MRI has been more established, the focus of research has shifted to assessment of inflammatory activity. However, when it comes to choosing the right sequences, there has only been one published original study (by Halligan et al[9]), which was performed back in 1998. Since then, there has been no study concerning the choice of sequences. There have been multiple review articles where experienced authors have mentioned their protocols, albeit with some differences between them[10-13].
The fistula-in-ano is notably different from other fistulae[14]. While detection of a fistula is the primary concern with other abdominal fistulae, for fistula-in-ano this is usually established by the surgeon before imaging. Indeed the most important clinical indication for imaging in fistula-in-ano is the assessment of fistula extension. The reason for this is that fistula-in-ano can affect the anal sphincter complex, with inadvertent damage to this complex possibly leading to impaired continence. This means that while fat-saturated sequences can increase the detection rate of thin fluid filled fistulae, sufficient anatomic details are still required in order to determine fistula extension.
The sequences used for fistula-in-ano imaging consist of T1-weighted images before and after gadolinium contrast and T2-weighted images. Both these sequences can be obtained with or without different forms of fat suppression. The use of gadolinium contrast is controversial[10].
T2 weighted imaging (T2WI): turbo-spin-echo (T2 TSE) has been used extensively for imaging in rectal cancer[15-18] and provides superb tumor-fat-neighboring tissue contrast. There are, however, other T2-weighted sequences that could replace traditional T2 TSE. 3D T2 TSE could, in fact, potentially replace all different imaging planes due to the possibility of multi-planar reconstruction and reformatting[9,19]. In the current study, we put this to the test for assessment of fistula-in-ano. The main purpose of this study is to compare 3D TSE with 2D TSE. However, we also compared different T2 weighted sequences with each other, different T1 weighted sequences after intravenous Gadolinium administration with each other, and finally T1 and the T2 sequences were compared to one another.
Thirty-nine consecutive patients (mean age 40 years; range 10-85 years, 22/17 male/female) requesting MRI of fistula-in-ano were included in this prospective clinical trial. The study was approved by the local ethical committee and informed patient consent was obtained.
Pelvic MRI-examinations were performed on a 1.5 T systems (Philips Intera, Best, The Netherlands) using a four-channel cardiac phased-array coil (Philips, The Netherlands). Following our routines, an anti-peristaltic agent was administrated prior to examination (normally Glucagon 1 mg intramuscularly, unless contraindications are present when Buscopan is administered). The images are started with T2 weighted images and concluded with T1 weighted images before and after intravenous gadolinium contrast agent (Dotarem) in a standard dose. Usual contraindications to contrast agents and MRI are applied. Sequence variables are almost the same regarding slice thickness (3-5 mm), gap (0-1 mm), matrix (256-520, 256 for T1WI and 512 for T2WI), and field of view (250-300 mm). Only the 3D T2 TSE is performed with a slice thickness of 1-2 mm. Relaxation time (TR) and echo time (TE) are mentioned for each sequence separately, as outlined below.
T2 weighted sequences: (1) 2D T2 TSE (two dimensional turbo-spin-echo) is performed in three planes: sagittal, oblique axial (perpendicular to anal canal), and oblique coronal (parallel to anal canal). TR is 4000-5000 ms, TE 120-130, and flip angle (FA) 90 degrees; (2) 3D T2 TSE. Volumetric ISotropic T2w Acquisition (VISTA) is the Philips vendor sequence name (known as SPACE in Siemens and CUBE in GE). VISTA (Philips, The Netherlands) stands for Volumetric ISotropic T2w Acquisition. TE is 275 ms, TR 1500 ms, and FA 90 degrees. For rectal cancer we use 3D T2 TSE in the sagittal plane, but for fistula-in-ano the imaging plane is perpendicular to anal canal; (3) Short tau inversion recovery (STIR) was applied with a TR of 1500 ms and TE of 15; and (4) T2 TSE with fat saturation was performed with a TE of 70 ms and TR of 2500 ms.
T1 weighted sequences: These sequences were performed the same way before and after intravenous gadolinium contrast agent administration: (1) 3D T1 weighted prepared gradient echo sequence with fat saturation [T1 High Resolution Isotropic Volume Excitation (THRIVE)] was used with a TR of 16 ms and TE 8 ms. FA is 10 degrees. THRIVE is the Philips vendor name; the corresponding sequence in Siemens is Volume Interpolated Breathhold Examination and Liver Acquisition with Volume Acceleration in GE; and (2) 2D T1 fat saturation or Spectral presaturation with inversion recovery (SPIR) has a TR of 9 ms and TE of 4-5 ms. FA is 10 degrees. The acquisition times were between 4 and 6 min each (6 min for 3D T2 TSE).
Only axial/oblique images are compared to each other. One radiologist (Torkzad MR) with more than 10 years of experience in pelvic imaging assessed each sequence separately and blindly. The extension of each fistula tract was noted in regard to pelvic landmarks (pelvic floor, sphincter muscles, and ischioanal fossa), internal openings (location clockwise, as well as height in regard to dentate line), and signs of activity (fluid-filled channels or avid contrast enhancement).
The reference test was comprised of surgical findings (25 cases), follow-up, EUS findings (21 cases), and assessment by an independent experienced radiologist (Ahlström H) with access to all sequences (all cases) whenever findings were incongruent.
The results were dichotomized based on detection of the entire fistula tract, correct assessment of internal openings (clockwise ± 1, and height wise ± 1 cm), and detection of inflammation. Cohen’s kappa was measured based on comparison to the set of reference tests. Agreement levels were defined as follows: poor (less than 0.2), fair (≥ 0.2 but < 0.4), moderate (≥ 0.4 but < 0.6), good (≥ 0.6 but < 0.8), strong (≥ 0.8 but < 0.95), and perfect (≥ 0.95).
A total of 56 fistula tracts were found in the studied patients. Two patients had only simple intersphincteric fistulae. The remaining 37 had trans-sphincteric tracts, of which 13 had simple tracts. Ten patients had an additional tract or abscess to the trans-sphincteric tract and 14 had multiple tracts. The results of comparisons between sequences is mentioned below and summarized in Table 1.
| Sequence | Tract extension | Internal opening | Active inflammation |
| T2 weighted | |||
| 2D TSE | 1.00 | 0.88 | 0.88 |
| 3D TSE | 0.88 | 0.70 | 0.62 |
| STIR | 0.19 | 0.31 | 0.63 |
| 2D TSE with fat sat | 0.54 | 0.54 | 0.54 |
| T1 weighted | |||
| 3D T1 weighted with fat saturation | 0.94 | 0.97 | 0.94 |
| SPIR | 0.71 | 0.66 | 0.87 |
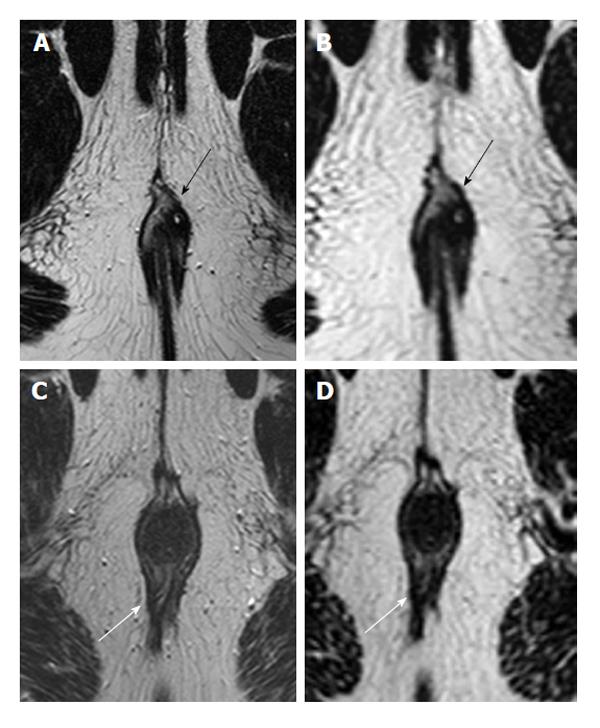
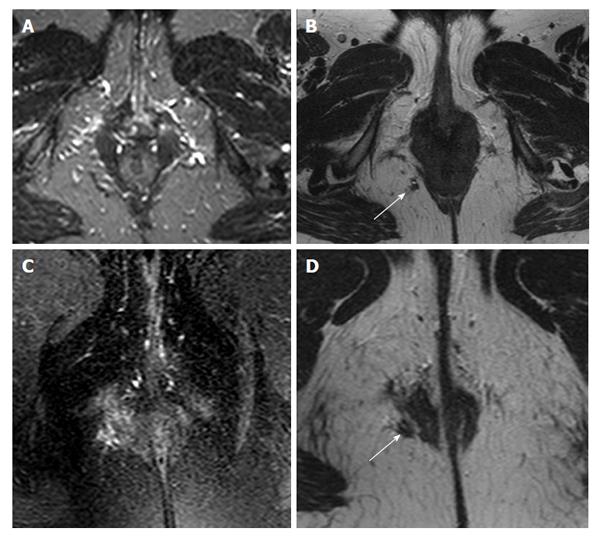
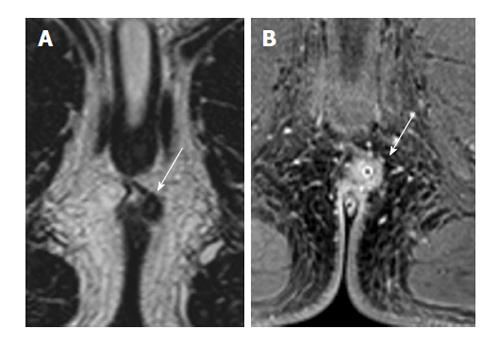
The best T2 weighted sequence for depiction of fistula tracts was 2D T2 TSE, where agreement levels for fistula tract depiction were perfect (Cohen’s kappa = 1.0). 3D T2 TSE showed a 0.88 agreement for the characterization of fistula tracts (i.e., strong agreement; Figure 1). Both STIR and T2 with fat saturation showed worse agreement levels, with a Cohen’s kappa of 0.19 (poor agreement) and 0.54 (moderate agreement), respectively (Figure 2). Internal openings showed somewhat less agreement on 2D TSE (Cohen’s kappa 0.88, defined as strong agreement) and with 3D T2 TSE 0.70 (good agreement). Cohen’s kappa was 0.31 for STIR (fair agreement) and 0.54 with T2 with fat saturation (moderate agreement). Detection of inflammation was concordant with Cohen’s kappa being 0.88 with 2D T2 TSE (strong agreement), 0.62 (good agreement) with 3D T2 TSE, 0.63 with STIR (good agreement), and 0.54 with T2 with fat saturation (moderate). STIR, 3D T2 TSE, and T2 with fat saturation did not contribute in any case compared to 2D T2 TSE (Figure 3).
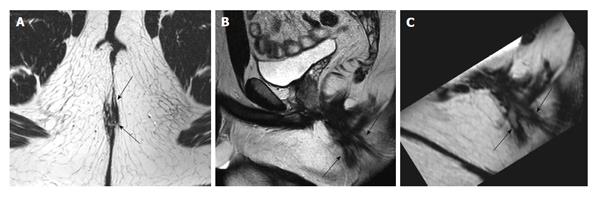
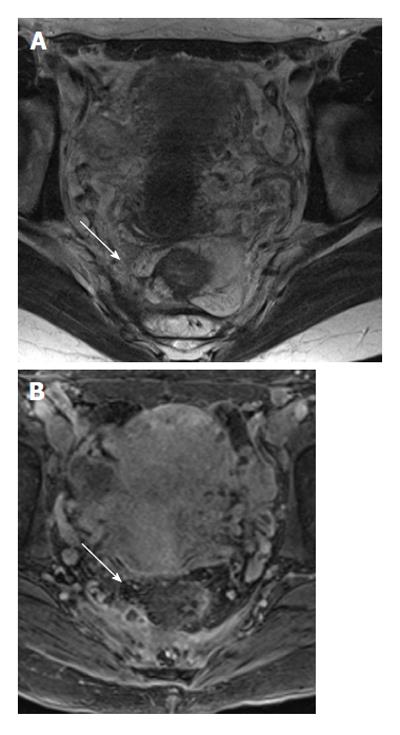
Post-contrast 3D T1 weighted prepared gradient echo sequence with fat saturation (THRIVE) showed better agreement in the depiction of fistulae (Cohen’s kappa = 0.94 or strong agreement), finding inner openings (Cohen’s kappa = 0.97 or perfect agreement), and evaluating inflammation (Cohen’s kappa = 0.94 or strong agreement) compared to post-contrast 2D T1 fat saturation or SPIR, where the corresponding figures were 0.71 (good agreement), 0.66 (good agreement), and 0.87 (strong agreement), respectively (Figure 4).
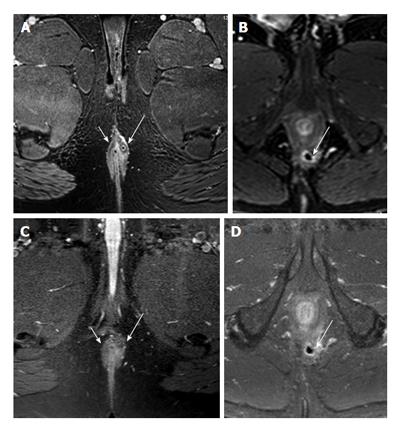
Comparing the best T1 and T2 sequences (i.e., 2D T2 TSE with 3D T1) showed that for the best results, both sequences were necessary (see figures mentioned above). While 2D T2 TSE was slightly better for the determination of fistula extension, 3D T1 enabled better depiction of internal openings and inflammation (Figures 5 and 6).
This study is one of the few that examine MRI protocols for the characterization of fistula-in-ano. We observed that among T2 sequences, 2D TSE provides the best evaluation. Along with other authors[10,13], we hoped that 3D T2 weighted TSE might be able to replace 2D TSE. However, we were unable to find support for this, and as a result have abandoned 3D TSE after our evaluations.
The use of T1 weighted imaging is somewhat controversial, with several works examining it. Mostly, these have looked at its role as a contrast-enhancement for the evaluation of the presence or degree of inflammation[20-22]. To our knowledge, there has never been a published work which has said that T1 sequence should be used. In our study, we did not subject each patient to both types of T1 weighted sequences; half of patients were imaged with different types. Despite the recommendations of some authors[11], we found THRIVE to give better results. We were not surprised that post-contrast imaging was superior in the depiction of active inflammation. However, we were surprised that THRIVE enabled better depiction of internal openings[23]. Our results suggest that contrast-enhanced T1 weighted imaging is beneficial.
Our study suffers several disadvantages. Firstly, we did not have many cases to examine, although it must be said that our numbers are not particularly small in comparison with the current radiologic literature concerning fistula-in ano. Also we had only one blind radiologist who evaluated the images; it would have been better if more radiologists had assessed the images blindly.
In our study we have used a novel method to compare different sequences. We did not look at sensitivity or specificity in diagnosis of fistula-in-ano. A recent meta-analysis looking into this from 2012 found the degree of heterogeneity between studies to be high, with sensitivities being reported as between 0.63 to 0.93, while the figures for specificity were 0.51 to 0.82[24].
The numbers of sequences that could be employed are many, with several that were not assessed by us. A recent Japanese study[25] employed diffusion weighted imaging in 24 patients for the assessment of disease activity and found it to be a feasible method. They employed b-values of 0 and 1000. Not using gadolinium is an interesting option which basically diminishes the semi-invasive character of post-contrast MRI. A Turkish study[26] employed a similar approach for the comparison of sequences in the depiction of fistulae in 26 patients. However, they only compared 2D TSE, STIR, and post-contrast 2D T1 weighted echo fast low angle shot images. Sequences such as 3D imaging were not used. Despite this the authors came to the same conclusion as us; both T2WI and post-contrast T1WI are necessary.
Both 2DT T2WI and contrast-enhanced 3D T1 weighted prepared gradient echo sequence with fat saturation seem necessary for the adequate depiction of fistula course, its internal openings, and the presence of active inflammation.
Treatment of fistula-in-ano requires careful assessment of the extension and activity of fistulae. Magnetic resonance imaging (MRI) has emerged as the dominant method for such assessment, especially for the more complicated fistulae. There are many available sequences, although the values of these sequences have not been tested against each other or for different properties of fistula-in-ano.
To test different MRI sequences for the best depiction of extension, internal openings, and inflammatory activity of fistula-in-ano.
The authors tested old and new sequences against each other. They found that T2 weighted imaging: turbo-spin-echo (T2 TSE), with its best depiction of sphincter muscles, is essential and necessary for the assessment of extensions in fistula-in-ano. Contrary to hopes that 3D T2 TSE would replace 2D T2 TSE, it was instead found not to add anything of value and thus incapable of replacing 2D T2 TSE. Fat saturated T2-weighted sequences and short tau inversion recovery were inferior to T2 TSE. Surprisingly, post-contrast T1 weighted imaging proved necessary for finding internal openings or evaluating inflammation activity. There were also differences between different post-contrast T1 sequences, with post-contrast 3D T1 weighted prepared gradient echo sequence with fat saturation providing the best results.
This study outlines that 2D TSE and post-contrast 3D T1 weighted prepared gradient echo sequence with fat saturation are both sufficient and necessary.
Fistula-in-ano is defined as the presence of fistulae (abnormal channels in hollow body organs; in this case the anorectal lumen and skin), sinus (blind channels), and their sequelae and nearby abscesses without any shown contact to anorectal lumen.
The authors evaluated different MRI sequences (T1, T2, and 3D) for assessing fistulas in 39 MRI patients. An excellent paper which was necessary for daily routine radiology, it has a clear message as well as a short overview of the literature.
P- Reviewers: Liu YY, Metwalli ZA, Razek AAKA, Schreyer AG, Sijens PE S- Editor: Ji FF L- Editor: Rutherford A E- Editor: Liu SQ
| 1. | Abcarian H. Anorectal infection: abscess-fistula. Clin Colon Rectal Surg. 2011;24:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 2. | Simpson JA, Banerjea A, Scholefield JH. Management of anal fistula. BMJ. 2012;345:e6705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Subhas G, Singh Bhullar J, Al-Omari A, Unawane A, Mittal VK, Pearlman R. Setons in the treatment of anal fistula: review of variations in materials and techniques. Dig Surg. 2012;29:292-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Tabry H, Farrands PA. Update on anal fistulae: surgical perspectives for the gastroenterologist. Can J Gastroenterol. 2011;25:675-680. [PubMed] |
| 5. | Schwartz DA, Wiersema MJ, Dudiak KM, Fletcher JG, Clain JE, Tremaine WJ, Zinsmeister AR, Norton ID, Boardman LA, Devine RM. A comparison of endoscopic ultrasound, magnetic resonance imaging, and exam under anesthesia for evaluation of Crohn’s perianal fistulas. Gastroenterology. 2001;121:1064-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 341] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 6. | Sun MR, Smith MP, Kane RA. Current techniques in imaging of fistula in ano: three-dimensional endoanal ultrasound and magnetic resonance imaging. Semin Ultrasound CT MR. 2008;29:454-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Szurowska E, Wypych J, Izycka-Swieszewska E. Perianal fistulas in Crohn’s disease: MRI diagnosis and surgical planning: MRI in fistulazing perianal Crohn’s disease. Abdom Imaging. 2007;32:705-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Wise PE, Schwartz DA. The evaluation and treatment of Crohn perianal fistulae: EUA, EUS, MRI, and other imaging modalities. Gastroenterol Clin North Am. 2012;41:379-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Halligan S, Healy JC, Bartram CI. Magnetic resonance imaging of fistula-in-ano: STIR or SPIR? Br J Radiol. 1998;71:141-145. [PubMed] |
| 10. | Torkzad MR, Karlbom U. MRI for assessment of anal fistula. Insights Imaging. 2010;1:62-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | de Miguel Criado J, del Salto LG, Rivas PF, del Hoyo LF, Velasco LG, de las Vacas MI, Marco Sanz AG, Paradela MM, Moreno EF. MR imaging evaluation of perianal fistulas: spectrum of imaging features. Radiographics. 2012;32:175-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Gage KL, Deshmukh S, Macura KJ, Kamel IR, Zaheer A. MRI of perianal fistulas: bridging the radiological-surgical divide. Abdom Imaging. 2013;38:1033-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | O’Malley RB, Al-Hawary MM, Kaza RK, Wasnik AP, Liu PS, Hussain HK. Rectal imaging: part 2, Perianal fistula evaluation on pelvic MRI--what the radiologist needs to know. AJR Am J Roentgenol. 2012;199:W43-W53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Lewis RT, Bleier JI. Surgical Treatment of Anorectal Crohn Disease. Clin Colon Rectal Surg. 2013;26:90-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Taylor FG, Quirke P, Heald RJ, Moran BJ, Blomqvist L, Swift IR, Sebag-Montefiore D, Tekkis P, Brown G. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol. 2014;32:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 443] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 16. | Torkzad MR, Påhlman L, Glimelius B. Magnetic resonance imaging (MRI) in rectal cancer: a comprehensive review. Insights Imaging. 2010;1:245-267. [PubMed] |
| 17. | Torkzad MR, Kamel I, Halappa VG, Beets-Tan RG. Magnetic resonance imaging of rectal and anal cancer. Magn Reson Imaging Clin N Am. 2014;22:85-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Beets-Tan RG, Lambregts DM, Maas M, Bipat S, Barbaro B, Caseiro-Alves F, Curvo-Semedo L, Fenlon HM, Gollub MJ, Gourtsoyianni S. Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2013;23:2522-2531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 19. | Torkzad MR, Wikström J, Hansen T, Bergman A, Bjerner T, Ahlström H. The Clinical Perspective on Value of 3D, Thin Slice T2-Weighted Images in 3T Pelvic MRI for Tumors. Current Medical Imaging Reviews. 2012;8:76-81. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Horsthuis K, Lavini C, Bipat S, Stokkers PC, Stoker J. Perianal Crohn disease: evaluation of dynamic contrast-enhanced MR imaging as an indicator of disease activity. Radiology. 2009;251:380-387. [PubMed] |
| 21. | Spencer JA, Ward J, Beckingham IJ, Adams C, Ambrose NS. Dynamic contrast-enhanced MR imaging of perianal fistulas. AJR Am J Roentgenol. 1996;167:735-741. [PubMed] |
| 22. | Szyszko TA, Bush J, Gishen P, Sellu D, Desouza NM. Endoanal magnetic resonance imaging of fistula-in-ano: a comparison of STIR with gadolinium-enhanced techniques. Acta Radiol. 2005;46:3-8. [PubMed] |
| 23. | Buchanan GN, Halligan S, Bartram CI, Williams AB, Tarroni D, Cohen CR. Clinical examination, endosonography, and MR imaging in preoperative assessment of fistula in ano: comparison with outcome-based reference standard. Radiology. 2004;233:674-681. [PubMed] |
| 24. | Siddiqui MR, Ashrafian H, Tozer P, Daulatzai N, Burling D, Hart A, Athanasiou T, Phillips RK. A diagnostic accuracy meta-analysis of endoanal ultrasound and MRI for perianal fistula assessment. Dis Colon Rectum. 2012;55:576-585. [PubMed] |
| 25. | Yoshizako T, Wada A, Takahara T, Kwee TC, Nakamura M, Uchida K, Hara S, Luijten PR, Kitagaki H. Diffusion-weighted MRI for evaluating perianal fistula activity: feasibility study. Eur J Radiol. 2012;81:2049-2053. [PubMed] |
| 26. | Yildirim N, Gökalp G, Öztürk E, Zorluoğlu A, Yilmazlar T, Ercan I, Savci G. Ideal combination of MRI sequences for perianal fistula classification and the evaluation of additional findings for readers with varying levels of experience. Diagn Interv Radiol. 2012;18:11-19. [PubMed] |