Published online Jun 28, 2025. doi: 10.4329/wjr.v17.i6.107522
Revised: April 23, 2025
Accepted: May 29, 2025
Published online: June 28, 2025
Processing time: 86 Days and 10.8 Hours
This pictorial review discusses the imaging approach to evaluate for proper placement or complications of pediatric gastrostomy tube (G-tube) placement and long-term use. G-tubes are crucial for long-term nutritional support in patients facing challenges with oral intake. The article depicts the role of imaging such as contrast radiography, fluoroscopy, ultrasound, and computed tomography scans for confirming G-tube position and evaluating complications, in addition to basic anatomical considerations and placement techniques. Complications discussed include malposition, intraperitoneal placement, buried bumper syndrome, and tube malfunction. Specific imaging techniques and checklists are provided to guide clinicians in assessing G-tube placement accurately. The latter half of the review is a comprehensive exploration of pearls and pitfalls of imaging when employed to detect complications to avoid false positives and negatives.
Core Tip: Gastrostomy tube (G-tube) complications can be evaluated using fluoroscopy or 2-view contrast radiography with positive contrast injection. Presence of intraluminal contrast with gastric contour and rugae, normal emptying into the duodenum and confirmation of balloon positioning are key indicators of appropriate positioning. Awareness of critical complications associated with G-tube helps in early recognition and better patient management. These conditions include intraperitoneal placement, bowel perforation, colonic or esophageal placement, buried bumper syndrome and local abscess.
- Citation: Patel DD, Schenker KE, Averill LW, May LA. Imaging of pediatric gastrostomy tube malposition: Pearls and pitfalls. World J Radiol 2025; 17(6): 107522
- URL: https://www.wjgnet.com/1949-8470/full/v17/i6/107522.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i6.107522
Enteral nutrition plays a vital role in ensuring adequate nutrition and quality of life for patients facing challenges in oral intake during and after hospitalization, offering a reliable and effective means of delivering essential nutrients directly into the gastrointestinal tract. In cases where impaired oral intake is expected to be less than four to six weeks, nasogastric tubes are often used. However, for patients with long-term needs or permanent feeding requirements, insertion of a gastrostomy tube (G-tube) is the preferred solution[1-4].
G-tube is helpful in cases of diminished oral intake or feeding aversion, especially in cases of neurologic injuries, and esophageal atresia. Additionally, children with increased nutritional requirements (such as burns, cystic fibrosis, and chronic infections) or with impaired gastrointestinal system, such as short gut syndrome, inflammatory bowel disease, and pancreatitis, also benefit from G-tube usage[3]. Feeding by a G-tube can mimic physiological feeding by utilizing bolus feeds that emulate natural eating patterns, making them suitable for patients who can tolerate periodic, larger feedings. On the other hand, G-tubes can be used when continuous feeds are necessary, particularly in cases where patients face risks such as gastroesophageal reflux disease, aspiration, or pancreatitis[2,5].
Children with an indwelling G-tube had a minor complication rate ranging from 16%-74% with skin granulomas, local site infection and dislodgement being the most frequent culprits[6,7].
A recent meta-analysis found a major complication rate in pediatric G-tubes of 3.6% with percutaneous endoscopic (PEG) showing more complications over laparoscopic insertion[8]. About half of the patients with an indwelling G-tube experience dislodgement at some point[9,10]. In addition, laparoscopically inserted G-tubes have lesser complications than image guided or endoscopically guided insertions[8,11]. This comprehensive review discusses the imaging tech
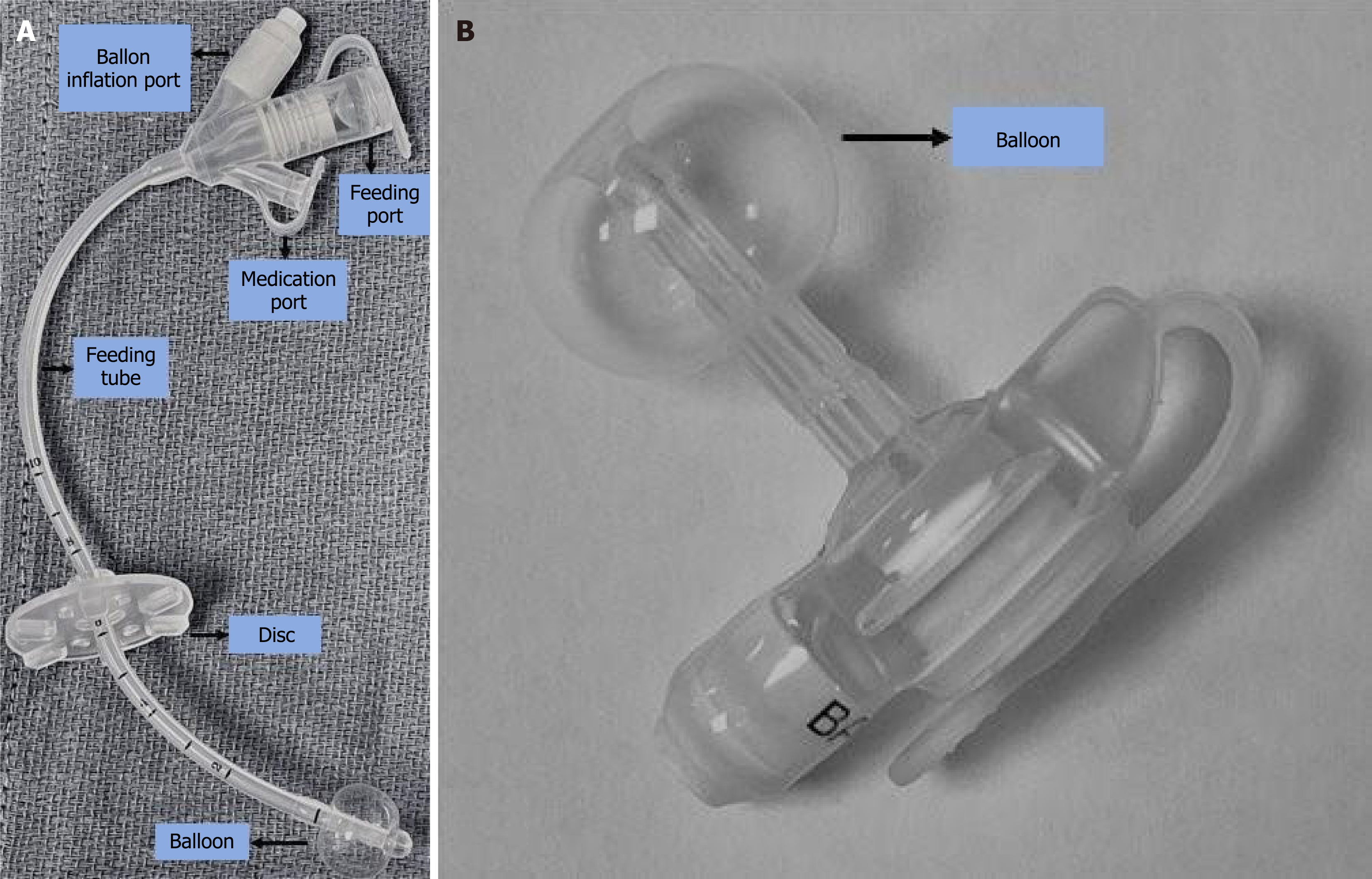
The standard G-tube comprises an external portion with a disk against the skin, an internal balloon in the stomach with the tube opening into the stomach (Figure 1A). In pediatrics, a preferred alternative is the low-profile G-tube, or gastrostomy-button, which minimizes the external profile (Figure 1B). It features a small, flush-to-skin port as the primary external component, reducing the risk of dislodgment, and still utilizes an internal balloon for stabilization. This design is advantageous for pediatric patients due to its reduced risk of dislodgement, easy stoma care and better cosmetic appearance[12]. Non-availability of smaller sizes for younger patients, painful insertion requiring sedation and external leakages are some its limitations[13]. The choice between standard and low-profile G-tubes depends on clinical factors such as the patient's age, medical condition, and lifestyle considerations.
G-tube placement techniques vary, with options ranging from surgical procedures to less invasive methods. Surgical, laparoscopic, PEG, ultrasound-guided, and fluoroscopic interventional radiology (IR) techniques are commonly employed based on the patient's condition and medical requirements[14]. In general, laparoscopy is preferred over PEG[4]. In some cases, G-tube to gastrojejunostomy tube (GJ) conversion procedure is performed in the IR setting.
The G-tube tract takes 2-4 weeks to mature. Thus, an early dislodgement predisposes to peritonitis and perforation and a blind attempt to reinsert the tube risks malposition. In contrast, late dislodgement can be corrected by reinsertion in the emergency department (ED) due to maturation of the tract[15]. Stenosis of a mature tract can occur if reinsertion of a dislodged G-tube is delayed (> 24 hours), increasing risk of complications. Thus, confirmatory imaging is warranted in scenarios such as reinsertion after early dislodgement, or traumatic or delayed reinsertion. Imaging can be skipped in timely reinsertion of late dislodgement or if clinical assessment ensures appropriate replacement[16,17].
Contrast abdominal radiography (radiographs performed after injection of water-soluble contrast) is the preferred initial imaging method for assessing G-tube placement. Traditionally, fluoroscopy was considered superior for evaluating G-tube complications. When using contrast radiography, both anteroposterior (AP) and lateral views are crucial for confirming proper positioning, particularly the retroperitoneal path of the GJ tube within the duodenal C-loop. However, Tompe et al[18] demonstrated that contrast radiography is equally effective in detecting tube malposition compared to fluoroscopy. Fluoroscopy may require on-site radiologist or additional personnel, is higher in both cost and radiation dose, and so should be reserved for cases with significant clinical concerns or other major complications. Despite its capability for real-time monitoring of G-tube position, these disadvantages have led to a decline in its preference.
Portable contrast abdominal radiography allows the patient to remain in the ED or inpatient unit, and thus quicker to perform. Water-soluble iodinated contrast (180-300 mgI/mL) should be used rather than barium due to the possibility of peritoneal leak or perforation.
For contrast radiography, our institutional protocol involves injecting 10 mL of contrast (reduced to 5 mL for infants under one year old). A cross-table lateral abdomen radiograph is obtained immediately, along with an AP supine radiograph (Figure 2 and Table 1).
| Checklist for gastrostomy tube contrast radiography | |
| Look for | Reason |
| Intraluminal contrast | Confirms proper flow and position |
| Contrast flows to dependent stomach and outlines rugal folds | Ensures tube position into the stomach |
| Contrast transits into duodenum | Rules out obstruction |
| Spherical shape of the catheter balloon | Confirm inflation |
| Intraperitoneal leak | Confirms perforation |
| Abdominal wall leak | Tube malposition |
| Tube wall continuity | Integrity/Breaks |
Fluoroscopy can be used when clinical findings are not explained by contrast radiography findings. An initial scout view is obtained to look for tube kinking, discontinuity, or gross malposition. Subsequently, both AP and lateral views of the opacified stomach are acquired during contrast administration. The last image hold technique generally provides sufficient detail for evaluation while minimizing radiation exposure. Standard radiographic exposures can be selectively employed as needed to detect subtle contrast extravasation into the peritoneal cavity. Real-time fluoroscopy facilitates dynamic assessment of many G-tube related issues.
Recent advances in point-of-care ultrasound have allowed use of ultrasound to replace and confirm G-tube positions in ED decreasing the need for radiography. The benefits include reduced length of stay in the ED and no radiation exposure but is dependent on operator experience and training[19]. Ultrasound is particularly effective in addressing abdominal wall concerns and evaluating fluid collections. In addition, ultrasound can be used as an imaging guidance for initial percutaneous placement of the G-tube and can be used bedside in critically ill patients[20].
CT serves as an adjunct in the assessment of G-tubes. It can be helpful in cases where the anatomy is complex or in cases where there is suspicion for injury to adjacent structures include the liver. Additionally, it can effectively assess fluid collections, abscesses, and the presence of pneumoperitoneum.
Confirm the presence of rugal folds and normal gastric contour on both AP and lateral views to ensure proper G-tube placement. The contrast should follow the expected path outlining the stomach.
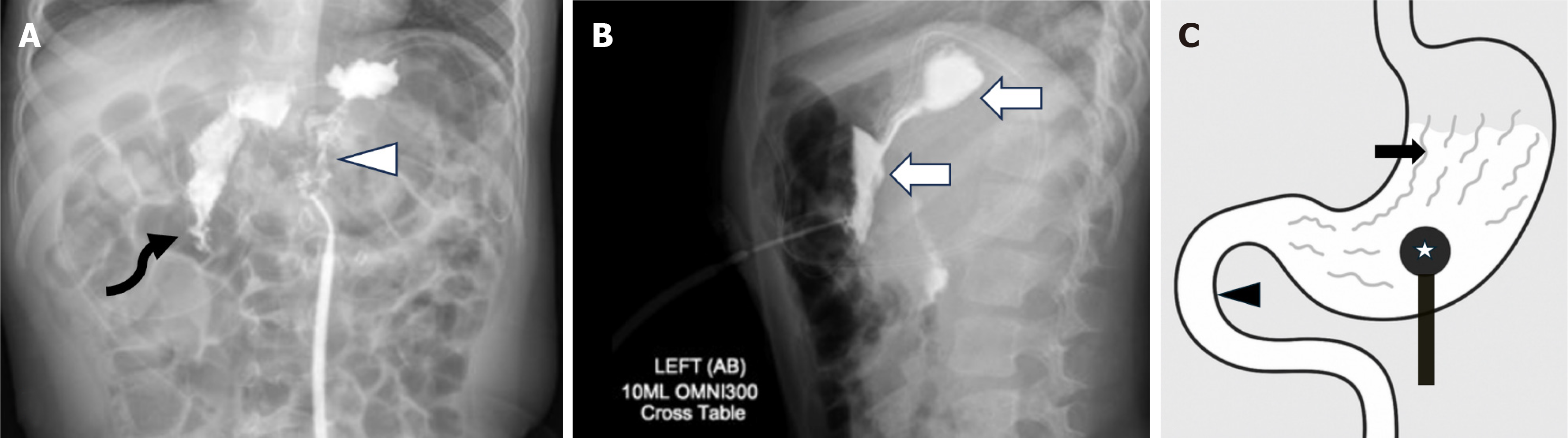
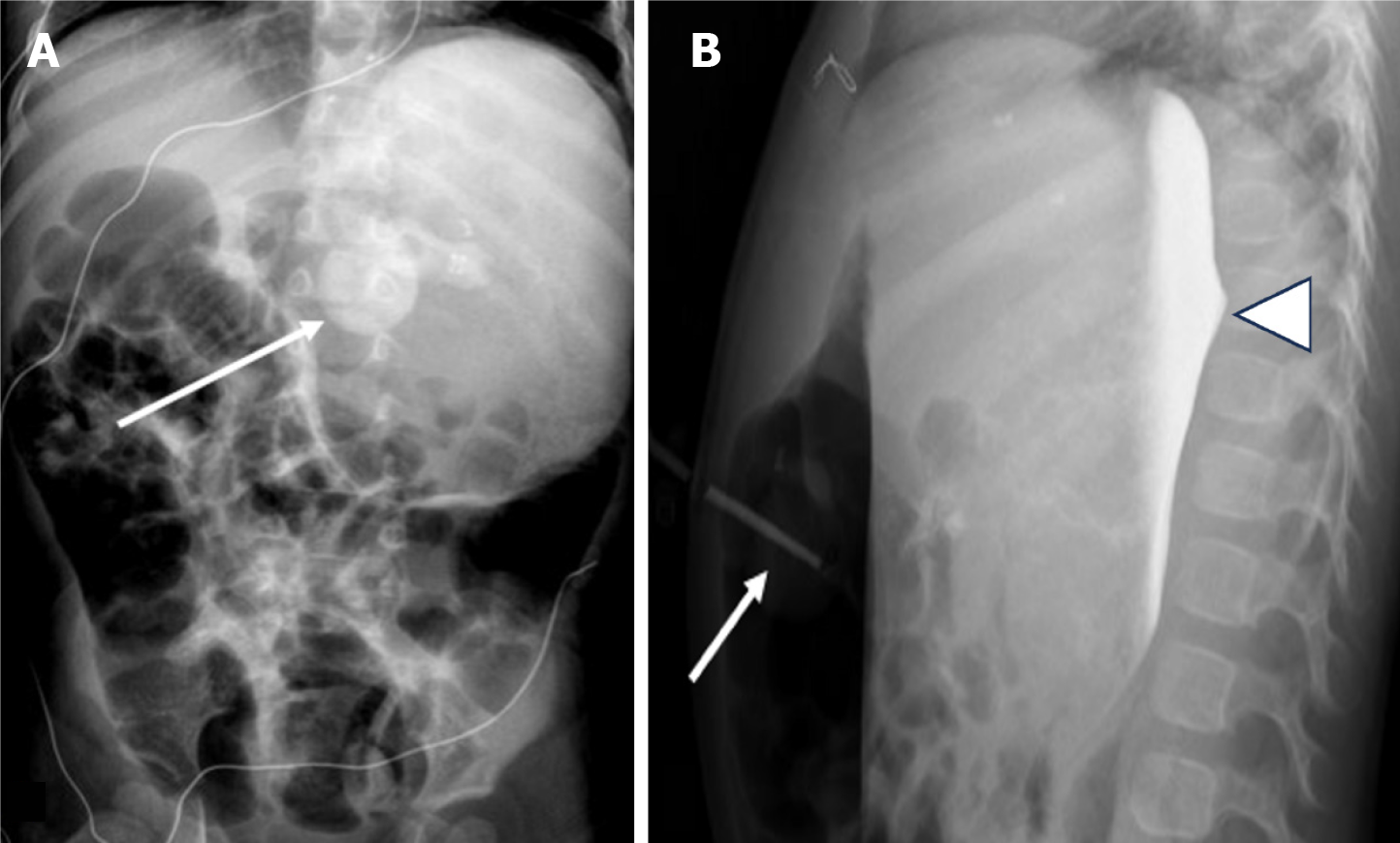
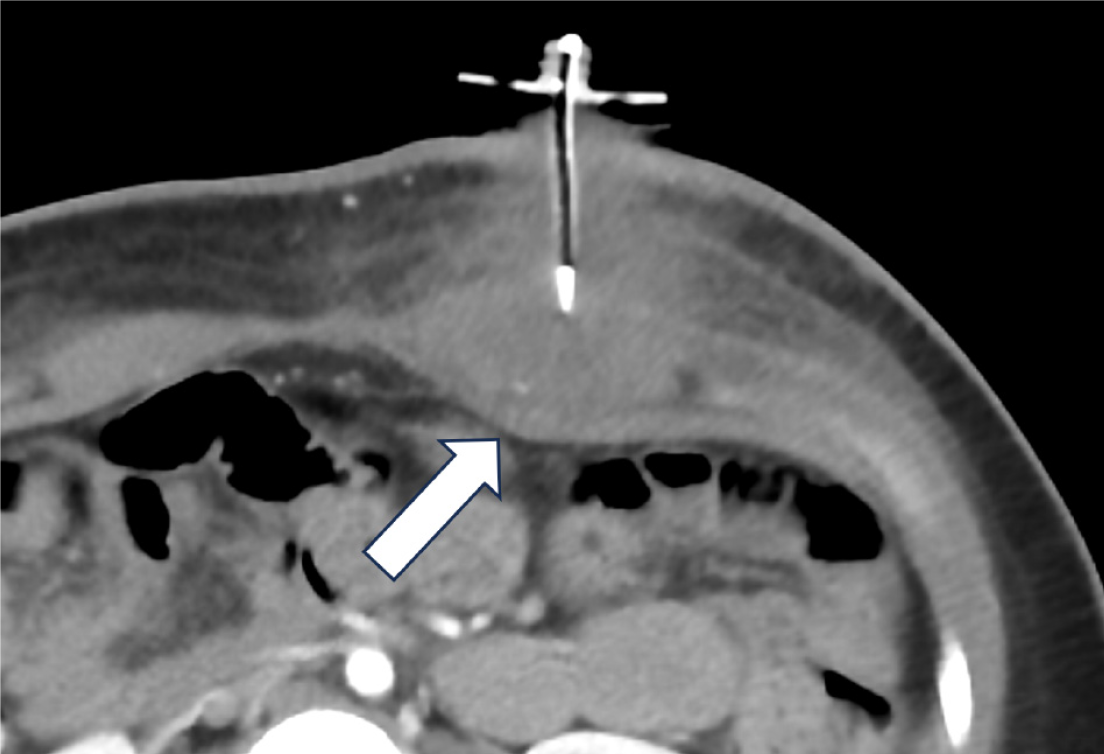
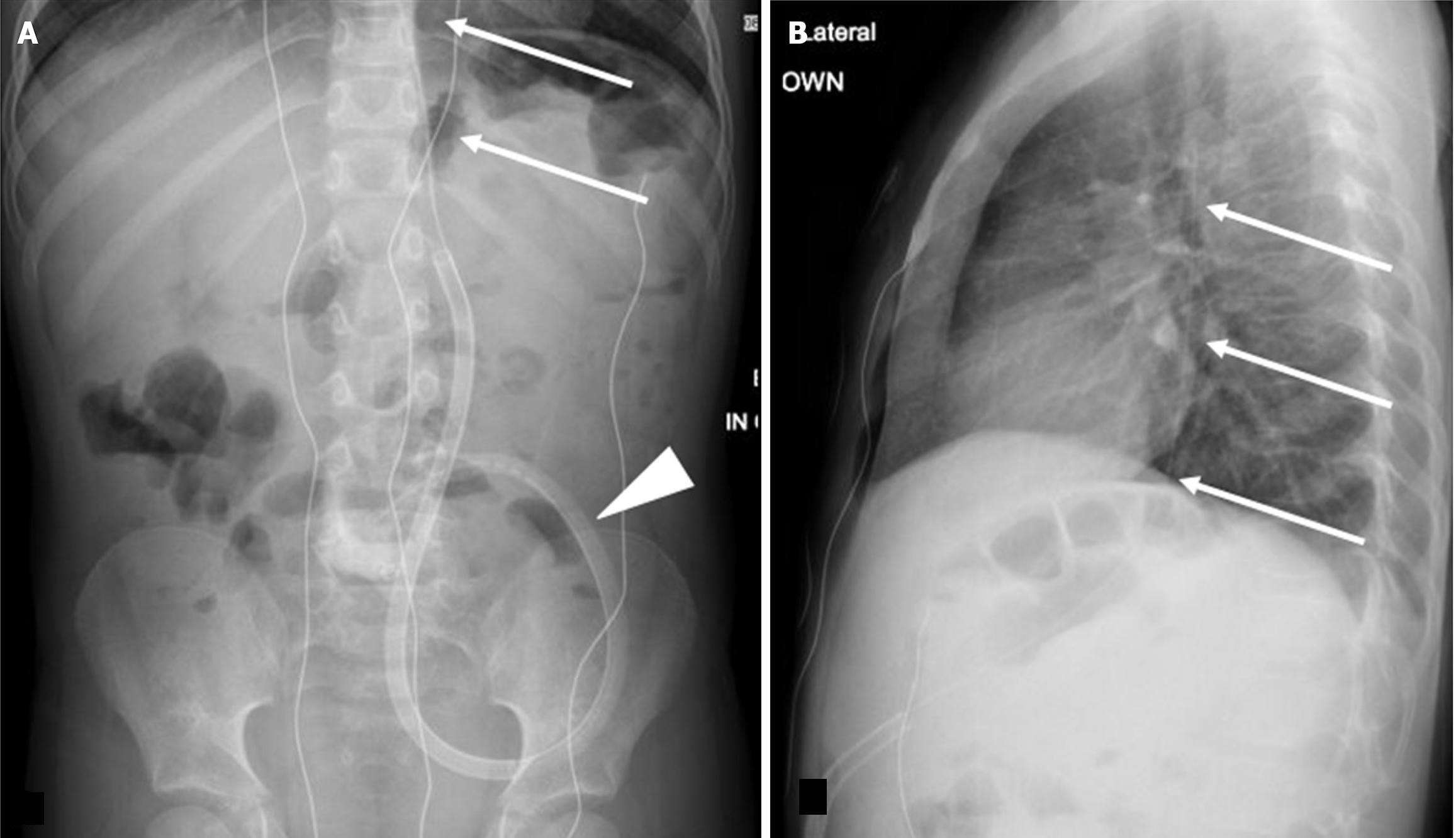
Inadvertent peritoneal placement of the G-tube can be easily missed. Contrast pooling in a dependent position can mimic contrast accumulation in the gastric fundus, leading to a false negative interpretation of appropriate placement. Carefully assess the contrast pattern and look for outlining of bowel loops, which indicates intraperitoneal placement (Figure 3). In cases of longstanding misplacement, a loculated collection may form, appearing as an abnormally shaped contrast accumulation without rugal folds.
Intraperitoneal placement of the G-tube occurs less frequently than migration of the G-tube back into the peritoneum[21]. Alternatively, if the tract is wider than the catheter, there can be trickling of gastric contents into the peritoneal cavity. These scenarios predispose to the development of peritonitis (Figure 4). Continued feeding into the peritoneal cavity may be life threatening with rapid clinical deterioration, and immediate consultation with the referring emergency or surgical team is recommended.
A small degree of pneumoperitoneum is expected after G-tube placement due to the inadvertent peritoneal puncture.
Maintain a high level of vigilance for excessive free air, especially along the liver or in subdiaphragmatic regions (Figure 4A). It may be dismissed as expected postoperative appearance. Looking for additional signs of peritonitis in such scenarios can help radiologists and clinicians guide further management[22].
Observe the path of the contrast carefully to determine the location of the G-tube balloon and its impact on contrast flow. In normal placement, intraluminal contrast should follow the gastric contour with visualization of gastric rugae and importantly a spherical filling defect of the G-tube balloon deep to the abdominal wall within the gastric lumen suggests appropriate placement.
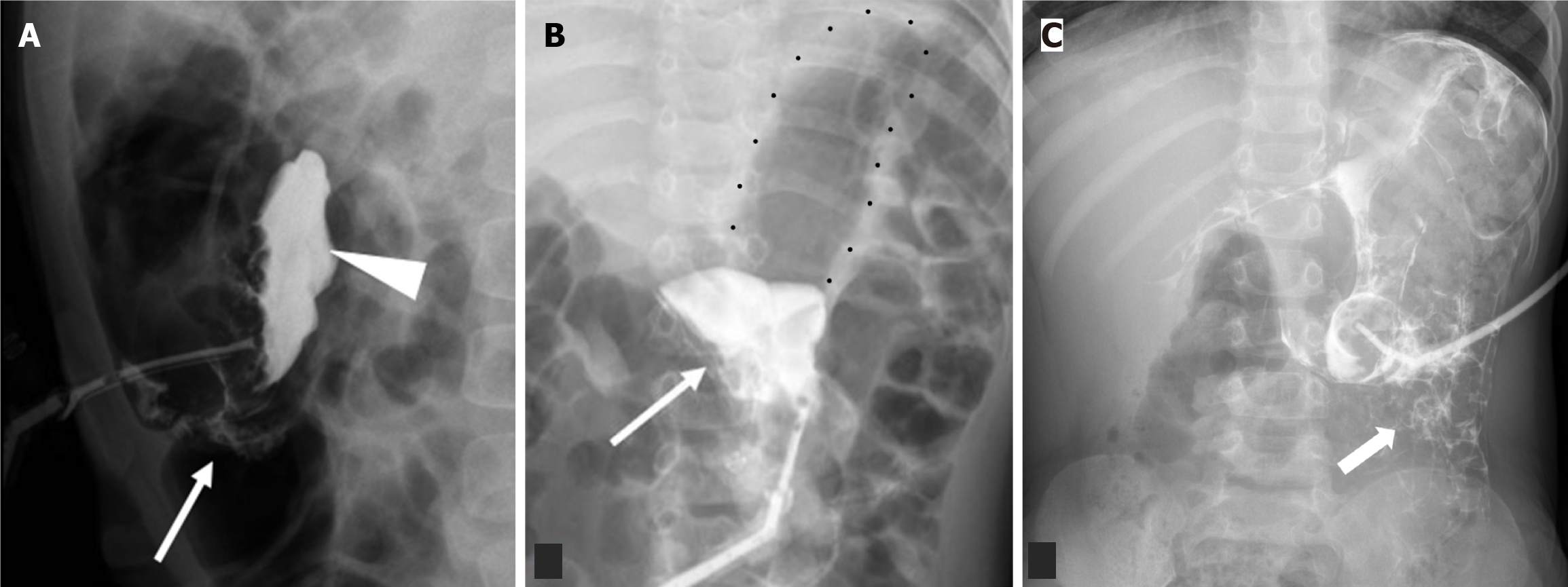
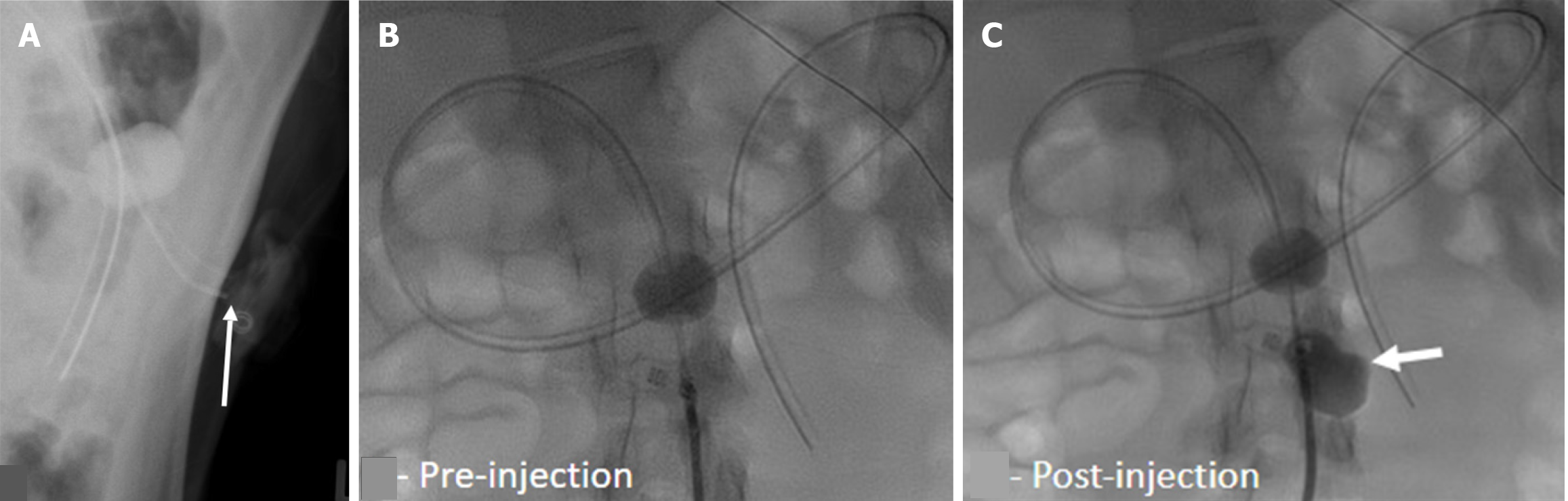
Misinterpreting the location of the G-tube balloon and its impact on contrast flow can lead to misdiagnosis and delayed management of complications. If the balloon migrates distally to the pylorus, it causes gastric outlet obstruction, and the gastric contour appears expanded with an air-fluid level and relative loss of rugal folds (Figure 5). This clinically presents vomiting and feeding intolerance. If the balloon is in the proximal duodenum, contrast only outlines the duodenal C-loop, and the stomach may not be visualized (Figure 6). Rarely, the G-tube balloon in the duodenum may also cause biliary or pancreatic drainage obstruction. Thus, visualization of gastric contour ensures proper G-tube placement and function. Contrast emptying from the stomach into the duodenum can be a normal supportive finding.
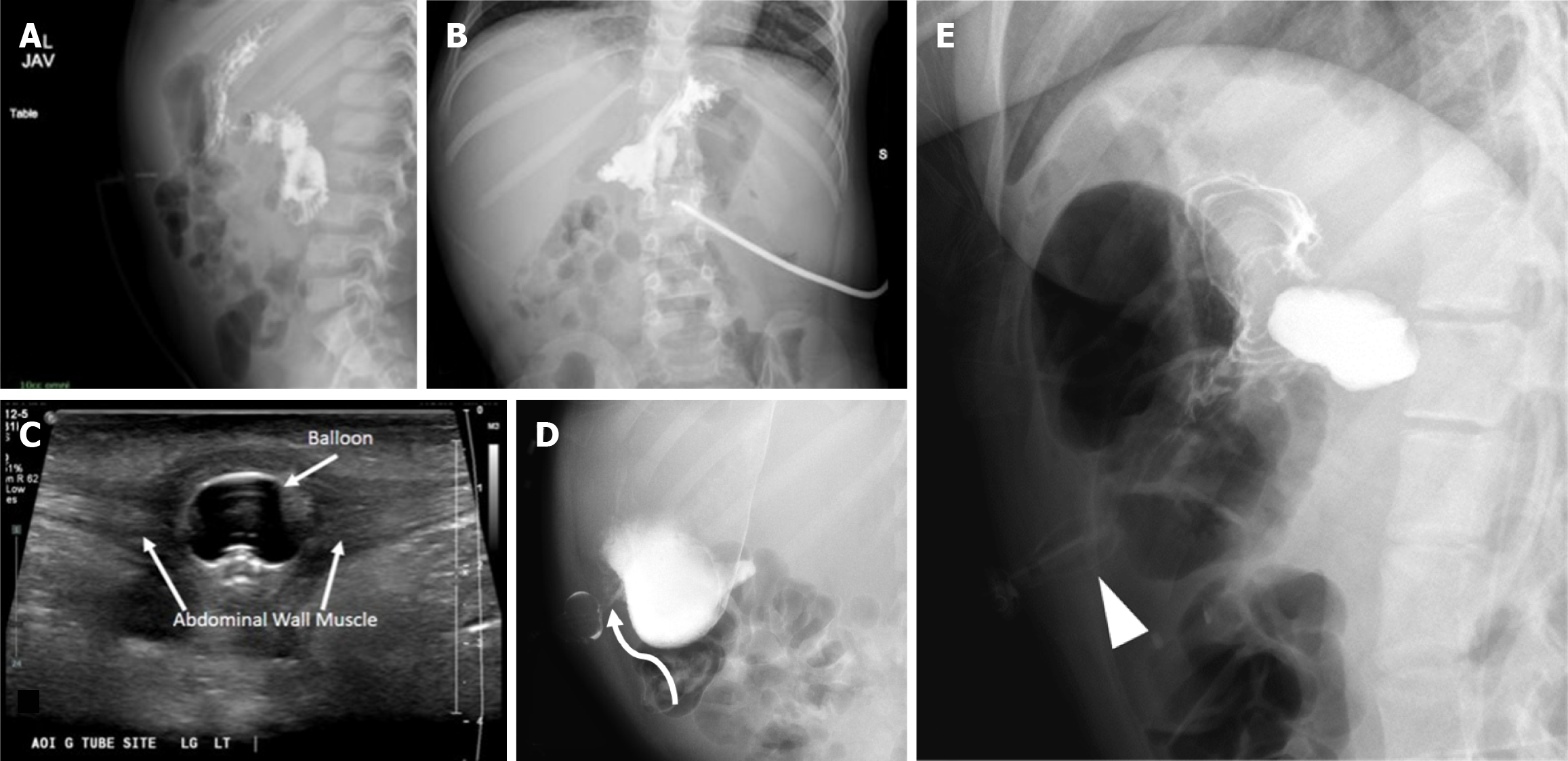
Buried bumper syndrome involves abnormal positioning of the inflated ballon into the gastric or abdominal wall. Contrast radiography will demonstrate normal gastric contour albeit without a round filling defect from the balloon. In addition, a deformed balloon may also be seen in the anterior abdominal wall on contrast radiography (Figure 7E).
Due to the frequent imaging encounters in patients with G/GJ tubes, establishing a standardized approach for assessing tube placement is essential. Regardless of the imaging modality or clinical reason for the study, the visualized portion of the G/GJ tube should be carefully evaluated on every image.
When using contrast radiography, both AP and lateral views are crucial for confirming proper positioning, particularly the retroperitoneal path of the GJ tube within the duodenal C-loop. Comparing current images with prior studies helps identify any subtle changes or deviations from the baseline tube position. This practice is especially valuable when reviewing studies where the G/GJ tube is incidentally visualized and might not trigger an automatic comparison by Picture Archiving and Communication Systems, such as scoliosis radiographs. By consistently implementing these steps, radiologists can ensure a thorough evaluation of G/GJ tube placement and promptly identify potential complications, ultimately optimizing patient care.
Patients with G/GJ tubes often undergo numerous imaging studies using various modalities (radiography, fluoroscopy, CT) for a range of clinical indications. While the G/GJ tube may be incidentally visualized within the field of view, meticulous evaluation of its positioning and course is frequently overlooked. This oversight can lead to missed complications such as tube malposition or migration, resulting in delayed diagnosis and management. For instance, a retracted GJ tube into the stomach presents as vomiting and feed intolerance since the feeds administered via the jejunostomy port are emerging into the stomach (Figure 8)[13].
Maintaining vigilance for any discontinuity and the potential accumulation of injected contrast into the abdominal wall is essential, as these are indicative of tube wall breaks. Additionally, associated soft tissue swelling in the area can serve as a subtle indicator of complications.
The continuity of the G/GJ tube, especially within the abdominal wall, is frequently overlooked due to increased soft tissue density and peripheral location on images. This oversight can lead to missed tube wall breaks and associated complications, delaying appropriate management.
Clinical presentation of G/GJ tube discontinuity varies depending on the location of the breach. When the tube wall break occurs within the skin and abdominal wall, it often leads to dermatitis and cutaneous breakdown. This can further predispose the patient to infection and abscess formation (Figure 9). If the breach is located within the peritoneum, more severe complications such as peritonitis, abscess formation, and sepsis can arise.
Importantly, a leak may also indicate the possibility of a distal obstruction within the G/GJ tube. Another potential complication is a rupture of the jejunal port into the stomach, which can manifest as increased vomiting and feed intolerance.
To definitively diagnose a leak and guide further management, contrast injection under fluoroscopic guidance can be employed. This allows for direct visualization of the leak site and assessment of the extent of the issue.
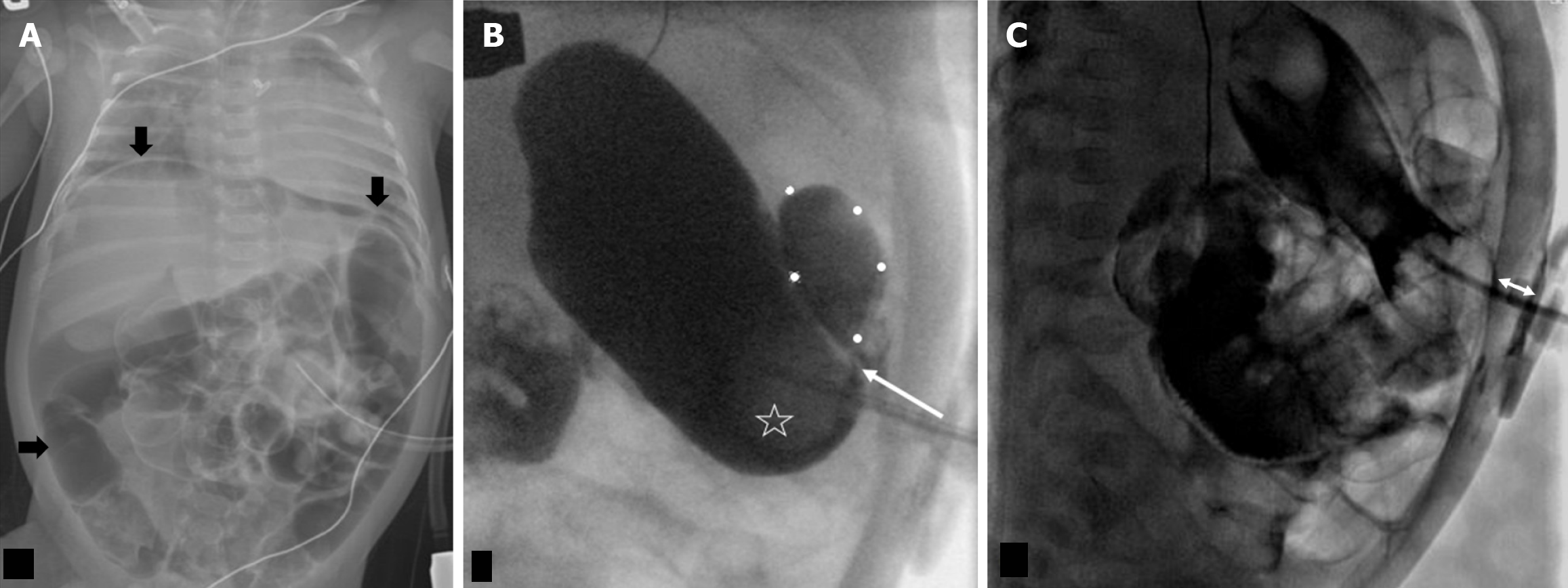
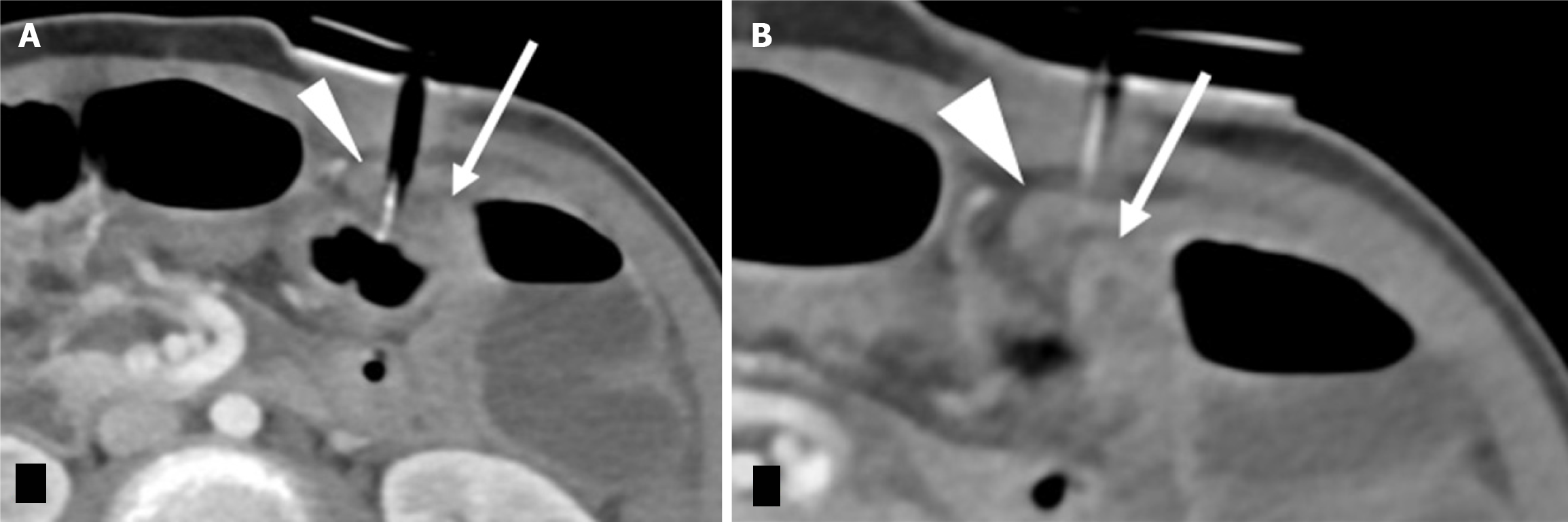
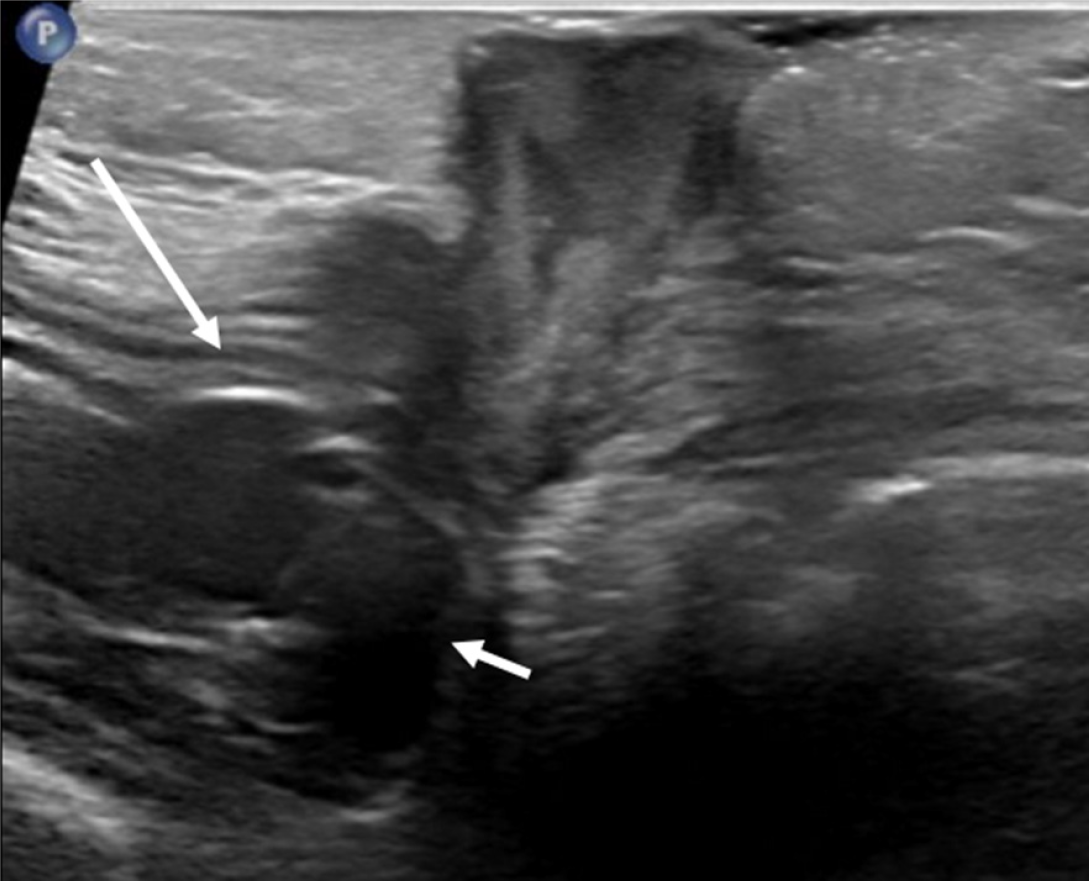
Continued symptoms or high suspicion of abnormal G-tube positioning warrants further imaging beyond initial contrast radiographs. For instance, in Buried Bumper syndrome, ultrasound or CT can clearly demonstrate the G-tube balloon embedded within the abdominal wall rather than the gastric lumen, as illustrated in Figure 10.
Over-reliance on contrast radiography when there's a high suspicion of G-tube malposition, such as Buried Bumper syndrome. Standard contrast radiographs may appear deceptively normal, with contrast filling the stomach and small bowel without obvious extravasation (Figure 7A and B). This is because the contrast follows the gastric contours and rugae, but the buried balloon doesn't create a filling defect. Depending on the G-tube tip location, contrast extravasation into the peritoneal cavity may occur (Figure 7D), but again, the key finding of the absent balloon filling defect can be easily missed.
On lateral contrast radiographs, an increased distance between the external bolster and the internal retention balloon should raise suspicion for bowel interposition between the abdominal wall and the stomach. CT imaging can definitively confirm this finding.
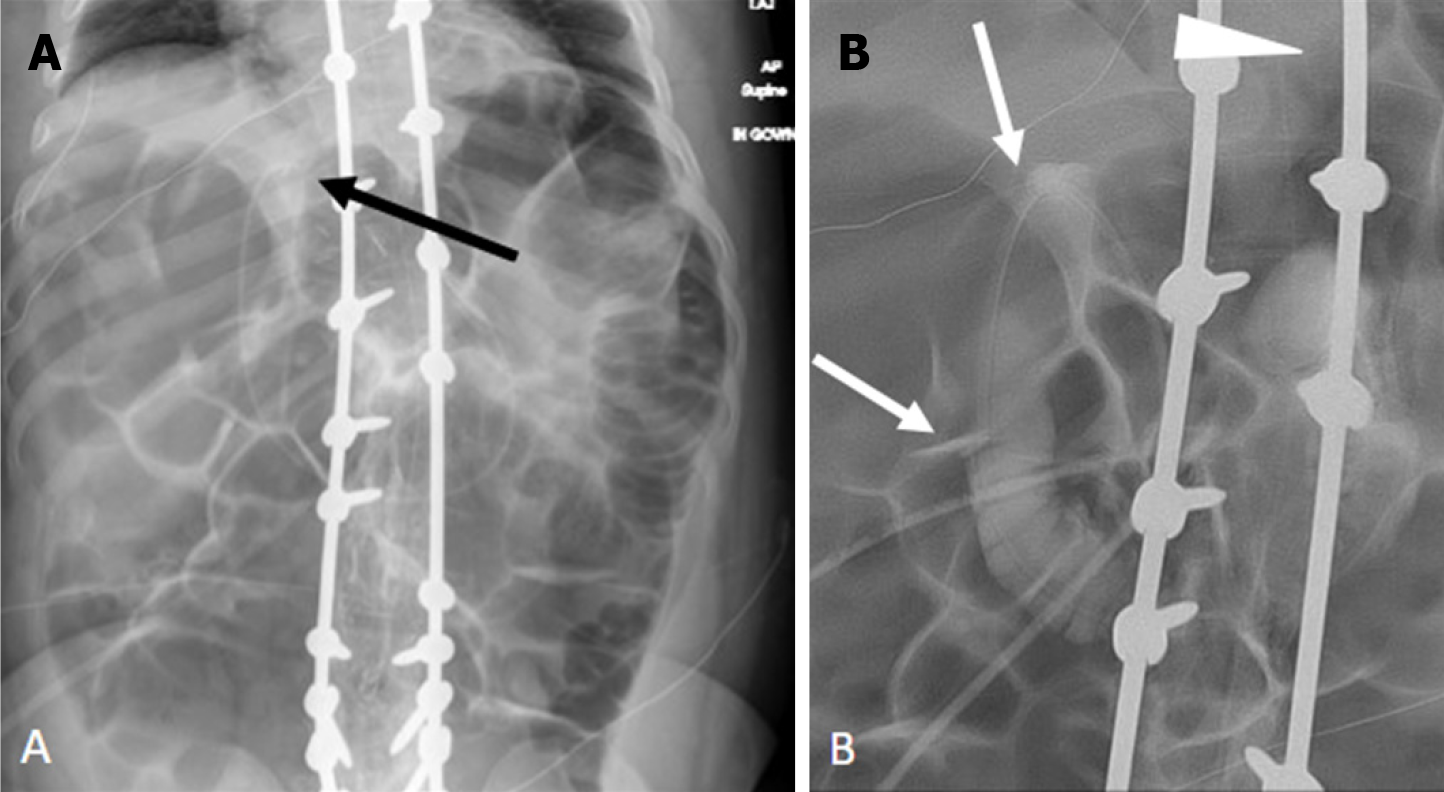
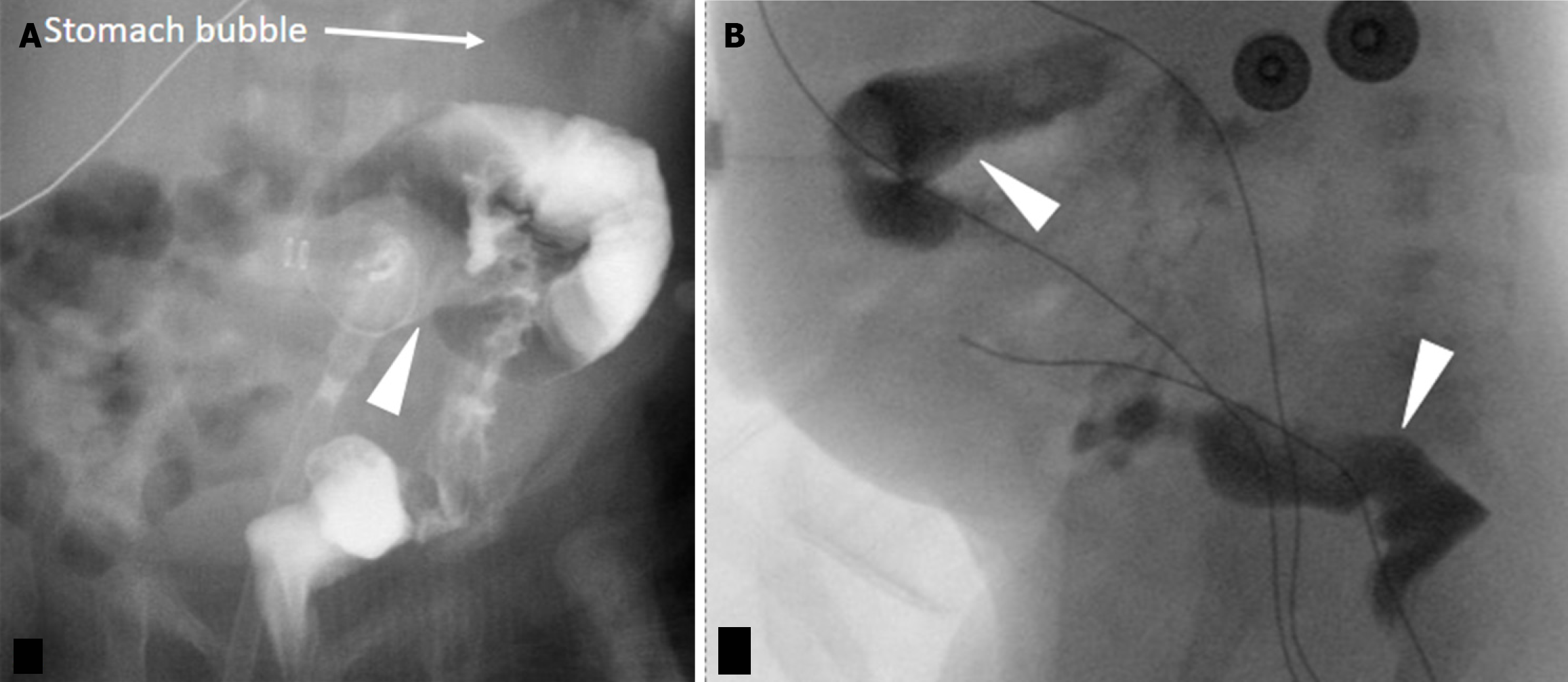
Failure to meticulously assess the G-tube course for interposed bowel loops during image interpretation. The presence of other pathology should not distract from scrutinizing G-tube positioning. Anatomically, the transverse colon's anterior location predisposes it to perforation during G-tube placement. While this can present acutely with severe pain and/or pneumoperitoneum, it may also be asymptomatic[23]. Lateral contrast radiographs may reveal the increased bolster-balloon distance, but CT provides superior clarity and should be employed when suspicion is high. Additionally, be aware of the potential for gastrocolic fistula formation, presenting with diarrhea after G-tube exchange[21]. Fluoroscopy can demonstrate the fistulous tract and/or colonic haustral markings (Figure 11). Delayed G-tube tip retraction into the colon can also manifest as diarrhea and halitosis[21] Less commonly, the G-tube may also be placed into the stomach through the liver or the small bowel (Figure 12)[24].
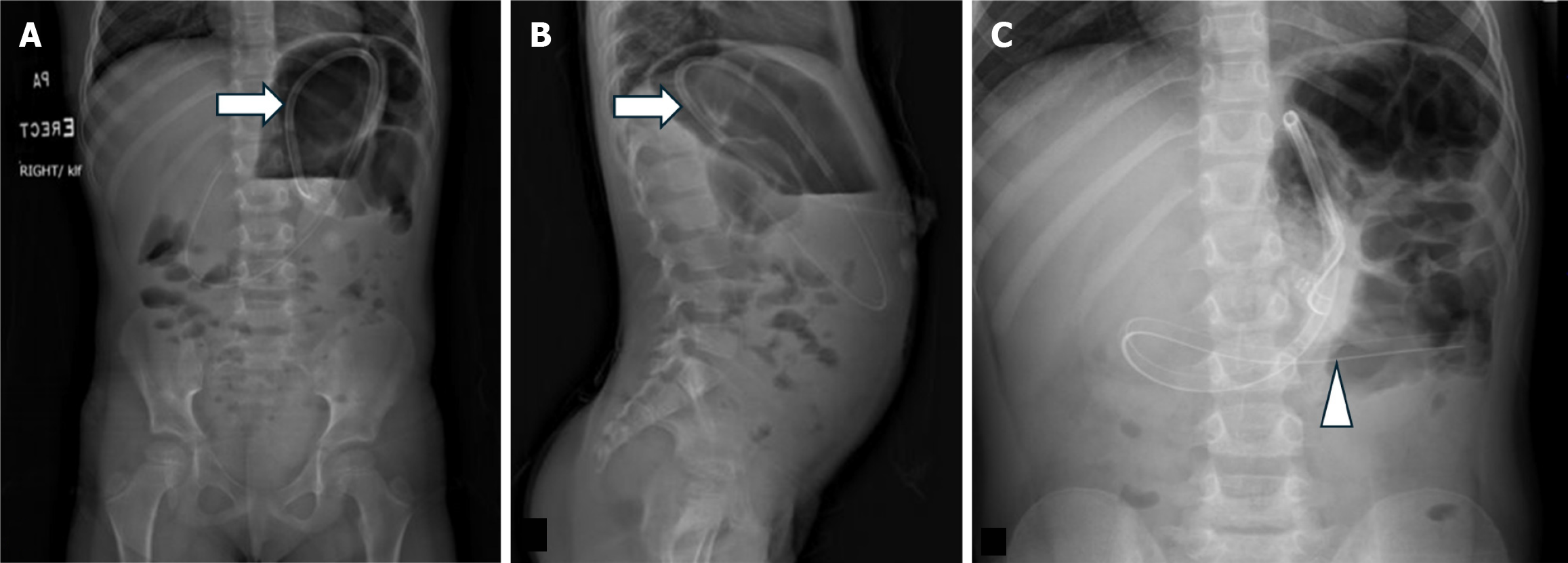
A rare complication associated with GJ tubes is esophageal migration because of longer length compared to G-tubes. Patients often present with vomiting and symptoms similar to gastroesophageal reflux. A radiograph without injected contrast into the GJ tube is typically sufficient to establish this analogous position since the tube will course cephalad into the esophagus (Figure 13).
Patients with an infection at the G-tube site typically exhibit symptoms such as pain, swelling, and redness. Ultrasound is a preferred modality whenever superficial abdominal wall infections are suspected while CT is preferred for intra-abdominal abscesses. CT may also be a better choice if the patient has a large body habitus which limits penetration of an ultrasound beam. Ultrasound imaging of cellulitis usually has hyperechoic subcutaneous fat locules separated by hypoechoic fluid. Abscesses, on the contrary, are seen as localized hypoechoic to isoechoic fluid collections with peripheral hyperemia[25]. Both abscesses and granulation tissue can appear hypoechoic on ultrasound, but clinical differentiation is possible. An abscess typically shows posterior acoustic enhancement on ultrasound, which on gentle compre
A G-tube can be prone to blockage as a result of chronic repeated use or administering dry/incompatible materials such as crushed tablets[27]. In this scenario, flushing or replacement of the tube helps in majority of the cases. If that fails, fluoroscopic evaluation should be considered to exclude the possibility of a malpositioned tube.
In conclusion, this review underscores the critical role of imaging in confirming the proper placement of pediatric G-tubes and assessing for complications. The choice of imaging modality in evaluating G-tubes depends on the clinical scenario and level of concern. The article provides a review of the available modalities and indication for each, tips for image optimization, and checklist for thorough assessment. Recognizing these complications early is essential for timely intervention and improved patient outcomes.
| 1. | Covarrubias DA, Covarrubias GM, Ho JM. Radiologic Percutaneous Gastrostomy for Enteral Access in Patients Requiring Long-Term Nutritional Support. In: Rajendram R, Preedy VR, Patel VB, editors. Diet and Nutrition in Critical Care. Berlin: Springer, 2014. [DOI] [Full Text] |
| 2. | López LG, Pedrón-giner CC, Martínez-costa C, Garrido CC. Home Enteral Nutrition. In: Rajendram R, Preedy VR, Patel VB, editor. Diet and Nutrition in Critical Care. Berlin: Springer, 2015. [DOI] [Full Text] |
| 3. | Blumenstein I, Shastri YM, Stein J. Gastroenteric tube feeding: techniques, problems and solutions. World J Gastroenterol. 2014;20:8505-8524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 325] [Cited by in RCA: 269] [Article Influence: 24.5] [Reference Citation Analysis (10)] |
| 4. | Berman L, Baird R, Sant'Anna A, Rosen R, Petrini M, Cellucci M, Fuchs L, Costa J, Lester J, Stevens J, Morrow M, Jaszczyszyn D, Amaral J, Goldin A. Gastrostomy Tube Use in Pediatrics: A Systematic Review. Pediatrics. 2022;149:e2021055213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Kumbhar SS, Plunk MR, Nikam R, Boyd KP, Thakrar PD. Complications of percutaneous gastrostomy and gastrojejunostomy tubes in children. Pediatr Radiol. 2020;50:404-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Semerci R, Pars H. Complications of pediatric enteral nutrition at home: a systematic review of quantitative research. Clin Sci Nutr. 2024;6:27-42. [DOI] [Full Text] |
| 7. | Hamilton EC, Curtin T, Slack RS, Ge C, Slade AD, Hayes-Jordan A, Lally KP, Austin MT. Surgical Feeding Tubes in Pediatric and Adolescent Cancer Patients: A Single-institution Retrospective Review. J Pediatr Hematol Oncol. 2017;39:e342-e348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Sandberg F, Viktorsdóttir MB, Salö M, Stenström P, Arnbjörnsson E. Comparison of major complications in children after laparoscopy-assisted gastrostomy and percutaneous endoscopic gastrostomy placement: a meta-analysis. Pediatr Surg Int. 2018;34:1321-1327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Fernandez-Pineda I, Sandoval JA, Jones RM, Boateng N, Wu J, Rao BN, Davidoff AM, Shochat SJ. Gastrostomy Complications in Pediatric Cancer Patients: A Retrospective Single-Institution Review. Pediatr Blood Cancer. 2016;63:1250-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Kably I, Acharya V, Richardson AJ, Bhatia S. Prostatic Artery Embolization in Refractory Hematuria of Prostatic Origin. Tech Vasc Interv Radiol. 2020;23:100694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Ahmed Z, Iqbal U, Aziz M, Arif SF, Badal J, Farooq U, Lee-Smith W, Gangwani MK, Kamal F, Kobeissy A, Mahmood A, Nawras A, Khara HS, Confer BD, Adler DG. Outcomes and Complications of Radiological Gastrostomy vs. Percutaneous Endoscopic Gastrostomy for Enteral Feeding: An Updated Systematic Review and Meta-Analysis. Gastroenterology Res. 2023;16:79-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Cortez AR, Warren PW, Goddard GR, Jenkins TM, Sauser JA, Gerrein BT, Rymeski BA. Primary Placement of a Low-Profile Gastrostomy Button Is Safe and Associated With Improved Outcomes in Children. J Surg Res. 2020;249:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Soscia J, Friedman JN. A guide to the management of common gastrostomy and gastrojejunostomy tube problems. Paediatr Child Health. 2011;16:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Rajan A, Wangrattanapranee P, Kessler J, Kidambi TD, Tabibian JH. Gastrostomy tubes: Fundamentals, periprocedural considerations, and best practices. World J Gastrointest Surg. 2022;14:286-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (3)] |
| 15. | Shah R, Shah M, Aleem A. Gastrostomy Tube Replacement. 2023 Apr 17. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 16. | Showalter CD, Kerrey B, Spellman-Kennebeck S, Timm N. Gastrostomy tube replacement in a pediatric ED: frequency of complications and impact of confirmatory imaging. Am J Emerg Med. 2012;30:1501-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Jacobson G, Brokish PA, Wrenn K. Percutaneous feeding tube replacement in the ED--are confirmatory x-rays necessary? Am J Emerg Med. 2009;27:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Tompe AP, Lee GM, Noel-Macdonnell JR, Chan SS. Retrospective cohort comparison between fluoroscopic and radiograph-only exams for evaluation of gastrostomy and gastrojejunostomy tubes. Pediatr Radiol. 2023;53:2021-2029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Alerhand S, Tay ET. Point-of-care ultrasound for confirmation of gastrostomy tube replacement in the pediatric emergency department. Intern Emerg Med. 2020;15:1075-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Reis SP, Brejt SZ, Weintraub JR, Ahmad N, Susman J, Mobley DG. Percutaneous Ultrasound Guided Gastrostomy Tube Placement: A Prospective Cohort Trial. J Intensive Care Med. 2022;37:641-646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Milanchi S, Wilson MT. Malposition of percutaneous endoscopic-guided gastrostomy: Guideline and management. J Minim Access Surg. 2008;4:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Brotherton T, Chhaparia A, Presti M, Sayuk G, Elwing J. Symptomatic Pneumoperitoneum After Gastrostomy Tube Placement Managed by Pneumocentesis. ACG Case Rep J. 2021;8:e00700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Ahmad J, Thomson S, McFall B, Scoffield J, Taylor M. Colonic injury following percutaneous endoscopic-guided gastrostomy insertion. BMJ Case Rep. 2010;2010:bcr0520102976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Chhaparia A, Hammami MB, Bassuner J, Hachem C. Trans-Hepatic Percutaneous Endoscopic Gastrostomy Tube Placement: A Case Report of A Rare Complication and Literature Review. Gastroenterology Res. 2018;11:145-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | O'Rourke K, Kibbee N, Stubbs A. Ultrasound for the Evaluation of Skin and Soft Tissue Infections. Mo Med. 2015;112:202-205. [PubMed] |
| 26. | Chau CL, Griffith JF. Musculoskeletal infections: ultrasound appearances. Clin Radiol. 2005;60:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Huang SY, Engstrom BI, Lungren MP, Kim CY. Management of dysfunctional catheters and tubes inserted by interventional radiology. Semin Intervent Radiol. 2015;32:67-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |