Published online May 26, 2025. doi: 10.4330/wjc.v17.i5.104839
Revised: April 1, 2025
Accepted: May 7, 2025
Published online: May 26, 2025
Processing time: 133 Days and 8.2 Hours
The clinical application of doxorubicin (DOX) is limited by its potential to cause cardiac cardiotoxicity.
To investigate the correlation between calumenin (CALU) and mitochondrial kinetic-related proteins in rats with DOX cardiomyopathy.
A rat model of DOX-induced cardiomyopathy was used to evaluate the effects of DOX. We observed the effect of DOX on electrical conduction in cardiomyocytes using the electromapping technique. Masson staining was performed to evaluate myocardium fibrosis. Electron microscopy was used to observe the changes in pathological ultrastructure of the myocardium. Western blotting and ELISAs were performed to detect protein levels and intracellular free Ca2+ concentration.
DOX slowed conduction and increased conduction dispersion in cardiomyocytes. The myocardial pathology in rats treated with DOX exhibited a significant deterioration, as demonstrated by an increase in mitochondrial Ca2+ concentration and a decrease in the expression of CALU, optic atrophy-1, and Bcl-2. Additionally, there was an increase in the expression of connexin 43 (Cx43) and the mitochondrial mitotic proteins dynamin-related protein 1, CHOP, Cytochrome C, and Bax in DOX rats. Decreased expression of CALU in cardiomyocytes triggered an increase in cytoplasmic free calcium concentration, which would normally be taken up by mitochondria, but de
Increased cytoplasmic free calcium ion concentration induces calcium overload in ventricular myocytes, leading to decreased Cx43 protein, slowed conduction in myocytes, and increased conduction dispersion, resulting in arrhy
Core Tip: Doxorubicin (DOX) is an antitumor drug, with the main side effect being cardiotoxicity. This study investigated the time course and mechanism of DOX-induced myocardial injury by injecting DOX into rats and conducting cardiac electrophysiological tests and ultrastructural observations of myocardium. The results showed that DOX began to damage myocardial mitochondria as early as the second week after administration. The study clearly demonstrated that DOX causes calcium overload in myocardial cells by reducing calumenin expression, which in turn leads to myocardial cell apoptosis and arrhythmia.
- Citation: Shi H, Yang SA, Bai LY, Du JJ, Wu Z, He ZH, Liu H, Cui JY, Zhao M. Mechanism of myocardial damage induced by doxorubicin via calumenin-regulated mitochondrial dynamics and the calcium–Cx43 pathway. World J Cardiol 2025; 17(5): 104839
- URL: https://www.wjgnet.com/1949-8462/full/v17/i5/104839.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i5.104839
Doxorubicin (DOX), a widely used anthracycline antitumor agent, has been a cornerstone in the treatment of various malignancies, including breast cancer, lymphoma, and sarcoma, since its introduction in the 1960s. Despite its potent anticancer efficacy, the clinical application of DOX is substantially limited by its dose-dependent cardiotoxicity, which may result in irreversible cardiomyopathy and congestive heart failure. This condition, known as DOX-induced car
Mitochondrial dynamics play an important role in the pathogenesis of various cardiomyopathies[7], and play an important role in maintaining the normal function of cardiomyocytes[8]. Due to the highly dynamic characteristics of mitochondria, they can continue the fusion–division cycle, which is known as mitochondrial dynamics. Mitochondrial fusion proteins include Mfn1 and Mfn2 and optic atrophy (OPA)-1, and mitochondrial division proteins include mito
Connexin 43 (Cx43) is one of the most important gap junction proteins in cardiomyocytes, responsible for intercellular electrical signaling and material exchange, and essential for maintaining normal electrophysiological function and structural integrity of the heart[11]. It was found that in DOX-induced cardiomyopathy, Cx43 expression was decreased and abnormally distributed. Normally, Cx43 is mainly located in the intercalated disc region of cardiomyocytes, but after DOX treatment[12], it is closely associated with the development of myocardial fibrosis, which may lead to inhomogeneous electrical conduction and further increase the risk of arrhythmias[13]. Cx43 is closely associated with the development of myocardial fibrosis, which may lead to heterogeneity of electrical conduction, further increasing the risk of arrhythmias. DOX exacerbates cardiomyocyte injury by disrupting the function of mitochondrial Cx43, leading to mitochondrial ROS overproduction and impaired energy metabolism[13].
Calumenin (CALU), a member of the CREC family, is commonly found in the endoplasmic reticulum/sarcoplasmic reticulum of mammalian cardiomyocytes[14]. Decreased expression of CALU protein causes intracellular Ca2+ overload and leads to endoplasmic reticulum stress; however, CALU can alleviate the endoplasmic reticulum stress response of cardiomyocytes to a certain extent, and reduce the apoptosis rate of cardiomyocytes due to endoplasmic reticulum stress[15]. In recent years, the role of CALU in regulating calcium homeostasis in the endoplasmic reticulum has been widely recognized in studies of DOX[16-18]. With the increasing number of studies on DOX cardiomyopathy, these and other studies have suggested that its occurrence and development mechanism are complex. However, the etiology remains to be established by in vivo and in vitro experiments to explore the role of CALU in the pathogenesis of DOX cardiomyopathy, and to provide a theoretical basis for its treatment.
In this study, we observed the effects of DOX on electrical conduction in cardiomyocytes, as well as its effects on the expression of CALU[19,20], mitochondrial calcium concentration, mitochondrial fusion proteins and split proteins in cardiomyocytes using an electric mapping technique, to clarify whether DOX-induced arrhythmias are caused by reduction of CALU expression and overload of mitochondrial calcium, and to provide a new target for the treatment of DOX-induced arrhythmias.
DOX was supplied by Solarbio Co. Ltd (Beijing, China). The antibodies of CALU, OPA-1, DRP-1, Bax, Bcl-2, CHOP, Cytochrome C, CX43, and GAPDH were purchased from Abcam Co. Ltd. An intracellular free Ca2+ concentration kit was obtained from Shanghai Haohai Biological Technology Co. Ltd. (Shanghai, China).
All rats were fed with a standard diet. The treatment of rats was in accordance with the NIH Guide for the Care and Use of Laboratory Animals and all protocols were approved by the Institutional Animal Care and Use Committee of Inner Mongolia University for Nationalities. Thirty-six clean grade male Sprague–Dawley rats aged 2–3 months and weighing 180–220 g (purchased from Changchun Yisi Experimental Animal Technology Co., China Ltd.) were randomly divided into the control (CON) group and DOX group at different time points (2, 4 and 8 weeks). The animals were fed at 20°C–25°C and 50%–65% relative humidity. The light and dark cycle was 12 hours, and there were no restrictions on diet. Before the experiment, the rats underwent 1 week of preconditioning. After 1 week of feeding, rats in the DOX groups were injected intraperitoneally with DOX (2 mg/kg) weekly. Rats in the CON group were gavaged with 0.9% NaCl weekly. This was done for 2, 4 and 8 consecutive weeks. The general state of the rats in the model group was observed, mainly the reaction of rats, diet, hair color, activity, excretion and body weight were recorded.
Rats were weighed, injected intraperitoneally with sodium heparin (3125 U/kg), anesthetized with isoflurane for 15 min, and then killed. The rats were placed on the experimental table and the chest was opened in an inverted "T" shape to expose the heart. The lungs were lifted up with forceps and the heart was quickly cut off along the back of the lungs and placed in a glass Petri dish with precooled benchtop solution[21]. The aorta was quickly located, excess tissue was cut away, the aorta was carefully placed at the bottom of the cannula, tied tightly with surgical sutures, and the pre-prepared KH fluid in the syringe was gently pushed into the heart to pump out the cardiac remnants for Langendorff perfusion. The perfusion rate was 10 mL/min and the perfusion temperature was 37°C ± 0.5°C. The residual blood in the heart was drained to restore the heart to normal rhythm with a heart rate > 250 bpm and stabilized for 15 minutes before the experiments[22].
The electrocardiogram (ECG) electrodes were placed on both sides of the heart and the ECG was recorded continuously; the pen test electrode was placed in the left ventricle and electrical signal conduction in the ventricular myocytes of rats in the model group was detected under sinusoidal conditions and compared with that of the CON group.
Rat left ventricular tissue was resected, fixed with paraformaldehyde solution, and Masson staining was performed. Changes in the degree of myocardial fibrosis of rats in each group were observed by microscopy (Nikon Eclipse CI).
Rat left ventricular tissues were excised and fixed in 4% glutaraldehyde solution in 2%–4.1% osmic acid and 0.1M PBS (pH 7.4) and fixed at 20°C for 2 hours. The tissue samples underwent dehydration, penetration, embedding, slicing and uranium lead double staining. The results were observed by electron microscopy (Tecnai G2 20 Twin).
Proteins were extracted from rat cardiomyocytes and newborn rat cardiomyocytes. After SDS-PAGE separation, the proteins were sealed with 5% BSA, and the primary anti-CALU (1:1000), anti-CHOP (1:1000), anti-DRP-1 (1:1000), anti-OPA-1 (1:1000), anti-CytC (1:1000), anti-Bax (1:1000), anti-Cx43 (1:1000) and anti-Bcl-2 (1:1000) antibodies were added and incubated at 4°C overnight. The secondary antibody was added and incubated for 1 h. Bound antibody was observed using enhanced chemiluminescence.
The intracellular free Ca2+ in rat cardiac tissues was detected with the intracellular free Ca2+ detection kit and fluorescence labeling instrument, which were obtained from Shanghai Haohai Biological Technology Co. Ltd. Cardiac tissues were digested to a single-cell suspension, which was treated with reaction reagent and a dye solution. The results were detected with a fluorescence microplate instrument (excitation = 550 nm, emission = 590 nm).
The experimental data were expressed as mean ± SD. Statistical analysis was conducted using SSPS version 11.5 software. The t test was used to detect significant differences between the two groups. Student's unpaired or paired t test was performed for comparisons between two groups and multiple group comparisons were carried out using one-way ANOVA with Bonferroni post hoc test.
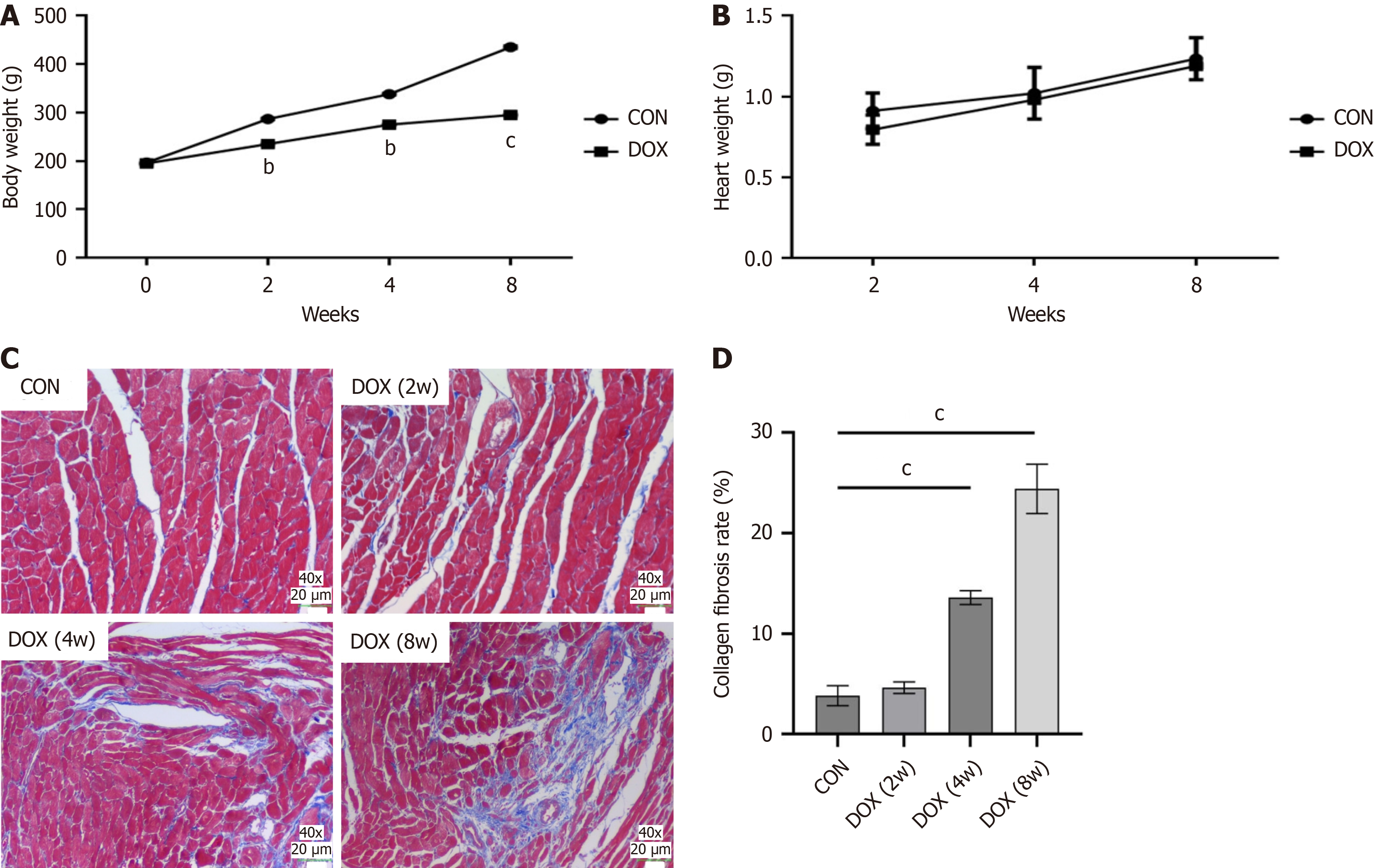
In the CON group, rats showed an increase in body weight over time, smooth and shiny fur and no mortality. However, the body weight of rats in the DOX-treated groups (2-week, 4-week, and 8-week groups) decreased significantly over time compared with the CON group (Figure 1A). The heart–body weight ratio was also significantly increased in the DOX-treated group, and this further increased with the duration of treatment (Figure 1B). These results suggest that DOX not only causes weight loss, but may also cause cardiac hypertrophy or edema.
Pathological analysis of myocardial tissue showed that cardiomyocytes in control rats were tightly arranged and orderly, with fewer intercellular collagen fibers, and no significant fibrosis was observed. However, in the DOX -treated group, cardiomyocytes were disorganized and significantly deformed, with increased cell spacing, a significant increase in intercellular blue collagen fibers, and worsening fibrosis. In particular, myocardial fibrosis was most severe in the 8-week DOX-treated group (Figure 1C). These results confirmed the direct damaging effect of DOX on myocardial tissue.
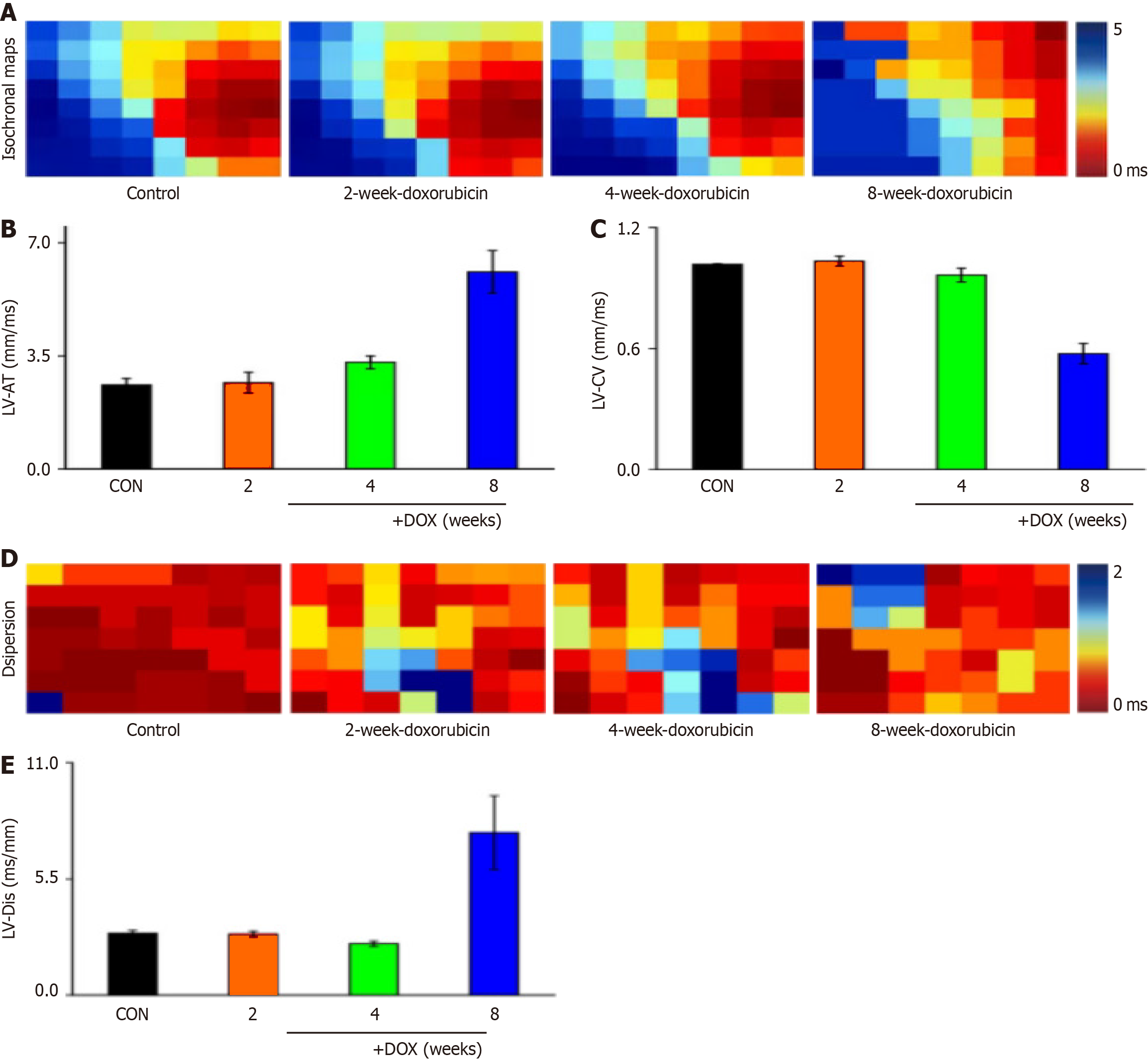
The left ventricular signals of rat hearts were recorded with 64-channel pen electrodes. Under sinus rhythm, the effect of ventricular conduction time was obvious in the model group (Figure 2A), and the conduction changes were significantly different. Analysis of the left ventricular conduction time revealed that there was a tendency to prolong the conduction time after drug administration (Figure 2B), and there was no significant change in conduction velocity (Figure 2C).
Conduction dispersion was a concern in the study of arrhythmic disease mechanisms, and examination of cardiac conduction dispersion in DOX model rats in sinus rhythm revealed a significant effect on cardiac conduction dispersion in the model group (Figure 2D), with a tendency for prolongation of ventricular conduction dispersion in sinus rhythm (Figure 2E).
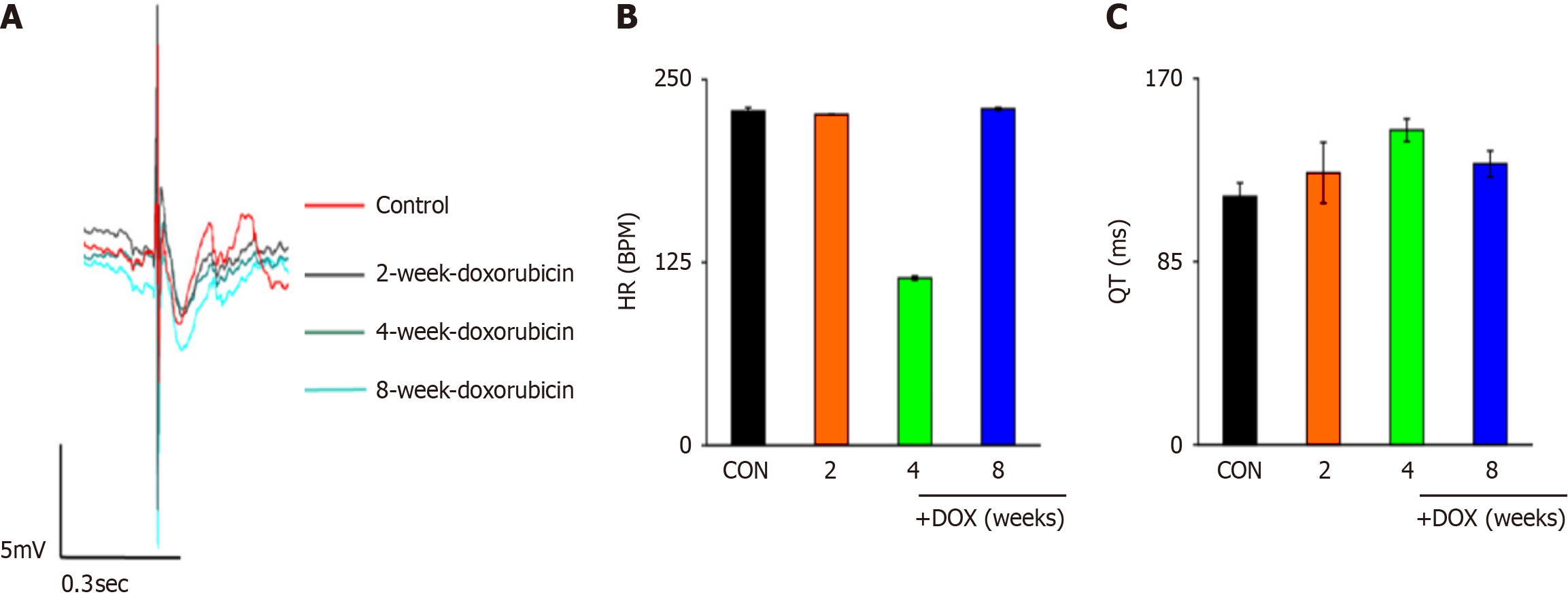
ECG is an important component of cardiac monitoring, and cardiac ECG changes were determined using multichannel electrophysiological labeling assays (Figure 3A). Heart rate slowed down after drug administration and all changes were significantly different (Figure 3B). Statistical analysis revealed prolongation of the QT interval (Figure 3C). When the QT interval was prolonged, the action potential timescale was abnormally prolonged, which may have led to increased Ca2+ inflow via L-type Ca2+ channels. Dysfunction of IK1 channels may have affected the function of other ion channels (e.g., IKr), which indirectly led to prolongation of the QT interval.
Mitochondrial Ca2+ levels rose significantly in the DOX group compared to the CON group, both in vivo and in vitro. Levels were also higher in the 8-week DOX group than in the 2-week and 4-week DOX groups (Tables 1 and 2).
| Group | The concentration of intracellular free Ca2+ (nmol/L) |
| CON | 20.14 ± 1.31 (nmol/L) |
| DOX | 90.89 ± 23.15 (nmol/L)c |
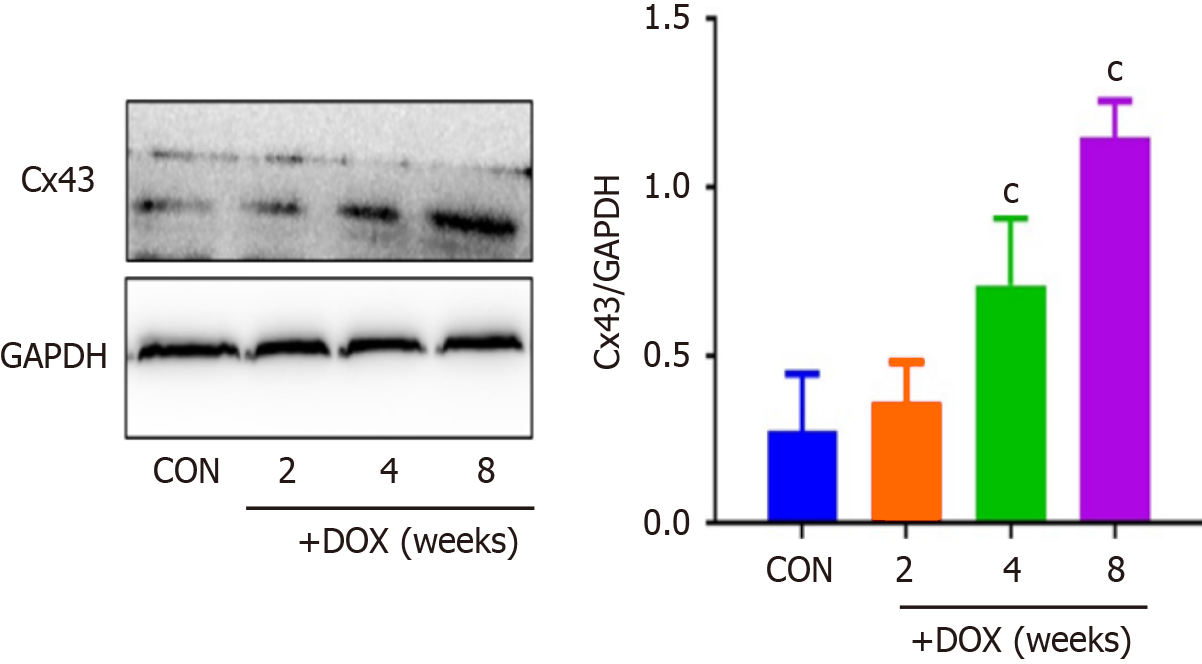
Western blotting revealed that the levels of Cx43 protein were significantly elevated in the DOX group compared with the CON group (Figure 4).
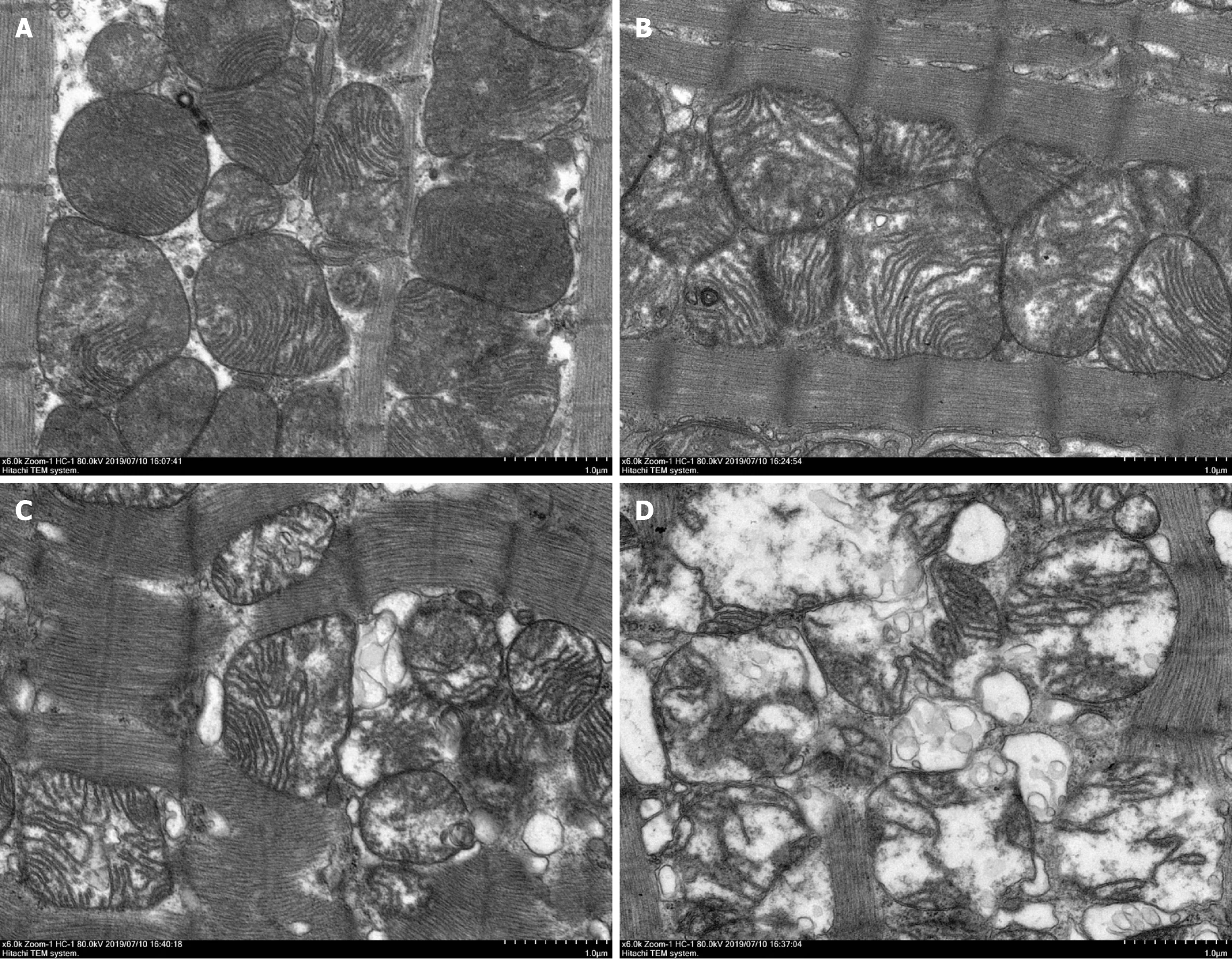
In the intact sarcomere, it was observed through the use of electron microscopy that the heart tissue of the CON rat exhibited a well-preserved structure, featuring clearly visible mitochondria and myocardial fibers that were neatly arranged in an orderly fashion. Ultrastructural changes in cardiomyocytes were observed in DOX-treated rats. Compared with the CON group, disordered myocardial fibers and vacuolation of mitochondria were found in the DOX groups. Vacuoles in mitochondria disappeared and myocardial fibers were fractured, few and even absent in the 8-week DOX group, compared with the 2-week and 4-week DOX groups (Figure 5).
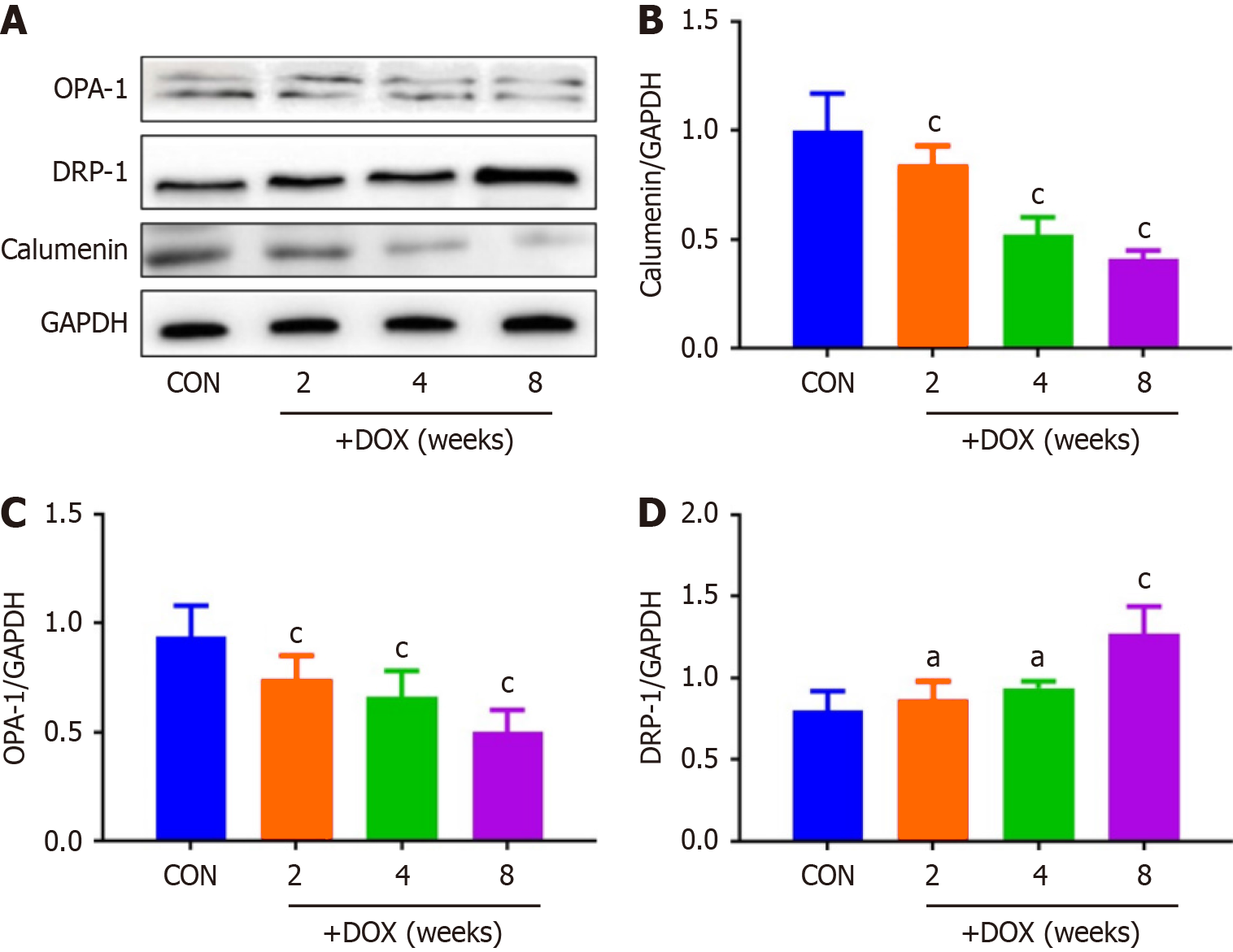
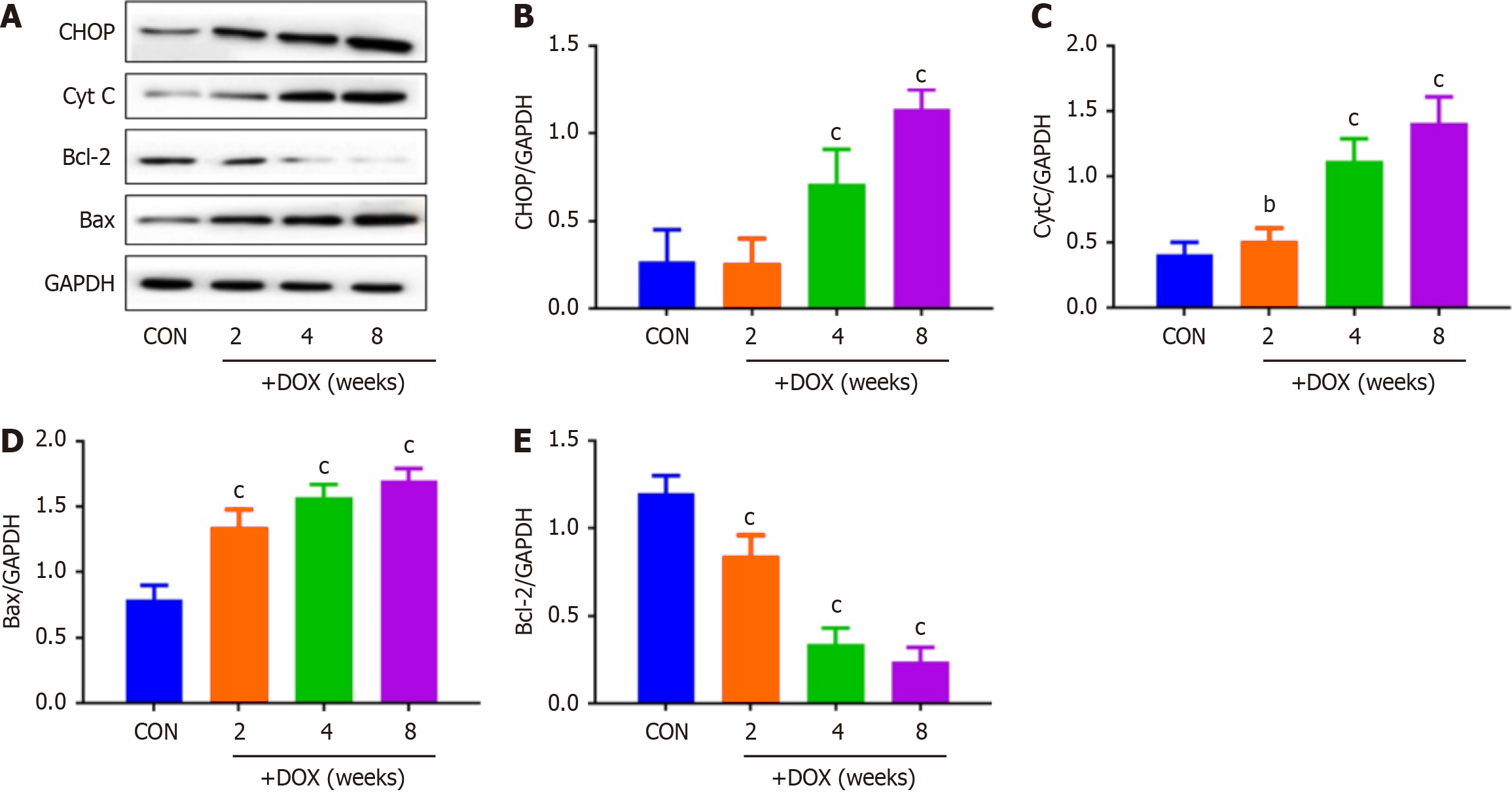
DOX reduced the expression of CALU in vivo in the DOX group, compared with the CON group (Figure 5). OPA-1 was decreased and DRP-1 was increased in the DOX group, compared with the CON group (Figure 6). To confirm the role of mitochondrial-induced apoptosis, we analyzed the expression of CytC, CHOP, Bax and Bcl-2. DOX increased expression of CytC, CHOP and Bax and reduced expression of Bcl-2 in the DOX group (Figure 7).
The current study demonstrates that in rat hearts, the effect of DOX in vivo may be associated with CALU-regulated mitochondrial dynamics and the calcium-Cx43 pathway. Related studies[20,23,24] have shown that DOX can cause pathological damage in the myocardium. In our study, we found that cardiomyocyte fibrosis was more severe in the DOX group than in the CON group, and mitochondrial vacuoles disappeared and myocardial fibers were fractured and fewer in rat hearts treated with DOX, compared with control rat hearts. These findings suggested that the effect of DOX on the heart was related to mitochondria. Mitochondrial dynamics play a crucial role in physiological functions, including the maintenance of mitochondrial DNA stability and critical life processes like energy synthesis and cell aging[19]. Mito
Calcium plays a necessary role in nerve impulse transmission, cell signaling, and maintaining calcium homeostasis in mammals. In our research, we discovered that DOX prolonged ventricular conduction time and conduction dispersion, and slowed heart rate. In addition, statistical analyses revealed that DOX prolonged the QT interval. The key structure for rapid electrical conduction between cardiomyocytes is the gap junction, a transmembrane channel for electrical and chemical coupling between cardiomyocytes, which is essential for maintaining cardiac rhythm, of which Cx-forming hemichannels are a major component. Cardiomyocytes express Cx40, Cx43 and Cx45, with Cx43 being the most widely distributed in the mammalian cardiovascular and nervous systems, and the main Cx expressed by ventricular myocytes. The mechanism by which DOX leads to the reduction of Cx43 protein involves several aspects, including oxidative stress, abnormal phosphorylation regulation, and activation of protein degradation pathways. DOX undergoes redox cycling in cells through its quinone structure, generating large amounts of ROS[29]. Excess ROS directly damage cardiomyocytes, and may also lead to structural and functional impairment of Cx43 by oxidizing key amino acid residues (e.g., cysteine and methionine) of the Cx43 protein[29]. DOX inhibits the activity of protein kinase (PK) A and PKC, which normally stabilize Cx43 by phosphorylating specific serine residues (e.g., Ser368 and Ser365) to stabilize Cx43 and promote its localization to the cell membrane[30]. In contrast, DOX may activate protein phosphatases (e.g., PP1 and PP2A), leading to dephosphorylation of Cx43, which in turn promotes its degradation via the ubiquitin–proteasome pathway[31]. It has been shown that DOX can upregulate the expression of E3 ubiquitin ligases (e.g. Nedd4 and ITCH) that recognize and ubiquitinate Cx43, thereby promoting its degradation by the proteasome[32]. DOX may also induce autophagic degra
It has been reported[22] that CALU plays an important role in the occurrence and development of acute myocardial ischemia, arrhythmia and other diseases. Our results also showed that expression of CALU was significantly decreased in the DOX group compared with the CON group. This indicated that expression of CALU was decreased by DOX. We found that DOX decreased rat body weight, and increased the heart weight–body weight ratio. DOX reduced expression of CALU due to Ca2+ overload, and increased apoptosis of myocardial cells. In vivo experiments showed that DOX reduced expression of CALU, OPA-1 and Bcl-2, and increased expression of DRP-1, CytC, CHOP and Bax in rats.
We found that DOX decreased expression of CALU and mitochondrial fusion proteins, increased expression of mitochondrial split proteins, and increased the cytoplasmic calcium concentration of cardiomyocytes. CALU is a calcium-binding protein found in the lumen of the endoplasmic reticulum. If the expression of CALU is reduced in cardiomyo
Future studies will include female rats and systematically compare the differences in DOX cardiotoxicity in male and female rats. This may involve the role of sex hormones (e.g., estrogen and androgen) in the regulation of CALU and Cx43 expression, as well as the effect of sex on mitochondrial dynamics and calcium homeostasis. We plan to design experiments with multiple dose groups (e.g., low, medium and high doses) and multiple time points (e.g., short-term and long-term) in future studies to assess the dose–response relationship and cumulative effects of DOX. This will contribute to a more comprehensive understanding of the dose-dependent and time-dependent characteristics of DOX cardioto
| 1. | Tadokoro T, Ikeda M, Ide T, Deguchi H, Ikeda S, Okabe K, Ishikita A, Matsushima S, Koumura T, Yamada KI, Imai H, Tsutsui H. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. 2020;5:e132747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 432] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 2. | Borchmann P, Goergen H, Kobe C, Lohri A, Greil R, Eichenauer DA, Zijlstra JM, Markova J, Meissner J, Feuring-Buske M, Hüttmann A, Dierlamm J, Soekler M, Beck HJ, Willenbacher W, Ludwig WD, Pabst T, Topp MS, Hitz F, Bentz M, Keller UB, Kühnhardt D, Ostermann H, Schmitz N, Hertenstein B, Aulitzky W, Maschmeyer G, Vieler T, Eich H, Baues C, Stein H, Fuchs M, Kuhnert G, Diehl V, Dietlein M, Engert A. PET-guided treatment in patients with advanced-stage Hodgkin's lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet. 2017;390:2790-2802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 249] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 3. | Verheijen M, Schrooders Y, Gmuender H, Nudischer R, Clayton O, Hynes J, Niederer S, Cordes H, Kuepfer L, Kleinjans J, Caiment F. Bringing in vitro analysis closer to in vivo: Studying doxorubicin toxicity and associated mechanisms in 3D human microtissues with PBPK-based dose modelling. Toxicol Lett. 2018;294:184-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Xie L, Xue F, Cheng C, Sui W, Zhang J, Meng L, Lu Y, Xiong W, Bu P, Xu F, Yu X, Xi B, Zhong L, Yang J, Zhang C, Zhang Y. Cardiomyocyte-specific knockout of ADAM17 alleviates doxorubicin-induced cardiomyopathy via inhibiting TNFα-TRAF3-TAK1-MAPK axis. Signal Transduct Target Ther. 2024;9:273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Hangas A, Aasumets K, Kekäläinen NJ, Paloheinä M, Pohjoismäki JL, Gerhold JM, Goffart S. Ciprofloxacin impairs mitochondrial DNA replication initiation through inhibition of Topoisomerase 2. Nucleic Acids Res. 2018;46:9625-9636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Wallace KB, Sardão VA, Oliveira PJ. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ Res. 2020;126:926-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 359] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 7. | Sekiguchi A, Motegi SI, Uchiyama A, Uehara A, Fujiwara C, Yamazaki S, Perera B, Nakamura H, Ogino S, Yokoyama Y, Akai R, Iwawaki T, Ishikawa O. Botulinum toxin B suppresses the pressure ulcer formation in cutaneous ischemia-reperfusion injury mouse model: Possible regulation of oxidative and endoplasmic reticulum stress. J Dermatol Sci. 2018;90:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Yu Y, Xing N, Xu X, Zhu Y, Wang S, Sun G, Sun X. Tournefolic acid B, derived from Clinopodium chinense (Benth.) Kuntze, protects against myocardial ischemia/reperfusion injury by inhibiting endoplasmic reticulum stress-regulated apoptosis via PI3K/AKT pathways. Phytomedicine. 2019;52:178-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Lee JH, Kwon EJ, Kim DH. Calumenin has a role in the alleviation of ER stress in neonatal rat cardiomyocytes. Biochem Biophys Res Commun. 2013;439:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Vasiljevic M, Heisler FF, Hausrat TJ, Fehr S, Milenkovic I, Kneussel M, Sieghart W. Spatio-temporal expression analysis of the calcium-binding protein calumenin in the rodent brain. Neuroscience. 2012;202:29-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Rusiecka OM, Montgomery J, Morel S, Batista-Almeida D, Van Campenhout R, Vinken M, Girao H, Kwak BR. Canonical and Non-Canonical Roles of Connexin43 in Cardioprotection. Biomolecules. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Pecoraro M, Rodríguez-Sinovas A, Marzocco S, Ciccarelli M, Iaccarino G, Pinto A, Popolo A. Cardiotoxic Effects of Short-Term Doxorubicin Administration: Involvement of Connexin 43 in Calcium Impairment. Int J Mol Sci. 2017;18:2121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Martins-Marques T, Ribeiro-Rodrigues T, de Jager SC, Zuzarte M, Ferreira C, Cruz P, Reis L, Baptista R, Gonçalves L, Sluijter JP, Girao H. Myocardial infarction affects Cx43 content of extracellular vesicles secreted by cardiomyocytes. Life Sci Alliance. 2020;3:e202000821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Mazzorana M, Hussain R, Sorensen T. Ca-Dependent Folding of Human Calumenin. PLoS One. 2016;11:e0151547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Guo JJ, Xu FQ, Li YH, Li J, Liu X, Wang XF, Hu LG, An Y. Alginate oligosaccharide alleviates myocardial reperfusion injury by inhibiting nitrative and oxidative stress and endoplasmic reticulum stress-mediated apoptosis. Drug Des Devel Ther. 2017;11:2387-2397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Sahoo SK, Kim T, Kang GB, Lee JG, Eom SH, Kim DH. Characterization of calumenin-SERCA2 interaction in mouse cardiac sarcoplasmic reticulum. J Biol Chem. 2009;284:31109-31121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Sahoo SK, Kim DH. Characterization of calumenin in mouse heart. BMB Rep. 2010;43:158-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Wang Y, Cui X, Wang Y, Fu Y, Guo X, Long J, Wei C, Zhao M. Protective effect of miR378* on doxorubicin-induced cardiomyocyte injury via calumenin. J Cell Physiol. 2018;233:6344-6351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Vásquez-Trincado C, García-Carvajal I, Pennanen C, Parra V, Hill JA, Rothermel BA, Lavandero S. Mitochondrial dynamics, mitophagy and cardiovascular disease. J Physiol. 2016;594:509-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 460] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 20. | Rovira-Llopis S, Bañuls C, Diaz-Morales N, Hernandez-Mijares A, Rocha M, Victor VM. Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox Biol. 2017;11:637-645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 478] [Cited by in RCA: 445] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 21. | Ding M, Dong Q, Liu Z, Liu Z, Qu Y, Li X, Huo C, Jia X, Fu F, Wang X. Inhibition of dynamin-related protein 1 protects against myocardial ischemia-reperfusion injury in diabetic mice. Cardiovasc Diabetol. 2017;16:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 22. | Wang Y, Sun Y, Fu Y, Guo X, Long J, Xuan LY, Wei CX, Zhao M. Calumenin relieves cardiac injury by inhibiting ERS-initiated apoptosis during viral myocarditis. Int J Clin Exp Pathol. 2017;10:7277-7284. [PubMed] |
| 23. | Wei S, Ma W, Li X, Jiang C, Sun T, Li Y, Zhang B, Li W. Involvement of ROS/NLRP3 Inflammasome Signaling Pathway in Doxorubicin-Induced Cardiotoxicity. Cardiovasc Toxicol. 2020;20:507-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 24. | Hao E, Mukhopadhyay P, Cao Z, Erdélyi K, Holovac E, Liaudet L, Lee WS, Haskó G, Mechoulam R, Pacher P. Cannabidiol Protects against Doxorubicin-Induced Cardiomyopathy by Modulating Mitochondrial Function and Biogenesis. Mol Med. 2015;21:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 25. | Brown DA, Perry JB, Allen ME, Sabbah HN, Stauffer BL, Shaikh SR, Cleland JG, Colucci WS, Butler J, Voors AA, Anker SD, Pitt B, Pieske B, Filippatos G, Greene SJ, Gheorghiade M. Expert consensus document: Mitochondrial function as a therapeutic target in heart failure. Nat Rev Cardiol. 2017;14:238-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 555] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 26. | Chang JY, Yu F, Shi L, Ko ML, Ko GY. Melatonin Affects Mitochondrial Fission/Fusion Dynamics in the Diabetic Retina. J Diabetes Res. 2019;2019:8463125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Zhao L, Zhuang J, Wang Y, Zhou D, Zhao D, Zhu S, Pu J, Zhang H, Yin M, Zhao W, Wang Z, Hong J. Propofol Ameliorates H9c2 Cells Apoptosis Induced by Oxygen Glucose Deprivation and Reperfusion Injury via Inhibiting High Levels of Mitochondrial Fusion and Fission. Front Pharmacol. 2019;10:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Yu S, Zhang L, Liu C, Yang J, Zhang J, Huang L. PACS2 is required for ox-LDL-induced endothelial cell apoptosis by regulating mitochondria-associated ER membrane formation and mitochondrial Ca(2+) elevation. Exp Cell Res. 2019;379:191-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 29. | Mongirdienė A, Skrodenis L, Varoneckaitė L, Mierkytė G, Gerulis J. Reactive Oxygen Species Induced Pathways in Heart Failure Pathogenesis and Potential Therapeutic Strategies. Biomedicines. 2022;10:602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 30. | Zou H, Zhang M, Yang X, Shou H, Chen Z, Zhu Q, Luo T, Mou X, Chen X. Cynaroside regulates the AMPK/SIRT3/Nrf2 pathway to inhibit doxorubicin-induced cardiomyocyte pyroptosis. J Zhejiang Univ Sci B. 2024;25:756-772. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Wang T, Wang W, Wang Q, Xie R, Landay A, Chen D. The E3 ubiquitin ligase CHIP in normal cell function and in disease conditions. Ann N Y Acad Sci. 2020;1460:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Huang Y, Wei C, Li P, Shao Y, Wang M, Wang F, Niu G, Sun K, Zhang Q, Gou Z, Yan X. FGF21 protects against doxorubicin-induced cardiotoxicity by inhibiting connexin 43 ubiquitination. Free Radic Biol Med. 2023;208:748-758. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Zhu Y, Dai J, Song B, Zhang Y, Yang T, Xu H, Xu X, Gao Y, Yan T, Shen W, Zhang W, Zhang S, Liu P. Connexin 43 Prevents Radiation-Induced Intestinal Damage via the Ca2+-Dependent PI3K/Akt Signaling Pathway. Radiat Res. 2024;201:294-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 34. | Xiong W, Li B, Pan J, Li D, Yuan H, Wan X, Zhang Y, Fu L, Zhang J, Lei M, Chang ACY. Mitochondrial Amount Determines Doxorubicin-Induced Cardiotoxicity in Cardiomyocytes. Adv Sci (Weinh). 2025;12:e2412017. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |