Published online Jun 27, 2025. doi: 10.4240/wjgs.v17.i6.107086
Revised: April 4, 2025
Accepted: May 14, 2025
Published online: June 27, 2025
Processing time: 73 Days and 11 Hours
Esophageal neuroendocrine carcinoma (NEC), a rare and aggressive malignancy with a poor prognosis, is often diagnosed at an advanced stage. The optimal treatment strategy for locally advanced and recurrent esophageal NEC remains unclear, and conversion surgery has only been reported for a few cases. Herein, we present the case of a 66-year-old male with locally advanced esophageal NEC initially diagnosed as squamous cell carcinoma.
The patient underwent induction chemotherapy with docetaxel, cisplatin, and 5-fluorouracil, followed by conversion surgery, including subtotal esophagectomy, three-field lymph node dissection, and distal pancreatectomy with splenectomy, due to infiltration of the pancreas by the No. 11p lymph node. Postoperative pathological findings revealed a large cell-type NEC without a squamous cell carcinoma component, suspected to be a mixed neuroendocrine/non-neuroendocrine neoplasm. Hepatic metastasis was diagnosed within one month of surgery. Despite the administration of four courses of irinotecan + cisplatin chemotherapy, the treatment effect was considered a ‘progressive disease’. After a multidisciplinary discussion, the patient underwent partial liver resection, followed by second-line chemotherapy with amrubicin. The patient achieved three-year survival with no new recurrence.
This case highlights the potential of multimodal treatment for long-term survival in advanced esophageal NEC.
Core Tip: We used a multimodal treatment approach for locally advanced unresectable esophageal neuroendocrine carcinoma (NEC). Two aspects of this case are noteworthy. First, because NEC was finally diagnosed through postoperative patho
- Citation: Okamoto K, Fujisawa K, Kono K, Ogawa Y, Shimoyama H, Haruta S, Takazawa Y, Ueno M, Udagawa H. Long-term survival with multimodal treatment including conversion surgery for locally advanced esophageal neuroendocrine carcinoma: A case report. World J Gastrointest Surg 2025; 17(6): 107086
- URL: https://www.wjgnet.com/1948-9366/full/v17/i6/107086.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i6.107086
Esophageal neuroendocrine carcinoma (NEC) is a rare esophageal malignancy characterized by a high histological grade and poor prognosis[1]. The incidence of NEC among esophageal malignancies ranges from 0.4% to 2.0%[2-4]. It is distinguished by its aggressive nature, rapid progression, and unfavorable prognosis[3]. Consequently, many cases of NEC are already unresectable during the first diagnosis, with distant lymph nodes and liver metastases being frequent[5-7]. The current guidelines recommend a multidisciplinary strategy including surgery, chemotherapy, and radiation therapy[8]. Although a surgical approach is generally considered effective for limited-stage NEC without lymph node metastasis, it reportedly results in an unfavorable prognosis in cases of advanced NEC because of its aggressive nature[7]. Particularly, because of its rarity and high malignancy rate, evidence to establish standard treatment protocols for unresectable and recurrent cases is insufficient, and reports on conversion surgery for esophageal NEC are limited[9]. Herein, we report a successful case of achieving long-term survival through multimodal treatment for locally advanced esophageal NEC, including conversion surgery and resection of liver metastasis.
A 66-year-old man with a chief complaint of dysphagia and abdominal discomfort underwent esophagogastroduodenoscopy (EGD).
Symptoms developed 6 months before presentation, marked by ongoing difficulty in swallowing and abdominal discomfort.
The patient was diagnosed with hypertension, and his blood pressure was satisfactorily controlled with an oral anti-hypertensive drug.
The patient smoked 20 cigarettes a day for 25 years and consumed excessive alcohol daily. There was no family history of malignant tumors.
The patient’s height and weight were 1.67 m and 63.1 kg, respectively, with a body mass index of 22.6 kg/m². No distinct physical signs were observed.
Serum tumor marker levels were normal [squamous cell carcinoma (SCC) antigen, 0.8 ng/mL; cytokeratin fragment, 1.2 ng/mL; carcinoembryonic antigen, 1.1 ng/mL], with the exception of the anti-p53 antibody level, which was increased to 100.8 U/mL. No abnormalities were found in routine blood analysis.
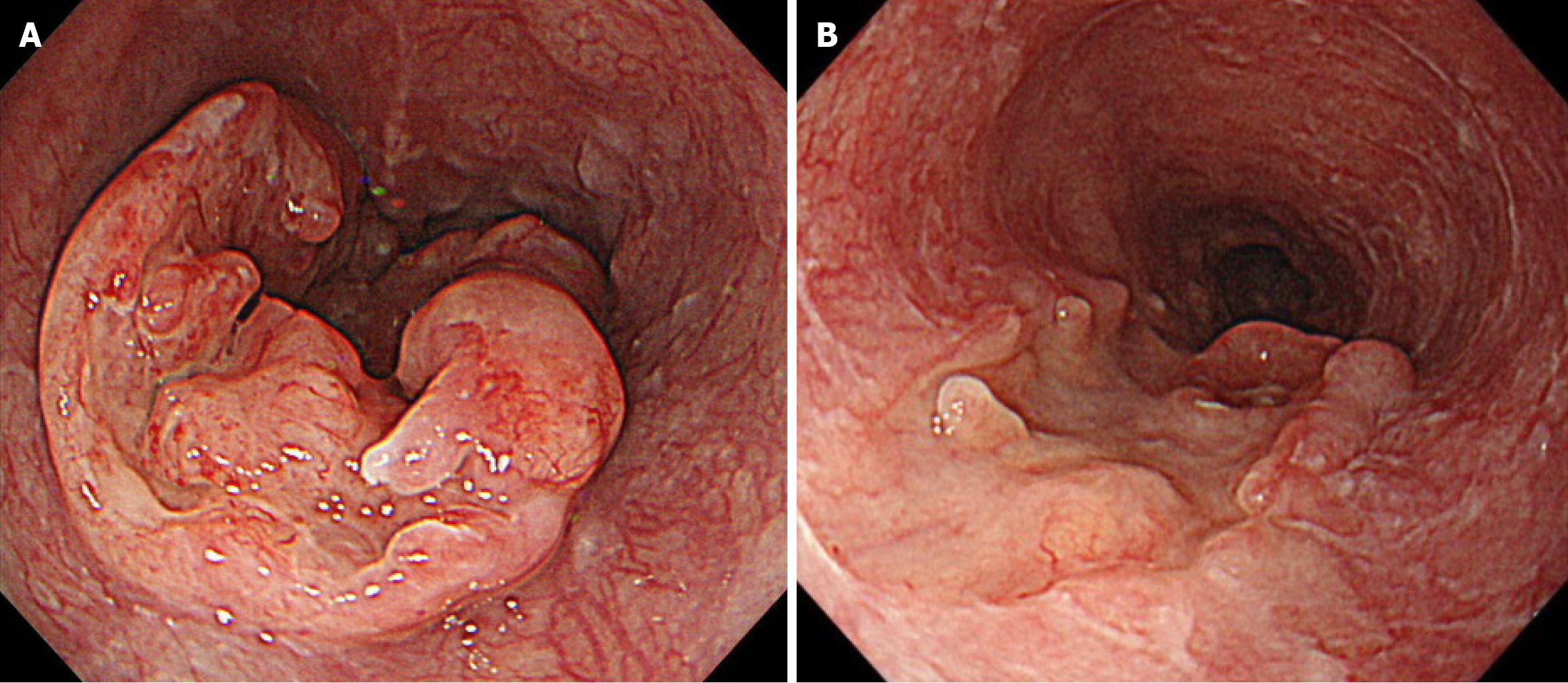
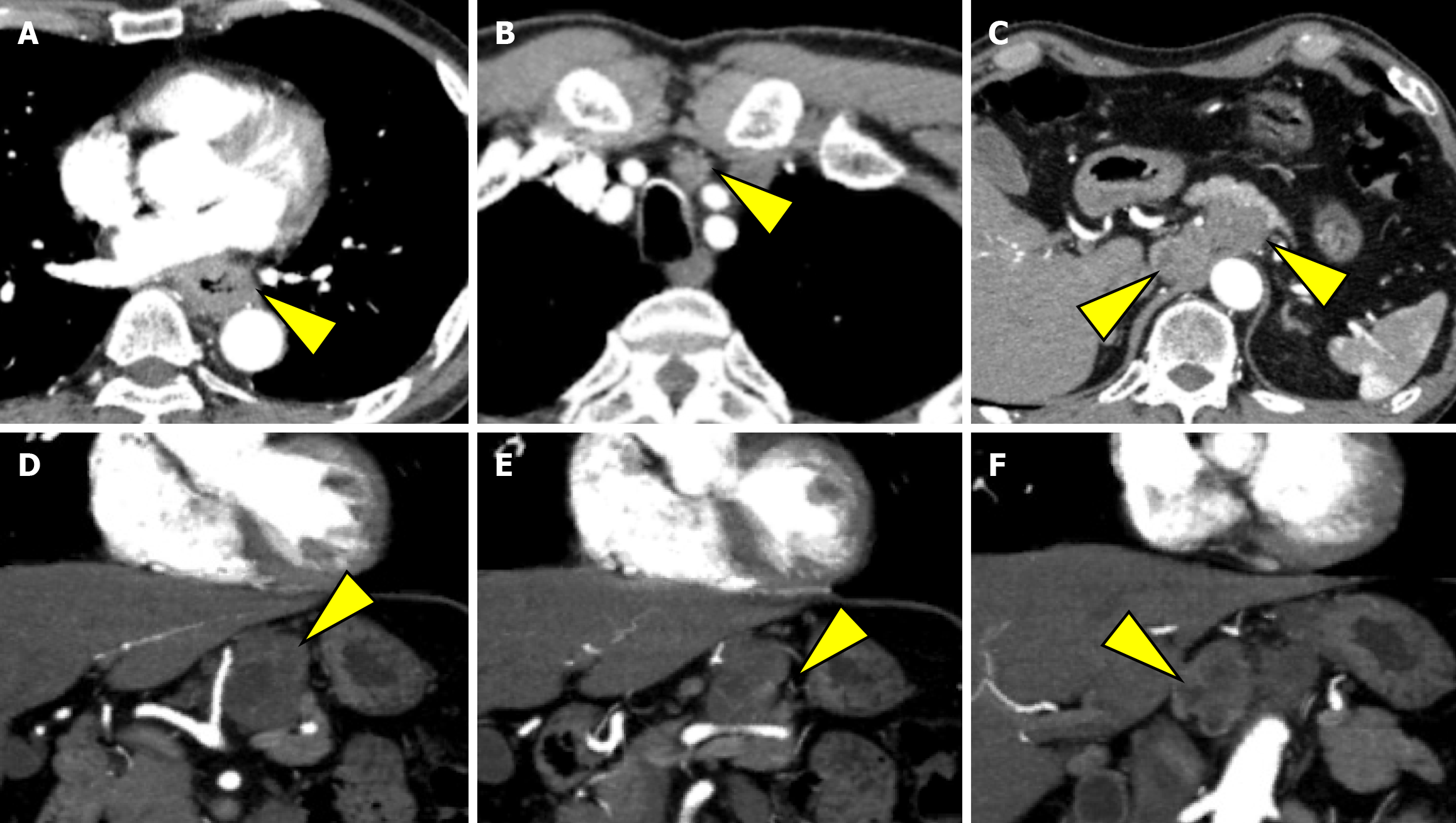
EGD revealed a semicircular, type 3 tumor in the middle thoracic esophagus, located 29-36 cm from the incisors (Figure 1A). Histopathological analysis of the biopsy specimen confirmed SCC. Computed tomography (CT) revealed irregular wall thickening from the middle to the lower thoracic esophagus, corresponding to the primary lesion (Figure 2A). In addition, an enlarged No. 101L lymph node measuring 15 mm was observed (Figure 2B), along with bulky suprapancreatic lymph node metastasis involving No. 8 and No. 9, forming a mass; furthermore, No. 11p was enlarged to 35 mm and showed direct invasion into the pancreatic body (Figure 2C-F). No other distant metastases were observed. On the basis of these results, the patient was diagnosed with locally advanced unresectable esophageal cancer of the middle thoracic esophagus [cT4a (No. 11p-pancreas) N4M0 cStage Iva][10,11].
The pretreatment diagnosis was locally advanced unresectable esophageal SCC of the middle thoracic esophagus [cT4a (No. 11p-pancreas) N4M0 cStage Iva]. The postoperative diagnosis was esophageal NEC, type 5, ypT4aN4 (No. 108, No. 11p) M0 ypStage IVa[10,11]. The final diagnosis was suspected mixed neuroendocrine non-neuroendocrine neoplasm (MiNEN).
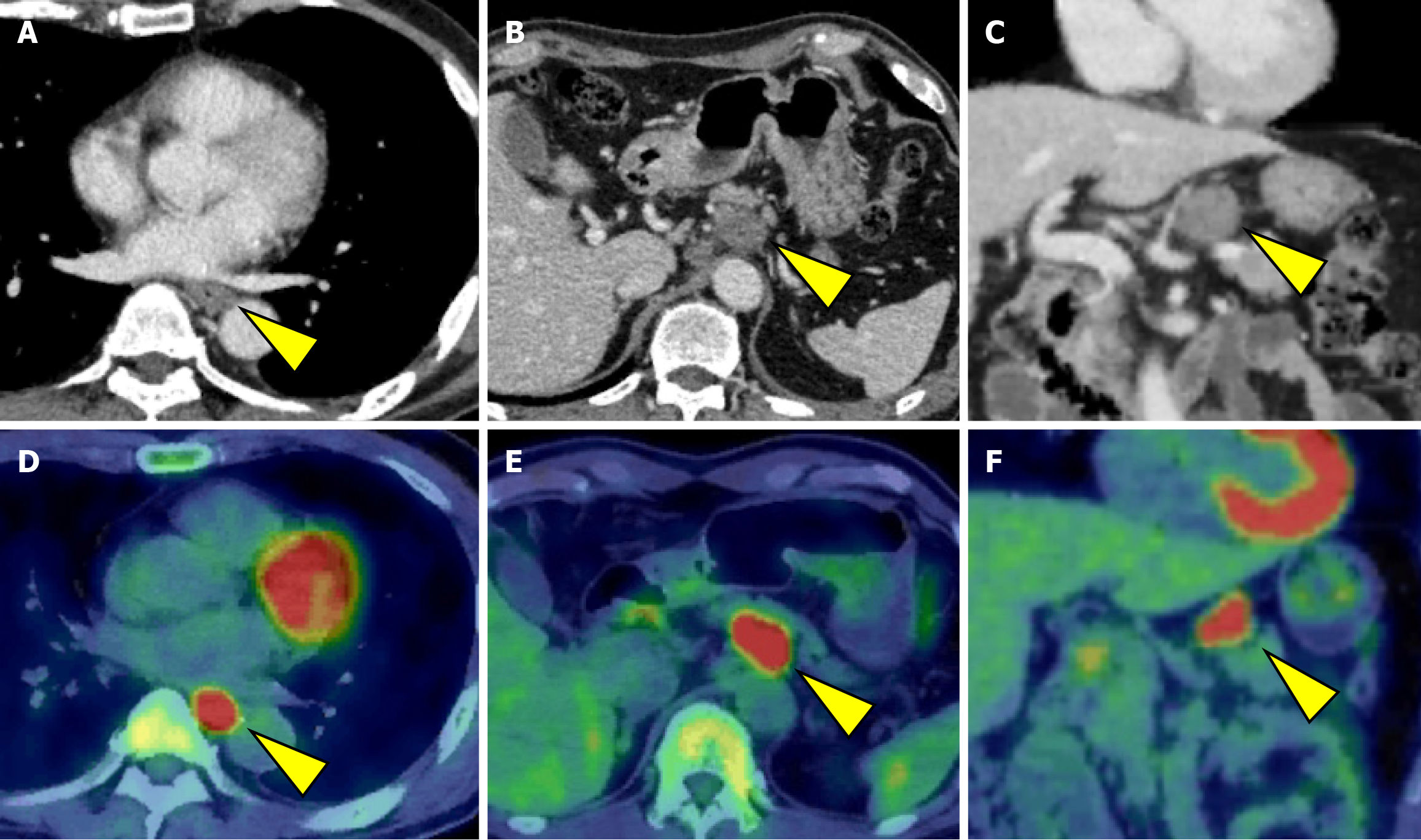
Initially, we planned to administer chemotherapy, and conversion surgery would be considered if the tumor became resectable following the chemotherapy. The induction chemotherapy involved docetaxel + cisplatin + 5-fluorouracil (DCF) (docetaxel 70 mg/m2/day on day 1, cisplatin 70 mg/m2/day on day 1, and 5-fluorouracil 750 mg/m2/day on days 1-5). After three courses of DCF chemotherapy, EGD and CT revealed shrinkage of the primary lesion (Figures 1B and 3A). Additionally, CT demonstrated a remarkable reduction in the size of the metastases involving No. 101L, No. 8, and No. 9. In contrast, the No. 11p lymph node showed only slight shrinkage (Figure 3B and C). Positron emission tomo
Therefore, we planned to perform laparotomy, thoracoscopic subtotal esophagectomy, gastric conduit reconstruction via the retrosternal route, and three-field lymph node dissection. The decision to begin with laparotomy was to assess the potential for noncurative outcomes due to possible dissemination and to evaluate the necessity of combined pancreatic and splenic resection, given the infiltration of the No. 11p lymph node into the pancreas. Intraoperatively, the No. 8 and No. 9 lymph nodes, which were enlarged before chemotherapy, could not be identified because of fibrosis. The No. 11p lymph node was directly adherent to the splenic artery and the body of the pancreas, making dissection impossible. Therefore, distal pancreatectomy and splenectomy were performed first. During mediastinal lymph node dissection via thoracoscopic surgery, en bloc dissection was performed while preserving the lymph nodes’ attachment to the esophagus, ensuring complete clearance. With regard to neck dissection, to ensure complete resection of the No. 101L lymph node, which was enlarged before chemotherapy, the left half of the trachea was fully exposed from the neck to the mediastinal inlet. The operative time was 778 minutes, with an estimated blood loss of 676 mL.
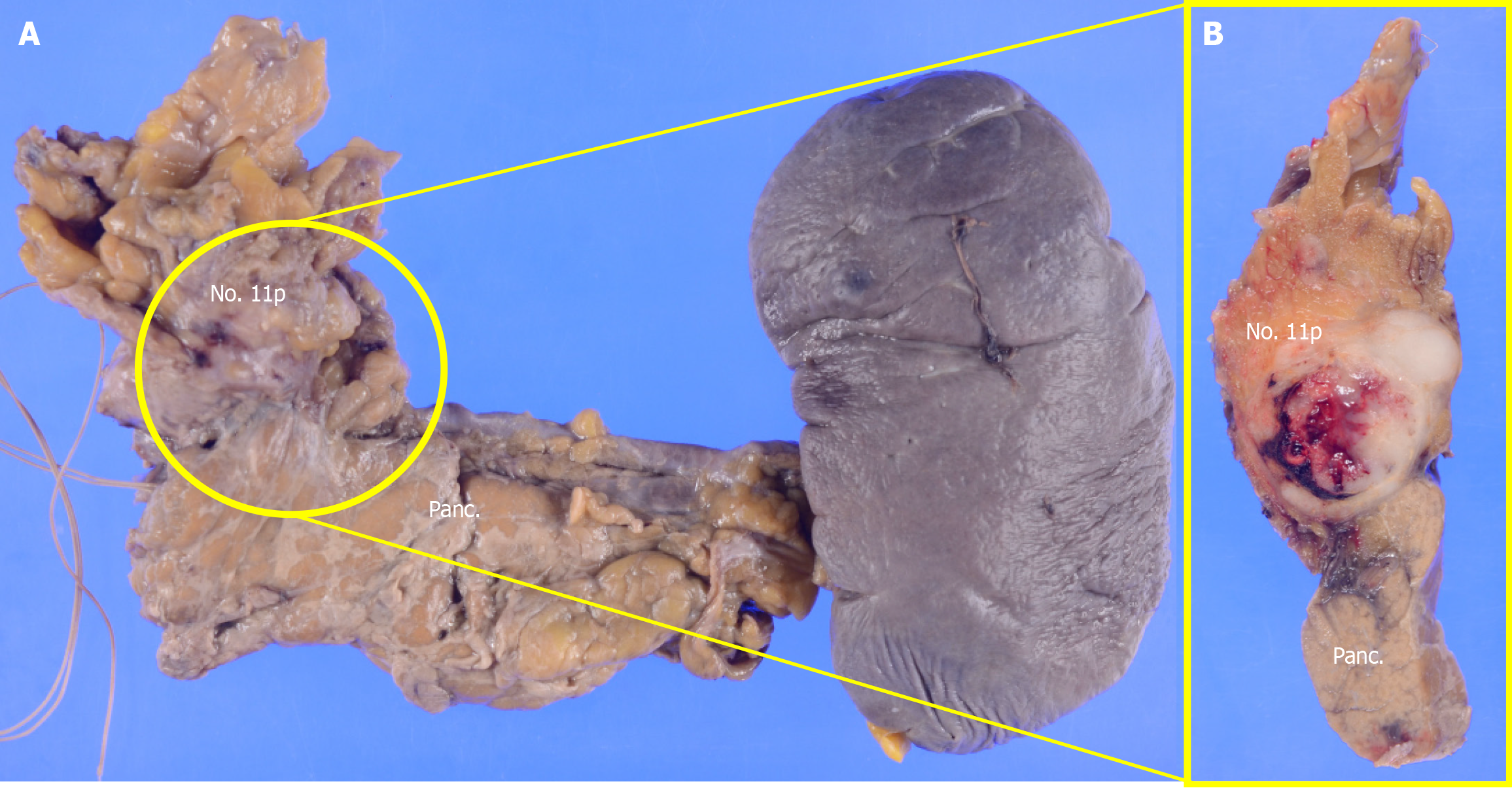
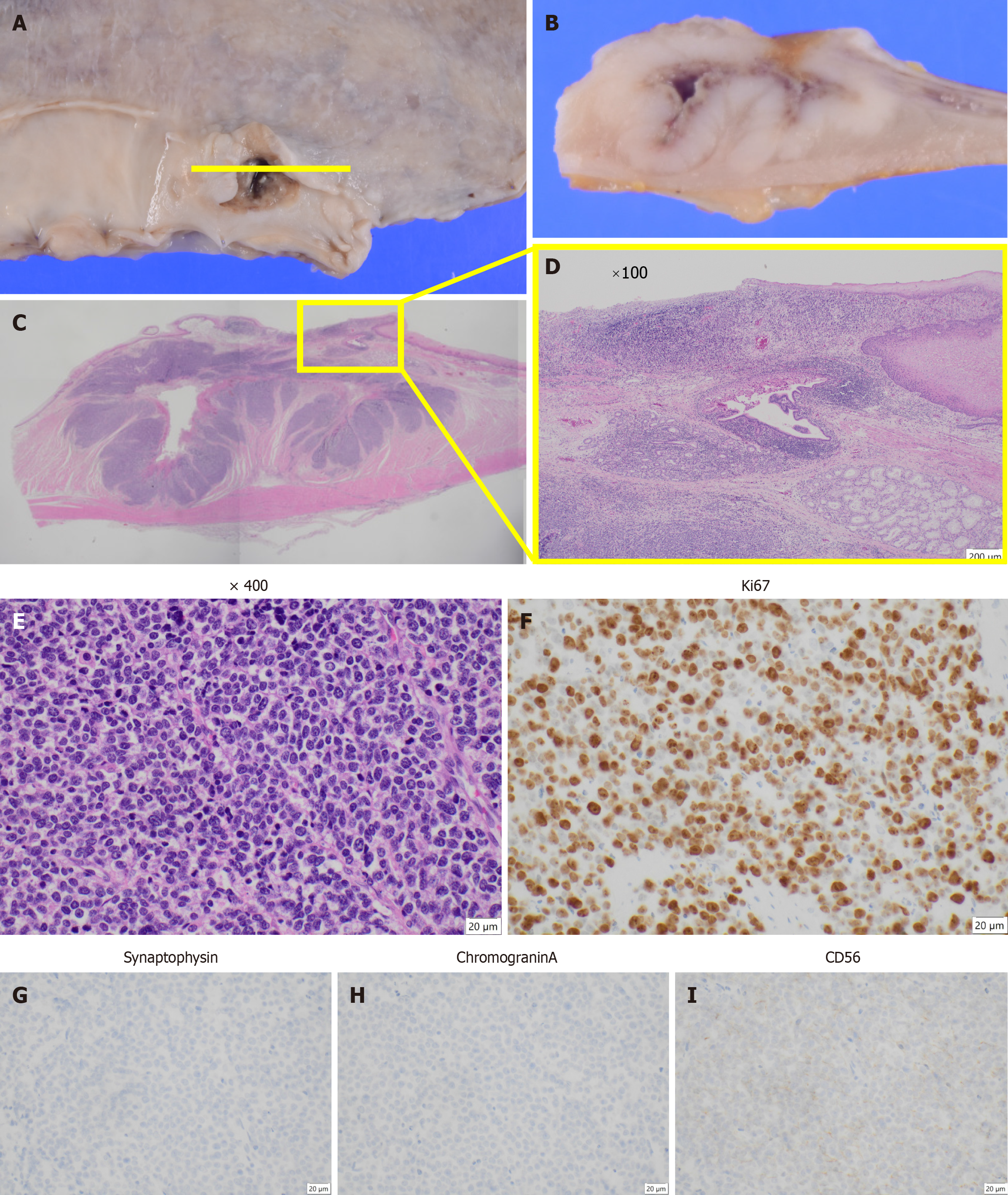
The postoperative pathological diagnosis was NEC, type 5, ypT4aN4 (No. 108, No. 11p) M0 ypStage IVa[10,11]. The resected pancreatic body and spleen showed that the No. 11p lymph node was macroscopically attached to the pancreas. However, pathological examination confirmed the absence of direct infiltration (Figure 4). Histopathological examination of the primary lesion revealed that while multinucleated giant cells and pleomorphic cells were present in the primary lesion, SCC detected before chemotherapy was not observed. Instead, large cell-type NEC was detected. Large pleomorphic cells with a high nucleus-to-cytoplasm ratio formed solid nests and proliferated (Figure 5A-E). Ki-67 staining was positive in more than 90% of the cells. Immunohistochemical staining revealed CD56 (+), chromogranin A
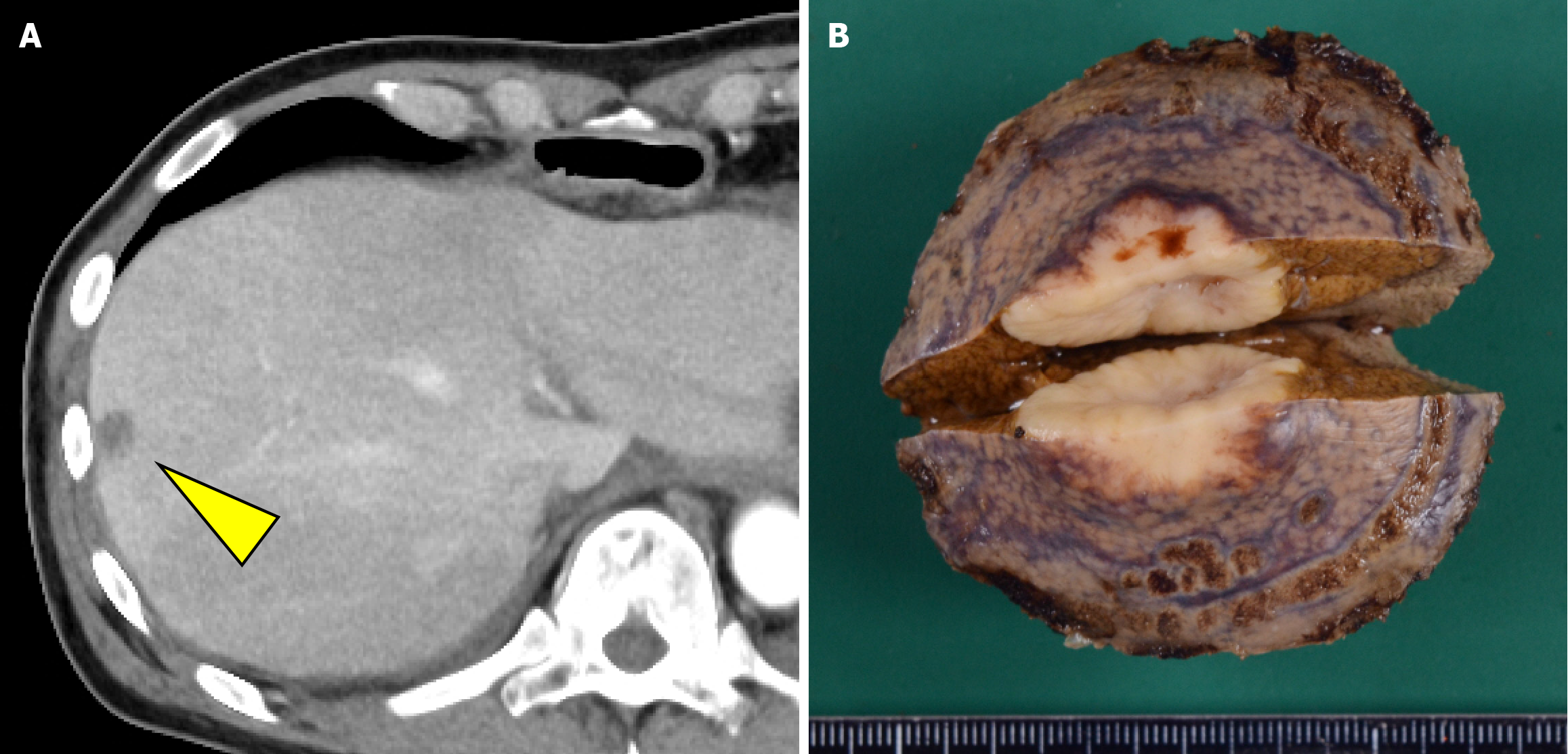
The patient resumed oral intake on postoperative day 7 and was discharged from the hospital on postoperative day 20. However, CT performed within 1 month postoperatively revealed a hypovascular nodule in liver segment 8 that was undetectable preoperatively (Figure 6). Metastatic hepatocellular carcinoma was suspected. Given that the histopathological findings of the primary lesion and metastatic lymph nodes were consistent with only NEC, four courses of irinotecan + cisplatin (IP) chemotherapy (IP: Irinotecan 60 mg/m²/day on days 1, 8, and 15 and cisplatin 60 mg/m²/day on day 1) were administered, and the cisplatin dose was reduced to 75% starting from the third course because of renal function decline. Despite treatment, the lesion increased in size from 15 mm to approximately 23 mm and was classified as progressive disease. After a multidisciplinary discussion, surgical treatment was planned, and partial segment 8 resection was performed. There were adhesions in the abdominal cavity because of the previous surgery, and the Pringle maneuver was not used. Histopathological examination confirmed metastasis of esophageal NEC. Subsequently, based on the efficacy of IP therapy, six courses of amrubicin (40 mg/m² on days 1-3) were administered as a second-line treatment.
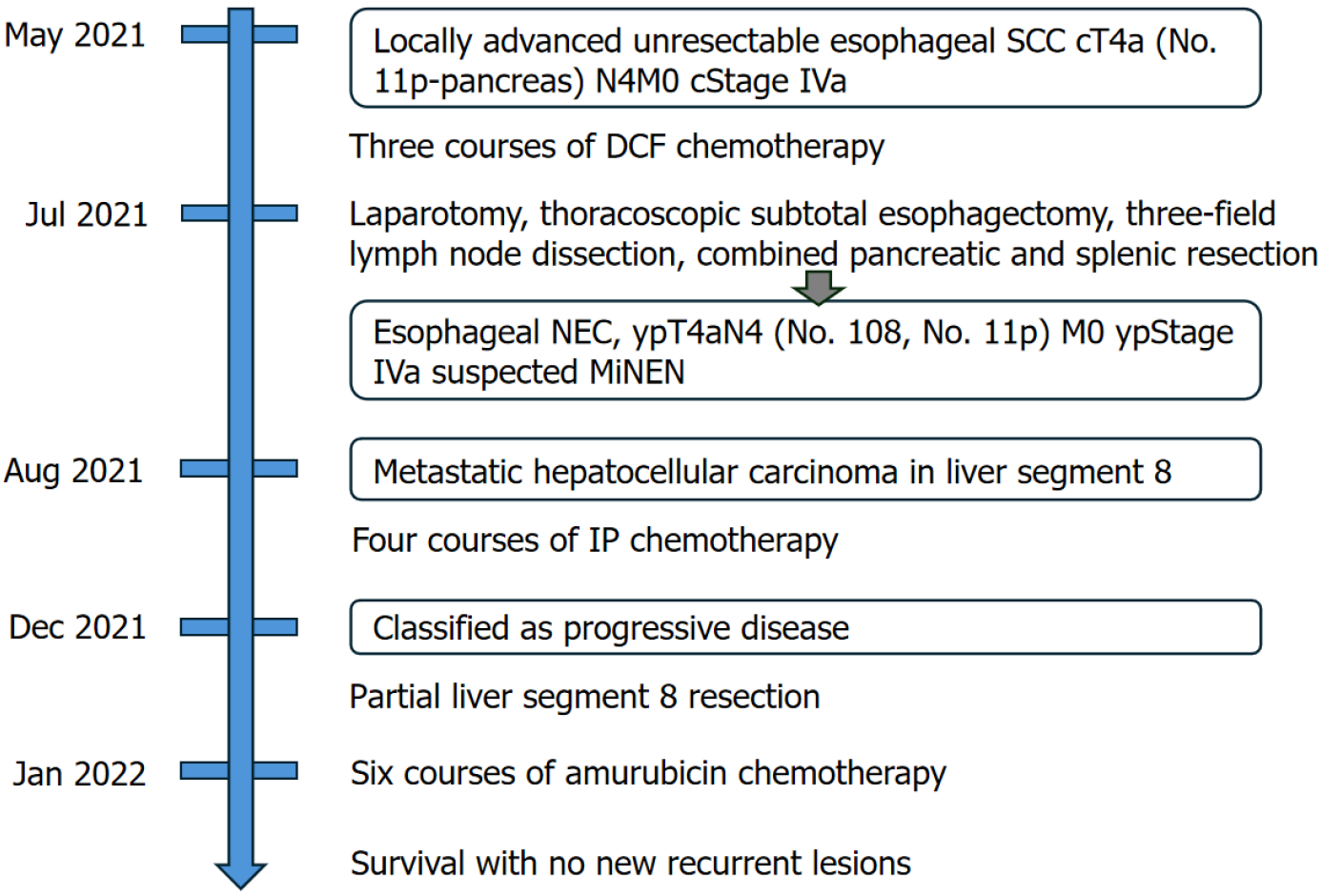
Following surgical resection of the liver metastasis, no new recurrent lesions were detected, and the patient survived for 3 years after surgery and was alive at the time of writing this report. Figure 7 shows a summary of the treatment course in this case.
We reported a case of locally advanced, unresectable esophageal cancer initially diagnosed as SCC but confirmed to be NEC after surgery; the final diagnosis was suspected MiNEN. The patient achieved long-term survival through a multidisciplinary treatment approach. Two aspects of this case are noteworthy. First was the difficulty in diagnosing NEC. Because NEC was finally diagnosed through postoperative pathological examination, chemotherapy with the DCF regimen was administered based on the initial presumption of SCC. This resulted in complete resolution of the SCC component, allowing for subsequent targeting of the remaining NEC component. Second was the effectiveness of surgical therapy for advanced NEC. Following chemotherapy, no new distant metastases were observed, and effective tumor control was achieved for the primary lesion. In such cases, surgery can be effective if resection is feasible, even for locally advanced NEC.
Regarding the diagnosis of NEC, preoperative biopsy often fails to identify this lesion; this is a significant challenges that may influence treatment planning. Histopathologically, NEC frequently coexists with SCC or adenocarcinoma (ADC) within the same tumor[2,12]. A retrospective review of nine surgical cases of NEC at our department between January 2012 and December 2023 revealed three patients who were not diagnosed with NEC preoperatively. Furthermore, postoperative pathology showed association with other histological types in five patients (three with SCC and two with ADC). Because treatment is typically guided by histological diagnosis of biopsy samples in cases where different cancer types are concurrent, treatment may be administered on the basis of non-NEC components, potentially leading to misdiagnosis, especially in advanced, non-surgical cases. Two possibilities can be considered. First, the tumor may have exhibited cellular heterogeneity before treatment, with neuroendocrine differentiated cells present but undetectable in the initial biopsy. These cells might have subsequently proliferated because of their resistance to treatment. Alternatively, neuroendocrine differentiation may have emerged from multipotent cells within the original neoplasm. In this case, histopathological examination of the primary lesion, resected pancreas, and metastatic lymph nodes revealed findings consistent with the therapeutic effects of chemotherapy for the SCC component. However, the minimal change in the size of the enlarged No. 11p lymph node and the therapeutic effect graded as 1a for the primary lesion indicated that the effect on the NEC component was minimal. This suggests the cellular heterogeneity of SCC and NEC.
Concerning the treatment approach for NEC, long-term survival rates with conversion surgery for esophageal NEC are limited[9]. Of note, conversion surgery for esophageal SCC following DCF induction chemotherapy has demonstrated promising results, with esophagectomy and R0 resection rates of 42% and 95%, respectively[13]. In this case, successful resectability was attributed to a significant reduction in the SCC component. However, in addition to the aggressive nature of the disease, the coexistence of SCC and ADC, as mentioned above, complicates treatment, and the therapeutic approach for NEC remains unclear. For NEC without lymph node metastasis, radical resection with adjuvant che
With regard to chemotherapy regimens, postoperative chemotherapeutic regimens specifically targeting NEC should be considered given the persistence of NEC in the primary lesions, metastatic lymph nodes, and liver metastases. The TOPIC-NEC trial demonstrated that IP and etoposide + cisplatin chemotherapy regimens are comparable and considered standard options for advanced or recurrent NEC[16]. Both regimens are used as neoadjuvant and adjuvant chemotherapeutic regimens at our institution. In the present case, systemic chemotherapy; specifically, IP chemotherapy, was initially administered for liver metastasis. The treatment strategy was determined according to the short interval between recurrence after the radical surgery and the high malignancy of NEC. However, the disease progressed after IP chemotherapy. Given the absence of other new recurrent lesions and limited tolerance to continued chemotherapy, surgical intervention was performed. After the surgery for liver metastasis, amrubicin was administered as a second-line therapy on the basis of its demonstrated efficacy against platinum-refractory gastrointestinal NEC[17,18]. Frizziero et al[19] reported that the median progression-free survival for advanced MiNEN with either locoregional advanced disease or distant metastasis was 5.6 months (95% confidence interval: 4.4-7.4), and the median overall survival was 9.0 months (95% confidence interval: 5.2-13.4). Accordingly, the survival of our patient without recurrence for 3 years after surgery for hepatic metastasis is a remarkably long-term survival outcome.
Given the rarity of esophageal NEC and the limited evidence base, this case report contributes to our understanding of the potential role of multimodal treatment, including conversion surgery. However, several limitations should be acknowledged. First, because this is a report of a single case, the findings lack generalizability and may not apply to all patients with advanced NEC, given the heterogeneity of the disease and individual factors. Second, initial use of the DCF regimen, based on the presumed diagnosis of SCC, may not have been optimal for the NEC component, which showed a limited response. NEC-specific chemotherapy regimens, such as IP or etoposide + cisplatin regimens, might have been more appropriate if the correct diagnosis was established preoperatively. Moreover, the early development of liver metastasis may indicate the presence of undetected micrometastases at the time of surgery and underscores the limitations of current staging techniques. The final diagnosis of suspected MiNEN also complicates the interpretation of the treatment response, given the heterogeneous biological behavior of this tumor. These factors highlight the need for improved preoperative diagnostic tools, such as molecular profiling and advanced imaging techniques, to enhance diagnosis and staging and guide more tailored treatment strategies. Larger, prospective studies are needed to establish standardized treatment protocols for advanced esophageal NEC and determine the role of conversion surgery and the optimal timing of systemic therapy.
In conclusion, we report the successful multimodal treatment of locally advanced, unresectable esophageal cancer with No. 11p lymph node metastasis invading the pancreas. Long-term survival was achieved through multidisciplinary treatment including conversion surgery and resection of liver metastasis, which was refractory to chemotherapy. The findings from this case suggest that even in advanced NEC, a multidisciplinary approach combining preoperative and postoperative chemotherapy with surgical therapy is valuable for achieving curability.
| 1. | Giannetta E, Guarnotta V, Rota F, de Cicco F, Grillo F, Colao A, Faggiano A; NIKE. A rare rarity: Neuroendocrine tumor of the esophagus. Crit Rev Oncol Hematol. 2019;137:92-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | Huang Q, Wu H, Nie L, Shi J, Lebenthal A, Chen J, Sun Q, Yang J, Huang L, Ye Q. Primary high-grade neuroendocrine carcinoma of the esophagus: a clinicopathologic and immunohistochemical study of 42 resection cases. Am J Surg Pathol. 2013;37:467-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Kukar M, Groman A, Malhotra U, Warren GW, Bogner P, Nwogu CE, Demmy TL, Yendamuri S. Small cell carcinoma of the esophagus: a SEER database analysis. Ann Surg Oncol. 2013;20:4239-4244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Yun JP, Zhang MF, Hou JH, Tian QH, Fu J, Liang XM, Wu QL, Rong TH. Primary small cell carcinoma of the esophagus: clinicopathological and immunohistochemical features of 21 cases. BMC Cancer. 2007;7:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Gray KD, Moore MD, Panjwani S, Elmously A, Afaneh C, Fahey TJ 3rd, Zarnegar R. Predicting Survival and Response to Treatment in Gastroesophageal Neuroendocrine Tumors: An Analysis of the National Cancer Database. Ann Surg Oncol. 2018;25:1418-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Egashira A, Morita M, Kumagai R, Taguchi KI, Ueda M, Yamaguchi S, Yamamoto M, Minami K, Ikeda Y, Toh Y. Neuroendocrine carcinoma of the esophagus: Clinicopathological and immunohistochemical features of 14 cases. PLoS One. 2017;12:e0173501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Morita M, Taguchi K, Kagawa M, Nakanoko T, Uehara H, Sugiyama M, Ota M, Ikebe M, Sugimachi K, Esaki T, Toh Y. Treatment strategies for neuroendocrine carcinoma of the upper digestive tract. Int J Clin Oncol. 2020;25:842-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Garcia-Carbonero R, Sorbye H, Baudin E, Raymond E, Wiedenmann B, Niederle B, Sedlackova E, Toumpanakis C, Anlauf M, Cwikla JB, Caplin M, O'Toole D, Perren A; Vienna Consensus Conference participants. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology. 2016;103:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 419] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 9. | Enjoji T, Kobayashi S, Hayashi K, Tetsuo H, Matsumoto R, Maruya Y, Araki T, Honda T, Akazawa Y, Kanetaka K, Nakao K, Eguchi S. Long-term survival after conversion surgery for an esophageal neuroendocrine carcinoma: a case report. Gen Thorac Cardiovasc Surg Cases. 2024;3:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Ito T, Masui T, Komoto I, Doi R, Osamura RY, Sakurai A, Ikeda M, Takano K, Igarashi H, Shimatsu A, Nakamura K, Nakamoto Y, Hijioka S, Morita K, Ishikawa Y, Ohike N, Kasajima A, Kushima R, Kojima M, Sasano H, Hirano S, Mizuno N, Aoki T, Aoki T, Ohtsuka T, Okumura T, Kimura Y, Kudo A, Konishi T, Matsumoto I, Kobayashi N, Fujimori N, Honma Y, Morizane C, Uchino S, Horiuchi K, Yamasaki M, Matsubayashi J, Sato Y, Sekiguchi M, Abe S, Okusaka T, Kida M, Kimura W, Tanaka M, Majima Y, Jensen RT, Hirata K, Imamura M, Uemoto S. JNETS clinical practice guidelines for gastroenteropancreatic neuroendocrine neoplasms: diagnosis, treatment, and follow-up: a synopsis. J Gastroenterol. 2021;56:1033-1044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 11. | Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus. 2017;14:1-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 708] [Article Influence: 88.5] [Reference Citation Analysis (1)] |
| 12. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2440] [Article Influence: 488.0] [Reference Citation Analysis (3)] |
| 13. | Yokota T, Kato K, Hamamoto Y, Tsubosa Y, Ogawa H, Ito Y, Hara H, Ura T, Kojima T, Chin K, Hironaka S, Kii T, Kojima Y, Akutsu Y, Matsushita H, Kawakami K, Mori K, Nagai Y, Asami C, Kitagawa Y. Phase II study of chemoselection with docetaxel plus cisplatin and 5-fluorouracil induction chemotherapy and subsequent conversion surgery for locally advanced unresectable oesophageal cancer. Br J Cancer. 2016;115:1328-1334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 14. | Meng MB, Zaorsky NG, Jiang C, Tian LJ, Wang HH, Liu CL, Wang J, Tao Z, Sun Y, Wang J, Pang QS, Zhao LJ, Yuan ZY, Ping W. Radiotherapy and chemotherapy are associated with improved outcomes over surgery and chemotherapy in the management of limited-stage small cell esophageal carcinoma. Radiother Oncol. 2013;106:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Xu L, Li Y, Liu X, Sun H, Zhang R, Zhang J, Zheng Y, Wang Z, Liu S, Chen X. Treatment Strategies and Prognostic Factors of Limited-Stage Primary Small Cell Carcinoma of the Esophagus. J Thorac Oncol. 2017;12:1834-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Morizane C, Machida N, Honma Y, Okusaka T, Boku N, Kato K, Nomura S, Hiraoka N, Sekine S, Taniguchi H, Okano N, Yamaguchi K, Sato T, Ikeda M, Mizuno N, Ozaka M, Kataoka T, Ueno M, Kitagawa Y, Terashima M, Furuse J; Japan Clinical Oncology Group (JCOG). Effectiveness of Etoposide and Cisplatin vs Irinotecan and Cisplatin Therapy for Patients With Advanced Neuroendocrine Carcinoma of the Digestive System: The TOPIC-NEC Phase 3 Randomized Clinical Trial. JAMA Oncol. 2022;8:1447-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 17. | Nio K, Arita S, Isobe T, Kusaba H, Kohashi K, Kajitani T, Tamura S, Hirano G, Mitsugi K, Makiyama A, Esaki T, Ariyama H, Oda Y, Akashi K, Baba E. Amrubicin monotherapy for patients with extrapulmonary neuroendocrine carcinoma after platinum-based chemotherapy. Cancer Chemother Pharmacol. 2015;75:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Kitagawa Y, Osumi H, Shinozaki E, Ota Y, Nakayama I, Suzuki T, Wakatsuki T, Ichimura T, Ogura M, Ooki A, Takahari D, Suenaga M, Chin K, Yamaguchi K. Safety and efficacy of amrubicin monotherapy in patients with platinum-refractory metastatic neuroendocrine carcinoma of the gastrointestinal tract: a single cancer center retrospective study. Cancer Manag Res. 2019;11:5757-5764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Frizziero M, Wang X, Chakrabarty B, Childs A, Luong TV, Walter T, Khan MS, Morgan M, Christian A, Elshafie M, Shah T, Minicozzi A, Mansoor W, Meyer T, Lamarca A, Hubner RA, Valle JW, McNamara MG. Retrospective study on mixed neuroendocrine non-neuroendocrine neoplasms from five European centres. World J Gastroenterol. 2019;25:5991-6005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |