Published online Jun 27, 2025. doi: 10.4240/wjgs.v17.i6.104545
Revised: February 23, 2025
Accepted: May 12, 2025
Published online: June 27, 2025
Processing time: 142 Days and 3.4 Hours
Gastric cancer (GC) remains a significant global health challenge, with high incidence and mortality rates. Neoadjuvant chemotherapy is increasingly used to improve surgical outcomes and long-term survival in advanced cases. However, individual responses to treatment vary widely, and current imaging methods often fall short in accurately predicting efficacy. Advanced imaging techniques, such as computed tomography (CT) 3D reconstruction and texture analysis, offer potential for more precise assessment of therapeutic response.
To explore the application value of CT 3D reconstruction volume change rate, texture feature analysis, and visual features in assessing the efficacy of neoad
A retrospective analysis was conducted on the clinical and imaging data of 97 patients with advanced GC who received S-1 plus Oxaliplatin combined chemo
The minimum misclassification rate of texture features in venous phase CT images (7.85%) was lower than in the arterial phase (13.92%). The volume change rate in the effective chemotherapy group (75.20%) was significantly higher than in the ineffective group (41.75%). There was a strong correlation between volume change rate and TRG grade (r = -0.886, P < 0.001). Multivariate analysis showed that gastric wall peristalsis (OR = 0.286) and thickness change rate ≥ 40% (OR = 0.265) were independent predictive factors. Receiver operating characteristic curve analysis indicated that the volume change rate [area under the curve (AUC) = 0.885] was superior to the CT visual feature model (AUC = 0.795). When the cutoff value was 82.56%, the sensitivity and specificity were 85.62% and 96.45%, respectively.
The CT 3D reconstruction volume change rate can serve as a preferred quantitative indicator for evaluating the efficacy of neoadjuvant chemotherapy in GC. Combining it with a CT visual feature predictive model can further improve the accuracy of efficacy evaluation.
Core Tip: This study highlights the value of computed tomography (CT) 3D reconstruction, texture analysis, and visual features in assessing neoadjuvant chemotherapy efficacy for gastric cancer (GC). The tumor volume change rate, derived from CT 3D reconstruction, showed a strong correlation with pathological tumor regression grade, outperforming CT visual features in predictive accuracy. Texture analysis, especially in the venous phase, demonstrated superior diagnostic capability. Combining these quantitative and qualitative imaging indicators provides a robust evaluation framework, aiding personalized treatment decisions. These findings emphasize the clinical utility of advanced imaging techniques for optimizing chemotherapy strategies and improving patient outcomes in GC management.
- Citation: Wang CY, Zhang L, Ma JW. Computed tomography 3D reconstruction and texture analysis for evaluating the efficacy of neoadjuvant chemotherapy in advanced gastric cancer. World J Gastrointest Surg 2025; 17(6): 104545
- URL: https://www.wjgnet.com/1948-9366/full/v17/i6/104545.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i6.104545
Gastric cancer (GC) is one of the most common malignant tumors worldwide, accounting for 6.8% of the global cancer population, with both incidence and mortality rates ranking high[1]. In recent years, neoadjuvant chemotherapy has become an important strategy for treating advanced GC and is widely used in clinical practice[2]. Studies have shown that neoadjuvant chemotherapy can downstage tumors, improve surgical resection rates, and enhance long-term survival outcomes[3]. However, there is significant individual variability in patient responses to neoadjuvant chemotherapy, making accurate assessment of chemotherapy efficacy crucial for formulating subsequent treatment plans and predicting prognosis[4].
Currently, imaging examinations are one of the main methods for evaluating the efficacy of neoadjuvant chemotherapy in GC[5]. The traditional Response Evaluation Criteria in Solid Tumors (RECIST) primarily assesses changes in the maximum diameter of the tumor, but this single-dimensional measurement method may not accurately reflect overall tumor regression, especially for lesions with irregular shapes or uneven shrinkage[6]. With advances in medical imaging technology, computed tomography (CT) 3D reconstruction offers new possibilities for precise tumor volume mea
In recent years, radiomics has emerged as a novel research method that extracts and analyzes a large number of imaging features, revealing tumor characteristics that are difficult to identify with conventional imaging examinations[8]. These features may be closely related to the biological behavior of tumors and their response to treatment. Previous studies have shown that CT-based radiomics analysis has potential value in predicting the treatment effects of various tumors[9]. However, research on the application of CT radiomic features in evaluating the efficacy of neoadjuvant chemotherapy for GC is relatively limited, and its predictive value needs further validation. On the other hand, CT visual features, as the most commonly used evaluation method in clinical practice, have the advantages of being simple to operate and easy to promote[10]. However, there is currently a lack of systematic research to assess and compare the value of CT 3D reconstruction measurements, texture feature analysis, and visual features in predicting the efficacy of neoadjuvant chemotherapy for GC. Establishing an accurate and reliable imaging evaluation system is of great importance for guiding clinical decision-making. CT radiomics has been increasingly applied in tumor diagnosis and treatment. Radiomics can reveal more detailed imaging phenotypic characteristics of tumors through high-throughput extraction and analysis of quantitative features from medical images[11,12]. Studies have shown that CT radiomics features not only reflect tumor heterogeneity but are also closely related to tumor molecular phenotypes, gene mutation status, and prognosis[13]. In non-small cell lung cancer, CT-based radiomics models have been proven to effectively predict EGFR mutation status and immunotherapy efficacy. In colorectal cancer research, CT texture features have been found to predict the efficacy of neoadjuvant chemoradiotherapy and are significantly correlated with pathological complete response[14].
Therefore, this study aims to retrospectively analyze the CT image data of patients with advanced GC who received the S-1 plus Oxaliplatin combined chemotherapy (SOX) regimen as neoadjuvant chemotherapy, to explore the application value of CT 3D reconstruction volume change rate, texture feature analysis, and visual features in predicting chemo
This study employs a retrospective design, and the research protocol has been reviewed and approved by the hospital's ethics committee. As the study is a retrospective analysis, informed consent from patients was waived. The collection and use of all patient data comply with the provisions of the Declaration of Helsinki, and the study strictly adheres to the principles of medical ethics, protecting patient privacy throughout the process.
We conducted a retrospective analysis of patients with advanced GC treated at our hospital from January 2022 to March 2024. The inclusion criteria are as follows: (1) Pathologically confirmed gastric adenocarcinoma by gastroscopy biopsy; (2) Clinical staging of II-III; (3) No antitumor treatment received at the initial diagnosis; (4) Received 2-4 cycles of SOX regimen neoadjuvant chemotherapy; (5) Underwent enhanced CT scans before and after chemotherapy, with image quality meeting diagnostic requirements; and (6) Underwent radical surgical treatment within 4-6 weeks after chemotherapy and obtained complete postoperative pathological results. The exclusion criteria are as follows: (1) History of other malignant tumors; (2) Severe cardiac, hepatic, or renal dysfunction or other significant organ dysfunction; (3) Interruption or change of the treatment plan due to severe adverse reactions during neoadjuvant chemotherapy; (4) Poor CT image quality or severe motion artifacts; (5) Failure to complete standardized surgical treatment for various reasons; and (6) Incomplete clinical or follow-up data.
The surgical treatment plan in this study is formulated based on the treatment strategy for patients with advanced GC after neoadjuvant chemotherapy. All enrolled patients underwent radical gastrectomy within 4-6 weeks after completing 2-4 cycles of the SOX regimen neoadjuvant chemotherapy, following discussion and assessment by a multidisciplinary team (MDT). The specific implementation of the surgical protocol adheres to the following principles and procedures: First, preoperative routine examinations are completed, including cardiac and pulmonary function assessment, coagulation function, blood routine, and laboratory tests for liver and kidney function, to ensure the patient's tolerance to surgery. Surgery is performed under general anesthesia, with the patient in the supine position, and an upper abdominal midline incision is made. After entering the abdomen, the abdominal cavity is explored for ascites, peritoneal metastatic nodules, and the local infiltration of the tumor and the extent of involvement of surrounding organs are assessed. The surgical resection range, including total gastrectomy or distal subtotal gastrectomy, is chosen based on the location, size, and infiltration range of the gastric primary lesion. The criteria for choosing the above surgical methods are: For cardia cancer, gastric body cancer, or diffuse infiltrating GC patients, total gastrectomy is performed; for antral cancer, distal subtotal gastrectomy can be performed as long as the proximal margin is ≥ 5 cm.
During surgery, the D2 lymph node dissection standard is strictly followed, which means systematic dissection of the 1st and 2nd group of lymph nodes, including: Infrapyloric lymph nodes (No. 6), suprapyloric lymph nodes (No. 5), right gastric artery lymph nodes (No. 12), common hepatic artery lymph nodes (No. 8), celiac artery lymph nodes (No. 9), splenic artery distal and proximal lymph nodes (No. 11p, 11 d), lesser curvature lymph nodes (No. 3), and greater curvature lymph nodes (No. 4). For total gastrectomy, the dissection of the esophageal hiatus lymph nodes (No. 1, 2) is also completed. Lymph node dissection must follow the principle of en bloc resection, pay attention to protect important blood vessels and nerves, especially the vagus nerve trunk and the celiac plexus. The method of gastrointestinal reconstruction is chosen based on the specific resection range: After total gastrectomy, Roux-en-Y esophagojejunostomy is performed for reconstruction; after distal subtotal gastrectomy, Billroth-II gastrojejunostomy is performed for reconstruction. Anastomosis is done using mechanical or manual methods to ensure that the anastomotic site is tension-free and well-vascularized.
Intraoperative considerations include: (1) Careful operation to avoid tumor tissue damage and prevent cancer cell implantation; (2) Accurate judgment of the resection range to ensure negative margins; (3) Thorough lymph node dissection to avoid omissions; (4) Reasonable use of electrocoagulation to prevent postoperative bleeding; and (5) Proper handling of the anastomotic site to prevent anastomotic fistula. A routine abdominal drainage tube is placed before the end of surgery to observe the nature of the drainage fluid. Postoperatively, patients are given anti-infection and nutritional support treatment, and vital signs and complications are closely monitored. After the patient has recovered well, the subsequent treatment plan is formulated based on the postoperative pathological results. All surgeries in this study are performed by a team of gastrointestinal surgery experts with rich experience to ensure the quality and safety of the surgery. Through the implementation of standardized surgical protocols, reliable pathological evidence is provided for the evaluation of the efficacy of neoadjuvant chemotherapy.
The SOX neoadjuvant chemotherapy regimen used in this study is a standard combination chemotherapy protocol for patients with advanced GC, consisting of two main drugs: Oxaliplatin and S-1[15]. The specific administration plan is as follows: On the first day, oxaliplatin is administered intravenously at a dose of 130 mg/m², and concurrently, S-1 is orally administered at a dose of 80 mg/m² twice daily (after breakfast and dinner) during the first two weeks, followed by a one-week rest period. The aforementioned medication schedule constitutes a complete treatment cycle (21 days), and patients are required to complete 2-4 cycles of neoadjuvant chemotherapy. During chemotherapy, the treatment team will adjust the chemotherapy dosage and cycles based on the patient's therapeutic response and tolerance to adverse reactions, ensuring the safety and efficacy of the treatment. After completing neoadjuvant chemotherapy, patients undergo a comprehensive assessment by the MDT, and radical gastrectomy and D2 lymph node dissection are performed upon confirmation of surgical indications. At the end of the postoperative recovery period, patients will continue to receive 2-4 cycles of SOX regimen adjuvant chemotherapy to consolidate the therapeutic effect and reduce the risk of recurrence and metastasis.
In this study, all enrolled patients underwent abdominal scans using a Siemens 64-slice 128-layer spiral CT scanner at two time points: Before neoadjuvant chemotherapy and before surgery. The standardized enhanced scanning protocol involved injecting the iodinated contrast agent iohexol (concentration of 320 mgI/mL) via a double-barreled high-pressure injector into the patient's elbow vein at a constant rate of 3 mL/s. Image acquisition phases were set for the arterial phase at 30 seconds and the venous phase at 70 seconds after contrast injection. The scanning technical parameters were set as follows: Tube voltage 120-140 kV, tube current 250-300 mA, pitch 0.6, tube rotation time of 0.5 seconds per rotation, and the slice thickness and slice interval for image reconstruction were uniformly set to 1mm to ensure high-quality thin-layer CT image data acquisition. Subsequently, the raw CT image data were imported into the ITK-snap medical image processing software for post-processing, and the three-dimensional reconstruction function of the software was used to segment the target area, ultimately obtaining three-dimensional stereo image information of the tumor lesion, providing basic data support for subsequent quantitative analysis.
The image post-processing and data analysis in this study mainly include texture feature extraction and three-dimensional reconstruction measurement. In the texture feature extraction process, MaZda software (version 4.7) is used to analyze CT images. First, the CT images are converted to bmp format and imported into the software, where the region of interest (ROI) is manually outlined on the largest lesion plane. To reduce the impact of image contrast and brightness changes on the analysis results, gray-level normalization is performed for each ROI using the dynamic thresholding method of μ ± 3SD (μ is the mean gray level, SD is the standard deviation). The extraction of texture features covers multiple dimensions: (1) Gray-level histogram co-occurrence matrix features, including parameters such as angular second moment, contrast, correlation, entropy, entropy and sum, square and, average and, variance and, inverse difference moment, and difference entropy, all calculated in four directions; (2) Run-length matrix features, including run-length non-uniformity, gray-level non-uniformity, long-term emphasis, short-term emphasis, and run-image fraction; (3) Absolute gradient features; (4) Autoregressive model parameters (theta1-4 and sigma); and (5) Wavelet transform features. Through the above analysis, a total of about 300 texture feature parameters are obtained. To select the most predictive parameters from these features, four feature selection methods are used: Fisher's coefficient method, probability of error plus average correlation coefficient (POE+ACC) method, mutual information (MI) method, and a combined method (Fisher, POE+ACC and MI combined method, FPM). Subsequently, the B11 software built into MaZda is used for classification analysis, successively employing raw data analysis (RDA), principal component analysis (PCA), linear discriminant analysis (LDA), and nonlinear discriminant analysis (NDA) to evaluate the predictive accuracy of each parameter for the efficacy of neoadjuvant chemotherapy in GC, with the misclassification rate as the evaluation indicator. The lower the misclassification rate, the higher the predictive value of the extracted texture features. For volumetric measurements, ITK-SNAP version 3.8.0 software was employed using a semi-automatic segmentation approach. The segmentation process involved specific parameter settings including pre-segmentation threshold ranges (set at -50 to +150 HU), bubble radius for region growing (2.0 mm), number of iterations (250), curvature force (0.2), and advection force (0.1). Following initial automatic segmentation, manual refinement was performed by experienced radiologists to ensure accuracy. The volume calculation utilized a voxel-counting method with tri-linear interpolation. Inter-observer validation was conducted with two independent radiologists performing measurements on a subset of 30 cases, with intraclass correlation coefficients (ICC) calculated to assess reliability (ICC > 0.85 indicating excellent agreement).
In terms of three-dimensional reconstruction measurement, the ITK-snap medical image processing software is used to process 122 sets of CT image data. First, the window level and width are adjusted to 40 and 300, respectively, to obtain the best display effect of the gastric wall. A 1 mm slice thickness is used for scanning, and the most prominent area of the mass is precisely located on the axial, sagittal, and coronal sections, and the lesion range is manually outlined with a red marker. To ensure measurement accuracy, the area of each segmented region is based on a 1mm unit, and the software automatically calculates the volume of the segmented region. Segmentation is performed layer by layer along the lesion boundary until the lesion disappears completely or the gastric wall thickness returns to normal. All segmented layers are superimposed on a three-dimensional reconstruction platform to obtain the total volume of the tumor and measure the maximum thickness of the tumor at the largest segment area. The above processing is performed on the images before and after neoadjuvant chemotherapy for 97 patients, and the volume and thickness changes before and after treatment are calculated. The evaluation indicators include: Tumor thickness reduction percentage = (pre-chemotherapy tumor thickness - post-chemotherapy tumor thickness)/pre-chemotherapy tumor thickness × 100%, and tumor volume reduction percentage = (pre-chemotherapy tumor volume - post-chemotherapy tumor volume)/pre-chemotherapy tumor volume × 100%. These quantitative indicators provide objective evidence for evaluating the efficacy of neoadjuvant chemotherapy.
In this study, the tumor regression grade (TRG) system is used to evaluate the efficacy of neoadjuvant chemotherapy, with postoperative pathological results as the gold standard[16]. According to the Becker criteria, the degree of tumor regression in patients is divided into four grades: TRG1a for complete response (no tumor residue), TRG1b for near-complete response (tumor residue < 10%), TRG2 for partial response (10% < tumor residue < 50%), and TRG3 for minimal response or no response (tumor residue > 50%). Based on the pathological assessment results, patients are divided into two groups: The chemotherapy effective group (tumor regression > 50%, including TRG1a, TRG1b, and TRG2) and the chemotherapy ineffective group (tumor regression ≤ 50%, i.e., TRG3) (Table 1).
| TRG grading | Content |
| TRG1a | (Complete remission): No residual tumor |
| TRG1b | (Subtle remission): Tumor residual < 10% |
| TRG2 | (Partial relief): 10%< tumor residue < 50% |
| TRG3 | (Minor or no relief): Tumor residual > 50% |
This study analyzes the clinical characteristics and chemotherapy effects in GC patients by evaluating CT image visual features. The main observation indicators include tumor maximum thickness, gastric wall peristalsis, lesion enhancement pattern, lymph node metastasis, abdominal lesion presentation, and distant metastasis. For tumor maximum thickness assessment, measurements are taken in axial, sagittal, and coronal planes to evaluate tumor volume load. Gastric wall peristalsis is assessed by comparing dynamic changes in gastric wall morphology at different scanning phases to determine the tumor's impact on gastric wall movement. Lesion enhancement patterns are classified into type A and type B based on the enhancement mode from the arterial to venous phase, reflecting tumor blood supply characteristics. Regional lymph node metastasis is evaluated by observing the size, shape, density, and enhancement characteristics of lymph nodes. Abdominal lesion presentation mainly observes the presence of ascites, peritoneal thickening, and nodular formation as signs of abdominal metastasis. Distant metastasis focuses on evaluating the presence of metastatic lesions in the liver and other organs.
Statistical analysis was performed using SPSS 26.0 software. The normality of continuous data was assessed with the Shapiro-Wilk test. Normally distributed data are expressed as mean ± SD, and comparisons between groups were made using independent sample t-tests. Non-normally distributed data are presented as median and interquartile range [M (P25, P75)], and comparisons were made using the Mann-Whitney U test. Categorical data are expressed as frequency and percentage, n (%), and comparisons were made using χ2 tests or Fisher's exact tests. Spearman correlation analysis was used to assess the correlation between tumor volume and thickness change rates and pathological TRG grading. Univariate analysis was used to identify statistically significant indicators (P < 0.05) among CT visual features, which were then included in a multivariate logistic regression analysis to construct a predictive model and create a nomogram. The diagnostic performance of the volume change rate and CT visual feature model was evaluated using receiver operating characteristic (ROC) curve analysis, calculating the area under the curve (AUC), sensitivity, and specificity. Internal validation of the model was performed using the Bootstrap method (1000 resamples), and model fit was assessed with a calibration curve. Decision curve analysis (DCA) was used to evaluate the clinical application value of the model. All statistical tests were two-sided, with P < 0.05 indicating statistical significance.
A total of 97 patients with advanced GC received 2-4 cycles of SOX regimen neoadjuvant chemotherapy. The results (Table 2) indicate that there were no statistically significant differences between the two groups in terms of baseline characteristics such as age, gender, clinical stage, tumor location, and Lauren classification (all P > 0.05), demonstrating the comparability of the two groups.
| Project | Chemotherapy ineffective group (n = 35) (tumor residual ≥ 50%) | Chemotherapy effective group (n = 62) (tumor residual < 50%) | t/χ2 | P value |
| Age | 62.35 + 8.42 | 61.87 + 9.13 | 0.258 | 0.797 |
| Gender | 0.342 | 0.559 | ||
| Male | 24 (68.57) | 39 (62.90) | ||
| Female | 11 (31.43) | 23 (37.10) | ||
| Staging | 0.876 | 0.349 | ||
| Stage II | 13 (37.14) | 29 (46.77) | ||
| Stage III | 22 (62.86) | 33 (53.23) | ||
| Location | 0.523 | 0.470 | ||
| Cardia | 15 (42.86) | 31 (50.00) | ||
| Gastric body | 20 (57.14) | 31 (50.00) | ||
| Lauren's classification | 1.247 | 0.536 | ||
| Diffuse type | 14 (40.00) | 21 (33.87) | ||
| Mixed type | 8 (22.86) | 19 (30.65) | ||
| Intestinal type | 13 (37.14) | 22 (35.48) |
Texture feature analysis of arterial and venous phase CT images before and after neoadjuvant chemotherapy for GC was performed using MaZda software. Feature selection was conducted using the Fisher coefficient method, POE + ACC method, MI method, and false positive nodule (FPN) combined method. The accuracy of predicting the efficacy of neoadjuvant chemotherapy was evaluated using four classification methods: RDA, PCA, LDA, and NDA. The results showed that the texture features predicting neoadjuvant chemotherapy efficacy mainly came from the venous phase, with a minimum misclassification rate of 7.85%, compared to 13.92% in the arterial phase (Table 3). The minimum misclassification rate in both phases occurred with the NDA method. The significantly lower misclassification rate in the venous phase indicates that its texture features have higher predictive value, making venous phase imaging more accurate for measuring tumor volume and thickness.
| Discrimination methods | RDA misclassification rate (%) | PCA misclassification rate (%) | LDA misclassification rate (%) | NDA misclassification rate (%) |
| Venous phase | ||||
| Fisher | 21.86 | 18.45 | 14.92 | 11.53 |
| POE + ACC | 19.75 | 16.82 | 13.45 | 9.86 |
| MI | 18.32 | 15.46 | 11.85 | 8.92 |
| FPN | 17.53 | 14.25 | 10.63 | 7.85 |
| Arterial phase | ||||
| Fisher | 25.92 | 22.85 | 19.46 | 16.85 |
| POE + ACC | 24.53 | 21.46 | 18.25 | 15.42 |
| MI | 22.85 | 19.75 | 16.92 | 14.53 |
| FPN | 21.46 | 18.53 | 15.86 | 13.92 |
The study results showed significant differences in volume and thickness change rates between the effective and ineffective chemotherapy groups (P1 < 0.001). In the effective group, the median volume change rate was 75.20%, significantly higher than the median thickness change rate of 40.65%. In the ineffective group, the median volume change rate was 41.75%, also higher than the median thickness change rate of 29.83%. The data indicate that in both groups, the median volume change rate was significantly higher than the corresponding thickness change rate (Table 4).
| Chemotherapy ineffective group (n = 35) | Chemotherapy effective group | Z | P1 | r | P2 | |
| Tumor volume change rate (%) | 41.75 (32.86, 48.52) | 75.20 (65.42, 82.35) | 7.246 | < 0.001 | 0.886 | < 0.001 |
| Tumor thickness change rate (%) | 29.83 (24.53, 35.62) | 40.65 (35.28, 46.92) | 5.835 | < 0.001 | 0.725 | < 0.001 |
To further assess the correlation between tumor volume and thickness change rates with postoperative pathological TRG, Spearman correlation analysis was used. The results showed a significant negative correlation for both indicators with pathological TRG grading. The volume change rate exhibited a stronger negative correlation (r = -0.886, P < 0.001), while the thickness change rate had a relatively weaker correlation (r = -0.725, P < 0.001). A larger absolute value of the negative correlation coefficient indicates a stronger correlation with TRG grading, suggesting that the volume change rate may be a more reliable imaging indicator for evaluating chemotherapy efficacy.
These results indicate that the tumor volume change rate measured by CT 3D reconstruction not only better distinguishes between effective and ineffective chemotherapy cases but also has a stronger correlation with the degree of pathological regression, making it a preferred quantitative indicator for evaluating the efficacy of neoadjuvant chemo
Univariate analysis showed significant differences in five CT visual features between the effective and ineffective chemotherapy groups (all P < 0.05). After assigning values to these significant indicators, stepwise regression analysis identified gastric wall peristalsis and thickness change rate as two independent predictive factors (Table 5).
| CT imaging features | Chemotherapy ineffective group (n = 35) | Chemotherapy effective group (n = 62) | χ2 | P value |
| Hickness change rate | 16.524 | < 0.001 | ||
| < 40% | 26 (74.29) | 23 (37.10) | ||
| 40% | 9 (25.71) | 39 (62.90) | ||
| Gastric wall motility | 13.682 | < 0.001 | ||
| Present | 11 (31.43) | 42 (67.74) | ||
| Absent | 24 (68.57) | 20 (32.26) | ||
| Lesion enhancement pattern | 7.845 | 0.005 | ||
| Type A | 13 (37.14) | 40 (64.52) | ||
| Type B | 22 (62.86) | 22 (35.48) | ||
| Lymph node metastasis | 2.246 | 0.134 | ||
| N0 | 15 (42.86) | 36 (58.06) | ||
| N+ | 20 (57.14) | 26 (41.94) | ||
| Peritoneal thickening and nodules | 6.235 | 0.013 | ||
| None | 16 (45.71) | 43 (69.35) | ||
| Present | 19 (54.29) | 19 (30.65) |
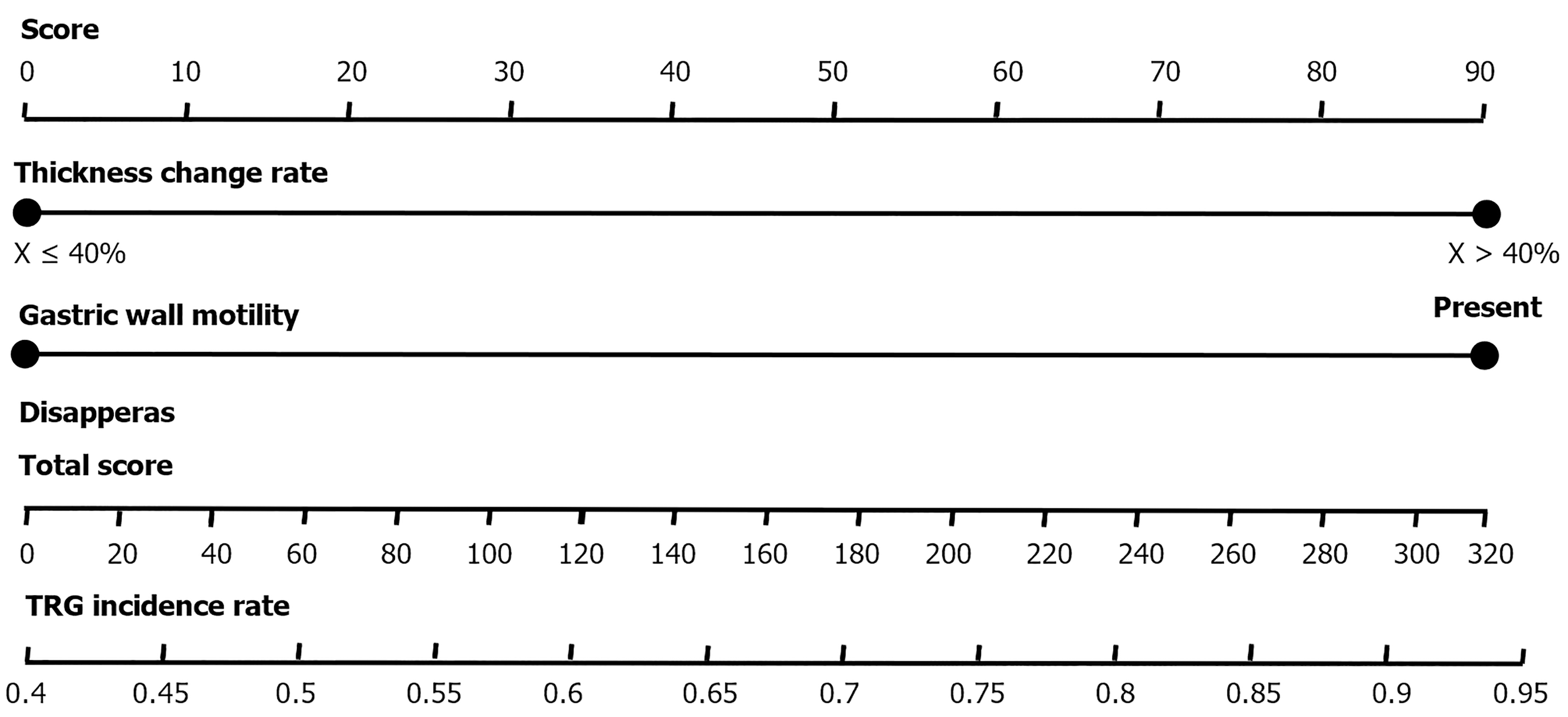
Multivariate logistic regression analysis results indicated that gastric wall peristalsis (OR = 0.286, 95%CI: 0.085-0.912) and thickness change rate ≥ 40% (OR = 0.265, 95%CI: 0.078-0.935) are independent factors predicting chemotherapy efficacy. Based on these two indicators, a CT subjective prediction model was constructed, and a nomogram was created: A thickness change rate ≥ 40% scores 100 points, and the presence of gastric wall peristalsis scores 98 points. The total score corresponds to different TRG grading prediction probabilities (Table 6; Figure 1).
| Item | Regression coefficient β | SE | Wald | P value | OR | 95%CI |
| Gastric wall motility | -1.22 | 0.56 | 8.856 | 0.003 | 0.286 | 0.085-0.912 |
| Thickness change rate > 40% | -1.32 | 0.58 | 10.245 | < 0.001 | 0.265 | 0.078-0.935 |
| Constant | 2.15 | 0.42 | 12.468 | < 0.001 | 8.625 |
These results suggest that gastric wall peristalsis and a thickness change rate ≥ 40% in CT visual features can serve as important predictive indicators for evaluating the efficacy of neoadjuvant chemotherapy in GC, with significant clinical application value.
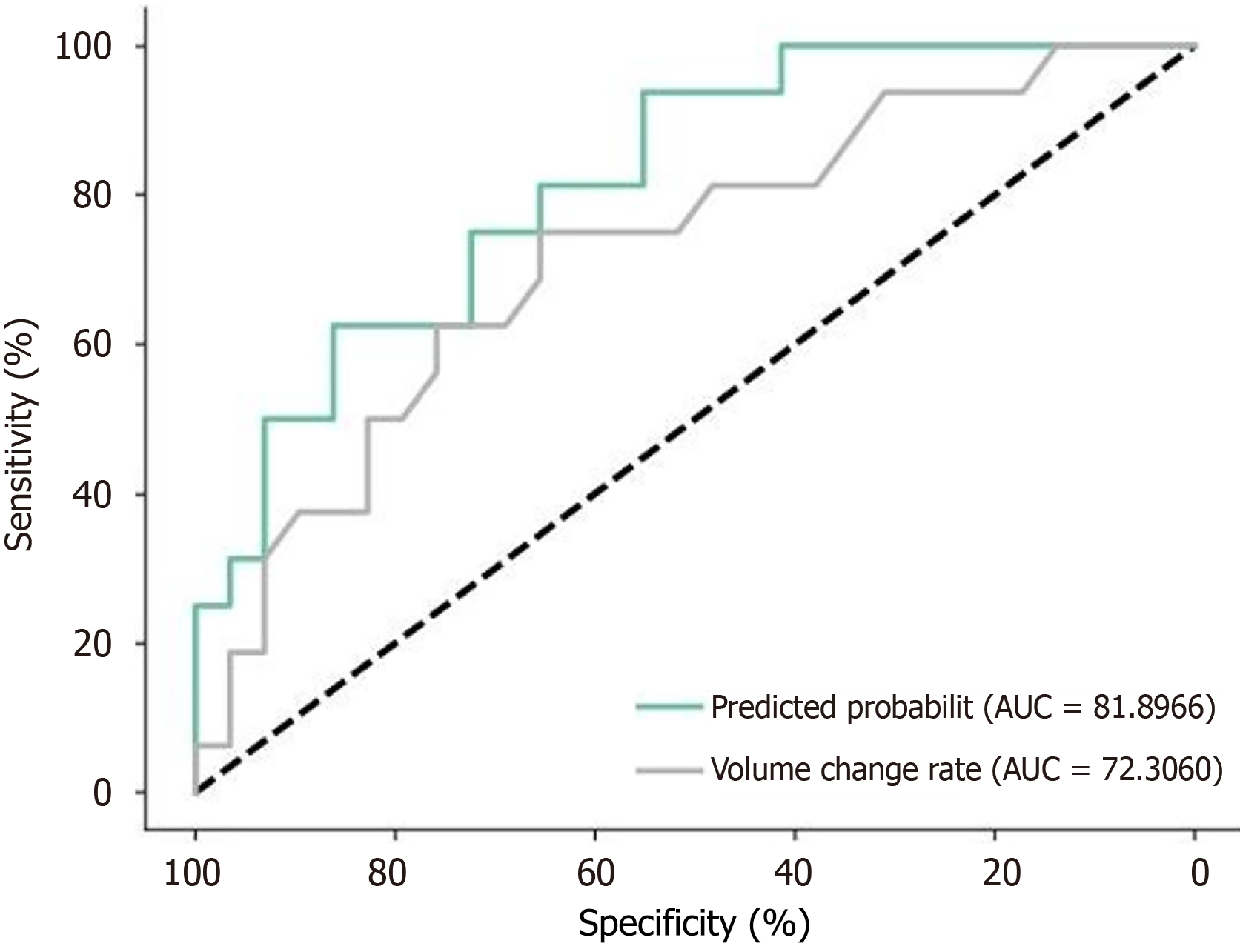
The ROC curve analysis results (Figure 2; Table 7) show: The AUC for tumor volume change rate is 0.885 (95%CI: 0.762-0.985). The optimal cutoff value calculated using the Youden index is 82.56%, with a sensitivity of 85.62% and specificity of 96.45%. The AUC for the CT visual feature model is 0.795 (95%CI: 0.685-0.912). The optimal cutoff value is 75.82%, with a sensitivity of 62.45% and specificity of 83.86%.
| AUC | 95%CI | Optimal cut-off value (%) | Sensitivity (%) | Specificity (%) | |
| Tumor volume change rate | 0.885 | 0.762-0.985 | 82.5 | 85.62 | 96.45 |
| CT subjective efficacy model | 0.795 | 0.685-0.912 | 75.82 | 62.45 | 83.86 |
Compared to the CT visual feature model, the tumor volume change rate has a higher AUC, sensitivity, and specificity, indicating superior diagnostic efficiency in predicting postoperative pathological regression. This result suggests that measuring the volume change rate before and after neoadjuvant chemotherapy in GC can more accurately assess chemotherapy efficacy than the CT visual feature binary regression model.
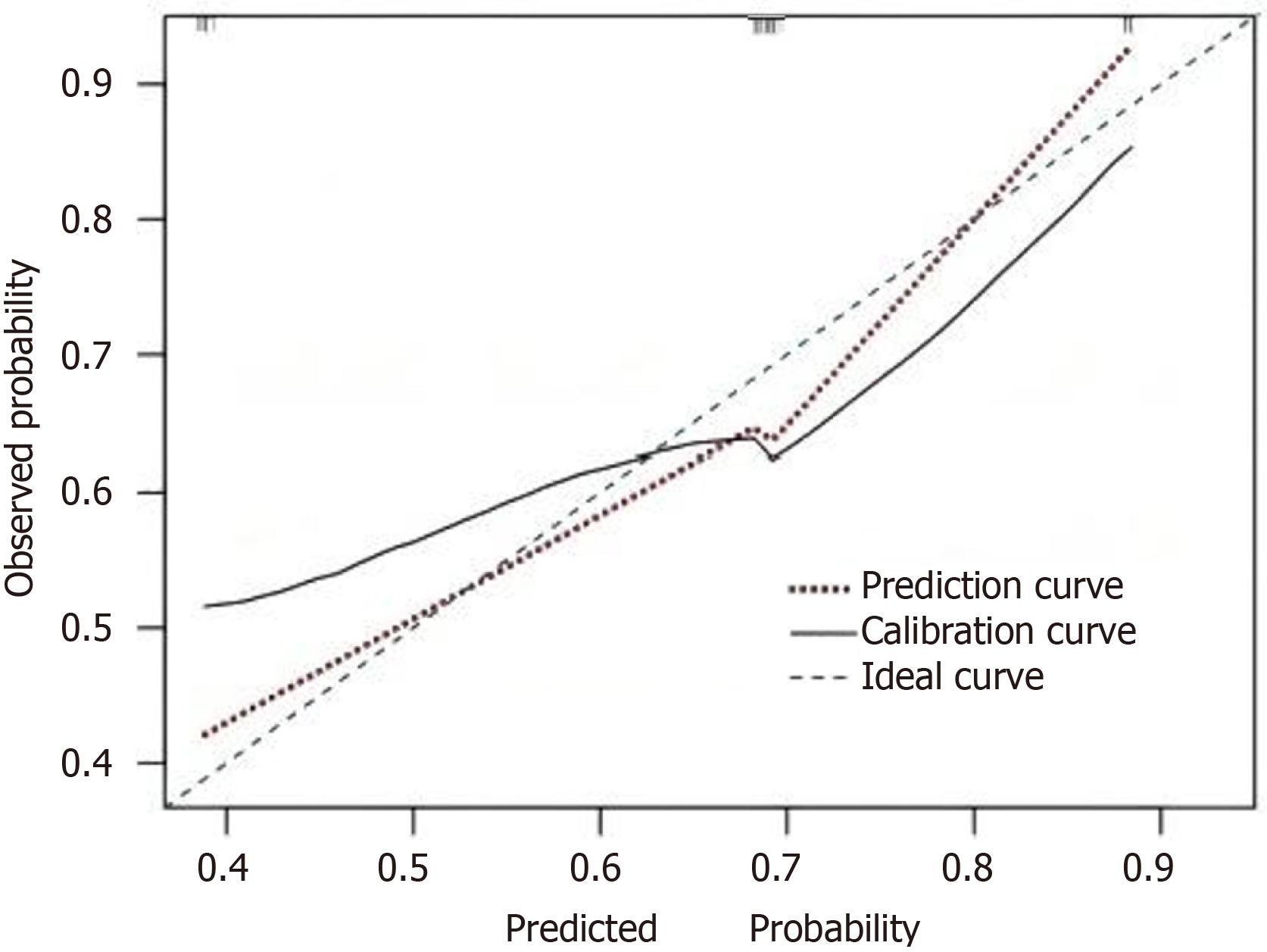
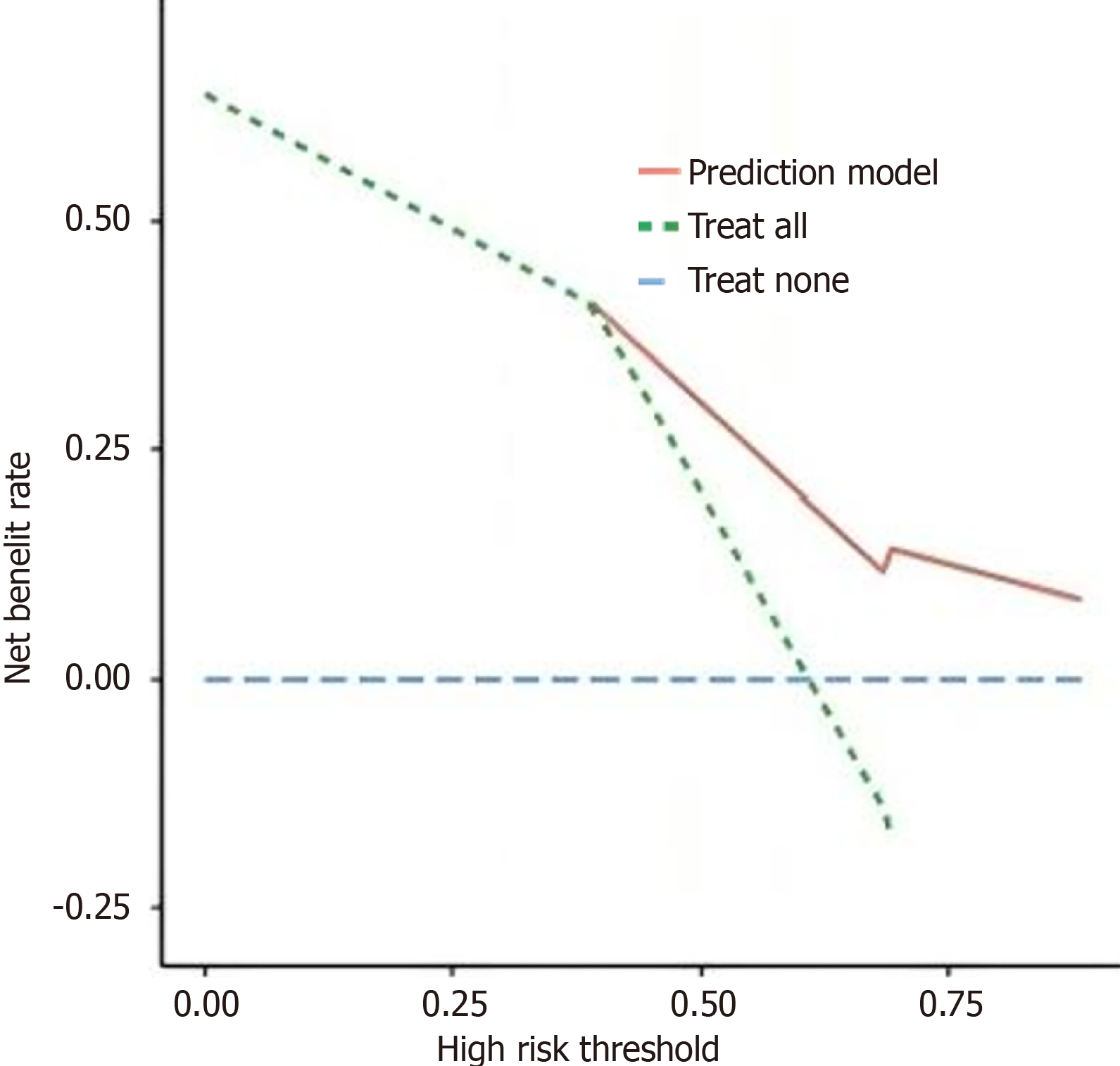
In this study, the Bootstrap method was used to randomly sample 1000 times to perform internal validation of the model, and an internal calibration plot was drawn. The results indicated that the mean absolute error between the calibration curve and the standard curve was 0.072, suggesting that the predicted results fit well with the actual results. The model has good discrimination. Based on the AUC, the predictive ability of the model was tested, with an AUC of 0.795 and a 95%CI of (0.685, 0.912), indicating that the nomogram model in this study has good predictive power. The clinical DCA method was used to assess the adaptability of the clinical model. The DCA curve showed that when the high-risk threshold was between 0.35 and 0.85, the higher the clinical net benefit rate for patients, the more patients could benefit from the model, indicating that the model has good clinical applicability (Figures 3 and 4).
This study analyzed the CT images of 97 advanced GC patients who underwent SOX regimen neoadjuvant chemo
Our findings build upon and extend previous research in several key ways. The venous phase texture analysis achieving a 7.85% misclassification rate aligns with earlier studies showing superior predictive value of venous phase imaging for tumor assessment[17]. The possible reason is that venous phase images better display the microenvironmental characteristics of tumor tissue, including tumor heterogeneity, degree of necrosis, and vascular distribution[18,19]. Additionally, by comparing four feature selection methods and classification analysis methods, the combined method of FPN (Fisher, POE + ACC and MI combined method) with NDA showed the best predictive performance. This suggests that in the process of texture feature selection, integrating multiple methods can more comprehensively reflect the changes in tumor tissue characteristics, thereby improving predictive accuracy[20,21].
Secondly, the comparative analysis of volume and thickness change rates revealed that the volume change rate is significantly higher than the thickness change rate in both the effective and ineffective chemotherapy groups. The median volume change rate in the effective chemotherapy group was 75.20%, significantly higher than the 41.75% in the ineffective group (P < 0.001). More importantly, the volume change rate showed a stronger negative correlation with postoperative pathological TRG grading (r = -0.886, P < 0.001). This finding is of significant clinical importance, indicating that volume change measured by three-dimensional reconstruction more accurately reflects the overall regression of the tumor[22,23]. Our volume change rate results (75.20% in effective group vs 41.75% in ineffective group, P < 0.001) demonstrate stronger correlation with pathological response (r = -0.886) compared to traditional one-dimensional measurements, supporting recent literature on the limitations of conventional RECIST criteria in capturing irregular tumor regression patterns[24]. Three-dimensional reconstruction technology, by assessing the lesion in all directions, can more comprehensively capture the dynamic changes in tumor volume[25].
In terms of CT intuitive sign analysis, this study used multivariate logistic regression analysis to identify two inde
In terms of diagnostic efficacy comparison, ROC curve analysis showed that the predictive performance of volume change rate (AUC = 0.885) is superior to the CT intuitive sign model (AUC = 0.795). When the optimal cut-off value for volume change rate is set at 82.56%, a high sensitivity (85.62%) and specificity (96.45%) can be achieved. This result further supports the use of volume change rate as the preferred indicator for assessing chemotherapy efficacy[28,29]. However, considering that precise three-dimensional reconstruction measurements may not be feasible in clinical practice, the CT intuitive sign model still has important supplementary value[30].
This study systematically compared the application value of various CT-based assessment methods in predicting the efficacy of GC neoadjuvant chemotherapy, providing a comprehensive assessment strategy for clinical practice. The study validated the significant advantages of volume change rate over traditional measurement methods using a large sample dataset and established a prediction model based on CT intuitive signs, confirming its clinical practical value through DCA.
However, as a single-center retrospective study, this study has the potential for selection bias. Additionally, due to the lack of an external validation dataset, the generalizability of the model needs further verification. At the same time, the process of three-dimensional reconstruction measurement is time-consuming, which may affect its promotion and application in clinical settings. On the other hand, this study did not delve into the potential impact of different numbers of chemotherapy cycles on assessment accuracy.
Based on the results, we recommend that when assessing the efficacy of GC neoadjuvant chemotherapy, priority should be given to using CT three-dimensional reconstruction to measure volume change rate, combined with CT intuitive signs for comprehensive judgment. For situations where precise volume measurement cannot be implemented, prediction and evaluation can be made based on the CT intuitive sign model established in this study.
Future studies should focus on developing automated segmentation algorithms to improve the efficiency of three-dimensional reconstruction, expanding the study sample size and conducting multi-center validation, exploring the application of artificial intelligence technology in radiomics analysis to enhance predictive accuracy, and studying the impact of different chemotherapy regimens and cycles on assessment criteria.
This study is a single-center study with a relatively limited sample size. Although the research results demonstrate that CT 3D reconstruction volume change rate has good predictive value in evaluating the efficacy of neoadjuvant chemo
In summary, this study confirms the superiority of CT three-dimensional reconstruction volume change rate in assessing the efficacy of GC neoadjuvant chemotherapy and constructs a CT intuitive sign prediction model with practical value. These research findings provide clinicians with more accurate tools for efficacy assessment, helping to optimize treatment strategies and improve patient outcomes.
| 1. | Shah R, Khaitan PG, Pandita TK, Rafiq A, Abrol D, Suri J, Kaul S, Kumar R, Sharma S. Gastric cancer in Jammu and Kashmir, India: A review of genetic perspectives. J Cancer Res Ther. 2022;18:873-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Piemonti L, Vettor L, Contro E. A Case Report of Metastatic Gastric Cancer Treated with Pembrolizumab during Pregnancy. Fetal Diagn Ther. 2024;51:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Fiflis S, Papakonstantinou M, Giakoustidis A, Christodoulidis G, Louri E, Papadopoulos VN, Giakoustidis D. Comparison between upfront surgery and neoadjuvant chemotherapy in patients with locally advanced gastric cancer: A systematic review. World J Gastrointest Surg. 2023;15:1808-1818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Fong C, Johnston E, Starling N. Neoadjuvant and Adjuvant Therapy Approaches to Gastric Cancer. Curr Treat Options Oncol. 2022;23:1247-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Li Y, Wu J, Han M, Li W, Bi Z. Modified Double-Tract Reconstruction in Gastrointestinal Reconstruction after Proximal Gastrectomy. J Coll Physicians Surg Pak. 2024;34:1374-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Zhao B, Obuchowski N, Yang H, Chou Y, Ma H, Guo P, Tang Y, Schwartz L, Sullivan D. Comparing quantitative imaging biomarker alliance volumetric CT classifications with RECIST response categories. Radiol Adv. 2025;2:umaf001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Huang S, Zhang Y, Wumener X, Lei Y, Liang Y. Case report: Dynamic (18)F-FDG PET/CT display of a bronchial mass as a second primary cancer mimicking mediastinal lymph node in a gastric carcinoma survivor. Front Oncol. 2024;14:1447843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Zhang W, Wang S, Dong Q, Chen W, Wang P, Zhu G, Chen X, Cai Y. Predictive nomogram for lymph node metastasis and survival in gastric cancer using contrast-enhanced computed tomography-based radiomics: a retrospective study. PeerJ. 2024;12:e17111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Wang G, Ding F, Chen K, Liang Z, Han P, Wang L, Cui F, Zhu Q, Cheng Z, Chen X, Huang C, Cheng H, Wang X, Zhao X. CT-based radiomics nomogram to predict proliferative hepatocellular carcinoma and explore the tumor microenvironment. J Transl Med. 2024;22:683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Zhou W, Zhou Y, Zhang X, Huang T, Zhang R, Li D, Xie X, Wang Y, Xu M. Development and Validation of an Explainable Machine Learning Model for Identification of Hyper-Functioning Parathyroid Glands from High-Frequency Ultrasonographic Images. Ultrasound Med Biol. 2024;50:1506-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Wang Y, Li Y, Chen S, Wen Z, Hu Y, Zhang H, Zhou P, Pang H. Development of a CT radiomics prognostic model for post renal tumor resection overall survival based on transformer enhanced K-means clustering. Med Phys. 2025;52:3243-3257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Zhang Z, Zhao X, Gu J, Chen X, Wang H, Zuo S, Zuo M, Wang J. Spectral CT radiomics features of the tumor and perigastric adipose tissue can predict lymph node metastasis in gastric cancer. Abdom Radiol (NY). 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Zhang R, Zheng H, Lin J, Wang J. Review of the application of dual-energy CT combined with radiomics in the diagnosis and analysis of lung cancer. J Appl Clin Med Phys. 2025;26:e70020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Oh G, Gi Y, Lee J, Kim H, Wu HG, Park JM, Choi E, Shin D, Yoon M, Lee B, Son J. Hybrid Approach to Classifying Histological Subtypes of Non-small Cell Lung Cancer (NSCLC): Combining Radiomics and Deep Learning Features from CT Images. J Imaging Inform Med. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Zhang M, Cuan J, Wang W, Guo Y, Zhao J. Effects of Yipi Huayu decoction on tumor markers, immune function, and adverse reactions during chemotherapy in gastric cancer patients: a retrospective propensity score-matched study. Am J Transl Res. 2024;16:3599-3613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 16. | Liu L, Cui WC, Sun Y, Wang H, Liang ZN, Wu W, Yan K, Ji YL, Dong L, Yang W. Classification of Neoadjuvant Therapy Response in Patients With Colorectal Liver Metastases Using Contrast-Enhanced Ultrasound-With Histological Pathology as the Gold Standard. Ultrasound Med Biol. 2025;51:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 17. | Ortega C, Anconina R, Joshi S, Metser U, Prica A, Johnson S, Liu ZA, Keshavarzi S, Veit-Haibach P. Combination of FDG PET/CT radiomics and clinical parameters for outcome prediction in patients with non-Hodgkin's lymphoma. Nucl Med Commun. 2024;45:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Czibor S, Csatlós Z, Fábián K, Piroska M, Györke T. Volumetric and textural analysis of PET/CT in patients with diffuse large B-cell lymphoma highlights the importance of novel MTVrate feature. Nucl Med Commun. 2024;45:931-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Li Z, Zhao M, Li Z, Huang YH, Chen Z, Pu Y, Zhao M, Liu X, Wang M, Wang K, Yeung MHY, Geng L, Cai J, Zhang W, Yang R, Ren G. Quantitative texture analysis using machine learning for predicting interpretable pulmonary perfusion from non-contrast computed tomography in pulmonary embolism patients. Respir Res. 2024;25:389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Virarkar M, Daoud T, Sun J, Montanarella M, Menendez-Santos M, Mahmoud H, Saleh M, Bhosale P. MRI Radiomics Data Analysis for Differentiation between Malignant Mixed Müllerian Tumors and Endometrial Carcinoma. Cancers (Basel). 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Leonhardi J, Sabanov A, Höhn AK, Sucher R, Seehofer D, Mehdorn M, Schnarkowski B, Ebel S, Denecke T, Meyer HJ. CT Texture Analysis of Perihilar Cholangiocarcinoma-Associations With Tumor Grading, Tumor Markers and Clinical Outcome. Cancer Rep (Hoboken). 2024;7:e2132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Saccomano G, Pinamonti M, Longo E, Marcuzzo T, Tromba G, Dreossi D, Brun F. The potential of x-ray virtual histology in the diagnosis of skin tumors. Skin Res Technol. 2024;30:e13801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Dupré A, Buiron C, De Crignis L, Bouroche G, Coutzac C, Mouton N. Laparoscopic Extended Segmentectomy VIII Guided by Three-Dimensional Reconstruction and Hepatic Veins with a Cranio-Caudal Approach. Ann Surg Oncol. 2024;31:6567-6568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Lai MY, Kang SY, Sun YT, Quan TT, Lu SX, He CY, Zhou ZW, Yang LQ, Luo HY, Wang FH, Li YH, Xu RH, Guan WL, Qiu MZ. Comparison of response evaluation criteria in solid tumors and tumor regression grade in evaluating the effect of preoperative systemic therapy of gastric cancer. BMC Cancer. 2022;22:1031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 25. | Xin S, Chen J, Dongming L, Wei X, Yiran H. Application of three-dimensional reconstruction of renal tumor vessels to guide laparoscopic partial nephrectomy of hilar tumors and non-hilar tumors under zero ischemia. Asian J Surg. 2024;47:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Li J, Li Z, Wang Y, Li Y, Zhang J, Li Z, Tang L. CT radiomics-based intratumoral and intertumoral heterogeneity indicators for prognosis prediction in gastric cancer patients receiving neoadjuvant chemotherapy. Eur Radiol. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Lim CY, Cha DI, Jeong WK, Cho YY, Hong S, Hong S, Kim K, Kim JH. Prediction of microsatellite-stable/epithelial-to-mesenchymal transition molecular subtype gastric cancer using CT radiomics and clinicopathologic factors. Eur J Radiol. 2025;185:111990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | He H, Wang P, Zhou H, Wei W, Lin J, Chen Y, Wang F, Liu S. The advantages of preoperative 3D reconstruction over 2D-CT in thoracoscopic segmentectomy. Updates Surg. 2024;76:2875-2883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Guo D, Zhu XY, Han S, Liu YS, Cui DP. Evaluating the use of three-dimensional reconstruction visualization technology for precise laparoscopic resection in gastroesophageal junction cancer. World J Gastrointest Surg. 2024;16:1311-1319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Prior O, Macarro C, Navarro V, Monreal C, Ligero M, Garcia-Ruiz A, Serna G, Simonetti S, Braña I, Vieito M, Escobar M, Capdevila J, Byrne AT, Dienstmann R, Toledo R, Nuciforo P, Garralda E, Grussu F, Bernatowicz K, Perez-Lopez R. Identification of Precise 3D CT Radiomics for Habitat Computation by Machine Learning in Cancer. Radiol Artif Intell. 2024;6:e230118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (5)] |