Published online Nov 15, 2017. doi: 10.4239/wjd.v8.i11.475
Peer-review started: May 19, 2017
First decision: July 20, 2017
Revised: August 8, 2017
Accepted: October 16, 2017
Article in press: October 17, 2017
Published online: November 15, 2017
Processing time: 186 Days and 1.4 Hours
To identify reproductive disturbances among adolescent girls and young women with type 1 diabetes mellitus (T1DM) in Saudi Arabia.
This cross sectional study was conducted among 102 female with T1DM, (aged 13-29 years) who attended the Diabetes Clinic at Diabetes Treatment Center, Prince Sultan Military Medical City, Saudi Arabia between April 2015 to March 2016. Clinical history, anthropometric characteristics and reproductive disturbance were collected through a questionnaire.
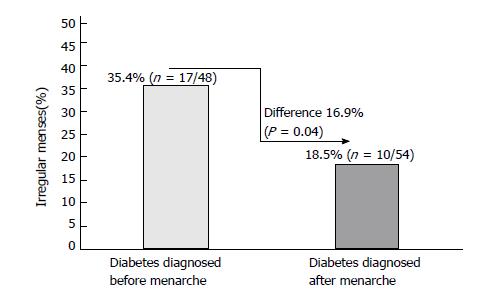
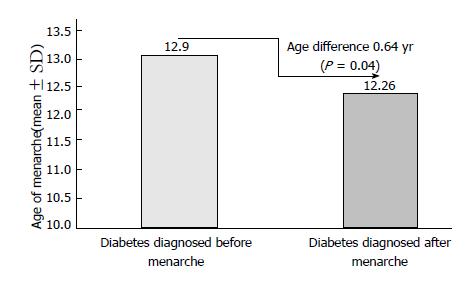
Of 102 patients included in this analysis, 26.5% (27/102) were reported that they experienced an irregular menses. Of these patients, when compared to whose diabetes was diagnosed before menarche (35.4%, 17/48), patients diagnosed with diabetes after menarche (18.5%, 10/54) showed significantly less irregular menses (difference 16.9%, P = 0.04). Similarly, compared to patients diagnosed with diabetes prior to menarche (mean age 12.9 years; n = 48), patients diagnosed with diabetes after menarche (mean age 12.26 years; n = 54) were found to have 0.64 years delay in the age of menarche (P = 0.04). Among the studied patients, 15.7% (16/102) had polycystic ovary syndrome (PCOS). Of these PCOS patients, 37.5% (6/16) had irregular menses, 6.3% (1/16) had Celiac disease, 37.5% (6/16) had Hashimoto thyroiditis and 18.7% (3/16) had acne.
More than one fourth of the study population with T1DM experiencing an irregular menses. Adolescent girls and young women diagnosed with diabetes prior to menarche showed higher menstrual irregularity and a delay in the age of menarche.
Core tip: The present study found more than one fourth of the adolescent girls and young women with type 1 diabetes experiencing an irregular menses. Adolescent girls and young women diagnosed with diabetes prior to menarche reported 16.9% higher menstrual irregularity and 0.64 years delay in the age of menarche.
- Citation: Braham R, Robert AA, Musallam MA, Alanazi A, Swedan NB, Al Dawish MA. Reproductive disturbances among Saudi adolescent girls and young women with type 1 diabetes mellitus. World J Diabetes 2017; 8(11): 475-483
- URL: https://www.wjgnet.com/1948-9358/full/v8/i11/475.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i11.475
The last few decades have shown a trend of a steady increase in the incidence of type 1 diabetes mellitus (T1DM) patients in most parts of the world[1-3]. Research has shown a rise in the incidence of T1DM in Saudi Arabia in the preceding 30 years as well as in the prevalence of T1DM among the children and adolescents in the Kingdom to be 109.5 per 100000, a figure higher than that of several advanced countries[4-6].
T1DM is a chronic autoimmune disease that represents a multi-faceted challenge to normal reproductive function throughout life. Despite the improvements in diabetes therapy, adolescent girls and young women with T1DM still face frequent disturbances in the reproductive system including infertility, delayed onset of puberty and menarche, menstrual irregularities (especially oligomenorrhoea) and premature ovarian failure (POF)[7-9]. Such females show a tendency, on average, to attain menarche a little later in life than non-diabetic women[10]. At the other extreme, diabetic women tend to experience menopause marginally sooner[8,11]. Furthermore, women with T1DM exhibit a high degree of hyperandrogenic disorders, like polycystic ovary syndrome (PCOS) and hirsutism[12]. Despite the previous, PCOS is commonly linked with conditions driven by insulin resistance, such as type 2 diabetes mellitus (T2DM)[13]. However, several studies demonstrated that T1DM patients may also experience insulin resistance, especially in obese individuals, and even PCOS[12,14].
Compared to studies performed in the developed countries, limited literature are available in Saudi Arabia on reproductive disturbances among adolescent girls and young woman due to the lack of appropriate studies performed in these specific aspects. Therefore, the current work was done as a cross-sectional study to identify the reproductive disturbances among adolescent girls and young women with T1DM in a tertiary care center, specifically attempting to isolate the factors that can cause such abnormalities.
This cross sectional study was conducted among 102 female with T1DM (aged 13-29 years) who attended the Diabetes Clinic at Diabetes Treatment Center, Prince Sultan Military Medical City (PSMMC), Saudi Arabia between April 2015 to March 2016. The PSMMC is a 1200-bed, tertiary medical center in Riyadh, Saudi Arabia, with almost 40000 annual admissions (950000 active patients files) per year from different region of the country.
The participants were conveniently selected according to their availability during their routine visit to the outpatient clinics. All patients provided written informed consent to participate the study, for adolescent patients consent form were collected from their parents/legal guardians.
Adolescent patients with T1DM and young women aged 13-29 years and Saudi nationals were included in the study, while patients with T2DM, double diabetes (expressing features resulting from both type 1 diabetes and type 2 diabetes), maturity onset diabetes of young (MODY), pregnant and patients using oral contraceptive pills were excluded. Also, patients with the other conditions with similar phenotypical characteristics to those associated with PCOS such as Hyperprolactinaemia states, Cushing’s syndrome, Acromegaly, Congenital adrenal hyperplasia, thyroid disorders, adrenal tumors (adrenal carcinoma, adrenal adenoma), ovarian tumors, androblastomas (Sertoli–Leydig cell tumors), granulosa cell tumors, Sertoli cell tumors, Hilus cell tumors, primitive neuro-ectodermal tumors (PNET) and HAIR-AN syndrome were excluded.
Anthropometric characteristics and a detailed clinical history were obtained through a questionnaire. A complete physical examination was performed for all patients. Body mass index (BMI) was calculated by dividing the weight in kilograms by the square of height in meters (BMI; kg/m2) and BMI z score (adjusted for child age and gender). The z score (or SD score) was calculated as per the formula (Xi-Mx)/SD, where Xi is the actual measurement, Mx is the mean value for that age and gender, and SD is the standard deviation corresponding to that age and gender [15].
History of recurrent diabetic ketoacidosis (RDKA) (defined as three or more episodes occurring within a period of four years as visiting the accident and emergency room or admitted in hospital), dyslipidemia, Hashimoto thyroiditis, Celiac disease and premature ovarian failure (POF) were also collected[16]. All patients were also screened for diabetic complications (neuropathy and retinopathy and nephropathy) if the duration of their diabetes 5 years or more.
Menstrual disturbances were recorded based on verbal information provided by the patients. Oligomenorrhea was defined by 3 or more cycles with a length of more than 36 d in the previous year, and amenorrhea was defined by lack of vaginal bleeding for the last 3 mo. Hirsutism was defined as excess terminal (thick pigmented) body hair in an androgen-dependent pattern, and which is commonly noted on the upper lip, chin, periareolar area, in the midsternum, and along the linea alba of the lower abdomen. It was estimated according to the modified Ferriman-Gallwey scale score of 8 or more, which was determined by a single experienced observer[17]. The presence of acne was also evaluated. The puberty was appreciated according to the Tanner Stage[18]. The POF was defined as the development of irregular menses or amenorrhea before the age of 40 years in association with follicle-stimulating hormone (FSH: IU/L) concentrations in the postmenopausal range (as defined by the measuring laboratory). Polycystic ovaries were diagnosed by pelvic or intravaginal sonography according to the Rotterdam consensus criteria. PCOS was defined by the presence of two out of three of the following criteria: (1) Oligo- and/or anovulation (irregular menses or amenorrhea); (2) Clinical and/or biochemical signs of hyperandrogenism; and (3) Polycystic ovaries (by pelvic ultrasound, include the presence of 12 or more follicles in each ovary measuring 2 to 9 mm in diameter and/or increased ovarian volume (>10 mL; calculated using the formula 0.5 × length × width ×thickness). One ovary fitting this definition is sufficient to define polycystic ovarian morphology (PCOM) and exclusion of other etiologies[19].
The glycosylated haemoglobin A1c (HbA1c) testing was performed in our laboratory using a method that is The National Glycohaemoglobin Standardization Program (NGSP), United States certified and standardized to the Diabetes Control and Complications Trial (DCCT) assay[20]. Total testosterone level was done for all the patients (normal level 0.69 to 2.1 nmol/L). Plasma testosterone was analyzed using Elecsys Testosterone II from Roche company where the electrochemiluminescence immunoassay “ECLIA” was intended for use on Elecsys and cobas e immunoassay analyzers. Regarding the testosterone level, 0.69 to 2.1 nmol/L considered as normal and testosterone > 2.1 mmol/L considered as high testosterone level.
In patients with irregular menses and no clinical or biochemical features of hyperandrogenism, we performed pelvic ultrasound to study the morphology of the ovaries. Pelvic ultrasound was also performed in patients with amenorrhea.
Data analysis was carried out using Microsoft Excel 2010 (Microsoft Corporation, Seattle, WA, United States) and Statistical Package for Social Sciences version 22 (SPSS Inc., Chicago, IL, United States). In addition to the descriptive analysis, χ2 test (for categorical variables) and independent t test (for continuous variables) were also performed to identify variables associated with reproductive disturbances before and after diagnosis menarche and patients with PCOS and without PCOS. Continuous variables are represented as mean values ± SD, while categorical variables are expressed as frequencies and percentages. A P-value of < 0.05 was considered as statistically significant.
The overall mean of the clinical parameters of the study population were as follows: Age 18.26 ± 4.05 (range 13-29 years), age at diagnosis of diabetes 11.5 ± 3.96 (range 1-21 years), duration of T1DM 6.8 ± 5.35 years (range 1-28 years), age at menarche 12.56 ± 0.96 (range 9-16 years), BMI 23.54 ± 3.3 kg/m2 (range 17.8-34.4) and HbA1c was 9.23 ± 1.92 (range 6-16).
Clinical and anthropometric characteristics of the study population are shown in Table 1. Among the studied population, 41.2% (42/102) possess family history of DM; 15.7% (16/102) had PCOS; 1% (1/102) had POF; and 2.95% (3/102) had amenorrhea. The study also found that 26.5% (27/102) of patients had irregular menses; 18.6% (19/102) had acne; 6.9% (7/102) had Celiac disease; 33.3% (34/102) had Hashimoto thyroiditis; 13.7% (14/102) had dyslipidemia, 24.5% (25/102) had hirsutism;12.7% (13/102) had RDKA and 11.8% (12/102) had diabetes complication (neuropathy and retinopathy and none of our patients had nephropathy).
| Variables | Frequency (n = 102) | % |
| Age (yr) | ||
| 13-19 | 71 (mean age 15.9 ± 2.72) | 69.6 |
| 20-29 | 31 (mean age 23.5 ± 2.41) | 30.4 |
| Family History of diabetes | ||
| No | 60 | 58.8 |
| Yes | 42 | 41.2 |
| Polycystic ovarian syndrome | ||
| No | 86 | 84.3 |
| Yes | 16 | 15.7 |
| Recurrent diabetic ketoacidosis | ||
| No | 89 | 87.3 |
| Yes | 13 | 12.7 |
| Complications of diabetes | ||
| No | 90 | 88.2 |
| Yes | 12 | 11.8 |
| Age of diagnosis (yr) | ||
| 1-10 | 34 (mean 7.1 ± 2.85) | 33.3 |
| 11-21 | 68 (mean 13.8 ± 1.99) | 66.7 |
| Age of menarche (yr) | ||
| 9-11 | 5 | 4.9 |
| 12 | 50 | 49 |
| 13-16 | 47 | 46.1 |
| Menses | ||
| Irregular | 27 | 26.5 |
| Regular | 75 | 73.5 |
| Premature ovarian failure | ||
| No | 101 | 99 |
| Yes | 1 | 1 |
| Celiac disease | ||
| No | 95 | 93.1 |
| Yes | 7 | 6.9 |
| Hashimoto thyroiditis | ||
| No | 68 | 66.7 |
| Yes | 34 | 33.3 |
| Dyslipidemia | ||
| No | 88 | 86.3 |
| Yes | 14 | 13.7 |
| Hirsutism | ||
| No | 77 | 75.5 |
| Yes | 25 | 24.5 |
| Acne | ||
| No | 83 | 81.4 |
| Yes | 19 | 18.6 |
| Pelvic ultrasound | ||
| Not done | 67 | 66.7 |
| Small ovaries | 3 | 2.9 |
| Normal | 11 | 10.8 |
| PCOS | 21 | 20.6 |
| Testosterone level | ||
| Normal | 77 | 75.5 |
| High | 25 | 24.5 |
| Hemoglobin A1C | ||
| ≤ 7% | 13 (mean 6.67 ± 0.57) | 12.7 |
| > 7% | 89 (mean 9.57 ± 1.78) | 87.3 |
Majority of the study population are in teen age group 13-19 (69.6%, 71/102; mean = 15.9). Among the 71 teenagers, 2.8% (2/71) underweight, 26.8% (19/71) overweight, 2.8% (2/71) obese and 67.6% (48/71) were normal. The mean age of the young women (20-29 years, n = 31) was 23.5 years. Among the young women, 3.2% (1/31) underweight, 48.4% (15/31) overweight, 3.2% (1/31) obese and 45.2% (14/31) were normal.
The factors associated with menarche before and after diagnosis of diabetes are shown in Table 2. A total of 47.1% (48/102) patients were diagnosed as having diabetes before menarche and 52.9% (54/102) were diagnosed as having diabetes after menarche. Age, duration of DM and acne showed statistically significant differences among the two groups. Similarly, when compared with those identified with diabetes before menarche (9.73%), the girls diagnosed with diabetes post menarche (8.78%) reported lower HbA1c levels (P = 0.007).
| Variables | Diabetes diagnosed before menarche (n = 48), n (%) | Diabetes diagnosed after menarche (n = 54), n (%) | P value |
| Age (yr) | |||
| 13-19 (n = 71) | 38 (53.5) | 33 (46.5) | 0.038 |
| 20-29 (n = 31) | 10 (32.3) | 21 (67.7) | |
| Family history of diabetes | |||
| No (n = 60) | 28 (46.7) | 32 (53.3) | 0.542 |
| Yes (n = 42) | 20 (47.6) | 22 (52.4) | |
| Polycystic ovarian syndrome | |||
| No (n = 86) | 42 (48.8) | 44 (51.2) | 0.289 |
| Yes (n = 16) | 6 (37.5) | 10 (62.5) | |
| Recurrent diabetic ketoacidosis | |||
| No (n = 89) | 42 (47.2) | 47 (52.8) | 0.591 |
| Yes (n = 13) | 6 (46.2) | 7 (53.8) | |
| Complications of diabetes | |||
| No (n = 90) | 41(45.6) | 49 (54.4) | 0.299 |
| Yes (n = 12) | 7 (58.3) | 5 (41.7) | |
| Menses | |||
| Irregular (n = 27) | 17 (63) | 10 (37) | 0.04 |
| Regular (n = 75) | 31 (41.3) | 44 (58.7) | |
| Premature ovarian failure | |||
| No (n = 101) | 48 (47.5) | 53 (52.5) | |
| Yes (n = 1) | 0 | 1 (100) | 0.529 |
| Celiac disease | |||
| No (n = 95) | 44 (46.3) | 51(53.7) | 0.434 |
| Yes (n = 7) | 4 (57.1) | 3 (42.9) | |
| Hashimoto thyroiditis | |||
| No (n = 68) | 31 (45.6) | 37 (54.4) | 0.416 |
| Yes (n = 34) | 17 (50) | 17 (50) | |
| Dyslipidemia | |||
| No (n = 88) | 42 (47.7) | 46 (52.3) | 0.482 |
| Yes (n = 14) | 6 (42.9) | 8 (57.1) | |
| Hirsutism | |||
| No (n = 77) | 37 (48.1) | 40 (51.9) | 0.452 |
| Yes (n = 25) | 11 (44) | 14 (56) | |
| Acne | |||
| No (n = 83) | 45 (54.2) | 38 (45.8) | 0.002 |
| Yes (n = 19) | 3 (15.8) | 16 (84.2) | |
| Pelvic ultrasound | |||
| Not done (n = 67) | 31 (46.3) | 36 (53.7) | 0.103 |
| Small ovaries (n = 3) | 3 (100) | 0 | |
| Normal (n = 11) | 7 (63.6) | 4 (36.4) | |
| Polycystic ovary syndrome (n = 21) | 7 (33.3) | 14 (66.7) | |
| Testosterone level | |||
| Normal (n = 77) | 40 (51.9) | 37 (48.1) | 0.065 |
| High (n = 25) | 8 (32) | 17 (68) | |
| Variables | mean ± SD | mean ± SD | P value |
| Body mass index | 23.83 ± 0.5 | 23.28 ± 0.3 | 0.123 |
| Duration of diabetes | 9.13 ± 0.8 | 4.74 ± 0.5 | 0.011 |
| Age at diabetes diagnosis | 8.42 ± 0.4 | 14.3 ± 0.2 | 0 |
| Insulin dose unit/kg | 0.84 ± 0.23 | 0.88 ± 0.23 | 0.794 |
| Hemoglobin A1c | 9.73 ± 0.3 | 8.78 ± 0.2 | 0.007 |
| Body mass index | 23.83 ± 0.5 | 23.28 ± 0.3 | 0.123 |
Figure 1 shows the percentage differences of irregular menses among patients diagnosed with diabetes before and after menarche. Compared to whose diabetes was diagnosed before menarche 35.4% (17/48), patients diagnosed with diabetes after menarche 18.5% (10/54) showed less irregular menses (differences 16.9%, P = 0.04). Similarly, compared with patients diagnosed with diabetes prior to menarche (mean age 12.9 years), patients diagnosed with diabetes post menarche (mean age 12.26 years) were found to have 0.64 years less in the age of menarche (P = 0.04) (Figure 2).
Results displayed as patients with PCOS (15.7%; 16/102) and without PCOS (84.3%; 86/102) are shown in Table 3. Out of 71 teenagers and out of 31 young women 15.5% (11/71) and 16.1% (5/31) had PCOS respectively. Of these PCOS patients, 37.5% (6/16) had irregular menses, 6.3% (1/16) had Celiac disease, 37.5% (6/16) had Hashimoto thyroiditis and 18.7% (3/16) had acne. There were no significant differences were observed among patients with PCOS and without PCOS except the variables family history (P = 0.001) and BMI (P = 0.008).
| Variables | No PCOS (n = 86) | PCOS (n = 16) | P value |
| Age (yr) | |||
| 13-19 yr (n = 71) | 60 (84.5) | 11 (15.5) | 0.574 |
| 20-29 yr (n = 31) | 26 (83.9) | 5 (16.1) | |
| Family History of diabetes | |||
| No (n = 60) | 57 (95) | 3 (5) | 0.001 |
| Yes (n = 71) | 29 (69) | 13 (31) | |
| Recurrent diabetic ketoacidosis | |||
| No (n = 89) | 75 (84.3) | 14 (15.7) | 0.43 |
| Yes (n = 13) | 11 (84.6) | 2 (15.4) | |
| Complications of diabetes | |||
| No (n = 90) | 79 (87.8) | 11 (12.2) | 0.092 |
| Yes (n = 12) | 7 (58.3) | 5 (41.7) | |
| Menses | |||
| Irregular (n = 27) | 21 (78) | 6 (16.7) | 0.214 |
| Regular (n = 75) | 65 (87) | 10 (15.4) | |
| Premature ovarian failure | |||
| No (n = 101) | 85 (84.2) | 16 (15.8) | 0.843 |
| Yes (n = 1) | 1 (100) | 0 | |
| Celiac disease | |||
| No (n = 95) | 80 (84.2) | 15 (15.8) | 0.698 |
| Yes (n = 7) | 6 (85.7) | 1 (14.3) | |
| Hashimoto thyroiditis | |||
| No (n = 68) | 58 (85.3) | 10 (14.7) | 0.453 |
| Yes (n = 34) | 28 (82.4) | 6 (17.6) | |
| Dyslipidemia | |||
| No (n = 88) | 76 (86.4) | 12 (13.6) | 0.15 |
| Yes (n = 14) | 10 (71.4) | 4 (28.6) | |
| Hirsutism | |||
| No (n = 77) | 62 (80.5) | 15 (19.5) | 0.054 |
| Yes (n = 25) | 24 (96) | 1 (4) | |
| Acne | |||
| No (n = 83) | 70 (84.3) | 13 (15.7) | 0.612 |
| Yes (n = 19) | 16 (84.2) | 3 (15.8) | |
| Pelvic ultrasound | |||
| Not done (n = 67) | 54 (80.6) | 13 (19.4) | 0.317 |
| Small ovaries (n = 3) | 2 (66.7) | 1 (33.3) | |
| Normal (n = 11) | 10 (91) | 1 (9) | |
| PCOS (n = 21) | 20 (95.2) | 1 (4.8) | |
| Testosterone level | |||
| Normal (n = 77) | 62 (80.5) | 15 (19.5) | 0.054 |
| High (n = 25) | 24 (96) | 1 (4) | |
| Variables | mean ± SD | mean ± SD | P value |
| Body mass index | 23.2 ± 2.86 | 25.3 ± 4.77 | 0.008 |
| Duration of diabetes | 6.59 ± 5.16 | 7.94 ± 6.45 | 0.53 |
| Age at diabetes diagnosis | 11.6 ± 3.82 | 10.8 ± 4.69 | 0.227 |
| Age of menarche | 12.5 ± 1 | 12.63 ± 0.69 | 0.118 |
| Insulin dose unit/kg | 0.8 ± 0.24 | 0.83 ± 0.20 | 0.493 |
| Hemoglobin A1c | 9.23 ± 1.98 | 9.19 ± 1.64 | 0.516 |
Reproductive disturbance has long been recognized as a prevalent problem among girls and young women with T1DM[21]. In the present study, we found that approximately one fourth (26.5%) of the study population experiencing an irregular menses. An earlier study reported that approximately one third of young women with T1DM suffer some kind of menstrual dysfunction[21]. However, other studies reported that the rates of menstrual irregularity are 50% higher among patients with T1DM than those without T1DM[10,22]. Furthermore, it is well demonstrated that T1DM decreased physical, psychological well-being (depression and diabetes-related distress) and decreased the quality of life of adolescent girls and young women[23]. Such disturbances combined with other dysfunctions that could influence the reproductive system, are highly significant for type 1 diabetic adolescent girls and young women; in particular, those diagnosed prior to puberty often have peripubertal disturbances[25-27].
A growing number of studies have investigated that globally, a secular trend of younger age at menarche has been well recognized[28-30]. The mean age of menarche varies from about 12.5 years in the United States to 12.72 in Canada, 12.9 in the United Kingdom and 13.06 years in Iceland[28-30]. A study done on girls in Istanbul, Turkey, identified the median age at menarche to be 12.74 years[31]. In Saudi Arabia, the age of menarche shows considerable declining patterns. In the past decade, a study reported that the mean age at menarche among Saudis was 13.05[32]. However, more recent data from a study in 2015 indicated that the mean age of menarche for Saudi school going girls was 11.5 ± 1.48 years[33]. The present study revealed the mean age of menarche was 12.56 ± 0.96 among adolescent girls and young women with T1DM, which is higher than previous study findings (11.5 ± 1.48 years) among the normal school going girls in Saudi Arabia[33].
Similar to others, this study also identified delayed menarche among girls with T1DM[34,35]. The results from this present study demonstrated, that when those diagnosed with diabetes prior to menarche (12.26 years), experienced a delay (0.64 years) in the mean age of onset of menstruation compared with girls diagnosed after menarche. Other studies reported a delay of about one year in girls with T1DM at the age of menarche if diabetes mellitus onset was before puberty[25-27]. Kjaer, similar to the earlier findings of Bergqvist and Burkart, reported that should diabetes develop during childhood, menarche is often delayed[25]. However, age of menarche is comparable to that of the non-diabetic controls if diabetes mellitus onset occurred post puberty. This implies that menarche is not influenced by a genetic predisposition to diabetes but is possibly affected by the presence of clinical diabetes and diabetic metabolic disturbances, specifically the high HbA1c levels and duration of diabetes[26,27]. In the present study, when compared to patients who diagnosed as having diabetes after menarche (9.73 ±0.3), patients who diagnosed as having diabetes before menarche (7.8 ± 0.2) had significantly lower HbA1c. Despite diagnostic and therapeutic advances of recent decades, delay at menarche and high prevalence of menstrual irregularity is still detected among adolescent females with T1DM[10,36]. The present study shows, that compared with those diagnosed with diabetes before menarche (35.4%), the girls diagnosed with diabetes after menarche (18.5%) experience less menstrual disturbance. Similarly, when compared with those identified with diabetes before menarche (9.73%), the girls diagnosed with diabetes post menarche (8.78%) had lower HbA1c levels. Studies also indicated that age at diabetes diagnosis and the HbA1c level were the risk factors for patients diagnosed prior menarche, which later in life resulted in the impairment of crucial life processes, including disturbances in menstruation and fertility, sexual and urinary tract dysfunctions[37]. An epidemiological study investigating menarche and menstrual disturbances, demonstrated menstrual dysfunction in 21.6% of women with T1DM compared with 10.8% in the nondiabetic controls, in 245 insulin-treated diabetic women and 253 healthy women. Therefore, it was evident that menstrual dysfunction occurred nearly twice as often in women with T1DM compared with the non-diabetic controls[26].
The fact that PCOS, which occurs quite frequently in women in the reproductive age, is related to reproductive and metabolic dysfunctions[38]. One of the main characteristics of PCOS[39,40] is obesity, ranging from 12.5%[41] to 100%[42], with a total estimated prevalence of 49%[43], as reported in a recent meta-analysis[44]. Obesity may exacerbate the metabolic and reproductive disorders linked to this syndrome[45], like insulin resistance, dyslipidemia, and metabolic syndrome[38]. The meta-analysis revealed that women with PCOS exhibit higher levels of triglycerides (TG), LDL-cholesterol and total cholesterol (TC), and lower levels of HDL-cholesterol when compared with the controls, irrespective of BMI[46]. This present study showed a significant difference in the BMI between those with PCOS (mean BMI 25.3) and those without PCOS (mean BMI 23.2). Similarly, a family history of DM also revealed a significant difference between those with PCOS and those without the condition, a fact supported by earlier studies which recorded significantly increased numbers of women with a positive family history of diabetes among those with PCOS[47].
This study has few limitations, mainly, it was performed in a single center examining only a specified number of risk factors, there was no control group with which to compare the study population, patients were taking oral contraceptive pills were excluded which can lead to underestimation of a reproductive disturbances proportion of female with T1DM especially PCOS and finally the details regarding the pelvic ultrasound report that in most cases does not include the measurement of the ovaries but defined only as small or normal. More research is necessary to address the limitation identified in this work. However, this study presents pertinent information on reproductive disturbance among Saudi adolescent girls and young women with T1DM.
In conclusion, more than one fourth of the study population with T1DM experiencing an irregular menses. Adolescent girls and young women diagnosed with diabetes prior to menarche reported higher menstrual irregularity and a delay in the age of menarche. More studies are required to confirm these findings among T1DM patients from different ethnic backgrounds.
Many studies have reported frequent disturbances in the reproductive system including infertility, delayed onset of menarche, menstrual irregularities and premature ovarian failure, in adolescent girls and young women with type 1 diabetes. Such females show a tendency, on average, to attain menarche a little later in life than non-diabetic women. Further, studies have found that there is a delay in the age of menarche and higher menstrual irregularity among type 1 diabetes if the onset of diabetes occurs before or near the onset of menarche and that this delay increases with poor glycemic control.
Reproductive disturbance has long been recognized as a prevalent problem among girls and young women with type 1 diabetes mellitus (T1DM). However, compared to studies performed in the developed countries, limited literatures are available in Saudi Arabia on reproductive disturbances among adolescent girls and young woman due to the lack of appropriate studies performed in these specific aspects.
The authors found more than one fourth of the adolescent girls and young women with type 1 diabetes experiencing an irregular menses. Adolescent girls and young women diagnosed with diabetes prior to menarche reported 16.9% higher menstrual irregularity and 0.64 years delay in the age of menarche.
A better understanding of the nature, evolution and underlying mechanisms of the reproductive disturbances will help to develop the improved diagnostic and therapeutic strategies for an imperative set of co-morbidities disturbing the adolescent girls and young women with type 1 diabetes.
This is a well-written manuscript on an interesting and important clinical issue namely the frequency of irregular menstruation and amenorrhea in young women and teenagers with type 1 diabetes.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Saudi Arabia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fatima SS, Gómez-Sáez JM, Hssan MMA, Vestergaard ET S- Editor: Kong JX L- Editor: A E- Editor: Zhao LM
| 1. | Daneman D. Type 1 diabetes. Lancet. 2006;367:847-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 596] [Article Influence: 31.4] [Reference Citation Analysis (1)] |
| 2. | Peczyńska J, Peczyńska J, Jamiołkowska M, Polkowska A, Zasim A, Łuczyński W, Głowińska-Olszewska B, Bossowski A. [Epidemiology of diabetes type 1 in children aged 0-14 in Podlasie Province in years 2005-2012]. Pediatr Endocrinol Diabetes Metab. 2016;22:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Brazeau AS, Nakhla M, Wright M, Panagiotopoulos C, Pacaud D, Henderson M, Rahme E, Da Costa D, Dasgupta K. Stigma and Its Impact on Glucose Control Among Youth With Diabetes: Protocol for a Canada-Wide Study. JMIR Res Protoc. 2016;5:e242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Al-Hayek AA, Robert AA, Abbas HM, Itani MB, Al-Saeed AH, Juhani AE, Al-Goudah HS, Al-Sabaan FS. Assessment of health-related quality of life among adolescents with type 1 diabetes mellitus in Saudi Arabia. Saudi Med J. 2014;35:712-717. [PubMed] |
| 5. | Al-Herbish AS, El-Mouzan MI, Al-Salloum AA, Al-Qurachi MM, Al-Omar AA. Prevalence of type 1 diabetes mellitus in Saudi Arabian children and adolescents. Saudi Med J. 2008;29:1285-1288. [PubMed] |
| 6. | Al Dawish MA, Robert AA, Braham R, Al Hayek AA, Al Saeed A, Ahmed RA, Al Sabaan FS. Diabetes Mellitus in Saudi Arabia: A Review of the Recent Literature. Curr Diabetes Rev. 2016;12:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 244] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 7. | Codner E, Merino PM, Tena-Sempere M. Female reproduction and type 1 diabetes: from mechanisms to clinical findings. Hum Reprod Update. 2012;18:568-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Morariu EM, Szuszkiewicz-Garcia M, Krug EI, Lemos BD, DeRiso L, Tedesco MB, Koerbel GL, Winters SJ, Korytkowski MT. MENSTRUAL AND REPRODUCTIVE FUNCTION IN WOMEN WITH TYPE 1 DIABETES. Endocr Pract. 2015;21:750-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Yoon HJ, Lee YH, Kim SR, Rim TH, Lee EY, Kang ES, Cha BS, Lee HC, Lee BW. Glycated albumin and the risk of micro- and macrovascular complications in subjects with type 1 diabetes. Cardiovasc Diabetol. 2015;14:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Schweiger BM, Snell-Bergeon JK, Roman R, McFann K, Klingensmith GJ. Menarche delay and menstrual irregularities persist in adolescents with type 1 diabetes. Reprod Biol Endocrinol. 2011;9:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Krause M, Vainio L, Zwetchkenbaum S, Inglehart MR. Dental education about patients with special needs: a survey of U.S. and Canadian dental schools. J Dent Educ. 2010;74:1179-1189. [PubMed] |
| 12. | Escobar-Morreale HF, Roldán B, Barrio R, Alonso M, Sancho J, de la Calle H, García-Robles R. High prevalence of the polycystic ovary syndrome and hirsutism in women with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2000;85:4182-4187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Utiger RD. Insulin and the polycystic ovary syndrome. N Engl J Med. 1996;335:657-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Pedersen O, Beck-Nielsen H. Insulin resistance and insulin-dependent diabetes mellitus. Diabetes Care. 1987;10:516-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Lukács A, Varga B, Kiss-Tóth E, Soós A, Barkai L. Factors influencing the diabetes-specific health-related quality of life in children and adolescents with type 1 diabetes mellitus. J Child Health Care. 2014;18:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Chapman J, Wright AD, Nattrass M, FitzGerald MG. Recurrent diabetic ketoacidosis. Diabet Med. 1988;5:659-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Hatch R, Rosenfield RL, Kim MH, Tredway D. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 667] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 18. | Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3911] [Cited by in RCA: 3971] [Article Influence: 70.9] [Reference Citation Analysis (0)] |
| 19. | Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3717] [Cited by in RCA: 4164] [Article Influence: 198.3] [Reference Citation Analysis (0)] |
| 20. | Al-Rubeaan K. National surveillance for type 1, type 2 diabetes and prediabetes among children and adolescents: a population-based study (SAUDI-DM). J Epidemiol Community Health. 2015;69:1045-1051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Griffin ML, South SA, Yankov VI, Booth RA Jr, Asplin CM, Veldhuis JD, Evans WS. Insulin-dependent diabetes mellitus and menstrual dysfunction. Ann Med. 1994;26:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Adcock CJ, Perry LA, Lindsell DR, Taylor AM, Holly JM, Jones J, Dunger DB. Menstrual irregularities are more common in adolescents with type 1 diabetes: association with poor glycaemic control and weight gain. Diabet Med. 1994;11:465-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Melin EO, Thunander M, Svensson R, Landin-Olsson M, Thulesius HO. Depression, obesity, and smoking were independently associated with inadequate glycemic control in patients with type 1 diabetes. Eur J Endocrinol. 2013;168:861-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Hapunda G, Abubakar A, van de Vijver F, Pouwer F. Living with type 1 diabetes is challenging for Zambian adolescents: qualitative data on stress, coping with stress and quality of care and life. BMC Endocr Disord. 2015;15:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | BERGQVIST N. The gonadal function in female diabetics. Acta Endocrinol Suppl (Copenh). 1954;19:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Kjaer K, Hagen C, Sandø SH, Eshøj O. Epidemiology of menarche and menstrual disturbances in an unselected group of women with insulin-dependent diabetes mellitus compared to controls. J Clin Endocrinol Metab. 1992;75:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Zarzycki W, Zieniewicz M. Reproductive disturbances in type 1 diabetic women. Neuro Endocrinol Lett. 2005;26:733-738. [PubMed] |
| 28. | Anderson SE, Dallal GE, Must A. Relative weight and race influence average age at menarche: results from two nationally representative surveys of US girls studied 25 years apart. Pediatrics. 2003;111:844-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 296] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 29. | Al-Sahab B, Ardern CI, Hamadeh MJ, Tamim H. Age at menarche in Canada: results from the National Longitudinal Survey of Children & Youth. BMC Public Health. 2010;10:736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Macgússon TE. Age at menarche in Iceland. Am J Phys Anthropol. 1978;48:511-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Atay Z, Turan S, Guran T, Furman A, Bereket A. Puberty and influencing factors in schoolgirls living in Istanbul: end of the secular trend? Pediatrics. 2011;128:e40-e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Babay ZA, Addar MH, Shahid K, Meriki N. Age at menarche and the reproductive performance of Saudi women. Ann Saudi Med. 2004;24:354-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Al-Agha AE, Alabbad S, Tatwany B, Aljahdali A. Menarche Age of Mothers and Daughters and Correlation between them in Saudi Arabia. Reprod Syst Sex Disord. 2015;4:1000153. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Raha O, Sarkar B, Godi S, GhoshRoy A, Pasumarthy V, Chowdhury S; JDRF-India, Vadlamudi RR. Menarcheal age of type 1 diabetic Bengali Indian females. Gynecol Endocrinol. 2013;29:963-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Zachurzok A, Deja G, Gawlik A, Drosdzol-Cop A, Małecka-Tendera E. Hyperandrogenism in adolescent girls with type 1 diabetes mellitus treated with intensive and continuous subcutaneous insulin therapy. Endokrynol Pol. 2013;64:121-128. [PubMed] |
| 36. | Harjutsalo V, Maric-Bilkan C, Forsblom C, Groop PH; FinnDiane Study Group. Age at menarche and the risk of diabetic microvascular complications in patients with type 1 diabetes. Diabetologia. 2016;59:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Danielson KK, Palta M, Allen C, D’Alessio DJ. The association of increased total glycosylated hemoglobin levels with delayed age at menarche in young women with type 1 diabetes. J Clin Endocrinol Metab. 2005;90:6466-6471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Spritzer PM. Polycystic ovary syndrome: reviewing diagnosis and management of metabolic disturbances. Arq Bras Endocrinol Metabol. 2014;58:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 39. | Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1929-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 560] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 40. | Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3363] [Cited by in RCA: 4017] [Article Influence: 191.3] [Reference Citation Analysis (0)] |
| 41. | de Vries L, Karasik A, Landau Z, Phillip M, Kiviti S, Goldberg-Stern H. Endocrine effects of valproate in adolescent girls with epilepsy. Epilepsia. 2007;48:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Peppard HR, Marfori J, Iuorno MJ, Nestler JE. Prevalence of polycystic ovary syndrome among premenopausal women with type 2 diabetes. Diabetes Care. 2001;24:1050-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Graff SK, Mário FM, Alves BC, Spritzer PM. Dietary glycemic index is associated with less favorable anthropometric and metabolic profiles in polycystic ovary syndrome women with different phenotypes. Fertil Steril. 2013;100:1081-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18:618-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 565] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 45. | Diamanti-Kandarakis E, Spritzer PM, Sir-Petermann T, Motta AB. Insulin resistance and polycystic ovary syndrome through life. Curr Pharm Des. 2012;18:5569-5576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Wild RA, Rizzo M, Clifton S, Carmina E. Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril. 2011;95:1073-9.e1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 47. | Moini A, Eslami B. Familial associations between polycystic ovarian syndrome and common diseases. J Assist Reprod Genet. 2009;26:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |