Published online Aug 15, 2025. doi: 10.4239/wjd.v16.i8.106967
Revised: May 14, 2025
Accepted: June 30, 2025
Published online: August 15, 2025
Processing time: 155 Days and 21.7 Hours
Maternal diabetes significantly increases the risk of adverse maternal and neo
To evaluate the performance of the FreeStyle Libre H FCGM against plasma glucose and its correlations with HbA1c and GA.
This prospective observational study involved 152 pregnant women with GDM or T2DM, with intermittent collection of venous plasma glucose, HbA1c, GA, and concurrent FCGM data at regular intervals at a single center. Relationships were evaluated using restricted cubic spline and mixed-effects models. Receiver operating characteristic curve analysis was performed to compare the ability of HbA1c and GA to detect suboptimal glycemic control.
Analysis of 507 FCGM-plasma glucose pairs revealed an overall mean absolute relative difference of 7.96%. Mean absolute relative differences were 9.22%, 7.75%, and 4.15% for low (3.5-4.4 mmol/L), medium (4.5-7.8 mmol/L), and high (7.9-13 mmol/L) glucose levels, respectively. Most values fell within zone A or zone B on the Clarke and Parkes Error Grids. Bland-Altman analysis indicated a slight underestimation by FCGM (-0.121 mmol/L). Restricted cubic spline analysis revealed significant linear or nonlinear associations between HbA1c/GA and mean glucose, time in range, time above range, and coefficient of variation, but not time below range. Both HbA1c and GA were influenced by gestational age and pregestational body mass index. Receiver operating characteristic analysis showed that HbA1c had comparable or superior performance to GA in detecting suboptimal glycemic control based on FCGM-derived thresholds.
The FCGM system served as a validated reference for evaluating glycemic markers in pregnant women with T2DM and GDM. HbA1c reliably assessed average glycemia, while GA provided complementary insight.
Core Tip: This study demonstrates the reliability of the FreeStyle Libre H flash continuous glucose monitoring (FCGM) system in pregnant women with gestational diabetes mellitus and type 2 diabetes mellitus, showing a low mean absolute relative difference of 7.96% compared to venous plasma glucose. Hemoglobin A1c (HbA1c) and glycated albumin (GA) showed significant linear or non-linear associations with FCGM metrics, although both were influenced by gestational age and pregestational body mass index. In this study, FCGM served as a validated reference for evaluating HbA1c and GA. HbA1c remained a reliable marker, while GA provided supplementary value for comprehensive glycemic assessment.
- Citation: Lyu L, Huang YL, Huang Y, Wu ZY, Ping F, Li YX. Performance of flash continuous glucose monitoring and glycemic marker correlations in Chinese pregnant women with non-type 1 diabetes. World J Diabetes 2025; 16(8): 106967
- URL: https://www.wjgnet.com/1948-9358/full/v16/i8/106967.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i8.106967
Maternal diabetes affects approximately one-sixth of pregnancies globally[1], increasing the risk of adverse obstetric and neonatal outcomes, including primary cesarean section, macrosomia, and neonatal hypoglycemia[2]. Although strict glycemic control can reduce these risks, it may also increase the likelihood of maternal hypoglycemia[3]. Currently, in clinical practice, glycemic monitoring for people with diabetes primarily relies on self-monitoring of blood glucose (SMBG) combined with biochemical markers including hemoglobin A1c (HbA1c) and glycated albumin (GA)[4]. SMBG has traditionally been the standard method for glucose tracking during pregnancy, but it is often associated with pain, invasiveness, and poor compliance, which may contribute to adverse pregnancy outcomes[5]. HbA1c and GA reflect average blood glucose levels over different time intervals[6], but neither can provide real-time glucose data, which are essential for pregnancy management. These limitations underscore the need for monitoring technologies capable of capturing real-time glucose dynamics during pregnancy.
Continuous glucose monitoring (CGM) offers an alternative perspective by providing continuous, real-time glucose data that SMBG may miss. The CONCEPTT study demonstrated that CGM use in pregnant women with type 1 diabetes mellitus (T1DM) improved time in range (TIR) and fetal outcomes[7], highlighting the potential of advanced glucose monitoring in managing maternal diabetes. The flash CGM (FCGM) system, an alternative to traditional CGM, monitors interstitial glucose levels for up to 14 days without the need for finger-prick testing. While its performance has been extensively studied in non-pregnant individuals[8-11], research on patients with diabetes during pregnancy remains limited[12-14].
This study aimed to evaluate the reliability of the FCGM system in pregnant women with gestational diabetes mellitus (GDM) and type 2 diabetes mellitus (T2DM), using pre-meal venous plasma glucose as the reference standard. While the American Diabetes Association (ADA) guidelines recommend CGM and specific TIR targets for individuals with T1DM[15], the performance and interpretability of CGM metrics in pregnancies affected by diabetes other than T1DM remain underexplored. In particular, the correlations between FCGM metrics and biochemical markers such as HbA1c and GA have not been well defined in this population, motivating the present investigation.
This prospective observational study was conducted within a single-center cohort. Participants were pregnant women attending routine prenatal follow-ups at the outpatient clinic of Peking Union Medical College Hospital. The inclusion criteria were as follows: (1) Age ≥ 18 years; (2) Pregnant women with GDM or T2DM who were referred to the De
Between November 2021 and January 2025, we enrolled 152 pregnant women with either GDM or T2DM. The T2DM cases included both women with pregestational T2DM and those who met the diagnostic criteria for diabetes during the first-trimester screening. Diabetes was diagnosed based on the ADA guidelines[16].
Participants wore the FreeStyle Libre H (Abbott Diabetes Care, Witney, Oxon, United Kingdom), a professional-mode FCGM system that does not require scanning every 8 hours. The timing of CGM insertion was determined by each participant’s initial visit to the Department of Endocrinology. The sensor, worn on the upper arm, continuously recorded glucose levels for up to 14 days. Participants were also provided with a reader to access real-time glucose readings from the sensor. During the 14-day sensor wear period, venous blood samples were collected once via standard venipuncture by trained clinical staff. These samples included pre-meal plasma glucose, HbA1c, GA, and hemoglobin (Hb) levels. Blood sampling was minimally invasive and conducted as part of routine clinical care. The study protocol was approved by the institutional ethics committee, approval No. I-24PJ2607.
All the sensor data were downloaded and exported to Microsoft Excel for analysis, with the first 24 hours of data excluded to ensure accuracy. FCGM metrics, including mean glucose (MG), coefficient of variation (CV), TIR (3.5-7.8 mmol/L), time above range (TAR: > 7.8 mmol/L), and time below range (TBR: < 3.5 mmol/L), were calculated. Venous glucose values were compared with the nearest corresponding glucose readings recorded by the sensor.
Mean absolute difference (MAD) and mean absolute relative difference (MARD) were calculated using reference plasma glucose measurements for the overall dataset and specific glucose ranges (3.5-4.4 mmol/L, 4.5-7.8 mmol/L and 7.9-13 mmol/L). In addition, MAD and MARD were calculated separately based on the days of device wear: D1-D3, D4-D6, D7-D8, D9-D12, and D13-D14, corresponding to the time intervals of venous blood glucose collection. Clarke and Parkes Error Grids, along with the Bland-Altman method, were used to assess the level of agreement[17]. The accuracy and reliability of the CGM measurements were also evaluated using the FDA performance standards for integrated CGM (iCGM)[18]. The specific standards applied are detailed in Supplementary Table 1.
Clinical characteristics between the GDM and T2DM groups were compared using the independent samples t test for normally distributed continuous variables (assessed by the Shapiro-Wilk test), and the Mann-Whitney U test for non–normally distributed continuous variables, and Fisher’s exact test for categorical variables. A P value < 0.05 was considered statistically significant.
Spearman correlation coefficients (r) were used to evaluate relationships not only between HbA1c and GA but also between HbA1c/GA and FCGM metrics. Unadjusted restricted cubic spline (RCS) analyses were conducted to evaluate the associations of MG, TIR, TAR, TBR, and CV with HbA1c and GA, with three knots placed at the 25th, 50th, and 75th percentiles of the respective independent variables. To address intra-subject correlations from multiple MG-HbA1c/GA pairs per participant, an intercept-only mixed-effects model was compared with a series of models incorporating random effects for pregestational body mass index (BMI), gestational age (defined as the gestational week at the time of data collection), Hb and serum iron. The Akaike Information Criterion, with lower values indicating better model fit, was used to select the optimal model, balancing fitting accuracy with model complexity. Receiver operating characteristic (ROC) curve analysis was used to compare the predictive performance of HbA1c and GA for TIR < 70%, TIR < 90%, and TAR > 25%. These thresholds were based on clinical consensus recommendations from the ADA[15]. The area under the curve (AUC), optimal cut-off values (based on the Youden index), sensitivity, and specificity were calculated. Statistical analyses were performed using IBM SPSS Statistics version 20 (IBM Corp., Armonk, NY, United States) and Stata version 18 (StataCorp., College Station, TX, United States). In this study, correlation strength was categorized as follows[19]: Negligible (0.00-0.10), weak (0.10-0.39), moderate (0.40-0.69), strong (0.70-0.89), and very strong (0.90-1.00).
Table 1 provides an overview of the characteristics of 152 pregnant women enrolled in this study, comprising 89 (58.6%) with GDM and 63 (41.4%) with T2DM. In the GDM group, 50 (56.2%) of the participants used insulin therapy, whereas in the T2DM group, 49 (77.7%) received medication, including 40 (63.5%) who used insulin, 1 (1.6%) who used metformin alone, and 8 (12.7%) who used both insulin and metformin.
| Characteristic | GDM | T2DM | P value |
| N | 89 (58.6%) | 63 (41.4%) | - |
| Age (years) | 35.3 ± 3.8 | 35.6 ± 3.9 | 0.717 |
| Age at diagnosis (years) | 35 (33-38) | 33 (29.5-36) | 0.002 |
| Gestational age at first visit (weeks) | 29.3 (26.6-32.7) | 10.7 (8.1-16.7) | < 0.001 |
| Pre-pregnancy BMI (kg/m2) | 23.3 (20.4-25.8) | 25.1 (22.1-29.0) | 0.003 |
| Gravidity | 2 (1-3) | 1 (1-2) | 0.04 |
| Parity | 0 (0-1) | 0 (0-0) | 0.072 |
| Mode of conception | |||
| Natural conception | 68 (76.4%) | 53 (84.1%) | 0.495 |
| IVF-ET | 16 (18.0%) | 8 (12.7%) | 0.488 |
| Ovulation induction by letrozole | 5 (5.6%) | 2 (3.2%) | 0.516 |
| Singleton pregnancy | 83 (93.3%) | 62 (98.4%) | 0.24 |
| Multiple pregnancy | 6 (6.7%) | 1 (1.6%) | 0.24 |
| HbA1c (%) | 5.2 (5.0-5.5) | 5.3 (5.0-5.9) | 0.02 |
| GA (%) | 13.3 (12.2-13.7) | 13.3 (12.6-14.6) | 0.364 |
| Hemoglobin (g/L) | 125.7 ± 10.1 | 124.9 ± 10.8 | 0.613 |
| Serum iron (μg/dL) | 92.0 (75.5-116.0) | 108.0 (82-132) | 0.049 |
| Serum ferritin (ng/mL) | 28.5 (16.0-52.8) | 53 (35-92) | < 0.001 |
| Serum vitamin B12 (pg/mL) | 312 (228-433) | 342 (281-476) | 0.072 |
| Pharmacotherapy | |||
| Insulin | 50 (56.2%) | 40 (63.5%) | 0.012 |
| Metformin | 0 (0%) | 1 (1.6%) | 0.349 |
| Insulin and metformin | 0 (0%) | 8 (12.7%) | < 0.001 |
| CGM profiles | 2 (1-3) | 2 (1-6) | 0.063 |
| CGM metrics | |||
| Number of days CGM worn (days)1 | 12.5 (11.3-12.8) | 12.9 (11.3-12.9) | 0.081 |
| Percentage of time CGM is active (%) | 100 (100-100) | 100 (100-100) | 0.636 |
| Mean glucose (mmol/L) | 5.5 (5.2-5.8) | 5.4 (5.0-5.9) | 0.214 |
| Time in range (%) (3.5-7.8 mmol/L) | 93.4 (87.5-96.8) | 91.1 (84.7-95.4) | 0.001 |
| Time above range (%) (> 7.8 mmol/L) | 3.9 (1.3-8.6) | 4.8 (1.6-10.7) | 0.045 |
| Time below range (%) (< 3.5 mmol/L) | 0.7 (0.2-3.1) | 1.4 (0.2-4.3) | 0.021 |
| Time below range (%) (< 3.0 mmol/L) | 0.1 (0-0.9) | 0.2 (0-1.1) | 0.215 |
| Coefficient of variation (%) | 19.9 (17.5-23.1) | 21.9 (19.0-25.5) | < 0.001 |
HbA1c levels were slightly lower in the GDM group compared to the T2DM group [5.2% (5.0-5.5) vs 5.3% (5.0-5.9), P = 0.02], while GA levels showed no significant difference between the two groups [13.3% (12.2-13.7) vs 13.3% (12.6-14.6), P = 0.364]. TIR was notably higher in the GDM group [93.4% (87.5-96.8) vs 91.1% (84.7-95.4), P = 0.001], while MG did not show a significant difference [5.5 (5.2-5.8) mmol/L vs 5.4 (5.0-5.9) mmol/L, P = 0.214].
A total of 507 matched pre-meal venous plasma glucose values were analyzed. Supplementary Table 2 shows that the overall MARD was 7.96%, and the MAD was 0.402 mmol/L. For plasma glucose levels of 3.5-4.4 mmol/L [n = 95 (18.74%)], the MARD was 9.22% (MAD = 0.384 mmol/L); for 4.5-7.8 mmol/L [n = 403 (79.49%)], the MARD was 7.75% (MAD = 0.407 mmol/L); and for 7.9-13 mmol/L [n = 9 (1.78%)], the MARD was 4.15% (MAD = 0.38 mmol/L). Detailed results for specific time intervals showed MARD values of 9.31% [D1-D3, n = 46 (9.07%)], 7.74% [D4-D6, n = 79 (15.58%)], 8.04% [D7-D8, n = 78 (15.38%)], 7.28% [D9-D12, n = 216 (42.60%)], and 9.04% [D13-D14, n = 88 (17.36%)], with corresponding MADs of 0.498, 0.397, 0.394, 0.365, and 0.453 mmol/L, respectively.
As shown in Supplementary Table 1, the FCGM system demonstrated a high level of overall accuracy, meeting the FDA’s iCGM standards in most respects. Nevertheless, it failed to meet the criteria in two key areas: (1) Only 80.87% of measurements fell within ± 20% of reference values, below the iCGM threshold of 87%; and (2) When CGM glucose levels were below 3.9 mmol/L, only 76.92% of measurements were within ± 0.83 mmol/L, also below the required threshold of 85%. Importantly, the system fully satisfied the performance requirements within both the iCGM-defined target range (3.9-10.0 mmol/L) and the hyperglycemic range (> 10.0 mmol/L).
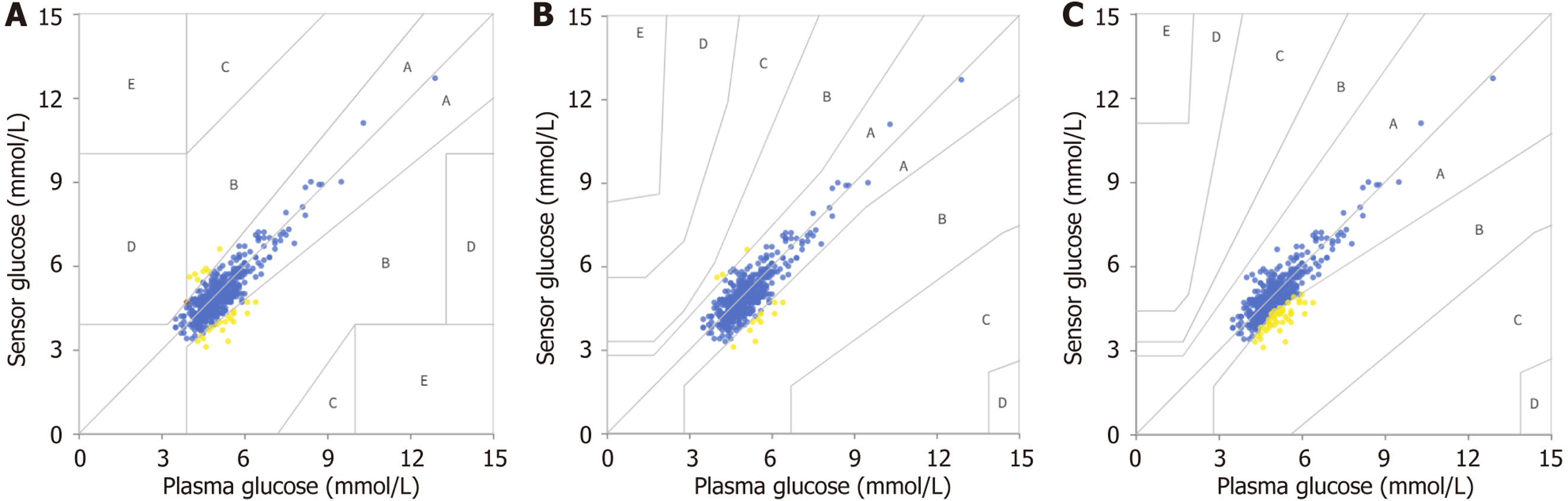
Figure 1A illustrates the distribution across Clarke Error Grid zones, with proportions in zone A, zone B, zone C, zone D and zone E being 94.48%, 5.33%, 0.00%, 0.20% and 0.00%, respectively. The Parkes 1 and Parkes 2 Error Grids similarly demonstrated high concordance. In Parkes 1, all glucose measurements fell within zone A (97.24%) or zone B (2.76%), whereas in Parkes 2, 90.14% of measurements were in zone A and 9.86% in zone B (Figure 1B and C).
As depicted in Supplementary Figure 1, the Bland-Altman plot indicated a mean bias of -0.121 mmol/L, with limits of agreement ranging from -1.119 to 0.878 mmol/L.
As shown in Supplementary Figure 2, the RCS analysis revealed a significant overall association between HbA1c and GA (482 pairs; P for overall < 0.001), with clear evidence of a nonlinear relationship (P for nonlinear = 0.002). The Spearman correlation coefficient between the two variables was 0.312 (P < 0.001). In subgroup analyses, the correlation between HbA1c and GA was not significant in the GDM group (228 pairs; r = 0.109, P = 0.101), but was moderately positive in the T2DM group (254 pairs; r = 0.462, P < 0.001).
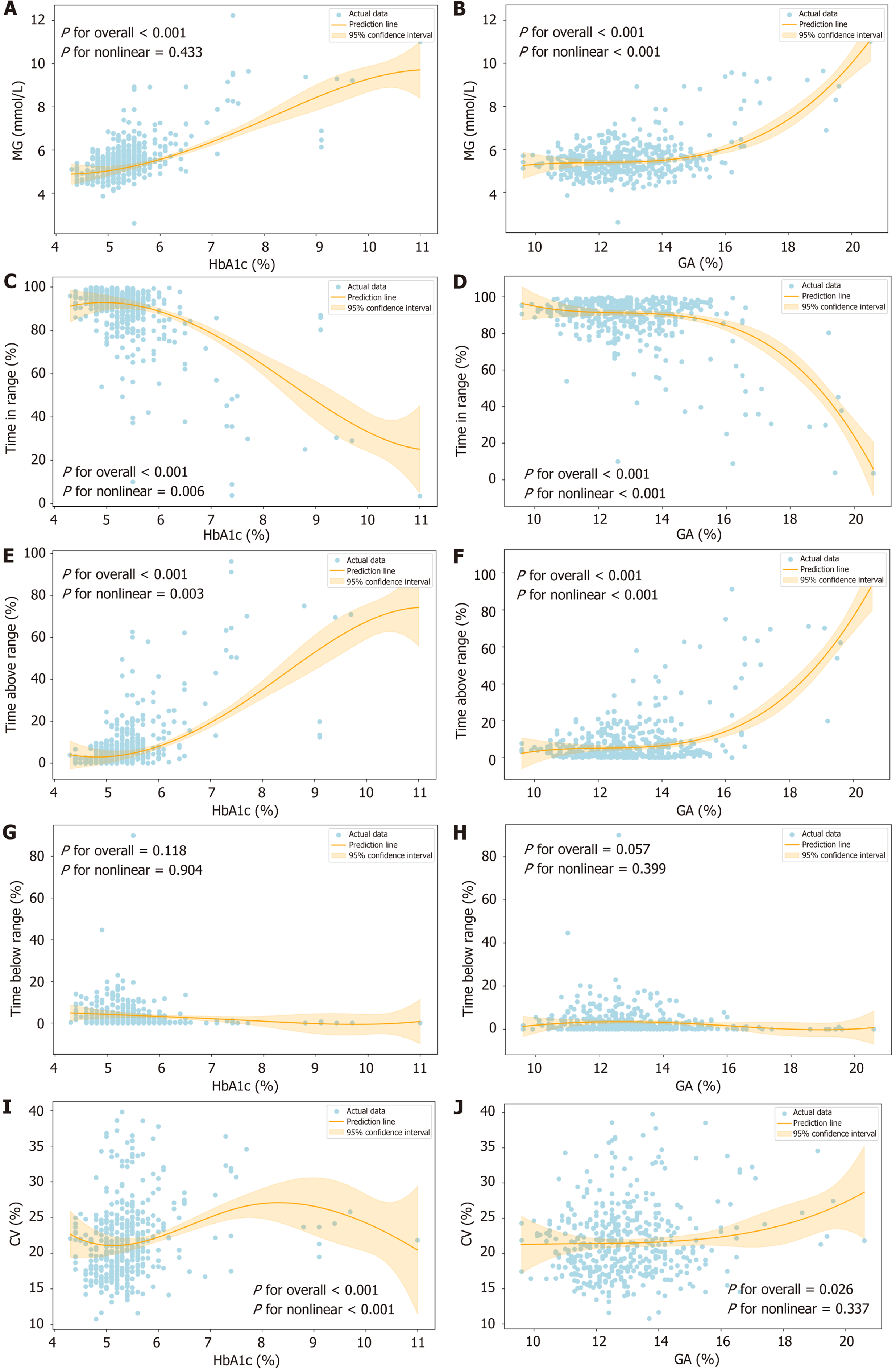
Figure 2 illustrates the relationship between FCGM metrics and HbA1c/GA during pregnancy. A total of 488 HbA1c-FCGM and 497 GA-FCGM matched pairs were included in the analysis. As shown in Figure 2A, HbA1c was positively and linearly associated with MG (P for overall < 0.001; P for nonlinear = 0.433). Significant correlations were also observed between HbA1c and TIR, TAR, and CV (P for overall < 0.001 for all). Notably, these associations were nonlinear, with P values for nonlinearity of 0.006, 0.003, and 0.006 for TIR, TAR, and CV, respectively (Figure 2C, E, and I). No significant correlation was found between HbA1c and TBR (P for overall = 0.118; P for nonlinear = 0.904) (Figure 2G).
GA was significantly associated with MG, TIR, TAR and CV (P for overall < 0.001) (Figure 2B, D, F, and J). While the relationship between GA and CV was linear (P for nonlinear = 0.337), the associations between GA and MG, TIR, and TAR were nonlinear (P for nonlinear < 0.001 for all). Additionally, no significant relationship was found between GA and TBR (P for overall = 0.057; P for nonlinear = 0.399), indicating no meaningful correlation between these two variables.
Supplementary Figure 3 presents a heatmap of Spearman correlations between HbA1c/GA and FCGM metrics in the overall study population, highlighting stronger correlations with HbA1c than with GA. The strongest correlation was observed between HbA1c and MG (r = 0.475).
Supplementary Table 3 provides additional analyses of the Spearman correlation coefficients between HbA1c/GA and FCGM-derived metric pairs (MG, TIR, TAR, TBR, and CV) in the GDM and T2DM groups. In the GDM group, 231 HbA1c-FCGM metric pairs and 238 GA-FCGM metric pairs were included. HbA1c showed weak correlations with four FCGM metrics (MG, TIR, TAR, and CV), while GA was significantly correlated only with TBR. In the T2DM group, 257 HbA1c-FCGM metric pairs and 259 GA-FCGM metric pairs were analyzed. FCGM metrics showed moderate to weak correlations with HbA1c, which were generally higher than those observed with GA, except for TBR, where GA showed a slightly stronger correlation.
As shown in Table 2, the inclusion of random slopes for HbA1c improved the model fit. The optimal model (model 7) predicted MG as a function of HbA1c, pregestational BMI, gestational age, Hb, and serum iron, with a coefficient of 0.692 for the association between HbA1c and MG. Gestational age (P = 0.010) and pregestational BMI (P = 0.018) were significant predictors, whereas Hb (P = 0.371) and serum iron (P = 0.876) were not.
| Model | AIC | Fixed effects | Intercept (95%CI) | P value | HbA1c | P value | Other covariates | P value |
| 1 | 1019.85 | Intercept only | 5.548 (5.412-5.683) | < 0.001 | - | - | - | - |
| 2 | 901.65 | HbA1c | 1.957 (1.345-2.569) | < 0.001 | 0.669 (0.557-0.782) | < 0.001 | - | - |
| 3 | 895.41 | HbA1c | 1.619 (0.962-2.277) | < 0.001 | 0.685 (0.573-0.798) | < 0.001 | - | - |
| Gestational age1 | 0.010 (0.003-0.017) | 0.004 | ||||||
| 4 | 899.29 | HbA1c | 1.529 (0.800-2.259) | < 0.001 | 0.639 (0.524-0.754) | < 0.001 | - | - |
| Prepregnancy BMI | 0.024 (0.002-0.047) | 0.036 | ||||||
| 5 | 689.21 | HbA1c | 1.956 (1.094-2.818) | < 0.001 | 0.715 (0.597-0.834) | < 0.001 | - | - |
| Hemoglobin | -0.002 (-0.007 to 0.004) | 0.492 | ||||||
| 6 | 889.26 | HbA1c | 2.093 (1.417-2.770) | < 0.001 | 0.665 (0.552-0.778) | < 0.001 | - | - |
| Serum iron | -0.001 (-0.003 to 0.001) | 0.349 | ||||||
| 7 | 676.89 | HbA1c | 1.165 (0.095-2.235) | 0.033 | 0.692 (0.568-0.816) | < 0.001 | - | - |
| Gestational age | 0.010 (0.002-0.017) | 0.010 | ||||||
| Prepregnancy BMI | 0.029 (0.005-0.054) | 0.018 | ||||||
| Hemoglobin | -0.002 (-0.008 to 0.003) | 0.371 | ||||||
| Serum iron | 0.000 (-0.002 to 0.003) | 0.876 |
Similarly, as shown in Table 3, the optimal model (model 7) provided the best fit for predicting MG based on GA, gestational age, pregestational BMI, Hb and serum iron, with a coefficient of 0.270 for the association between GA and MG. Both gestational age (P = 0.021) and pregestational BMI (P < 0.001) remained significant predictors, while Hb and serum iron continued to show no significant associations.
| Model | AIC | Fixed effects | Intercept (95%CI) | P value | GA | P value | Other covariates | P value |
| 1 | 1019.85 | Intercept only | 5.548 (5.412-5.683) | < 0.001 | - | - | - | - |
| 2 | 937.08 | GA | 2.518 (1.853-3.184) | < 0.001 | 0.234 (0.183-0.284) | < 0.001 | - | - |
| 3 | 933.63 | GA | 2.273 (1.579-2.968) | < 0.001 | 0.237 (0.187-0.288) | < 0.001 | - | - |
| Gestational age1 | 0.008 (0.001-0.015) | 0.019 | ||||||
| 4 | 908.75 | GA | 0.523 (-0.393 to 1.438) | 0.263 | 0.253 (0.205-0.302) | < 0.001 | - | - |
| Prepregnancy BMI | 0.072 (0.047-0.096) | < 0.001 | ||||||
| 5 | 747.13 | GA | 2.169 (1.143-3.195) | < 0.001 | 0.232 (0.174-0.289) | < 0.001 | - | - |
| Hemoglobin | 0.003 (-0.002 to 0.009) | 0.269 | ||||||
| 6 | 914.80 | GA | 2.777 (2.076-3.478) | < 0.001 | 0.242 (0.191-0.293) | < 0.001 | - | - |
| Serum iron | -0.003 (-0.006 to -0.001) | 0.015 | ||||||
| 7 | 701.73 | GA | 0.160 (-1.441 to 1.212) | 0.807 | 0.270 (0.215-0.325) | < 0.001 | - | - |
| Gestational age | 0.010 (0.001-0.016) | 0.021 | ||||||
| Prepregnancy BMI | 0.076 (0.050-0.102) | < 0.001 | ||||||
| Hemoglobin | 0.002 (-0.003 to 0.008) | 0.373 | ||||||
| Serum iron | -0.001 (-0.004 to 0.001) | 0.242 |
To further compare the predictive performance of HbA1c and GA in detecting glycemic abnormalities, we performed ROC curve analyses for three binary outcomes based on FCGM metrics: TIR < 70%, TIR < 90%, and TAR > 25%. As shown in Supplementary Figure 4, HbA1c consistently demonstrated equal or superior discriminative ability compared to GA. For identifying TIR < 70%, the AUC for HbA1c was 0.847 [95% confidence interval (CI): 0.776-0.919] at a cut-off value of 5.75%, yielding 62.5% sensitivity and 89.8% specificity, whereas GA had a comparable AUC of 0.832 (95%CI: 0.751-0.914) at a cut-off of 13.75%, with 75% sensitivity and 77.6% specificity. In detecting TIR < 90%, HbA1c outperformed GA with an AUC of 0.728 (95%CI: 0.681-0.774) vs 0.590 (95%CI: 0.537-0.642). At the optimal cut-offs (5.25% for HbA1c and 13.05% for GA), HbA1c showed markedly higher sensitivity (75.6% vs 47.1%) and comparable specificity (59.7% vs 67.1%). For TAR > 25%, both markers showed excellent predictive performance, with AUCs of 0.899 (95%CI: 0.847-0.951) for HbA1c and 0.884 (95%CI: 0.828-0.940) for GA. HbA1c again demonstrated higher specificity (90.2% vs 78%) at the same respective cut-offs (5.75% and 13.75%).
This prospective observational study is the first to evaluate the effectiveness and practicality of the FreeStyle Libre H FCGM System in pregnant women with non-T1DM, using venous plasma glucose as the reference standard. We conducted dynamic monitoring of glucose-related indicators and investigated their associations throughout pregnancy. Correlations between FCGM, HbA1c, and GA in non-T1DM pregnancies remain underexplored compared to traditional CGM studies in T1DM. This research gap motivated us to organize the present study.
The performance of the FCGM system in pregnant participants was considered satisfactory. Although approximately two–thirds of participants received insulin therapy, the mean TIR exceeded 90%, and the mean CV remained below 24%, indicating good glycemic control. This may be attributed to stricter glycemic targets during pregnancy (as recommended by guidelines[4,15]) and the exclusion of T1DM participants, who are known to typically exhibit higher CV values[20], thereby potentially reducing the overall CV in our study population.
Despite its overall satisfactory performance, the FCGM system did not fully meet the FDA’s iCGM standards in certain parameters. These discrepancies may be attributed to the physiological complexities of glucose regulation during pregnancy, particularly the lag between interstitial and plasma glucose levels. Future studies with larger sample sizes and more frequent glucose measurements are warranted to further validate the accuracy of FCGM systems in pregnant populations.
Not all FCGM readings could be paired with venous plasma glucose measurements, highlighting real-world challenges, such as sensor disruptions, non–adherence, and mismatched timing of venous blood sampling, which are common in outpatient settings. Despite these limitations, the FCGM system demonstrated strong agreement with plasma glucose, achieving a MARD of 7.96%, with nearly all glucose values falling within Zone A or Zone B. This level of per
We used pre-meal plasma glucose as a reference instead of capillary glucose to minimize variability from sampling inconsistencies and rapid glucose fluctuations[21,22]. While venous plasma glucose remains the gold standard for gly
Research on the FCGM system in pregnant populations remains limited, particularly studies that use plasma glucose as the reference standard, which often report higher MARD values[12-14]. For instance, a study conducted in sub-Saharan Africa involving 28 pregnant individuals (20 with GDM and 8 controls) reported a MARD of 11.9% using oral glucose tolerance test results as the reference[13]. However, this study did not include Consensus and Clarke Error Grid analyses - likely due to its small sample size - and also reported a mean underestimation of plasma glucose by 0.78 mmol/L under high ambient temperatures. In comparison, our study demonstrated a smaller difference of 0.121 mmol/L, indicating more consistent performance under varying environmental conditions.
Studies of other CGM systems in pregnant populations have reported comparable findings[23,24]. For example, Polsky et al[24] evaluated the Dexcom G7 CGM system in pregnant women with T1DM, T2DM, and GDM, reporting a MARD of 9.5%, and 99.8% of values falling within zone A or zone B of the Parkes Error Grid. While the Dexcom G7 CGM system demonstrated similar reliability in providing accurate glucose values, it is not yet available for clinical use in China.
The observed variations in MARD across different time intervals reveal important performance trends of the FCGM system. The higher MARD during the initial days (D1-D3, 9.31%) suggests a potential stabilization period, while improved accuracy in the middle phase (D9-D12, 7.28%) indicates optimal performance after adaptation. However, the increased MARD in the final days (D13-D14, 9.04%) may reflect sensor aging or physiological fluctuations. These findings emphasize the need for further optimization of the FCGM system, particularly during the initial and final phases of use, to ensure consistent accuracy throughout the device’s functional lifespan.
Our findings indicate that MARD was higher at lower plasma glucose concentrations (9.22% for 3.5-4.4 mmol/L) compared to higher levels (7.75% for 4.5-7.8 mmol/L and 4.15% for 7.9-13 mmol/L). This suggests reduced sensor accuracy at lower glucose concentrations, potentially due to the smaller number of matched pairs at these levels, leading to increased variability. In addition to its sensitivity to the proportion of low glucose values, MARD has several limi
To address these limitations, the Bland-Altman plot was used to evaluate the agreement between FCGM and plasma glucose measurements, providing insights into both the magnitude and direction of measurement errors. It demonstrated a general underestimation of plasma glucose by the FCGM system, although occasional overestimations were observed. This bias is likely attributed to inherent differences between interstitial and plasma glucose levels[21]. Episodes of hypoglycemia reported by the FCGM system without corresponding symptoms do not necessarily warrant immediate correction unless symptoms are present. However, in the presence of adverse symptoms, patients should confirm hypo
Compared to previous studies, our analysis revealed a notably weaker and non–significant association between GA and HbA1c in the GDM group (r = 0.109, P = 0.101), in contrast to a prior report that found a stronger correlation (r = 0.405, P = 0.033) in a similar population[25]. This discrepancy may be explained by the better glycemic control observed in our population compared to earlier reports, which likely reduced variability and weakened the relationship between these two biomarkers. This finding prompted a more in-depth examination of the limitations of GA as a glycemic marker during pregnancy.
It is widely recognized that GA reflects short-term glycemic status due to its shorter lifespan of 2-3 weeks[6], aligning with the interval between blood collections in our study. However, our RCS models (Figure 2) and heatmap (Supplementary Figure 3) indicated that GA, while reflecting short-term glycemia, showed only weak correlations with FCGM-derived metrics, particularly in the GDM group. Its limited association with FCGM parameters may be partly attributed to lower glycemic variability and tighter glucose control in our study population.
Furthermore, several studies have suggested that GA may be unsuitable for diagnosing diabetes in pregnancy[25-28]. One limitation of GA is its sensitivity to BMI, as demonstrated in previous studies[27-29] and further supported by our mixed-effects models, despite showing a negligible correlation (Table 3). Additionally, renal physiology - such as changes in estimated glomerular filtration rate during pregnancy - may also influence GA levels[27]. While GA may still serve as a supplementary biomarker, our findings suggest it lacks sufficient reliability on its own for guiding glycemic management during pregnancy.
Although HbA1c showed stronger correlations with FCGM-derived metrics than GA, these associations were only moderate, reinforcing the importance of CGM as a valuable tool for capturing dynamic glycemic patterns. Given the physiological changes in pregnancy - such as altered erythrocyte turnover, tighter glycemic targets and altered glucose metabolism[30] - HbA1c should be interpreted cautiously and ideally in conjunction with GA and CGM metrics to ensure a comprehensive glucose assessment of glycemic control in pregnant women. We specifically recommend more frequent HbA1c monitoring (e.g., monthly as recommended by ADA[15]) to compensate for its inherently limited temporal resolution when used in isolation. Taken together, our findings support the use of HbA1c as a reliable biomarker for glycemic assessment during pregnancy, while GA may provide additional value as a complementary, but not sub
Subgroup analyses revealed stronger correlations between FCGM metrics and glycemic biomarkers in the T2DM group compared to the GDM group (Supplementary Table 3). This difference may be attributed to the chronic hyperglycemia and longer-term metabolic disruptions observed in T2DM, as opposed to the milder, transient hyperglycemia typical of GDM. These findings underscore the importance of tailoring glycemic management strategies to the distinct physiological characteristics and needs of different subgroups.
Previous studies have shown that MG is closely linked to neonatal outcomes during pregnancy. Sanusi et al[31] reported that both MG and TIR were associated with neonatal outcomes in pregnant patients with T1DM and T2DM, with MG demonstrating slightly better predictive performance than TIR. Our study found that HbA1c exhibited the strongest correlation with MG (r = 0.475, P < 0.01) among all FCGM metrics (Supplementary Figure 3), and this association was confirmed to be linear based on RCS models (Figure 2). These findings highlight the clinical importance of MG, which is why our study specifically focused on this variable when examining its relationship with HbA1c and GA in the mixed-effects models.
Gestational age and pregestational BMI were identified as significant factors influencing the HbA1c-MG relationship through mixed-effects models, consistent with previous studies[32,33]. The effect of gestational age might be partially explained by diagnostic timing: In our study, women with GDM were typically diagnosed later in pregnancy (mean gestational age at FCGM initiation: 29.3 weeks), whereas those with T2DM were managed much earlier (mean gestational age: 10.7 weeks). This systematic difference in gestational timing likely contributed to differences in glycemic physiology and influenced the behavior and interpretation of glycemic markers such as HbA1c and GA in relation to FCGM-derived metrics. These findings underscore the importance of considering diagnostic timing when evaluating biomarker performance across diabetes subtypes. However, in contrast to prior research[34-36], Hb and serum iron did not demonstrate statistical significance in our study, likely due to the low prevalence of anemia and iron deficiency in our study population.
This study has several limitations. First, it was conducted at a single center with a modest sample size, which limits statistical power and underscores the need for larger studies to validate the observed associations, including the influence of BMI and gestational age on the relationship between FCGM metrics and glycemic markers. Second, the inclusion of primarily Chinese participants limits generalizability. Third, the overall good glycemic control and the relatively stable HbA1c range among participants may have biased the favorable performance of FCGM, as this homogeneity could limit the ability to detect variations in the impact of FCGM metrics on glycemic control. Fourth, although MG reflects approximately 14 days of glucose exposure and is physiologically relevant in pregnancy, direct comparisons with markers reflecting longer timeframes - such as GA (2-3 weeks) and HbA1c (8-12 weeks in non-pregnant individuals) - may introduce inherent limitations due to mismatched temporal windows. Finally, the absence of pregnancy outcome data precludes assessing the clinical impact of FCGM use. Future studies should include multi-ethnic cohorts with broader glycemic variability, incorporate maternal-fetal outcomes, and consider real-world implementation factors to comprehensively validate the clinical utility of CGM and biochemical markers in pregnancy.
In conclusion, the FreeStyle Libre H FCGM system exhibited strong concordance with plasma glucose measurements, supporting its analytical validity in pregnant women with non-T1DM. HbA1c emerged as a more reliable marker of glucose metabolism than GA, although both were influenced by factors such as BMI and gestational age. Rather than serving as a replacement for or superior alternative to biochemical markers, FCGM provided a validated, temporally detailed reference framework against which the clinical relevance of HbA1c and GA could be evaluated. Its CGM capability supports more precise and timely management of diabetes during pregnancy.
The authors thank all study participants and acknowledge the staff of Peking Union Medical College Hospital for their invaluable assistance.
| 1. | Magliano DJ, Boyko EJ; IDF Diabetes Atlas 10th Edition Scientific Committee. IDF DIABETES ATLAS [Internet]. Brussels: International Diabetes Federation; 2021–. [PubMed] |
| 2. | HAPO Study Cooperative Research Group; Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3783] [Cited by in RCA: 3693] [Article Influence: 217.2] [Reference Citation Analysis (0)] |
| 3. | Rayburn W, Piehl E, Jacober S, Schork A, Ploughman L. Severe hypoglycemia during pregnancy: its frequency and predisposing factors in diabetic women. Int J Gynaecol Obstet. 1986;24:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | American Diabetes Association Professional Practice Committee. 6. Glycemic Goals and Hypoglycemia: Standards of Care in Diabetes-2024. Diabetes Care. 2024;47:S111-S125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 203] [Article Influence: 203.0] [Reference Citation Analysis (0)] |
| 5. | Cosson E, Baz B, Gary F, Pharisien I, Nguyen MT, Sandre-Banon D, Jaber Y, Cussac-Pillegand C, Banu I, Carbillon L, Valensi P. Poor Reliability and Poor Adherence to Self-Monitoring of Blood Glucose Are Common in Women With Gestational Diabetes Mellitus and May Be Associated With Poor Pregnancy Outcomes. Diabetes Care. 2017;40:1181-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Lee JE. Alternative biomarkers for assessing glycemic control in diabetes: fructosamine, glycated albumin, and 1,5-anhydroglucitol. Ann Pediatr Endocrinol Metab. 2015;20:74-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Feig DS, Donovan LE, Corcoy R, Murphy KE, Amiel SA, Hunt KF, Asztalos E, Barrett JFR, Sanchez JJ, de Leiva A, Hod M, Jovanovic L, Keely E, McManus R, Hutton EK, Meek CL, Stewart ZA, Wysocki T, O'Brien R, Ruedy K, Kollman C, Tomlinson G, Murphy HR; CONCEPTT Collaborative Group. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet. 2017;390:2347-2359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 557] [Cited by in RCA: 491] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 8. | Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The Performance and Usability of a Factory-Calibrated Flash Glucose Monitoring System. Diabetes Technol Ther. 2015;17:787-794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 459] [Cited by in RCA: 500] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 9. | Fokkert MJ, van Dijk PR, Edens MA, Abbes S, de Jong D, Slingerland RJ, Bilo HJ. Performance of the FreeStyle Libre Flash glucose monitoring system in patients with type 1 and 2 diabetes mellitus. BMJ Open Diabetes Res Care. 2017;5:e000320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 130] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 10. | Ji L, Guo X, Guo L, Ren Q, Yu N, Zhang J. A Multicenter Evaluation of the Performance and Usability of a Novel Glucose Monitoring System in Chinese Adults With Diabetes. J Diabetes Sci Technol. 2017;11:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Yan R, Li H, Kong X, Zhai X, Chen M, Sun Y, Ye L, Su X, Ma J. The Accuracy and Precision of the Continuously Stored Data from Flash Glucose Monitoring System in Type 2 Diabetes Patients during Standard Meal Tolerance Test. Int J Endocrinol. 2020;2020:5947680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Scott EM, Bilous RW, Kautzky-Willer A. Accuracy, User Acceptability, and Safety Evaluation for the FreeStyle Libre Flash Glucose Monitoring System When Used by Pregnant Women with Diabetes. Diabetes Technol Ther. 2018;20:180-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 13. | Milln JM, Walugembe E, Ssentayi S, Nkabura H, Jones AG, Nyirenda MJ. Comparison of oral glucose tolerance test and ambulatory glycaemic profiles in pregnant women in Uganda with gestational diabetes using the FreeStyle Libre flash glucose monitoring system. BMC Pregnancy Childbirth. 2020;20:635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Campos Lopes S, Brito AI, Barbosa M, Matos AC, Lopes Pereira M, Monteiro AM, Fernandes V. Flash glucose monitoring system in gestational diabetes: a study of accuracy and usability. Hormones (Athens). 2023;22:703-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | American Diabetes Association Professional Practice Committee. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes-2024. Diabetes Care. 2024;47:S282-S294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 96] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 16. | American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care. 2024;47:S20-S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 662] [Article Influence: 662.0] [Reference Citation Analysis (1)] |
| 17. | Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23:1143-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 402] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 18. | Freckmann G, Pleus S, Grady M, Setford S, Levy B. Measures of Accuracy for Continuous Glucose Monitoring and Blood Glucose Monitoring Devices. J Diabetes Sci Technol. 2019;13:575-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 19. | Schober P, Boer C, Schwarte LA. Correlation Coefficients: Appropriate Use and Interpretation. Anesth Analg. 2018;126:1763-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1999] [Cited by in RCA: 3888] [Article Influence: 555.4] [Reference Citation Analysis (0)] |
| 20. | Dunn TC, Ajjan RA, Bergenstal RM, Xu Y. Is It Time to Move Beyond TIR to TITR? Real-World Data from Over 20,000 Users of Continuous Glucose Monitoring in Patients with Type 1 and Type 2 Diabetes. Diabetes Technol Ther. 2024;26:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 21. | Cengiz E, Tamborlane WV. A tale of two compartments: interstitial versus blood glucose monitoring. Diabetes Technol Ther. 2009;11 Suppl 1:S11-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 22. | Kimberly MM, Vesper HW, Caudill SP, Ethridge SF, Archibold E, Porter KH, Myers GL. Variability among five over-the-counter blood glucose monitors. Clin Chim Acta. 2006;364:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Castorino K, Polsky S, O'Malley G, Levister C, Nelson K, Farfan C, Brackett S, Puhr S, Levy CJ. Performance of the Dexcom G6 Continuous Glucose Monitoring System in Pregnant Women with Diabetes. Diabetes Technol Ther. 2020;22:943-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 24. | Polsky S, Valent AM, Isganaitis E, Castorino K, O'Malley G, Beck SE, Gao P, Laffel LM, Brown FM, Levy CJ. Performance of the Dexcom G7 Continuous Glucose Monitoring System in Pregnant Women with Diabetes. Diabetes Technol Ther. 2024;26:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Chume FC, Renz PB, Hernandez MK, Freitas PAC, Camargo JL. Is there a role for glycated albumin in the diagnosis of gestational diabetes mellitus? Endocrine. 2021;72:681-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Zhu J, Chen Y, Li C, Tao M, Teng Y. The diagnostic value of glycated albumin in gestational diabetes mellitus. J Endocrinol Invest. 2018;41:121-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Dong Y, Zhai Y, Wang J, Chen Y, Xie X, Zhang C, Liu J, Lu Y, Tang G, Han L, Li L, Cao Z. Glycated albumin in pregnancy: reference intervals establishment and its predictive value in adverse pregnancy outcomes. BMC Pregnancy Childbirth. 2020;20:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Rooney MR, Zhang S, Fang M, Minhas AS, Wallace AS, Grams ME, Echouffo-Tcheugui JB, Christenson RH, Selvin E. Performance of glycated albumin as a biomarker of hyperglycemia in pregnancy: Results from the National Health and Nutrition Examination Survey 1999-2004. Clin Biochem. 2023;112:67-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Soffer MD, James KE, Thaweethai T, Callahan M, Barth WH Jr, Powe CE. Glycated Albumin and Glycemia in Pregnancy and Postpartum: A Pilot Study. Am J Perinatol. 2024;41:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Maresh MJ, Holmes VA, Patterson CC, Young IS, Pearson DW, Walker JD, McCance DR; Diabetes and Pre-eclampsia Intervention Trial Study Group. Glycemic targets in the second and third trimester of pregnancy for women with type 1 diabetes. Diabetes Care. 2015;38:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 31. | Sanusi AA, Xue Y, McIlwraith C, Howard H, Brocato BE, Casey B, Szychowski JM, Battarbee AN. Association of Continuous Glucose Monitoring Metrics With Pregnancy Outcomes in Patients With Preexisting Diabetes. Diabetes Care. 2024;47:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 32. | Law GR, Gilthorpe MS, Secher AL, Temple R, Bilous R, Mathiesen ER, Murphy HR, Scott EM. Translating HbA(1c) measurements into estimated average glucose values in pregnant women with diabetes. Diabetologia. 2017;60:618-624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Chen X, Zhang J, Tang Y, Zhang Y, Ma Z, Hu Y. Characteristics of Glucose-Lipid Metabolism in Early Pregnancy Among Overweight and Obese Women and Their Predictive Value for Gestational Diabetes Mellitus. Diabetes Metab Syndr Obes. 2024;17:3711-3723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 34. | Hashimoto K, Osugi T, Noguchi S, Morimoto Y, Wasada K, Imai S, Waguri M, Toyoda R, Fujita T, Kasayama S, Koga M. A1C but not serum glycated albumin is elevated because of iron deficiency in late pregnancy in diabetic women. Diabetes Care. 2010;33:509-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Rafat D, Ahmad J. HbA1c in pregnancy. Diabetes Metab Syndr. 2012;6:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Guo ZH, Tian HL, Zhang XQ, Zhang DH, Wang ZM, Wang K, Su WW, Chen F. Effect of anemia and erythrocyte indices on hemoglobin A1c levels among pregnant women. Clin Chim Acta. 2022;534:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |