Published online Jun 15, 2025. doi: 10.4239/wjd.v16.i6.105173
Revised: March 29, 2025
Accepted: May 8, 2025
Published online: June 15, 2025
Processing time: 150 Days and 4.7 Hours
Epidermal growth factor receptor (EGFR) is a transmembrane protein that is differentially expressed in gestational diabetes mellitus (GDM). Endothelial dy
To explore the molecular mechanism by which EGFR influences endothelial cell dysfunction in GDM at the transcriptional and protein levels.
Quantitative real-time polymerase chain reaction was used to detect the ex
In this study, EGFR was upregulated in clinical samples, GDM animal models and GDM cell models, and the knockdown of EGFR could mitigate the effect of streptozotocin (STZ) and high glucose (HG); promoted the proliferation, migration and vascularization of human umbilical vein endothelial cells (HUVECs); inhibited cell apoptosis and the expression of endothelial cell dysfunction markers (vascular cell adhesion molecule-1, tumor necrosis factor-α, vascular endothelial growth factor-A, and intercellular cell adhesion molecule-1); and alleviated the process of GDM in vivo. Mechanistically, EIF4A3 binding to long noncoding RNA H19 increased the stability of EGFR messenger RNA, thereby promoting HG-induced HUVECs dysfunction or STZ-induced endothelial cell dysfunction in GDM mice. In addition, ERRFI1 also regulated the expression of EGFR, and ERRFI1 inhibited EGFR activity by binding to EGFR, thereby inhibiting HG-induced HUVECs dysfunction.
Our study revealed that EGFR can accelerate the development of GDM by promoting endothelial cell dysfunction.
Core Tip: This study reveals that epidermal growth factor receptor (EGFR) drives endothelial cell dysfunction in gestational diabetes mellitus (GDM). EGFR upregulation in GDM models exacerbates dysfunction, while its knockdown enhances the proliferation, migration, and vascularization of human umbilical vein endothelial cells, and reduces the occurrence of apoptosis and the expression of dysfunction markers. Mechanistically, EIF4A3 stabilizes EGFR messenger RNA via long non-coding RNA H19, and ERRFI1 inhibits EGFR activity. EGFR emerges as a key therapeutic target for GDM.
- Citation: Tang D, Wang CF, Wang J, Jing XT, Ma J. Mechanism of the epidermal growth factor receptor in promoting endothelial cell dysfunction in gestational diabetes mellitus. World J Diabetes 2025; 16(6): 105173
- URL: https://www.wjgnet.com/1948-9358/full/v16/i6/105173.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i6.105173
Gestational diabetes mellitus (GDM) is a common metabolic issue during pregnancy and recognized as a significant hurdle to both maternal and fetal health[1]. The primary characteristics of GDM include maternal insulin resistance, dysfunction of endothelial cells, and chronic low-grade inflammation that typically occurs between the 24th and 28th week of gestation[2]. The impact of GDM on the vascular endothelium of both the fetus and placenta is associated with alterations in the expression and signaling pathways of insulin, adenosine and adipokine receptors[3]. Vascular endothelial cells (VECs) serve as the principal targets for insulin action within the body. When these cells experience dysfunction, their ability to effectively bind to insulin diminishes, impairing signal transduction and consequently disrupting blood flow regulation, as well as the delivery of insulin to target organs and tissues. This dysfunction exacerbates insulin resistance, further contributing to the progression of GDM[4]. Despite the critical role of endothelial dysfunction in GDM pathogenesis, the underlying molecular mechanisms remain elusive.
Several studies of GDM have indicated a link between the epidermal growth factor receptor (EGFR) and the onset of GDM[5,6]. As a member of the ERBB family of receptor tyrosine kinases (RTKs), EGFR activation triggers the autophosphorylation of RTKs, which, in turn, initiates a cascade of downstream signaling pathways[7]. EGFR signaling plays a crucial role in regulating several processes, including cell proliferation, differentiation, division, survival as well as angiogenesis and tumor development[8]. Furthermore, EGFR is implicated in the emergence and advancement of numerous cancers, making it a promising target for the management of various malignancies[9,10]. Notably, the ex
Long noncoding RNAs (lncRNAs), which exceed 200 base pairs in length, cannot encode proteins and play a significant role in sustaining normal physiological functions and modulating pathological alterations[14]. Research indicates that lncRNAs are crucial in the regulation of GDM. For example, a study by Wang et al[15] revealed that silencing the lncRNA PVT1 can influence the development of GDM by decreasing the proliferation, migration and invasion of trophoblast cells. The lncRNA H19, expressed from a maternally imprinted gene, is located on chromosome 11 in humans and is vital for mammalian development[16,17]. In addition to contributing to metabolic diseases during pregnancy[18] and GDM[19], studies have also indicated that H19 regulates EGFR expression[20]. Furthermore, EIF4A3, which acts as an RNA helicase, can directly influence the structure of RNA and interact with proteins that bind RNA, playing a significant role in various gene expression regulatory processes driven by RNA[21]. Additionally, research has demonstrated that EIF4A3 interacts with the lncRNA H19 to increase tumor proliferation[22]. We hypothesized that the RNA helicases EIF4A3 and H19 are involved in stabilizing EGFR mRNA in GDM. Whether this regulatory axis is dysregulated in GDM and how it leads to EGFR overexpression in endothelial cells is also unclear. Consequently, this investigation examined the roles of EIF4A3 and lncRNAs, specifically how the interaction between H19 affects the progression of GDM through the regulation of EGFR mRNA expression.
Additionally, the protein known as ERBB receptor feedback inhibitor 1 (ERRFI1 or MIG6) functions within the cytoplasm and is crucial for cell signal transduction. It inhibits the activity of several RTKs, thus influencing biological processes such as cell proliferation, differentiation and survival. ERRFI1 can bind to the kinase domains of EGFR and ERBB2, locking them into a conformation that is catalytically inactive, which leads to the suppression of EGFR signal transduction[23]. In a mouse model of lung adenocarcinoma driven by EGFR, reducing ERRFI1 expression resulted in an extended activation period of EGFR signaling, facilitating tumor development[24]. Furthermore, ERRFI1 also plays a role in the progression of streptozotocin (STZ)-induced diabetes by negatively modulating the EGFR signaling pathway[25]. Consequently, this study aimed to explore how ERRFI1 influences the progression of GDM through the modulation of EGFR protein expression.
In summary, we aimed to comprehensively elucidate the molecular mechanism by which EGFR affects GDM-related endothelial cell dysfunction at the transcriptional and protein levels. Our study is the first to link the roles of H19, EIF4A3, ERRFI1 and EGFR in the context of GDM, filling the research gap in this field, providing a new perspective for understanding the molecular basis of GDM. Furthermore, this study also provides potential molecular targets for the diagnosis and treatment of GDM, which has important clinical translational value.
Sixty pregnant women who underwent prenatal examinations at the hospital from January 2021 to August 2022 were selected for this study. This group consisted of 30 individuals diagnosed with GDM (GDM group) and 30 normal pregnant women (normal group), with a mean age of 27.02 ± 3.17 years. Placental samples were collected within 15 minutes following delivery and preserved at -80 °C. The inclusion criteria for the GDM group required patients who had a confirmed diagnosis of GDM that met specific established guidelines[26]. Glucose screening is currently recommended at 24-28 weeks of gestation[27]; therefore, patients diagnosed during pregnancy were included. The exclusion criteria were individuals with preexisting diabetes, those who experienced other complications during pregnancy, those with multiple pregnancies, those with cancer or severe infectious disease, those who had previously used glucocorticoids, those with significant liver or kidney impairments, and those who opted not to take part in the study. Prior to any study procedures, informed consent was secured from all participants, along with approval from the Hospital’s Ethics Committee.
Forty female C57BL/6J mice, aged 8 weeks and weighing between 20 and 22 g, were obtained from Hunan SJA Laboratory Animal Co., Ltd. After one week of acclimatization, the mice were randomly sorted into four groups, each consisting of ten mice. Prior to the experiment, blood samples were drawn from the tail vein to assess fasting blood glucose levels, with normal values ranging from 3 to 5 mmol/L. The estrous cycle was evaluated using the vaginal smear technique. Mating occurred between male and female mice in the early estrus phase. Mating was confirmed the following morning through vaginal examination, marking the initial day of pregnancy. On the sixth day of pregnancy, the model group received an intraperitoneal injection of STZ (40 mg/kg) for five consecutive days to induce a standard diabetes model[28], whereas the control group received an intraperitoneal injection of normal saline. On the 7th day after the first injection, the GDM mouse model was established with fasting blood glucose ≥ 11.1 mmol/L. To explore the regulatory mechanisms of H19 and EIF4A34 in GDM progression, lentiviruses (1 × 108 UT/50 μL, GenePharma) overexpressing H19 (OE-H19) or knocking down EIF4A34 (sh-RNA) were injected into mice via the tail vein. The respective negative controls were OE-negative control and short hairpin-negative control, which were injected in the same manner. The mice were delivered by cesarean section on the 18.5th day of pregnancy, the placenta was collected, and the relevant indices were measured.
Human umbilical vein endothelial cells (HUVECs) were sourced from Shenzhen Otwo Biotech Co., Ltd. They were then grown in Dulbecco’s modified eagle medium (DMEM) enriched with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Gibco, 15070063) inside an incubator set to 37 °C with 5% carbon dioxide (CO2) for general maintenance. To create the GDM cell model, cultured HUVECs were treated with 25 mmol/L glucose for 24 hours[29].
HUVECs were placed in 24-well plates and incubated overnight. When the cell density reached approximately 60%-70%, H19 knockdown or overexpression [short hairpin RNA targeting H19 (sh-H19) or OE-H19], EIF4A3 knockdown or overexpression (si-EIF4A3 or OE-EIF4A3) and EGFR overexpression (OE-EGFR) vectors were transfected into HUVECs according to the instructions of the Lipofectamine 3000 reagent (Invitrogen, Grand Island, NY, United States). The cells were then cultured at 37 °C in a 5% CO2 incubator. After 48 hours of incubation, the expression of H19 was detected via quantitative real-time polymerase chain reaction (RT-qPCR), and the expression levels of EIF4A3 and EGFR were detected by Western blotting to confirm the transfection efficiency.
Total RNA was extracted from placental tissue and HUVECs using TRIzol reagent (Invitrogen, 15596026). A first-strand cDNA synthesis kit (Genenode, China) was used for the reverse transcription of RNA to produce cDNA. cDNA was subsequently quantified through real-time PCR using a SYBR Green fluorescence quantitative PCR kit (Solarbio, China); Glyceraldehyde-3-phosphate dehydrogenase served as the internal control. The outcomes were analyzed using the 2-ΔΔCt method. The primer sequences are detailed in Table 1.
| Genes | Sequence |
| LncRNA H19 (human) | F: 5’-TCAGGAATCGGCTCTGGAAGGT-3’ |
| R: 5’-ATGTGGTGGCTGGTGGTCAAC-3’ | |
| LncRNA H19 (mouse) | F: 5’-ACGGAGCAGTGATCGGTGTCT-3’ |
| R: 5’-ACCTGTCATCCTCGCCTTCAGT-3’ | |
| EGFR (human) | F: 5’-TGAAGGCTGTCCAACGAATG-3’ |
| R: 5’-GGGTGTAAGAGGCTCCACAAG-3’ | |
| EGFR (mouse) | F: 5’-AATGTTCCCATCGCTGTCGT-3’ |
| R: 5’-TTGCATGTGGCCTCATCTTG-3’ | |
| EIF4A3 (human) | F: 5’-AAGGGAGAGATGTCATCGCAC-3’ |
| R: 5’-GCTTGAGTTTCACGAACCTGA-3’ | |
| EIF4A3 (mouse) | F: 5’-AGGAGGACATGACCAAAGTGG-3’ |
| R: 5’-TGCTGAATCGCTGAAGGTTTTT-3’ | |
| GAPDH (human) | F: 5’-ACCCACTCCTCCACCTTTGA-3’ |
| R: 5’-ACCACCCTGTTGCTGTAGCC-3’ | |
| GAPDH (mouse) | F: 5’-AGAGTGTTTCCTCGTCCCGTAG-3’ |
| R: 5’-GACTGTGCCGTTGAATTTGC-3’ |
Proteins were extracted from placental tissue and HUVECs using radio immunoprecipitation assay buffer (Sigma-Aldrich, United States) containing 1% protease and phosphatase inhibitors. The protein concentration was measured based on the guidelines provided in the manual of a bicinconic acid (BCA) detection kit (Thermo Scientific, United States). The total protein was subsequently separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After separation, the proteins were transferred onto polyvinylidene fluoride membranes (Millipore, United States) and blocked with a 5% skim milk powder solution at room temperature for 1.5 hours. The membranes were incubated with diluted primary antibodies against EGFR (1:2000, ab25894, Abcam, United Kingdom), vascular cell adhesion molecule-1 (VCAM-1) (1:2000, ab134047, Abcam, United Kingdom), tumor necrosis factor-α (TNF-α) (1:1000, ab183218, Abcam, United Kingdom), vascular endothelial growth factor-A (VEGF-A) (1:1000, ab214424, Abcam, United Kingdom), intercellular cell adhesion molecule-1 (ICAM-1) (1:500, ab171123, Abcam, United Kingdom), EIF4A3 (1:2000, ab32485, Abcam, United Kingdom), and ERRFI1 (1:1000, ab227944, Abcam, United Kingdom) overnight at 4 °C. The membranes were then treated with secondary antibodies (1:4000, ab97051, Abcam, United Kingdom) at room temperature for 1 hour, and color was developed with an enhanced chemiluminescence kit (Millipore, United States). Finally, the bands were semiquantitatively analyzed by ImageJ software.
Paraffin-embedded placental tissue was deparaffinized and rehydrated, followed by antigen retrieval in 0.01 M citrate buffer (potential of hydrogen = 6.0). The slides were treated with anti-EGFR (1:200, ab25894, Abcam, United Kingdom) and anti- cluster of differentiation (CD) 31 (1:500, ab182981, Abcam, United Kingdom) antibodies and incubated at 4 °C overnight. The proteins were detected via a horseradish peroxidase-conjugated secondary antibody along with a diaminobenzidine chromogenic reagent.
HUVECs (5 × 103 cells/well) were plated in 96-well plates and cultured at 37 °C in a 5% CO2 environment for 24 hours. Each group of cells was treated on the basis of their specific group assignments. Following treatment, 10 μL from each well was combined with cell counting kit 8 (CCK-8) reagent. A microplate reader was used to measure the absorbance of each well at a wavelength of 450 nm.
Following treatment, HUVECs were collected, rinsed twice with phosphate-buffered saline (PBS), and resuspended in 200 μL of PBS. The rate of apoptosis was assessed using an Annexin-V-fluorescein isothiocyanate/propidium iodide (FITC/PI) apoptosis kit (Absin, China). According to the instructions provided by the manufacturer, 5 μL of Annexin V-FITC and 5 μL of PI were added to each well. The mixture was then incubated in the dark for 15 minutes, after which flow cytometry was used to evaluate cell apoptosis.
HUVECs in the logarithmic growth phase were chosen for the experiments. Following standard digestion and passage, 24-well plates were inoculated with the cells. Once the cell density reached 90%, a pipette tip was used to scratch the cell layer. After a 24-hour incubation period, cell migration was examined under a microscope, and images were captured.
The concentration of HUVECs in all groups was adjusted to 1 × 105 cells/mL using serum-free DMEM. To each upper chamber of the Transwell system, 200 μL of the cell suspension was added, whereas 600 μL of DMEM supplemented with 10% FBS was added to the lower chamber of the 24-well plate. After 24 hours of culture, the cells that had not migrated from the upper layer of the chamber were washed with PBS, and the chamber was then stained with 1% crystal violet (Solarbio, China). The cells in the fixed position in each well were counted under an inverted microscope (CKX53, OLYMPUS, Japan), and 3 fields of view were selected for counting and photography.
To a prechilled 96-well plate, 50 μL/well of Matrigel gel was added, and the plate was placed in a 37 °C incubator for 30 minutes to solidify the gel. The HUVECs were trypsinized, and 2 × 104 cells/well were seeded in 96-well plates coated with MatrigeI gel. Finally, the cells were incubated in a 5% CO2 incubator at 37 °C for 12 hours and then observed under an inverted microscope and photographed.
LncRNA H19 was constructed in vitro by GenePharma, and the biotinylated probe Bio-LncRNA H19 was then constructed with a biotin RNA labeling mixture. The biotinylated probe and Pierce™ streptavidin agarose beads were mixed and incubated overnight at 4 °C. The cell lysates and RNase inhibitors were then added. After 1 hour of incubation on ice, the eluted proteins were analyzed with Western blotting.
Once the cell density reached 90%, approximately 1 × 107 cells were harvested, resuspended in an equal volume of 1 × passive lysis buffer lysis buffer, gently aspirated, and placed on ice for 5 minutes. For the prepared protein A/G agarose beads, 5 μg of the specific antibody or control IgG antibody was added, followed by incubation at room temperature for one hour. A mixture consisting of 100 μL of lysis buffer and the supernatant was combined with 900 μL of protein A/G along with the relevant antibody mixture, and the mixture was incubated at 4 °C overnight. After being spun down at 4000 rpm for 1 minute at 4 °C, the supernatant was removed, 1 mL of NT-2 solution was added, and the mixture was thoroughly shaken. The supernatant was then removed by centrifugation, after which the protein A/G complex was resuspended in 150 μL of proteinase K buffer and incubated at 55 °C for 30 minutes. For RNA extraction, 1 mL of TRIzol was included, and an equivalent volume of RNA was used for reverse transcription and qPCR analysis.
Placental tissue from the mice was collected, sectioned, and deparaffinized. The samples were then stained with hematoxylin for 5 minutes, differentiated with 5% acetic acid for 1 minute, and subsequently treated with a blue returning solution for one to two minutes. The tissue was stained with an eosin solution for one minute, dehydrated in ethanol, and finally mounted with neutral gum for observation and analysis.
Each tissue sample from the placenta was rinsed twice with PBS. Then, 50 μL of terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-nick end labelling (TUNEL) detection solution (consisting of 2 μL of terminal deoxynucleotidyl transferase enzyme + 48 μL of fluorescein-labeled 2’-deoxyuridine 5’-triphosphate solution) (C1089, Beyotime, China) was carefully added to the sample in a dropwise manner, and the sample was coated with slides and incubated in the dark in a humidified chamber at 37 °C for 1 hour. Subsequently, the cells and tissue samples were rinsed with PBS 3 times, mounted with glycerol, and finally observed under a microscope and photographed.
The cells were harvested and lysed using IP lysis buffer, and the supernatant was extracted by centrifugation. Protein A/G Sepharose (Santa Cruz Biotechnology) was preincubated with an anti-ERRFI1 antibody (1:300, Novus Biologicals) for 60 minutes at 4 °C with slow shaking, followed by two washes. All IP beads were incubated overnight at 4 °C with slow shaking, centrifuged to collect the beads, and then washed three times with lysis buffer. The immunoprecipitates were analyzed via western blotting.
The cell cultures were treated with the cell-permeable proteasome inhibitor MG132 (Beyotime, China) at a concentration of 20 μM for 6 hours. Next, immunoprecipitation lysis buffer containing both protease and phosphatase inhibitors was added, and the mixture was incubated for 30 minutes. The resulting lysate was subjected to immunoprecipitation with an anti-ERRFI1 antibody (1:300, Novus Biologicals) and was placed on a rotary shaker overnight at 4 °C. Ubiquitination of ERRFI1 was assessed using an anti-ubiquitin antibody, and the immunoprecipitated proteins were quantified via western blot analysis.
HUVECs were treated with cycloheximide (50 μg/mL). Following treatment, proteins were extracted and measured using a BCA protein quantification kit (Thermo Scientific, Waltham, MA, United States). The proteins were further analyzed by western blotting.
Data were analyzed using GraphPad Prism version 8 software (GraphPad, United States). A minimum of three repe
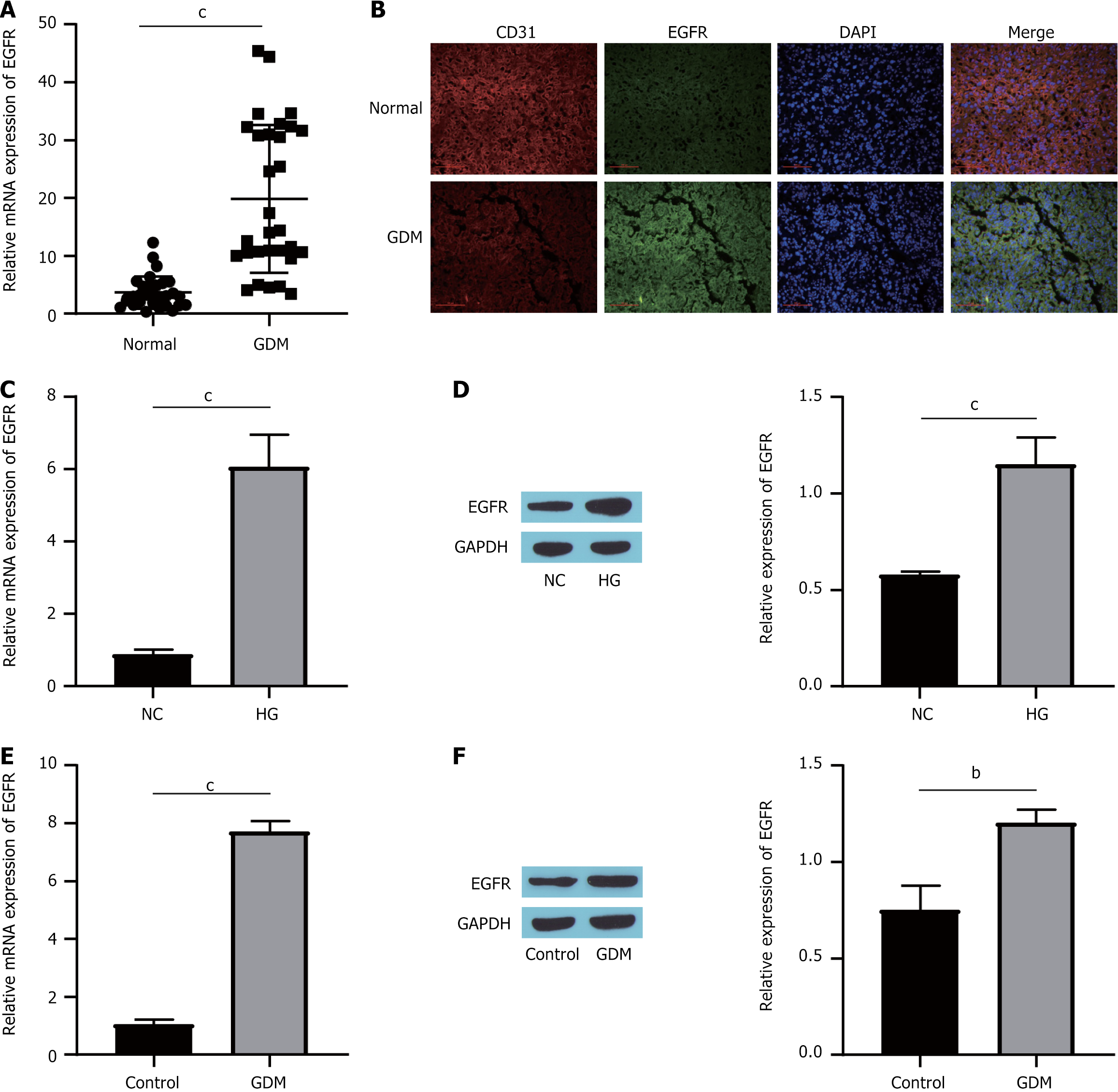
The initial step of the study involved assessing EGFR expression in patients with GDM. Analysis of clinical samples revealed a notable increase in EGFR levels within the placental tissues of GDM patients compared with those in the normal group (Figure 1A). Additionally, the findings demonstrated the colocalization of EGFR and CD31 (Figure 1B). This evidence suggested that EGFR expression was predominantly increased in endothelial cells. Similar findings were noted in high glucose (HG)-induced HUVECs and in STZ-induced GDM mice. Treatment with either HG or STZ significantly elevated the expression of both EGFR mRNA and protein (Figure 1C-F). Overall, these outcomes indicated that GDM patients presented markedly elevated EGFR levels.
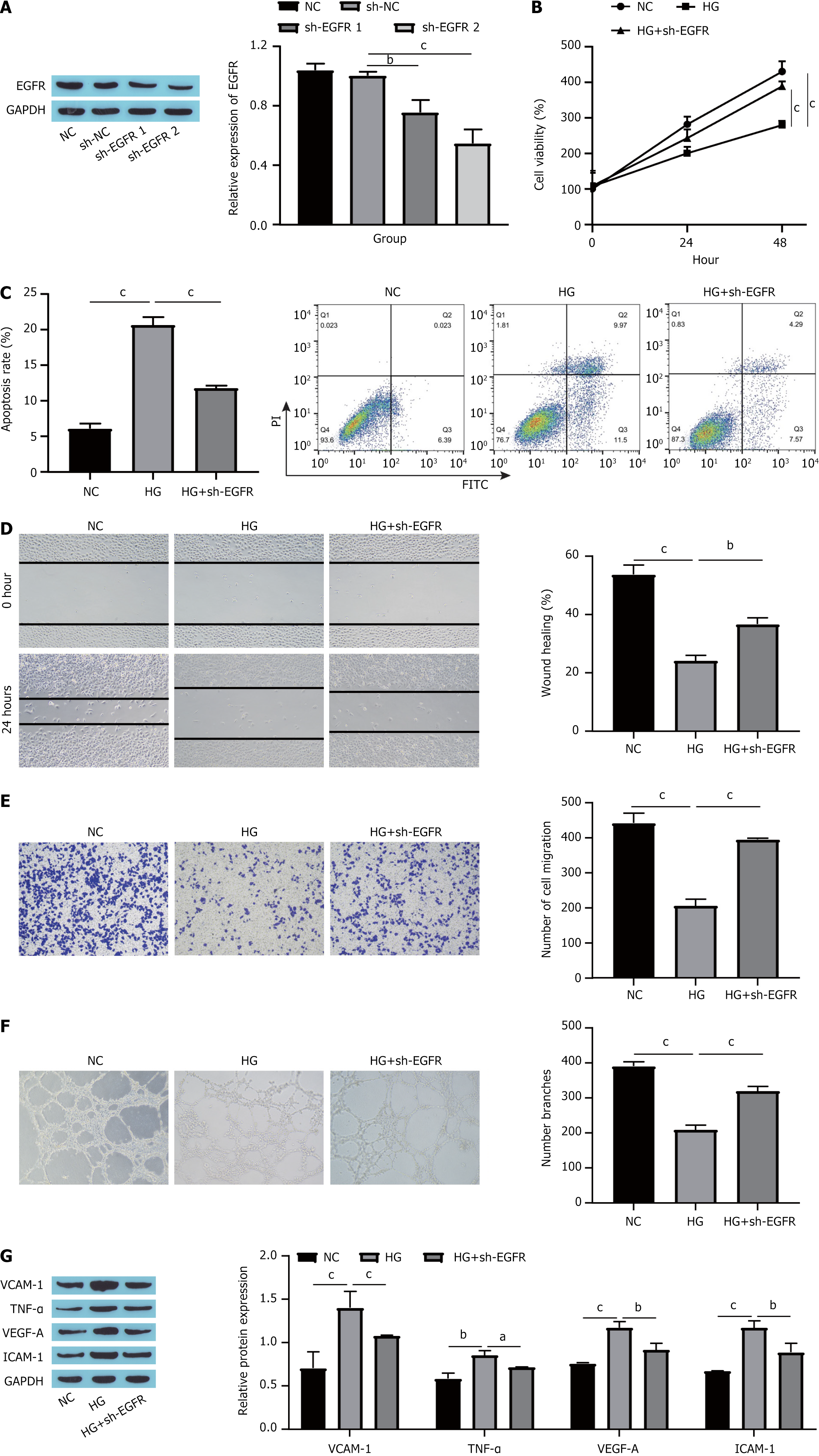
To investigate the influence of EGFR on HUVECs induced by HG, we initially knocked down EGFR in HUVECs, which resulted in a significant decrease in EGFR expression (Figure 2A). We subsequently assessed changes in cell proliferation, apoptosis and migration. Compared with the normal control (NC) group, HG treatment markedly reduced both the proliferation and migration capabilities of HUVECs while increasing their degree of apoptosis. Additionally, the knockdown of EGFR partially counteracted the effects of HG, facilitating the proliferation and migration of HUVECs while decreasing apoptosis (Figure 2B-E). Angiogenesis assays demonstrated that HG treatment reduced HUVECs angiogenesis; however, further silencing of EGFR increased angiogenesis in HUVECs (Figure 2F). Ultimately, we measured the expression levels of markers indicative of endothelial cell dysfunction. Compared with the NC group, the expression levels of VCAM-1, TNF-α, VEGF-A, and ICAM-1 were significantly increased in the HG group, and this effect was partially mitigated by additional EGFR knockdown (Figure 2G). Collectively, these findings suggest that EGFR knockdown can mitigate HG-induced dysfunction in HUVECs.
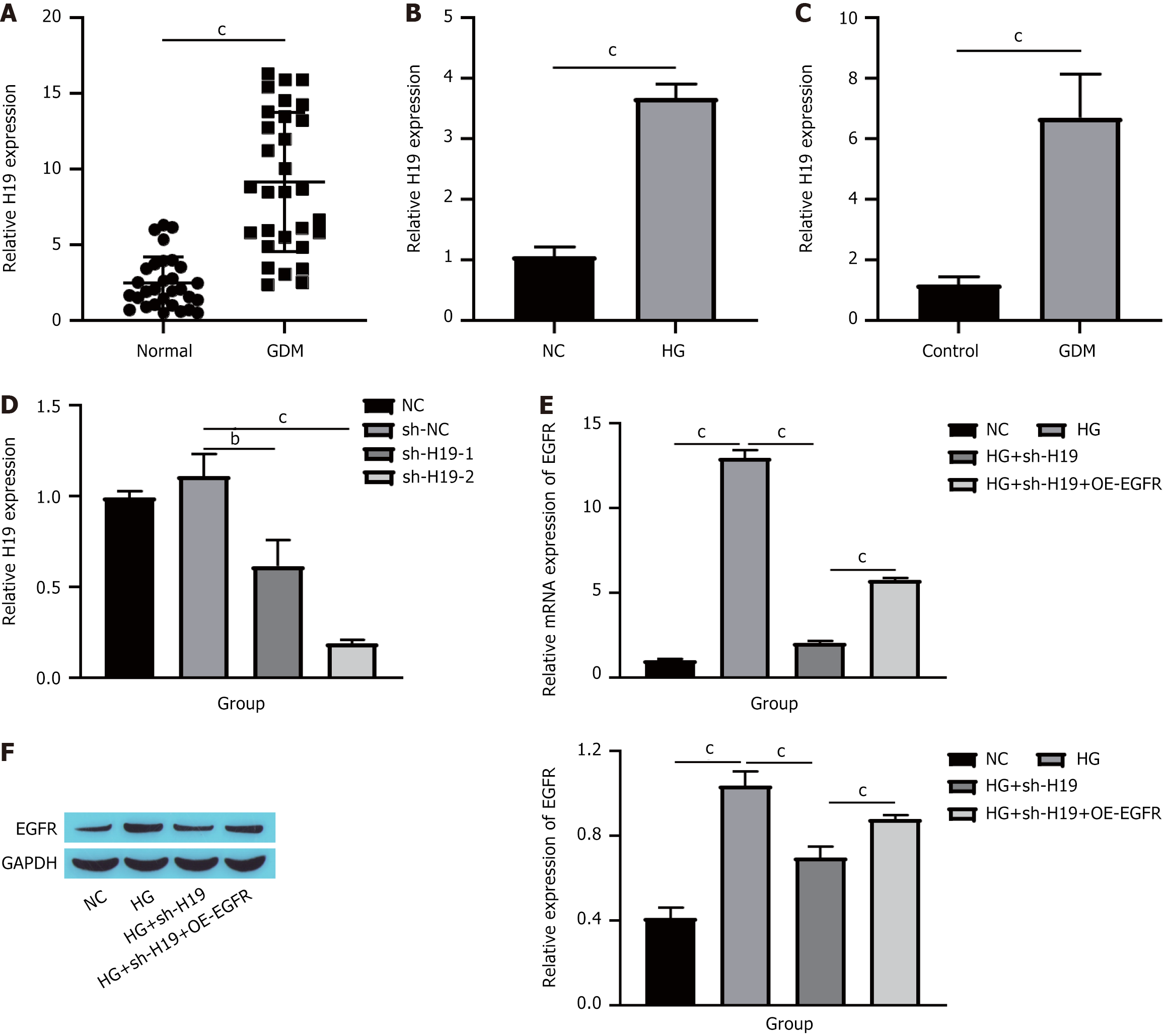
As previously noted, H19 not only has a critical function in the progression of GDM[19] but also influences the expression of EGFR[20]. Consequently, we investigated whether the impact of EGFR on HUVECs dysfunction is modulated by H19. Initially, we assessed H19 expression levels in patients with GDM. Compared with those in the normal group, H19 levels were elevated in the placental tissue of individuals with GDM (Figure 3A). A similar trend was observed in HUVECs stimulated with HG and in STZ-induced GDM mice (Figure 3B and C). We subsequently knocked down H19 in HUVECs, which led to a significant reduction in H19 expression (Figure 3D). Finally, we measured EGFR expression. Compared with the HG group, H19 knockdown markedly decreased both the EGFR mRNA and protein levels, whereas further overexpression of EGFR resulted in increases in the EGFR mRNA and protein levels (Figure 3E and F). These findings suggest that H19 is elevated in GDM and can increase EGFR expression.
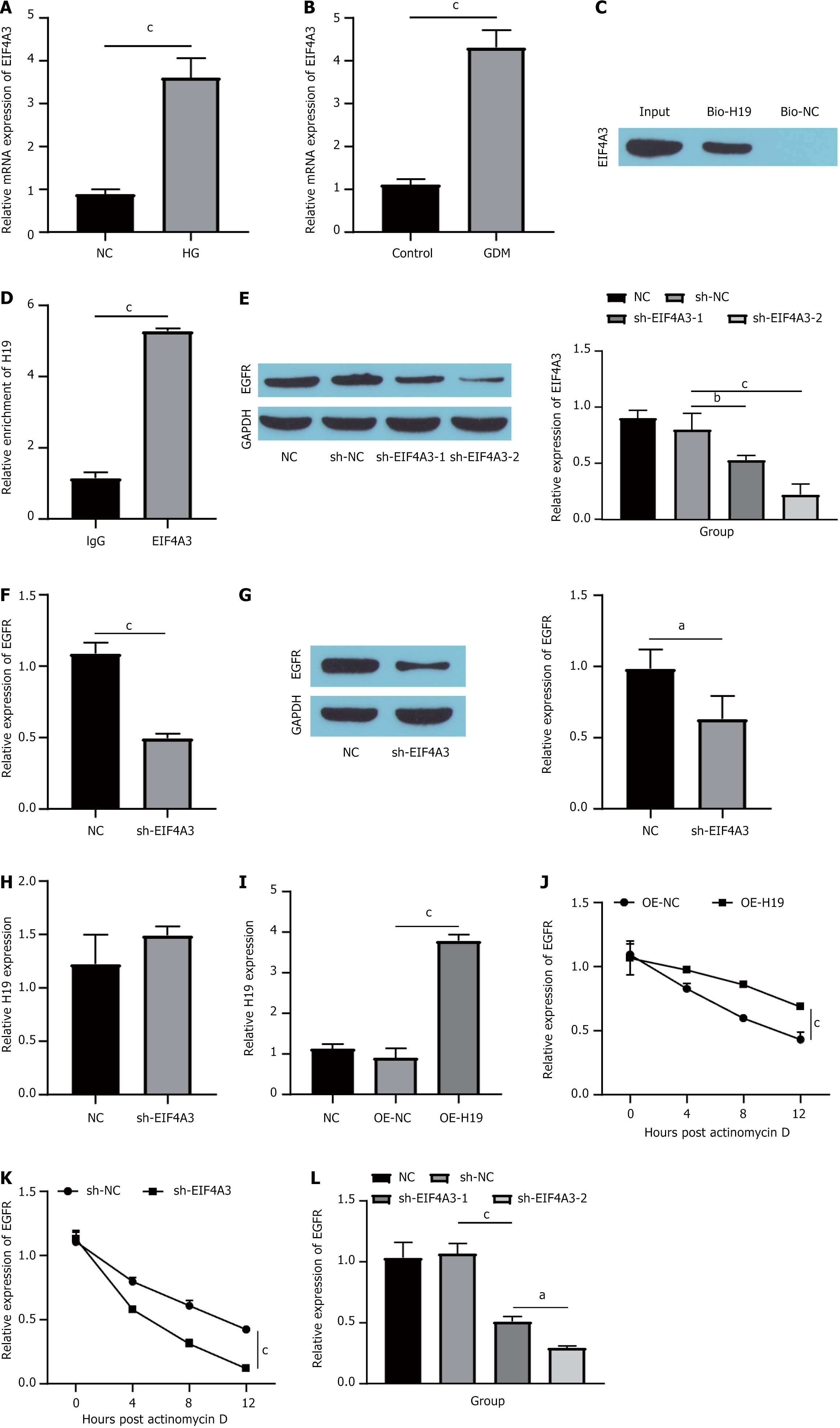
With respect to the mechanism by which H19 regulates EGFR, recent research has indicated that numerous lncRNAs play crucial roles in molecular regulatory pathways through their associations with various proteins[30]. This mechanism identified by Han et al[22], who demonstrated that H19 can influence the proliferation of colorectal cancer cells via its interaction with EIF4A3. In this study, we performed experiments to assess whether H19 regulates EGFR by binding to EIF4A3. First, we examined the expression of EIF4A3 in HG-induced HUVECs and STZ-induced GDM mouse placentas. The RT-qPCR results revealed that EIF4A3 expression was upregulated in both the HG and GDM groups (Figure 4A and B). Next, RNA pull-down and western blot analyses revealed significant enrichment of EIF4A3 within the H19 pull-down products (Figure 4C). Subsequent RNA immunoprecipitation (RIP) further confirmed the binding affinity between EIF4A3 and H19 (Figure 4D). In addition, we knocked down EIF4A3 in HUVECs and detected a significant decrease in the expression of EIF4A3, indicating that the knockdown was successful (Figure 4E). Notably, the depletion of EIF4A3 led to a marked decrease in both EGFR mRNA and protein expression in HUVECs (Figure 4F and G). Importantly, the reduction in EIF4A3 did not influence H19 expression levels in these cells (Figure 4H). Then, we assessed the extent of H19 overexpression (Figure 4I). The overexpression of H19 mitigated the impact of actinomycin D treatment on the stability of EGFR mRNA in HUVECs (Figure 4J). Conversely, the knockdown of EIF4A3 enhanced the effect of actinomycin D on EGFR mRNA stability in HUVECs (Figure 4K). Finally, we compared the effects of H19 knockdown alone (sh-H19) or in conjunction with EIF4A3 inhibition (sh-H19 + sh-EIF4A3) on EGFR mRNA expression. Compared with that in the NC group, the expression of EGFR mRNA in the sh-H19 group was downregulated, and simultaneous treatment with sh-H19 + sh-EIF4A3 further inhibited the expression of EGFR mRNA (Figure 4L). These findings suggest that H19 plays a role in increasing the stability of EGFR mRNA by binding to EIF4A3.
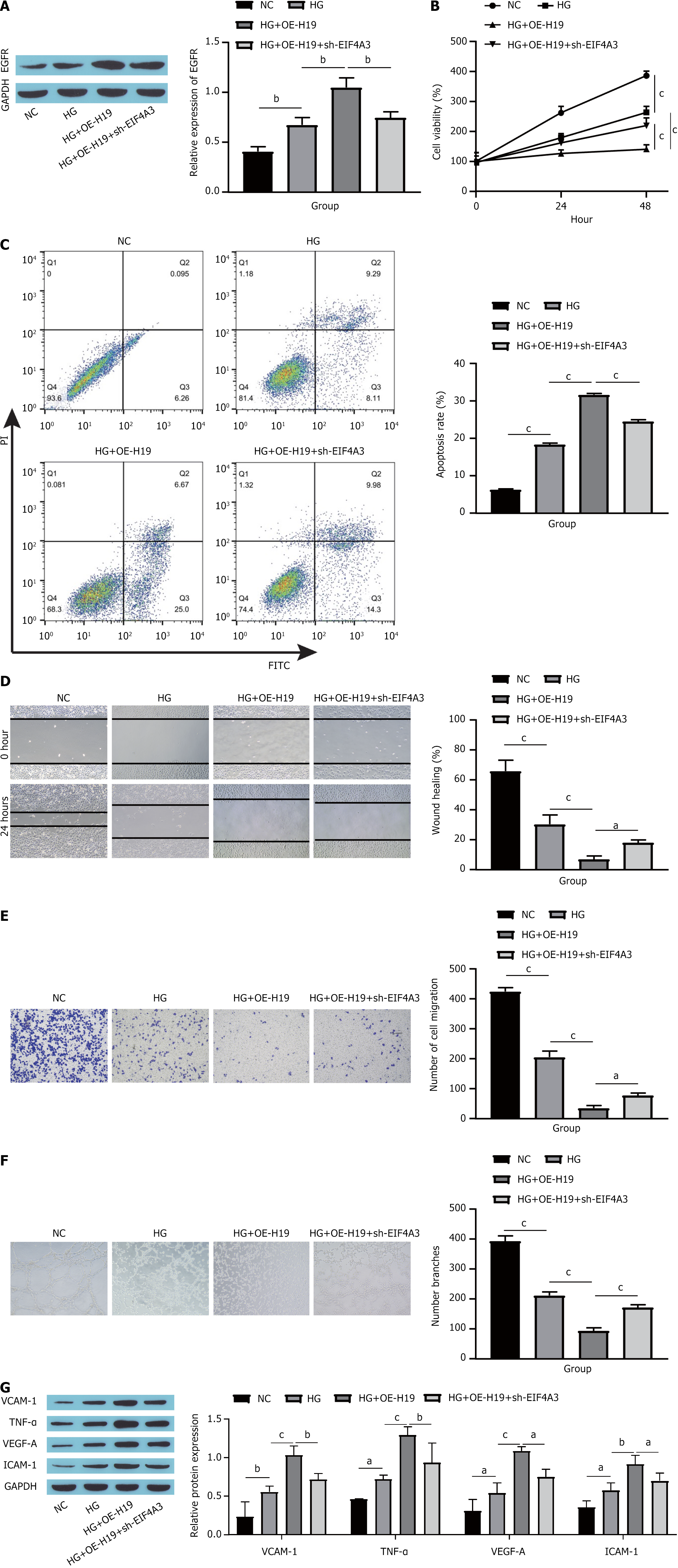
The impact of the EIF4A3/H19/EGFR pathway on HUVECs subjected to HG conditions was investigated. Initially, EGFR expression was assessed. Compared with HG conditions, H19 overexpression increased EGFR levels, whereas EIF4A3 silencing led to a reduction in EGFR expression (Figure 5A). Next, cell proliferation, apoptosis and migration were measured. Compared with the HG group, H19 overexpression inhibited the proliferation and migration of HUVECs and promoted apoptosis, whereas EIF4A3 knockdown partially reversed the effects of H19 overexpression, i.e., promoted the proliferation and migration of HUVECs and inhibited apoptosis (Figure 5B-E). Angiogenesis assays indicated that H19 overexpression suppressed angiogenesis in HUVECs, whereas further silencing of EIF4A3 increased angiogenesis in these cells (Figure 5F). Finally, markers indicative of endothelial cell dysfunction were evaluated. Compared with the HG group, H19 overexpression was associated with increases in VCAM-1, TNF-α, VEGF-A, and ICAM-1; meanwhile, EIF4A3 was knocked down, which partially reversed the influence of H19 overexpression (Figure 5G). These results indicated that EIF4A3 binding to H19 inhibited the proliferation, migration and vascularization of HUVECs by promoting the expression of EGFR and promoted apoptosis and dysfunction.
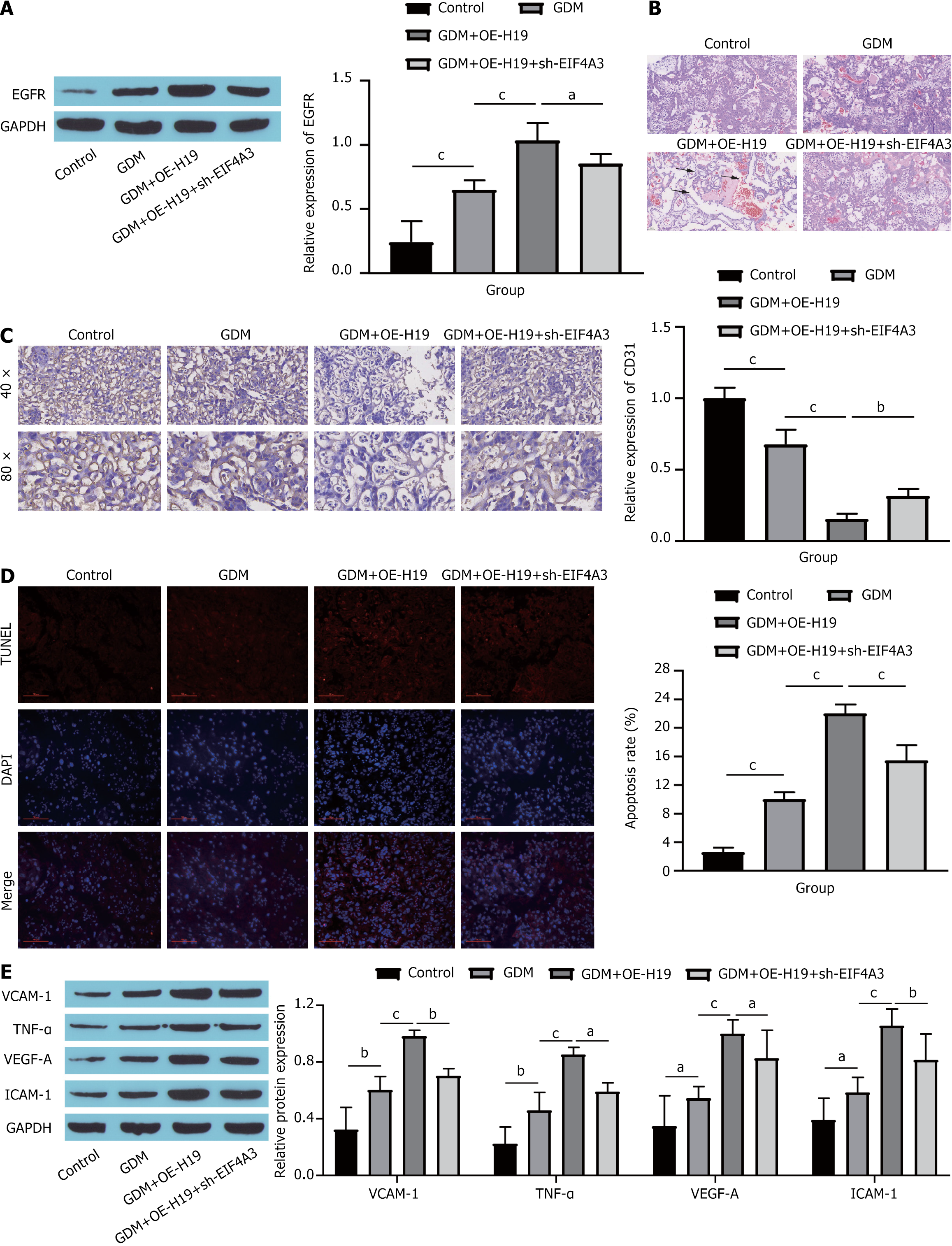
Subsequently, the impact of the EIF4A3/H19/EGFR axis on the endothelial cells of GDM mice was verified in vivo. Consistent with the findings of in vitro studies, H19 overexpression markedly increased EGFR expression, whereas further silencing of EIF4A3 resulted in a decrease in EGFR levels (Figure 6A). Compared with those of the GDM group, the tissue stratification boundaries in the placental tissue of the GDM + OE-H19 group were not obvious, the cell distribution was loose and disordered, the intercellular space was enlarged, the capillary distribution was reduced, and the cell structure and morphology of the placental tissue were restored to a certain extent after further knockdown of EIF4A3 (Figure 6B). Immunohistochemical analysis revealed a significant reduction in the VEC-specific marker CD31 in GDM mice; moreover, H19 overexpression further decreased CD31 levels, whereas EIF4A3 knockdown somewhat increased CD31 expression (Figure 6C). Assessment of apoptosis via TUNEL staining revealed a marked increase in the apoptosis rate among GDM mice. In addition, H19 overexpression further exacerbated cell apoptosis, whereas EIF4A3 silencing resulted in a decrease in the apoptosis rate (Figure 6D). Finally, the expression of endothelial cell dysfunction markers was detected. The overexpression of H19 significantly promoted the expression of VCAM-1, TNF-α, VEGF-A and ICAM-1, whereas further knockdown of EIF4A3 reversed the effect of H19 overexpression to a certain extent (Figure 6E). Collectively, these findings suggest that the binding of EIF4A3 to H19 contributes to endothelial cell dysfunction in GDM mice by increasing EGFR expression.
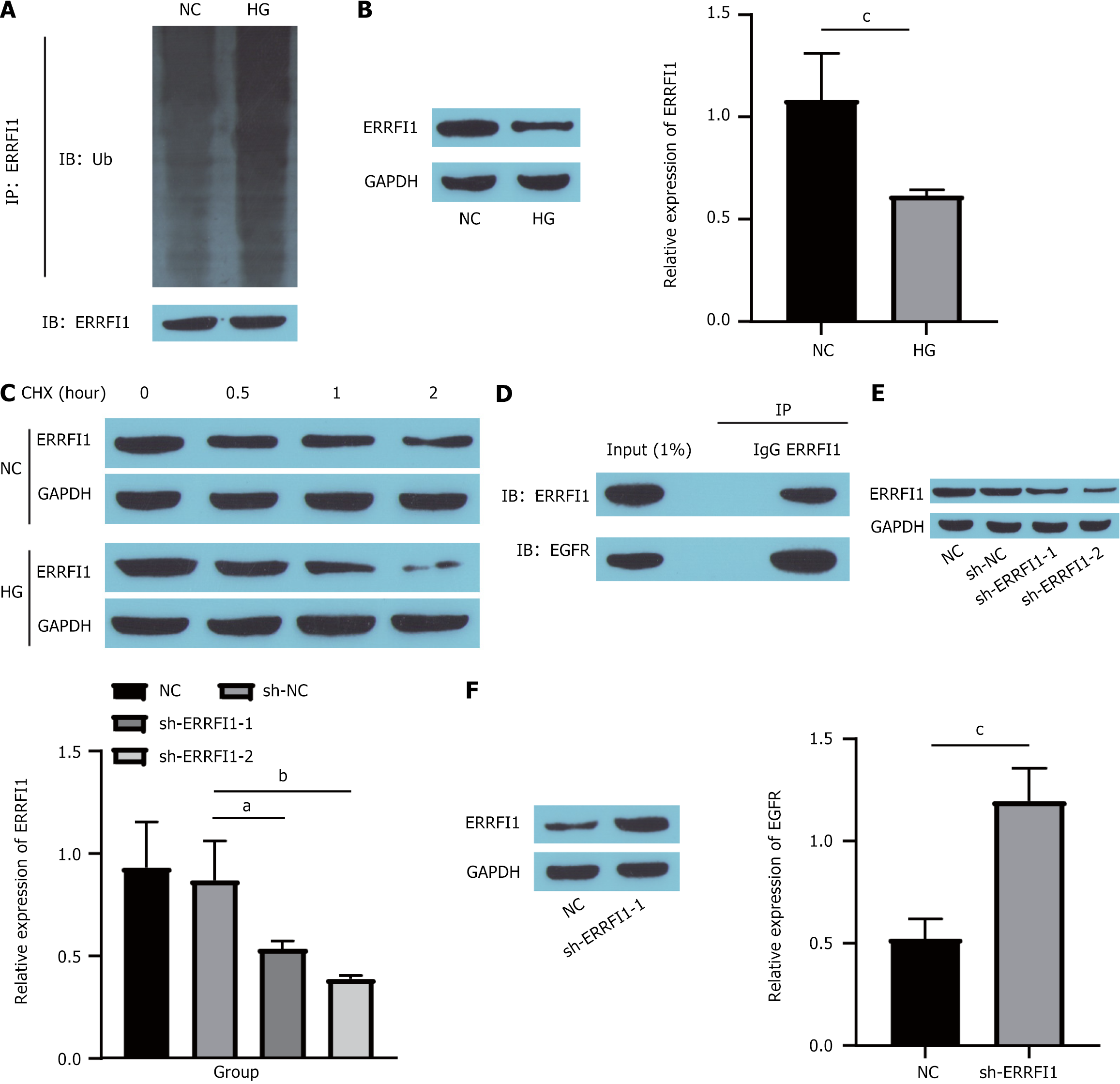
Moreover, we conducted a literature review, which indicated that ERRFI1 plays a role in regulating EGFR expression[31] and impacts diabetes progression[25]. Consequently, we investigated the influence of ERRFI1 on EGFR. First, we assessed the effect of HG on ERRFI1. Compared with the NC group, the ubiquitination of ERRFI1 (Figure 7A) was markedly increased in the HC group, which reduced its expression (Figure 7B). Next, the cells were exposed to cycloheximide (a protein synthesis inhibitor) to evaluate ERRFI1 expression. These findings demonstrated that the degradation rate of the ERRFI1 protein was significantly greater in the HG-treated group than in the NC group (Figure 7C), indicating that HG treatment notably undermined the stability of the ERRFI1 protein. Furthermore, coimmunoprecipitation studies revealed an interaction between ERRFI1 and EGFR (Figure 7D). Finally, we knocked down ERRFI1 in HUVECs (Figure 7E), which effectively increased EGFR expression (Figure 7F). Collectively, these findings suggest that high-glucose conditions can increase EGFR expression by inhibiting ERRFI1.
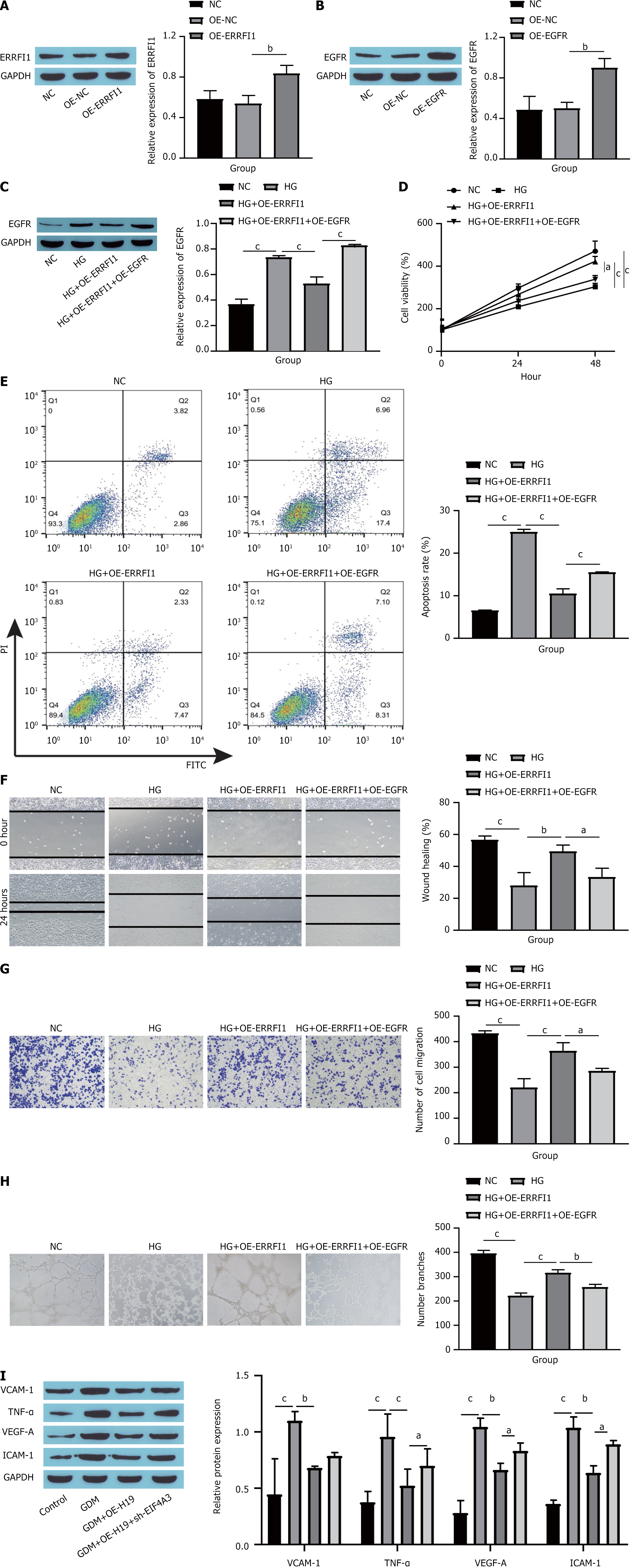
Ultimately, the influence of the ERRFI1/EGFR axis on HG-induced dysfunction in HUVECs was confirmed. First, we measured the overexpression efficiency of both ERRFI1 and EGFR. Compared with the NC group, the OE-ERRFI1 group presented a notable increase in ERRFI1 expression (Figure 8A), whereas the OE-EGFR group presented a significant increase in EGFR levels (Figure 8B). After OE-ERRFI1 and OE-EGFR were transfected into cells, the expression of EGFR in the cells was detected by Western blotting. The results revealed that transfection of OE-ERRFI1 significantly inhibited EGFR protein expression, and further transfection of OE-EGFR reversed the inhibitory effect of OE-ERRFI1 on EGFR protein expression (Figure 8C). Cell viability assessment via the CCK-8 assay demonstrated that ERRFI1 overexpression effectively increased the proliferation of HUVECs, but the addition of EGFR hindered this proliferation (Figure 8D). Flow cytometry revealed that, compared with HG treatment, ERRFI1 overexpression inhibited the apoptosis of HUVECs, and further overexpression of EGFR promoted the apoptosis of HUVECs (Figure 8E). The findings from the scratch assays and Transwell experiments indicated that ERRFI1 overexpression facilitated HUVECs migration, whereas further EGFR overexpression impeded this migration (Figure 8F and G). Angiogenesis assays revealed that ERRFI1 overexpression promoted angiogenesis in HUVECs, whereas subsequent EGFR overexpression inhibited this process (Figure 8H). Furthermore, Western blot analyses revealed that increased ERRFI1 expression substantially reduced the levels of endothelial cell dysfunction markers, including VCAM-1, TNF-α, VEGF-A, and ICAM-1, with additional EGFR overexpression partially negating the impacts of ERRFI1 overexpression (Figure 8I). These results indicate that ERRFI1 binds to EGFR to promote HUVECs proliferation, migration and vascularization and inhibits apoptosis and dysfunction by inhibiting EGFR expression.
Our research revealed that EGFR acts as a novel factor connecting endothelial cell impairment with the onset of GDM. Initially, we reported that EGFR expression was significantly elevated in human clinical samples of GDM, as well as in both the STZ-induced GDM mouse model and the HG-induced cellular model. Furthermore, under HG conditions, reducing EGFR levels effectively mitigated HG-induced dysfunction in HUVECs. Moreover, we discovered that the expression of EGFR was modulated by the combined influence of EIF4A3/H19 and ERRFI1. EIF4A3 binding to H19 enhances the stability of EGFR mRNA, and ERRFI1 binding to EGFR inhibits EGFR activity.
The endothelium consists of a vast layer of cells that line the inner surfaces of blood vessels, serving as the boundary between the blood and surrounding tissues[32]. It is involved in numerous biological processes, such as regulating vasomotor tone, maintaining hemostatic equilibrium, facilitating cell movement, controlling permeability, and influencing cell growth, survival and immune responses[33]. During pregnancy, certain endocrine factors need to be modified to accommodate unique physiological conditions; consequently, various systems, including the cardiovascular system, undergo alterations[34,35]. Nevertheless, these adaptations can trigger metabolic changes that directly impact endothelial function and metabolism[34,35]. Endothelial cell dysfunction is recognized as a central factor contributing to metabolic disorders during pregnancy and has been pinpointed as a primary feature of GDM[36]. Our investigation revealed that the expression levels of markers indicative of endothelial dysfunction, such as VCAM-1, TNF-α, VEGF-A, and ICAM-1, significantly increased throughout the progression of GDM. This observation aligns with earlier research findings, suggesting that endothelial impairment may contribute to the onset of GDM. Furthermore, Sun et al[37] noted that HG exposure suppressed migration and angiogenesis in HUVECs, whereas increased HUVECs migration and angiogenesis mitigated GDM progression. Additionally, Lei et al[38] reported that HG exposure inhibited the proliferation and invasion of HUVECs while promoting their apoptosis and oxidative stress. Our findings bolster prior research findings, indicating that a series of alterations in HUVECs could be major factors driving the progression of GDM.
EGFR is a membrane-associated protein that is crucial for cell growth, proliferation and differentiation. Abnormal EGFR signaling pathways involving EGFR are linked to the development and progression of various types of cancers and metabolic disorders[39]. Duquenne et al[40] reported that the leptin receptor-EGFR complex facilitates the entry of leptin into the hypothalamus, consequently decreasing food consumption and lipogenesis, which are vital for understanding diabetes pathophysiology. Qiu et al[41] reported that baicalin positively influences GDM partially by regulating EGFR expression. In addition, Alur et al[42] similarly noted a relationship between the EGFR gene and the onset of GDM. Thus, EGFR represents a significant target for therapeutic intervention in GDM. In the present study, we observed the upregulation of EGFR in GDM patients, and the silencing of EGFR expression appeared to mitigate HG-induced dysfunction in HUVECs. While our study demonstrates specific EGFR knockdown effects, we acknowledge potential off-target consequences of small interfering RNA (siRNA)-mediated silencing. The scrambled siRNA control (Figure 2A) helped minimize nonspecific effects. However, compensatory activation of alternative pathways might occur during prolonged EGFR inhibition. Therefore, future studies should employ pharmacological inhibitors combined with genetic approaches to evaluate such adaptive responses in GDM contexts. Our findings revealed that increased EGFR expression accelerated the progression of GDM in C57BL/6J mice induced by STZ. As an effective inducer of GDM, STZ injection served as a reliable and feasible model for our research. This study suggests that EGFR may serve as a novel biomarker and the
The mechanisms that regulate EGFR expression are also crucial for the treatment of GDM. LncRNAs, a category of noncoding RNAs, are essential for normal physiological functions and controlling pathological changes. The significant role of these genes in the progression of GDM has garnered increased attention[43]. For example, Li et al[44] reported that the expression of the lncRNA RPL13p5 exacerbated insulin resistance in patients with GDM, thus advancing the condition. Additionally, Ye et al[45] reported that the lncRNA MEG3 facilitates apoptosis in HUVECs while suppressing their proliferation, migration and angiogenesis via the protein kinase B pathway, ultimately impairing fetal endothelial function affected by GDM. H19 has been investigated in relation to metabolic disorders in pregnant women[18] and is known to regulate EGFR expression[20]. However, the role of H19 in GDM is poorly understood. Our findings indicate that H19 expression is elevated in GDM and that reducing H19 levels leads to decreased EGFR expression. Furthermore, numerous studies have indicated that lncRNAs can engage in molecular regulatory networks through protein in
Furthermore, with respect to the mechanism regulating EGFR expression, a literature search indicated that ERRFI1, an early response gene, inhibits EGFR activation through its ERBB-binding domain and EGFR kinase domain[47]. More specifically, ERRFI1 attaches to the kinase domain of EGFR, preventing the assembly of an asymmetric kinase dimer and thus stabilizing the EGFR kinase in a state that is catalytically inactive[47]. ERRFI1 has also been found to be associated with the developmental programming of systemic metabolic syndrome[48]. Because ERRFI1 is involved in EGFR family signal transduction, we verified the mechanism of action of the ERRFI1/EGFR axis in GDM. Our findings revealed that hyperglycemia increased EGFR expression through the ubiquitination of ERRFI1, subsequently leading to dysfunction in HUVECs. With respect to regulatory mechanisms, our results largely aligned with those of previous studies, but crucially, we adapted our research to GDM to clarify how protein regulation contributes to the progression of GDM. We found that ERRFI1 affects the activity of the EGFR signaling pathway by regulating the protein expression of EGFR. This result reveals the regulatory mechanism of EGFR at the protein level and further contributes to our understanding of EGFR function.
In summary, our research indicated that inhibiting EGFR might hinder the progression of GDM by safeguarding en
| 1. | Alejandro EU, Mamerto TP, Chung G, Villavieja A, Gaus NL, Morgan E, Pineda-Cortel MRB. Gestational Diabetes Mellitus: A Harbinger of the Vicious Cycle of Diabetes. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 169] [Article Influence: 33.8] [Reference Citation Analysis (1)] |
| 2. | ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol. 2018;131:e49-e64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 1152] [Article Influence: 164.6] [Reference Citation Analysis (0)] |
| 3. | Subiabre M, Villalobos-Labra R, Silva L, Fuentes G, Toledo F, Sobrevia L. Role of insulin, adenosine, and adipokine receptors in the foetoplacental vascular dysfunction in gestational diabetes mellitus. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | He Y, Wu N. Research Progress on Gestational Diabetes Mellitus and Endothelial Dysfunction Markers. Diabetes Metab Syndr Obes. 2021;14:983-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Zheng Y, Zhu N, Wang J, Zhao N, Yuan C. Crocetin suppresses gestational diabetes in streptozotocin-induced diabetes mellitus rats via suppression of inflammatory reaction. J Food Biochem. 2021;45:e13857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Li J, Song L, Zhou L, Wu J, Sheng C, Chen H, Liu Y, Gao S, Huang W. A MicroRNA Signature in Gestational Diabetes Mellitus Associated with Risk of Macrosomia. Cell Physiol Biochem. 2015;37:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Singh D, Attri BK, Gill RK, Bariwal J. Review on EGFR Inhibitors: Critical Updates. Mini Rev Med Chem. 2016;16:1134-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 8. | Sabbah DA, Hajjo R, Sweidan K. Review on Epidermal Growth Factor Receptor (EGFR) Structure, Signaling Pathways, Interactions, and Recent Updates of EGFR Inhibitors. Curr Top Med Chem. 2020;20:815-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 313] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 9. | Reda M, Ngamcherdtrakul W, Gu S, Bejan DS, Siriwon N, Gray JW, Yantasee W. PLK1 and EGFR targeted nanoparticle as a radiation sensitizer for non-small cell lung cancer. Cancer Lett. 2019;467:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | De Pauw I, Lardon F, Van den Bossche J, Baysal H, Pauwels P, Peeters M, Vermorken JB, Wouters A. Overcoming Intrinsic and Acquired Cetuximab Resistance in RAS Wild-Type Colorectal Cancer: An In Vitro Study on the Expression of HER Receptors and the Potential of Afatinib. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Grissa O, Yessoufou A, Mrisak I, Hichami A, Amoussou-Guenou D, Grissa A, Djrolo F, Moutairou K, Miled A, Khairi H, Zaouali M, Bougmiza I, Zbidi A, Tabka Z, Khan NA. Growth factor concentrations and their placental mRNA expression are modulated in gestational diabetes mellitus: possible interactions with macrosomia. BMC Pregnancy Childbirth. 2010;10:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Clemente L, Boeldt DS, Grummer MA, Morita M, Morgan TK, Wiepz GJ, Bertics PJ, Bird IM. Adenoviral transduction of EGFR into pregnancy-adapted uterine artery endothelial cells remaps growth factor induction of endothelial dysfunction. Mol Cell Endocrinol. 2020;499:110590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Shi M, Sun L, Wei J, Shen Y, Wang J, Zhang P, Yang X, Ding Y, Yin W, Lu X, Yang X, Wang G, Li R. Quercetin alleviates endothelial dysfunction in preeclampsia by inhibiting ferroptosis and inflammation through EGFR binding. Commun Biol. 2025;8:90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Bridges MC, Daulagala AC, Kourtidis A. LNCcation: lncRNA localization and function. J Cell Biol. 2021;220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 528] [Cited by in RCA: 976] [Article Influence: 244.0] [Reference Citation Analysis (0)] |
| 15. | Wang Q, Lu X, Li C, Zhang W, Lv Y, Wang L, Wu L, Meng L, Fan Y, Ding H, Long W, Lv M. Down-regulated long non-coding RNA PVT1 contributes to gestational diabetes mellitus and preeclampsia via regulation of human trophoblast cells. Biomed Pharmacother. 2019;120:109501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Steinhoff C, Paulsen M, Kielbasa S, Walter J, Vingron M. Expression profile and transcription factor binding site exploration of imprinted genes in human and mouse. BMC Genomics. 2009;10:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Engemann S, Strödicke M, Paulsen M, Franck O, Reinhardt R, Lane N, Reik W, Walter J. Sequence and functional comparison in the Beckwith-Wiedemann region: implications for a novel imprinting centre and extended imprinting. Hum Mol Genet. 2000;9:2691-2706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Zeng Y, Wu Y, Zhang Q, Xiao X. Non-coding RNAs: The link between maternal malnutrition and offspring metabolism. Front Nutr. 2022;9:1022784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Ding GL, Wang FF, Shu J, Tian S, Jiang Y, Zhang D, Wang N, Luo Q, Zhang Y, Jin F, Leung PC, Sheng JZ, Huang HF. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia. Diabetes. 2012;61:1133-1142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 20. | Wang L, Sun Y, Yi J, Wang X, Liang J, Pan Z, Li L, Jiang G. Targeting H19 by lentivirus-mediated RNA interference increases A549 cell migration and invasion. Exp Lung Res. 2016;42:346-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Sakellariou D, Frankel LB. EIF4A3: a gatekeeper of autophagy. Autophagy. 2021;17:4504-4505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Han D, Gao X, Wang M, Qiao Y, Xu Y, Yang J, Dong N, He J, Sun Q, Lv G, Xu C, Tao J, Ma N. Long noncoding RNA H19 indicates a poor prognosis of colorectal cancer and promotes tumor growth by recruiting and binding to eIF4A3. Oncotarget. 2016;7:22159-22173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 167] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 23. | Frosi Y, Anastasi S, Ballarò C, Varsano G, Castellani L, Maspero E, Polo S, Alemà S, Segatto O. A two-tiered mechanism of EGFR inhibition by RALT/MIG6 via kinase suppression and receptor degradation. J Cell Biol. 2010;189:557-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 24. | Maity TK, Venugopalan A, Linnoila I, Cultraro CM, Giannakou A, Nemati R, Zhang X, Webster JD, Ritt D, Ghosal S, Hoschuetzky H, Simpson RM, Biswas R, Politi K, Morrison DK, Varmus HE, Guha U. Loss of MIG6 Accelerates Initiation and Progression of Mutant Epidermal Growth Factor Receptor-Driven Lung Adenocarcinoma. Cancer Discov. 2015;5:534-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Chen YC, Colvin ES, Griffin KE, Maier BF, Fueger PT. Mig6 haploinsufficiency protects mice against streptozotocin-induced diabetes. Diabetologia. 2014;57:2066-2075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | International Association of Diabetes and Pregnancy Study Groups Consensus Panel; Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva Ad, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2777] [Cited by in RCA: 3175] [Article Influence: 211.7] [Reference Citation Analysis (1)] |
| 27. | Demir E, Ozkan H, Seckin KD, Sahtiyancı B, Demir B, Tabak O, Kumbasar A, Uzun H. Plasma Zonulin Levels as a Non-Invasive Biomarker of Intestinal Permeability in Women with Gestational Diabetes Mellitus. Biomolecules. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Li T, Wang W, Li S, Gong C. Lusianthridin Exerts Streptozotocin-Induced Gestational Diabetes Mellitus in Female Rats via Alteration of TLR4/MyD88/NF-κB Signaling Pathway. J Oleo Sci. 2023;72:775-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Sáez T, de Vos P, Kuipers J, Sobrevia L, Faas MM. Fetoplacental endothelial exosomes modulate high d-glucose-induced endothelial dysfunction. Placenta. 2018;66:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 30. | Yang F, Huo XS, Yuan SX, Zhang L, Zhou WP, Wang F, Sun SH. Repression of the long noncoding RNA-LET by histone deacetylase 3 contributes to hypoxia-mediated metastasis. Mol Cell. 2013;49:1083-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 415] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 31. | Cairns J, Fridley BL, Jenkins GD, Zhuang Y, Yu J, Wang L. Differential roles of ERRFI1 in EGFR and AKT pathway regulation affect cancer proliferation. EMBO Rep. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Reglero-Real N, Colom B, Bodkin JV, Nourshargh S. Endothelial Cell Junctional Adhesion Molecules: Role and Regulation of Expression in Inflammation. Arterioscler Thromb Vasc Biol. 2016;36:2048-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 33. | Kornacki J, Gutaj P, Kalantarova A, Sibiak R, Jankowski M, Wender-Ozegowska E. Endothelial Dysfunction in Pregnancy Complications. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Henriques AC, Carvalho FH, Feitosa HN, Macena RH, Mota RM, Alencar JC. Endothelial dysfunction after pregnancy-induced hypertension. Int J Gynaecol Obstet. 2014;124:230-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Sobrevia L, Abarzúa F, Nien JK, Salomón C, Westermeier F, Puebla C, Cifuentes F, Guzmán-Gutiérrez E, Leiva A, Casanello P. Review: Differential placental macrovascular and microvascular endothelial dysfunction in gestational diabetes. Placenta. 2011;32 Suppl 2:S159-S164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Echeverria C, Eltit F, Santibanez JF, Gatica S, Cabello-Verrugio C, Simon F. Endothelial dysfunction in pregnancy metabolic disorders. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Sun CC, Lai YN, Wang WH, Xu XM, Li XQ, Wang H, Zheng JY, Zheng JQ. Metformin Ameliorates Gestational Diabetes Mellitus-Induced Endothelial Dysfunction via Downregulation of p65 and Upregulation of Nrf2. Front Pharmacol. 2020;11:575390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Lei T, Gao Y, Duan Y, Cui C, Zhang L, Si M. Panax notoginseng saponins improves healing of high glucose-induced wound through the GSK-3β/β-catenin pathway. Environ Toxicol. 2022;37:1867-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Mimeault M, Batra SK. Recent progress on normal and malignant pancreatic stem/progenitor cell research: therapeutic implications for the treatment of type 1 or 2 diabetes mellitus and aggressive pancreatic cancer. Gut. 2008;57:1456-1468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Duquenne M, Folgueira C, Bourouh C, Millet M, Silva A, Clasadonte J, Imbernon M, Fernandois D, Martinez-Corral I, Kusumakshi S, Caron E, Rasika S, Deliglia E, Jouy N, Oishi A, Mazzone M, Trinquet E, Tavernier J, Kim YB, Ory S, Jockers R, Schwaninger M, Boehm U, Nogueiras R, Annicotte JS, Gasman S, Dam J, Prévot V. Leptin brain entry via a tanycytic LepR-EGFR shuttle controls lipid metabolism and pancreas function. Nat Metab. 2021;3:1071-1090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 41. | Qiu S, Wu X, Wu Q, Jin X, Li H, Roy R. Pharmacological Action of Baicalin on Gestational Diabetes Mellitus in Pregnant Animals Induced by Streptozotocin via AGE-RAGE Signaling Pathway. Appl Biochem Biotechnol. 2024;196:1636-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 42. | Alur V, Raju V, Vastrad B, Tengli A, Vastrad C, Kotturshetti S. Integrated bioinformatics analysis reveals novel key biomarkers and potential candidate small molecule drugs in gestational diabetes mellitus. Biosci Rep. 2021;41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Filardi T, Catanzaro G, Mardente S, Zicari A, Santangelo C, Lenzi A, Morano S, Ferretti E. Non-Coding RNA: Role in Gestational Diabetes Pathophysiology and Complications. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 44. | Li Y, Cheng X, Li D. LncRNA RPL13p5 gene expression promotes insulin resistance in patients with gestational diabetes. Ann Palliat Med. 2021;10:11024-11034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Ye HH, Yang SH, Zhang Y. MEG3 damages fetal endothelial function induced by gestational diabetes mellitus via AKT pathway. Eur Rev Med Pharmacol Sci. 2018;22:8553-8560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 46. | Ballut L, Marchadier B, Baguet A, Tomasetto C, Séraphin B, Le Hir H. The exon junction core complex is locked onto RNA by inhibition of eIF4AIII ATPase activity. Nat Struct Mol Biol. 2005;12:861-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 264] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 47. | Zhang X, Pickin KA, Bose R, Jura N, Cole PA, Kuriyan J. Inhibition of the EGF receptor by binding of MIG6 to an activating kinase domain interface. Nature. 2007;450:741-744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 292] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 48. | Chao YM, Tain YL, Leu S, Wu KL, Lee WC, Chan JY. Developmental programming of the metabolic syndrome: Next-generation sequencing analysis of transcriptome expression in a rat model of maternal high fructose intake. Sheng Li Xue Bao. 2016;68:557-567. [PubMed] |