Published online Jun 15, 2025. doi: 10.4239/wjd.v16.i6.103616
Revised: March 13, 2025
Accepted: April 25, 2025
Published online: June 15, 2025
Processing time: 181 Days and 6.3 Hours
Traditional Chinese medicine offers many valuable remedies for maintaining blood glucose homeostasis in patients with type 2 diabetes mellitus. Bile powder (BP) is a powdered form of bile derived from pigs. It has been used historically in various medicinal applications. Currently, the therapeutic potential of BP in regulating glucose homeostasis remains unclear. Bile acids (BAs) are increasingly recognized for their role in glucose metabolism particularly through the modu
To investigate BP effects on glucose homeostasis and elucidate its mechanistic role through GLP-1 and farnesoid X receptor (FXR) signaling.
A diabetic mouse model was established using a high-fat diet and streptozotocin administration. Mice were treated with BP at doses of 25, 50, or 75 mg/kg/day for 45 days. Glucose homeostasis was assessed via the oral glucose tolerance test and insulin tolerance test. Serum GLP-1 levels were measured by enzyme-linked immunosorbent assay. A GLP-1 receptor antagonist and an FXR agonist were used to clarify the underlying mechanisms. In vitro STC-1 murine enteroendocrine cells were treated with a BP-mimicking BA mixture to assess GLP-1 secretion and proglucagon gene expression.
BP treatment significantly improved glucose homeostasis in the diabetic mouse model as indicated by lower blood glucose (P < 0.05) and improved insulin sensitivity. BP enhanced GLP-1 secretion (P < 0.05), which was an effect abolished by the GLP-1 receptor antagonist. This observation confirmed its dependence on GLP-1 signaling. In STC-1 cells, BP-derived BA mixtures stimulated GLP-1 secretion and upregulated proglucagon expression (P < 0.05). Mechanistically, BP inhibited FXR signaling as evidenced by the reversal of its effects upon fexaramine administration. In addition, long-term BP treatment suppressed FXR signaling, resulting in elevated GLP-1 levels and preventing glucose dysregulation.
BP improved glucose homeostasis by promoting GLP-1 secretion via FXR inhibition, highlighting its potential as a therapeutic strategy for metabolic disorders.
Core Tip: Traditional Chinese medicine has several substances that regulate blood glucose in the treatment of type 2 diabetes mellitus. One of these substances is pig bile powder (BP). However, its mechanism of action remains unclear. This study demonstrated that BP treatment improved glucose tolerance and insulin sensitivity in a diabetic mouse model. Bile acids in the BP act as farnesoid X receptor antagonists and enhance glucagon-like peptide-1 production. Glucagon-like peptide-1 is a key hormone for glucose regulation. Our findings furthered the understanding of the therapeutic potential of BP for type 2 diabetes mellitus treatment.
- Citation: Sun YM, Kuang JL, Zhang HH, Xia XX, Wang JY, Zheng D, Zhou KJ, Tang YJ, Zhao AH, Jia W, Xie GX, Zheng XJ. Pig bile powder maintains blood glucose homeostasis by promoting glucagon-like peptide-1 secretion via inhibiting farnesoid X receptor. World J Diabetes 2025; 16(6): 103616
- URL: https://www.wjgnet.com/1948-9358/full/v16/i6/103616.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i6.103616
Pig bile powder (BP), also known as Pulvis Fellis Suis, is a type of traditional Chinese medicine (TCM) documented in the Chinese Pharmacopoeia. Pig bile is typically collected, filtered, and dried to form a powdered substance. As a traditional remedy, BP has been prescribed for digestive health, detoxification, and inflammatory conditions[1,2]. BP continues to be used in modern medical practice for digestive disorders, liver conditions, and related ailments[3]. Our previous research investigated the potential benefits of BP in managing metabolic disorders such as metabolic-associated fatty liver disease[4], and we found that the main components of BP could regulate blood glucose[5]. However, we still need to determine whether BP can be used as a hypoglycemic agent, and its specific hypoglycemic mechanism remains unknown.
Bile acids (BAs), the main components in bile, serve as important signaling molecules by participating in the regulation of blood glucose[6-10] and lipid metabolism[11-15]. BAs and their receptors are potential targets for therapeutic in
Glucagon-like peptide-1 (GLP-1), a hormone secreted by intestinal enteroendocrine L cells, has the ability to decrease blood glucose in a glucose-dependent manner by enhancing insulin secretion[16]. In recent years, GLP-1 receptor agonists have emerged as the most promising agents for treating diabetes and obesity[17,18]. Notably, BAs are well-recognized as stimulants of GLP-1 through their interaction with BA receptors[19]. Our previous studies found that HCA species could upregulate GLP-1 production and secretion[5], which may be a key mechanism by which BP regulates glucose meta
To investigate how BP ameliorates impaired blood glucose, we treated a diabetic mouse model induced by a high-fat diet (HFD) and streptozotocin (STZ) with BP. A GLP-1 receptor antagonist (GLP-1-RA) and an farnesoid X receptor (FXR) agonist were used to clarify the underlying mechanisms. We also applied a BP-mimicking BA mixture in vitro to confirm the effects of BP.
All animal experiments were conducted in strict accordance with national regulations and received approval from the Institutional Animal Care and Use Committee of the Center for Laboratory Animals, Shanghai Sixth People’s Hospital, affiliated with Shanghai Jiao Tong University School of Medicine. Male wildtype C57BL/6J mice (6-weeks-old) were sourced from Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China). The mice were housed in a specific-pathogen-free environment and maintained under controlled conditions with a 12-hour light/dark cycle, a temperature of 20-22 °C, and a humidity level of 45% ± 5%. They had unrestricted access to purified rodent feed and ultrapure water. Before experimentation, the mice underwent a 1-week acclimation period in the animal facility where they were provided with chow diet ad libitum. For the study, a standard chow diet (TP23522; Trophic Animal Feed High-tech Co., Ltd, Nantong, Jiangsu Province, China) and a HFD (HFD: 60% kcal from fat; D12492; Research Diets, Inc., New Brunswick, NJ, United States) were utilized. Body weight, food intake, and water consumption were recorded weekly throughout the experiment.
Animal experiment 1 (HFD + STZ diabetic model): C57BL/6J mice (male, 6-weeks-old) were fed with a HFD for 12 weeks. In the 6th week, animals were fasted for 4 hours to measure fasting blood glucose, which was used as the basic blood glucose value of this cohort of animals. Then the animals were fasted for 24 hours (free drinking water) and were injected with a single dose of STZ (V900890; Sigma-Aldrich, St Louis, MO, United States) (50 mg/kg, intravenous) as a freshly prepared solution in sodium citrate, potential of hydrogen = 5.5 (S4641; Sigma-Aldrich) (0.1 mmol/L). After 5 days, the animals were fasted for 4 hours, and blood glucose was measured to ensure the models were successfully established. The STZ-treated mice exhibited a glucose level of 11.1 mmol/L.
Animal experiment 2 (BP treatment): BP (Lot G20210901) was provided by Henan Liwei Biological Pharmaceutical Co., Ltd. (Jiaozuo, Henan Province, China). Twenty HFD + STZ diabetic mice were randomly divided into four groups. They were orally administered the following agents with a HFD for 45 days: (1) HFD + STZ group: Mice (n = 5) were orally administered with 6 % sodium bicarbonate (NaHCO3) (S6014, Sigma-Aldrich) as the control vehicle; (2) Low-dose BP group: Mice (n = 5) were orally administered with 25 mg/kg/day of BP; (3) Medium-dose BP group: Mice (n = 5) were orally administered with 50 mg/kg/day of BP; and (4) High-dose BP group: Mice (n = 5) were orally administered with 75 mg/kg/day of BP. The oral glucose tolerance test (OGTT) was performed after 30 days and 45 days of treatment. The insulin tolerance test (ITT) was performed after 45 days of treatment.
Animal experiment 3 (GLP-1-RA treatment): Fifteen HFD + STZ diabetic mice were randomly divided into three groups. They were administered the following agents with a HFD for 30 days: (1) HFD + STZ group: Mice (n = 5) were administered the control vehicle [6% NaHCO3 (intragastric gavage)]; (2) BP group: Mice (n = 5) were administered BP (75 mg/kg/day, intragastric gavage); and (3) BP + GLP-1-RA group: Mice (n = 5) were administered BP (75 mg/kg/day, intragastric gavage) and exendin-3 (9-39) amide (GLP-1-RA; 2081; RD Systems, Minneapolis, MN, United States) in saline (25 nmol/kg/day, intraperitoneal).
Animal experiment 4 (Intestinal FXR agonist treatment): Fifteen HFD + STZ diabetic mice were randomly divided into three groups. They were orally administered the following agents with a HFD for 30 days: (1) HFD + STZ group: Mice (n = 5) were administered the control vehicle, 6% NaHCO3; (2) BP group: Mice (n = 5) were administered BP (75 mg/kg/day); and (3) BP + fexaramine (FEX) group: Mice (n = 5) were administered BP (75 mg/kg/day) and FEX (BCP15784; BioChemPartner, Shanghai, China) in 0.5 % sodium carboxymethyl cellulose (100 mg/kg/day).
Animal experiment 5 (BP preventive treatment): Ten C57BL/6J mice (male, 6-weeks-old) were randomly divided into two groups. They were orally administered the following agents with a HFD for 12 weeks: (1): HFD + STZ group: Mice (n = 5) were administered with 6% NaHCO3 as the control vehicle; and (2) BP group: Mice (n = 5) were administered with 75 mg/kg/day of BP. In the 6th week all mice were injected with STZ (50 mg/kg, intravenous).
The murine enteroendocrine cell line STC-1 (sex unknown; CRL-3254; ATCC, Manassas, VA, United States) was utilized for in vitro studies. STC-1 cells were cultured in Dulbecco’s modified eagle medium (Invitrogen, Waltham, MA, United States) containing 10% fetal bovine serum (Gibco, Waltham, MA, United States) and 1% penicillin-streptomycin, at 37 °C and 5% carbon dioxide. The cell line was negative for mycoplasma contamination detection before conducting the experiments.
The composition and corresponding proportions of BA in BP were simulated to construct a BA mixture for cell intervention. This BA mixture was composed of two species of BA. The most abundant was the HCA species, accounting for 51.1% and included 3.1% HCA, 5.7% hyodeoxycholic acid, 10.3% glycohyocholic acid, 26.5% glycohyodeoxycholic acid, 0.4% taurochenodeoxycholic acid, and 5.1% taurohyodeoxycholic acid. The second most abundant was the chenodeoxycholic acid (CDCA) species accounting for 27.9% of the mixture and included 21.8% glycochenodeoxycholic acid, 3.3% taurochenodeoxycholic acid, and 2.8% CDCA. phosphate-buffered saline accounted for 21% of the mixture.
The cells were plated into 12-well plates with an initial cell density of 2 × 105 cells/well. They were treated with different doses (0, 10, and 20 μg/mL) of the BA mixture for 1 hour and 24 hours for determination of the GLP-1 level in the supernatant and the expression level of the proglucagon (Gcg) gene.
The cell lines were treated with different doses (0, 10, and 20 μg/mL) of the BA mixture for 1 hour and 24 hours. The cell media was obtained and centrifuged at 2500 g for 8 minutes to remove cell debris. The supernatant was collected. Active GLP-1 was measured by an active GLP-1 assay kit (EZGLPHS-35K; Millipore, Burlington, MA, United States).
After a 12-hour overnight fast, mice were administered the dipeptidyl peptidase-4 inhibitor sitagliptin (3 mg/kg; MK0431; MedChemExpress, Monmouth Junction, NJ, United States) via gavage 1 hour before receiving a liquid diet (10 mL/kg; Ensure Plus; Abbott, Chicago, IL, United States). Fifteen minutes post-gavage, retro-orbital blood was collected for analysis. Active GLP-1 levels were measured using an assay kit (EZGLPHS-35K; Millipore).
Following a 12-hour overnight fast, mice were given an oral glucose load (1 g/kg). Blood glucose levels from tail vein samples were analyzed using a glucose meter (OneTouch Ultra; LifeScan, Johnson and Johnson, Malvern, PA, United States) at 0, 15, 30, 60, and 120 minutes.
After a 4-hour fast mice received an intraperitoneal injection of insulin (2 U/kg). Blood glucose levels were measured at 0, 15, 30, 60, and 120 minutes post-injection.
Total RNA from STC-1 cell lysates was extracted using Trizol reagent (Invitrogen) per the manufacturer’s instructions. RNA concentration was determined using a NanoDrop spectrophotometer. Reverse transcription was performed with the Primer Script RT reagent kit (Takara, Kusatsu, Japan), and quantitative polymerase chain reaction (PCR) primers were synthesized by Sangon Biotech (Shanghai, China). Quantitative reverse transcription PCR was conducted using PowerUp SYBR Green PCR master mix (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, United States). Expression levels of Gcg, Fxr, and Fgf15 were normalized to glyceraldehyde-3-phosphate dehydrogenase. Results were presented as fold changes relative to the control group.
Intestinal tissues were fixed in 4% paraformaldehyde and embedded in optimal cutting temperature compound (Sakura Finetek, Torrance, CA, United States). Sections were deparaffinized, rehydrated, and stained with an FXR antibody (1:200; orb156973; Biorbyt, Cambridge, United Kingdom) using an immunohistochemistry kit (D601037-0020; Sangon Biotech, Shanghai, China). Images were captured with a Nikon digital microscope (Tokyo, Japan) and analyzed using ImageJ software.
Serum FGF15 levels were quantified using an enzyme-linked immunosorbent assay kit (CSB-EL522052MO; CUSABIO, Wuhan, Hubei Province, China).
Results were summarized as mean ± SEM. All the bar plots in this study were generated by GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, United States). Differential significance analysis using the Student’s t-test was performed by SPSS 24.0 (IBM SPSS, Armonk, NY, United States). A P value < 0.05 indicated significance.
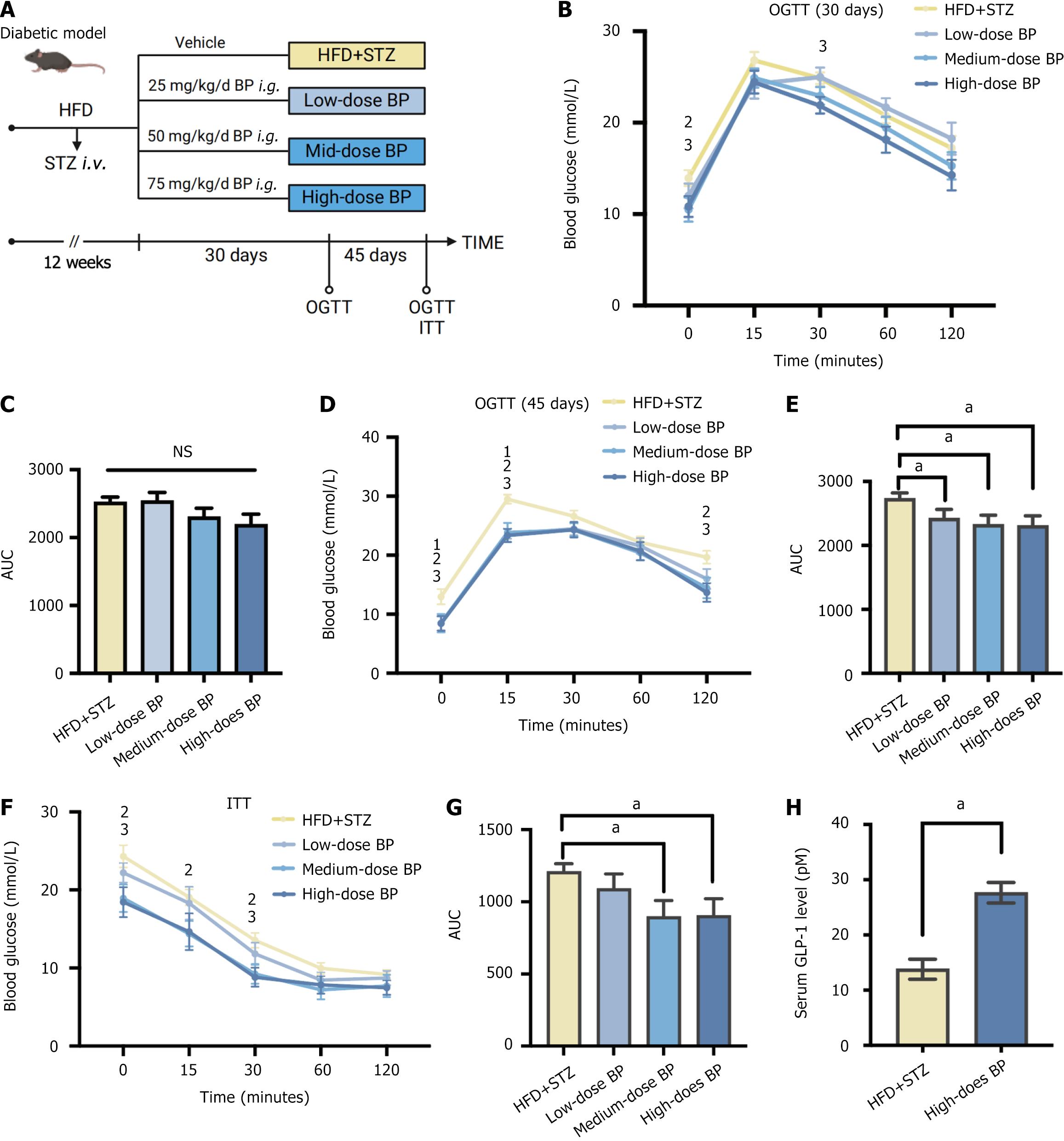
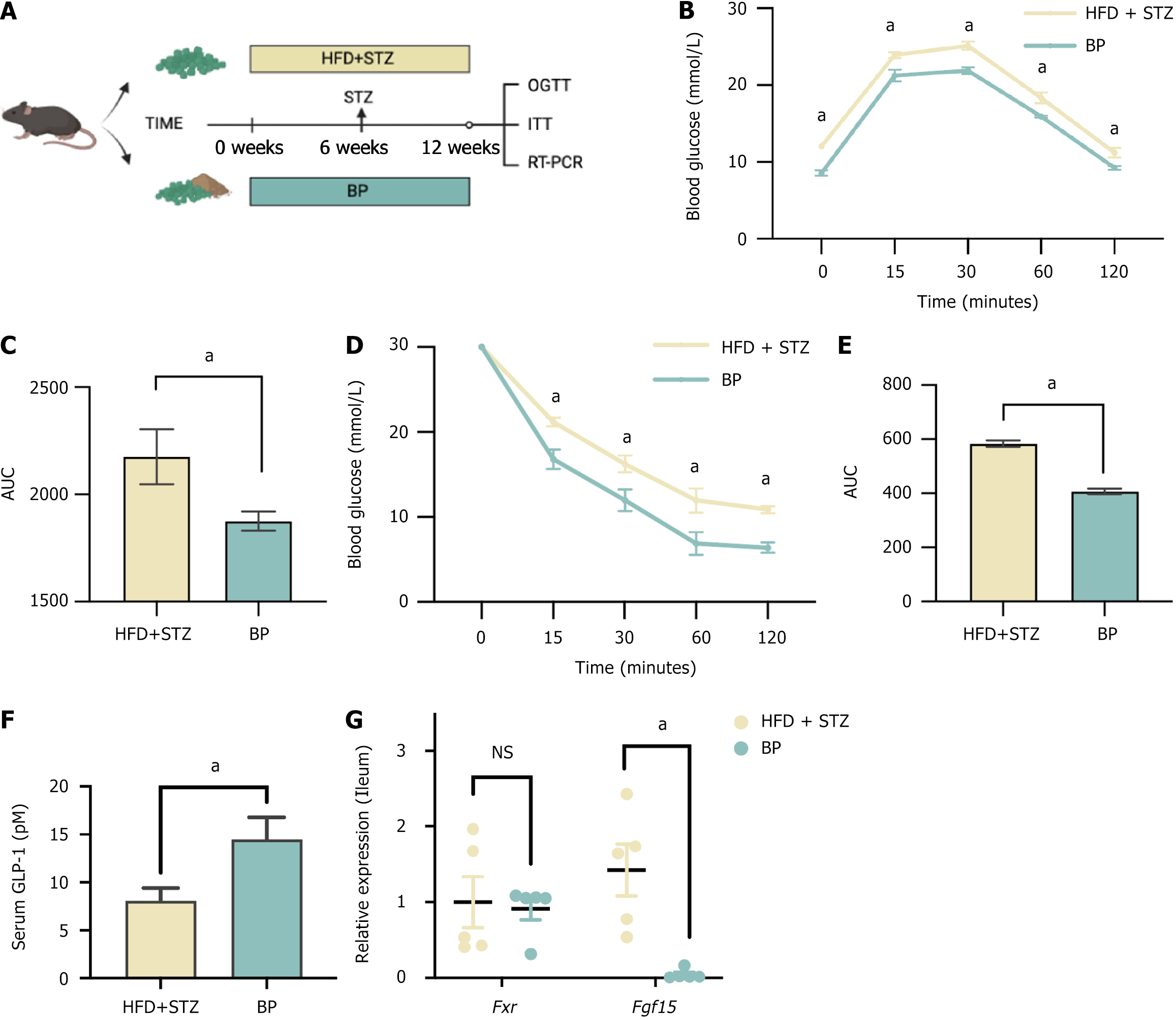
To investigate the effect of BP on glucose homeostasis, we administered BP to the diabetic mouse model induced by HFD and STZ (HFD + STZ). We used low, medium, and high doses of BP at 25, 50, and 75 mg/kg/day, respectively. The OGTT was performed on day 30 and 45 after BP intervention, and the ITT was performed on day 45 (Figure 1A). The mice treated with BP at the medium and high doses for 30 days had lower blood glucose levels (Figure 1B and C). After oral administration of BP for 45 days, a significant reduction of glucose levels was observed (Figure 1D and E). In addition to the consistently lower blood glucose levels in the medium-dose and high-dose BP groups, the mice in the low-dose BP group also showed improved blood glucose compared with the HFD + STZ group. The area under the curve (AUC) was significantly lower in the three BP treatment groups compared with the HFD + STZ group.
Mice treated with medium and high doses of BP also showed significant improvement in ITT performance. There was a reduction in the AUC area, which indicated improved insulin resistance (Figure 1F and G). In addition, we tested serum GLP-1 levels in the HFD + STZ group and the high-dose BP group. GLP-1 levels significantly increased after BP treatment (Figure 1H). These results showed that BP could maintain glucose homeostasis potentially via GLP-1 promotion.
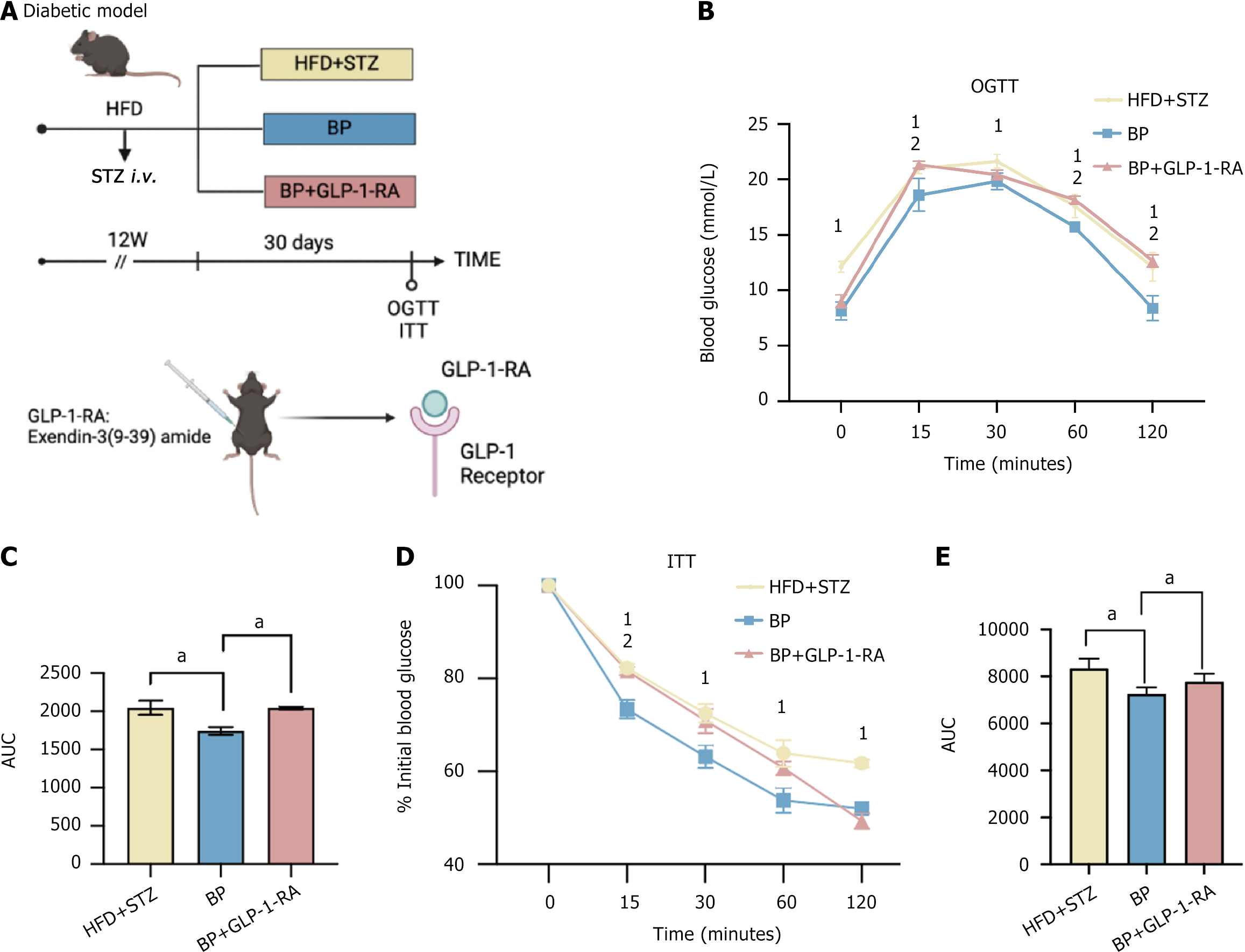
GLP-1, as the GLP-1-R ligand, activates intracellular signaling pathways that regulate insulin secretion, glucose metabolism, and satiety. To investigate whether the hyperglycemic effect of BP depended on GLP-1, we inhibited GLP-1 downstream signaling using the GLP-1-RA, exendin-3 (9-39) amide. HFD + STZ diabetic mice were treated with BP and BP + GLP-1-RA for 30 days (Figure 2A). Consistent with the previous observation, the OGTT (Figure 2B and C) and ITT (Figure 2D and E) were improved with the BP treatment compared with the HFD + STZ group. This improvement was not observed when GLP-1 signaling was inhibited by the GLP-1-RA. The blood glucose levels and AUCs for the OGTT and ITT were similar when comparing the BP + GLP-1-RA and the HFD + STZ groups. GLP-1-RA eliminated the improvement in blood glucose observed after BP administration, which suggests that BP ameliorated blood sugar disorder via GLP-1 signaling.
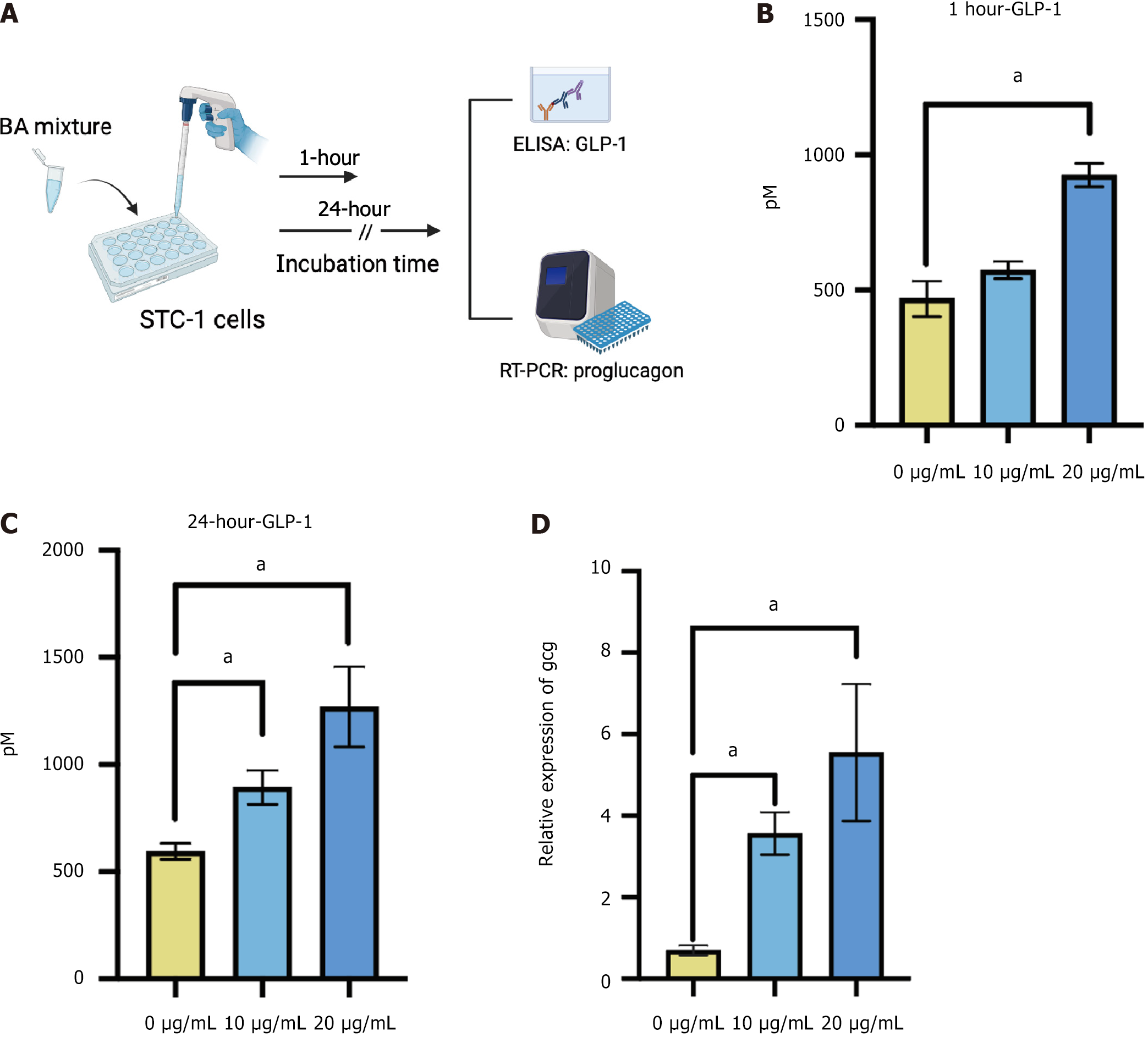
After observing GLP-1 enhancement after BP treatment in the mouse models, we further investigated the effect of BAs predominant in BP on GLP-1 release in vitro. The BA composition of BP was characterized as 51.1% HCA species and 27.9% CDCA species, with BAs constituting 83.0% of the total BP[20]. We treated STC-1 cells with the BA mixture at doses of 0, 10, and 20 μg/mL. After 1-hour and 24-hour incubations, the cell medium was collected for GLP-1 measurement, and the cells were collected for the expression of the Gcg gene (Figure 3A). The results showed that GLP-1 levels sig
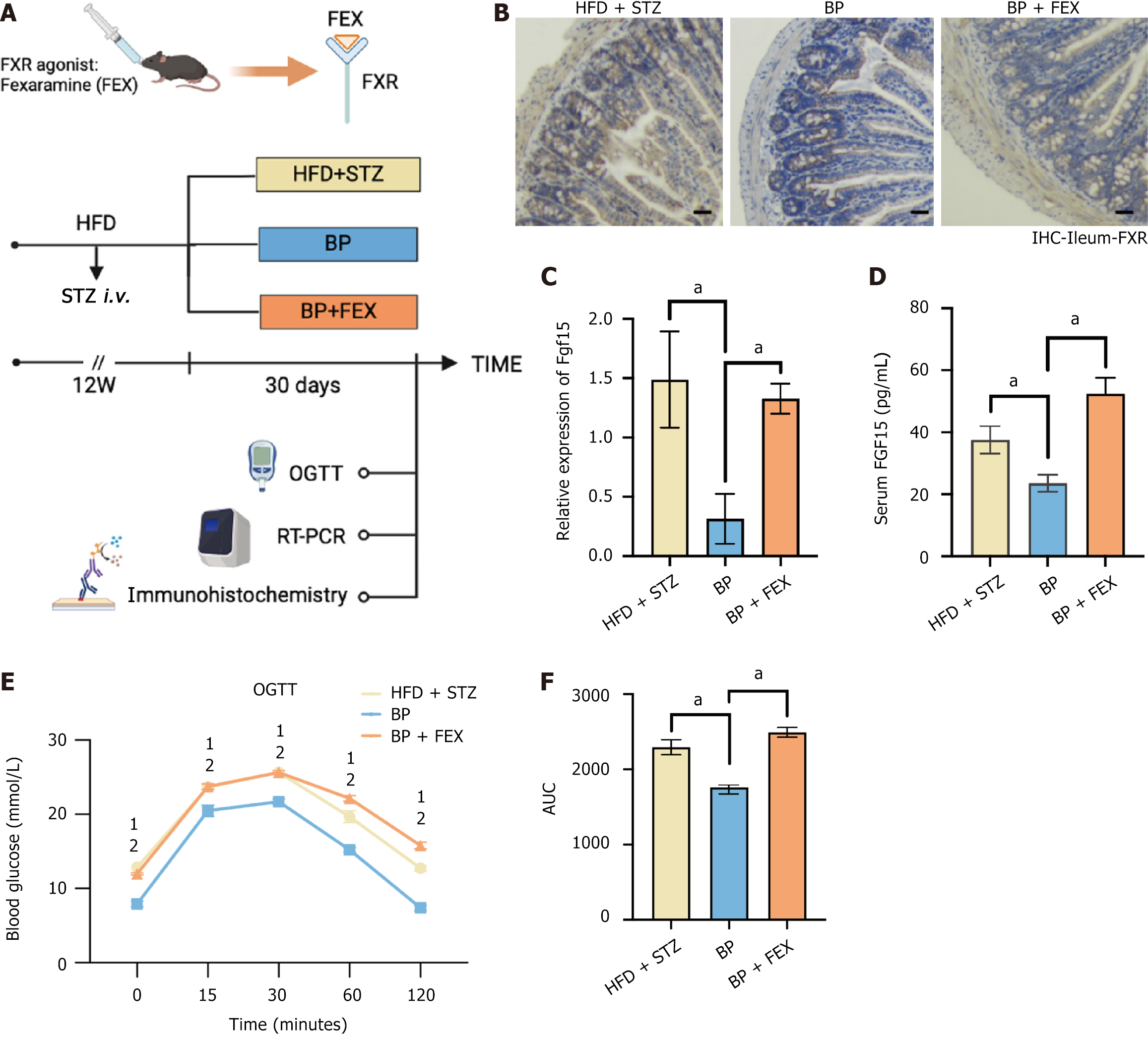
We then explored the mechanism by which BP upregulated GLP-1. Our previous studies highlighted the role of the BA nuclear receptor FXR in GLP-1 production in intestinal L cells. To investigate whether the increase in GLP-1 by BP was due to intestinal FXR inhibition, we treated mice with FEX, an intestinal FXR agonist (Figure 4A). We observed downregulated expression of Fxr-related downstream secreted peptide FGF15 with the BP treatment compared with the HFD + STZ group. There was no significant difference between the HFD + STZ group and the BP + FEX group (Figure 4B-D). We then performed the OGTT to evaluate the blood glucose status. The results showed that FEX administration weakened the effectiveness of BP in maintaining blood glucose homeostasis. The blood glucose levels before and after glucose loading and the AUC of the OGTT were significantly increased after FEX treatment and were comparable to the HFD + STZ group (Figure 4E and F). Taken together, these findings suggested that BP could regulate glucose homeostasis and promote GLP-1 production by inhibiting the intestinal FXR signaling pathway.
To further determine the potential preventive effects of BP, we treated mice with BP for 12 weeks along with HFD and STZ treatment (Figure 5A). The results showed that BP had a strong ability to prevent glucose disorders. The OGTT results indicated that BP decreased serum glucose levels and the AUC of the OGTT compared with the model group, suggesting improved glucose tolerance (Figure 5B and C). In addition, insulin resistance was significantly improved after BP preventative treatment (Figure 5D and E). The promotion of BP on serum GLP-1 and inhibition of FXR-FGF15 were also observed (Figure 5F and G). Thus, BP demonstrated preventive potential for dysglycemia by inhibiting intestinal FXR signaling and promoting the secretion of GLP-1.
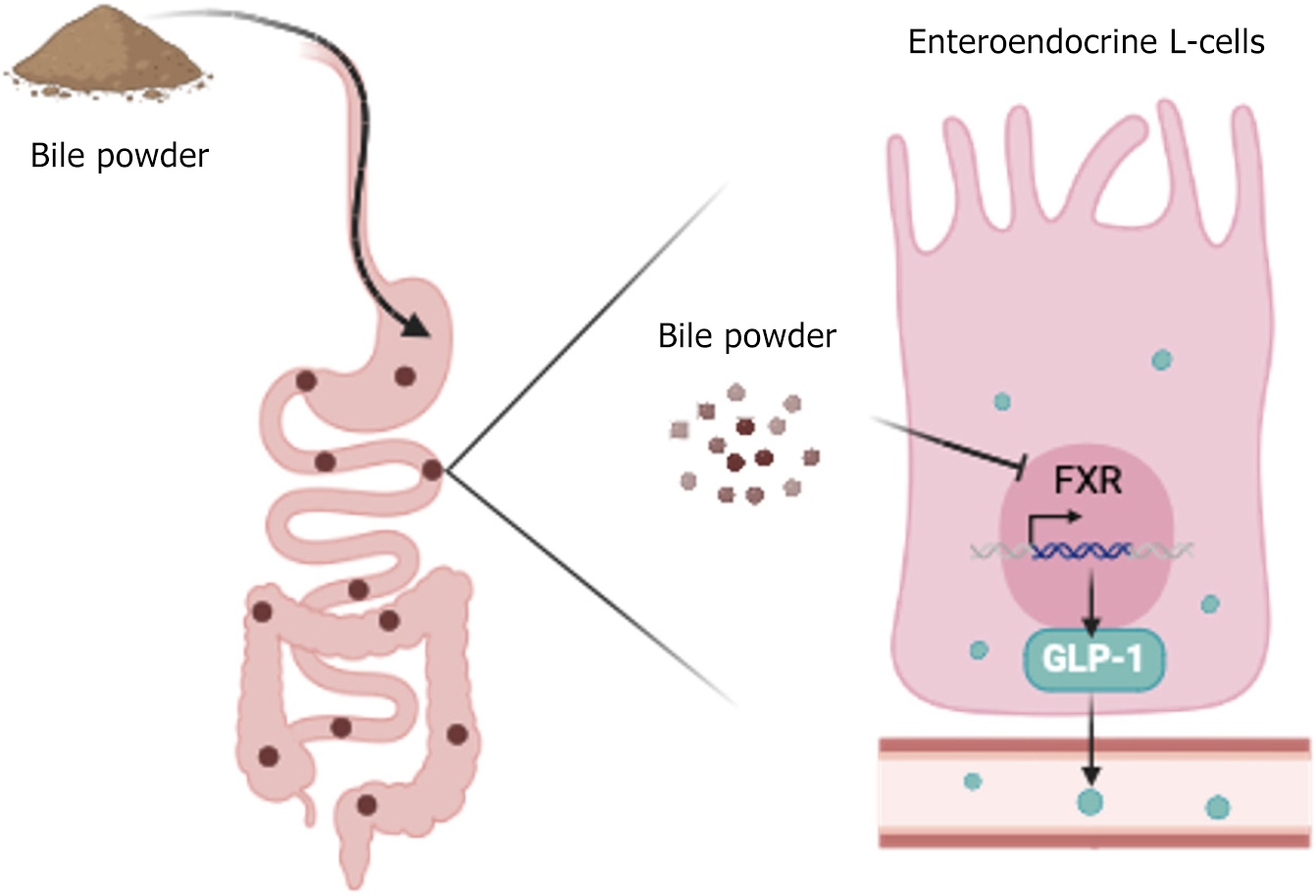
We demonstrated that BP treatment significantly alleviated hyperglycemia and insulin resistance in diabetic mice models. Mechanistically BP inhibited intestinal FXR signaling and promoted the secretion of GLP-1 by enteroendocrine cells, contributing to metabolic improvements (Figure 6). These findings provide a novel perspective and basis for the potential application of BP in T2DM treatment.
BP has a long history and has been widely used in TCM. Its earliest records date back to the “Compendium of Materia Medica”. This ancient text described the properties of BP as clearing heat, detoxifying, promoting bile secretion, and calming the mind. Historically, BP has been applied in the treatment of gastrointestinal, respiratory, and dermatological ailments[1-3]. Notably, concerns have been raised about the therapeutic effects of BP in metabolic diseases related to lipid and glucose metabolic dysfunction[4,5]. However, it is still unknown whether BP can alleviate a hyperglycemic phe
BAs are significant signaling molecules and are pivotal in the regulation of lipid and blood glucose metabolism[8]. Our previous studies demonstrated that HCA species, which account for the majority of BAs in BP and act as a metabolic biomarker, are inversely correlated with glucose levels in patients with T2DM[5]. Mechanistically, HCA species improved glucose homeostasis by promoting GLP-1 production and secretion through synergistically modulating the Takeda G-protein-coupled receptor 5 and FXR signaling pathways in enteroendocrine cells. Additionally, we found that BP ameliorated metabolic-associated steatotic liver disease-related phenotypes by modulating the gut-liver axis and si
Intestinal FXR plays a crucial role in the treatment of metabolic diseases[21-23]. FXR is a BA receptor. Its activation or inhibition in enteroendocrine cells can influence the production and secretion of GLP-1 and thereby regulating glucose metabolism[24]. Additionally, other mechanisms exist regarding the role of intestinal FXR in the regulation of glucose metabolism. Inhibiting intestinal FXR has been linked to decreased ceramide levels, which are associated with improved hepatic glucose metabolism and insulin resistance[25]. Treating diabetic mice with FEX and BP showed that the the
Recently gut-targeted FXR modulators have shown promise in treating metabolic diseases. Several FXR antagonists have entered clinical trials. SIPI-7623[26] is currently in a phase 1 trial for the treatment of mixed hyperlipidemia. HPG1860[27] has advanced to a phase 2 trial. However, its results have not yet been disclosed. Several FXR agonists, including obeticholic acid, tropifexor, and EDP-305, have progressed to clinical trials and have shown promise in slowing nonalcoholic steatohepatitis progression[28-30]. These findings highlight the therapeutic potential of FXR modulators in metabolic diseases.
In preclinical studies, the FXR antagonist, HS218, lowered blood glucose by inhibiting hepatic gluconeogenesis and reducing hepatic glucose output[31]. Another gut-specific FXR antagonist, F6, demonstrated greater efficacy than obeticholic acid in a nonalcoholic steatohepatitis model[32]. F6 significantly improved hepatic steatosis and inflammation through microbiota modulation. While clinical validation is still ongoing, these preclinical findings support the potential of BP as an FXR antagonist for metabolic disease treatment.
BP displays significant potential for clinical translation due to key advantages that align with modern therapeutic needs, particularly in the treatment of T2DM. BP provides broad metabolic regulatory benefits. Our previous studies showed that HCA species effectively reduced blood glucose, attenuated lipid accumulation, and exerted anti-inflammatory effects[4,5]. Besides GLP-1 regulation, the therapeutic targets of BP also included the gut microbiome and immunity regulation.
The safety of BP has already been proven. It is a TCM documented in the Chinese pharmacopoeia. The established standardization of the preparation and quality control of BP ensures its quality and clinical safety. We have also comprehensively tested the toxicity of BP in accordance with the National Food Safety Standards (GB 15193)[20]. The results demonstrated that BP exhibited no acute oral toxicity at doses up to 15 g/kg body weight and no subchronic oral toxicity within the dose range of 0.75-2.25 g/kg body weight. Additionally, no genotoxicity, chromosomal toxicity, or reproductive cell toxicity was observed at doses up to 10 g/kg body weight, and no teratogenic effects were detected within the dose range of 0.75-2.25 g/kg body weight. These findings further support the safety of BP and its potential for clinical application.
BP shows promising metabolic benefits due to its active BA components. BP modulates enteroendocrine pathways, contributing to glucose homeostasis. Mechanistically, BP intervention significantly alleviates hyperglycemia and insulin resistance in diabetic mice by inhibiting intestinal FXR signaling and thereby enhancing GLP-1 secretion. Its dual function as an FXR antagonist and GLP-1 enhancer offers a broader metabolic regulatory effect. These findings provide a novel perspective on the potential therapeutic role of BP in T2DM treatment.
| 1. | He J, Lv L, Wang Z, Huo C, Zheng Z, Yin B, Jiang P, Yang Y, Li J, Gao Y, Xue J. Pulvis Fellis Suis extract attenuates ovalbumin-induced airway inflammation in murine model of asthma. J Ethnopharmacol. 2017;207:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | He J, Liang J, Zhu S, Li J, Zhang Y, Sun W. Anti-inflammatory effects of Pulvis Fellis Suis extract in mice with ulcerative colitis. J Ethnopharmacol. 2011;138:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Qiao X, Ye M, Pan DL, Miao WJ, Xiang C, Han J, Guo DA. Differentiation of various traditional Chinese medicines derived from animal bile and gallstone: simultaneous determination of bile acids by liquid chromatography coupled with triple quadrupole mass spectrometry. J Chromatogr A. 2011;1218:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Kuang J, Wang J, Li Y, Li M, Zhao M, Ge K, Zheng D, Cheung KCP, Liao B, Wang S, Chen T, Zhang Y, Wang C, Ji G, Chen P, Zhou H, Xie C, Zhao A, Jia W, Zheng X, Jia W. Hyodeoxycholic acid alleviates non-alcoholic fatty liver disease through modulating the gut-liver axis. Cell Metab. 2023;35:1752-1766.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 122] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 5. | Zheng X, Chen T, Jiang R, Zhao A, Wu Q, Kuang J, Sun D, Ren Z, Li M, Zhao M, Wang S, Bao Y, Li H, Hu C, Dong B, Li D, Wu J, Xia J, Wang X, Lan K, Rajani C, Xie G, Lu A, Jia W, Jiang C, Jia W. Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism. Cell Metab. 2021;33:791-803.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 259] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 6. | Chen B, Bai Y, Tong F, Yan J, Zhang R, Zhong Y, Tan H, Ma X. Glycoursodeoxycholic acid regulates bile acids level and alters gut microbiota and glycolipid metabolism to attenuate diabetes. Gut Microbes. 2023;15:2192155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 7. | Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1044] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 8. | Chávez-Talavera O, Tailleux A, Lefebvre P, Staels B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152:1679-1694.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 677] [Article Influence: 84.6] [Reference Citation Analysis (1)] |
| 9. | Lee JS, Han P, Chaudhury R, Khan S, Bickerton S, McHugh MD, Park HB, Siefert AL, Rea G, Carballido JM, Horwitz DA, Criscione J, Perica K, Samstein R, Ragheb R, Kim D, Fahmy TM. Metabolic and immunomodulatory control of type 1 diabetes via orally delivered bile-acid-polymer nanocarriers of insulin or rapamycin. Nat Biomed Eng. 2021;5:983-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1223] [Cited by in RCA: 1392] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 11. | Clifford BL, Sedgeman LR, Williams KJ, Morand P, Cheng A, Jarrett KE, Chan AP, Brearley-Sholto MC, Wahlström A, Ashby JW, Barshop W, Wohlschlegel J, Calkin AC, Liu Y, Thorell A, Meikle PJ, Drew BG, Mack JJ, Marschall HU, Tarling EJ, Edwards PA, de Aguiar Vallim TQ. FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption. Cell Metab. 2021;33:1671-1684.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 285] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 12. | Xu H, Fang F, Wu K, Song J, Li Y, Lu X, Liu J, Zhou L, Yu W, Yu F, Gao J. Gut microbiota-bile acid crosstalk regulates murine lipid metabolism via the intestinal FXR-FGF19 axis in diet-induced humanized dyslipidemia. Microbiome. 2023;11:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 13. | Nie Q, Luo X, Wang K, Ding Y, Jia S, Zhao Q, Li M, Zhang J, Zhuo Y, Lin J, Guo C, Zhang Z, Liu H, Zeng G, You J, Sun L, Lu H, Ma M, Jia Y, Zheng MH, Pang Y, Qiao J, Jiang C. Gut symbionts alleviate MASH through a secondary bile acid biosynthetic pathway. Cell. 2024;187:2717-2734.e33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Reference Citation Analysis (0)] |
| 14. | Jiao N, Baker SS, Chapa-Rodriguez A, Liu W, Nugent CA, Tsompana M, Mastrandrea L, Buck MJ, Baker RD, Genco RJ, Zhu R, Zhu L. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67:1881-1891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 515] [Article Influence: 73.6] [Reference Citation Analysis (1)] |
| 15. | Da Dalt L, Moregola A, Svecla M, Pedretti S, Fantini F, Ronzio M, Uboldi P, Dolfini D, Donetti E, Baragetti A, Mitro N, Scorrano L, Norata GD. The inhibition of inner mitochondrial fusion in hepatocytes reduces non-alcoholic fatty liver and improves metabolic profile during obesity by modulating bile acid conjugation. Cardiovasc Res. 2024;119:2917-2929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (1)] |
| 16. | Drucker DJ. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018;27:740-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 1104] [Article Influence: 157.7] [Reference Citation Analysis (1)] |
| 17. | Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 811] [Cited by in RCA: 958] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 18. | Yao H, Zhang A, Li D, Wu Y, Wang CZ, Wan JY, Yuan CS. Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: systematic review and network meta-analysis. BMJ. 2024;384:e076410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 155] [Reference Citation Analysis (12)] |
| 19. | Wang Q, Lin H, Shen C, Zhang M, Wang X, Yuan M, Yuan M, Jia S, Cao Z, Wu C, Chen B, Gao A, Bi Y, Ning G, Wang W, Wang J, Liu R. Gut microbiota regulates postprandial GLP-1 response via ileal bile acid-TGR5 signaling. Gut Microbes. 2023;15:2274124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 20. | Wu L, Wang J, Lei J, Ge K, Qu C, Liu J, Huang F, Sun D, Chao X, Chen T, Zhao A, Jia W, Zheng X, Xie G. Toxicological evaluation of porcine bile powder in Kunming mice and Sprague-Dawley rats. Front Pharmacol. 2024;15:1424940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 21. | Avila MA, Moschetta A. The FXR-FGF19 Gut-Liver Axis as a Novel "Hepatostat". Gastroenterology. 2015;149:537-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116:1102-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 697] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 23. | Adorini L, Pruzanski M, Shapiro D. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug Discov Today. 2012;17:988-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 24. | Wang YD, Chen WD, Moore DD, Huang W. FXR: a metabolic regulator and cell protector. Cell Res. 2008;18:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 314] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 25. | Xie C, Jiang C, Shi J, Gao X, Sun D, Sun L, Wang T, Takahashi S, Anitha M, Krausz KW, Patterson AD, Gonzalez FJ. An Intestinal Farnesoid X Receptor-Ceramide Signaling Axis Modulates Hepatic Gluconeogenesis in Mice. Diabetes. 2017;66:613-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 163] [Article Influence: 20.4] [Reference Citation Analysis (1)] |
| 26. | Nian SY, Wang GP, Jiang ZL, Xiao Y, Huang MH, Zhou YH, Tan XD. Synthesis and biological evaluation of novel SIPI-7623 derivatives as farnesoid X receptor (FXR) antagonists. Mol Divers. 2019;23:19-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Mo C, Xu X, Zhang P, Peng Y, Zhao X, Chen S, Guo F, Xiong Y, Chu XJ, Xu X. Discovery of HPG1860, a Structurally Novel Nonbile Acid FXR Agonist Currently in Clinical Development for the Treatment of Nonalcoholic Steatohepatitis. J Med Chem. 2023;66:9363-9375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (1)] |
| 28. | Ratziu V, Rinella ME, Neuschwander-Tetri BA, Lawitz E, Denham D, Kayali Z, Sheikh A, Kowdley KV, Desta T, Elkhashab M, DeGrauw J, Goodwin B, Ahmad A, Adda N. EDP-305 in patients with NASH: A phase II double-blind placebo-controlled dose-ranging study. J Hepatol. 2022;76:506-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 83] [Article Influence: 27.7] [Reference Citation Analysis (1)] |
| 29. | Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, Bedossa P, Geier A, Beckebaum S, Newsome PN, Sheridan D, Sheikh MY, Trotter J, Knapple W, Lawitz E, Abdelmalek MF, Kowdley KV, Montano-Loza AJ, Boursier J, Mathurin P, Bugianesi E, Mazzella G, Olveira A, Cortez-Pinto H, Graupera I, Orr D, Gluud LL, Dufour JF, Shapiro D, Campagna J, Zaru L, MacConell L, Shringarpure R, Harrison S, Sanyal AJ; REGENERATE Study Investigators. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 940] [Cited by in RCA: 925] [Article Influence: 154.2] [Reference Citation Analysis (0)] |
| 30. | Pedrosa M, Seyedkazemi S, Francque S, Sanyal A, Rinella M, Charlton M, Loomba R, Ratziu V, Kochuparampil J, Fischer L, Vaidyanathan S, Anstee QM. A randomized, double-blind, multicenter, phase 2b study to evaluate the safety and efficacy of a combination of tropifexor and cenicriviroc in patients with nonalcoholic steatohepatitis and liver fibrosis: Study design of the TANDEM trial. Contemp Clin Trials. 2020;88:105889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 31. | Xu X, Shi X, Chen Y, Zhou T, Wang J, Xu X, Chen L, Hu L, Shen X. HS218 as an FXR antagonist suppresses gluconeogenesis by inhibiting FXR binding to PGC-1α promoter. Metabolism. 2018;85:126-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 32. | Zhang C, Liu Y, Wang Y, Ge X, Jiao T, Yin J, Wang K, Li C, Guo S, Xie X, Xie C, Nan F. Discovery of Betulinic Acid Derivatives as Potent Intestinal Farnesoid X Receptor Antagonists to Ameliorate Nonalcoholic Steatohepatitis. J Med Chem. 2022;65:13452-13472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |