Published online May 15, 2025. doi: 10.4251/wjgo.v17.i5.105933
Revised: March 19, 2025
Accepted: March 20, 2025
Published online: May 15, 2025
Processing time: 94 Days and 1.2 Hours
Circular RNAs (circRNAs) are critical regulators in tumorigenesis, functioning as microRNA sponges or protein decoys. Although numerous circRNAs have been implicated in gastric cancer progression, the role of hsa_circRNA_101996 remains unclear. This study hypothesizes that hsa_circRNA_101996 promotes gastric cancer cell proliferation and apoptosis via the microRNA-577 (miR-577)/high mobility group nucleosome binding domain 5 (HMGN5) axis.
To investigate the role of hsa_circRNA_101996 in gastric cancer proliferation and apoptosis through the miR-577/HMGN5 axis.
Forty-one paired gastric cancer tissues and adjacent non-cancerous tissues were analyzed. Differential circRNA expression was identified using GSE83521 and GSE89143 datasets. miR-577 and HMGN5 were predicted via CircInteractome and TargetScan. Functional experiments (MTT, colony formation, Western blot) and dual-luciferase reporter assays were performed in gastric cancer cell lines (OCUM-1, HSC-39). In vivo tumorigenesis was validated in nude mice. Statistical analysis included Student’s t-test and one-way ANOVA (P < 0.05).
Hsa_circRNA_101996 was significantly upregulated in gastric cancer tissues and cell lines compared to adjacent non-cancerous tissues (P < 0.05). Dual-luciferase reporter assays validated the interactions among hsa_circRNA_101996, miR-577, and HMGN5. In vitro, gastric cancer cells overexpressing hsa_circRNA_101996 showed significantly increased proliferation and decreased apoptosis compared to controls (P < 0.05). Cells transfected with miR-577 mimics exhibited reduced proliferation and increased apoptosis
Hsa_circRNA_101996 promotes gastric cancer progression by sponging miR-577 to upregulate HMGN5, suggesting a novel therapeutic target for gastric cancer.
Core Tip: This study reveals that circRNA_101996 promotes gastric cancer progression by sponging microRNA-577 (miR-577) to upregulate high mobility group nucleosome binding domain 5, thereby enhancing cell proliferation and inhibiting apoptosis. This study identifies a novel regulatory mechanism of circRNA_101996 in gastric cancer pathogenesis.
- Citation: Wang XL, Zhang L, Shang Q. Circular RNA hsa_circRNA_101996 modulates gastric cancer cell proliferation and apoptosis through the miR-577/HMGN5 axis. World J Gastrointest Oncol 2025; 17(5): 105933
- URL: https://www.wjgnet.com/1948-5204/full/v17/i5/105933.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i5.105933
Gastric cancer ranks as the fifth most common malignancy globally and the third leading cause of cancer-related mortality[1]. Data from the International Agency for Research on Cancer in 2020 reported approximately 1,089,103 new cases and 768793 deaths worldwide[2]. Current treatment strategies primarily encompass surgery, chemotherapy, and targeted therapy. The 5-year survival rate varies significantly by stage, ranging from 60% to 80% for stage IA and IB tumors, and dropping to 18% to 50% for stage III tumors, underscoring the overall poor prognosis[3,4]. This low survival rate is primarily due to the advanced stage at diagnosis, tumor metastasis, heterogeneity, and resistance to radiotherapy and chemotherapy[5-8]. Recent discoveries of novel tumor markers and therapeutic targets have enhanced early diagnosis and treatment of gastric cancer. Additionally, various non-coding RNAs have been identified as key players in the pathogenesis and progression of gastric cancer[9-11].
Circular RNAs (circRNAs) are a recently discovered class of endogenous non-coding RNAs characterized by a closed-loop structure formed via reverse splicing of introns or exons, linking the 5' and 3' ends covalently[12,13]. Emerging evidence suggests that circRNAs play a crucial role in the pathogenesis of various malignancies, presenting them as potential biomarkers and therapeutic targets[14]. Unlike linear mRNA and microRNA (miRNA), circRNAs possess a distinct closed-loop configuration[14,15] and exhibit increased stability due to resistance against RNAase degradation, contributing to their extended half-life within cells[15,16]. Recent investigations have implicated circRNAs in modulating diverse behaviors of gastric cancer cells, encompassing cell proliferation[17], migration[18], invasion[19,20], and apoptosis[19,21,22]. In a previous study, researchers identified hsa_circRNA_101996 as a promoter of migration and invasion in gastric cancer cells through the upregulation of MMP2/MMP9 via the miR-143/TET2 pathway[23]. Notably, circRNA_101996 has also been implicated in the regulation of tumor cell proliferation and apoptosis in cervical cancer[24,25] and prostate cancer[26]. Nevertheless, the precise mechanism underlying the role of circRNA_101996 in regulating gastric cancer cell proliferation and apoptosis remains elusive.
CircRNAs function as "sponges" that modulate miRNA expression levels[14]. These molecules possess multiple miRNA binding sites, enabling them to sequester specific miRNAs within cells. This sequestration reduces the inhibitory impact of miRNAs on other target genes, thereby influencing related signaling pathways[14-16]. This competitive titration process can significantly impact cell growth, differentiation, and apoptosis, thereby regulating the onset and progression of gastric cancer[15]. This study aims to explore the role of circRNA hsa_circRNA_101996 in regulating gastric cancer cell proliferation and apoptosis. We seek to elucidate its regulatory mechanism through the miR-577/high mobility group nucleosome binding domain 5 (HMGN5) axis. Through systematic experimental design and bioinformatics analysis, we aim to unveil the significance of this circRNA-miRNA-mRNA regulatory network in the development and advancement of gastric cancer. The discoveries from this research hold substantial theoretical and clinical implications, fostering a deeper understanding of gastric cancer's molecular mechanisms, identifying novel therapeutic targets, and devising personalized treatment strategies. The insights gained from this study are expected to revolutionize the approach to treating and managing gastric cancer, leading to improved patient prognoses and survival rates. Ultimately, this research will provide compelling evidence and support for implementing personalized therapy in future clinical settings.
Antibodies used in this study included HMGN5 (Catalog ab186001, Abcam, Cambridge, United Kingdom), Bax (Catalog ET1603-34, HUABIO, Hangzhou, China), Bcl-2 (Catalog ET1702-53, HUABIO, Hangzhou, China), and β-actin (Catalog M1210-2, HUABIO, Hangzhou, China). 3-(4,5-Dimethylthiahiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Catalog M5655) and dimethyl sulfoxide (DMSO, Catalog D5879) were procured from Sigma-Aldrich (United States). RIPA lysis buffer (Catalog P0013B), Phenylmethanesulfonyl fluoride (PMSF, Catalog ST2573), and BCA protein assay kit (Catalog P0009) were purchased from Beyotime (China). The transfection reagent Lipofectamine™ 3000 (Catalog L3000001) was obtained from Thermo Fisher Scientific (USA). Super ECL prime (Catalog S6008) was purchased from United Staes Everbright®Inc. (China).
We collected 41 paired samples of gastric cancer tissues and adjacent tissues (located at a distance of > 5 cm from the tumor) from patients diagnosed with gastric cancer. Among these samples, 18 were from male patients, and 23 were from female patients. The average age of the patients was 54.8 ± 5.6 years. The study was reviewed and approved by the Xinxiang Central Hospital Institutional Review Board (Approval No. 2023-227). All participants provided informed consent before participating in the study. The research protocols and subsequent procedures were carried out by the Code of Ethics of the World Medical Association (Declaration of Helsinki) as printed in the British Medical Journal on 18 July 1964.
Sixteen male BALB/c nude mice (6 weeks old) were provided by the SLAC Laboratory Animal Co., Ltd (Shanghai) and kept under specific pathogen-free conditions. For the subcutaneous injection-induced tumor model establishment, pLV-CircRNA_101996-transfected OCUM-1 cells (5 × 106 cells/100μL) were carefully subcutaneously injected into the flanks of the mice, and each experimental group consisted of 8 animals. Tumor size was measured every 3 days for a total of 3 weeks. After the 3 weeks, the mice were euthanized, and tumor weight was measured. All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Ethical Committees of Xinxiang Central Hospital [IACUC protocol number: (Protocol No. 2024-KY-0723-001)].
We chose two datasets, GSE89143 and GSE83521, from the Gene Expression Omnibus (GEO) database. Subsequently, we performed differential analysis using the Gene Expression Omnibus 2R tool and screened for differentially expressed circRNAs.
Immunohistochemistry (IHC) staining was performed following previously described protocols[27]. Briefly, tissue sections were deparaffinized and rehydrated using graded ethanol solutions. Antigen retrieval was achieved by boiling the deparaffinized tissue specimens in 10 mmol/L citrate buffer (pH = 6.0) for 20 minutes. To block endogenous peroxidase activity, the sections were incubated with a 3% H2O2 solution. Subsequently, the sections were exposed to an anti-HMGN5 protein antibody and developed using 3, 3'-diaminobenzidine, followed by counterstaining with hematoxylin. Negative control (NC) sections were included, where parallel incubation with the non-specific IgG (1:300) was performed instead of the HMGN5-specific antibody.
Evaluation of IHC staining was conducted by two experienced pathologists employing a semi-quantitative scoring system based on both the intensity of staining and the percentage of positively stained cells. The staining intensity of each specimen was scored on a scale of 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). To obtain the final staining scores for all samples, the staining intensity score was multiplied by the positive HMGN5 expression score.
Gastric cancer cell lines, including MGC-803, BGC-823, OCUM-1, SGC-7901, and HSC-39, were cultured in Dulbecco's Modified Eagle's Medium (Thermo Fisher Scientific, United States) supplemented with 10% fetal bovine serum from Clark, United States. Additionally, the human normal gastric epithelium cell line GES-1 was cultured in DMEM containing 10% FBS. All cells were maintained in an incubator at 37 °C with 5% CO2.
To knock down the target transcription, short hairpin RNA for hsa_circRNA_101996 (shCircRNA_101996) was purchased from Hippobio, China. To overexpress miR-577, a miR-577 mimic was also obtained from Hippobio, China. Transfection was performed using Lipofectamine 3000 Transfection Reagent (Invitrogen, United States) following the manufacturers' instructions.
For permanent overexpression of hsa_circRNA_101996 and HMGN5, pLV-CircRNA_101996 and pLV-HMGN5 vectors were utilized, respectively. An empty vector served as the NC. Lentivirus was used to package all these vectors, and viral supernatants were used for transduction. Stable transductions were selected by growth in media containing ampicillin.
Gastric cancer cells (300 cells per well) were transfected with respective vectors and cultured in 6-well plates at 37 °C for two weeks. Afterward, the colonies were stained using 0.1% crystal violet.
The MTT assay was conducted to assess cell viability, following the established protocol[26]. Briefly, HSC-39 and OCUM-1 cells with stable transfections of each vector were cultured in logarithmic phase, counted, and then seeded in 96-well plates at a density of 800 cells in 200 μL of medium per well. The cells were allowed to attach overnight, and DMSO was used as the control. At specified time points, MTT solution (5 mg/mL, 20 μL per well) was added to the culture medium, followed by incubation at 37 °C for 2 hours. The formazan crystals were subsequently dissolved using 200 μL of DMSO. The microplate reader (Thermo Fisher, Waltham, MA, United States) was utilized to measure the absorbance at 450 nm. Each experiment was performed independently in triplicates to ensure reliability and consistency.
The cells were collected and lysed in a RIPA lysis buffer containing PMSF, following the protocol described previously[26]. The protein concentration was determined using a BCA protein assay kit. Subsequently, the proteins were separated on 8%-12% SDS-PAGE gels and transferred to a polyvinylidene fluoride (PVDF) membrane. After blocking the PVDF membrane with 5% BSA at room temperature for 2 hours, it was incubated overnight at 4 °C with a diluted primary antibody. On the following day, the PVDF membranes were treated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 2 hours. Finally, the results were analyzed using the Super ECL Prime and Western blotting detection system.
Samples were collected, and cells were isolated using Trizol reagent following the manufacturer's instructions, as previously described[21,26]. Total RNA was extracted and reverse-transcribed to cDNA using M-MLV reverse transcriptase (Promega, Wisconsin, United States). For qRT-PCR, a 20 μL reaction mixture was prepared, comprising 2 μL of cDNA template, 10 μL of 2 × GoTaq® qPCR Master Mix (Promega, United States), 0.5 μL of forward primers (Huada Gene, China), 0.5 μL of reverse primers (Huada Gene, China), and 7 μL of nuclease-free water. The amplification program consisted of initial denaturation at 95 °C for 5 minutes, followed by 45 cycles of denaturation at 95 °C for 15 seconds, annealing at 60 °C for 30 seconds, and extension at 72 °C for 90 seconds, with a final extension step at 72 °C for 10 minutes. The normalized expression control was determined using GAPDH/U6 value. The primer sequences are provided in Table 1.
| Name | Forward/sense (from 5’ to 3’) | Reverse/antisense (from 5’ to 3’) |
| circRNA_101996 | GAGTGGGAGTGTTGGAAGAAG | TTACTAAAGGCAAACGGTGAA |
| miR-577 | GCGGCGGTAGATAAAATATTGG | ATCCAGTGCAGGGTCCGAGG |
| HMGN5 | GGTTGTCTGCTATGCTTGTG | ACTGCTTCTTGCTTGGTTTC |
| GAPDH | GGAGAGTGTTTCCTCGTCCC | ACTGTGCCGTTGAATTTGCC |
| U6 | GCGCGTCGTGAAGCGTTC | GTGCAGGGTCCGAGGT |
Firstly, we cloned has_circRNA_101996 and HMGN5 into the downstream region of the firefly luciferase gene, pGL3 vector (Invitrogen, United States), separately. Then, HEK293T cells were co-transfected with a wild-type vector (150 ng) or mutant vector (150 ng). Subsequently, miR-577 mimic or NC (2 ng) was transfected into the HEK293T cells using lipofectamine 3000 (Invitrogen). After 48 hours of transfection, the luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega, United States) and normalized to Renilla luciferase activity.
The statistical analyses were conducted using GraphPad Prism 6.0 (GraphPad Software, United States). Student's t-test was employed to assess the difference between the two groups, while one-way ANOVA was used for comparisons involving multiple groups. The χ2 test was utilized to analyze categorical variables. Notably, the results presented in this study were obtained from a minimum of three independent experiments. P < 0.05 was considered to be statistically significant.
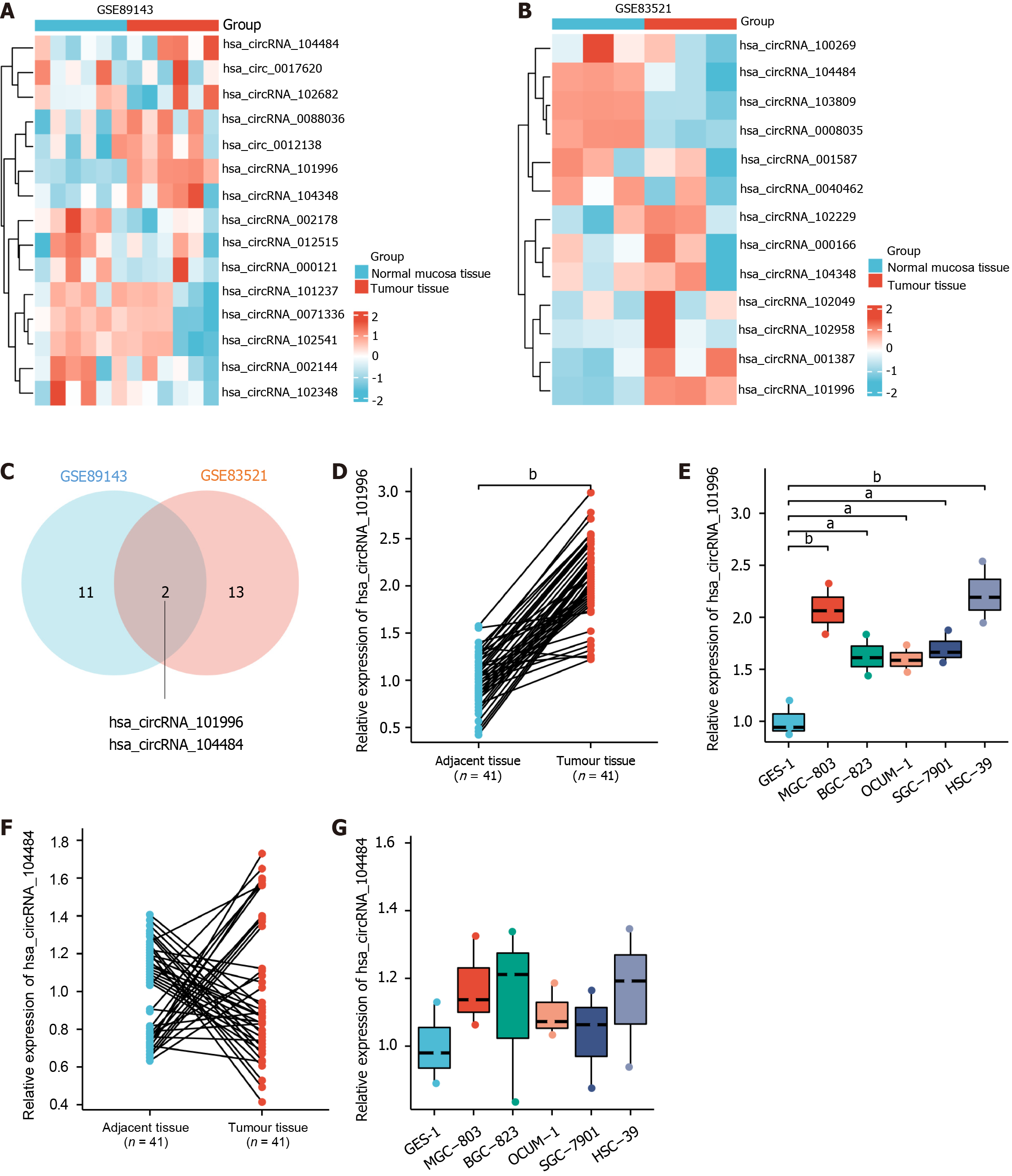
Based on the analysis of GEO databases (GSE83521 and GSE89143) as shown in Figure 1A-C, two commonly differentially expressed genes were identified. Among them, hsa_circRNA_101996 was found to be upregulated in gastric cancer tissues, while hsa_circRNA_104484 was downregulated in gastric cancer tissues. The qRT-PCR results confirmed that compared to adjacent non-cancerous tissues, hsa_circRNA_101996 was significantly upregulated in gastric cancer tissues (Figure 1D) and in MGC-803, BGC-823, OCUM-1, SGC-7901, and HSC-39 cell lines (Figure 1E, P < 0.05). On the other hand, there was no significant difference in the expression of hsa_circRNA_104484 between gastric cancer tissues and cell lines compared to adjacent non-cancerous tissues and GES-1 cell line (Figure 1F and G, P > 0.05). Due to the lowest expression level of hsa_circRNA_101996 in the OCUM-1 cell line and the highest in the HSC-39 cell line, OCUM-1 and HSC-39 cell lines were selected for subsequent in vitro experiments.
Furthermore, we analyzed the association of circRNA_101996 with the clinical characteristics of gastric cancer patients, as shown in Table 2. It was observed that patients with high expression of circRNA_101996 had larger tumor sizes, but there was no significant association with other clinical features, suggesting the involvement of circRNA_101996 in gastric cancer proliferation and apoptosis.
| Clinical characteristics | hsa_circRNA_101996 | miR-577 | HMGN5 | ||||||
| Low expression group (≤ median) | High expression group (> median) | P value | Low expression group (≤ median) | High expression group (> median) | P value | Low expression group (≤ median) | High expression group (> median) | P value | |
| Gender | 0.443 | 0.890 | 0.162 | ||||||
| Male | 8 | 10 | 9 | 9 | 7 | 11 | |||
| Female | 13 | 10 | 12 | 11 | 14 | 9 | |||
| Age | 0.890 | 0.262 | 0.443 | ||||||
| > 50 | 12 | 11 | 10 | 13 | 13 | 10 | |||
| ≤ 50 | 9 | 9 | 11 | 7 | 8 | 10 | |||
| Body mass index (kg/m2) | 0.837 | 0.393 | 0.275 | ||||||
| > 24 | 13 | 13 | 12 | 14 | 15 | 11 | |||
| ≤ 24 | 8 | 7 | 9 | 6 | 6 | 9 | |||
| Tumor size (cm) | < 0.001 | 0.043 | 0.003 | ||||||
| > 3 | 5 | 16 | 14 | 7 | 6 | 15 | |||
| ≤ 3 | 16 | 4 | 7 | 13 | 15 | 5 | |||
| TNM stage | 0.654 | 0.086 | 0.412 | ||||||
| 1-2 | 8 | 9 | 6 | 11 | 10 | 7 | |||
| 3-4 | 13 | 11 | 15 | 9 | 11 | 13 | |||
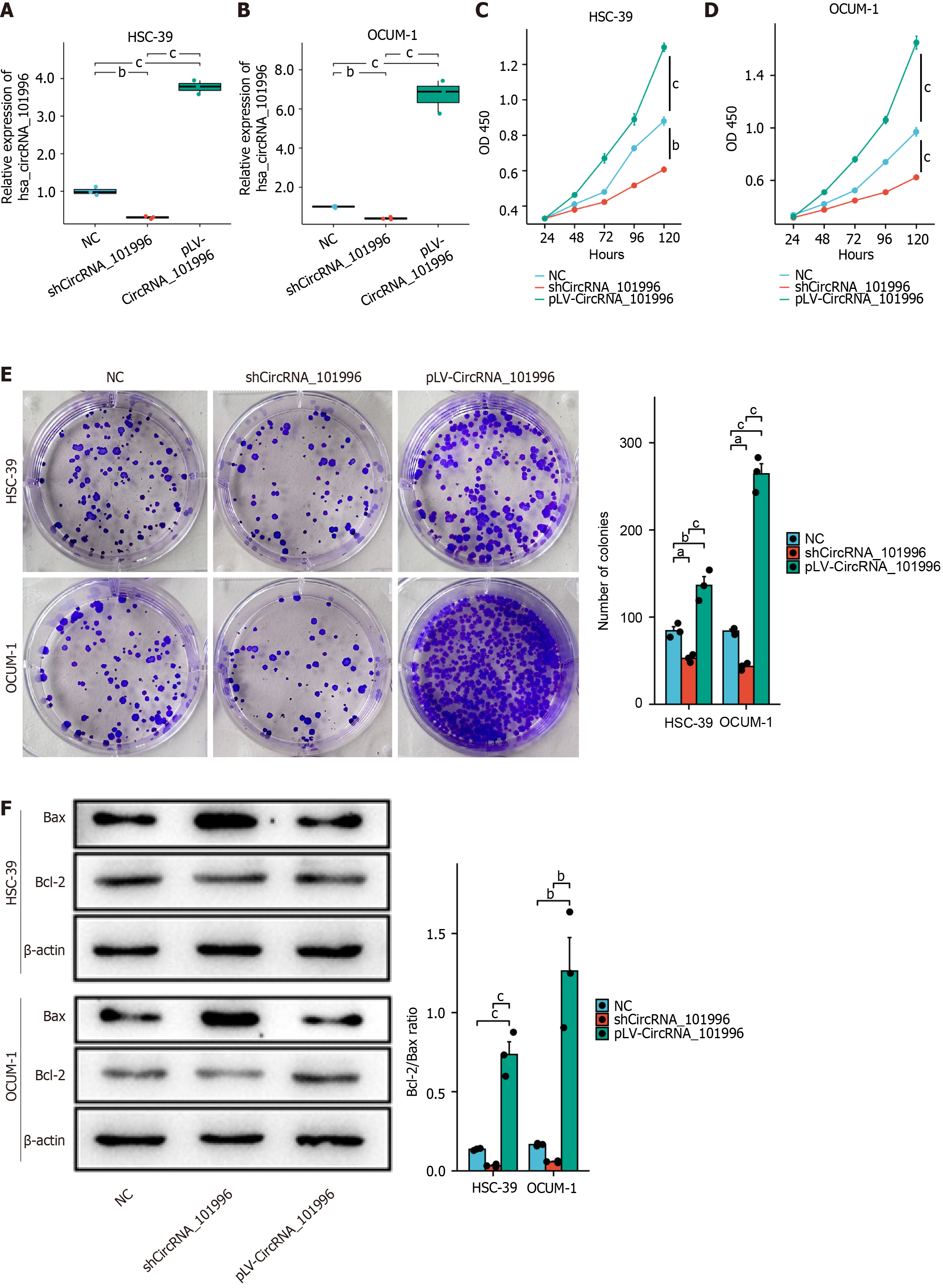
Firstly, we validated the efficiency of circRNA_101996 knockdown and overexpression in the OCUM-1 and HSC-39 cell lines, respectively. Compared to the NC group, the expression of circRNA_101996 was significantly reduced by 70% in the shCircRNA_101996 group (Figure 2A) and increased 4-6 times in the pLV-CircRNA_101996 group (Figure 2B).
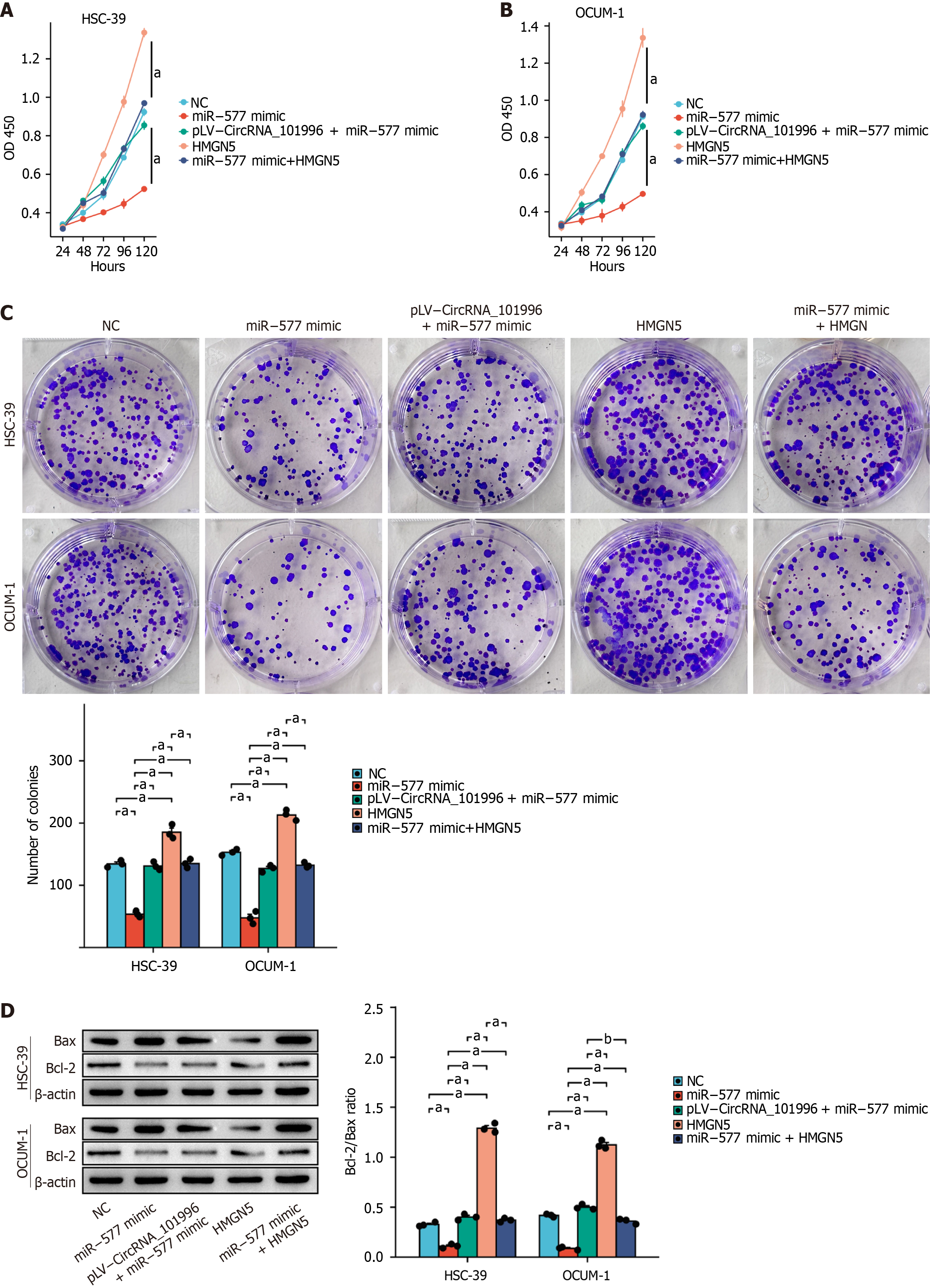
MTT and colony formation analyses demonstrated that the proliferation ability of OCUM-1 and HSC-39 cells was significantly decreased in the shCircRNA_101996 group compared to the NC group (Figure 2C-E). Conversely, the proliferation ability of cells in the pLV-CircRNA_101996 group was significantly increased (Figure 2C-E). Western blot results revealed that the Bcl-2/Bax ratio was significantly decreased in OCUM-1 and HSC-39 cells of the shCircRNA_101996 group, while the Bcl-2/Bax ratio was significantly increased in cells of the pLV-CircRNA_101996 group. Our findings indicate that circRNA_101996 promotes gastric cancer proliferation and inhibits apoptosis (Figure 2F).
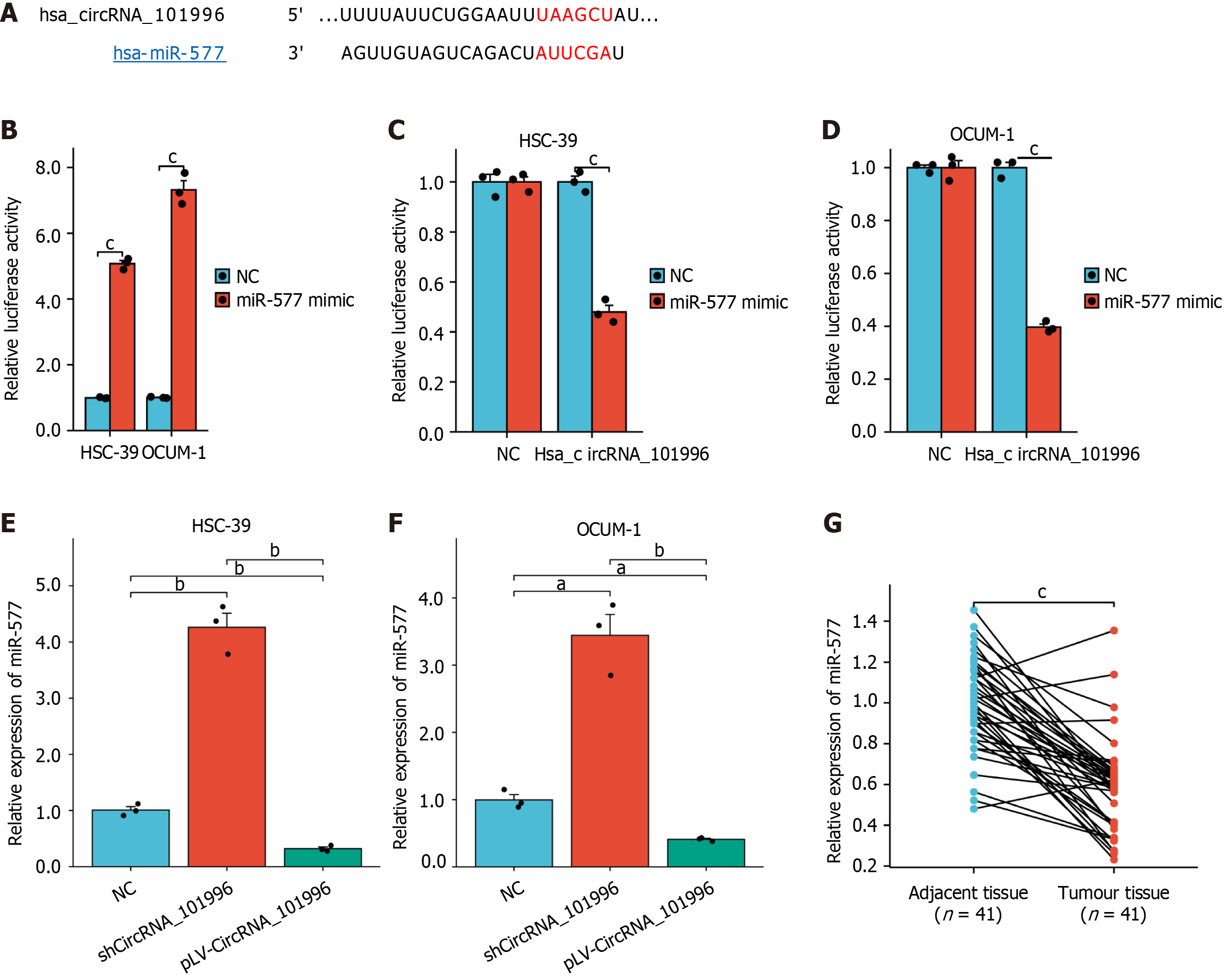
Further, CircInteractome was utilized to predict the binding of circRNA_101996 with miR-577, and the binding site was identified, as shown in Figure 3A. Following transfection of cells with miR-577 mimic, the expression of miR-577 was significantly increased (Figure 3B). Luciferase reporter gene assays confirmed the target relationship between hsa_circRNA_101996 and miR-577 (Figure 3C and D). Moreover, upon knockdown of hsa_circRNA_101996, miR-577 expression was significantly elevated compared to the NC group, exhibiting a statistically significant difference (P < 0.05, Figure 3E and F). Additionally, miR-577 expression was notably downregulated in gastric cancer tissues (Figure 3G), and patients with low miR-577 expression presented larger tumor sizes (Table 2).
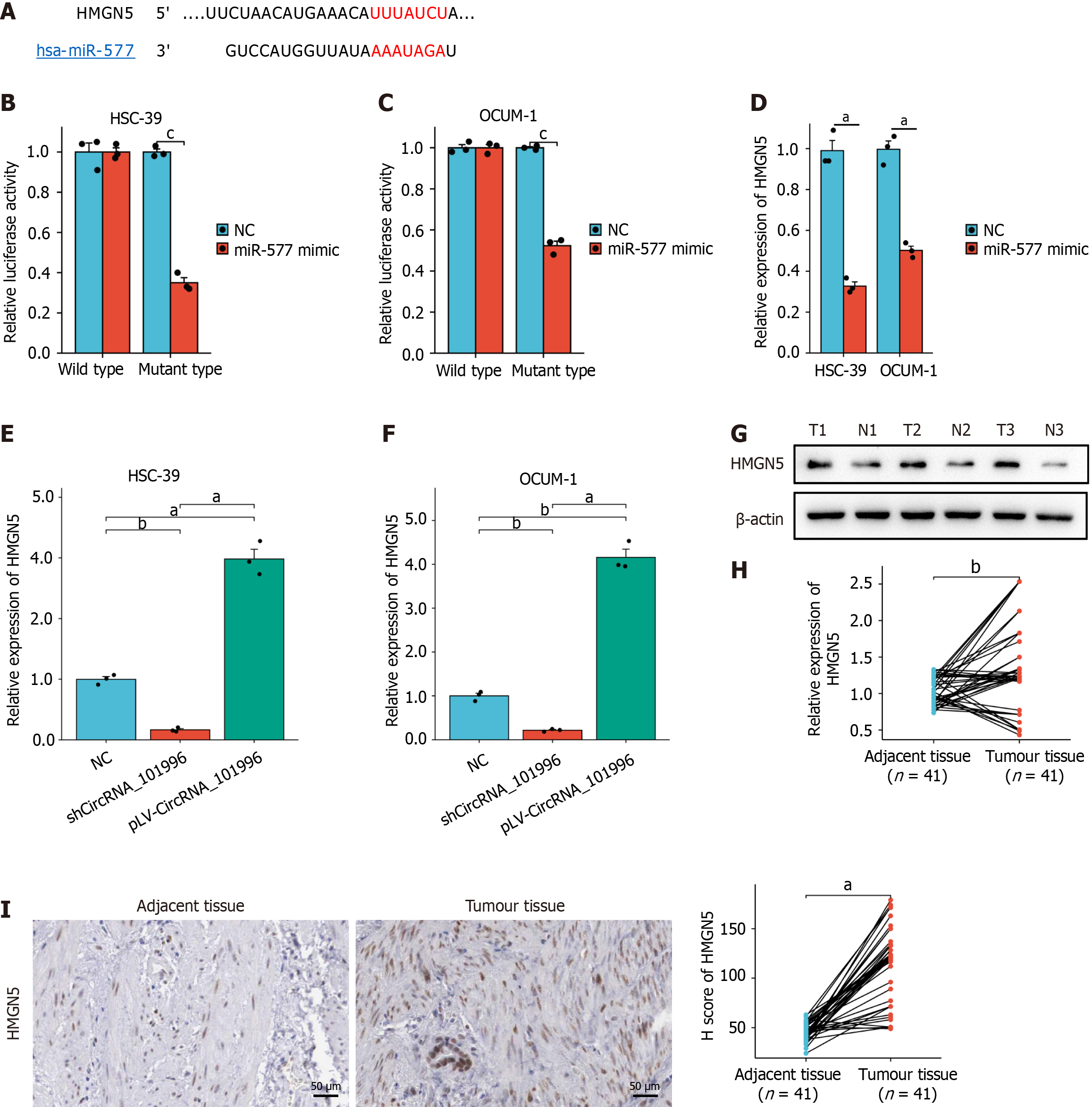
Using TargetScan, binding sites between miR-577 and HMGN5 were identified as shown in Figure 4A. Luciferase reporter gene assays confirmed the targeting relationship (Figure 4B and C). Upon transfection of cells with miR-577 mimic, the expression of HMGN5 was significantly reduced (Figure 4D). Knockdown of hsa_circRNA_101996 Led to a significant decrease in HMGN5 expression compared to the NC group, displaying a statistically significant difference (P < 0.05, Figure 4E and F). Furthermore, HMGN5 protein and mRNA levels were significantly upregulated in gastric cancer tissues (Figure 4G-I), and patients with high HMGN5 expression showed larger tumor sizes (P < 0.05, Table 2).
In vitro experiments showed that the analysis of MTT, colony formation, and Western blot results indicated a significant decrease in the proliferation capability (P < 0.05, Figure 5A-C) and a significant increase in the apoptosis capability (Figure 5D) of cells in the miR-577 mimic group compared to the NC group. However, co-transfection of pLV-CircRNA_101996 or pLV-HMGN5 reversed the effects of the miR-577 mimic. Furthermore, overexpression of HMGN5 significantly promoted the proliferation capability (P < 0.05, Figure 5) and suppressed apoptosis in OCUM-1 and HSC-39 cells.
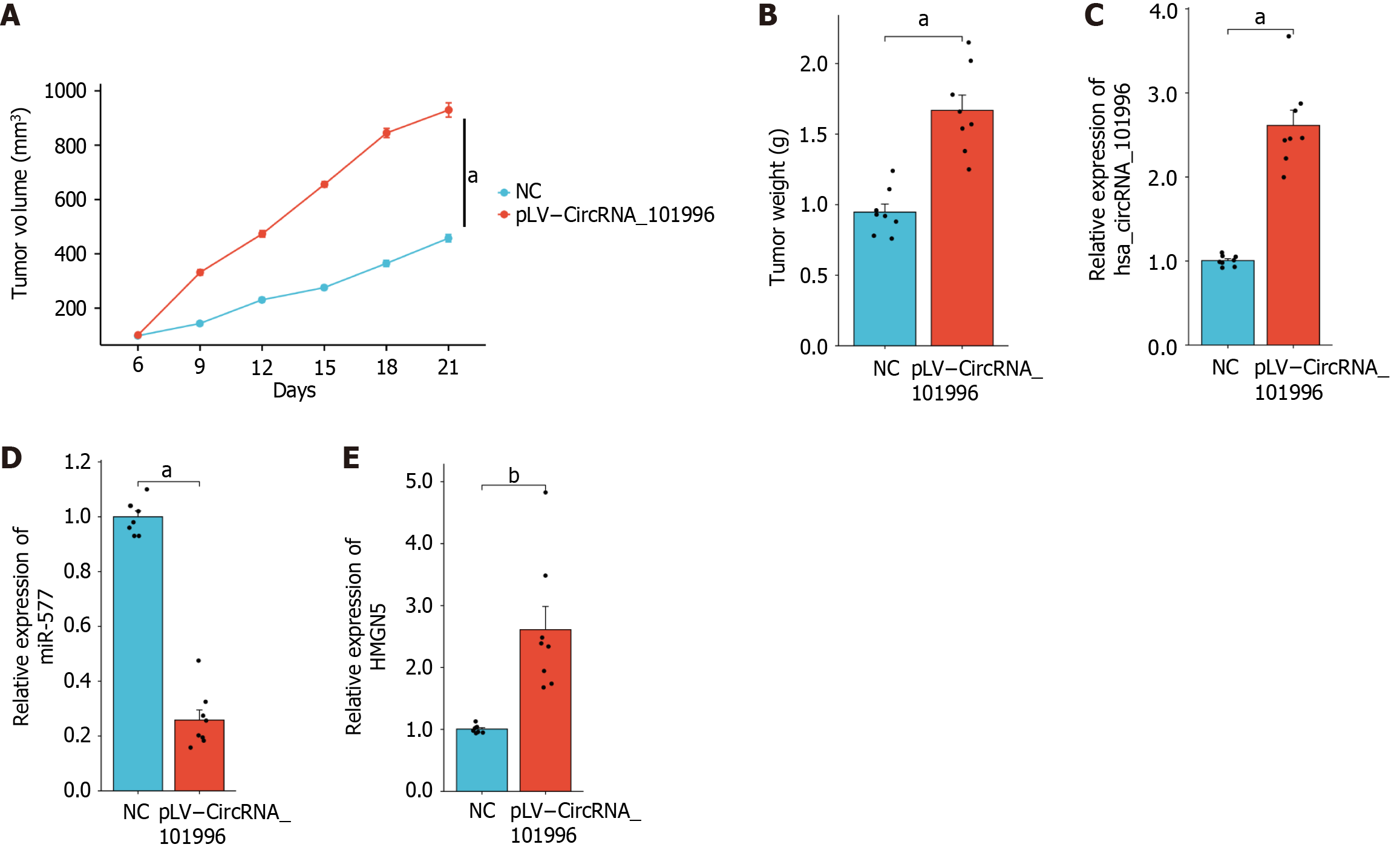
Based on the aforementioned in vitro experiments, the proliferation effect of OCUM-1 was more pronounced compared to HSC-39. Therefore, OCUM-1 was used for the in vivo experiments. The experiments measuring tumor volume and weight demonstrated that pLV-CircRNA_101996 significantly promoted tumor growth (P < 0.05, Figure 6A and B). Additionally, qRT-PCR confirmed that compared to the NC group, the expression levels of circRNA_101996 and HMGN5 were significantly upregulated in the tumor tissues of the pLV-CircRNA_101996 group, while the expression of miR-577 was significantly downregulated (P < 0.05, Figure 6C-E).
CircRNAs play crucial regulatory roles in various cellular biological processes, especially in the occurrence and progression of tumors[21]. Currently, the regulatory effects of circRNAs on malignant tumors are mainly exerted through the following two mechanisms: (1) As competing endogenous RNAs, circRNAs act as "molecular sponges" for miRNAs, sequestering miRNAs and inhibiting their degradation of downstream mRNAs, which leads to increased translation and ribosome binding of the target mRNAs, thereby modulating the biological behavior of tumor cells[18]. For instance, hsa_circ_0009172 regulates gastric cancer by inhibiting NTRK3 through mediation by miRNA-485-3p, while hsa_circ_0000670 promotes gastric cancer progression through the microRNA-384/SIX4 axis[28]; and (2) CircRNAs can function as protein sponges or decoys similar to certain known linear RNAs[21]. They can sequester protein-binding sites, thereby secluding specific proteins, and competitively bind to block the activity of proteins, affecting tumor proliferation and invasion capabilities[4]. Although numerous circRNAs have been found to participate in gastric cancer progression, the specific mechanism of circRNA_101996 in gastric cancer remains unclear. In a previous study, researchers identified hsa_circRNA_101996 as a key regulator that promotes migration and invasion of gastric cancer cells by upregulating MMP2 and MMP9 via the miR-143/TET2 pathway[23]. However, the precise mechanisms by which circRNA_101996 influences gastric cancer cell proliferation and apoptosis remain unclear.
To elucidate the potential mechanism of circRNA_101996 in influencing gastric cancer progression, we constructed a circRNA_101996 dual-luciferase reporter plasmid. The dual-luciferase assay results revealed that circRNA_101996 can competitively bind to miR-577, thus preventing miR-577 from binding to its target gene HMGN5. MiR-577 has been found to play a critical role in various tumors[4]. For instance, miR-577 inhibits the migration and invasion of hepatocellular carcinoma (HCC) cells by targeting HOXA1[29]. Wang et al[30] reported that low expression of miR-577 is associated with malignant clinicopathological features in HCC tissues, and miR-577 may suppress HCC growth by down-regulating β-catenin. Another study indicated that miR-577 inhibits colorectal cancer proliferation and metastasis through the HSP27-mediated feedback loop[31]. To screen for target genes of the circRNA_101996/miR-577 axis, we performed qRT-PCR and found that silencing circRNA_101996 significantly inhibited miR-577 expression and promoted HMGN5 mRNA expression. It has been reported that HMGN5 is involved in the occurrence and development of various tumors[32,33]. For example, HMGN5 can increase the proliferative and migratory capacity of colorectal cancer cells by targeting binding to FGF12[33]. In pancreatic ductal adenocarcinoma, HMGN5 promotes proliferation and invasion through the activation of the Wnt/β-catenin signaling pathway[32]. To confirm whether HMGN5 is a target of miR-577, we constructed HMGN5 3'-UTR-WT/MUT plasmids. Dual-luciferase reporter analysis suggested that HMGN5 is a potential target gene of miR-577. Furthermore, qRT-PCR demonstrated that the regulatory effect of circRNA_101996 on HMGN5 expression can be reversed by miR-577. Rescue experiments indicated that the effects of circRNA_101996 on gastric cancer cell proliferation and apoptosis can be partially reversed by miR-577. These results suggest that circRNA_101996 may exert a tumor-suppressive role through the miR-577/HMGN5 axis, indicating that circRNA_101996 regulates gastric cancer progression through the miR-577/HMGN5 axis.
This study has several limitations that need to be addressed. First, the specific circularization mechanism of hsa_circRNA_101996 remains unclear, and further investigation is required to better understand its biogenesis. Second, while this study demonstrates the regulatory effects of the hsa_circRNA_101996/miR-577/HMGN5 axis on gastric cancer proliferation and apoptosis, the potential role of hsa_circRNA_101996 in regulating gastric cancer cell biology through interactions with RNA-binding proteins or translation factors is still unknown. Further research, such as RNA pull-down combined with mass spectrometry to identify RNA-binding proteins, or transcriptomic profiling and polysome fractionation to explore its role in translational regulation, will be essential for a more comprehensive understanding of the functional versatility of hsa_circRNA_101996 in gastric cancer. Third, the translational potential of hsa_circRNA_101996 remains unvalidated in clinical settings, as its prognostic utility has not been evaluated in large-scale patient cohorts or liquid biopsy specimens. Additionally, while in vitro/in vivo evidence supports its oncogenic role, the feasibility and safety of siRNA/ASO-mediated therapeutic strategies targeting this circRNA have not been systematically explored. Furthermore, the clinical relevance of the proposed hsa_circRNA_101996/miR-577/HMGN5 axis for precision medicine applications remains hypothetical, as no prospective trials have validated its predictive value for treatment outcomes. Future studies incorporating multi-omics validation in diverse patient populations and preclinical models are essential to address these translational gaps.
In summary, we propose that circRNA_101996 may regulate HMGN5 expression by competitively binding to miR-577, thereby modulating malignant biological behaviors of gastric cancer cells, such as proliferation and apoptosis. This study provides a novel target for gastric cancer therapy and introduces new biological indicators for monitoring gastric cancer occurrence and recurrence.
| 1. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 960] [Article Influence: 320.0] [Reference Citation Analysis (0)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64647] [Article Influence: 16161.8] [Reference Citation Analysis (176)] |
| 3. | Norwood DA, Montalvan-Sanchez E, Dominguez RL, Morgan DR. Gastric Cancer: Emerging Trends in Prevention, Diagnosis, and Treatment. Gastroenterol Clin North Am. 2022;51:501-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 4. | Hall CE, Maegawa F, Patel AD, Lin E. Management of Gastric Cancer. Am Surg. 2023;89:2713-2720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 5. | Chang CW, Chen CY. Prognostic factors of advanced gastric cancer. J Chin Med Assoc. 2021;84:557-558. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Maharjan U, Kauppila JH. Survival trends in gastric cancer patients between 1987 and 2016: a population-based cohort study in Finland. Gastric Cancer. 2022;25:989-1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 7. | Ye M, Xiu LJ, Ji QQ, Zhang YC, Sun YW, Zhao Y, Wang D, Li YJ, Wang XW, Yue XQ, Sun DZ. Research progress in targeted therapies for gastric cancer. Int J Clin Pharmacol Ther. 2022;60:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Du G, Zeng Y, Chen D, Zhan W, Zhan Y. Application of radiomics in precision prediction of diagnosis and treatment of gastric cancer. Jpn J Radiol. 2023;41:245-257. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Gong YQ, Lu TL, Hou FT, Chen CW. Antisense long non-coding RNAs in gastric cancer. Clin Chim Acta. 2022;534:128-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Lu L, Chen B, Xu Y, Zhang X, Jin L, Qian H, Wang Y, Liang ZF. Role of ferroptosis and ferroptosis-related non-coding RNAs in the occurrence and development of gastric cancer. Front Pharmacol. 2022;13:902302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Chen X, Wei C, Huang L, Syrigos K, Li Y, Li P. Non-coding RNAs regulate mitochondrial dynamics in the development of gastric cancer. Front Mol Biosci. 2023;10:1107651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Roy S, Kanda M, Nomura S, Zhu Z, Toiyama Y, Taketomi A, Goldenring J, Baba H, Kodera Y, Goel A. Diagnostic efficacy of circular RNAs as noninvasive, liquid biopsy biomarkers for early detection of gastric cancer. Mol Cancer. 2022;21:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 13. | Wang X, Zhang J, Cao G, Hua J, Shan G, Lin W. Emerging roles of circular RNAs in gastric cancer metastasis and drug resistance. J Exp Clin Cancer Res. 2022;41:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 14. | Li W, Liu JQ, Chen M, Xu J, Zhu D. Circular RNA in cancer development and immune regulation. J Cell Mol Med. 2022;26:1785-1798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Kristensen LS, Jakobsen T, Hager H, Kjems J. The emerging roles of circRNAs in cancer and oncology. Nat Rev Clin Oncol. 2022;19:188-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 706] [Article Influence: 235.3] [Reference Citation Analysis (0)] |
| 16. | Wang C, Liu WR, Tan S, Zhou JK, Xu X, Ming Y, Cheng J, Li J, Zeng Z, Zuo Y, He J, Peng Y, Li W. Characterization of distinct circular RNA signatures in solid tumors. Mol Cancer. 2022;21:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 17. | Ma Q, Yang F, Huang B, Pan X, Li W, Yu T, Wang X, Ran L, Qian K, Li H, Li H, Liu Y, Liang C, Ren J, Zhang Y, Wang S, Xiao B. CircARID1A binds to IGF2BP3 in gastric cancer and promotes cancer proliferation by forming a circARID1A-IGF2BP3-SLC7A5 RNA-protein ternary complex. J Exp Clin Cancer Res. 2022;41:251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 53] [Reference Citation Analysis (0)] |
| 18. | Qiao L, Pan W, Yang J, Cheng Y, Han Y, Zhu Q, Liu R, Zhang H, Ba Y. Inhibitory effects of circR-127aa on gastric cancer progression and tumor growth. Cell Signal. 2025;125:111520. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Ding L, Zhao Y, Dang S, Wang Y, Li X, Yu X, Li Z, Wei J, Liu M, Li G. Circular RNA circ-DONSON facilitates gastric cancer growth and invasion via NURF complex dependent activation of transcription factor SOX4. Mol Cancer. 2019;18:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 20. | Shen X, Kong S, Ma S, Shen L, Zheng M, Qin S, Qi J, Wang Q, Cui X, Ju S. Hsa_circ_0000437 promotes pathogenesis of gastric cancer and lymph node metastasis. Oncogene. 2022;41:4724-4735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 21. | Ma C, Wang X, Yang F, Zang Y, Liu J, Wang X, Xu X, Li W, Jia J, Liu Z. Circular RNA hsa_circ_0004872 inhibits gastric cancer progression via the miR-224/Smad4/ADAR1 successive regulatory circuit. Mol Cancer. 2020;19:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 22. | Lu C, Wu J, Li X, Huang W, Fang Y, Huang Y. Hsa_circ_0003356 suppresses gastric cancer progression via miR-556-5p/FKBP5 axis. Toxicol In Vitro. 2024;97:105787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 23. | Huang F, Jiang J, Yao Y, Hu S, Wang H, Zhu M, Yu L, Liu Q, Jia H, Xu W. Erratum: Circular RNA Hsa_circRNA_101996 promotes the development of Gastric Cancer via Upregulating Matrix Metalloproteinases-2/Matrix Metalloproteinases-9 through MicroRNA-143/Ten-eleven translocation-2 Pathway: Erratum. J Cancer. 2024;15:2805-2809. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Song T, Xu A, Zhang Z, Gao F, Zhao L, Chen X, Gao J, Kong X. CircRNA hsa_circRNA_101996 increases cervical cancer proliferation and invasion through activating TPX2 expression by restraining miR-8075. J Cell Physiol. 2019;234:14296-14305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 25. | Song TF, Xu AL, Chen XH, Gao JY, Gao F, Kong XC. Circular RNA circRNA_101996 promoted cervical cancer development by regulating miR-1236-3p/TRIM37 axis. Kaohsiung J Med Sci. 2021;37:547-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | He JH, Han ZP, Zhou JB, Chen WM, Lv YB, He ML, Li YG. MiR-145 affected the circular RNA expression in prostate cancer LNCaP cells. J Cell Biochem. 2018;119:9168-9177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Shen DD, Pang JR, Bi YP, Zhao LF, Li YR, Zhao LJ, Gao Y, Wang B, Wang N, Wei L, Guo H, Liu HM, Zheng YC. LSD1 deletion decreases exosomal PD-L1 and restores T-cell response in gastric cancer. Mol Cancer. 2022;21:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 127] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 28. | Ding W, Li Z, Liu X, Wang J, Wang J, Jiang G, Yu H, Wang T. Hsa_circ_0008667 promotes progression and improves the prognosis of gastric cancer by inhibiting miR-9-5p. Arab J Gastroenterol. 2024;25:349-355. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Han S, Liu Z, Wang Y, Wang L, Yao B, Guo C, Song T, Tu K, Liu Q. MicroRNA‑577 inhibits the migration and invasion of hepatocellular carcinoma cells by targeting homeobox A1. Oncol Rep. 2018;39:2987-2995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Wang LY, Li B, Jiang HH, Zhuang LW, Liu Y. Inhibition effect of miR-577 on hepatocellular carcinoma cell growth via targeting β-catenin. Asian Pac J Trop Med. 2015;8:923-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Chu LY, Peng YH, Weng XF, Xie JJ, Xu YW. Blood-based biomarkers for early detection of esophageal squamous cell carcinoma. World J Gastroenterol. 2020;26:1708-1725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (2)] |
| 32. | Zhao J, Wang Y, Wu X. HMGN5 promotes proliferation and invasion via the activation of Wnt/β-catenin signaling pathway in pancreatic ductal adenocarcinoma. Oncol Lett. 2018;16:4013-4019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Zhu GJ, Liu F, Xu YG, Zhao CX, Zhao JG, Sun C. HMGN5 promotes invasion and migration of colorectal cancer through activating FGF/FGFR pathway. Eur Rev Med Pharmacol Sci. 2021;25:1330-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |