Published online May 15, 2025. doi: 10.4251/wjgo.v17.i5.105311
Revised: March 6, 2025
Accepted: March 18, 2025
Published online: May 15, 2025
Processing time: 118 Days and 0.4 Hours
Hepatocellular carcinoma (HCC) is the most common pathological type of liver cancer and was the third leading cause of cancer-related deaths worldwide in 2020.
To evaluate the diagnostic potential of key tumor markers in serum, bile, and fecal samples for detecting HCC.
Blood, bile, and fecal samples were collected from patients (n = 265) with HCC and cholecystitis from Guangxi Medical University’s First Affiliated Hospital. Immunohistochemistry was performed on 69 HCC samples, and 16S ribosomal RNA sequencing was conducted on 166 fecal samples. Tumor marker cut-off values in bile and feces were determined using the Youden index, while serum biomarkers followed hospital standards. Diagnostic performance was evaluated using receiver operating characteristic analysis.
The areas under the curve (AUCs) for distinguishing HCC were 0.898, 0.904, and 0.859 for serum alpha-fetoprotein (AFP), prothrombin induced by vitamin K absence-II (PIVKA-II), and bile AFP, respectively. Serum AFP had the highest diagnostic value (80%) for early-stage HCC. Combination analysis found that bile AFP and serum PIVKA-II achieved the highest AUC of 0.965 (P < 0.001), suggesting that bile AFP may serve as a valuable complementary biomarker, particularly in cases where serum AFP is not significantly elevated. Additionally, bile AFP was positively correlated with Actinomyces, which plays a significant role in promoting tumorigenesis; and was negatively correlated with Faecalibacterium, which was associated with robust anticancer immune responses (P < 0.05). These findings suggest the potential role of gut microbiota in modulating AFP levels and HCC progression.
Bile AFP improved the sensitivity of HCC detection, with the combination of bile AFP and PIVKA-II demon
Core Tip: This study evaluated the diagnostic potential of various biomarkers for hepatocellular carcinoma (HCC), with serum prothrombin induced by vitamin K absence-II (PIVKA-II) showing the highest diagnostic accuracy. However, serum alpha-fetoprotein (AFP) was more accurate for early HCC diagnosis. Bile AFP improved diagnostic performance and its combination with PIVKA-II achieved the highest area under the curve for HCC detection. Additionally, bile AFP was associated with poorer HCC clinical outcomes.
- Citation: Chen ZJ, Wang XK, Han CY, He YF, Liang TY, Mo ST, Zhu GZ, Yang CK, Ye XP, Lv ZL, Pang SF, Chen XD, Wang P, Peng T. Diagnostic value of alpha-fetoprotein and prothrombin induced by vitamin K absence-II in serum, bile, and feces in hepatocellular carcinoma. World J Gastrointest Oncol 2025; 17(5): 105311
- URL: https://www.wjgnet.com/1948-5204/full/v17/i5/105311.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i5.105311
Hepatocellular carcinoma (HCC) is the most common pathological type of liver cancer (LC)[1]. In 2020, HCC became the third leading cause of cancer-related deaths worldwide, a sharp rise from its rank as the ninth in 2000[2]. Consequently, most patients are diagnosed at an advanced stage with a poor prognosis, highlighting the urgent need for improved screening and early detection strategies[3,4].
Evidence suggests that tumor markers can increase 0.5-1.5 years before HCC becomes detectable on imaging[5]. Typically, biomarkers including alpha-fetoprotein (AFP), prothrombin induced by vitamin K absence-II (PIVKA-II)[6], and AFP-L3 are used for HCC detection[7]. Additionally, the gut microbiome may influence the progression of HCC through the gut-liver axis, potentially altering the composition of bile and fecal samples[8]. Other tumor markers such as carcinoembryonic antigen (CEA), cancer antigen 125 (CA125), CA153, and CA199 have also been associated with various gastrointestinal cancers. Unlike previous studies that primarily focused on serum biomarkers, the present study comprehensively evaluated multiple biomarkers in serum, bile, and fecal samples. Furthermore, since AFP expression promotes HCC development, microbiome analysis was combined with biomarker profiling to explore how the gut microbiota (GM) influences tumor markers and HCC progression. It is anticipated that the proposed innovative and comprehensive strategy for HCC detection will address the critical limitations of current diagnostic methods.
From August 2018 to August 2020, a total of 265 eligible patients were admitted to the Department of Hepatobiliary Surgery at the First Affiliated Hospital of Guangxi Medical University (Nanning, China). Among them, 225 were diagnosed with HCC and 40 had chronic cholecystitis. This study was ethically approved by the hospital’s ethics committee (Approval No. 2020 [No. KY-E-118]). All patients voluntarily participated and provided written informed consent.
Inclusion criteria for the HCC group were as follows: (1) Patients between 18 and 80 years of age; (2) Patients with a diagnosis of HCC confirmed by postoperative pathology; and (3) Patients who have not undergone any previous treatments for HCC. Inclusion criteria for the control group were as follows: (1) Patients between 18 and 80 years old; and (2) Patients with a diagnosis of chronic cholecystitis confirmed by postoperative pathology. Patients with cholecystitis who had intrahepatic or extrahepatic bile duct stones, hepatic hemangioma, or liver function grade B-C.
HCC staging was determined using both the Barcelona clinic LC (BCLC) staging system and the China LC (CNLC) staging system[9,10].
Serum samples were obtained from all 265 patients before surgery, bile samples were collected from 118 patients during surgery, and fecal samples were collected from 166 patients before surgery. Fecal supernatant collection was performed with strict precision. Patients were instructed to collect midsection feces in a sterile 50 mL centrifuge tube, which was then stored at -80 °C until analysis. To prepare the fecal supernatant, a 5 g fecal sample was mixed with high-pressure distilled phosphate-buffered saline at a 1 g: 1 mL ratio. The mixture was centrifuged at 12000 × g for 5 minutes, and 5 mL of the supernatant was carefully extracted and filtered through a 40 μm filter. The filtrate was then transferred to a sterile blood collection tube and promptly submitted for analysis. All steps were meticulously performed to ensure the quality and integrity of the samples.
CEA, CA125, CA153, and CA199 are common tumor markers associated with various gastrointestinal cancers, while AFP and PIVKA-II are widely used for the diagnosis of HCC. Therefore, all samples were analyzed for AFP, PIVKA-II, CEA, CA125, CA153, and CA199 using a chemiluminescence microparticle immunoassay.
The main reagents included the EP209 alpha-1-fetoprotein monoclonal antibody (ZSGB-BIO, Beijing, China), cytokeratin 7 monoclonal antibody, clone UMAB161 (ZSGB-BIO), and polymerized horseradish peroxidase-anti MS/Rb IgG SD3003 (Celnovte Biotechnology, Rockville, MD, United States).
The experimental procedures are detailed in the Supplementary Methods section, available online. Immunohistochemical analyses were performed by expert pathologists in our department, who were blinded to the patients’ clinical and pathological information. A marker was considered positive if more than 10% of tumor cells exhibited moderate or intense AFP expression. By contrast, it was considered negative if no protein markers were present or if AFP expression was observed in less than 10% of tumor cells.
DNA was extracted from the fecal samples of all patients. The extracted DNA was then amplified, amplicon libraries were constructed, and sequencing was performed using the Illumina MiSeq Platform (AIage Life Science Co. Ltd., Guangxi, China). Raw sequencing data underwent quality control and taxonomic annotation. The experimental methods followed those described by Pang et al[11], who explored the correlation between gut microbiome composition and the longevity of centenarians. Detailed procedures are provided in the online supplementary methods.
Statistical analysis was performed using SPSS version 22.0. Quantitative variables are expressed as the mean ± SD, while categorical variables are presented as proportions. For normally distributed data, comparisons were made using the Student’s t-test, whereas non-normally distributed data were analyzed using the Wilcoxon rank-sum test. Correlations between different biomarkers were assessed using Spearman’s rank correlation test.
Binary logistic regression analysis was performed to determine the coefficient (β) of each marker. The combined predictor of two markers was calculated using the following formula[12,13], where β1 and β2 are the regression coefficients for marker 1 and marker 2, respectively. This combined predictor enhances diagnostic accuracy by integrating both markers.
The optimal cut-off value for each biomarker was determined using Youden’s index[14], a metric that reflects both sensitivity and specificity. For bile and fecal biomarkers, the optimal cut-off values were selected based on the maximum Youden’s index, whereas hospital-standard cut-off values were used for serum biomarkers. Receiver operating characteristic (ROC) curves were plotted to evaluate the diagnostic performance of AFP and PIVKA-II, with area under the curve (AUC) values calculated[15,16]. AUC measures the discriminative ability of a biomarker, with higher values indicating better diagnostic performance. Sensitivity and specificity for all six markers in HCC diagnosis were calculated. The Kappa consistency test and paired χ² test (McNemar test) were used to compare sensitivity and specificity between different groups[17,18]. Furthermore, Kaplan-Meier analysis with the log-rank tests was conducted to compare recurrence-free survival (RFS) between patients with HCC with positive and negative AFP immunohistochemistry results. Statistical significance was set at P < 0.05 for all analyses.
The study included patients aged 18 to 80 years. There was no statistically significant difference in age between the HCC and cholecystitis groups (P > 0.05). The incidence of HCC was higher in males, whereas cholecystitis was more prevalent in females, with a statistically significant difference between groups (P < 0.001). The infection rate of hepatitis B virus (HBV) was significantly higher in the HCC group compared to the cholecystitis group (P < 0.001). No significant difference in body mass index was observed between the groups (P > 0.05). All patients in the HCC group were newly diagnosed and had not received prior treatment. The cholecystitis group included patients with gallstone- or polyp-induced cholecystitis but no liver diseases such as intrahepatic or extrahepatic bile duct stones or hepatic hemangiomas. Neither group had major comorbidities.
Among the 225 patients with HCC, 156 (69.3%) were classified as BCLC stage A. Besides, according to the CNLC staging system, 97 (43.1%) were classified as stage Ia and 80 (35.6%) as stage Ib, indicating that the study primarily focuses on early-stage HCC.
Analysis of bile samples revealed that among 103 specimens from patients with HCC, 5 (4.9%) were negative for serum AFP but positive for bile AFP, and 1 (1.0%) was negative for serum PIVKA-II but positive for bile PIVKA-II. These findings suggest that bile biomarker analysis may enhance the detection of early-stage HCC.
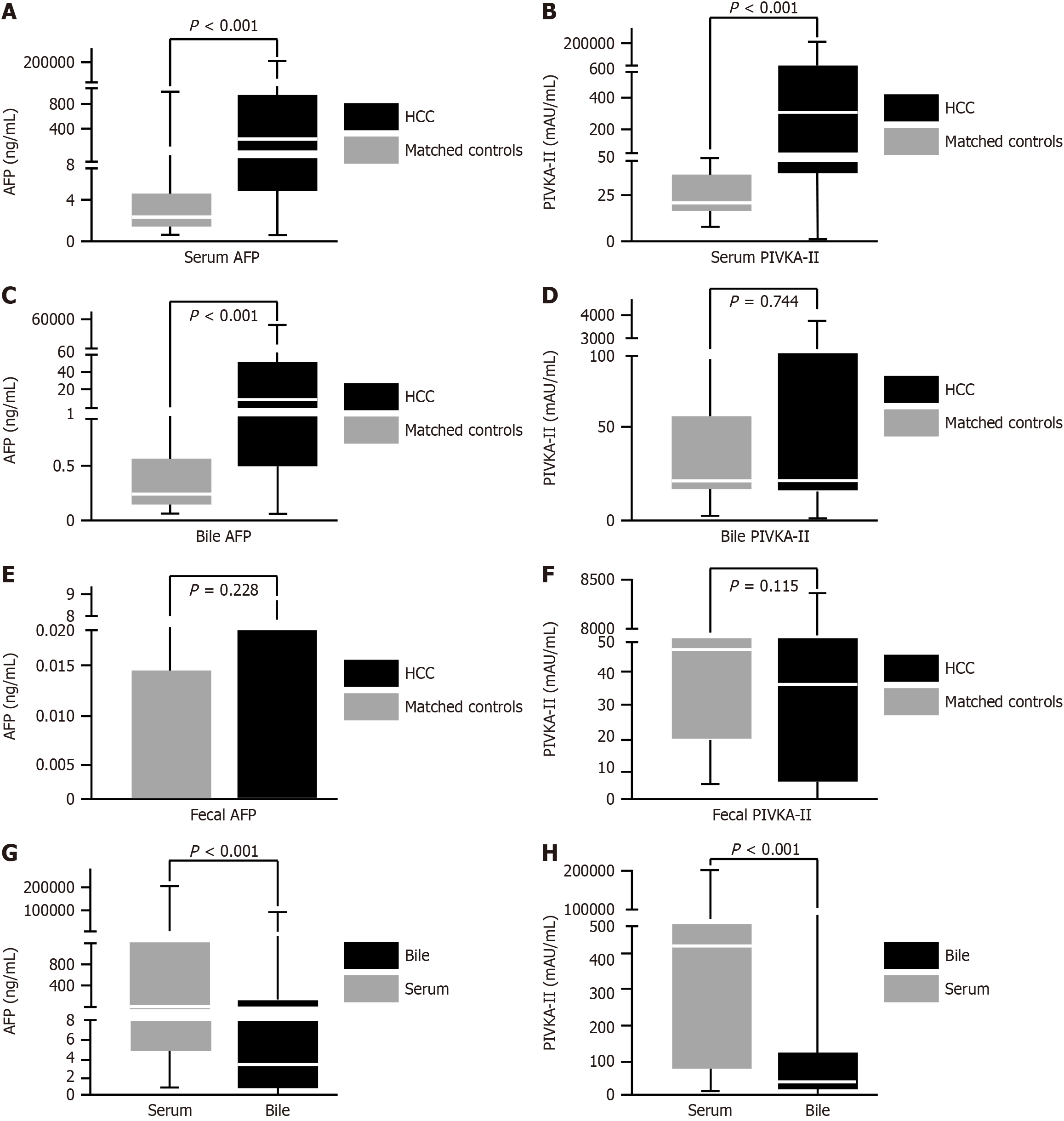
Figure 1 illustrates significant differences in serum AFP, PIVKA-II levels, and bile AFP levels between the HCC and control groups (P < 0.001). These findings suggest that serum AFP, PIVKA-II, and bile AFP hold strong potential as diagnostic biomarkers for HCC.
Among the 225 patients included in this study, 214 (95.1%) did not exhibit portal vein tumor thrombus, and 195 (86.7%) had only a single tumor. The maximum tumor diameter (d) was ≤ 5 cm in 145 (64.5%) cases. Additionally, distant metastasis was observed in only 4 (1.8%) patients. A total of 198 (88%) patients had moderate differentiation, and microvascular invasion (MVI) was absent (MVI = 0) in 169 (75.1%) cases. These pathological features suggest that the study predominantly focuses on early-stage patients with HCC.
We conducted a correlation analysis using the Spearman correlation coefficient. The results revealed a significant positive correlation between serum AFP and bile AFP, with a correlation coefficient of 0.9 (P < 0.001). In addition, serum AFP, serum PIVKA-II, and bile AFP also showed significant positive correlations with tumor BCLC staging and MVI grade (P < 0.05), as shown in Supplementary Table 1.
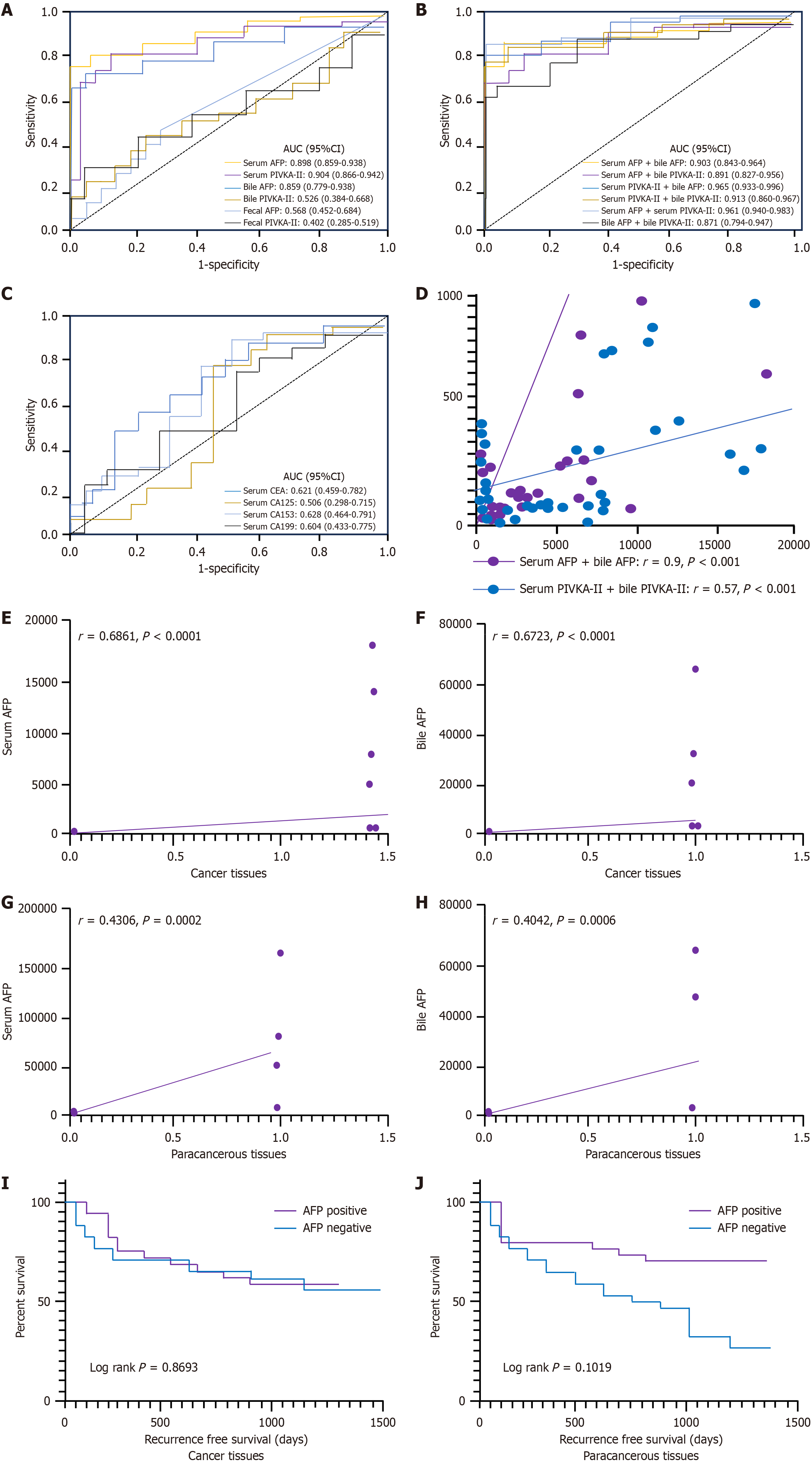
CEA, CA125, CA153, and CA199 were not particularly effective at distinguishing between patients with HCC and non-HCC (P > 0.001). Similarly, all of the fecal supernatants were not particularly effective at distinguishing between patients with HCC and non-HCC counterparts (P > 0.001). The maximum AUCs for distinguishing HCC from chronic cholecystitis in serum were 0.898 for AFP and 0.904 for PIVKA-II. The maximum AUCs for distinguishing HCC from cholecystitis in bile were 0.8559 for AFP and 0.526 for PIVKA-II. Additionally, serum PIVKA-II had the highest AUC for distinguishing HCC from cholecystitis in all samples (P < 0.001) (Table 1 and Figure 2A-C).
| Samples | Indicators | Cut-off value | Sensitivity (%) | Specificity (%) | AUC (95%CI) | P value |
| Serum | AFP, ng/mL | 8.78 | 65.8 | 100.0 | 0.898 (0.859-0.938) | < 0.001 |
| PIVKA-II, mAU/mL | 40.00 | 78.2 | 90.9 | 0.904 (0.866-0.942) | < 0.001 | |
| CEA, ng/mL | 5.00 | 10.5 | 100.0 | 0.621 (0.459-0.782) | 0.159 | |
| CA125, U/mL | 35.00 | 7.6 | 66.7 | 0.506 (0.298-0.715) | 0.940 | |
| CA153, U/mL | 31.3 | 1.9 | 100.0 | 0.628 (0.464-0.791) | 0.137 | |
| CA199, U/mL | 37.00 | 11.4 | 100.0 | 0.604 (0.433-0.775) | 0.225 | |
| Bile | AFP, ng/mL | 1.32 | 61.2 | 100.0 | 0.859 (0.779-0.938) | < 0.001 |
| PIVKA-II, mAU/mL | 59.50 | 35.9 | 80.0 | 0.526 (0.384-0.668) | 0.725 | |
| CEA, ng/mL | 84.26 | 9.4 | 100.0 | 0.371 (0.179-0.563) | 0.219 | |
| CA125, U/mL | 123.75 | 3.8 | 100.0 | 0.211 (0.048-0.374) | 0.006 | |
| CA153, U/mL | 1.35 | 56.6 | 66.7 | 0.596 (0.407-0.786) | 0.358 | |
| CA199, U/mL | 4.31 | 92.3 | 44.4 | 0.622 (0.394-0.850) | 0.246 | |
| Feces | Fecal AFP, ng/mL | 0.04 | 16.4 | 96.2 | 0.568 (0.452-0.684) | 0.272 |
| Fecal PIVKA-II, mAU/mL | 1469.20 | 5.7 | 96.2 | 0.402 (0.285-0.519) | 0.115 | |
| CEA, ng/mL | 15001.00 | 0.0 | 100.0 | 0.431 (0.156-0.706) | 0.659 | |
| CA125, U/mL | 23.25 | 6.9 | 100.0 | 0.297 (0.000-0.611) | 0.195 | |
| CA153, U/mL | 1.85 | 17.2 | 100.0 | 0.522 (0.229-0.814) | 0.890 | |
| CA199, U/mL | 8.64 | 62.1 | 75.0 | 0.575 (0.462-0.689) | 0.659 | |
| Combination | Fecal AFP + fecal PIVKA-II | -0.0016 | 56.4 | 77.0 | 0.631 (0.514-0.748) | 0.034 |
| Serum AFP + bile AFP | 9.34 | 70.0 | 100.0 | 0.903 (0.843-0.964) | < 0.001 | |
| Serum AFP + serum PIVKA-II | 10.84 | 90.7 | 97.0 | 0.961 (0.940-0.983) | < 0.001 | |
| Serum PIVKA-II + bile AFP | 113.30 | 88.3 | 100.0 | 0.965 (0.933-0.996) | < 0.001 | |
| Serum PIVKA-II + bile PIVKA-II | 29.90 | 86.3 | 92.3 | 0.913 (0.860-0.967) | < 0.001 | |
| Serum AFP + bile PIVKA-II | 4.35 | 81.4 | 92.3 | 0.891 (0.827-0.956) | < 0.001 | |
| Bile AFP+ bile PIVKA-II | 1.09 | 60.2 | 100.0 | 0.871 (0.794-0.947) | < 0.001 |
Regarding combinations of biomarkers, the combination of “bile AFP and serum PIVKA-II” showed the highest AUC of 0.965 (P < 0.001) compared to the other combinations (Table 1 and Figure 2B). Additionally, serum AFP and bile AFP exhibited a strong correlation (r = 0.9, P < 0.001; Figure 2D). These findings collectively suggest that bile AFP may be useful for differentiating between patients with HCC and non-HCC.
The sensitivities, specificities, and cut-off values of the six independent markers for all patients are shown in Table 1. In bile samples, AFP had a sensitivity of 61.2% with a cut-off of 1.32 ng/mL, whereas in serum samples, it had a sensitivity of 65.8% at a cut-off value of 8.78 ng/mL. PIVKA-II, with a cut-off value of 40 mAU/mL, demonstrated an increased sensitivity of 78.2%. Table 2 shows that bile AFP had a diagnostic efficacy of 4.9% (5 of 103 cases) in the serum AFP-negative but PIVKA-II-positive group and 10.7% (11 of 103 cases) in the serum PIVKA-II-negative but AFP-positive group.
| Group | Indicators | Sensitivity (%) | AUC (95%CI) | P value |
| Serum AFP negative group | Serum PIVKA-II | 75.3 | 0.887 (0.827-0.947) | < 0.001 |
| Bile AFP | 13.9 | 0.657 (0.487-0.828) | 0.079 | |
| Bile PIVKA-II | 38.9 | 0.537 (0.369-0.705) | 0.679 | |
| Fecal AFP | 0.0 | 0.512 (0.347-0.649) | 0.868 | |
| Fecal PIVKA-II | 4.3 | 0.386 (0.250-0.521) | 0.105 | |
| Serum PIVKA-II negative group | Serum AFP | 61.2 | 0.848 (0.765-0.931) | < 0.001 |
| Bile AFP | 55.0 | 0.842 (0.714-0.969) | 0.001 | |
| Bile PIVKA-II | 5.0 | 0.270 (0.088-0.452) | 0.021 | |
| Fecal AFP | 6.0 | 0.561 (0.415-0.708) | 0.418 | |
| Fecal PIVKA-II | 6.0 | 0.352 (0.214-0.491) | 0.051 | |
| Both serum AFP and serum PIVKA-II negative group | Bile AFP | 0.0 | 0.648 (0.426-0.871) | 0.233 |
| Bile PIVKA-II | 11.1 | 0.230 (0.018-0.441) | 0.030 | |
| Fecal AFP | 18.2 | 0.483 (0.274-0.692) | 0.872 | |
| Fecal PIVKA-II | 9.0 | 0.414 (0.209-0.619) | 0.412 |
In addition, to enhance the diagnostic value for HCC detection, we combined AFP and PIVKA-II and assessed the sensitivities and specificities of different combinations (Table 1). The combination of AFP and PIVKA-II in serum yielded the highest sensitivity of 90.7% and a specificity of 97%. The combination of bile AFP and serum PIVKA-II achieved a sensitivity of 88.3% and the highest specificity of 100%.
We used the Kappa consistency test and paired χ² test to compare the sensitivity and specificity of the combined markers. The combination of “AFP in bile and PIVKA-II in serum” demonstrated significantly higher sensitivity compared to serum PIVKA-II (P = 0.035) or bile AFP alone (P < 0.001) for HCC detection.
As shown in Table 3, the HCC population was divided into subgroups based on various staging systems. According to the BCLC staging system, the HCC population was categorized into four subgroups: BCLC 0, BCLC A, BCLC B, and BCLC C + D. The CNLC staging system further divided the HCC population into four subgroups: CNLC Ia, CNLC Ib, CNLC II, and CNLC III + IV. Based on tumor diameter, the HCC population was categorized into four subgroups: “Tumor diameter ≤ 2 cm”, “2 cm < tumor diameter ≤ 5 cm”, “5 cm < tumor diameter ≤ 10 cm”, and “tumor diameter > 10 cm”. According to the number of tumors, the HCC population was divided into two subgroups: “single tumor” and “multiple tumors”.
| Characteristic | Serum | Bile | Fecal supernatant | |||||||
| Sensitivity (%) | AUC (95%CI) | P value | Sensitivity (%) | AUC (95%CI) | P value | Sensitivity (%) | AUC (95%CI) | P value | ||
| Tumor staging (CNLC) | ||||||||||
| Ia | AFP, ng/mL | 65.1 | 0.900 (0.848-0.952) | < 0.001 | 46.5 | 0.805 (0.688-0.923) | < 0.001 | 11.1 | 0.555 (0.424-0.686) | 0.419 |
| PIVKA-II, mAU/mL | 63.5 | 0.835 (0.768-0.903) | < 0.001 | 18.6 | 0.383 (0.209-0.557) | 0.180 | 12.7 | 0.466 (0.337-0.596) | 0.620 | |
| Ib | AFP, ng/mL | 63.8 | 0.898 (0.842-0.954) | < 0.001 | 67.5 | 0.882 (0.797-0.968) | < 0.001 | 18.8 | 0.571 (0.436-0.706) | 0.316 |
| PIVKA-II, mAU/mL | 91.3 | 0.960 (0.925-0.994) | < 0.001 | 41.9 | 0.609 (0.458-0.761) | 0.211 | 0.0 | 0.333 (0.202-0.463) | 0.018 | |
| II | AFP, ng/mL | 71.0 | 0.878 (0.777-0.978) | < 0.001 | 66.7 | 0.874 (0.728-1) | 0.003 | 27.3 | 0.607 (0.443-0.770) | 0.207 |
| PIVKA-II, mAU/mL | 80.6 | 0.929 (0.858-0.999) | < 0.001 | 55.6 | 0.652 (0.418-0.886) | 0.222 | 0.0 | 0.392 (0.231-0.552) | 0.200 | |
| III + IV | AFP, ng/mL | 88.2 | 0.929 (0.818-1) | < 0.001 | 100.0 | 1.00 (1-1) | < 0.001 | 14.3 | 0.544 (0.296-0.792) | 0.725 |
| PIVKA-II, mAU/mL | 94.1 | 0.989 (0.966-1) | < 0.001 | 75.0 | 0.708 (0.453-0.963) | 0.107 | 0.0 | 0.342 (0.095-0.587) | 0.202 | |
| Tumor staging (BCLC) | ||||||||||
| 0 | AFP, ng/mL | 80.0 | 0.924 (0.817-1) | < 0.001 | 50.0 | 0.850 (0.649-1) | 0.036 | 16.7 | 0.644 (0.401-0.887) | 0.277 |
| PIVKA-II, mAU/mL | 40.0 | 0.679 (0.448-0.910) | 0.09 | 0.0 | 0.267 (0.047-0.486) | 0.162 | 50.0 | 0.622 (0.3-0.944) | 0.359 | |
| A | AFP, ng/mL | 59.0 | 0.884 (0.836-0.932) | < 0.001 | 57.3 | 0.837 (0.747-0.927) | < 0.001 | 15.5 | 0.560 (0.439-0.680) | 0.351 |
| PIVKA-II, mAU/mL | 77.6 | 0.900 (0.856-0.943) | < 0.001 | 33.3 | 0.505 (0.358-0.652) | 0.953 | 4.1 | 0.398 (0.275-0.521) | 0.111 | |
| B | AFP, ng/mL | 77.1 | 0.926 (0.852-1) | < 0.001 | 64.3 | 0.900 (0.792-1) | < 0.001 | 21.7 | 0.614 (0.454-0.773) | 0.173 |
| PIVKA-II, mAU/mL | 82.9 | 0.932 (0.868-0.997) | < 0.001 | 28.6 | 0.557 (0.344-0.770) | 0.600 | 4.3 | 0.390 (0.23-0.549) | 0.186 | |
| C + D | AFP, ng/mL | 87.5 | 0.941 (0.859-1) | < 0.001 | 90.0 | 0.967 (0.897-1) | < 0.001 | 0.0 | 0.516 (0.324-0.709) | 0.865 |
| PIVKA-II, mAU/mL | 91.7 | 0.985 (0.961-1) | < 0.001 | 80.0 | 0.747 (0.524-0.970) | 0.040 | 0.0 | 0.360 (0.181-0.538) | 0.148 | |
| Tumor diameter (cm) | ||||||||||
| d ≤ 2 | AFP, ng/mL | 73.8 | 0.898 (0.822-0.937) | < 0.001 | 62.5 | 0.817 (0.663-0.970) | 0.003 | 16.7 | 0.547 (0.395-0.699) | 0.549 |
| PIVKA-II, mAU/mL | 52.4 | 0.792 (0.691-0.893) | < 0.001 | 43.8 | 0.575 (0.369-0.781) | 0.477 | 6.7 | 0.347 (0.201-0.494) | 0.051 | |
| 2< d ≤ 5 | AFP, ng/mL | 60.2 | 0.912 (0.863-0.961) | < 0.001 | 57.8 | 0.841 (0.740-0.941) | < 0.001 | 12.3 | 0.581 (0.453-0.709) | 0.223 |
| PIVKA-II, mAU/mL | 75.7 | 0.887 (0.834-0.941) | < 0.001 | 33.3 | 0.542 (0.382-0.702) | 0.627 | 6.2 | 0.435 (0.303-0.567) | 0.330 | |
| 5 < d ≤ 10 | AFP, ng/mL | 67.3 | 0.884 (0.814-0.955) | < 0.001 | 58.8 | 0.88 (0.785-0.976) | < 0.001 | 0.0 | 0.502 (0.346-0.658) | 0.979 |
| PIVKA-II, mAU/mL | 94.5 | 0.980 (0.952-1) | < 0.001 | 35.3 | 0.486 (0.311-0.661) | 0.879 | 7.1 | 0.371 (0.222-0.520) | 0.104 | |
| d > 10 | AFP, ng/mL | 72.0 | 0.874 (0.760-0.988) | < 0.001 | 87.5 | 0.950 (0.849-1) | < 0.001 | 35.3 | 0.689 (0.52-0.858) | 0.038 |
| PIVKA-II, mAU/mL | 96.0 | 0.993 (0.977-1) | < 0.001 | 37.5 | 0.508 (0.224-0.793) | 0.949 | 0.0 | 0.362 (0.188-0.536) | 0.130 | |
| Number of tumors | ||||||||||
| Single | AFP, ng/mL | 65.6 | 0.901 (0.86-0.9441) | < 0.001 | 58.9 | 0.849 (0.765-0.934) | < 0.001 | 14.0 | 0.555 (0.435-0.674) | 0.381 |
| PIVKA-II, mAU/mL | 77.4 | 0.896 (0.856-0.937) | < 0.001 | 36.7 | 0.539 (0.394-0.683) | 0.634 | 6.6 | 0.409 (0.289-0.529) | 0.148 | |
| Multiple | AFP, ng/mL | 66.7 | 0.883 (0.785-0.980) | < 0.001 | 76.9 | 0.923 (0.825-1) | < 0.001 | 31.6 | 0.651 (0.483-0.818) | 0.087 |
| PIVKA-II, mAU/mL | 83.3 | 0.954 (0.910-0.997) | < 0.001 | 30.8 | 0.441 (0.215-0.667) | 0.596 | 0.0 | 0.358 (0.191-0.526) | 0.108 | |
In BCLC stage 0, CNLC stage Ia, and HCC with a tumor diameter ≤ 2 cm, the diagnostic sensitivity of serum AFP (80%, 73.8%) was higher than that of serum PIVKA-II (40%, 52.4%). Bile AFP was significantly more effective than serum AFP in diagnosing multiple HCCs (sensitivity 87.5%, AUC = 0.950) or HCCs with a diameter > 10 cm (sensitivity 76.9%, AUC = 0.923) (P < 0.001).
Of the 72 LC tissue samples, 17 tested positive for AFP, achieving a positivity rate of 23.6%. By contrast, only 6 of 72 paraneoplastic tissue samples were positive for AFP. The positivity rate of AFP was significantly higher in LC tissues compared to paracancerous tissues, and the staining was primarily localized in the cytoplasm of the cells, as shown in Figure 3A and B. By contrast, a negative AFP immunohistochemistry result is displayed in Figure 3C. Elevated levels of serum or bile AFP (including the serum AFP-negative but bile AFP-positive group) were observed in AFP immunohistochemistry-positive patients. Further analysis revealed that the rates of positive AFP immunohistochemistry in tumor tissues were significantly correlated with serum (r = 0.686, P < 0.001) and bile (r = 0.672, P < 0.001) AFP levels, in contrast, this correlation was markedly weaker in paracancerous tissues, as depicted in Figure 2E-H.
To explore the presence of bile AFP in patients with serum AFP-negative HCC and to investigate the spatial distribution of AFP in tumor tissue relative to bile ducts, we used tissue sections adjacent to the tumor. Bile duct epithelial cells were labeled using cytokeratin 7 monoclonal antibody and compared with adjacent tumor tissues previously stained for AFP (Figure 3D-E).
Further analysis revealed that there was no significant difference in RFS between AFP immunohistochemistry-negative and AFP immunohistochemistry-positive patients with HCC in tumor tissues (P = 0.869, hazard ratio [HR] = 1.085, 95% confidence interval [CI]: 0.4069-2.893) and adjacent tissues (P = 0.102, HR = 4.584, 95%CI: 1.441-14.58), as demonstrated in Figure 2I and J.
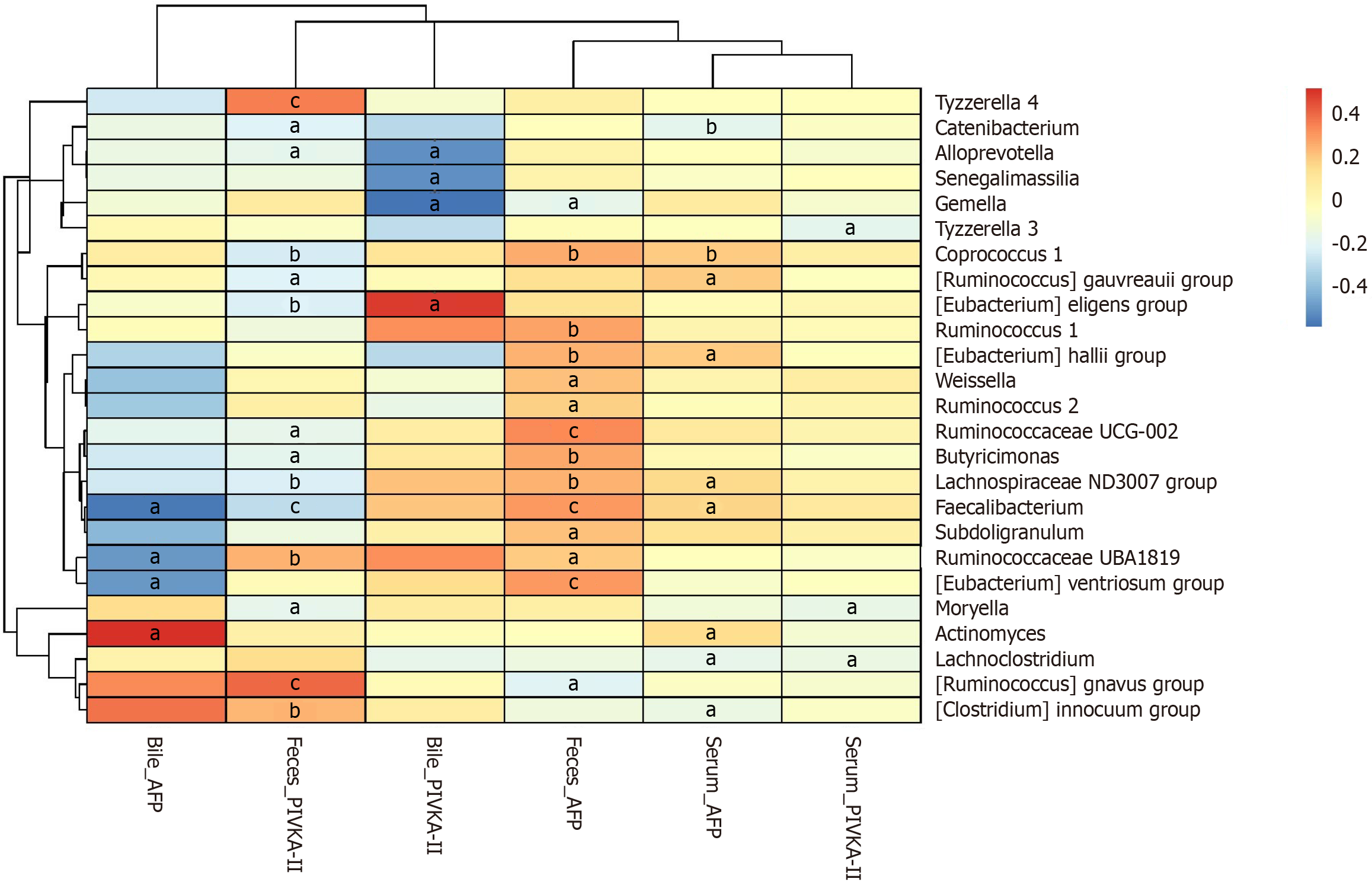
To investigate the relationship between the gut microbiome and tumor markers in patients with HCC, we identified the top 25 bacterial genera that showed a significant correlation with the tumor markers, as represented in Figure 4.
Serum AFP was positively correlated with Coprococcus 1, Ruminococcus gauvreauii group, Eubacterium hallii group, Lachnospiraceae ND3007 group, Faecalibacterium, and Actinomyces (P < 0.05). Serum PIVKA-II was negatively correlated with Tyzzerella 3, Moryella, and Lachnoclostridium (P < 0.05). Bile AFP was positively correlated with Actinomyces and negatively correlated with Faecalibacterium (P < 0.05). Bile PIVKA-II was positively correlated with the Eubacterium eligens group (P < 0.05), as demonstrated in Figure 4.
This study evaluated the diagnostic potential of various biomarkers for HCC, including CEA, CA125, CA153, CA199, AFP, and PIVKA-II in serum, bile, and fecal samples. CEA is widely used in the detection of colorectal cancer but its utility for early-stage detection is limited. Its primary advantage lies in its strong association with poor prognosis and its high sensitivity in detecting recurrent colorectal cancer, providing an approximate lead time of 5 months[19]. As a marker for the early diagnosis of ovarian cancer in high-risk populations, CA125 is used for diagnosing ascites in patients with liver cirrhosis[20]. CA153 is a specific breast cancer marker, playing a significant role in its diagnosis and postoperative monitoring[21], although elevated levels can also be observed in other tumors[22]. CA199 is a sensitive marker for pancreatic cancer and has moderate sensitivity in gastrointestinal cancers including ovarian, colorectal, and gastric cancers[23-25]. Since fecal samples are commonly used to study the impact of microbiota on diseases, particularly colorectal cancer, fecal supernatants were innovatively used to detect tumor marker levels. However, CEA, CA125, CA153, and CA199 levels in the blood, bile, and feces were not effective in distinguishing patients with and without HCC, consistent with previous studies indicating that CA125 does not significantly distinguish HCC[26].
Compared to other tumor markers, CA125 is more closely associated with tumors with a diameter greater than 5 cm and a poorer Child-Pugh classification, especially in those patients with ascites. However, its diagnostic value for early-stage HCC remains limited[27]. Yang et al’s study suggests that CA153 might have some diagnostic value for LC but it was significantly elevated in the advanced disease stages[26] and is primarily associated with liver metastasis of breast cancer and ocular metastasis of LC[28,29]. In addition, it has been reported that CA199 has a diagnostic sensitivity of 64.28%, while CEA has a sensitivity of 83.67% in HCC[30]. However, since most of our participants were in the early HCC stage, these markers had limited utility in distinguishing HCC.
As one of the earliest tumor markers identified, AFP was initially recognized as a serum marker for HCC and later found to be associated with germ cell tumors[31]. Our study showed that serum AFP had a sensitivity of 65.8% and a specificity of 100% for diagnosing HCC, aligning with the findings of Wong et al[32] who reported a sensitivity range of 39% to 65% and a specificity range of 76% to 94% for serum AFP. Moreover, previous studies have shown that AFP levels do not significantly increase in 80% of small LCs[33,34]. However, in contrast to previous studies, the sensitivity of serum AFP was higher, reaching 73.8% for HCC tumors smaller than 2 cm. Since AFP can also be elevated in conditions such as active hepatitis, liver cirrhosis, and certain non-HCC tumors[35,36], it is crucial to incorporate additional tumor markers to enhance its diagnostic value.
PIVKA-II is a diagnostic marker for HCC[37], with a reported sensitivity of 62% and a specificity of 95% for diagnosing HCC using a cut-off value of 40 mAU/mL[32]. In the present study, serum PIVKA-II showed a sensitivity of 78.2% and specificity of 90.9% when using the same cut-off value, higher than those reported in previous studies. Furthermore, the sensitivity of PIVKA-II was superior to that of AFP, highlighting the improved diagnostic performance of PIVKA-II in this study. It has been reported that AFP has an advantage over PIVKA-II in diagnosing early-stage LC, particularly when tumors are smaller than 3 cm or 2 cm[36,38], but the overall diagnostic value of PIVKA-II was superior to that of AFP in the present study. However, AFP levels may begin to rise as early as 6 months before an HCC diagnosis[39], explaining why serum AFP was more effective than serum PIVKA-II for detecting early-stage HCC.
Emerging research has highlighted the release of extracellular vesicles (EVs) by malignant tumors into surrounding fluids, which has shown promise in cancer diagnosis. Indeed, bile EVs could be used to differentiate between malignant and non-malignant common bile duct strictures with 63.3% accuracy[24]. Also, CA125, either alone or combined with CEA, could effectively distinguish between benign and malignant causes of biliary obstruction[40]. In the present study, bile AFP could diagnose 61.2% of patients with HCC, with even higher sensitivity in cases of tumors smaller than 2 cm, indicating the diagnostic potential of bile for LC. AFP-producing human cholangiocarcinoma cells exhibited enhanced tumorigenic and malignant potential in vitro and in vivo[41]. Interestingly, although many AFP-enriched hepatocytes were identified, they were not located near the bile ducts, consistent with our focus on HCC rather than cholangiocarcinoma. Additionally, bile AFP levels were positively correlated with AFP expression in tumor tissues, leading us to hypothesize that AFP produced by hepatocytes is secreted into the bile via the canalicular system.
The combination of serum AFP with bile AFP increased sensitivity from 65.8% to 70%. Although serum AFP combined with serum PIVKA-II showed the highest sensitivity for diagnosing HCC (90.7%), serum PIVKA-II combined with bile AFP had the highest AUC of 0.965. This suggests that bile AFP, when combined with serum PIVKA-II, can significantly improve the diagnostic sensitivity for differentiating patients with and without HCC, particularly in cases where AFP levels alone are insufficient for diagnosis. The improved AUC value indicates a potential clinical advantage as it could contribute to earlier detection and more accurate HCC diagnosis, especially in high-risk populations. Given the critical importance of early detection in improving patient outcomes, these combined biomarkers present a promising diagnostic strategy that could complement current clinical methods, offering more reliable results in challenging diagnostic situations.
There was also a strong correlation between AFP levels and cancer tissues, particularly in cases with poor pathological types. It has been reported that AFP is more closely associated with poorly differentiated LC[41] and can enhance human LC cell proliferation[42]. When AFP expression was silenced in the HCC cell line Huh7, cell proliferation was inhibited by 46.15%[43]. In addition, the GM can influence the tumor immune microenvironment and contribute to the development of LC through the gut-liver axis[44,45]. AFP is associated with tumor malignancy and the GM composition is linked to tumor formation. In the present study, there was a strong negative correlation between bile AFP and Faecalibacterium as well as a strong positive correlation with Actinomyces. Previous studies have shown that Faecalibacterium is associated with robust anticancer immune responses in patients with cancer[46], while Actinomyces plays a significant role in promoting tumorigenesis[47]. These findings confirm the relationship among the GM, tumor markers, and tumor malignancy but further studies are required to determine the underlying mechanisms. Although AFP correlates with HCC malignancy, our immunohistochemical analysis did not reveal a significant correlation between AFP positivity in tumor tissues and patient RFS. However, we suggest that patients with HCC with positive serum and bile AFP levels should be closely monitored for potential tumor recurrence.
Our findings have significant implications for the early diagnosis and clinical management of HCC. First, since serum AFP demonstrated a sensitivity of 80% in early HCC diagnosis, outperforming PIVKA-II, it should be prioritized in HCC screening given its affordability and diagnostic efficiency. For high-risk populations, such as patients with chronic hepatitis B or liver cirrhosis, a combined test of AFP and PIVKA-II is recommended when feasible, as it enhances diagnostic accuracy and facilitates early detection. Second, the combination of serum PIVKA-II and bile AFP exceeded the diagnostic performance of AFP or PIVKA-II alone, suggesting that bile AFP testing could serve as a valuable supplementary tool, improving both sensitivity and specificity for patients with suspected HCC but without significantly elevated serum AFP. For patients undergoing biliary procedures such as endoscopic retrograde cholangiopancreatography or biliary drainage, bile AFP may provide additional diagnostic value, potentially increasing the early detection of HCC. Third, our study demonstrated a strong correlation between AFP levels and HCC histological subtypes, with notably high AFP expression in poorly differentiated HCC cases. Moreover, silencing AFP expression reduced HCC cell proliferation, supporting its role in HCC malignancy and progression. Thus, AFP not only serves as a diagnostic biomarker but also aids in assessing disease progression and recurrence risk and may serve as a potential therapeutic target. Finally, this study is the first to reveal a potential link between bile AFP levels and GM with bile AFP negatively correlated with Faecalibacterium and positively correlated with Actinomyces. Since Faecalibacterium is linked to anti-cancer immune responses, whereas Actinomyces contributes to tumorigenesis, the GM may influence the tumor microenvironment and regulate AFP expression and HCC progression but further studies are required to determine the underlying mechanisms.
The study had several limitations. First, most participants were infected with HBV, which may limit the generalizability of the findings to other etiological groups, such as those with cirrhosis related to hepatitis C, alcoholic or non-alcoholic steatohepatitis, or type 2 diabetes. Second, the control group consisted of patients with chronic cholecystitis rather than cirrhosis, mainly due to easier access to bile samples. Although this may introduce potential bias, it is unlikely to significantly affect the results. Third, since most HCC surgical patients were in the early stages of cancer, there might be potential for selection bias, meaning that the sampled population may not fully represent all patients with LC, which could impact the generalizability of our findings. Finally, our study population was primarily derived from the Guangxi region of China, and variations in ethnicity, geographic location, and clinical characteristics might lead to differences in outcomes. Therefore, the applicability of our findings to broader populations requires further validation with multicenter data. In the future, we plan to expand our clinical sample collection and collaborate in multicenter studies to enhance the reliability and generalizability of our research.
This study represents a significant advancement by applying classical tumor markers for HCC to unconventional specimens, such as bile and feces. Importantly, the combination of serum AFP, PIVKA-II, and bile AFP significantly enhanced HCC detection efficiency, with serum AFP proving to be a more effective diagnostic marker for early-stage HCC compared to serum PIVKA-II. Moreover, AFP was linked to the malignancy of HCC. Therefore, patients with dual positivity for serum and bile AFP, in particular, require enhanced monitoring to prevent tumor progression and recurrence.
We would like to express our gratitude to all those who contributed to the successful completion of this research. We extend our appreciation to the Key Laboratory of Early Prevention and Treatment for Regional High-Frequency Tumors (Guangxi Medical University)-Ministry of Education. Their cooperation was essential to the accomplishment of this work.
| 1. | Kim E, Kim D, Lee JS, Yoe J, Park J, Kim CJ, Jeong D, Kim S, Lee Y. Capicua suppresses hepatocellular carcinoma progression by controlling the ETV4-MMP1 axis. Hepatology. 2018;67:2287-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 2. | Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 1048] [Article Influence: 349.3] [Reference Citation Analysis (0)] |
| 3. | Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1630] [Cited by in RCA: 1765] [Article Influence: 252.1] [Reference Citation Analysis (0)] |
| 4. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4517] [Article Influence: 347.5] [Reference Citation Analysis (2)] |
| 5. | Yu R, Tan Z, Xiang X, Dan Y, Deng G. Effectiveness of PIVKA-II in the detection of hepatocellular carcinoma based on real-world clinical data. BMC Cancer. 2017;17:608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Notarpaolo A, Layese R, Magistri P, Gambato M, Colledan M, Magini G, Miglioresi L, Vitale A, Vennarecci G, Ambrosio CD, Burra P, Di Benedetto F, Fagiuoli S, Colasanti M, Maria Ettorre G, Andreoli A, Cillo U, Laurent A, Katsahian S, Audureau E, Roudot-Thoraval F, Duvoux C. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCC. J Hepatol. 2017;66:552-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 7. | Zhao Y, Gao Q, Pei L, Wang C, Jin L, Liao F. Current status and future prospects of biomarkers in the diagnosis of hepatocellular carcinoma. Int J Biol Markers. 2017;32:e361-e369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Li J, Sung CY, Lee N, Ni Y, Pihlajamäki J, Panagiotou G, El-Nezami H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci U S A. 2016;113:E1306-E1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 425] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 9. | Hsu CY, Liu PH, Ho SY, Huang YH, Lee YH, Lee RC, Nagaria TS, Hou MC, Huo TI. Metastasis in patients with hepatocellular carcinoma: Prevalence, determinants, prognostic impact and ability to improve the Barcelona Clinic Liver Cancer system. Liver Int. 2018;38:1803-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Selby LK, Tay RX, Woon WW, Low JK, Bei W, Shelat VG, Pang TC, Junnarkar SP. Validity of the Barcelona Clinic Liver Cancer and Hong Kong Liver Cancer staging systems for hepatocellular carcinoma in Singapore. J Hepatobiliary Pancreat Sci. 2017;24:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Pang S, Chen X, Lu Z, Meng L, Huang Y, Yu X, Huang L, Ye P, Chen X, Liang J, Peng T, Luo W, Wang S. Longevity of centenarians is reflected by the gut microbiome with youth-associated signatures. Nat Aging. 2023;3:436-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 67] [Reference Citation Analysis (0)] |
| 12. | Lasko TA, Bhagwat JG, Zou KH, Ohno-Machado L. The use of receiver operating characteristic curves in biomedical informatics. J Biomed Inform. 2005;38:404-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 486] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 13. | Ma S, Huang J. Combining multiple markers for classification using ROC. Biometrics. 2007;63:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Garg K, Campolonghi S. A Step-by-Step Guide to Selecting an Optimal Cut-Off Value Based on the Receiver Operating Characteristic and Youden Index in Methods Designed to Diagnose Lyme Disease. Methods Mol Biol. 2024;2742:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Carter JV, Pan J, Rai SN, Galandiuk S. ROC-ing along: Evaluation and interpretation of receiver operating characteristic curves. Surgery. 2016;159:1638-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 412] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 16. | Kamarudin AN, Cox T, Kolamunnage-Dona R. Time-dependent ROC curve analysis in medical research: current methods and applications. BMC Med Res Methodol. 2017;17:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 530] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 17. | Li DL, Shen F, Yin Y, Peng JX, Chen PY. Weighted Youden index and its two-independent-sample comparison based on weighted sensitivity and specificity. Chin Med J (Engl). 2013;126:1150-1154. [PubMed] |
| 18. | Hawass NE. Comparing the sensitivities and specificities of two diagnostic procedures performed on the same group of patients. Br J Radiol. 1997;70:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 186] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Duffy MJ. Carcinoembryonic antigen as a marker for colorectal cancer: is it clinically useful? Clin Chem. 2001;47:624-630. [PubMed] |
| 20. | Zuckerman E, Lanir A, Sabo E, Rosenvald-Zuckerman T, Matter I, Yeshurun D, Eldar S. Cancer antigen 125: a sensitive marker of ascites in patients with liver cirrhosis. Am J Gastroenterol. 1999;94:1613-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Safi F, Kohler I, Röttinger E, Beger H. The value of the tumor marker CA 15-3 in diagnosing and monitoring breast cancer. A comparative study with carcinoembryonic antigen. Cancer. 1991;68:574-582. [PubMed] [DOI] [Full Text] |
| 22. | Schutter EM, Davelaar EM, van Kamp GJ, Verstraeten RA, Kenemans P, Verheijen RH. The differential diagnostic potential of a panel of tumor markers (CA 125, CA 15-3, and CA 72-4 antigens) in patients with a pelvic mass. Am J Obstet Gynecol. 2002;187:385-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Luo G, Jin K, Deng S, Cheng H, Fan Z, Gong Y, Qian Y, Huang Q, Ni Q, Liu C, Yu X. Roles of CA19-9 in pancreatic cancer: Biomarker, predictor and promoter. Biochim Biophys Acta Rev Cancer. 2021;1875:188409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 194] [Article Influence: 48.5] [Reference Citation Analysis (1)] |
| 24. | Bast RC Jr, Klug TL, Schaetzl E, Lavin P, Niloff JM, Greber TF, Zurawski VR Jr, Knapp RC. Monitoring human ovarian carcinoma with a combination of CA 125, CA 19-9, and carcinoembryonic antigen. Am J Obstet Gynecol. 1984;149:553-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 117] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Quentmeier A, Möller P, Schwarz V, Abel U, Schlag P. Carcinoembryonic antigen, CA 19-9, and CA 125 in normal and carcinomatous human colorectal tissue. Cancer. 1987;60:2261-2266. [PubMed] [DOI] [Full Text] |
| 26. | Yang C, Geng H, Zhu S, Zheng X, Li T, Duan L. Multiple Diagnostic Indicators in the Development of Chronic Hepatitis B, Liver Cirrhosis, and Liver Cancer. Altern Ther Health Med. 2023;29:153-159. [PubMed] |
| 27. | Zhou S, Wang Z, Li M, Wu L. Elevated Preoperative Serum CA125 Predicts Larger Tumor Diameter in Patients with Hepatocellular Carcinoma and Low AFP Levels. Biomed Res Int. 2019;2019:6959637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Tang J, Zhang LJ, Kang M, Huang R, Shu HY, Wei H, Zou J, Pan YC, Ling Q, Shao Y. AFP and CA-125 as an accurate risk factor to predict eye metastasis in hypertension patients with liver carcinoma: A STROBE-compliant article. Front Genet. 2022;13:1010903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Cao R, Wang LP. Serological diagnosis of liver metastasis in patients with breast cancer. Cancer Biol Med. 2012;9:57-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 30. | Verma N, Vinocha A. Role of CA 19.9 and CEA in predicting diagnosis in hepatocellular carcinoma. J Cancer Res Ther. 2023;19:1356-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 31. | Abelev GI, Eraiser TL. Cellular aspects of alpha-fetoprotein reexpression in tumors. Semin Cancer Biol. 1999;9:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Wong GL, Chan HL, Tse YK, Chan HY, Tse CH, Lo AO, Wong VW. On-treatment alpha-fetoprotein is a specific tumor marker for hepatocellular carcinoma in patients with chronic hepatitis B receiving entecavir. Hepatology. 2014;59:986-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 33. | Saffroy R, Pham P, Reffas M, Takka M, Lemoine A, Debuire B. New perspectives and strategy research biomarkers for hepatocellular carcinoma. Clin Chem Lab Med. 2007;45:1169-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Johnson PJ. The role of serum alpha-fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin Liver Dis. 2001;5:145-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 265] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 35. | Yang JD, Dai J, Singal AG, Gopal P, Addissie BD, Nguyen MH, Befeler AS, Reddy KR, Schwartz M, Harnois DM, Yamada H, Gores GJ, Feng Z, Marrero JA, Roberts LR. Improved Performance of Serum Alpha-Fetoprotein for Hepatocellular Carcinoma Diagnosis in HCV Cirrhosis with Normal Alanine Transaminase. Cancer Epidemiol Biomarkers Prev. 2017;26:1085-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 36. | Di Bisceglie AM, Sterling RK, Chung RT, Everhart JE, Dienstag JL, Bonkovsky HL, Wright EC, Everson GT, Lindsay KL, Lok AS, Lee WM, Morgan TR, Ghany MG, Gretch DR; HALT-C Trial Group. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol. 2005;43:434-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 245] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 37. | Abelev GI, Perova SD, Khramkova NI, Postnikova ZA, Irlin IS. Production of embryonal alpha-globulin by transplantable mouse hepatomas. Transplantation. 1963;1:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 373] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 38. | Fujiyama S, Morishita T, Shibata J, Sato T. [Clinical usefulness of plasma PIVKA-II assay and its limitations in patients with hepatocellular carcinoma]. Gan To Kagaku Ryoho. 1989;16:1129-1138. [PubMed] |
| 39. | Choi J, Kim GA, Han S, Lee W, Chun S, Lim YS. Longitudinal Assessment of Three Serum Biomarkers to Detect Very Early-Stage Hepatocellular Carcinoma. Hepatology. 2019;69:1983-1994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 40. | Chen CY, Shiesh SC, Tsao HC, Lin XZ. The assessment of biliary CA 125, CA 19-9 and CEA in diagnosing cholangiocarcinoma--the influence of sampling time and hepatolithiasis. Hepatogastroenterology. 2002;49:616-620. [PubMed] |
| 41. | Ishii T, Yasuchika K, Suemori H, Nakatsuji N, Ikai I, Uemoto S. Alpha-fetoprotein producing cells act as cancer progenitor cells in human cholangiocarcinoma. Cancer Lett. 2010;294:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Wang XW, Xie H. Alpha-fetoprotein enhances the proliferation of human hepatoma cells in vitro. Life Sci. 1999;64:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Yang X, Zhang Y, Zhang L, Zhang L, Mao J. Silencing alpha-fetoprotein expression induces growth arrest and apoptosis in human hepatocellular cancer cell. Cancer Lett. 2008;271:281-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Xiaoyu P, Chao G, Lihua D, Pengyu C. Gut bacteria affect the tumoral immune milieu: distorting the efficacy of immunotherapy or not? Gut Microbes. 2020;11:691-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Ohtani N, Hara E. Gut-liver axis-mediated mechanism of liver cancer: A special focus on the role of gut microbiota. Cancer Sci. 2021;112:4433-4443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 46. | Routy B, Gopalakrishnan V, Daillère R, Zitvogel L, Wargo JA, Kroemer G. The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol. 2018;15:382-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 389] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 47. | Xu Z, Lv Z, Chen F, Zhang Y, Xu Z, Huo J, Liu W, Yu S, Tuersun A, Zhao J, Zong Y, Shen X, Feng W, Lu A. Dysbiosis of human tumor microbiome and aberrant residence of Actinomyces in tumor-associated fibroblasts in young-onset colorectal cancer. Front Immunol. 2022;13:1008975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |