Published online May 15, 2025. doi: 10.4251/wjgo.v17.i5.104341
Revised: February 2, 2025
Accepted: February 28, 2025
Published online: May 15, 2025
Processing time: 148 Days and 21.7 Hours
Metastatic colorectal cancer (mCRC) is a global health challenge with a poor prognosis. Prognostic markers are critical for survival prediction.
To evaluate a novel tumor marker index (TMI) combining carcinoembryonic antigen and carbohydrate antigen 19-9.
This multicenter, retrospective study measured baseline carcinoembryonic antigen and carbohydrate antigen 19-9 levels to calculate a TMI as the geometric mean of values normalized to their upper limits of normal. Receiver operating characteristic curve analysis assessed TMI’s prognostic accuracy, and patients were stratified into high-TMI (≥ 1.39) and low-TMI (< 1.39) groups. The primary endpoint was overall survival (OS), with progression-free survival and treatment response as secondary endpoints.
The study included 305 mCRC patients with a median follow-up of 22.9 months. The median OS for high-TMI patients was 29.5 months, significantly lower than the 45.6 months observed in the low-TMI group (P = 0.02). The 2-year OS rates for the high- and low-TMI groups were 59.4% and 72.9%, respectively. Median progression-free survival was also shorter for the high-TMI group (14.0 vs 16.0 months, P = 0.84). High TMI is an independent prognostic factor for worse OS.
TMI is a simple, cost-effective prognostic tool for mCRC, with high TMI associated with poorer survival outcomes.
Core Tip: The tumor marker index (TMI), derived from the combination of carcinoembryonic antigen and carbohydrate antigen 19-9, offers a novel and cost-effective approach to prognostication in metastatic colorectal cancer. This study demonstrates that a high TMI is independently associated with significantly shorter overall survival, with potential implications for risk stratification and individualized patient management. These findings highlight the clinical value of integrating TMI into routine practice for better outcome prediction.
- Citation: Ilhan Y, Balcik OY, Guzel HG, Onder AH, Demir B, Baser MN, Karadag I, Ozbay MF, Genc TB, Uzuntas S, Poyrazoglu O, Beypinar I, Ergun Y, Ozturk B. Novel tumor marker index combining carcinoembryonic antigen and carbohydrate antigen 19-9: New prognostic factor for metastatic colorectal cancer. World J Gastrointest Oncol 2025; 17(5): 104341
- URL: https://www.wjgnet.com/1948-5204/full/v17/i5/104341.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i5.104341
Colorectal cancers (CRC) rank as the third most common cancers in both men and women worldwide, and their increasing prevalence in younger age groups in recent years has amplified their significance as a major cause of morbidity and mortality[1]. In metastatic CRC (mCRC), where surgical treatment is not feasible, systemic therapies form the cornerstone of primary treatment. These include cytotoxic chemotherapies, various biological therapies targeting growth factors, immunotherapies, and their combinations. Clinical trials completed over the past five years have demonstrated that certain therapeutic agents improve overall survival (OS) based on the molecular and pathological characteristics of the tumor. Despite these advancements, approximately 70%-75% of patients with mCRC survive longer than one year, 30%-35% survive beyond three years, and less than 20% survive beyond five years[2]. Therefore, identifying prognostic factors early in mCRC is crucial for predicting treatment responses and estimating survival, which are critical aspects of clinical practice. The identification of prognostic factors and the development of prognostic scoring systems that predict survival may aid clinicians in patient communication and in making informed treatment decisions.
Various prognostic factors in mCRC play a critical role in predicting treatment responses and survival outcomes. Among these, mutations in genes such as KRAS, NRAS, and BRAF are generally associated with poorer prognosis. Additionally, the location of the primary tumor (with left-sided tumors typically having a better prognosis than right-sided ones), levels of carcinoembryonic antigen (CEA), a commonly used tumor marker, and the patient’s performance status (PS) are key prognostic indicators. Elevated CEA levels, low PS scores, and decreased albumin levels, an inflammatory marker, have been reported in multiple studies to correlate with worse survival outcomes[3-5]. In recent years, particularly in early-stage CRCs, persistently elevated circulating tumor DNA levels following surgery ± adjuvant therapy have also been associated with poor prognosis[6]. However, methods such as circulating tumor DNA analysis, though advanced, remain limited in clinical practice due to challenges in accessibility and cost-effectiveness, especially in developing countries. Therefore, the development of easily accessible, cost-effective, and reliable prognostic markers remains a pressing need.
As highlighted above, the prognostic significance of CEA, one of the tumor markers frequently used in routine practice, is well-established in CRC. Another commonly utilized, cost-effective, and accessible tumor marker is carbohydrate antigen 19-9 (CA 19-9), which also holds importance in CRC. Several studies have demonstrated the potential prognostic value of combining these two tumor markers, particularly in the perioperative period[7,8]. However, the sensitivity and specificity of combined tumor marker usage remain limited, warranting further research. In recent years, interest in this area has grown, with notable studies emphasizing the role of combined tumor marker indices. For instance, a tumor marker index (TMI) based on cytokeratin 19 fragment (CYFRA21-1) and squamous cell carcinoma antigen has been reported as a prognostic factor in squamous esophageal cancer. Similarly, a TMI derived from CYFRA21-1 and CEA has shown prognostic value in non-small cell lung cancer (NSCLC)[9,10]. Additionally, Kamada et al[8] in a retrospective study published in 2024, evaluated the prognostic significance of a newly developed TMI combining CEA and CA 19-9 in 306 patients with stage 1-3 CRC who underwent surgery. Their findings demonstrated that this TMI is a simple, accessible, cost-effective, and valuable prognostic index for early-stage colon cancer[8]. This study aims to evaluate the efficacy of an innovative TMI, consisting of CEA and CA 19-9, in predicting treatment responses and long-term disease prognosis in mCRC, compared to other established prognostic factors. To the best of our knowledge, this is the first study conducted on this topic in patients with mCRC.
Our study was designed as a multicenter, retrospective study. Patients over the age of 18 with histopathologically confirmed mCRC were included. Both de novo metastatic and recurrent metastatic cases were eligible for inclusion. Patients younger than 18 years, those without a pathological diagnosis, those who had not received any treatment in the metastatic stage, or those whose data could not be reliably retrieved retrospectively were excluded from the study. In addition to basic demographic information such as age and gender, clinically significant details such as disease pattern, tumor location, treatments received, and sites of metastasis were meticulously recorded. Baseline laboratory parameters, including CEA and CA 19-9 levels obtained immediately before the initiation of first-line therapy at the time of metastatic diagnosis, were recorded. Additional inflammatory indices, such as the neutrophil-to-lymphocyte ratio (NLR), as well as progression and final outcomes, were comprehensively recorded through a review of hospital databases and patient files.
The primary endpoint of our study was OS, while the secondary endpoints were progression-free survival (PFS) and treatment response rates. Right-sided colon tumors were defined as those located from the proximal rectum to the splenic flexure, whereas left-sided colon tumors were defined as those located from the splenic flexure to the cecum. PFS was defined as the time from the date of metastatic disease diagnosis to the date of progression, death in the absence of progression, or the last follow-up, whichever occurred first. OS was defined as the time from the date of diagnosis to the date of death or the last follow-up for surviving patients.
The cut-off values for CEA and CA 19-9 were 5.0 ng/mL and 37.0 U/mL, respectively. TMI was defined as the geometric mean of the normalized values of CEA and CA 19-9. Normalization was performed by dividing the individual tumor marker values by the respective laboratory cut-off values. In summary, TMI was calculated using the following formula, as described in previous literature[8,9].
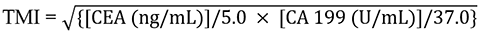
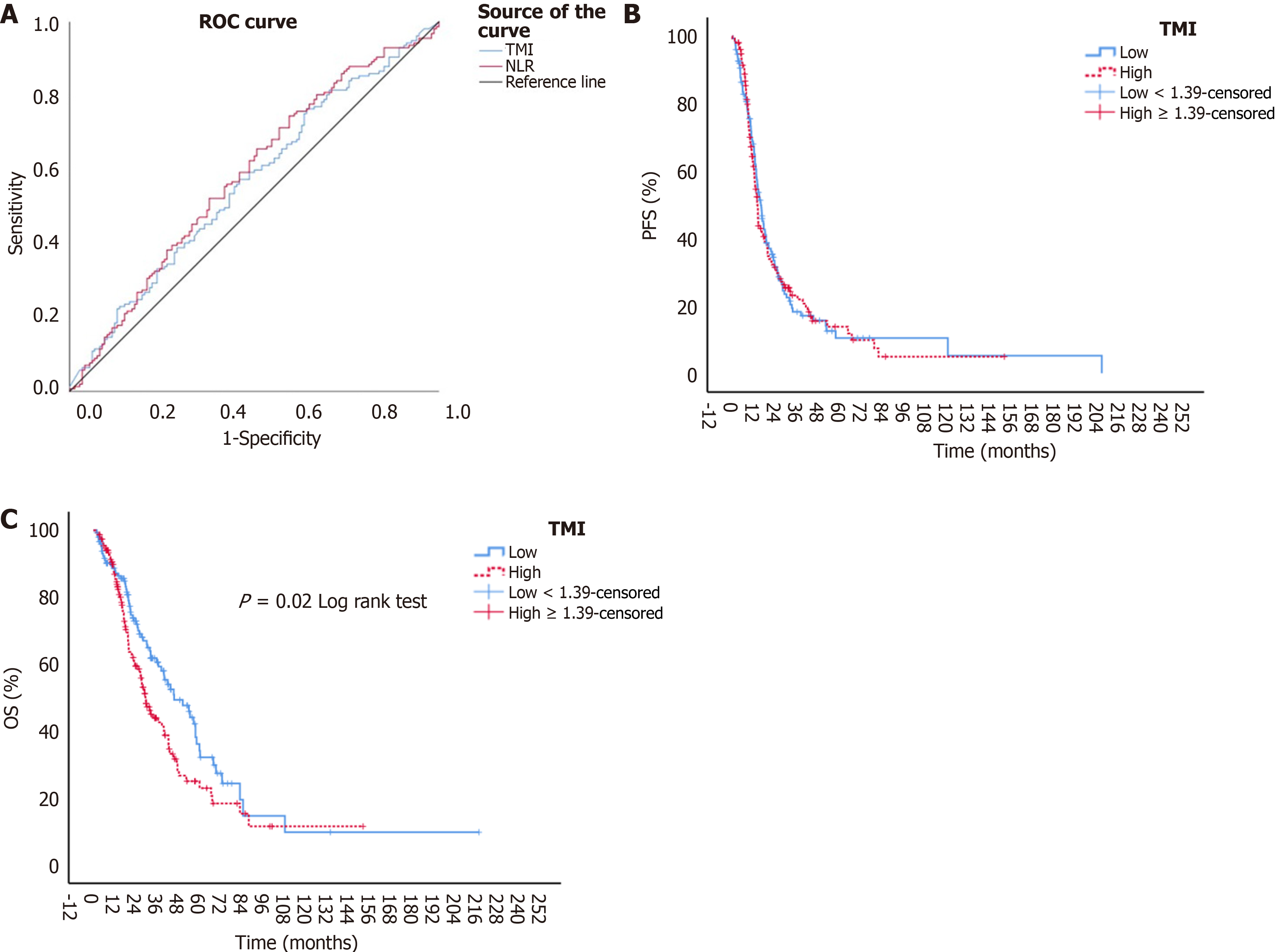
In our study, the TMI cut-off value was determined using receiver operating characteristic (ROC) analysis at an optimal specificity and sensitivity. A cut-off value of ≥ 1.39 was established for TMI [area under the curve (AUC): 0.574; 95% confidence interval (CI): 0.499-0.628, P = 0.049, sensitivity: 55.4%, specificity: 55.4%] (Figure 1A). Based on this cut-off, patients were categorized into two primary groups: TMI-high (TMI ≥ 1.39) and TMI-low (TMI < 1.39), and analyses were conducted accordingly. Additionally, other nutritional indices were evaluated in the study. The NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count before the initiation of first-line therapy in the metastatic phase. For NLR, ROC analysis identified an optimal cut-off of ≥ 2.76 (AUC: 0.583; 95%CI: 0.519-0.647, P = 0.012, sensitivity: 56.1%, specificity: 56.1%) (Figure 1A). The Glasgow prognostic score (GPS) was calculated based on pre-treatment C-reactive protein and albumin levels, as previously defined in the literature[11].
Statistical analysis was performed using “IBM SPSS Statistics for Windows, Version 25.0 (Statistical Package for the Social Sciences, IBM Corp., Armonk, NY, United States)”. Descriptive statistics were presented as frequency (n) and percentage (%) for categorical variables and as mean ± SD and median (min-max) for continuous variables. For binary group comparisons, the independent t-test was used. ROC curve analysis was employed to determine the optimal cut-off values for various indices in predicting mortality. The Pearson χ2 test and Fisher’s exact test were used for comparisons of categorical variables. Survival times between clinical groups were compared using the Kaplan-Meier method. In univariate models, statistically significant variables were further assessed for their prognostic impact on progression and mortality risk using multivariate Cox regression analysis. P value of < 0.05 was considered statistically significant.
This research was designed and conducted following Good Clinical Practice and the Declaration of Helsinki. It was approved by the Clinical Research Ethics Committee of the Faculty of Medicine, Alanya Alaaddin Keykubat University (approval No. 10354421-2024/23-04).
A total of 305 patients were included in the study. When the entire population was evaluated, the mean age of the patients was 64.5 ± 12.4 years. Of the patients, 192 (63.0%) were male and 113 (37.0%) were female. In the overall population, 209 patients (68.5%) were classified as having de novo metastatic disease, while 96 patients (31.5%) had recurrent metastatic disease. In our study, patients were divided into two main groups according to TMI categories. The TMI-low group consisted of 151 patients, and the TMI-high group consisted of 154 patients. Baseline demographic characteristics and disease-related variables were thoroughly analyzed between the TMI groups, and in general, the distributions between the groups were observed to be homogeneous. Notably, the proportion of patients with liver metastasis was significantly higher in the TMI-high group compared to the TMI-low group (76.6% vs 62.9%, P = 0.01). In contrast, the proportion of patients with peritoneal metastasis was lower in the TMI-high group compared to the TMI-low group (17.5% vs 29.8%, P = 0.01). Additionally, molecular analyses showed that the frequency of KRAS mutations was higher in the TMI-high group than in the TMI-low group (37.7% vs 23.2%, P = 0.02). A detailed comparison of the baseline demographic and clinical characteristics, as well as the core molecular features, of all patients and those in the TMI-high and TMI-low groups, is presented in Table 1.
| Total (n = 305) | TMI-low (n = 151) | TMI-high (n = 154) | P value | |
| Age, mean ± SD | 64.5 ± 12.4 | 64.1 ± 12.8 | 64.9 ± 12.0 | 0.56a |
| ≤ 65 years | 150 (49.2) | 76 (50.3) | 74 (48.1) | 0.69b |
| > 65 years | 155 (50.8) | 75 (49.7) | 80 (51.9) | |
| Sex | ||||
| Female | 113 (37.0) | 58 (38.4) | 55 (35.7) | 0.63b |
| Male | 192 (63.0) | 93 (61.6) | 99 (64.3) | |
| ECOG-PS | ||||
| 0 | 108 (35.4) | 54 (35.8) | 54 (35.1) | 0.18c |
| 1 | 156 (51.1) | 83 (55) | 73 (47.4) | |
| 2 | 36 (11.8) | 12 (7.9) | 24 (15.6) | |
| 3 | 5 (1.6) | 2 (1.3) | 3 (1.9) | |
| BMI | ||||
| ≤ 25 kg/m2 | 201 (65.9) | 109 (72.2) | 92 (59.7) | 0.02b |
| > 25 kg/m2 | 104 (34.1) | 42 (27.8) | 62 (40.3) | |
| Smoking history | ||||
| No | 172 (56.4) | 81 (53.6) | 91 (59.1) | 0.34b |
| Yes | 133 (43.6) | 70 (46.4) | 63 (40.9) | |
| Alcohol using | ||||
| No | 280 (91.8) | 138 (91.4) | 142 (92.2) | 0.79b |
| Yes | 25 (8.2) | 13 (8.6) | 12 (7.8) | |
| DM | ||||
| No | 244 (80.0) | 123 (81.5) | 121 (78.6) | 0.53b |
| Yes | 61 (20.0) | 28 (18.5) | 33 (21.4) | |
| HT | ||||
| No | 199 (65.2) | 102 (67.5) | 97 (63) | 0.40b |
| Yes | 106 (34.8) | 49 (32.5) | 57 (37) | |
| CAD | ||||
| No | 265 (86.9) | 132 (87.4) | 133 (86.4) | 0.78b |
| Yes | 40 (13.1) | 19 (12.6) | 21 (13.6) | |
| Disease status | ||||
| De-novo metastatic | 209 (68.5) | 96 (63.6) | 113 (73.4) | 0.06b |
| Recurrent metastatic | 96 (31.5) | 55 (36.4) | 41 (26.6) | |
| History of adjuvant CT | ||||
| No | 206 (67.5) | 94 (62.3) | 112 (72.7) | 0.05b |
| Yes | 99 (32.5) | 57 (37.7) | 42 (27.3) | |
| Liver metastasis | ||||
| No | 92 (31.2) | 56 (37.1) | 36 (23.4) | 0.01b |
| Yes | 213 (69.8) | 95 (62.9) | 118 (76.6) | |
| Lung metastasis | ||||
| No | 201 (65.9) | 104 (68.9) | 97 (63) | 0.28b |
| Yes | 104 (34.1) | 47 (31.1) | 57 (37) | |
| Bone metastasis | ||||
| No | 281 (92.1) | 142 (94) | 139 (90.3) | 0.22b |
| Yes | 24 (7.9) | 9 (6) | 15 (9.7) | |
| Peritoneal metastasis | ||||
| No | 233 (76.4) | 106 (70.2) | 127 (82.5) | 0.01b |
| Yes | 72 (23.6) | 45 (29.8) | 27 (17.5) | |
| Number of metastatic site | ||||
| 1 | 157 (51.5) | 80 (53) | 77 (50) | 0.35b |
| 2 | 91 (29.8) | 48 (31.8) | 43 (27.9) | |
| 3 | 38 (12.5) | 17 (11.3) | 21 (13.6) | |
| 4 or more | 19 (6.2) | 6 (4) | 13 (8.4) | |
| Isolated liver metastasis | ||||
| No | 207 (67.9) | 106 (70.2) | 101 (65.6) | 0.39b |
| Yes | 98 (32.1) | 45 (29.8) | 53 (34.4) | |
| History of metastasectomy | ||||
| No | 242 (79.3) | 110 (72.8) | 132 (85.7) | 0.006b |
| Yes | 63 (20.7) | 41 (27.2) | 22 (14.3) | |
| Tumor location | ||||
| Right side colon cancer | 65 (21.3) | 27 (17.9) | 38 (24.7) | 0.29b |
| Left side colon cancer | 140 (45.9) | 70 (46.4) | 70 (45.5) | |
| Rectum cancer | 100 (32.8) | 54 (35.8) | 46 (29.9) | |
| KRAS | ||||
| Wild | 182 (59.7) | 98 (64.9) | 84 (54.5) | 0.02b |
| Mutant | 93 (30.5) | 35 (23.2) | 58 (37.7) | |
| Unknown | 30 (9.8) | 18 (11.9) | 12 (7.8) | |
| NRAS | ||||
| Wild | 236 (77.4) | 114 (75.5) | 122 (79.2) | 0.26b |
| Mutant | 20 (6.6) | 8 (5.3) | 12 (7.8) | |
| Unknown | 49 (16.1) | 29 (19.2) | 20 (13) | |
| BRAF | ||||
| Wild | 241 (79.0) | 118 (78.1) | 123 (79.9) | 0.79b |
| Mutant | 10 (3.3) | 6 (4) | 4 (2.6) | |
| Unknown | 54 (17.7) | 27 (17.9) | 27 (17.5) | |
| MSI status | ||||
| Microsatellite stable | 183 (60.0) | 85 (56.3) | 98 (63.6) | 0.42b |
| MSI-high | 11 (3.6) | 6 (4) | 5 (3.2) | |
| Unknown | 111 (36.4) | 60 (39.7) | 51 (33.1) | |
| Metastatic line(s) of CT | ||||
| 1 | 120 (40.0) | 61 (40.9) | 59 (39.1) | 0.66b |
| 2 | 87 (29.0) | 46 (30.9) | 41 (27.2) | |
| 3 | 41 (13.7) | 17 (11.4) | 24 (15.9) | |
| 4 and more | 52 (17.3) | 25 (16.8) | 27 (17.9) | |
| Using immunotherapy | ||||
| No | 302 (99.0) | 150 (99.3) | 152 (98.7) | 0.99c |
| Yes | 3 (1.0) | 1 (0.7) | 2 (1.3) | |
| GPS | ||||
| 0 | 134 (43.9) | 78 (51.7) | 56 (36.4) | 0.03b |
| 1 | 132 (43.3) | 57 (37.7) | 75 (48.7) | |
| 2 | 39 (12.8) | 16 (10.6) | 23 (14.9) | |
| NLR, mean ± SD | 3.45 ± 2.55 | 3.15 ± 1.98 | 3.76 ± 2.98 | 0.04c |
When the treatment response rates for first-line therapy were evaluated, a complete response was achieved in 20 patients (6.6%) in the entire patient group. The objective response rate (ORR) and disease control rate were 44.6% and 78.0%, respectively, for the entire population. When patients were assessed according to their TMI scores, the ORR in the TMI-high and TMI-low groups were 42.2% and 47.0%, respectively (P = 0.40). Similarly, the disease control rate in the TMI-high and TMI-low groups were 78.6% and 77.5%, respectively (P = 0.82). No statistically significant difference was observed between the groups in terms of treatment response rates. A detailed comparison of the response to first-line treatment for all patients, as well as for the TMI-high and TMI-low groups, is presented in Table 2.
| Total (n = 305) | TMI-low (n = 151) | TMI-high (n = 154) | P value | |
| Best response | 0.19 | |||
| CR | 20 (6.6) | 14 (9.2) | 6 (3.9) | |
| PR | 116 (38) | 57 (37.7) | 59 (38.3) | |
| SD | 102 (33.4) | 46 (30.5) | 56 (36.4) | |
| PD | 40 (13.1) | 17 (11.3) | 23 (14.9) | |
| Missing/unknown | 27 (8.9) | 17 (11.3) | 10 (6.5) | |
| Objective response rate (CR + PR) | 136 (44.6) | 71 (47.0) | 65 (42.2) | 0.40 |
| Disease control rate (CR + PR + SD) | 238 (78) | 117 (77.5) | 121 (78.6) | 0.82 |
In our study, the median follow-up time for patients was 22.9 months (min-max, 1.40-217.5 months). The median PFS (mPFS) and median OS (mOS) for all patients were 14.5 (95%CI: 12.8-16.1) months and 38.7 (95%CI: 32.0-45.3) months, respectively. When assessed according to TMI scores, the mPFS for the TMI-high and TMI-low groups were 14.0 (95%CI: 12.7-15.2) months and 16.0 (95%CI: 13.5-18.4) months, respectively. Although the mPFS was numerically shorter in the TMI-high group, no statistically significant difference was found between the groups (P = 0.84) (Figure 1B). The estimated 2-year PFS rates for the TMI-high and TMI-low groups were 31.4% and 34.4%, respectively, and the 5-year estimated PFS rates were 13.8% and 10.4%, respectively. The mOS for the TMI-high and TMI-low groups were 29.5 (95%CI: 24.4-34.5) months and 45.6 (95%CI: 32.4-58.8) months, respectively, with the mOS in the TMI-high group being statistically significantly lower (P = 0.02) (Figure 1C). The estimated 2-year OS rates for the TMI-high and TMI-low groups were 59.4% and 72.9%, respectively, and the 5-year estimated OS rates were 22.9% and 34.1%, respectively.
Although not the primary endpoint of our study, survival analyses related to patient and tumor characteristics were also performed. In terms of mPFS, disease status, history of adjuvant chemotherapy, number of metastatic sites, history of metastasectomy, and KRAS, NRAS, and BRAF status were identified as variables that significantly influenced survival in univariate analyses. The variables that showed a significant survival difference for mPFS in the univariate models were included in the multivariate analysis using a Cox regression model. Having metastases in two or three different regions in mCRC was independently associated with poor prognosis when compared to patients with metastases in a single region, as separate independent poor prognostic factors {for two different metastatic regions [hazard ratio (HR) = 1.92, 95%CI: 1.34-2.75, P < 0.001]; for three different metastatic regions (HR = 2.20, 95%CI: 1.44-3.36, P < 0.001)}. The results of the univariate and multivariate analyses for PFS are detailed in Table 3.
| Variable | Univariate analyses | P value | Multivariate analyses | P value |
| Median PFS (95%CI), month | HR (95%CI) | |||
| Age | ||||
| ≤ 65 years | 15.9 (13.5-18.3) | 0.29 | ||
| > 65 years | 13.9 (12.2-15.7) | |||
| Gender | ||||
| Female | 14.3 (11.1-17.5) | 0.77 | ||
| Male | 14.5 (12.8-16.3) | |||
| ECOG-PS | ||||
| 0 | 14.7 (11.5-17.9) | 0.18 | ||
| 1 | 15.9 (14.0-17.7) | |||
| 2 | 10.1 (7.8-12.3) | |||
| 3 | 25.1 (0.3-49.9) | |||
| BMI | ||||
| ≤ 25 kg/m2 | 13.9 (13.0-14.8) | 0.08 | ||
| > 25 kg/m2 | 19.9 (14.4-25.4) | |||
| Smoking history | ||||
| No | 14.5 (12.4-16.5) | 0.69 | ||
| Yes | 14.4 (11.0-17.8) | |||
| Disease status | ||||
| De novo metastatic | 13.2 (12.5-14.6) | 0.002a | Ref | 0.71 |
| Recurrent metastatic | 23.7 (16.5-30.9) | 0.90 (0.53-1.52) | ||
| History of adjuvant CT | ||||
| No | 13.5 (12.3-14.6) | 0.004a | Ref | 0.30 |
| Yes | 21.7 (16.0-27.4) | 0.79 (0.44-1.22) | ||
| Liver metastasis | ||||
| No | 17.6 (8.7-26.5) | 0.05 | ||
| Yes | 14.3 (13.1-15.5) | |||
| Lung metastasis | ||||
| No | 14.5 (12.5-16.5) | 0.30 | ||
| Yes | 14.4 (11.9-16.9) | |||
| Bone metastasis | ||||
| No | 14.4 (12.8-16.0) | 0.73 | ||
| Yes | 19.4 (11.1-27.7) | |||
| Peritoneal metastasis | ||||
| No | 14.4 (12.8-15,9) | 0.28 | ||
| Yes | 17.5 (11.0-23.9) | |||
| Number of metastatic site | ||||
| 1 | 15.0 (13.0-17.0) | 0.004a | Ref | |
| 2 | 15.8 (12.1-19.4) | 1.92 (1.34-2.75) | < 0.001a | |
| 3 | 10.3 (8.4-12.1) | 2.20 (1.44-3.36) | < 0.001a | |
| 4 and more | 14.2 (3.4-24.9) | 1.39 (0.93-2.09) | 0.10 | |
| Isolated liver metastasis | ||||
| No | 14.5 (12.6-16.6) | 0.68 | ||
| Yes | 14.3 (11.7-17.0) | |||
| History of metastasectomy | ||||
| No | 14.0 (11.9-16.0) | 0.01a | Ref | 0.06 |
| Yes | 16.9 (12.7-21.1) | 0.71 (0.50-1.01) | ||
| Tumor location | ||||
| Right side colon cancer | 13.4 (11.4-15.4) | 0.54 | ||
| Left side colon cancer | 17.3 (13.3-21.3) | |||
| Rectum cancer | 13.5 (11.6-15.4) | |||
| KRAS | ||||
| Wild | 13.9 (12.6-15.2) | 0.003a | Ref | |
| Mutant | 14.6 (12.3-16.8) | 0.91 (0.66-1.23) | 0.52 | |
| Unknown | 28.7 (5.8-51.6) | 0.51 (0.21-1.21) | 0.13 | |
| NRAS | ||||
| Wild | 13.9 (12.8-15.0) | 0.001a | Ref | |
| Mutant | 17.5 (0.00-47.5) | 0.67 (0.36-1.26) | 0.22 | |
| Unknown | 25.1 (17.1-33.1) | 0.77 (0.39-1.54) | 0.47 | |
| BRAF | ||||
| Wild | 14.4 (12.8-15.9) | 0.01a | Ref | |
| Mutant | 8.4 (0.0-17.0) | 1.57 (0.81-3.04) | 0.18 | |
| Unknown | 20.4 (11.6-29.2) | 1.21 (0.71-2.07) | 0.48 | |
| MSI status | ||||
| Microsatellite stable | 14.3 (12.8-15.9) | 0.30 | ||
| MSI-high | - (-) | |||
| Unknown | 16.0 (13.4-18.5) | |||
| GPS | ||||
| 0 | 18.3 (15.5-21.0) | 0.07 | ||
| 1 | 13.9 (13.0-14.9) | |||
| 2 | 12.6 (9.1-16.1) | |||
| NLR | ||||
| < 2.76 | 16.5 (12.9-20.1) | 0.17 | ||
| ≥ 2.76 | 13.9 (12.6-15.2) | |||
| TMI | ||||
| < 1.39 | 16.0 (13.4-18.4) | 0.84 | ||
| ≥ 1.39 | 14.0 (12.7-15.2) |
In addition to the TMI score, when OS was examined, the Eastern Cooperative Oncology Group PS (ECOG-PS), history of metastasectomy, response to first-line treatment, GPS, and NLR were found to be significant in the univariate analysis. The variables that were found to be significant for OS were included in the Cox regression model. When compared to patients with ECOG-PS 0, those with ECOG-PS 2 had a significantly higher risk of death (HR = 3.52, 95%CI: 2.00-6.22, P < 0.001). Additionally, when compared to patients who had a complete response to first-line treatment, those with a partial response (HR = 5.19, 95%CI: 1.60-16.80, P = 0.006), stable disease (HR = 6.29, 95%CI: 1.92-20.59, P = 0.002), and progressive disease (HR = 15.73, 95%CI: 4.71-52.46, P < 0.001) had a significantly worse prognosis. Furthermore, an NLR ≥ 2.76 (HR = 1.46, 95%CI: 1.03-2.08, P = 0.033) and a high TMI score (≥ 1.39) (HR = 1.43, 95%CI: 0.99-2.06, P = 0.049) were identified as independent poor prognostic factors for mCRC patients. The results of the univariate and multivariate analyses for OS are detailed in Table 4.
| Variable | Univariate analyses | P value | Multivariate analyses | P value |
| Median OS (95%CI), month | HR (95%CI) | |||
| Age | ||||
| ≤ 65 years | 42.4 (33.7-51.0) | 0.09 | ||
| > 65 years | 32.0 (22.2-41.7) | |||
| Gender | ||||
| Female | 41.9 (31.9-52.0) | 0.17 | ||
| Male | 36.5 (27.8-45.1) | |||
| ECOG-PS | ||||
| 0 | 47.5 (32.2-62.7) | < 0.001a | Ref | |
| 1 | 36.7 (28.4-45.0) | 1.31 (0.88-1.97) | 0.18 | |
| 2 | 18.2 (13.7-22.7) | 3.52 (2.00-6.22) | < 0.001a | |
| 3 | 19.6 (16.2-23.0) | 1.56 (0.53-4.51) | 0.41 | |
| BMI | ||||
| ≤ 25 kg/m2 | 32.4 (24.5-40.3) | 0.09 | ||
| > 25 kg/m2 | 47.1 (37.0-57.0) | |||
| Smoking history | ||||
| No | 42.4 (33.8-51.1) | 0.13 | ||
| Yes | 30.1 (20.8-39.4) | |||
| Disease status | ||||
| De novo metastatic | 38.7 (30.7-46.6) | 0.24 | ||
| Recurrent metastatic | 36.5 (19.8-53.2) | |||
| History of adjuvant CT | ||||
| No | 32.3 (23.1-41.6) | 0.06 | ||
| Yes | 43.5 (30.0-57.0) | |||
| Liver metastasis | ||||
| No | 35.7 (28.3-43.0) | 0.78 | ||
| Yes | 39.9 (29.1-50.5) | |||
| Lung metastasis | ||||
| No | 40.2 (32.8-47.4) | 0.42 | ||
| Yes | 32.3 (23.2-41.5) | |||
| Bone metastasis | ||||
| No | 40.0 (32.7-47.3) | 0.26 | ||
| Yes | 31.9 (18.5-45.2) | |||
| Peritoneal metastasis | ||||
| No | 38.6 (30.0-47.3) | 0.83 | ||
| Yes | 38.3 (29.2-47.4) | |||
| Number of metastatic site | ||||
| 1 | 40.1 (32.3-48.1) | 0.09 | ||
| 2 | 43.5 (32.0-55.0) | |||
| 3 | 22.9 (13.2-32.5) | |||
| 4 and more | 27.7 (15.6-39.8) | |||
| Isolated liver metastasis | ||||
| No | 35.7 (28.8-42.6) | 0.22 | ||
| Yes | 45.3 (37.6-53.0) | |||
| History of metastasectomy | ||||
| No | 32.0 (25.0-38.9) | 0.02a | Ref | |
| Yes | 50.4 (39.1-61.7) | 0.92 (0.59-1.43) | 0.72 | |
| Tumor location | ||||
| Right side colon cancer | 31.9 (17.1-46.6) | 0.29 | ||
| Left side colon cancer | 45.2 (30.6-59.8) | |||
| Rectum cancer | 38.3 (28.7-47.9) | |||
| KRAS | ||||
| Wild | 30.3 (22.2-38.4) | 0.05 | ||
| Mutant | 47.1 (35.6-58.6) | |||
| Unknown | 48.3 (33.8-62.8) | |||
| NRAS | ||||
| Wild | 32.4 (25.4-39.3) | 0.004a | Ref | |
| Mutant | 59.9 (-) | 0.50 (0.22-1.10) | 0.09 | |
| Unknown | 56.6 (44.3-68.8) | 0.54 (0.29-1.02) | 0.06 | |
| BRAF | ||||
| Wild | 38.7 (30.4-46.9) | 0.06 | ||
| Mutant | 19.6 (5.2-33.9) | |||
| Unknown | 43.0 (25.8-60.2) | |||
| MSI status | ||||
| Microsatellite stable | 39.8 (31.5-48.0) | 0.79 | ||
| MSI-high | 45.6 (0.0-91.9) | |||
| Unknown | 35.7 (24.8-46.6) | |||
| Best treatment response | ||||
| CR | - (-) | < 0.001a | Ref | |
| PR | 45.3 (35.5-55.1) | 5.19 (1.60-16.80) | 0.006a | |
| SD | 36.7 (24.7-48.7) | 6.29 (1.92-20.59) | 0.002a | |
| PD | 20.9 (17.7-24.2) | 15.73 (4.71-20.59) | < 0.001a | |
| GPS | ||||
| 0 | 43.5 (34.2-52.8) | 0.006a | Ref | |
| 1 | 28.7 (21.2-36.2) | 1.37 (0.94-1.99) | 0.10 | |
| 2 | 59.9 (9.9-109.9) | 0.69 (0.37-1.27) | 0.24 | |
| NLR | ||||
| < 2.76 | 45.3 (35.0-55.6) | 0.005a | Ref | |
| ≥ 2.76 | 29.4 (24.8-33.9) | 1.46 (1.03-2.08) | 0.03a | |
| TMI | ||||
| < 1.39 | 45.6 (32.4-58.8) | 0.02a | Ref | |
| ≥ 1.39 | 29.5 (24.3-34.5) | 1.43 (0.99-2.06) | 0.049a |
This study aimed to evaluate the prognostic significance of the TMI, an innovative index that is low-cost, easy to implement, and suitable for routine clinical practice, in patients diagnosed with mCRC. It has been demonstrated that the TMI, created based on the combination of CEA and CA 19-9 tumor markers, holds significant prognostic value for patients’ OS. Patients with metastatic mCRC and a TMI-high (≥ 1.39) exhibited a worse prognosis compared to those with TMI-low. These findings support the potential use of TMI as a clinical tool for improving survival predictions in mCRC.
Both TMI-high and TMI-low groups contained a similar number of patients, and apart from the presence of liver metastasis, which could potentially affect survival, the baseline demographic and disease characteristics were mostly distributed similarly. In our patient population, advanced age and male gender, which are more commonly observed in mCRC, and in line with the literature, the most frequently observed site of metastasis was liver metastasis, followed by lung and peritoneal metastases[12]. In our study, the mutation frequencies of KRAS, NRAS, and BRAF, which are important in CRC prognosis, were found to be 30.5%, 6%, and 3.3%, respectively. In a recent study by Bożyk et al[13], KRAS mutation frequency was reported as 38.0%, NRAS mutation frequency as 4%, and BRAF mutation frequency as 4.8%, and the results are consistent with the molecular findings in our study and reliable according to the literature. Therefore, we believe that the patient population in our study well represents real-world clinical practice.
CEA is a tumor marker identified in CRC tissue, first described by Gold and Freedman[14] in 1965. It is a glycoprotein typically found in the embryonic endodermal epithelium and is expressed in various epithelial tumors[14,15]. Due to its low sensitivity and specificity in the diagnosis of CRC, CEA does not have a role in the diagnostic process. However, several studies have reported an association between preoperative serum CEA > 5.0 ng/mL and poor prognosis, particularly in patients with early-stage CRC. Additionally, it can be used in the post-operative follow-up of patients in remission[16]. CEA is extensively utilized not only for post-operative monitoring after curative resection but also for assessing prognosis and tracking treatment response in patients with mCRC. Its role is crucial in managing advanced stages of CRC, helping to guide therapeutic decisions and evaluate disease progression[17,18]. Despite various findings in the literature, there is currently insufficient evidence to support the use of CEA alone as a reliable marker for prognosis in mCRC, as it lacks adequate specificity or sensitivity.
Another important biomarker, CA 19-9, was developed by Koprowski et al[19] in 1979 and is frequently used in the monitoring of CRC, though it is not universally included in routine clinical practice as a standard marker for all patients. In addition to CRC, CA 19-9 is known to be elevated in various cancers, particularly pancreatic and other gastrointestinal cancers, as well as in benign conditions. Compared to CEA, it is less sensitive in several studies, and subsequent research has demonstrated that combining these two markers may lead to better clinical interpretations. For example, in a study assessing the sensitivity and specificity of CEA and CA 19-9 in patients diagnosed with CRC, the sensitivity and specificity for CEA were 64.5% and 89.2%, respectively, while for CA 19-9, they were 47.8% and 90.1%. When these two tests were combined, sensitivity increased (71.7%), but specificity decreased (82.9%). Although some studies have suggested that combining CEA and CA 19-9 could enhance diagnostic accuracy, their use in routine clinical practice for diagnosis is still not recommended. Furthermore, there are conflicting and limited results in the literature regarding the combined use of these biomarkers for predicting prognosis in early-stage or mCRC patients[8,20-22]. In summary, while CEA and CA 19-9 have been individually used as biomarkers in mCRC, few studies have explored a combined index incorporating both markers. In this study, we aimed to investigate the prognostic value of a novel TMI, which merges CEA and CA 19-9 into a single calculated score, addressing the need for a more integrated and potentially more accurate prognostic tool in CRC.
The concept of TMI was first introduced by Muley et al[10] in the context of resected early-stage NSCLC, where it was based on CYFRA21-1 and CEA, and its prognostic significance was evaluated using the geometric mean of these markers. This study demonstrated that elevated TMI serves as a strong negative prognostic factor for survival in resected NSCLC patients[10]. Subsequent studies across various types of cancer have investigated the use of CYFRA21-1 as a basis for evaluating TMI. For instance, a study conducted by Yin and Liu[9] in 2020 on esophageal squamous cell carcinoma showed that a high TMI, derived from the geometric mean of preoperative CYFRA21-1 and squamous cell carcinoma antigen, was an independent prognostic factor for radical resection in esophageal squamous cell carcinoma, compared to lower TMI[9]. In light of these findings, based on the hypothesis of a synergistic effect of tumor markers, Kamada et al[8] first investigated the prognostic importance of a newly developed TMI in operable stages 1-3 CRC, based on CEA and CA 19-9. In this study, the 5-year relapse-free survival rate (65.8% vs 89.7%, respectively) and the 5-year cancer-specific survival rate (77.3% vs 94.9%) were significantly lower in the high-TMI group compared to the TMI-low group (P < 0.001 for both). The study demonstrated that pre-treatment TMI is an important and useful prognostic indicator for resectable CRC[8]. In our study, which is the first to investigate TMI in patients with mCRC, similar findings were observed, and our study reached its primary endpoint. In our study, mOS was significantly lower in the TMI-high group compared to the TMI-low group (29.5 months vs 45.6 months, P = 0.02). Furthermore, although there was no statistical significance, the ORRs (42.2% vs 47.0%, P = 0.40) and mPFS (14.0 months vs 16.0 months, P = 0.84) were numerically worse in the TMI-high group. Additionally, multivariate analyses showed that high-TMI is an independent negative prognostic factor for OS. In conclusion, we believe that the combination of CEA and CA 19-9 tumor markers to form TMI is an important, simple, inexpensive, and easily applicable prognostic factor in clinical practice.
Although the optimal cut-off level for the newly developed TMI, based on CEA and CA 19-9, is not yet established, our study of 305 patients identified a significant cut-off value of 1.39. This value showed sensitivity and specificity both greater than 50%, suggesting its potential as a prognostic factor in CRC at both early and advanced stages. The predictive power of the TMI for prognosis in CRC, as indicated by the AUC value of 0.574, is moderate at best. We acknowledge this limitation and emphasize the need for caution when interpreting the clinical utility of TMI. While it shows some prognostic potential, further validation and studies with larger cohorts are necessary to better understand its role and refine its predictive accuracy in conjunction with other established biomarkers.
In addition to TMI, other variables that were statistically significant for PFS and OS in univariate analysis were further analyzed in separate multivariate models. As mentioned above, patients with metastases in two or three different regions had worse mPFS compared to those with a single metastasis, as expected due to the higher tumor burden. Additionally, although not the primary endpoint of our study, clinical conditions such as poor ECOG-PS, lack of response to first-line treatment, or disease progression were found to be associated with poor OS. The NLR, an inflammatory index calculated by dividing the absolute neutrophil count by the absolute lymphocyte count, which has been shown to have prognostic significance in numerous studies in the past decade, was also evaluated in our study. In univariate analysis, the mOS was 29.4 months in the NLR-high group and 45.3 months in the NLR-low group. Furthermore, multivariate analysis revealed that an NLR ≥ 2.76, measured immediately before treatment during the metastatic phase, was an independent poor prognostic factor for patients with mCRC (HR = 1.46, 95%CI: 1.03-2.08, P = 0.033). Previous studies in the literature have also shown that patients with high NLR have a worse prognosis[23,24]. In our study, the prognostic significance of the GPS, calculated based on albumin and C-reactive protein, could not be demonstrated. Our results are consistent with the literature, and NLR remains an important, simple, and accessible inflammatory parameter that can provide valuable prognostic insights in clinical practice. Additionally, studies have shown that low prognostic immune-nutritional indices calculated from albumin and monocyte count, are independent poor prognostic factors in mCRC[25]. These indices, similar to TMI, are based on easily accessible clinical parameters and may offer valuable prognostic insights, particularly in assessing the patient’s overall immune and nutritional status during the metastatic phase. It is important to note that our study represents, to our knowledge, the first investigation of TMI specifically in mCRC. Given the novel nature of TMI, it is essential to acknowledge that while it may provide valuable prognostic insights, direct comparisons with other established indices, such as NLR, GPS, prognostic immune-nutritional indices require further research. Our study demonstrates the potential of TMI as a cost-effective and accessible prognostic tool, similar to other simple indices derived from routine clinical parameters. However, additional studies comparing the sensitivity and specificity of these markers are needed to better define which index offers the most robust prognostic value in mCRC.
Our study is the first to be conducted in a population of patients with mCRC in the literature. The large sample size, multi-center design, the general consistency of the basic demographic and clinical characteristics with existing literature data, and the introduction of a newly developed index that can be calculated easily, non-invasively, without additional cost, and that can be readily applied in real-world clinical practice, can be considered as the strengths of our study.
Our study has several limitations. First, the unequal distribution of factors that could potentially affect survival, such as liver metastasis, metastasectomy status, and KRAS mutation status, due to the retrospective nature of the study, is one of the most important limitations. Second, while response rates to first-line treatments are presented, the chemotherapy and targeted therapy regimens, as well as the number of cycles patients received, are not clearly defined. This should be kept in mind as these factors could potentially influence survival outcomes. Finally, due to the lack of prior prospective studies and larger-scale trials in the literature, although we consider the cut-off value of 1.39 to be potentially suitable for TMI, the optimal cut-off value remains uncertain. The validity and reliability of this simple and potentially valuable index for clinical practice must be confirmed through larger, well-designed prospective studies with more appropriate populations.
In conclusion, this study demonstrates that survival predictions can be improved using an innovative TMI created by combining the tumor markers CEA and CA 19-9 in patients with mCRC. The TMI could be integrated into clinical practice as a valuable prognostic tool. Its simplicity, low cost, and ease of application may provide significant clinical benefits, especially in settings with limited resources and in developing countries.
| 1. | Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2279] [Cited by in RCA: 4724] [Article Influence: 4724.0] [Reference Citation Analysis (3)] |
| 2. | Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 1441] [Article Influence: 360.3] [Reference Citation Analysis (0)] |
| 3. | Marschner N, Frank M, Vach W, Ladda E, Karcher A, Winter S, Jänicke M, Trarbach T. Development and validation of a novel prognostic score to predict survival in patients with metastatic colorectal cancer: the metastatic colorectal cancer score (mCCS). Colorectal Dis. 2019;21:816-826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Hisada H, Takahashi Y, Kubota M, Shimura H, Itobayashi E, Shimura K, Nakamura A. Clinical and therapeutic features and prognostic factors of metastatic colorectal cancer over age 80: a retrospective study. BMC Gastroenterol. 2021;21:199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Bourakkadi Idrissi M, El Bouhaddouti H, Mouaqit O, Ousadden A, Ait Taleb K, Benjelloun EB. Left-Sided Colon Cancer and Right-Sided Colon Cancer: Are They the Same Cancer or Two Different Entities? Cureus. 2023;15:e37563. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Krell M, Llera B, Brown ZJ. Circulating Tumor DNA and Management of Colorectal Cancer. Cancers (Basel). 2023;16:21. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Lakemeyer L, Sander S, Wittau M, Henne-Bruns D, Kornmann M, Lemke J. Diagnostic and Prognostic Value of CEA and CA19-9 in Colorectal Cancer. Diseases. 2021;9:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 8. | Kamada T, Ohdaira H, Takahashi J, Aida T, Nakashima K, Ito E, Hata T, Yoshida M, Eto K, Suzuki Y. Novel tumor marker index using carcinoembryonic antigen and carbohydrate antigen 19-9 is a significant prognostic factor for resectable colorectal cancer. Sci Rep. 2024;14:4192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 9. | Yin N, Liu W. Clinical Value of Tumor Marker Index Based on Preoperative CYFRA 21-1 and SCC-Ag in the Evaluation of Prognosis and Treatment Effectiveness in Patients with Esophageal Squamous Cell Carcinoma. Onco Targets Ther. 2020;13:4135-4143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Muley T, Fetz TH, Dienemann H, Hoffmann H, Herth FJ, Meister M, Ebert W. Tumor volume and tumor marker index based on CYFRA 21-1 and CEA are strong prognostic factors in operated early stage NSCLC. Lung Cancer. 2008;60:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Nozoe T, Matono R, Ijichi H, Ohga T, Ezaki T. Glasgow Prognostic Score (GPS) can be a useful indicator to determine prognosis of patients with colorectal carcinoma. Int Surg. 2014;99:512-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Hugen N, van de Velde CJH, de Wilt JHW, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. 2014;25:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 351] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 13. | Bożyk A, Krawczyk P, Reszka K, Krukowska K, Kolak A, Mańdziuk S, Wojas-Krawczyk K, Ramlau R, Milanowski J. Correlation between KRAS, NRAS and BRAF mutations and tumor localizations in patients with primary and metastatic colorectal cancer. Arch Med Sci. 2022;18:1221-1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965;122:467-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1291] [Cited by in RCA: 1294] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 15. | Quentmeier A, Möller P, Schwarz V, Abel U, Schlag P. Carcinoembryonic antigen, CA 19-9, and CA 125 in normal and carcinomatous human colorectal tissue. Cancer. 1987;60:2261-2266. [PubMed] [DOI] [Full Text] |
| 16. | Konishi T, Shimada Y, Hsu M, Tufts L, Jimenez-Rodriguez R, Cercek A, Yaeger R, Saltz L, Smith JJ, Nash GM, Guillem JG, Paty PB, Garcia-Aguilar J, Gonen M, Weiser MR. Association of Preoperative and Postoperative Serum Carcinoembryonic Antigen and Colon Cancer Outcome. JAMA Oncol. 2018;4:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 17. | Duffy MJ, van Dalen A, Haglund C, Hansson L, Holinski-Feder E, Klapdor R, Lamerz R, Peltomaki P, Sturgeon C, Topolcan O. Tumour markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines for clinical use. Eur J Cancer. 2007;43:1348-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 342] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 18. | Hall C, Clarke L, Pal A, Buchwald P, Eglinton T, Wakeman C, Frizelle F. A Review of the Role of Carcinoembryonic Antigen in Clinical Practice. Ann Coloproctol. 2019;35:294-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 19. | Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5:957-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 865] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 20. | Vukobrat-Bijedic Z, Husic-Selimovic A, Sofic A, Bijedic N, Bjelogrlic I, Gogov B, Mehmedovic A. Cancer Antigens (CEA and CA 19-9) as Markers of Advanced Stage of Colorectal Carcinoma. Med Arch. 2013;67:397-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Jelski W, Mroczko B. Biochemical Markers of Colorectal Cancer - Present and Future. Cancer Manag Res. 2020;12:4789-4797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 22. | Zhang SY, Lin M, Zhang HB. Diagnostic value of carcinoembryonic antigen and carcinoma antigen 19-9 for colorectal carcinoma. Int J Clin Exp Pathol. 2015;8:9404-9409. [PubMed] |
| 23. | Nogueira-Costa G, Fernandes I, Gameiro R, Gramaça J, Xavier AT, Pina I. Prognostic utility of neutrophil-to-lymphocyte ratio in patients with metastatic colorectal cancer treated using different modalities. Curr Oncol. 2020;27:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Kim JH, Lee JY, Kim HK, Lee JW, Jung SG, Jung K, Kim SE, Moon W, Park MI, Park SJ. Prognostic significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with stage III and IV colorectal cancer. World J Gastroenterol. 2017;23:505-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Kayikcioglu E, Iscan G. A Novel Prognostic Index for Metastatic Colon Cancer: The Prognostic Immune Nutritional Index. Cureus. 2023;15:e33808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |