Published online May 15, 2025. doi: 10.4251/wjgo.v17.i5.103809
Revised: January 23, 2025
Accepted: February 27, 2025
Published online: May 15, 2025
Processing time: 153 Days and 0.5 Hours
Rectal cancer requires accurate preoperative assessment of T stage and differentiation grade for treatment planning. Traditional imaging and serum markers have limitations in diagnostic accuracy.
To evaluate the predictive value of dynamic contrast-enhanced-magnetic reso
We conducted a retrospective review of clinical data from 126 patients who were pathologically diagnosed with rectal cancer between January 2021 to June 2024. Each patient underwent DCE-MRI scans and serum tests for CA19-9 and CA125. Receiver operating characteristic curves were utilized to assess the diagnostic value of DCE-MRI parameters, including volume transfer constant (Ktrans), rate constant (Kep), and volume fraction of extravascular extracellular space (Ve), as well as serum biomarkers for staging and grading rectal cancer. The DeLong test algorithm was employed to evaluate differences in diagnostic performance among the various indicators.
There were statistically higher levels of Ktrans, Ve, CA19-9, and CA125 serum concentrations of patients with advanced T stages and on poorly differentiated tumors than that in patients with low stages and moderate to high differentiation (P < 0.05). Combined use of Ktrans and Ve for T stage diagnosis showed an area under the curve (AUC) of 0.892 [95% confidence interval (CI): 0.832-0.952], which increased to 0.923 (95%CI: 0.865-0.981) when combined with serum biomarkers. For grades differentiation, the combined DCE-MRI parameters had an AUC of 0.883 (95%CI: 0.821-0.945), which rose to 0.912 (95%CI: 0.855-0.969) when combined with serum markers. According to the Delong test, the combined diagnostic method performed better than a single diagnostic method (P < 0.05).
The combined application of DCE-MRI functional parameters and serum tumor markers can significantly improve the diagnostic accuracy of T staging and differentiation degree of rectal cancer, providing a new approach to improve the preoperative assessment system of rectal cancer. This combined diagnostic model has important clinical application value, but further validation is needed through large-scale multicenter studies.
Core Tip: This study evaluates the diagnostic efficacy of dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) parameters (volume transfer constant, volume fraction of extravascular extracellular space) and serum tumor markers [carbohydrate antigen (CA) 19-9, CA125] in rectal cancer. Results show that combined analysis significantly improves accuracy in assessing T staging and differentiation degree, surpassing individual methods. DCE-MRI captures tumor microenvironment characteristics, while serum markers reflect systemic tumor activity, providing complementary insights. The combined approach achieved area under the curves of 0.923 and 0.912 for T staging and differentiation, respectively, highlighting its clinical utility. These findings suggest that integrating functional imaging with serum biomarkers can enhance preoperative assessment and guide individualized treatment strategies for rectal cancer.
- Citation: Wang Q, Zhang XY, Yang JF, Tao YL. Comparison of dynamic contrast-enhanced-magnetic resonance imaging parameters and serum markers in preoperative rectal cancer evaluation: Combined diagnostic value. World J Gastrointest Oncol 2025; 17(5): 103809
- URL: https://www.wjgnet.com/1948-5204/full/v17/i5/103809.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i5.103809
Rectal cancer is among the most prevalent malignant tumors in the digestive system, with both incidence and mortality rates increasing worldwide[1]. Accurate preoperative staging and differentiation assessment are of great significance for formulating individualized treatment plans, predicting prognosis, and guiding clinical decisions. Currently, preoperative assessment mainly relies on imaging examinations and serological indicators, but there are still certain limitations to the diagnostic value of a single examination method[2].
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is a non-invasive functional imaging technique that can quantitatively evaluate tumor blood perfusion and microvascular permeability characteristics[3]. By analyzing the time-signal intensity curve and quantitative parameters, such as volume transfer constant (Ktrans), rate constant (Kep), and volume fraction of extravascular extracellular space (Ve), it can reflect the formation of tumor neovascularization and biological behavior characteristics[4-6]. Previous studies have shown that DCE-MRI parameters are closely related to tumor invasive depth, angiogenesis, and prognosis[7]. However, the application value of DCE-MRI parameters in the preoperative staging and differentiation assessment of rectal cancer still needs further verification.
Serum tumor marker detection has the advantages of being simple, economical, and having good repeatability, and is widely used in tumor diagnosis and monitoring[8]. Carbohydrate antigen 19-9 (CA19-9) and carbohydrate antigen 125 (CA125) are commonly used tumor markers in clinical practice, and their expression levels may be related to the progression and prognosis of rectal cancer[9]. Some studies have shown that the positive rate of CA19-9 and CA125 in colorectal cancer patients increases with the elevation of tumor node metastasis (TNM) staging[10], but their diagnostic efficacy in assessing tumor local infiltration depth and differentiation degree still needs to be explored.
In recent years, multi-parameter joint diagnostic models have received widespread attention in the field of tumor diagnosis[11]. The combination of DCE-MRI functional parameters and serum tumor markers may provide more comprehensive information for the preoperative assessment of rectal cancer, which is helpful to improve diagnostic accuracy[12].
Based on the above background, this study aims to analyze the expression characteristics of DCE-MRI quantitative parameters (Ktrans, Kep, Ve) and serum tumor markers (CA19-9, CA125) in patients with rectal cancer, evaluate their correlation with tumor T staging and differentiation degree, and explore the diagnostic efficacy of the two examination methods alone and in combination. The results of the study may provide new ideas and basis for improving the preoperative assessment system of rectal cancer and optimizing diagnostic strategies, which has important clinical application value.
This study is a single-center retrospective study that collected clinical data from patients with rectal cancer who underwent surgical treatment at our hospital from January 2021 to June 2024.
Inclusion criteria: (1) Aged 18-75 years, pathologically diagnosed with rectal adenocarcinoma by biopsy or surgical pathology; (2) Completed DCE-MRI examination within 2 weeks before surgery, with image quality meeting diagnostic requirements; (3) Completed serum CA19-9 and CA125 testing within 1 week before surgery; (4) Complete clinical data, including TNM staging and pathological differentiation information; (5) No prior anti-tumor treatments such as radiotherapy or chemotherapy; and (6) Karnofsky performance status score ≥ 70.
Exclusion criteria: (1) Presence of other malignancies or significant organ dysfunction; (2) Concurrent autoimmune diseases, active infections, severe coagulation disorders, or other conditions that may affect serum marker levels; (3) Contraindications to MRI examination or failure to complete dynamic enhancement scanning for various reasons; (4) Poor image quality, preventing accurate measurement and analysis; (5) Prior anti-tumor treatments such as radiotherapy, chemotherapy, or targeted therapy before surgery; (6) Pregnant or lactating women; (7) Mental disorders that prevent cooperation with examinations or follow-ups; and (8) Refusal of surgical treatment or loss to follow-up.
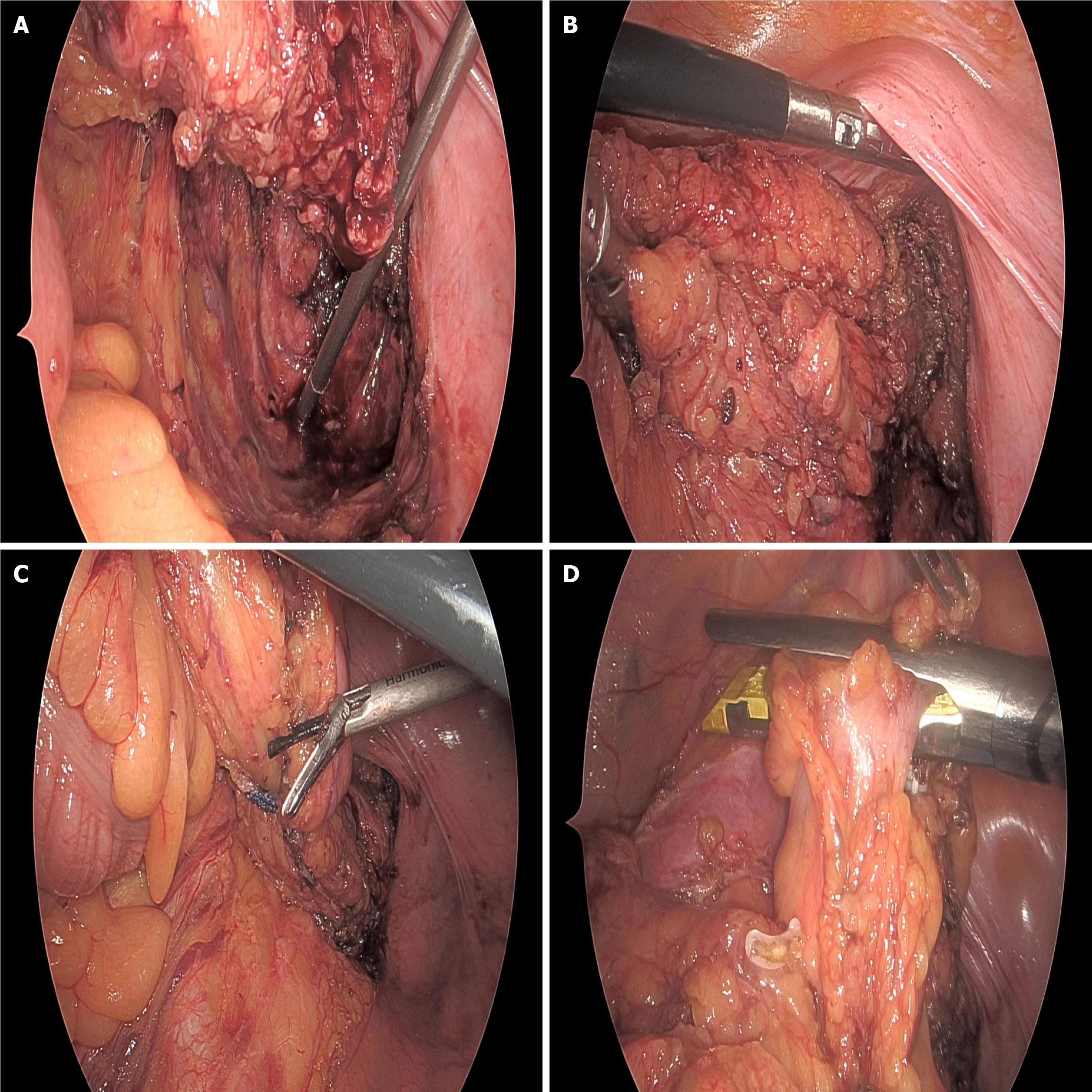
Surgical methods: Surgical approaches were selected based on tumor location, clinical staging, and patient-specific conditions. For mid and high rectal cancer patients with tumors > 5 cm from the anal verge, laparoscopic-assisted resection was preferentially performed, strictly adhering to the total mesorectal excision principle to ensure negative circumferential margins and thorough lymph node dissection. The surgery employed a five-port technique, including one observation port (10 mm above the umbilicus) and four operating ports (5 mm-12 mm in the lower abdomen and both lateral abdominal regions). Ultrasonic scalpels and laparoscopic linear cutters were used to ensure surgical precision when mobilizing the colon and transecting mesenteric vessels. For low rectal cancer patients ≤ 5 cm from the anal verge, after fully assessing the relationship between the tumor lower margin and the anal sphincter, a laparoscopic low anterior resection (Dixon procedure) was performed for those likely to preserve anal sphincter function, with prophylactic ileostomy when necessary; for those unable to preserve anal function, abdominoperineal resection (miles procedure) was adopted. All patients underwent D2/D3 lymph node dissection as recommended by the Japanese Society for Cancer of the Colon and Rectum. Surgical specimens were marked with distal, proximal, and circumferential margins to ensure radical surgery. Intraoperative records included surgery time, blood loss, incision length, and other surgery-related indicators. Anastomosis was chosen based on tumor location, with end-to-end or side-to-end anastomosis completed using directional stapling technology staplers or manual sutures. For cases with local infiltration or severe adhesions found intraoperatively, the resection range was appropriately expanded under the premise of surgical safety, and multi-organ resection was performed when necessary (Figure 1).
Postoperative recovery management: Postoperative recovery management adheres to the enhanced recovery after surgery philosophy, creating personalized recovery plans. Patients are encouraged to engage in active bed activities, deep breathing exercises, and lower limb venous thrombosis prevention movements 6-8 hours after surgery. Within 24 hours postoperatively, patients are assisted in getting out of bed for activities, with no less than 4 times a day, each lasting 15-30 minutes. Pain management employs a multimodal analgesic strategy, adjusting the medication plan based on the visual analogue scale (VAS) to maintain a VAS score ≤ 3. In terms of enteral nutrition, patients start with small amounts of warm water for mouth rinsing 6-12 hours postoperatively, and after 24-48 hours, gastrointestinal function is assessed. A liquid diet is initiated after passing gas, transitioning to a regular diet following bowel movements. For patients with a prophylactic ileostomy, stoma care training and guidance are provided. Vital signs and drainage fluid characteristics and volume are monitored, with daily food intake, flatulence, and bowel movement records maintained. Laboratory tests include monitoring of complete blood count, electrolytes, liver and kidney function, and other indicators. Functional exercise assessment systems evaluate postoperative functional recovery, including the activities of daily living (ADL) score and the 6-minute walk distance. Discharge criteria include normal body temperature without signs of infection, good incision healing, tolerance of a regular diet, restoration of bowel function, ability to move independently, and an ADL score ≥ 85.
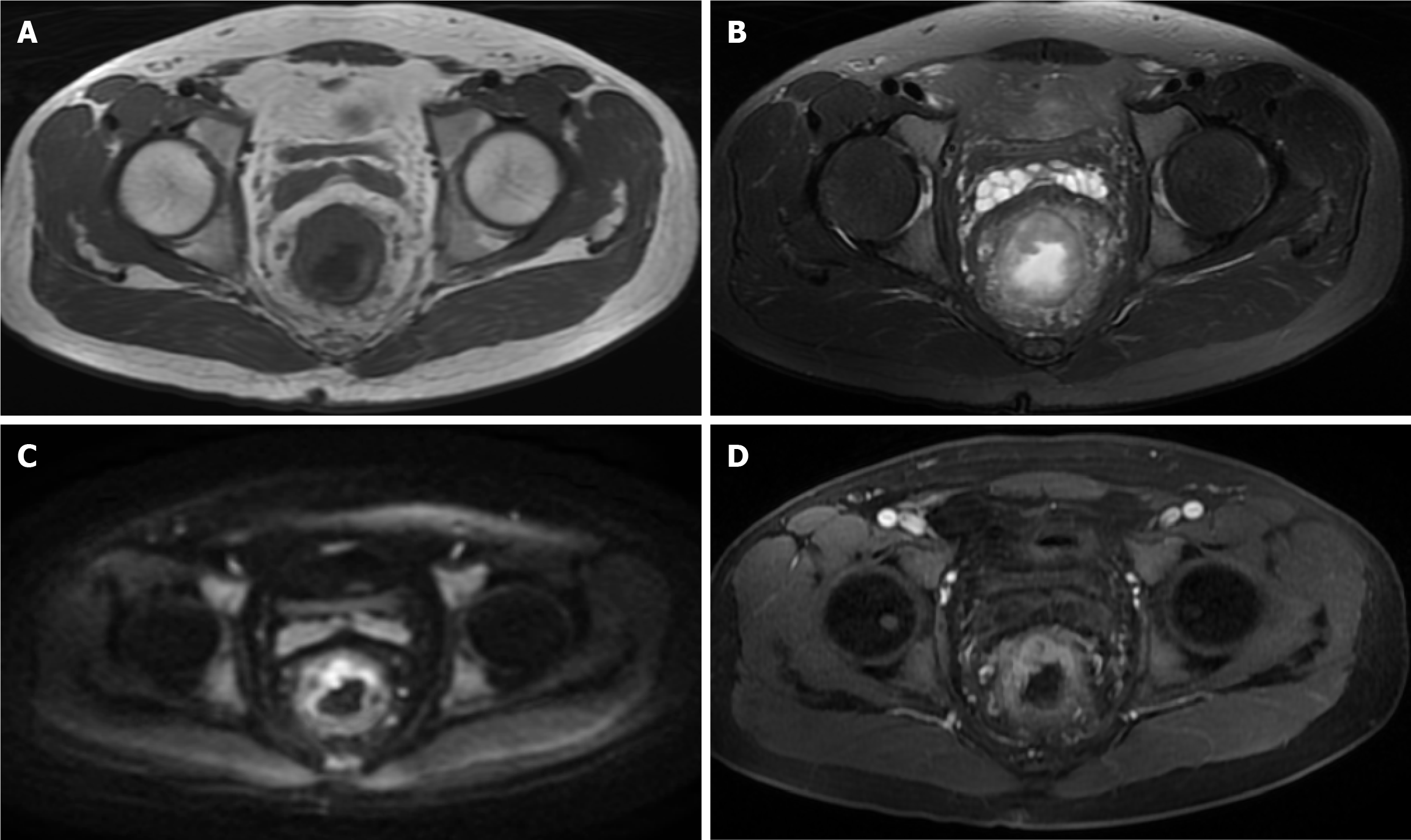
DCE-MRI examination: All patients underwent DCE-MRI examinations using the GE SIGNA Voyager 1.5T MRI system with a body phased-array coil. Prior to the examination, patients fasted for 4 hours, emptied intestinal gas, and were scanned in the supine position. Routine scanning included axial T1 weighted image, T2 weighted image, and sagittal T2 weighted image sequences. Dynamic contrast-enhanced scanning was performed using the liver acquisition with volume acceleration sequence, with scanning carried out at the end of expiration while the patient held their breath. The scan parameters were set as follows: Repetition time 3.5 ms, echo time 1.3 ms, flip angle 15°, slice thickness 3 mm, no gap, matrix 256 × 192, field of view 260 mm × 195 mm. Gadoteric acid contrast agent (0.1 mmol/kg) was injected via the antecubital vein at a rate of 3.0 mL/second, followed by a 20 mL saline flush. Three-dimensional volumetric scans were performed, acquiring baseline images before contrast injection and continuous acquisition of 10 time points post-injection, each with a scan time of 12 seconds. Images were processed using the workstation and the accompanying DCE-MRI post-processing software to obtain the time-signal intensity curves and quantitative perfusion parameters for the region of interest (ROI). The ROI selection avoided areas of necrosis, cystic changes, and large vessels. All image acquisition and analysis strictly followed standard operating procedures to ensure data accuracy and reproducibility (Figure 2).
Serum marker detection: All patients underwent serum marker detection with fasting venous blood samples. Patients were instructed to fast and abstain from drinking for more than 8 hours before blood collection. Early morning, 5 mL of venous blood was collected from the antecubital vein using coagulation tubes. Within 30 minutes of sample collection, centrifugation was completed at 3000 rpm for 10 minutes to separate the serum, which was then tested promptly. The Abbott Anility I fully automated chemiluminescence immunoassay system was used to detect levels of CA19-9 and CA125. All detection reagent kits were original Abbott reagents, and operations were strictly performed according to the instructions. Quality control was conducted using Technopath control reagents to ensure the accuracy and reliability of the detection results. CA19-9 was detected using a two-step immunodetection method with a detection range of 0-1200 U/mL and a reference value of ≤ 37 U/mL; CA125 was detected using a two-step immunodetection method with a detection range of 0-1000 U/mL and a reference value of ≤ 35 U/mL. All tests were completed in our hospital’s laboratory department by professionally trained and qualified personnel. Each batch of tests included high, medium, and low-level control reagents to ensure the accuracy and reproducibility of the results. Samples with abnormal results were retested for verification. All test data were recorded through the laboratory information system and interfaced with the clinical information system to ensure data integrity and traceability. To reduce detection errors, all samples were tested within 3 hours after collection, with strict control of room temperature (20-25 °C) and relative humidity (45%-75%).
Statistical analyses were conducted utilizing statistical product and service solutions version 26.0. For continuous variables, means and standard deviations were calculated, and group differences were assessed using independent samples t-tests. Categorical variables were presented as frequencies and percentages, with group comparisons performed using χ2 tests. This analysis involved determining the area under the curve (AUC), sensitivity, specificity, and Youden index. The Delong test was applied to assess differences in AUC values among various diagnostic indicators. All statistical assessments were conducted as two-tailed tests, with significance set at P < 0.05.
Our analysis (as detailed in Tables 1 and 2) included a total of 126 individuals diagnosed with rectal cancer, categorized into two groups based on T-stage: A low T-stage group comprising 72 patients and a high T-stage group with 54 patients. Comparative assessments between these groups regarding age, gender, body mass index, Karnofsky performance status, smoking habits, alcohol use, and histories of hypertension, diabetes, and familial cancer revealed no significant disparities (all P > 0.05). Further categorization based on the degree of tumor differentiation into a poorly differentiated group (49 patients) and a moderately to well-differentiated group (77 patients) also showed no significant variations in demographic and foundational clinical profiles (all P > 0.05). Additionally, the tumor location across the upper, middle, and lower bowel segments was uniformly distributed without significant variation across the different T-stage and differentiation groups (P > 0.05).
| Group | Early-stage group (n = 72) | Late-stage group (n = 54) | t/χ² | P value |
| Age (year) | 58.25 ± 10.32 | 59.46 ± 11.24 | 0.629 | 0.531 |
| Sex | 0.382 | 0.537 | ||
| Male | 42 (58.33) | 29 (53.70) | ||
| Female | 30 (41.67) | 25 (46.30) | ||
| BMI (kg/m²) | 23.45 ± 3.21 | 23.82 ± 3.15 | 0.643 | 0.521 |
| KPS score | 85.62 ± 7.85 | 84.93 ± 8.12 | 0.483 | 0.630 |
| Smoke | 0.295 | 0.587 | ||
| Yes | 31 (43.06) | 26 (48.15) | ||
| No | 41 (56.94) | 28 (51.85) | ||
| Alcohol | 0.428 | 0.513 | ||
| Yes | 28 (38.89) | 24 (44.44) | ||
| No | 44 (61.11) | 30 (55.56) | ||
| Hypertension | 0.317 | 0.573 | ||
| Yes | 25 (34.72) | 22 (40.74) | ||
| No | 47 (65.28) | 32 (59.26) | ||
| Diabetes | 0.394 | 0.530 | ||
| Yes | 18 (25.00) | 16 (29.63) | ||
| No | 54 (75.00) | 38 (70.37) | ||
| Family history | 0.273 | 0.601 | ||
| Yes | 12 (16.67) | 11 (20.37) | ||
| No | 60 (83.33) | 43 (79.63) | ||
| Tumor location | 0.518 | 0.772 | ||
| Upper section | 24 (33.33) | 16 (29.63) | ||
| Middle section | 28 (38.89) | 24 (44.44) | ||
| Lower section | 20 (27.78) | 14 (25.93) | ||
| Differentiation degree | 1.386 | 0.239 | ||
| Low differentiation | 25 (34.72) | 24 (44.44) | ||
| Medium to high differentiation | 47 (65.28) | 30 (55.56) |
| Group | Early-stage group (n = 49) | Late-stage group (n = 77) | t/χ² | P value |
| Age (year) | 58.92 ± 10.85 | 58.63 ± 10.68 | 0.147 | 0.883 |
| Sex | 0.286 | 0.593 | ||
| Male | 29 (59.18) | 42 (54.55) | ||
| Female | 20 (40.82) | 35 (45.45) | ||
| BMI (kg/m²) | 23.56 ± 3.18 | 23.64 ± 3.20 | 0.138 | 0.891 |
| KPS score | 85.12 ± 8.02 | 85.48 ± 7.92 | 0.252 | 0.801 |
| Smoke | 0.317 | 0.573 | ||
| Yes | 24 (48.98) | 33 (42.86) | ||
| No | 25 (51.02) | 44 (57.14) | ||
| Alcohol | 0.394 | 0.530 | ||
| Yes | 22 (44.90) | 30 (38.96) | ||
| No | 27 (55.10) | 47 (61.04) | ||
| Hypertension | 0.273 | 0.601 | ||
| Yes | 19 (38.78) | 28 (36.36) | ||
| No | 30 (61.22) | 49 (63.64) | ||
| Hypertension | 0.328 | 0.567 | ||
| Yes | 14 (28.57) | 20 (25.97) | ||
| No | 35 (71.43) | 57 (74.03) | ||
| Family history | 0.295 | 0.587 | ||
| Yes | 10 (20.41) | 13 (16.88) | ||
| No | 39 (79.59) | 64 (83.12) | ||
| Tumor location | 0.482 | 0.786 | ||
| Upper section | 15 (30.61) | 25 (32.47) | ||
| Middle section | 21 (42.86) | 31 (40.26) | ||
| Lower section | 13 (26.53) | 21 (27.27) |
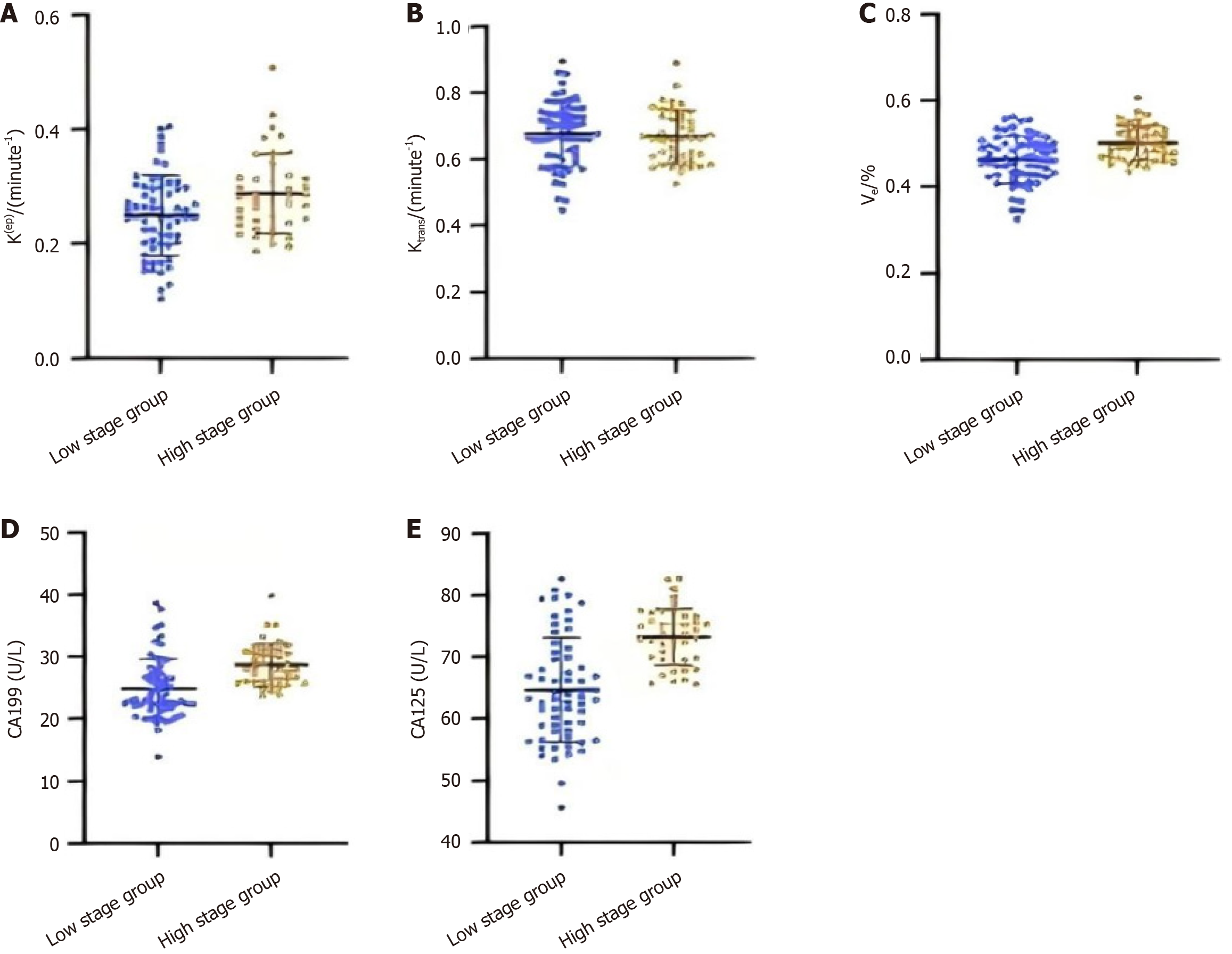
The findings from the study, as depicted in Figure 3, demonstrate that the Ktrans and Ve measurements are markedly elevated in the advanced T-stage cohort compared to the early T-stage cohort (P < 0.05). However, no significant discrepancy was observed in Kep values when comparing the two cohorts (P > 0.05). Concurrently, the serum concentrations of CA19-9 and CA125 were found to be considerably higher in the advanced T-stage group vs the early T-stage group (P < 0.05). These results may indicate a possible relationship between the T stage of rectal cancer and the DCE-MRI parameters (Ktrans and Ve) and serum markers (CA19-9 and CA125).
The findings presented in Figure 4 demonstrate that individuals with poorly differentiated rectal cancer exhibit significantly elevated Ktrans and Ve values compared to those with moderate to well-differentiated tumors (P < 0.05). Kep of the two differentiation groups had no difference (P > 0.05). In addition, serum levels of CA19-9 and CA125 are significantly elevated in the poorly differentiated group compared to moderately to well-differentiated group (P < 0.05). These findings suggest a potential relationship between DCE-MRI parameters and serum tumor markers with differentiation of rectal cancer.
The receiver operating characteristic (ROC) curve analysis shows (Table 3) that for T staging diagnosis, the AUC for Ktrans is 0.835 [95% confidence interval (CI): 0.762-0.908], and the AUC for Ve is 0.812 (95%CI: 0.736-0.888). When these two parameters are combined, the AUC increases to 0.892 (95%CI: 0.832-0.952). For differentiation degree diagnosis, the AUC for Ktrans is 0.821 (95%CI: 0.748-0.894), and the AUC for Ve is 0.803 (95%CI: 0.728-0.878). When combined, the AUC rises to 0.883 (95%CI: 0.821-0.945). These results indicate that DCE-MRI parameters have high diagnostic value in assessing T staging and differentiation degree of rectal cancer, and their combined application can further enhance diagnostic efficacy.
| Evaluation factors | AUC | 95%CI | Threshold value | True positive rate | Selectivity | Yoden |
| T installment | ||||||
| Ktrans | 0.835 | 0.762-0.908 | 0.286 | 79.825 | 75.632 | 0.555 |
| Ve | 0.812 | 0.736-0.888 | 0.375 | 77.234 | 73.521 | 0.508 |
| DCE-MRI parameters | 0.892 | 0.832-0.952 | 0.462 | 85.763 | 81.924 | 0.677 |
| Degree of differentiation | ||||||
| Ktrans | 0.821 | 0.748-0.894 | 0.273 | 78.632 | 74.825 | 0.535 |
| Ve | 0.803 | 0.728-0.878 | 0.362 | 76.523 | 72.934 | 0.495 |
| DCE-MRI parameters | 0.883 | 0.821-0.945 | 0.453 | 84.925 | 80.763 | 0.657 |
The ROC curve analysis shows (Table 4) that for T staging diagnosis, the AUC for CA19-9 and CA125 are 0.812 (95%CI: 0.736-0.888) and 0.798 (95%CI: 0.723-0.873), respectively. When DCE-MRI parameters are combined with tumor markers, the AUC increases to 0.923 (95%CI: 0.865-0.981), with sensitivity and specificity reaching 89.734% and 85.623%, respectively. For differentiation degree diagnosis, the AUC for CA19-9 and CA125 are 0.803 (95%CI: 0.728-0.878) and 0.785 (95%CI: 0.711-0.859), respectively. After combination, the AUC increases to 0.912 (95%CI: 0.855-0.969), with sensitivity and specificity of 88.653% and 84.372%.
| Evaluation factors | AUC | 95%CI | Threshold | True positive rate | Selectivity | Yoden |
| T installment | ||||||
| CA19-9 | 0.812 | 0.736-0.888 | 37.253 | 78.523 | 73.465 | 0.520 |
| CA125 | 0.798 | 0.723-0.873 | 32.864 | 76.352 | 71.923 | 0.483 |
| DCE-MRI indicators and cancer markers | 0.923 | 0.865-0.981 | 0.526 | 89.734 | 85.623 | 0.754 |
| Degree of differentiation | ||||||
| CA19-9 | 0.803 | 0.728-0.878 | 35.921 | 77.462 | 72.845 | 0.503 |
| CA125 | 0.785 | 0.711-0.859 | 31.532 | 75.283 | 70.942 | 0.462 |
| DCE-MRI parameters + tumor markers | 0.912 | 0.855-0.969 | 0.512 | 88.653 | 84.372 | 0.730 |
The results of the DeLong test (Table 5) indicate that there is no statistically significant difference in diagnostic efficacy between CA19-9 and CA125 (P > 0.05). However, the diagnostic efficacy of the combined DCE-MRI parameters and tumor markers is significantly superior to that of using CA19-9 or CA125 alone (both P < 0.05). This suggests that the combined application of DCE-MRI parameters and serum tumor markers can significantly enhance the diagnostic accuracy for T staging and differentiation degree of rectal cancer.
| Inspection results | Z-score | Significance level | AUC | Mean error | 95%CI |
| T installment | |||||
| CA19-9 and CA125 serum biomarkers | 0.862 | 0.389 | 0.014 | 0.016 | -0.018 to 0.046 |
| CA19-9 alongside DCE-MRI measurements and cancer markers | 4.253 | < 0.05 | 0.111 | 0.026 | 0.060 to 0.162 |
| CA125 in conjunction with DCE-MRI parameters and tumor markers | 4.682 | < 0.05 | 0.125 | 0.027 | 0.072 to 0.178 |
| Degree of differentiation | |||||
| CA19-9 and CA125 serum biomarkers | 0.923 | 0.356 | 0.018 | 0.020 | -0.021 to 0.057 |
| CA19-9 alongside DCE-MRI measurements and cancer markers | 4.132 | < 0.05 | 0.109 | 0.026 | 0.058 to 0.160 |
| CA125 in conjunction with DCE-MRI parameters and tumor markers | 4.521 | < 0.05 | 0.127 | 0.028 | 0.072 to 0.182 |
This study analyzed the expression characteristics and diagnostic value of DCE-MRI functional parameters and serum tumor markers in rectal cancer patients. The findings indicate that the combined application of these two diagnostic methods significantly improves the accuracy of assessing T staging and differentiation degree in rectal cancer, providing new insights for enhancing the preoperative evaluation system.
Firstly, the study found that the Ktrans and Ve values in the high-stage group were significantly higher than those in the low-stage group, which is consistent with previous research[13]. Klaassen et al[14] demonstrated that Ktrans is positively correlated with tumor angiogenesis and microvascular density; as tumor staging increases, the formation of new blood vessels also increases, leading to elevated Ktrans values[15]. The Ve value reflects the volume fraction of the extracellular space, and its increase suggests a greater tumor stromal component and more severe tissue structural disorganization, closely related to increased tumor invasion depth. However, the Kep values did not show statistically significant differences between different T staging groups, which may be related to tumor tissue heterogeneity and sample size limitations, necessitating further validation with a larger sample size.
Secondly, regarding differentiation assessment, the study found that the Ktrans and Ve values in the poorly differentiated group were significantly higher than those in the moderately to well-differentiated group, aligning with the biological behavior characteristics of tumors. Poorly differentiated tumors often exhibit greater invasiveness and enhanced angiogenic capacity, resulting in increased microvascular permeability and expanded interstitial spaces, thus presenting higher Ktrans and Ve values[16]. This finding provides important evidence for the use of DCE-MRI parameters in assessing the malignancy of rectal cancer. Notably, the Kep values also did not show significant differences between different differentiation groups, which may be related to the biological significance of the parameter itself and limitations in measurement methods.
In terms of serum tumor markers, the study found that levels of CA19-9 and CA125 increased with higher T staging and lower differentiation degree, consistent with the theory of increased tumor burden and worsening malignancy[17,18]. However, the diagnostic efficacy of individual tumor markers is relatively limited, which may be attributed to several factors: (1) Serum marker levels can be influenced by various non-tumor factors, such as inflammation and autoimmune diseases; (2) Some early-stage or well-differentiated tumors may present normal marker levels; and (3) There is also individual variability in marker expression[19,20].
ROC curve analysis showed that the combined application of DCE-MRI parameters (Ktrans, Ve) demonstrated high diagnostic efficacy in assessing T staging and differentiation degree, with AUCs reaching 0.892 and 0.883, respectively. The advantage of this combined application may stem from the fact that different parameters reflect various aspects of the tumor microenvironment: Ktrans primarily reflects vascular permeability, while Ve reflects extracellular space characteristics. The combination of both provides a more comprehensive assessment of the tumor’s biological behavior[21].
Notably, when DCE-MRI parameters are combined with serum tumor markers, the diagnostic efficacy is further enhanced, with AUCs reaching 0.923 and 0.912 for T staging and differentiation degree assessments, respectively. This significant improvement may be attributed to several factors: (1) DCE-MRI provides functional information about the local tumor microenvironment, while serum markers reflect the overall metabolic state of the tumor; (2) The complementary advantages of both methods can reduce the limitations of using a single diagnostic approach; and (3) The combined application offers multidimensional assessment metrics, which help improve diagnostic accuracy and reliability[22].
The results of the DeLong test further validate the superiority of the combined diagnostic model, showing that the joint application of DCE-MRI parameters and tumor markers has a significant advantage over the use of either method alone. This suggests that clinical practice should consider adopting a multiparameter evaluation strategy to obtain more accurate preoperative staging and differentiation information[23].
The innovations of this study are reflected in several aspects: (1) It is the first systematic evaluation of the combined application of DCE-MRI functional parameters and serum tumor markers in the preoperative assessment of rectal cancer; (2) It establishes a multiparameter-based diagnostic model that provides a more reliable basis for clinical decision-making; and (3) It quantifies the diagnostic efficacy of different assessment indicators, aiding in the optimization of examination protocols. However, this study does have some limitations: (1) As a single-center retrospective study, it may be subject to selection bias; (2) The sample size is relatively limited, necessitating validation through larger-scale multicenter studies; (3) Additionally, the reproducibility of DCE-MRI parameter measurements was not assessed; and (4) There is a lack of long-term follow-up data on patient prognosis.
The study demonstrates that integrating different diagnostic approaches, such as DCE-MRI functional parameters and serum tumor markers, has practical significance in clinical settings. This integrated approach can significantly improve the accuracy of T-staging and differentiation grading assessment for rectal cancer patients, thereby providing new insights for developing personalized treatment strategies[24-26]. Furthermore, this combined diagnostic method also helps to enhance the prognostic evaluation system for patients, offering valuable information to strengthen the preoperative assessment system.
Based on the findings of this study, we propose the following recommendations: (1) Where feasible, it is advisable to combine DCE-MRI functional imaging with serum tumor marker testing to enhance the accuracy of preoperative assessments; (2) Further exploration of standardized image post-processing and analysis workflows should be conducted to improve the reproducibility of parameter measurements; (3) Prospective studies should be initiated to evaluate the prognostic value of the combined diagnostic model; and (4) New technologies, such as artificial intelligence, should be explored for integration into multiparameter analyses to enhance diagnostic efficiency.
This study has made some progress in exploring the application of artificial intelligence and machine learning in medical diagnostics, but there are still some limitations. First, as a single-center retrospective study, there may be selection bias, which could affect the universality and scalability of the results. Secondly, the sample size is relatively limited, and larger-scale multicenter studies are needed to verify these findings. Additionally, the reproducibility of DCE-MRI parameter measurements was not assessed, and there is a lack of long-term follow-up data on patient prognosis.
Despite these limitations, this study provides new insights into the preoperative assessment of rectal cancer and confirms the significant value of combining DCE-MRI functional parameters and serum tumor markers in improving the accuracy of T-staging and differentiation grading assessment. Future research should focus on larger-scale multicenter studies to further validate the clinical applicability of this diagnostic model. Meanwhile, efforts should be made to explore the integration of new technologies, such as artificial intelligence, into multiparametric analysis to improve diagnostic efficiency.
In conclusion, this study confirms the significant value of combining DCE-MRI functional parameters with serum tumor markers in the preoperative assessment of T staging and differentiation degree in rectal cancer. This finding provides new insights and evidence for optimizing rectal cancer diagnosis and treatment strategies. Future research should focus on larger-scale multicenter studies to further validate the clinical applicability of this diagnostic model.
| 1. | Leeds IL, Coppersmith NA, Moore MS, Saleh A, Cruickshank K, Pantel H, Reddy V, Mongiu AK. By Any Other Name: Bowel Dysfunction After Proctectomy for Cancer and Its Predictive Factors in Administrative Databases. J Surg Res. 2024;303:342-351. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Yang L, Jinchang L, Wanjian G. Should we Continue to Monitor or Choose to Ignore the Mild Increase in CA19-9 after Surgery. Clin Lab. 2024;70. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Fonnegra RD, Mera C, Díaz GM, Hernández L. LA-Breast: A Latin American multiparametric breast DCE-MRI dataset with benign and malignant annotations. Data Brief. 2024;57:110995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Lim C, Lee H, Moon Y, Han SH, Kim HJ, Chung HW, Moon WJ. Volume and Permeability of White Matter Hyperintensity on Cognition: A DCE Imaging Study of an Older Cohort With and Without Cognitive Impairment. J Magn Reson Imaging. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Sui C, Nie Z, Xie X, Wang Y, Kong L, Ni SQ, Zhan J. Mn/S diatomic sites in C(3)N(4) to enhance O(2) activation for photocatalytic elimination of emerging pollutants. J Environ Sci (China). 2025;149:512-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Tepe M, Sevin E, Inan I, Aktan A, Ayaz M, Ibrahim Ali H, Senturk S. Utilizing CT and MRI in Assessing Peritumoral Neovascularization in Renal Cell Carcinoma: A Comprehensive Analysis of Histological Subtypes and Tumor Characteristics by Imaging. Curr Med Imaging. 2024;20:e15734056303423. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Zhang J, Zheng Y, Li L, Wang R, Jiang W, Ai K, Gan T, Wang P. Combination of IVIM with DCE-MRI for diagnostic and prognostic evaluation of breast cancer. Magn Reson Imaging. 2024;113:110204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Zhang B, Kapur P, Koduru PR, Jia L. Retroperitoneal Sarcomatoid Yolk Sac Tumor in a Chemotherapy-Naive Patient With Testicular Postpubertal Type Teratoma: A Rare Case Report With Emphasis on Molecular Features. Int J Surg Pathol. 2024;32:1537-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 9. | Ma S, Lu H, Jing G, Li Z, Zhang Q, Ma X, Chen F, Shao C, Lu Y, Wang H, Shen F. Deep learning-based clinical-radiomics nomogram for preoperative prediction of lymph node metastasis in patients with rectal cancer: a two-center study. Front Med (Lausanne). 2023;10:1276672. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Zheng Z, Wang X, Huang Y, Lu X, Chi P. Predictive value of changes in the level of carbohydrate antigen 19-9 in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Colorectal Dis. 2020;22:2068-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Shi J, Chen L, Wang B, Zhang H, Xu L, Ye J, Liu Y, Shao Y, Sun X, Zou Y. The value of ultrasound elastography combined with multi-parameters in the diagnosis of BI-RADS 4 breast lesions. Technol Health Care. 2022;30:1077-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Făgărășan V, Bințințan V, Seicean R, Caziuc A, AIlioaie R, Făgărășan G, Ilie-Ene A, Dindelegan G, Căinap C. Lymphocyte-to-monocyte, platelet-to-albumin and platelet-to-lymphocyte ratios as prognostic biomarkers for neoadjuvant treatment response in rectal cancer patients. Surg Oncol. 2024;56:102126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 13. | Zhou M, Huang H, Fan Y, Chen M, Wang Y, Gao F. Golden-angle radial sparse parallel magnetic resonance imaging of rectal perfusion: utility in the diagnosis of poorly differentiated rectal cancer. Quant Imaging Med Surg. 2023;13:4826-4838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Klaassen L, Jaarsma-Coes MG, Marinkovic M, Luyten GPM, Rasch CRN, Ferreira TA, Beenakker JM. Quantitative Perfusion-Weighted Magnetic Resonance Imaging in Uveal Melanoma. Invest Ophthalmol Vis Sci. 2024;65:17. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Shen H, Liu M, Zhou H, Li Y, Guo Y, Yin Y, Zhang F, Wang J. Differential expression and significance of cytokines in cerebrospinal fluid of patients with viral encephalitis. Neuroscience. 2024;561:11-19. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Cherbanyk F, Burgard M, Widmer L, Pugin F, Egger B. Risk factors for local recurrence of rectal cancer after curative surgery: A single-center retrospective study. J Visc Surg. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Pitoia F, Trimboli P, Abelleira E. Primary resistance to selpercatinib in a patient with advanced medullary thyroid cancer. Endocrine. 2024;86:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 18. | Sharma M, Devi P, Kaushal S, Ul-Ahsan A, Mehra S, Budhwar M, Chopra M. Cyto and Genoprotective Potential of Tannic Acid Against Cadmium and Nickel Co-exposure Induced Hepato-Renal Toxicity in BALB/c Mice. Biol Trace Elem Res. 2024;202:5624-5636. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Sun M, Yang Y, Zhao J. Establishment of a novel survival assessment and prediction model for advanced gastric cancer patients receiving immunotherapy. Oncol Lett. 2023;26:451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Yang X, Xu S, Qian Y, Xiao Q. Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain Behav Immun. 2017;64:162-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 308] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 21. | Mooshage CM, Tsilingiris D, Schimpfle L, Kender Z, Aziz-Safaie T, Hohmann A, Szendroedi J, Nawroth P, Sturm V, Heiland S, Bendszus M, Kopf S, Kurz FT, Jende JME. Insulin Resistance Is Associated With Reduced Capillary Permeability of Thigh Muscles in Patients With Type 2 Diabetes. J Clin Endocrinol Metab. 2023;109:e137-e144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 22. | Kataoka M. Ultrafast DCE-MRI as a new tool for treatment response prediction in neoadjuvant chemotherapy of breast cancer. Diagn Interv Imaging. 2023;104:565-566. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Ziegenfeuter J, Delbridge C, Bernhardt D, Gempt J, Schmidt-Graf F, Griessmair M, Thomas M, Meyer HS, Zimmer C, Meyer B, Combs SE, Yakushev I, Wiestler B, Metz MC. Sequential and Hybrid PET/MRI Acquisition in Follow-Up Examination of Glioblastoma Show Similar Diagnostic Performance. Cancers (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 24. | Arian A, Taher HJ, Suhail Najm Alareer H, Aghili M. Value of Conventional MRI, DCE-MRI, and DWI-MRI in the Discrimination of Metastatic from Non-Metastatic Lymph Nodes in Rectal Cancer: A Systematic Review and Meta-Analysis Study. Asian Pac J Cancer Prev. 2023;24:401-410. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Zheng J, Du PZ, Yang C, Tao YY, Li L, Li ZM, Yang L. DCE-MRI-based radiomics in predicting angiopoietin-2 expression in hepatocellular carcinoma. Abdom Radiol (NY). 2023;48:3343-3352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 26. | Zong R, Ma X, Shi Y, Geng L. The assessment of pathological response to neoadjuvant chemotherapy in muscle-invasive bladder cancer patients with DCE-MRI and DWI: a systematic review and meta-analysis. Br J Radiol. 2023;96:20230239. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |