Published online Jun 16, 2025. doi: 10.4253/wjge.v17.i6.107142
Revised: April 7, 2025
Accepted: May 8, 2025
Published online: June 16, 2025
Processing time: 87 Days and 3.4 Hours
Non variceal upper gastrointestinal bleeding (NVUGIB) is a life-threatening condition requiring prompt and effective hemostasis. Various endoscopic inter
To evaluate the efficacy and safety of novel hemostatic interventions compared to conventional endoscopic techniques for managing UGIB.
Cochrane, MEDLINE, PubMed and Scopus libraries were searched for rando
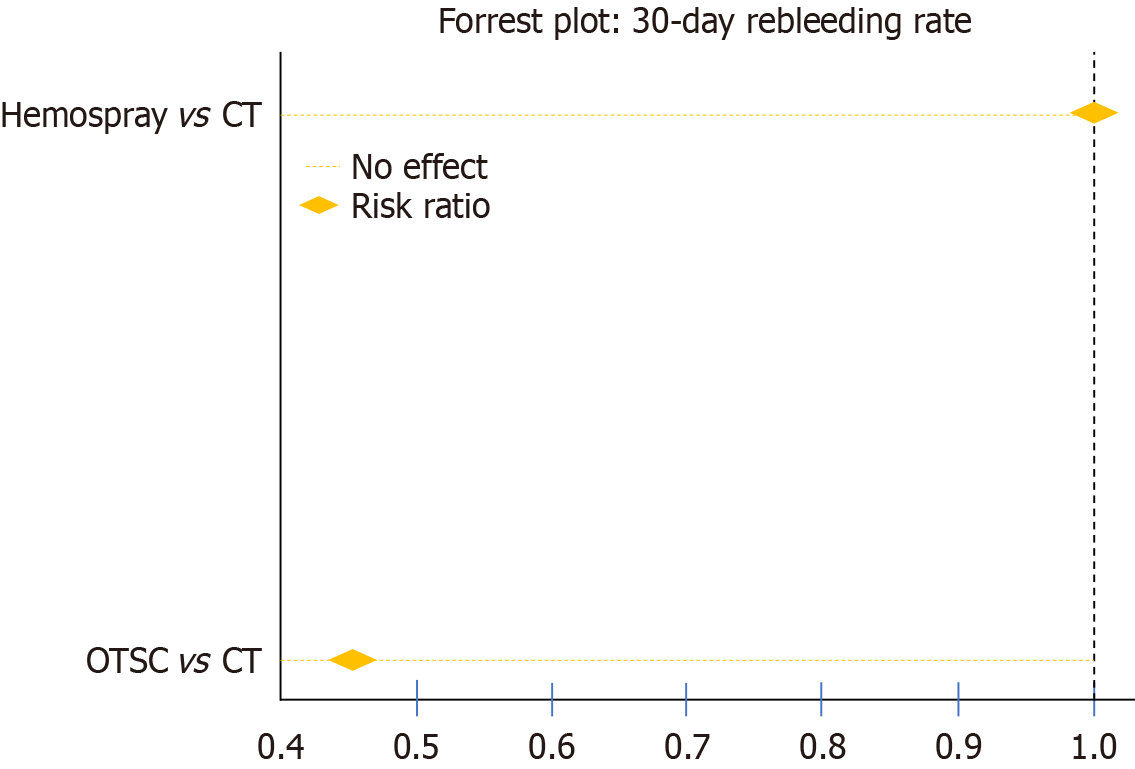
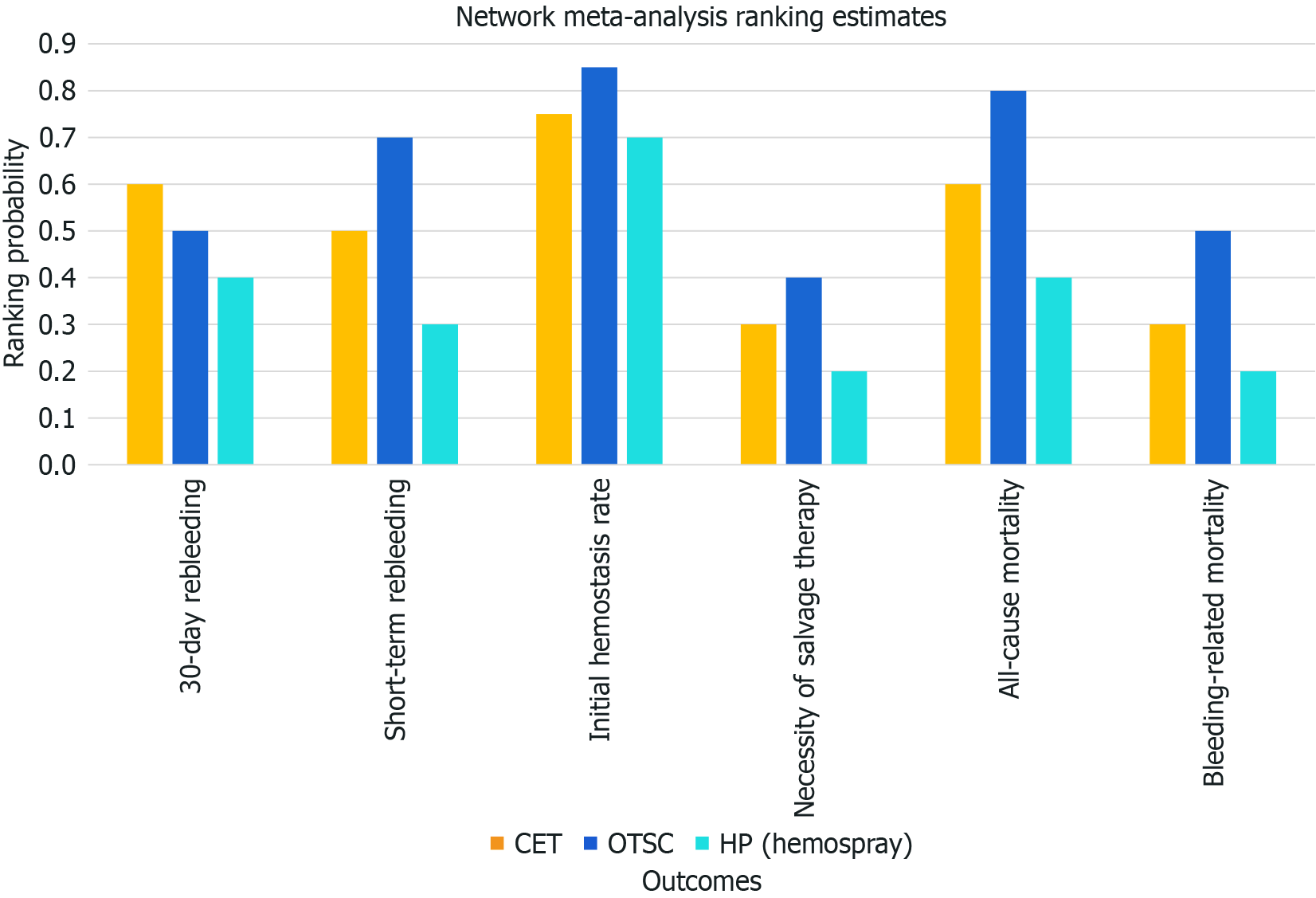
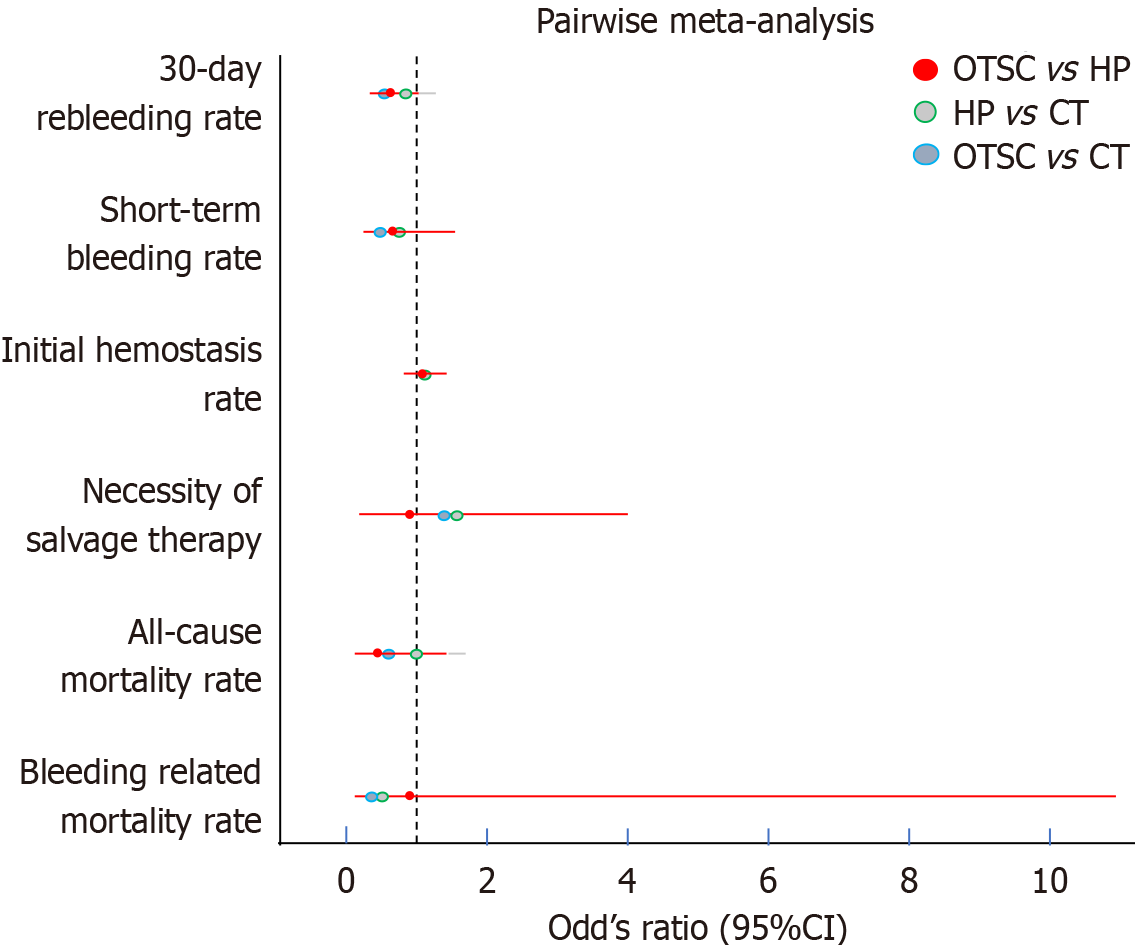
Seventeen studies were included in this analysis. Regarding the 30-day rebleeding rate, OTSC and HP showed superior efficacy compared with CT [OTSC vs CT: Relative risk (RR): 0.47, 95% confidence interval (CI): 0.33-0.65; HP vs CT: RR: 0.73, 95%CI: 0.45-1.13], while OTSC and HP had comparable efficacy (RR: 0.56, 95%CI: 0.30-1.05). OTSC ranked the highest in the network ranking estimate for this outcome. For the secondary outcomes, OTSC demonstrated superior efficacy for the short-term rebleeding rate (OTSC vs CT: RR: 0.35, 95%CI: 0.14-0.74; HP vs CT: RR: 0.62, 95%CI: 0.28-1.35; OTSC vs HP: RR: 0.59, 95%CI: 0.17-1.67). Regarding the initial hemostasis rate, OTSC was slightly more effective than CT (OTSC vs CT: RR: 1.20, 95%CI: 1.06-1.57) and comparable to HP (OTSC vs HP: RR: 1.08, 95%CI: 0.89-1.40). There were no significant differences among treatments for all-cause mortality, bleeding-related mortality, or the necessity of surgical or angiographic salvage therapy. OTSC consistently ranked highest across most outcomes in the network ranking estimate.
This meta-analysis highlights OTSC as the most effective intervention for reducing 30-day and short-term rebleeding rates in NVUGIB, surpassing both CT and HP, supporting OTSC as a preferred first-line treatment for NVUGIB, while HP and CT remain viable alternatives. Further studies are needed to explore long-term outcomes and cost-effectiveness.
Core Tip: This systematic review and network meta-analysis compares the efficacy of novel hemostatic interventions-over-the-scope clips (OTSC) and hemostatic powders (HP)-to conventional endoscopic therapy computed tomography (CT) for non variceal upper gastrointestinal bleeding (NVUGIB). OTSC demonstrated superior efficacy in reducing 30-day and short-term rebleeding rates, ranking as the most effective intervention. HP provided a moderate benefit but did not surpass CT. These findings support OTSC as a first-line treatment for high-risk NVUGIB, while HP remains a viable adjunct.
- Citation: Duggal S, Kalra I, Kalra K, Bhagat V. Advancing hemostasis: A meta-analysis of novel vs conventional endoscopic therapies for non variceal upper gastrointestinal bleeding. World J Gastrointest Endosc 2025; 17(6): 107142
- URL: https://www.wjgnet.com/1948-5190/full/v17/i6/107142.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i6.107142
Upper gastrointestinal bleeding (UGIB) is a significant clinical concern, with incidence rates estimated to range between 50 and 100 cases per 100000 individuals annually[1]. Acute non variceal UGIB (NVUGIB) represents a prevalent reason for hospital admissions in the United States, with a reported incidence of 66.7 cases per 100000 individuals[2]. The mortality rate associated with UGIB varies between 2% and 14%[3]. Common causes of NVUGIB include peptic ulcer disease, gastroduodenal erosions, Mallory-Weiss tears, and esophagitis, which collectively account for over 80% of cases. Less common etiologies include Cameron ulcers, upper gastrointestinal malignancies, angiodysplasia, polyps, gastric antral vascular ectasia, and Dieulafoy’s lesions[4].
Current management strategies for severe NVUGIB primarily rely on endoscopic interventions guided by the identification of stigmata of recent hemorrhage[5]. Standard therapeutic modalities include thermocoagulation techniques such as argon plasma coagulation (APC) and multipolar electrocautery, as well as through-the-scope (TTS) hemoclips, either as standalone treatments or in combination with epinephrine injection[6]. Over the past decade, novel therapeutic techniques have emerged, offering promising avenues to enhance hemostatic efficacy and improve clinical outcomes and reduce rebleeding rates. Over-the-scope clips (OTSC) and hemostatic powders (HP), including TC-325 (Hemospray, Cook Medical, Winston-Salem, NC), which can be directly applied to active bleeding sites, represent some of the latest advancements in the field. Established endoscopic interventions, including metallic clips, adrenaline injections, and thermal probes, continue to be widely utilized for the management of bleeding related to peptic ulcer disease[7]. Additionally, APC remains a key treatment modality for hemorrhage secondary to malignancies and diffuse vascular lesions[8].
This systematic review aims to provide a comprehensive comparison between conventional and emerging non-conventional hemostatic interventions for NVUGIB. We aim to critically assess the scientific evidence supporting these strategies, focusing on their effectiveness in achieving definitive hemostasis, defined as the successful control of active bleeding and prevention of rebleeding in high-risk patients.
The present systematic review and network meta-analysis was conducted according to the PRISMA guidelines and the PRISMA Network Meta-analyses Extension Statement. A literature search was conducted on Cochrane, MEDLINE, PubMed and Scopus libraries for all articles published up to October 2024. Only English language articles were included. The complete search strategies for each database are summarized in Supplementary Table 1.
The Patient, Intervention, Comparison, Outcome principle was employed for study inclusion. The inclusion criteria were as follows: (1) Patient: Adult patients (age ≥ 18 years) with NVUGIB; (2) Intervention: OTSC or Padlock, HP [including TC-325 (Hemospray, Cook Medical, Winston-Salem, NC, United States), Ankaferd Blood Stopper (Ankaferd Health Products, Istanbul, Turkey), CEGP-003 [CGBio, Seongnam, South Korea], UI-EWD (NexPowder; Next Biomedical, Incheon, South Korea), and EndoClot (Vitramed, Sydney, Australia and Kuala Lumpur, Malaysia)] with or without other conventional endoscopic therapeutic computed tomography (CT) modalities; (3) Comparison: OTSC or Padlock, and HP compared with each other or with CT (CT was defined as hemostasis with heater probe, bipolar electrocoagulation, hemostatic grasper, APC, radiofrequency ablation, through- the-scope clip, diluted epinephrine injection therapy, and the combination therapy of the aforementioned methods); and (4) Outcome: 30-day rebleeding rate.
Exclusion criteria were: (1) Noncomparative studies or comparative studies in the same category (i.e., intragroup comparison among different types of HP or between OTSC and padlock); (2) Non-English-language articles; (3) Insufficient information for the primary outcome; (4) Prospective studies, reviews, meta-analyses, letters, case reports or case series, editorials, comments, or animal studies; and (5) Studies including less than 10 patients in each arm. Three authors independently identified articles that were eligible for inclusion. Any disagreement was resolved by consensus.
The primary outcome was the 30-day rebleeding rate. The secondary outcomes included short-term rebleeding rate, initial hemostasis rate, the necessity of surgical or angiographic salvage therapy, all-cause mortality rate, and the bleeding-related mortality.
The 30-day rebleeding rate was defined as the presence of hypotension (systolic blood pressure < 90 mmHg), shock, tachycardia (> 110 beats per minute), melena, and hematemesis along with hemoglobin ≤ 90 g or a decrease in hemoglobin of ≥ 20 g from baseline within 30 days after primary successful endoscopic hemostasis.
Short-term rebleeding was defined as recurrent bleeding within 7 days after successful endoscopic hemostasis. The initial hemostasis rate was defined as the absence of continuous bleeding after endoscopic therapy. The necessity of surgical or angiographic salvage therapy was defined as continuous bleeding after endoscopic therapy that required to be treated by surgical or angiographic salvage therapy. All-cause mortality was defined as death from any cause within 30 days after endoscopic hemostasis. And the bleeding-related mortality was defined as death from hemorrhage within 30 days after endoscopic therapy.
The following information was extracted from each study in a standardized form by three authors independently: First author, year of publication, country or region, study design and study period, sample size, patients’ demographics, etiologies for bleeding and related outcomes. Studies were excluded if the data was insufficient. The quality of the studies was evaluated by three authors independently. All randomized controlled trials (RCTs) were assessed using the Cochrane Risk of Bias tool.
The effect size was summarized with traditional pairwise meta-analysis and network meta-analysis. Relative risk (RR) and 95% confidence interval (CI) were used to depict the summary effect size. Statistical heterogeneity was assessed using the I2 measure, which ranges from 0% to 100%, and values of 25%-49%, 50%-74%, and ≥ 75% represented low, moderate, and high heterogeneity, respectively.
Pairwise meta-analyses and the network meta-analysis were con- ducted using RevMan version 5.4 (The Nordic Cochrane Centre, Copenhagen, Denmark) and R v.4.1.3 (gemtc 1.0-1; R Foundation, Vienna, Austria). Data comparing the three endoscopic hemostasis methods were pooled using a random-effects model within a Bayesian framework. The CT was used as the reference in the network meta- analysis models since it is the most used control and represents the standard treatment for now. Inconsistency was defined as the disagreement between direct and indirect evidence. Global inconsistency and local inconsistency were evaluated using a design by treatment interaction model and intervention group-splitting methods. The network plots were used to explore the geometry of the network meta-analysis. The extent of the circle indicated the sample size of each modality, and the thickness of the line indicated the number of studies included in each comparison. A P value of less than 0.05 was regarded as statistically significant.
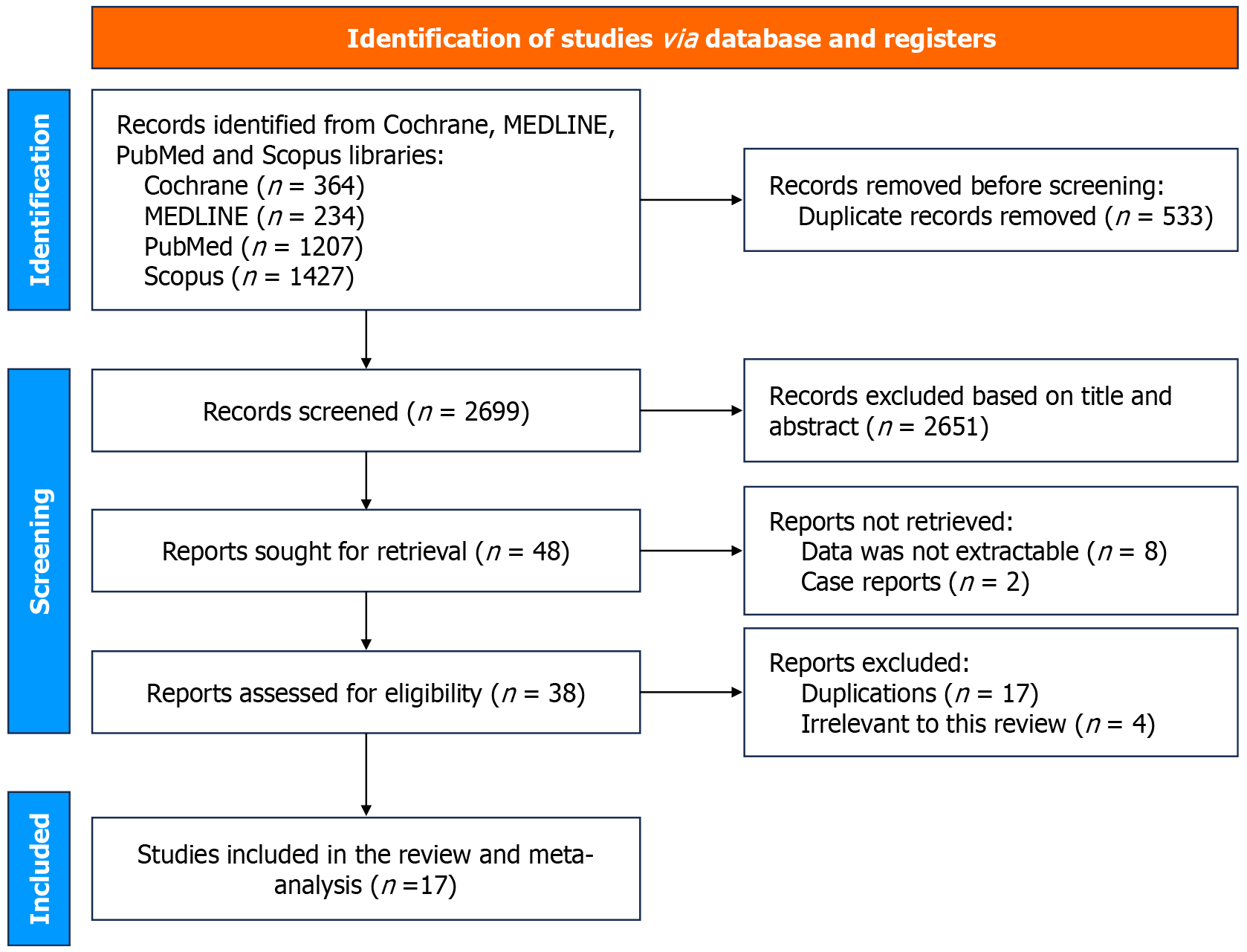
The flow diagram of study selection for the systematic review and network meta-analysis is shown in Figure 1. A total of 3232 were identified from primary literature search, out of which 533 duplicate articles were removed, leaving 2699 articles. After screening titles and abstracts, 2651 studies were excluded because they were irrelevant to the current analysis. 48 relevant articles were retrieved for further review, out of which 31 were excluded based on publication type: Case reports (n = 2), data not extractable (n = 8), analysis irrelevant to this review (n = 4), duplications (n = 17). Finally, 17 full text articles were included for the systemic review and meta-analysis including 1416 patients, all included studies were RCTs only. 11 studies compared HP with conventional therapy, in which 6 studies used TC-325 (Hemospray; Cook Medical), 2 studies used CEGP-003, 1 study used polysaccharide HP, 1 study used Hemostasis powder® (Iran) and another study used Hemostatic pad (ES-H-01). 6 studies compared OTSC with CT. Etiologies for bleeding reported included peptic ulcer disease, Mallory–Weiss tears, Dieulafoy lesion, and neoplasm, etc. The identified studies and their characteristics are summarized in Supplementary Table 2.
The randomization process was associated with some concerns in RCT4 and RCT14 due to incomplete reporting of allocation concealment. Deviations from intended interventions were noted in RCT2 and RCT12, raising concerns regarding potential protocol deviations. Most studies exhibited a low risk of bias concerning missing outcome data, except for RCT8, where missing data could have influenced the results. The measurement of outcomes was consistently rated as low risk across all studies. No significant issues were identified in the selection of reported results, as all outcomes were reported in accordance with the study protocols (Table 1).
| Study ID | Randomization process | Deviations from intended interventions | Missing outcome data | Measurement of outcomes | Selection of reported results | Overall risk of bias |
| RCT1 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| RCT2 | Low risk | Some concerns | Low risk | Low risk | Low risk | Some concerns |
| RCT3 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| RCT4 | Some concerns | Low risk | Low risk | Low risk | Low risk | Some concerns |
| RCT5 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| RCT6 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| RCT7 | Some concerns | Low risk | Low risk | Low risk | Low risk | Some concerns |
| RCT8 | Low risk | Low risk | Some concerns | Low risk | Low risk | Some concerns |
| RCT9 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| RCT10 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| RCT11 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| RCT12 | Low risk | Some concerns | Low risk | Low risk | Low risk | Some concerns |
| RCT13 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| RCT14 | Some concerns | Low risk | Low risk | Low risk | Low risk | Some concerns |
| RCT15 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| RCT16 | Low Risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| RCT17 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
Seventeen studies were included in this analysis. The forest plot (Figure 2) presents the 30-day rebleeding rates, com
Table 2 provides a detailed summary of the findings across various clinical outcomes, including short-term and 30-day rebleeding rates, initial hemostasis rate, necessity for salvage therapy, and mortality rates. OTSC demonstrated superior efficacy for the short-term rebleeding rate (OTSC vs CT: RR: 0.35, 95%CI: 0.14-0.74; HP vs CT: RR: 0.62, 95%CI: 0.28-1.35; OTSC vs HP: RR: 0.59, 95%CI: 0.17-1.67). In terms of initial hemostasis, OTSC showed an advantage with a network estimate of 1.20 (95%CI: 1.06-1.57), while HP had a risk ratio of 1.13 (95%CI: 0.99-1.35). There were no significant differences among treatments for all-cause mortality, bleeding-related mortality, or the necessity of surgical or angiographic salvage therapy. OTSC consistently ranked highest across most outcomes in the network ranking estimate (Figure 4).
| Outcome | Number of studies | Pairwise meta-analysis (OTSC vs CT) | Pairwise meta-analysis (HP vs CT) | Pairwise meta-analysis (OTSC vs HP) | I² (%) | Network meta-analysis (OTSC vs CT) | Network meta-analysis (HP vs CT) | Network meta-analysis (OTSC vs HP) |
| 30-day rebleeding rate | 8 | 0.47 (0.33-0.65) | 0.73 (0.45-1.13) | 0.56 (0.30-1.05) | 0.0 | 0.41 (0.25-0.63) | 0.73 (0.45-1.13) | 0.56 (0.30-1.05) |
| Short-term rebleeding rate | 8 | 0.43 (0.23-0.80) | 0.62 (0.28-1.35) | 0.59 (0.17-1.67) | 0.0 | 0.35 (0.14-0.74) | 0.62 (0.28-1.35) | 0.59 (0.17-1.67) |
| Initial hemostasis rate | 8 | 1.09 (0.99-1.20) | 1.13 (0.99-1.35) | 1.08 (0.89-1.40) | 0.0 | 1.20 (1.06-1.57) | 1.13 (0.99-1.35) | 1.08 (0.89-1.40) |
| Necessity of salvage therapy | 8 | 1.31 (0.66-2.60) | 1.41 (0.50-3.95) | 0.95 (0.24-3.98) | 0.0 | 1.34 (0.55-3.53) | 1.41 (0.50-3.95) | 0.95 (0.24-3.98) |
| All-cause mortality rate | 8 | 0.61 (0.35-1.06) | 0.99 (0.51-1.77) | 0.55 (0.22-1.45) | 0.0 | 0.54 (0.27-1.06) | 0.99 (0.51-1.77) | 0.55 (0.22-1.45) |
| Bleeding-related mortality rate | 8 | 0.37 (0.10-1.34) | 0.44 (0.10-1.92) | 0.90 (0.07-10.89) | 0.0 | 0.32 (0.05-1.45) | 0.44 (0.10-1.92) | 0.90 (0.07-10.89) |
The analysis of treatment modalities for NVUGIB revealed notable differences across various outcomes. Regarding the 30-day rebleeding rate, OTSC demonstrated superior effectiveness compared to CT, with a RR of 0.44, whereas HP showed a slightly higher risk compared to CT (RR = 1.02), favoring CT over HP. When comparing HP to OTSC, OTSC emerged as the more effective option with an indirect estimated risk ratio of 2.31 (Figure 5). In the short-term rebleeding rate analysis, OTSC again outperformed CT with an RR of 0.54, indicating significant effectiveness, while HP provided a moderate reduction in short-term rebleeding compared to CT (RR = 0.90). The comparative analysis between HP and OTSC indicated OTSC's superior performance with an RR of 1.68.
In terms of initial hemostasis, both OTSC and HP showed improvement over CT, with HP demonstrating a slight advantage (RR = 1.18) compared to OTSC (RR = 1.17), suggesting nearly equivalent effectiveness. The necessity of salvage therapy differed across treatments, with HP reducing the need compared to CT (RR = 0.65), while OTSC was associated with an increased need for salvage therapy (RR = 1.29). Comparing HP to OTSC, the former significantly reduced the necessity for salvage therapy (RR = 0.50).
Regarding all-cause mortality, OTSC proved to be the most effective intervention, significantly reducing mortality compared to CT (RR = 0.59), whereas HP was associated with a higher mortality risk (RR = 1.76), favoring CT over HP. A similar trend was observed for bleeding-related mortality, where CT significantly reduced mortality compared to HP (RR = 2.26), and OTSC showed no observed bleeding-related mortality events in the dataset, indicating it as the most favorable treatment in this domain.
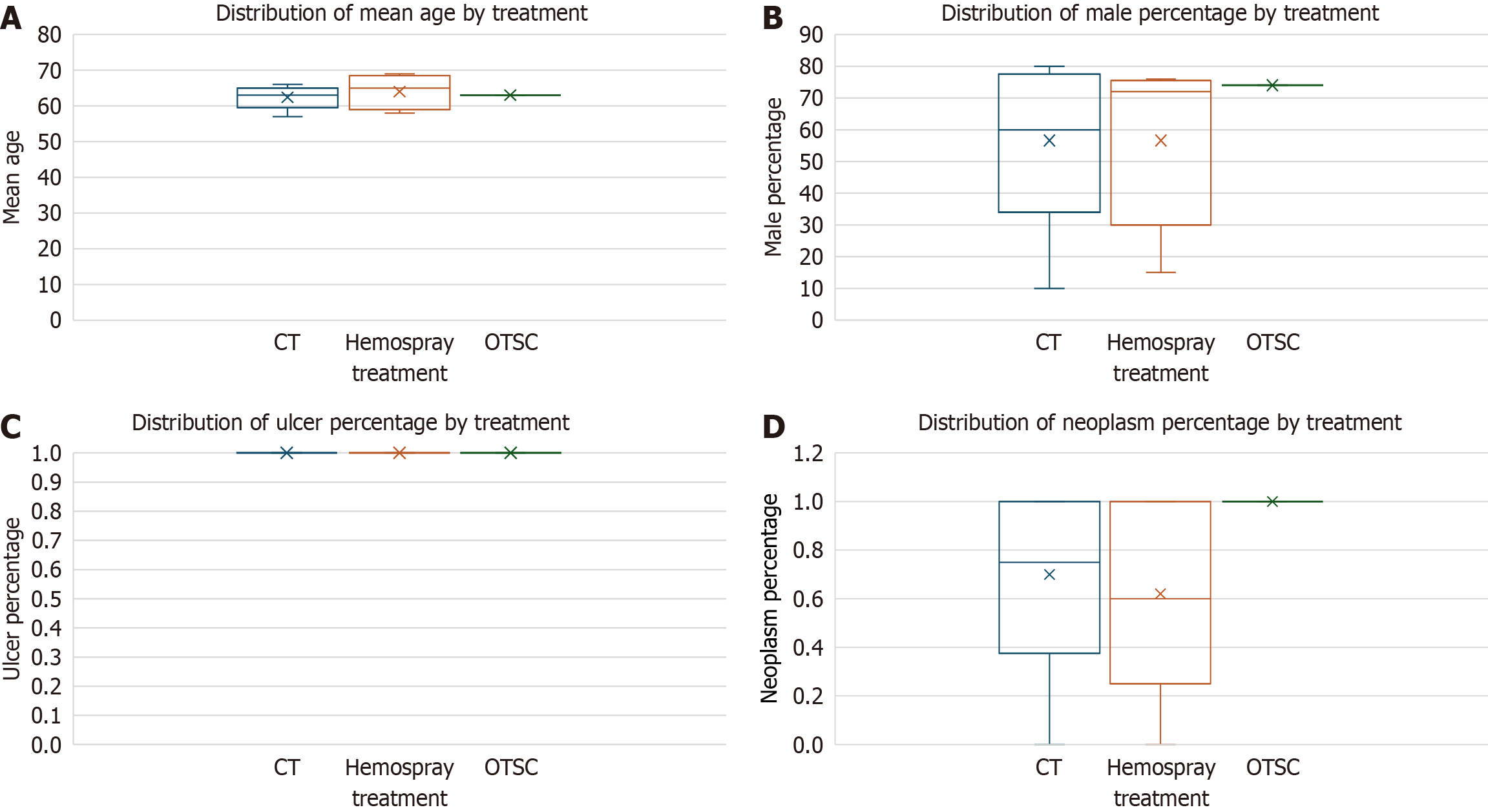
The meta-regression analysis of the 30-day rebleeding rate revealed that none of the examined covariates, including mean age (P = 0.546), male percentage (P = 0.196), ulcer percentage (P = 0.757), and neoplasm percentage (P = 0.742), had a statistically significant influence on the outcome (Figure 6). The model's performance, indicated by an R-squared value of 0.609, suggests that approximately 60.9% of the variance in 30-day rebleeding rates can be explained by these variables; however, the model itself was not statistically significant (P = 0.247). Sensitivity analysis results showed an overall pooled 30-day rebleeding event rate of 9.23%, with studies focusing on neoplastic etiologies exhibiting a slightly higher rebleeding rate (9.69%) compared to those with non-neoplastic etiologies (8.26%). For short-term rebleeding rates, the analysis found that mean age (P = 0.402), ulcer percentage (P = 0.693), and neoplasm percentage (P = 0.331) were not significant predictors, while male percentage (P = 0.082) approached statistical significance, suggesting a potential protective effect. The model for short-term rebleeding demonstrated stronger explanatory power with an R-squared of 0.766. In contrast, the analysis of initial hemostasis rates indicated that none of the covariates had significant effects, with an R-squared of 0.017, signifying minimal explanatory capability. Due to insufficient variability and missing data, results for the necessity of salvage therapy, all-cause mortality, and bleeding-related mortality could not be computed, leading to inconclusive findings. In summary, male percentage showed a potential trend towards reducing short-term rebleeding risk, while other covariates did not demonstrate statistical significance across the outcomes, and challenges in data availability limited further analysis of key mortality-related outcomes.
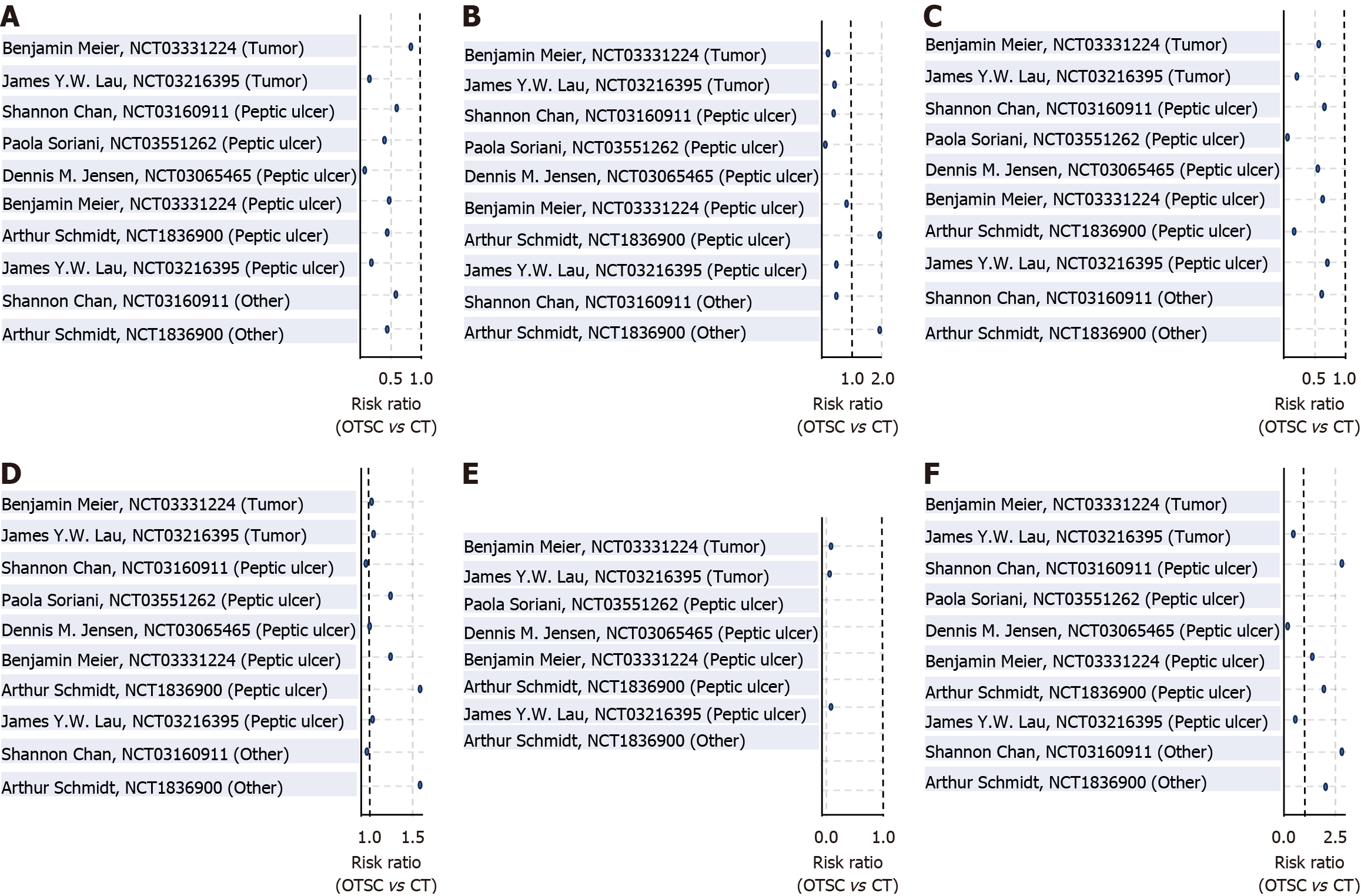
A detailed subgroup analysis was conducted to evaluate the efficacy of OTSC therapy vs CT across different bleeding etiologies, including peptic ulcer, tumor-related, and other causes (e.g., Mallory-Weiss tear, Dieulafoy’s lesion). In patients with peptic ulcer bleeding, OTSC significantly reduced both 30-day rebleeding (pooled RR: 0.404; 95%CI: 0.292-0.559; P = 0.0001) and short-term rebleeding rates (RR: 0.434; 95%CI: 0.286-0.66; P = 0.0001), without significant differences in initial hemostasis, need for surgical or transarterial embolization, or mortality. In the tumor-related bleeding subgroup, OTSC was associated with a significant reduction in short-term rebleeding (RR: 0.47; 95%CI: 0.237-0.934; P = 0.0312) and 30-day mortality (RR: 0.361; 95%CI: 0.173-0.753; P = 0.0066), whereas other outcomes did not reach statistical significance. Among other bleeding sources, OTSC significantly reduced 30-day rebleeding (RR: 0.5; 95%CI: 0.315-0.792; P = 0.0032), though its effect on short-term rebleeding, hemostasis, and mortality was not statistically significant. These findings highlight the potential benefit of OTSC in specific bleeding etiologies, particularly in peptic ulcer and tumor-related bleeding (Table 3; Figure 7).
| Subgroup | Outcome | Pooled RR (OTSC/CT) | 95%CI | P value |
| Peptic ulcer | 30-day rebleeding | 0.404 | 0.292-0.559 | 0.0001 |
| Short-term rebleeding | 0.434 | 0.286-0.66 | 0.0001 | |
| Initial hemostasis | 1.016 | 0.998-1.034 | 0.0774 | |
| Surgical/TAE necessity | 1.122 | 0.603-2.087 | 0.7171 | |
| 30-day mortality | 0.844 | 0.506-1.406 | 0.5138 | |
| Bleeding mortality | 0.741 | 0.14-3.922 | 0.7241 | |
| Tumor | 30-day rebleeding | 0.581 | 0.322-1.05 | 0.0719 |
| Short-term rebleeding | 0.47 | 0.237-0.934 | 0.0312 | |
| Initial hemostasis | 1.04 | 0.987-1.095 | 0.1447 | |
| Surgical/TAE necessity | 0.697 | 0.228-2.13 | 0.5263 | |
| 30-day mortality | 0.361 | 0.173-0.753 | 0.0066 | |
| Bleeding mortality | 0.163 | 0.019-1.383 | 0.0964 | |
| Other | 30-day rebleeding | 0.5 | 0.315-0.792 | 0.0032 |
| Short-term rebleeding | 0.635 | 0.336-1.201 | 0.1628 | |
| Initial hemostasis | 1.033 | 0.966-1.105 | 0.34 | |
| Surgical/TAE necessity | 2.154 | 0.833-5.57 | 0.1136 | |
| 30-day mortality | 1.147 | 0.568-2.314 | 0.7021 | |
| Bleeding mortality | 1.0 | 0.02-49.913 | 1.0 |
Determining the most effective strategies to prevent rebleeding is crucial in minimizing hospital readmissions and alleviating the financial burden on patients, their families, and the healthcare system. Thus, we conducted this systematic review and network meta-analysis to identify the optimal hemostatic approach for NVUGIB and to evaluate the comparative effectiveness of various treatment modalities. This analysis incorporates data from 17 studies with a substantial sample size, enhancing the reliability of the findings.
This meta-analysis highlights OTSC as the most effective intervention for reducing 30-day and short-term rebleeding rates in NVUGIB, surpassing both CT and HP, supporting OTSC as a preferred first-line treatment for NVUGIB. The (OTSC; Ovesco Endoscopy, Tuebingen, Germany) is an innovative endoscopic clipping device first introduced in 2007, initially reported in a case series demonstrating its successful use in repairing gastrointestinal perforations and treating GI bleeding[9]. The OTSC system consists of an applicator cap that attaches to the tip of the endoscope, similar to a band ligation device, and is deployed by suctioning the targeted tissue into the cap before releasing the clip to achieve secure tissue compression. It is available in multiple sizes, ranging from 11 to 14 mm, depending on the endoscope size, and comes in different tooth configurations-blunt and sharp versions—to accommodate various clinical applications. The clip's strong compression force enables it to embed deeply into the tissue, making it particularly effective for large and fibrotic ulcers that are resistant to standard hemostatic measures[10,11].
The FLETRock study further validated its efficacy, demonstrating a high success rate of 92.54% in achieving hemostasis in NVUGIB cases, with primary clinical success in 90.8% of patients and an additional 1.7% achieving hemostasis with adjunctive measures[12]. Jensen et al[12] monitored the rates of persistent arterial blood flow and rebleeding in NVUGIB cases using Doppler Endoscopic Probe and found that the rates of residual blood flow after standard visually guided endoscopic hemostasis of major NVUGIB were significantly higher than OTSC-treated lesions (24% vs 5%) and the rebleeding rates were also significantly higher (26% vs 5%)[13]. In contrast to TTS clips, which may require multiple applications and struggle with large vessels or fibrotic ulcer bases, OTSC's larger jaw size and higher compression force allow for better mucosal approximation and deeper tissue capture, resulting in more effective mechanical tamponade[14]. Recognizing its efficacy, the European Society of Gastrointestinal Endoscopy recommends OTSC as a first-line treatment for selected actively bleeding ulcers, particularly those larger than 2 cm, with large visible vessels greater than 2 mm, and located in high-risk vascular areas[15]. Conversely, the American College of Gastroenterology guidelines suggest OTSC primarily for patients experiencing recurrent bleeding after previous successful endoscopic hemostasis[16].
Despite its numerous advantages, the OTSC has several limitations that must be considered in clinical practice. One of the primary drawbacks is the need to remove the endoscope to attach the applicator cap, which can delay treatment, especially in emergent situations. This process may also require the use of a smaller endoscope, which can limit maneuverability and visualization. Additionally, OTSC deployment can be technically challenging, particularly in cases where lesions are located in difficult-to-access areas such as the upper esophageal sphincter, strictures, or stenotic regions. Proper positioning and deployment require optimal endoscope angulation, ideally greater than 120 degrees in each quadrant, to ensure accurate targeting and successful clip application. Furthermore, OTSC may not be suitable for tangentially positioned lesions, and misplacement can necessitate additional procedures for repositioning or removal. Cost is another factor to consider; while OTSC has been shown to be cost-effective in cases of recurrent bleeding, the initial cost of the device ($400-$450 USD) is relatively high compared to standard hemoclips, which may limit its routine use in some healthcare settings. However, a cost analysis based on the STING study results concluded that OTSC was cost effective in recurrent peptic ulcer bleeding, despite the increased cost of these clips[17,18]. The incremental cost-effectiveness ratio showed cost savings of €468.18 for total treatment and €236.49 for hemostasis-related costs per additional successful hemostasis when compared to conventional therapy. These findings emphasize that despite a higher upfront device cost, OTSC therapy prevents rebleeding events efficiently and reduces the need for costly rescue interventions, hospital readmissions, and prolonged intensive care unit stays[18]. Lastly, while OTSC provides strong compression, excessive force application can lead to tissue ischemia or injury in delicate areas, necessitating careful patient selection and procedural expertise[10,18].
HP has emerged as a promising treatment modality for NVUGIB, with several commercially available products, including TC-325 (Hemospray), EndoClot polysaccharide hemostatic system, CEGP-003, UI-EWD, and Ankaferd Bloodstopper. These powders differ in their chemical compositions but share a common mechanism of action, which involves water absorption, stimulation of the physiological clotting cascade, and formation of a mechanical barrier to control bleeding[19,20]. In our meta-analysis, two types of HPs, TC-325 and CEGP-003, were analyzed. TC-325, the most widely studied HP, is composed of bentonite-based aluminum phyllosilicate clay and is delivered through a specialized device consisting of an application catheter, a carbon dioxide (CO2) cartridge, and a syringe containing the powder. The deployment process involves identifying the bleeding site, flushing the adjacent area, and applying the powder in short bursts until complete coverage is achieved, forming an adhesive mechanical barrier that aids in hemostasis[21].
Several studies have demonstrated the advantages of TC-325 in achieving initial hemostasis, particularly in cases of diffuse mucosal bleeding and tumors where conventional hemostatic methods are challenging[22-24]. Our meta-analysis found that HP provides a moderate benefit over CT, with a risk ratio of 0.73 (95%CI: 0.45-1.13), suggesting a potentially comparable but not superior efficacy in preventing rebleeding. The largest RCT to date, which included 224 patients with active NVUGIB, reported 30-day rebleeding-free probabilities of 89.8% for TC-325 and 81.1% for standard therapy, demonstrating non-inferiority of TC-325 to conventional treatments[22]. Similarly, smaller studies, such as the randomized trial by Baracat et al[20], found no significant differences between TC-325 and standard hemoclips in achieving primary hemostasis (94.9% vs 90%, P = 0.487), though a non-significant trend towards higher rebleeding rates was observed in the TC-325 group (27.9% vs 15.8%)[23,24].
Despite its benefits, HP has several limitations. One of the major drawbacks is its short residency time within the gastrointestinal tract, typically lasting less than 24 hours, which can result in a higher likelihood of rebleeding. Unlike mechanical therapies such as OTSC, HP does not obliterate arterial blood flow beneath the stigmata of recent hemorrhage, often necessitating additional endoscopic interventions for definitive hemostasis. Moreover, the cost of TC-325 treatment is relatively high, with each application costing approximately $2500 USD, which may limit its widespread use, particularly in resource-limited settings. Another limitation is the lack of absorption by the gastrointestinal tract, which, while ensuring safety, also means it provides only temporary hemostasis without addressing the underlying pathology. This method works best when the area is dry, so it tends to be less effective during active spurting bleeding, such as in Forrest 1A ulcers[25,26]. Overall, while HP offers a valuable and easily deployable option for achieving temporary hemostasis, particularly in diffuse or neoplastic bleeding, its role as a definitive therapy remains limited. Our findings suggest that HP should be considered as an adjunctive therapy rather than a primary treatment for high-risk NVUGIB cases where arterial bleeding is prominent.
The strengths of this meta-analysis include its comprehensive approach, incorporating data from 17 RCTs with a substantial sample size, thereby enhancing the robustness and generalizability of the findings. The use of network meta-analysis allowed for indirect comparisons between different hemostatic techniques, providing a hierarchical ranking of their efficacy. Additionally, adherence to rigorous methodology, including PRISMA guidelines, and detailed assessment of bias contribute to the reliability of the results. The inclusion of multiple HP formulations and the comparative evaluation with OTSC and CT further provide valuable insights into the relative efficacy of different therapeutic options.
Despite its strengths, this meta-analysis has some limitations. The heterogeneity among included studies in terms of patient populations, bleeding etiologies, and treatment protocols may introduce variability in the results. The inclusion of only English-language studies could have introduced publication bias, potentially excluding relevant data from non-English sources, particularly studies on HPs like Ankaferd Blood Stopper, which are frequently conducted in non-English-speaking regions such as Turkey. Furthermore, while no significant differences in mortality were observed, this analysis may be underpowered to detect such differences, especially for bleeding-related mortality where events were rare. These factors warrant caution in interpreting the mortality outcomes. Future research with standardized protocols, inclusion of non-English literature, and extended follow-up is needed to validate these findings and inform clinical practice.
In conclusion, we provide a comprehensive comparison of conventional and emerging hemostatic interventions for NVUGIB. The findings indicate that OTSC demonstrate superior efficacy in reducing both short-term and 30-day rebleeding rates compared to CT and HP. OTSC's ability to provide durable hemostasis, particularly in cases involving large ulcer bases and fibrotic lesions, supports its role as a preferred first-line therapy. While HP offers a convenient and easily deployable option, its efficacy remains comparable but not superior to CT, highlighting its role as an adjunct rather than a standalone treatment. Our analysis reinforces the need for individualized treatment approaches, considering the advantages and limitations of each modality to optimize patient outcomes and reduce the burden of rebleeding.
| 1. | El-Tawil AM. Trends on gastrointestinal bleeding and mortality: where are we standing? World J Gastroenterol. 2012;18:1154-1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Wuerth BA, Rockey DC. Changing Epidemiology of Upper Gastrointestinal Hemorrhage in the Last Decade: A Nationwide Analysis. Dig Dis Sci. 2018;63:1286-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 170] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 3. | van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008;22:209-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 259] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 4. | Nulsen B, Jensen DM. Hemostasis Techniques for Non-variceal Upper GI Hemorrhage: Beyond Injection and Cautery. Dig Dis Sci. 2022;67:1431-1441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (39)] |
| 5. | Gralnek IM, Dumonceau JM, Kuipers EJ, Lanas A, Sanders DS, Kurien M, Rotondano G, Hucl T, Dinis-Ribeiro M, Marmo R, Racz I, Arezzo A, Hoffmann RT, Lesur G, de Franchis R, Aabakken L, Veitch A, Radaelli F, Salgueiro P, Cardoso R, Maia L, Zullo A, Cipolletta L, Hassan C. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 496] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 6. | Barkun AN, Almadi M, Kuipers EJ, Laine L, Sung J, Tse F, Leontiadis GI, Abraham NS, Calvet X, Chan FKL, Douketis J, Enns R, Gralnek IM, Jairath V, Jensen D, Lau J, Lip GYH, Loffroy R, Maluf-Filho F, Meltzer AC, Reddy N, Saltzman JR, Marshall JK, Bardou M. Management of Nonvariceal Upper Gastrointestinal Bleeding: Guideline Recommendations From the International Consensus Group. Ann Intern Med. 2019;171:805-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 327] [Article Influence: 54.5] [Reference Citation Analysis (16)] |
| 7. | Laine L, Barkun AN, Saltzman JR, Martel M, Leontiadis GI. ACG Clinical Guideline: Upper Gastrointestinal and Ulcer Bleeding. Am J Gastroenterol. 2021;116:899-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 279] [Article Influence: 69.8] [Reference Citation Analysis (36)] |
| 8. | Cochrane Handbook for Systematic Reviews of Interventions. 2019. . [RCA] [DOI] [Full Text] [Cited by in Crossref: 5433] [Cited by in RCA: 7245] [Article Influence: 1207.5] [Reference Citation Analysis (0)] |
| 9. | Kirschniak A, Kratt T, Stüker D, Braun A, Schurr MO, Königsrainer A. A new endoscopic over-the-scope clip system for treatment of lesions and bleeding in the GI tract: first clinical experiences. Gastrointest Endosc. 2007;66:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 241] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 10. | Asokkumar R, Soetikno R, Sanchez-Yague A, Kim Wei L, Salazar E, Ngu JH. Use of over-the-scope-clip (OTSC) improves outcomes of high-risk adverse outcome (HR-AO) non-variceal upper gastrointestinal bleeding (NVUGIB). Endosc Int Open. 2018;6:E789-E796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Wedi E, Fischer A, Hochberger J, Jung C, Orkut S, Richter-Schrag HJ. Multicenter evaluation of first-line endoscopic treatment with the OTSC in acute non-variceal upper gastrointestinal bleeding and comparison with the Rockall cohort: the FLETRock study. Surg Endosc. 2018;32:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Jensen DM, Kovacs TOG, Ghassemi KA, Kaneshiro M, Gornbein J. Why over-the-scope-clip is potentially more effective than standard endoscopic hemostasis as primary treatment of severe non-variceal upper gastrointestinal bleeding. Gastrointest Endosc. 2019;89:72. [DOI] [Full Text] |
| 13. | Wedi E, Gonzalez S, Menke D, Kruse E, Matthes K, Hochberger J. One hundred and one over-the-scope-clip applications for severe gastrointestinal bleeding, leaks and fistulas. World J Gastroenterol. 2016;22:1844-1853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Gralnek IM, Stanley AJ, Morris AJ, Camus M, Lau J, Lanas A, Laursen SB, Radaelli F, Papanikolaou IS, Cúrdia Gonçalves T, Dinis-Ribeiro M, Awadie H, Braun G, de Groot N, Udd M, Sanchez-Yague A, Neeman Z, van Hooft JE. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2021. Endoscopy. 2021;53:300-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 262] [Article Influence: 65.5] [Reference Citation Analysis (1)] |
| 15. | Ni Y, Ali K, Tang P, Hayat K, Cheng Z, Xu B, Qin Z, Zhang W. Over-the-scope clips for Nonvariceal upper gastrointestinal bleeding: a systematic review and meta-analysis of randomized studies. Postgrad Med J. 2025;101:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Kuellmer A, Behn J, Meier B, Wannhoff A, Bettinger D, Thimme R, Caca K, Schmidt A. Over-the-scope clips are cost-effective in recurrent peptic ulcer bleeding. United European Gastroenterol J. 2019;7:1226-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 17. | Chen Y, Zhao X, Wang D, Liu X, Chen J, Song J, Bai T, Hou X. Endoscopic Delivery of Polymers Reduces Delayed Bleeding after Gastric Endoscopic Submucosal Dissection: A Systematic Review and Meta-Analysis. Polymers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Chen YI, Barkun A, Nolan S. Hemostatic powder TC-325 in the management of upper and lower gastrointestinal bleeding: a two-year experience at a single institution. Endoscopy. 2015;47:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Shah MP, Saleem S, Attar B, et al. Hemospray versus conventional therapy for non-variceal upper gastrointestinal bleeding: a systematic review and meta-analysis. Cureus. 2024;16:e55079. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Baracat FI, de Moura DTH, Brunaldi VO, Tranquillini CV, Baracat R, Sakai P, de Moura EGH. Randomized controlled trial of hemostatic powder versus endoscopic clipping for non-variceal upper gastrointestinal bleeding. Surg Endosc. 2020;34:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Chen YI, Wyse J, Lu Y, Martel M, Barkun AN. TC-325 hemostatic powder versus current standard of care in managing malignant GI bleeding: a pilot randomized clinical trial. Gastrointest Endosc. 2020;91:321-328.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Chahal D, Tandon P, Jogendran M, Zhao B, Donnellan F. Hemospray for gastrointestinal bleeding: a meta-analysis. Gastrointest Endosc. 2020;91:AB281. [DOI] [Full Text] |
| 23. | Chahal D, Lee JGH, Ali-Mohamad N, Donnellan F. High rate of re-bleeding after application of Hemospray for upper and lower gastrointestinal bleeds. Dig Liver Dis. 2020;52:768-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Kwek BEA, Ang TL, Ong PLJ, Tan YLJ, Ang SWD, Law NM, Thurairajah PH, Fock KM. TC-325 versus the conventional combined technique for endoscopic treatment of peptic ulcers with high-risk bleeding stigmata: A randomized pilot study. J Dig Dis. 2017;18:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Smith LA, Stanley AJ, Bergman JJ, Kiesslich R, Hoffman A, Tjwa ET, Kuipers EJ, von Holstein CS, Oberg S, Brullet E, Schmidt PN, Iqbal T, Mangiavillano B, Masci E, Prat F, Morris AJ. Hemospray application in nonvariceal upper gastrointestinal bleeding: results of the Survey to Evaluate the Application of Hemospray in the Luminal Tract. J Clin Gastroenterol. 2014;48:e89-e92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Hussein M, Alzoubaidi D, Lopez MF, Weaver M, Ortiz-Fernandez-Sordo J, Bassett P, Rey JW, Hayee BH, Despott E, Murino A, Moreea S, Boger P, Dunn J, Mainie I, Graham D, Mullady DK, Early DS, Ragunath K, Anderson JT, Bhandari P, Goetz M, Kiesslich R, Coron E, Lovat LB, Haidry R. Hemostatic spray powder TC-325 in the primary endoscopic treatment of peptic ulcer-related bleeding: multicenter international registry. Endoscopy. 2021;53:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |