Published online Jun 27, 2025. doi: 10.4254/wjh.v17.i6.108096
Revised: May 6, 2025
Accepted: May 28, 2025
Published online: June 27, 2025
Processing time: 82 Days and 18.8 Hours
Metabolic dysfunction-associated fatty liver disease (MASLD) is a chronic liver disease characterized by hepatocellular steatosis, which is closely related to metabolic syndrome, with annually increasing morbidity in recent years. Gynecomastia (GYN), an abnormal proliferation of breast tissue in males, is common in males with sex hormone imbalance. Currently, there is insufficient research on the morbidity of GYN and its correlation among MASLD patients.
To investigate the prevalence of GYN and its associated clinical features in patients with MASLD and determine the prevalence of GYN in patients with MASLD and analyze the predictive effect of sex hormones on GYN using receiver operating characteristic (ROC) curves.
A cross-sectional study was conducted in a tertiary care hospital. Among them, 997 patients met the inclusion criteria and underwent breast ultrasonography to determine the presence of GYN. Anthropometric data, laboratory test data [estradiol (E2), androgens, liver function, glucose, lipids, low-density lipoprotein, high-density lipoprotein, creatinine, and uric acid, etc.], as well as information on medical history, severity of fatty liver (mild, moderate, and severe), and duration of the disease were collected. Package for the Social Sciences version 27 and R software (version 4.3.1) were used for data analysis.
The prevalence of GYN increased with the severity of fatty liver (27.6% for mild, 33.5% for moderate, and 58% for severe, P < 0.001); compared with non-GYN patients, GYN patients were older (54.11 ± 9.71 years vs 47.89 ± 9.92 years, P < 0.001), with significantly higher E2 levels, higher estrogen to androgen ratio (P < 0.001) and significantly lower androgen levels (P < 0.001). In ROC curve analysis, the combined model of testosterone and E2 had a high diagnostic value in predicting GYN in MASLD patients, surpassing a single indicator.
The presence of GYN may suggest more severe metabolic abnormalities in patients with MASLD. Therefore, early recognition of GYN may be crucial for early intervention in metabolic syndrome and endocrine abnormalities in patients with MASLD.
Core Tip: This pioneering study investigated the unexplored link between the severity/duration of metabolic dysfunction-associated fatty liver disease (MASLD) and gynecomastia (GYN) in adult males, addressing acritical research gap. By integrating MASLD with body mass index and sex hormone imbalances, we revealed GYN as a novel marker for metabolic disorders (hyperglycemia, hypertension, dyslipidemia), shedding light on MASLD-driven endocrine disruption. Our findings underscore the necessity of holistic metabolic management—targeting obesity, MASLD, and hormonal imbalance—to mitigate GYN progression. This first-of-its-kind analysis provides a transformative clinical framework for early screening and intervention, positioning GYN as a sentinel for underlying metabolic dysfunction, thereby enhancing preventive strategies and therapeutic outcomes for MASLD-related comorbidities.
- Citation: Zhang MH, Meng N, Zhang KH, Yu JK, Huang CH, Yang S, Zhu DY. Correlation between gynecomastia and endocrine regulation in patients with metabolic dysfunction-associated fatty liver disease: A cross-sectional study. World J Hepatol 2025; 17(6): 108096
- URL: https://www.wjgnet.com/1948-5182/full/v17/i6/108096.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i6.108096
Metabolic dysfunction-associated fatty liver disease (MASLD) is a major component of chronic liver disease, with a global prevalence of approximately 20%-30%[1]. MASLD is the leading cause of chronic liver disease, which is burdensome for significantly increased extra-hepatic disease[2]. The incidence of MASLD increases with obesity, significantly increasing medical and economic burden on this population[3,4]. MASLD is defined as the accumulation of more than 5% lipids in liver cells of individuals not who do not consuming excessive alcohol, excluding other known diseases (autoimmune, viral, etc.)[5]. It is chronic liver disease caused by obesity, diabetes mellitus (DM), and metabolic syndrome[6,7]. MASLD has multiple pathogenic factors such as insulin resistance, DM, hypertension, dyslipidemia, and obesity. It is not only considered a result of abnormal fat metabolism, but also a hepatic component of metabolic syndrome[8,9]. In recent years, an international panel of experts has renamed metabolism-associated fatty liver disease as MASLD. It is widely believed that MASLD is a metabolic disease, of which development can be affected by certain metabolic factors[10].
Gynecomastia (GYN) is described as an enlargement of male breast tissue, characterized by a mass (bilaterally or rarely unilaterally) extending from the center of nipple, accompanied by a pushable mass palpable under the areola, which may be painful in some patients[11]. The prevalence of GYN ranges from 32% to 65%, depending on diagnosis, age, and lifestyle[12]. GYN is associated with an imbalance in the estrogen/androgen balance, which can be caused by various factors including medications, endocrine disorders, and liver disease[13,14]. After 12 months or more, fibrosis and vitreous degeneration may occur, which is unlikely to resolve spontaneously even after removing the causative factors, and may have a significant impact on the patient's physical and mental health, as well as on social interaction[15].
Studies on the relationship between fatty liver and metabolic abnormalities and sex hormone imbalance has been widely recognized, and the relationship between metabolic abnormalities and imbalanced estrogen/androgen ratios and GYN has been well established. However, there is still a lack of research on the relationship between metabolic abnormalities and imbalanced estrogen/androgen ratios and GYN. Therefore, this study aims to investigate GYN in patients with fatty liver and analyze its correlation at different dimensions and levels, providing new ideas and bases for the prevention and treatment of GYN.
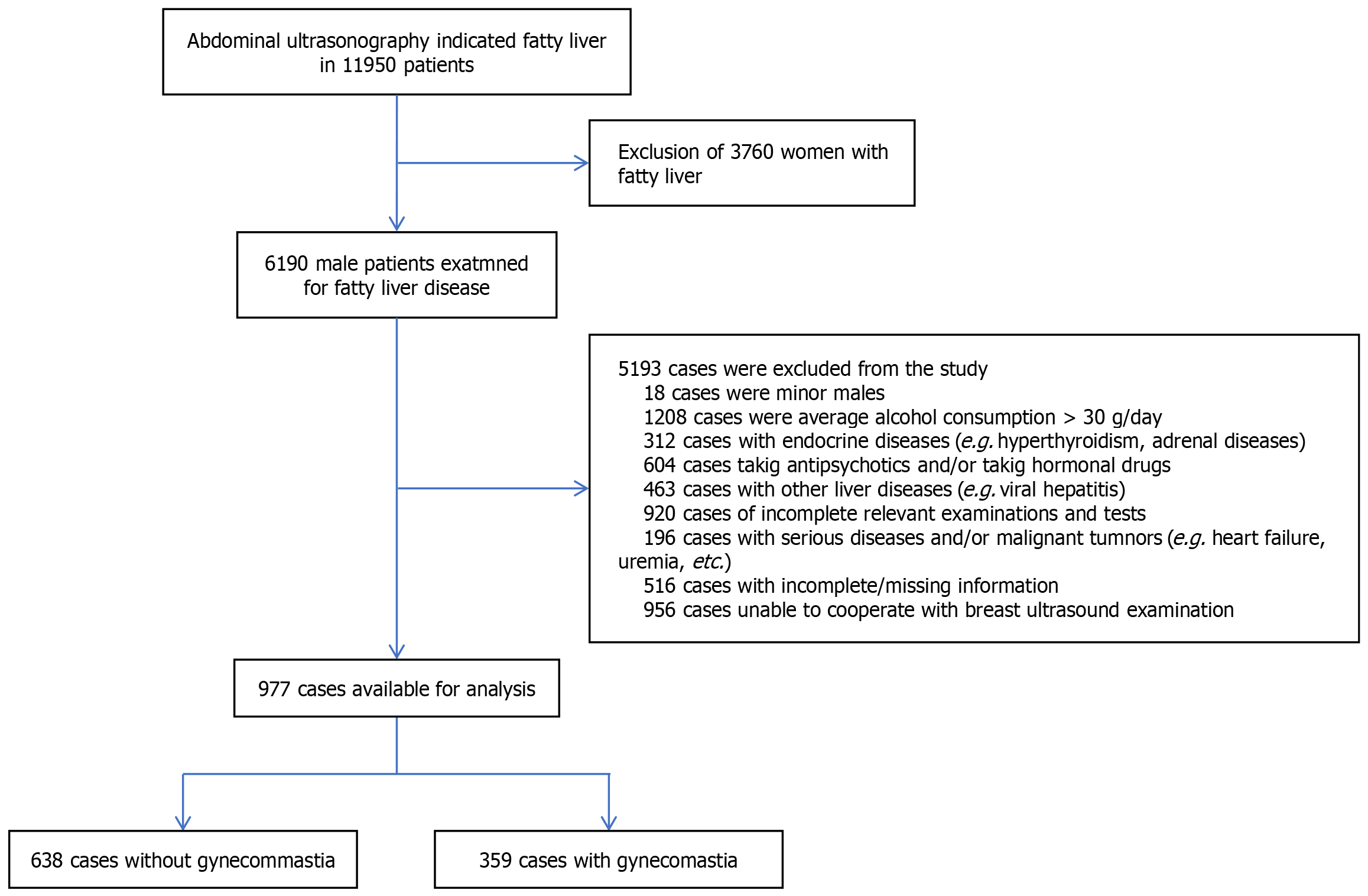
We retrospectively identified patients diagnosed with MASLD through ultrasound at our physical examination center from January 2024 to December 2024. Adult male patients who met the following criteria were included: (1) Diagnosed with steatohepatopathy by abdominal ultrasound; (2) Age ≥ 18 years; (3) No history of significant alcohol consumption (alcohol intake < 30 g/day) {according to the Zhonghua Neifenmi Zazhi and Metabolism 2018 Consensus on the Diagnosis and Treatment of Nonalcoholic Steatohepatopathies and Associated Metabolic Disorders (Second Edition) Chinese Medical Association Endocrinology Branch, Drinking volume: (1) Male alcohol consumption of converted ethanol < 30 g/day (< 210 g/week); and (2) Female < 20 g/day (< 140 g/week) [calculation method: Ethanol intake (g), women < 20 g/day (< 140 g/week)]. Male intake < 30 g/day (< 210 g/week) and female intake < 20 g/day (< 140 g/week) [calculation method: Ethanol intake (g) = volume (mL) × alcoholic strength (%) × 0.8]}; (4) No endocrine disorders (e.g., hyperthyroidism, adrenal disease, etc.); (5) No history of medication that may lead to breast development disorders (e.g., certain antipsychotic drugs, hormonal drugs, etc.); (6) Excluding other liver diseases (e.g. viral hepatitis, autoimmune liver disease, etc.); and (7) Completing relevant examinations and tests. Exclusion criteria: (1) Female MASLD patients; (2) Previous breast surgery or endocrine therapy; (3) Diseases known to affect the level of sex hormones (such as Crohn's syndrome, hyperthyroidism); (4) Patients with malignant tumors or other serious systemic diseases; (5) Patients with missing or incomplete data; and (6) Patients who do not cooperate with breast ultrasound.
After screening, 997 subjects were enrolled in our study. The flow chart for personnel screening is shown in Figure 1. Researchers performed free breast ultrasound examinations on all eligible subjects determine the presence of GYN. (1) Ultrasound showed hypoechoic glandular tissue behind the nipple-areola, distributed in a disk or fan shape, distinguishing it from surrounding fatty tissue[16]; and (2) Clinically, a thickness of > 0.5 cm of glandular tissue in male breast is usually recognized as the diagnostic criterion for this disease[17].
Diagnostic criteria for fatty liver: (1) Liver parenchyma with fine punctate hyperechoicity (liver echo intensity > spleen and kidney echoes); (2) Attenuation of liver far-field echoes; and (3) Poorly displayed intrahepatic ducts. The presence of item 1 of the above three, plus any of items 2 or 3, can be diagnostic of fatty liver[18].
Liver echogenicity: Slight enhancement, significant compared with the spleen, but the internal structure of the liver is still clear.
Vascular structure: The definition of the vessel walls is essentially normal,and vessel edges are still visible.
Contrast: Increased contrast between the liver and kidney, but the blood vessels of the liver and the structure of the hilar region are still clearly visible.
Liver echogenicity: Markedly enhanced, more pronounced compared with the spleen, and the fine structures within the liver begin to blur.
Vascular structures: The definition of the vessel walls decreases and the edges of the vessels begin to blur.
Contrast: Further increase in liver-kidney contrast, the blood vessels of the liver and the structures of the hilar region become blurred.
Liver echogenicity: Extremely enhanced, the liver shows marked hyperechoicity, prominent comppared with the spleen.
Vascular structures: The definition of the vessel walls is severely reduced and the vessels are barely visible.
Contrast: Extremely high contrast between the liver and kidney, detailed structures within the liver are not visible, including structures in the hilar area.
Breast ultrasonography was performed using Philips IU22 (Philips, Netherlands) and GE LOGIQ7 and 9 (General Electric, United States) ultrasound diagnostic equipment in the Department of Ultrasound of the hospital.
Due to the anonymity of the data, informed written consent was not required. The study was approved by the Ethics Committee of the Affiliated Hospital of Hangzhou Normal University (Ethics No. 2025(E2)-KS-045) and followed the ethical guidelines of the Declaration of Helsinki (1964).
Demographic characteristics such as age, height, weight, blood pressure, waist circumference (WC), hip circumference, exercise habit, smoking habit, severity of fatty liver, and duration of fatty liver were collected from all study subjects.
Blood samples for laboratory testing were collected through venipuncture in the early morning after an overnight fasting. Laboratory tests include: (1) Estradiol (E2); (2) Testosterone (T); (3) Alanine aminotransferase (ALT); (4) Aspartate aminotransferase (AST); (5) Gamma-glutamyltransferase (GGT); (6) Creatinine (Cr); (7) Uric acid (UA); (8) Total cholesterol (TC); (9) Triglycerides (TG); (10) High-density lipoprotein (HDL); (11) Low-density lipoprotein (LDL); (12) Fasting glucose (Glu); (13) Total bilirubin (TBil); and (14) Total protein (TP), etc.
The classification standard of body mass index (BMI) adopts the general standard of our country: (1) BMI < 18.5 kg/m2 is low body mass; (2) 18.5 kg/m2 ≤ BMI < 24 kg/m2 is normal body mass; (3) 24 kg/m2 ≤ BMI < 28 kg/m2 is super body mass; and (4) BMI ≥ 28 is obese. BMI = weight (kg)/height squared (m2).
Individuals taking antihypertensive medication or taking systolic pressure ≥ 140 mmHg and/ or diastolic pressure ≥ 90 mmHg were defined as hypertension[20].
DM was diagnosed based on random blood glucose levels ≥ 11.1 mmol/L, fasting plasma glucose values ≥ 7.0 mmol/L, and/or taking antidiabetic drugs[21].
Exercise group: ≥ 150 minutes of moderate-intensity exercise per week (World Health Organization exercise recommendations: At least 150 minutes of moderate-intensity aerobic exercise or 75 minutes of vigorous-intensity exercise per week for adults)[22]. Non-exercise group: notmeeting the above criteria, or not exercising on a regular basis.
Smoking: Individuals smoking more than one cigarette a day in the past year were considered smokers[23].
Fatty liver disease duration: Obtained based on the patient's self-report and/or past medical history and ultrasound records.
Patients were categorized into GYN and non-GYN groups based on breast ultrasound findings. Patients were categorized into mild, moderate, and severe MASLD subgroups based on the severity of fatty liver.
Descriptive statistics: Statistical software Statistical Package for the Social Sciences 27.0 was used. Normally distributed continuous variables were expressed as mean ± SD, and compared between groups through t-test and analysis of variance. Non normally distributed variables were expressed as [M (P25, P75)], and compared between groups through the rank-sum test. Rank data: For ordered categorical variables, differences in the distribution of component ratios were analyzed by χ² or Fisher's exact test according to the expected value of T frequency. Count data were expressed as frequencies and percentages, and comparisons between two groups were expressed using the χ² test or Fisher's test.
One-way logistic regression analysis: To assess the correlation between variables and GYN, a one-way logistic regression analysis was used to evaluate the relationship between GYN, patients' demographic characteristics (e.g., BMI, WC, age, etc.), and laboratory indices (e.g., sex hormone levels, liver function indices, lipid metabolism indices, etc.).
Multivariate logistic regression analysis: Multivariate logistic regression analysis was used to assess the correlation between fatty liver severity and sex hormone imbalance and GYN. Logistic regression models were constructed to assess the independent correlation between MASLD and GYN under different models.
Receiver operating characteristic curve: To assess the diagnostic ability of E2, estrogen to androgen ratio (ETR), and their combined metrics in predicting GYN in patients with MASLD, receiver operating characteristic (ROC) curve analysis was conducted in the study. The area under the curve (AUC), optimal threshold, sensitivity, and specificity were calculated for each variable.
Subgroup analyses: Subgroup analyses of mild to moderate to severe MASLD were performed to further explore the occurrence of GYN among patients with different severities of fatty liver disease. To facilitate comparison of the effect of duration of MASLD, the duration of MASLD was categorized into four groups according to 1-5 years, 5-10 years, 10-20 years, and ≥ 20 years.
For all analyses, P value < 0.05 was considered statistically significant and P value < 0.001 was considered highly significant.
Finally, 997 subjects met the criteria and were categorized into a GYN group (n = 359) and a non-GYN group (n = 638). The overall incidence of GYN in this study was 36% (n = 359). Table 1 presents the demographic and clinical characteristics of the study population. Table 1 represent demographic and body measurements, biochemical parameters, hormonal parameters and clinical and lifestyle factors. The incidence of GYN increased with the severity of steatosis (mild 27.6%, moderate 33.5%, severe 58%, P < 0.001); compared with non-GYN patients, GYN patients were older (54.11± 9.71 years vs 47.89± 9.92 years, P < 0.001), with higher E2 levels, higher E2 ratios were than those of patients without GYN (P < 0.001), and lower androgen levels were (P < 0.001). Furthermore, their BMI, WC, hip circumference, ALT, AST, Glu, systolic blood pressure (SBP), diastolic blood pressure (DBP), LDL, TC, and TG levels increased, while HDL levels decreased (P < 0.05). GYN patients showed higher prevalence of hypertension and diabetes.
| Non-GYN (n = 638) | GYN (n = 359) | P value | |
| Demographic and body measurements | |||
| Age (years) (mean ± SD) | 47.89 ± 9.92 | 54.± 9.71 | < 0.001 |
| Height (m) (mean ± SD) | 1.73 ± 0.06 | 1.72 ± 0.06 | 0.121 |
| Weight (kg) [M (P25, P75)] | 76.50 (71.00, 84.53) | 79.50 (72.60, 84.80) | < 0.001 |
| Body mass index (kg/m2) [M (P25, P75)] | 25.74 (24.16, 27.29) | 26.82 (24.96, 29.00) | < 0.001 |
| Waist circumference (cm) [M (P25, P75)] | 89.70 (85.08, 93.40) | 90.20 (85.60, 97.70) | 0.020 |
| Hip circumference (cm) [M (P25, P75)] | 102.35 (97.00, 106.63) | 103.30 (99.90, 106.70) | 0.018 |
| Waist-to-hip ratio | 0.87 (0.86, 0.90) | 0.87 (0.85, 0.92) | 0.422 |
| Systolic blood pressure (mmHg) [M (P25, P75)] | 127.00 (120.00, 134.00) | 133 (127.00, 143.00) | < 0.001 |
| Diastolic blood pressure (mmHg) [M (P25, P75)] | 76.00 (70.75, 83.00) | 81.00 (75.00, 87.00) | < 0.001 |
| Biochemical Parameters | |||
| Alanine aminotransferase (U/L) [M (P25, P75)] | 27.00 (21.00, 34.00) | 50.00 (36.00, 65.00) | < 0.001 |
| Aspartate aminotransferase (U/L) [M (P25, P75)] | 28.00 (21.00, 32.00) | 46.00 (35.00, 60.00) | < 0.001 |
| Gamma-glutamyltransferase (U/L) [M (P25, P75)] | 30.00 (22.00, 42.00) | 56.00 (39.00, 76.00) | < 0.001 |
| Creatinine (μmol/L) [M (P25, P75)] | 78.80 (71.30, 85.70) | 78.10 (70.50, 87.65) | 0.902 |
| Uric acid (μmol/L) [M (P25, P75)] | 384.00 (345.00, 414.00) | 439.00 (388.00, 486.00) | < 0.001 |
| Fasting blood glucose (mmol/L) [M (P25, P75)] | 5.31 (4.95, 5.70) | 5.82 (5.03, 6.60) | < 0.001 |
| Total cholesterol (mmol/L) [M (P25, P75)] | 4.98 (4.31, 5.60) | 6.06 (5.09, 6.96) | < 0.001 |
| Triglycerides (mmol/L) [M (P25, P75)] | 1.66 (1.26, 2.39) | 2.99 (1.82, 4.37) | < 0.001 |
| Low-density lipoprotein (mmol/L) [M (P25, P75)] | 2.82 (2.41, 3.22) | 3.18 (2.87, 3.66) | < 0.001 |
| High-density lipoprotein (mmol/L) [M (P25, P75)] | 1.26 (1.10, 1.39) | 1.12 (1.00, 1.26) | < 0.001 |
| Total bilirubin (μmol/L) [M (P25, P75)] | 17.60 (14.60, 20.30) | 18.7 (16.00, 21.70) | < 0.001 |
| Total protein (g/L) [M (P25, P75)] | 72.20 (69.80, 74.30) | 71.60 (69.70, 73.90) | 0.036 |
| Hormonal parameters | |||
| Estradiol (pmol/L) [M (P25, P75)] | 84.67 (69.71, 100.03) | 112.12 (99.98, 130.00) | < 0.001 |
| Testosterone (nmol/L) [M (P25, P75)] | 15.08 (11.36, 19.71) | 10.49 (6.80, 14.04) | < 0.001 |
| Estrogen to androgen ratio | 5.48 (3.74, 8.39) | 10.90 (7.45, 19.03) | < 0.001 |
| Clinical and lifestyle factors | |||
| Level | < 0.001 | ||
| Mild | 255 (72.4) | 97 (27.6) | |
| Moderate | 304 (66.5) | 153 (33.5) | |
| Severe | 79 (42) | 109 (58) | |
| Hypertension | < 0.001 | ||
| No | 540 (70) | 228 (30) | |
| Yes | 98 (42.8) | 131 (57.2) | |
| Diabetes mellitus | < 0.001 | ||
| No | 535 (72.3) | 205 (27.7) | |
| Yes | 103 (40) | 154 (60) | |
| Sporting | < 0.001 | ||
| No | 288 (51.8) | 268 (48.2) | |
| Yes | 350 (79.4) | 91 (20.6) | |
| Smoking | < 0.001 | ||
| No | 343 (70.1) | 146 (29.9) | |
| Yes | 295 (58.1) | 213 (41.9) | |
| Time | < 0.001 | ||
| 1-5 years | 185 (78.7) | 50 (21.3) | |
| 5-10 years | 229 (75.3) | 75 (24.7) | |
| 10-20 years | 159 (51.5) | 150 (48.5) | |
| ≥ 20 years | 65 (43.6) | 84 (56.4) |
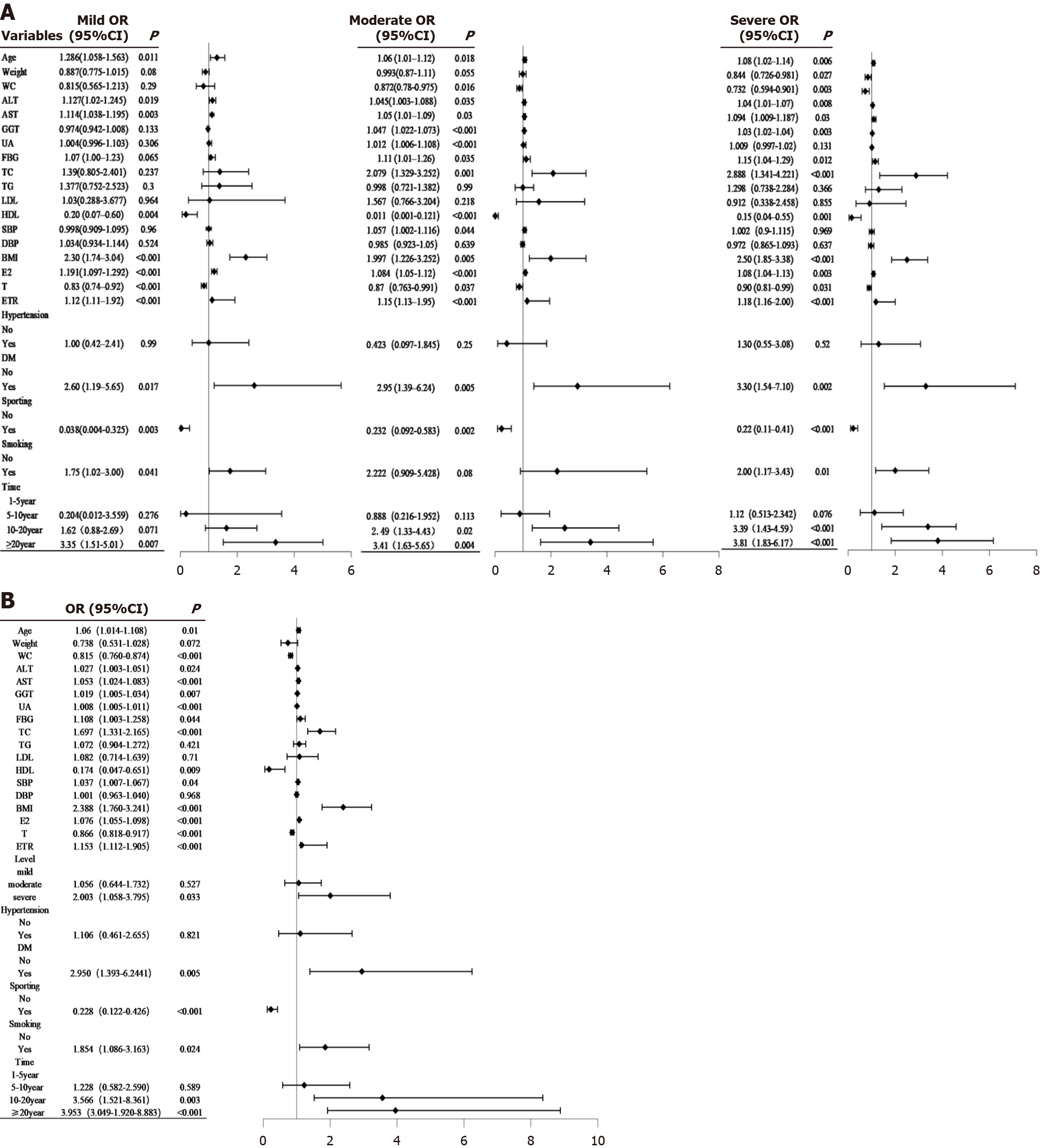
In this study, clinical and lifestyle data on hypertension, DM, exercise habits, smoking, and duration of MASLD were systematically collected, and their associations with GYN were verified by univariate and multivariate analyses (Table 2). For example: (1) Hypertension [odds ratio (OR) = 3.166, P < 0.001] and diabetes (OR = 3.902, P < 0.001) were significantly associated with the risk of GYN; (2) Physical inactivity (OR = 1/0.279, P < 0.001) and smoking (OR = 1.696, P < 0.001) were also independent risk factors; and (3) The risk of GYN was significantly higher in patients with MASLD disease duration ≥ 20 years (OR = 4.782, P < 0.001).
| P value | Odds ratio | 95%CI | |
| Clinical and lifestyle factors | |||
| Level | |||
| Mild | |||
| Moderate | 0.040 | 1.523 | 1.176-1.793 |
| Severe | < 0.001 | 3.627 | 2.500-5.263 |
| Hypertension | |||
| No | |||
| Yes | < 0.001 | 3.166 | 2.336-4.291 |
| Diabetes mellitus | |||
| No | |||
| Yes | < 0.001 | 3.902 | 2.900-5.291 |
| Sporting | |||
| No | |||
| Yes | < 0.001 | 0.279 | 0.210-0.371 |
| Smoking | |||
| No | |||
| Yes | < 0.001 | 1.696 | 1.305-2.204 |
| Time | |||
| 1-5 years | |||
| 5-10 years | 0.355 | 1.212 | 0.807-1.820 |
| 10-20 years | < 0.001 | 3.491 | 2.378-5.124 |
| ≥ 20 years | < 0.001 | 4.782 | 3.049-7.498 |
| Demographic and body measurements | |||
| Age | < 0.001 | 1.056 | 1.042-1.070 |
| Weight | < 0.001 | 1.025 | 1.012-1.037 |
| Body mass index | < 0.001 | 1.158 | 1.106-1.213 |
| Waist circumference | 0.003 | 1.020 | 1.007-1.033 |
| Hip circumference | 0.513 | 0.999 | 0.995-1.003 |
| Waist-to-hip ratio | 0.009 | 18.955 | 2.119-169.572 |
| Systolic blood pressure | < 0.001 | 1.048 | 1.036-1.059 |
| diastolic blood pressure | < 0.001 | 1.049 | 1.034-1.063 |
| Biochemical parameters | |||
| Alanine aminotransferase | < 0.001 | 1.084 | 1.072-1.096 |
| Aspartate aminotransferase | < 0.001 | 1.119 | 1.102-1.136 |
| Gamma-glutamyltransferase | < 0.001 | 1.054 | 1.045-1.062 |
| Creatinine | 0.753 | 1.002 | 0.991-1.012 |
| Uric acid | < 0.001 | 1.010 | 1.008-1.012 |
| Fasting blood glucose | < 0.001 | 1.268 | 1.151-1.398 |
| Total cholesterol | < 0.001 | 1.987 | 1.755-2.250 |
| Triglycerides | < 0.001 | 1.709 | 1.545-1.891 |
| Low-density lipoprotein | < 0.001 | 2.356 | 1.911-2.904 |
| High-density lipoprotein | < 0.001 | 0.062 | 0.031-0.126 |
| Total bilirubin | 0.053 | 1.023 | 1.000-1.046 |
| Total protein | 0.446 | 0.987 | 0.954-1.021 |
| Hormonal parameters | |||
| Estradiol | < 0.001 | 1.063 | 1.054-1.072 |
| Testosterone | < 0.001 | 0.851 | 0.828-0.875 |
| Estrogen to androgen ratio | < 0.001 | 1.173 | 1.142-1.205 |
In addition, model II further adjusted for potential confounders such as liver function,lipids and hormone levels (Table 3), and the results showed that the association between MASLD severity and GYN was still significant (OR = 1.873, P = 0.037 in the severe group), further supporting the reliability of the findings.
| Metabolic dysfunction-associated fatty liver disease | Nonadjusted | Model I | Model II | |||
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| Mild | Reference | Reference | Reference | |||
| Moderate | 1.523 (1.176-1.793) | 0.040 | 1.600 (1.107-1.835) | 0.046 | 1.256 (0.874-1.732) | 0.229 |
| Severe | 3.627 (2.500-5.263) | < 0.001 | 3.157 (2.056-4.847) | < 0.001 | 1.873 (1.038-3.381) | 0.037 |
Univariate analysis of MASLD in patients with GYN is displayed in Table 2. Table 2 represent demographic and body measurements, biochemical parameters, hormonal parameters and clinical and lifestyle factors. The results showed that age, WC, ALT, AST, GGT, UA, Glu, TC, TG, LDL, SBP, DBP, BMI, waist-to-hip ratio (WHR), E2, ETR, smoking, physical inactivity, and duration and severity of MASLD were positively correlated with GYN (P < 0.05). Androgen and serum HDL levels were negatively associated with GYN (P < 0.05).
MASLD duration greater than or equal to 20 years and 10-20 years were associated with an increased risk of GYN compared with the duration of 1-5 years (OR = 4.782, 95%CI: 3.049-7.498; OR = 3.491, 95%CI: 2.378-5.124, P < 0.001). Moderate fatty liver and severe fatty liver had an increased risk of GYN compared with mild fatty liver (OR = 1.523, 95%CI: 1.176-1.793, P = 0.04; OR = 3.627, 95%CI: 2.500-5.263, P < 0.001).
Based on one-way logistic regression analysis, unadjusted model I and model II were established for multivariate analysis, as detailed in Table 3. The mild group was set as the reference group, the correlation between MASLD and GYN was statistically significant in the moderate group compared with the mild group in the unadjusted model (OR = 1.523, 95%CI: 1.176-1.793, P = 0.04) and model I (OR = 1.600, 95%CI: 1.107-1.835, P = 0.046), while the correlation between MASLD and GYN was not statistically significant in model II (OR = 1.256, 95%CI: 0.874-1.732, P = 0.229). Compared with the mild group, the correlation between MASLD and GYN in the unadjusted model, model I, and model II were statistically significant in the severe group(P < 0.001, P < 0.001, P = 0.037).
Model I included: (1) Age; (2) Hypertension; (3) Diabetes; (4) Exercise; (5) Smoking; (6) BMI; and (7) WHR. Model II included: (1) Age; (2) Hypertension; (3) Diabetes; (4) Exercise; (5) Smoking; (6) BMI; (7) WHR; (8) ALT; (9) AST; (10) GGT; (11) Cr; (12) UA; (13) LDL; (14) HDL; (15) Tbil; (16) TP; (17) E2; (18) T; and (19) ETR.
The effect of MASLD on GYN was further explored by grouping MASLD according to its severity. The study was categorized into three groups according to the severity of MASLD: (1) Mild; (2) Moderate; and (3) Severe, with 352 (35.3%), 457(45.8%) and 188 (18.9%) patients in each group, respectively. The general characteristics and laboratory indicators between the different groups were statistically analyzed, as shown in Table 4. Table 4 represent demographic and body measurements, biochemical parameters, hormonal parameters and clinical and lifestyle factors. Among them, age, WC, ALT, AST, GGT, fasting blood glucose, TG, LDL, TBil, SBP, DBP, BMI, WHR, E2, and ETR increased with the severity of fatty liver (P < 0.05); HDL, T, and TP decreased with the severity of fatty liver (P < 0.05).
| Metabolic dysfunction-associated fatty liver disease | P value | |||
| Mild (n = 352) | Moderate (n = 457) | Severe (n = 188) | ||
| Demographic and body measurements | ||||
| Age (years) (mean ± SD) | 47.59 ± 11.007 | 51.25 ± 11.200 | 52.49 ± 10.635 | < 0.001 |
| Height (m) (mean ± SD) | 1.73 ± 0.06 | 1.72 ± 0.06 | 1.72 ± 0.06 | 0.084 |
| Weight (kg) [M (P25, P75)] | 77.5 (71.8, 83.9) | 77.0 (71.1, 84.7) | 77.7 (71.4, 84.7) | 0.750 |
| Waist circumference (cm) [M (P25, P75)] | 89.8 (85.1, 93.2) | 89.6 (85.4, 94.0) | 91.8 (85.6, 102.2) | 0.013 |
| Hip circumference (cm) [M (P25, P75)] | 103.3 (98.9, 106.7) | 102.6 (98.0, 106.6) | 101.7 (97.0, 107.0) | 0.369 |
| Body mass index (kg/m2) [M (P25, P75)] | 25.89 (24.47, 27.62) | 26.13 (24.3, 27.99) | 26.50 (24.5, 28.78) | 0.041 |
| Waist-to-hip ratio | 0.87 (0.85, 0.89) | 0.87 (0.86, 0.89) | 0.90 (0.86, 0.93) | < 0.001 |
| Systolic blood pressure (mmHg) [M (P25, P75)] | 129 (121, 136) | 128 (121, 136) | 132 (127, 142) | < 0.001 |
| Diastolic blood pressure (mmHg) [M (P25, P75)] | 78 (71, 84) | 77 (71, 84) | 80 (75, 86) | < 0.001 |
| Biochemical parameters | ||||
| Alanine aminotransferase (U/L) [M (P25, P75)] | 28 (22, 40) | 33 (23, 48) | 42 (29, 61) | < 0.001 |
| Aspartate aminotransferase (U/L) [M (P25, P75)] | 29 (22, 36) | 30 (23, 42) | 38 (29, 49) | < 0.001 |
| Gamma-glutamyltransferase (U/L) [M (P25, P75)] | 34 (24, 50) | 36 (25, 55) | 44 (30, 63) | < 0.001 |
| Creatinine (μmol/L) [M (P25, P75)] | 78.6 (72.0, 85.9) | 78.8 (70.6, 87.9) | 77.9 (71.3, 85.4) | 0.983 |
| Uric acid (μmol/L) [M (P25, P75)] | 389 (343, 432) | 400 (357, 449) | 410 (372, 460) | < 0.001 |
| Fasting blood glucose (mmol/L) [M (P25, P75)] | 5.32 (4.95, 5.98) | 5.39 (4.98, 5.99) | 5.58 (5.02, 6.33) | 0.029 |
| Total cholesterol (mmol/L) [M (P25, P75)] | 5.16 (4.38, 6.02) | 5.40 (4.47, 6.06) | 5.27 (4.57, 6.32) | 0.290 |
| Triglycerides (mmol/L) [M (P25, P75)] | 1.73 (1.31, 3.01) | 2.08 (1.41, 3.18) | 2.22 (1.52, 3.61) | 0.002 |
| Low-density lipoprotein (mmol/L) [M (P25, P75)] | 2.88 (2.45, 3.32) | 2.99 (2.52, 3.31) | 3.14 (2.75, 3.70) | < 0.001 |
| High-density lipoprotein (mmol/L) [M (P25, P75)] | 1.22 (1.06, 1.36) | 1.22 (1.08, 1.33) | 1.15 (1.04, 1.31) | 0.018 |
| Total bilirubin (μmol/L) [M (P25, P75)] | 17.4 (14.5, 20.3) | 17.9 (15.0, 21.3) | 18.9 (16.7, 21.5) | 0.002 |
| Total protein (g/L) [M (P25, P75)] | 72.4 (70.2, 74.7) | 71.9 (69.5, 73.9) | 71.5 (69.5, 74.0) | 0.002 |
| Hormonal parameters | ||||
| Estradiol (pmol/L) [M (P25, P75)] | 79.52 ± 20.10 | 96.02 ± 19.40 | 128.38 ± 20.37 | < 0.001 |
| Testosterone (nmol/L) [M (P25, P75)] | 16.46 (12.99, 20.47) | 12.81 (9.10, 16.90) | 8.66 (5.64, 12.45) | < 0.001 |
| Estrogen to androgen ratio | 4.74 (3.31, 6.93) | 7.40 (5.27, 11.26) | 14.20 (9.87, 24.19) | < 0.001 |
| Clinical and lifestyle factors | ||||
| Gynecomastia | < 0.001 | |||
| No | 255 (72.4) | 304 (66.5) | 79 (42) | |
| Yes | 97 (27.6) | 153 (33.5) | 109 (58) | |
| Hypertension | 0.099 | |||
| No | 279 (79.3) | 355 (77.7) | 134 (71.3) | |
| Yes | 73 (20.7) | 102 (22.3) | 54 (28.7) | |
| Diabetes mellitus | 0.016 | |||
| No | 268 (76.1) | 348 (76.1) | 124 (66) | |
| Yes | 84 (23.9) | 109 (23.9) | 64 (34) | |
| Sporting | 0.017 | |||
| No | 185 (52.6) | 249 (54.5) | 122 (64.9) | |
| Yes | 167 (47.4) | 208 (45.5) | 66 (35.1) | |
| Smoking | 0.949 | |||
| No | 175 (49.7) | 223 (51) | 91 (48.4) | |
| Yes | 177 (50.3) | 234 (49) | 97 (51.6) | |
| Time | 0.004 | |||
| 1-5 years | 85 (24.1) | 113(24.7) | 37 (19.7) | |
| 5-10 years | 115 (32.7) | 140 (30.6) | 49 (26.1) | |
| 10-20 years | 102 (29) | 151 (33) | 56 (29.8) | |
| ≥ 20 years | 50 (14.2) | 53 (11.6) | 46 (24.4) | |
Regarding GYN, the incidence of GYN in the severe group (58%) was significantly higher than that in the moderate group (33.5%) and the mild group (27.6%) (P < 0.001), indicating that the incidence of GYN increases with the severity of fatty liver.
Therefore, subgroup analyses of MASLD severity indicates that as fatty liver becomes serious, abnormalities in liver function, lipid metabolism, and sex hormones become more pronounced, which in turn promote the development of GYN in patients with MASLD.
The forest plot of the impact values of each MASLD subgroup on GYN are shown in Figure 2A. Figure 2B is a forest plot of the overall impact of MASLD on GYN values, which more visually and concretely reveals the magnitude of the impact values of variables in each subgroup. Forest mapping was carried out using R software (version 4.3.1). The forestplot package was employed for forest plot construction.
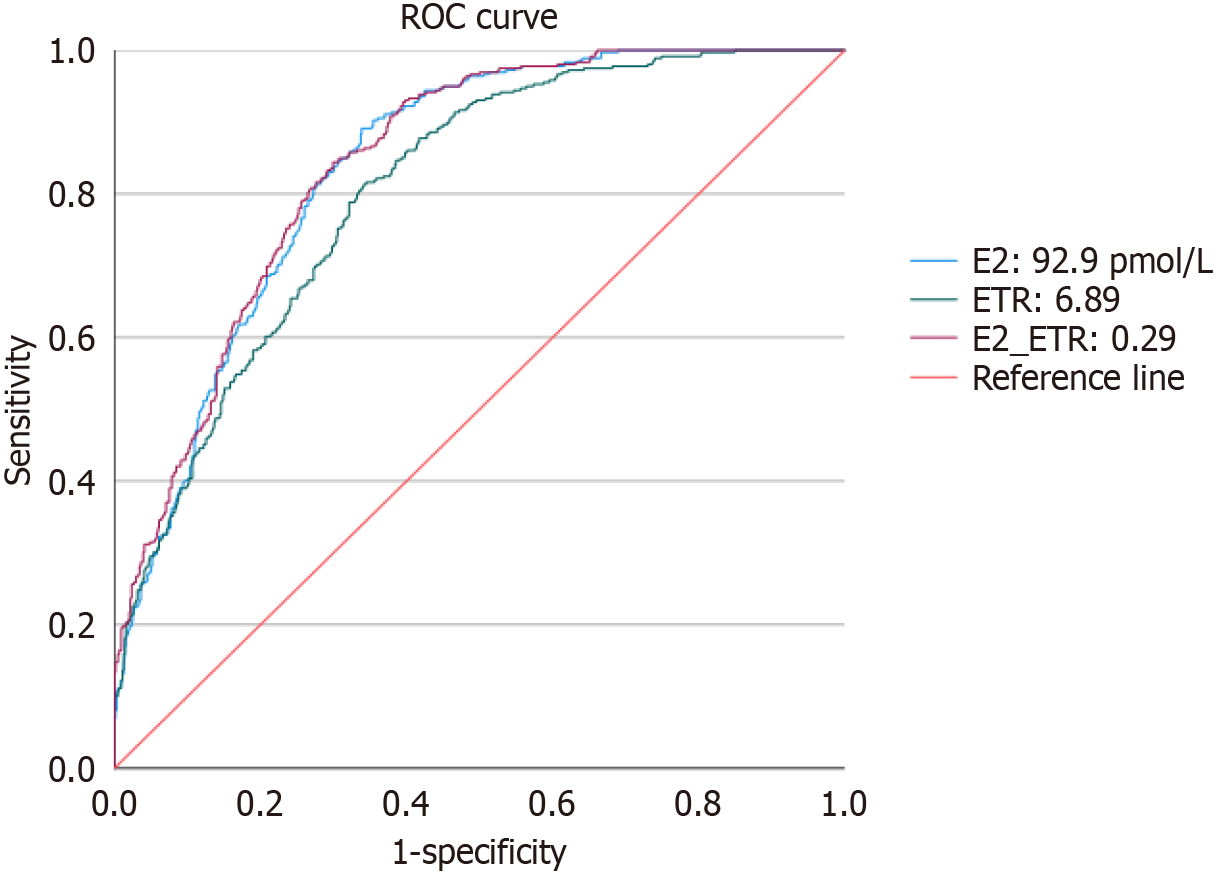
To assess the diagnostic efficacy of E2, ETR, and their combined metrics in predicting GYN, the study performed ROC curve analysis, and the results are shown in Table 5 and Figure 3. In univariate analysis, E2 showed higher diagnostic efficacy, with an AUC of 0.837, an optimal cutoff value of 92.9 pmol/L, a sensitivity of 89.1%, and a specificity of 66.1%. Inmultivariate joint analysis, the combined predictor of E2 and ETR showed an improved AUC of 0.843 (95%CI: 0.819-0.866) in predicting the development of GYN, with a Jordon index of 0.545, an optimal cut-off value of 0.29, and a sensitivity and specificity of 84.4% and 70.1%, respectively.
| Area under the curve | Youden’s index | Cut-off value | P value | 95%Cl | Sensitivity | Specificity | |
| E2 | 0.837 | 0.552 | 92.9 | < 0.001 | 0.813-0.861 | 89.1% | 66.1% |
| ETR | 0.804 | 0.471 | 6.89 | < 0.001 | 0.777-0.831 | 81.1% | 66% |
| E2_ETR | 0.843 | 0.545 | 0.29 | < 0.001 | 0.819-0.866 | 84.4% | 70.1% |
The diagnostic efficacy of the combined index was superior to that of the single index, exhibiting a higher AUC.
It can be seen that E2 has a higher predictive efficacy among the single indicators, but the AUC of the combined indicators is superior to that of every single indicator, suggesting the stronger diagnostic value of combined analysis of multiple indicators in predicting GYN and a better balance between sensitivity and specificity of combined indicators.
In this study, the incidence of GYN was 36% in patients with MASLD, and gradually increased with the severity of MASLD and the duration of the disease. It was found that the severity of MASLD and its duration remained risk factors for GYN after adjusting relevant confounders in multivariate logistic regression analysis.
In our study, we found that the severity of MASLD was significantly associated with the incidence of GYN, which increased with the severity and duration of fatty liver. This study included 352 patients with mild MASLD (35.3%), and the prevalence of GYN in the mild group was 27.6%, which was significantly lower than that in the moderate and severe groups (33.5% and 58%), and their sex hormone imbalances (e.g., the E2/T ratio) showed a gradient correlation with the severity of MASLD (Table 4). Despite the lower degree of metabolic abnormalities in patients with mild MASLD, through multiple logistic regression analysis (Table 3), we found that even after adjusting for confounders such as age, BMI, hypertension, and DM, the association between MASLD severity and GYN was still statistically significant (OR = 1.523 in the moderate group and OR = 3.627 in the severe group). This suggests that even though the metabolic disturbances of mild MASLD are mild, its association with GYN can still be effectively captured by a large sample size (n = 997) and rigorous statistical methods. Future studies could further refine the mechanism of the effect of mild MASLD by extending the follow-up time or incorporating more sensitive metabolic markers.
Studies have shown that patients with MASLD are more likely to experience sex hormone imbalances and estrogen/androgen ratio imbalances underlying breast tissue hyperplasia[24,25]. It has been noted that insulin resistance and chronic inflammation in patients with fatty liver disease inhibit T synthesis and increase levels of sex hormone-binding globulin, thereby reducing the effective concentration of free androgens[26]. Sex hormone imbalance is the key pathomechanism of GYN, which is believed to be primarily caused by enhanced stimulatory effect of estrogen on breast tissue and the diminished inhibitory effect of androgens on breast tissue[27,28]. The results of the present study are consistent with this finding, as we observed a particularly significant increase in the E2/T ratio in patients with GYN. In addition, our study also found that the severity of MASLD is closely related to the imbalance of sex hormones, especially as the severity of fatty liver increases, the E2 level gradually increases, the T level decreases, and the E2/T ratio significantly increases, making GYN more frequent in patients with severe fatty liver disease than in patients with mild to moderate fatty liver[29]. In this study, regardless of the severity of fatty liver, GYN patients showed significantly higher E2 levels, and ETR than non-GYN patients, while androgen levels were significantly lower[30]. There is no relevant literature that directly addresses the correlation of GYN in patients with MASLD.
However, GYN also occurs in patients with mild MASLD, whose changes in sex hormone levels are not as dramatic as those with moderate or severe MASLD. The E2 levels and ETR in moderate or severe MASLD patients are normal or only mildly elevated, while androgen levels are slightly decreased or decreased compared with non-MASLD patients. Possible explanation is as follows: (1) First, even in patients with mild fatty liver disease, metabolic disturbances (e.g., insulin resistance, abnormal lipid metabolism) may have already begun to act on the gonadal axis, but have not yet progressed to a stage of severe sex hormone imbalance[31]; (2) Second, individual differences exist, age, body fat distribution, and genetic background of patients may determine the extent to which fatty liver affects sex hormone metabolism[32]; and (3) Third, the local sensitivity of breast tissue may be more sensitive to E2 levels in certain patients, leading to breast development[33,34].
We also found that the longer the duration of MASLD, the more significant the metabolic disorders caused, and the more severe the degree of sex hormone disruption. A gradual increase in E2 levels and the ETR, and a gradual decrease in androgen levels, suggest that prolonged fatty liver disease may have a cumulative effect on the imbalance of sex hormones, which is consistent with the long-term effects of fatty liver disease on systemic metabolism. Related studies have shown that chronic inflammation and insulin resistance induced by fatty liver over time can have long-term inhibitory effects on the hypothalamic-pituitary-gonadal axis[35,36]. The increased incidence of GYN in patients with a longer duration of MASLD may be related to the cumulative effect of sex hormone disorders, especially in patients with a longer duration, the E2/T ratio is significantly higher than that in patients with a shorter duration[37].
In the present study, we found that the severity of fatty liver was positively correlated with E2 levels and E2-to-androgen ratio. The more severe the fatty liver, the higher the E2 levels and the E2-to-androgen ratio, both are crucial in GYN, which is consistent with existing studies. Studies have shown that increased aromatase activity in adipose tissue is the main cause of elevated E2 levels in patients with fatty liver disease. Patients with moderate-to-severe fatty liver disease typically have systemic fat accumulation, and abnormal liver function can reduce estrogen inactivation, thereby exacerbating the increase in E2 levels[38,39]. In contrast to the studies, estrogen is considered a protective factor against fatty liver and may resist its formation[40]. Contrary to the results of the present study, it may be due to the fact that their subjects were non-fatty liver patients, which further confirms the dual effect of estrogen on fatty liver[41].
In this study, we found that MASLD patients had significantly higher E2 levels than those without GYN. E2, as the main estrogen, acts directly on estrogen receptors in breast tissue to activate the proliferation of mammary epithelial cells and induce mastocytosis[42]. E2 stimulates the proliferation of mammary epithelial cells by down-regulating the inhibitory effects of androgens in breast stromal cells while stimulating the proliferation of mammary epithelial cells[43]. In addition, E2 may further promote breast development by altering the microenvironment of local breast tissue (e.g., stimulating angiogenesis and local inflammation)[44]. In short, the mechanism of action of estrogen includes enhancing receptor binding, increasing signaling, and decreasing androgen-mediated inhibition, thereby further shifting the balance toward estrogen dominance.
Androgen levels in MASLD patients with GYN are significantly lower than those in non-GYN patients. Androgens have an inhibitory effect on breast tissue through mechanisms such as inhibiting the proliferation of breast epithelial cells through the androgen receptor and limiting the pro-proliferative effects of estrogen[45,46].
MASLD patients with GYN have significantly higher ETR than non-GYN patients. Previous studies have shown that both elevated estrogen levels and decreased androgen levels lead to an elevated ETR, disrupting the dynamic balance of sex hormones in breast tissue[47]. Elevated ETR may lead to a state of persistent stimulation of breast tissue, allowing for accelerated proliferation of ductal and epithelial cells while inhibiting the supportive and reparative effects of stromal cells on breast tissue; in addition, this sex hormone imbalance is associated with alterations in the microenvironment of the breast tissue, which further promotes cell proliferation[48,49].
In the present study, some non-GYN patients also showed higher E2 levels while not developing breast dysplasia. According to related studies, this may be related to the different sensitivity of breast tissue to E2 in individuals, as well as the enhancement of androgen receptor activity or local anti-estrogenic effects[50]. It has been suggested that the expression level of estrogen receptor in breast tissue may determine whether E2 is associated with mastocytosis[51]. Studies have shown that estrogen receptor-α signaling plays a key role in estrogen-dependent gene expression and its impact on breast tissue development[52].
Some GYN patients do not have a significant decrease in T levels, only elevated E2 levels. According to related studies, this may be due to accelerated local androgen conversion. Even if the overall androgen level is normal, androgens in the breast tissue may be rapidly converted to E2 through the aromatase pathway, resulting in a local hyperestrogenic state[53,54]. Secondly, some patients may suffer androgen receptor dysfunction due to androgen dysfunction, and their inhibitory effect on breast tissue may be diminished regardless of normal androgen levels[55].
Pro-inflammatory factors such as interleukin-6, tumor necrosis factor-alpha, and adipokines such as leptin released from adipose tissue can act on male breast tissue, increasing aromatase activity and activating estrogen receptors in breast tissue, thereby promoting male breast hyperplasia, which is more common in patients with MASLD[56-58].
In addition, recent studies suggest that there may be a bidirectional relationship between GYN and metabolic syndrome. On the one hand, insulin resistance may inhibit the gonadal axis, leading to decreased T levels and relatively elevated estrogen[59] to promote abnormal development of breast tissue. On the other hand, GYN may reflect underlying endocrine disorders, suggesting that patients are more likely to have metabolic disturbances such as dyslipidemia and insulin resistance[60,61], which further emphasizes the importance of assessing metabolic risk in patients with GYN.
In this study, we evaluated the predictive value of sex hormone indicators (E2, T and their ratio ETR) for the occurrence of GYN in MASLD patients using ROC curves. E2 showed the highest predictive efficacy for a single indicator (AUC = 0.837). The model of combining E2 and T further improved the diagnostic value (AUC = 0.843). Therefore, the combined model (E2 + T) can help clinicians identify the high-risk group of GYN in MASLD patients more accurately. Serum E2 showed a critical value of 92.9 pmol/L, and ETR showed a critical value of 6.89. Several previous studies have been conducted to discuss the correlation between E2 and ETR and GYN. However, there is no study investigating the correlation between the value of E2 content and ETR and GYN, let alone analyzing the correlation of ROC curves.
Therefore, in patients with MASLD, if E2 approaches or exceeds this threshold (92.9 pmol/L) and/or ETR is close to or above 6.89, even without obvious breast enlargement, early GYN should be alerted and metabolic indexes (e.g., blood glucose, blood lipids) should be monitored to guide early interventions and reduce further progression of metabolic and hormonal abnormalities.
This study focused on a gender-specific male population of adult MASLD patients and analyzed their association with GYN, filling a research gap in the field. The relationship between the severity and duration of MASLD and GYN was explored for the first time. Currently, studies on GYN focus on endocrine diseases, pubertal development, or drug-induced GYN, while there is little research on MASLD-related GYN. The present study is the first to take MASLD, a metabolic liver disease, as an entry point, and combine it with the analysis of BMI and sex hormone imbalance to reveal the potential links between MASLD, sex hormones metabolic disorders, and GYN.
This study first attempted to take GYN as a potential marker of MASLD-related metabolic disorders and analyze the association between GYN and metabolic disorders (e.g., hyperglycemia, hypertension, and abnormalities of lipid metabolism), shedding new light on the early screening and intervention of clinical metabolic disorders.
Based on the results of this study, the treatment of adult GYN should focus on the comprehensive management of metabolic disorders, such as adjusting sex hormone balance by improving MASLD and obesity to reduce the occurrence and progression of GYN.
The study population consists of adult male MASLD patients from a physical examination center of a tertiary care hospital, and there may have been some selection bias. The relatively good health status of the physical examination population may underestimate the prevalence of GYN among patients with more severe metabolic disorders. Results of this study may not be directly generalizable to other groups since the study population only includes adult males(e.g., minors).
The sample size of this study is relatively limited, and the findings of this study can be further validated in future large-scale studies to improve the validity of the findings. Comparative studies with different racial, geographic, and cultural backgrounds can also help to reveal racial and geographic differences in the relationship between MASLD and GYN.
The small sample sizes of some subgroups may have affected statistical validity, but the significance results for key variables were validated by multiple models. These limitations do not diminish the core findings of this study that MASLD severity and disease duration are significantly associated with GYN risk and that sex hormone imbalance (E2/T ratio) is an important mediating mechanism. Further validation could be done in the future through prospective cohort studies or expanded sample sizes.
The study was a cross-sectional study design that only revealed correlations between MASLD, GYN, and related metabolic markers, but could not infer causality. For example, whether GYN is a consequence of MASLD development or a marker of metabolic disorders in patients with MASLD needs to be further verified in prospective cohort studies.
In this study, the duration of fatty liver disease was obtained based on patients' self-report and previous medical history records, which may have some recall bias and make it difficult to accurately assess the impact of fatty liver disease duration on GYN.
The diagnosis of MASLD cannot be derived from liver biopsy results due to cost and risk. The advantages of ultrasonography are its non-invasive and accessible nature, as well asits low cost compared with other screening modalities, making it still the most feasible mass screening tool. However, ultrasonography has some important limitations, such as the inability of ultrasonography to differentiate simple steatosis from non-alcoholic steatohepatitis, as well as its dependence on operator's skill and subjective interpretation.
In the included adult male MASLD patients, as the fatty liver progressed from mild to moderate or severe, the incidence of GYN increased progressively, with prolonged disease duration, and progressively increased estrogen-to-hormone ratio, suggesting that the severity and duration of MASLD may increase the risk of breast tissue hyperplasia by promoting metabolic disturbances and sex hormone imbalance. The presence of GYN may be a clinical marker of metabolic disturbances and sex hormone imbalance caused by MASLD patients. Therefore, early detection of GYN may be crucial for early intervention in metabolic syndrome and endocrine abnormalities in MASLD patients.
| 1. | Mantovani A, Valenti L. A call to action for fatty liver disease. Liver Int. 2021;41:1182-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59:1174-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 436] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 3. | Le MH, Le DM, Baez TC, Dang H, Nguyen VH, Lee K, Stave CD, Ito T, Wu Y, Yeo YH, Ji F, Cheung R, Nguyen MH. Global incidence of adverse clinical events in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Clin Mol Hepatol. 2024;30:235-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 4. | Younossi ZM, Henry L. Economic and Quality-of-Life Implications of Non-Alcoholic Fatty Liver Disease. Pharmacoeconomics. 2015;33:1245-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 5. | Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, Swain MG, Congly SE, Kaplan GG, Shaheen AA. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 1190] [Article Influence: 396.7] [Reference Citation Analysis (1)] |
| 6. | Viganò L, Lleo A, Aghemo A. Non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, metabolic syndrome and hepatocellular carcinoma-a composite scenario. Hepatobiliary Surg Nutr. 2018;7:130-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Hobeika C, Ronot M, Beaufrere A, Paradis V, Soubrane O, Cauchy F. Metabolic syndrome and hepatic surgery. J Visc Surg. 2020;157:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Reeves HL, Zaki MY, Day CP. Hepatocellular Carcinoma in Obesity, Type 2 Diabetes, and NAFLD. Dig Dis Sci. 2016;61:1234-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 9. | Ferguson D, Finck BN. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17:484-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 325] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 10. | Zhu JQ, Liu JZ, Yang SW, Ren ZY, Ye XY, Liu Z, Li XL, Han DD, He Q. Impact of the diagnosis of metabolic dysfunction-associated fatty liver disease and non-alcoholic fatty liver disease in patients undergoing liver transplantation for hepatocellular carcinoma. Front Endocrinol (Lausanne). 2024;15:1306091. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Yeonhee Pyo, Ki Han Kwon. Estrogen receptors, hormonal imbalance, GYN, hyperestrogenemia, and male breast cancer: A literature review. JOMH. 1-9. [DOI] [Full Text] |
| 12. | Mathur R, Braunstein GD. Gynecomastia: pathomechanisms and treatment strategies. Horm Res. 1997;48:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Neong SF, Billington EO, Congly SE. Sexual Dysfunction and Sex Hormone Abnormalities in Patients With Cirrhosis: Review of Pathogenesis and Management. Hepatology. 2019;69:2683-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Ismail AA, Barth JH. Endocrinology of gynaecomastia. Ann Clin Biochem. 2001;38:596-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Xu L, Yuan Y, Che Z, Tan X, Wu B, Wang C, Xu C, Xiao J. The Hepatoprotective and Hepatotoxic Roles of Sex and Sex-Related Hormones. Front Immunol. 2022;13:939631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 16. | Telegrafo M, Introna T, Coi L, Cornacchia I, Rella L, Stabile Ianora AA, Angelelli G, Moschetta M. Breast US as primary imaging modality for diagnosing gynecomastia. G Chir. 2016;37:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Dialani V, Baum J, Mehta TS. Sonographic features of gynecomastia. J Ultrasound Med. 2010;29:539-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Lee SS, Park SH. Radiologic evaluation of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:7392-7402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 266] [Cited by in RCA: 307] [Article Influence: 27.9] [Reference Citation Analysis (2)] |
| 19. | Khov N, Sharma A, Riley TR. Bedside ultrasound in the diagnosis of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:6821-6825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 93] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 20. | Narkiewicz K. Diagnosis and management of hypertension in obesity. Obes Rev. 2006;7:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S14-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 2132] [Article Influence: 426.4] [Reference Citation Analysis (0)] |
| 22. | Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The Physical Activity Guidelines for Americans. JAMA. 2018;320:2020-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3623] [Cited by in RCA: 3769] [Article Influence: 538.4] [Reference Citation Analysis (0)] |
| 23. | Bover Manderski MT, Delnevo CD, Warner KE. Toward a More Comprehensive Index of Youth Cigarette Smoking: Average Number of Cigarettes Smoked per Day among Students in the United States over Two Decades. Int J Environ Res Public Health. 2021;18:478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Papadimitriou K, Mousiolis AC, Mintziori G, Tarenidou C, Polyzos SA, Goulis DG. Hypogonadism and nonalcoholic fatty liver disease. Endocrine. 2024;86:28-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 25. | Pal P, Pasricha N, Sud R, Baluni P, Singh AK. Exploring Gonadal Functions in Patients With Alcoholic Liver Disease: A Correlative Analysis With Disease Severity. Cureus. 2024;16:e73178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Marino L, Jornayvaz FR. Endocrine causes of nonalcoholic fatty liver disease. World J Gastroenterol. 2015;21:11053-11076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 27. | Quiroz-Aldave JE, Gamarra-Osorio ER, Durand-Vásquez MDC, Rafael-Robles LDP, Gonzáles-Yovera JG, Quispe-Flores MA, Concepción-Urteaga LA, Román-González A, Paz-Ibarra J, Concepción-Zavaleta MJ. From liver to hormones: The endocrine consequences of cirrhosis. World J Gastroenterol. 2024;30:1073-1095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Reference Citation Analysis (0)] |
| 28. | Romano L, Granata L, Fusco F, Napolitano L, Cerbone R, Priadko K, Sciorio C, Mirone V, Romano M. Sexual Dysfunction in Patients With Chronic Gastrointestinal and Liver Diseases: A neglected Issue. Sex Med Rev. 2022;10:620-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 29. | Maruyama Y, Adachi Y, Aoki N, Suzuki Y, Shinohara H, Yamamoto T. Mechanism of feminization in male patients with non-alcoholic liver cirrhosis: role of sex hormone-binding globulin. Gastroenterol Jpn. 1991;26:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Weng C, Shao Z, Xiao M, Song M, Zhao Y, Li A, Pang Y, Huang T, Yu C, Lv J, Li L, Sun D. Association of sex hormones with non-alcoholic fatty liver disease: An observational and Mendelian randomization study. Liver Int. 2024;44:1154-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Hammerstad SS, Blackard JT, Lombardi A, Owen RP, Concepcion E, Yi Z, Zhang W, Tomer Y. Hepatitis C Virus Infection of Human Thyrocytes: Metabolic, Hormonal, and Immunological Implications. J Clin Endocrinol Metab. 2020;105:1157-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Cuhaci N, Polat SB, Evranos B, Ersoy R, Cakir B. Gynecomastia: Clinical evaluation and management. Indian J Endocrinol Metab. 2014;18:150-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 33. | Vehmas AP, Adam M, Laajala TD, Kastenmüller G, Prehn C, Rozman J, Ohlsson C, Fuchs H, Hrabě de Angelis M, Gailus-Durner V, Elo LL, Aittokallio T, Adamski J, Corthals G, Poutanen M, Strauss L. Liver lipid metabolism is altered by increased circulating estrogen to androgen ratio in male mouse. J Proteomics. 2016;133:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Liang B, Cheung AS, Nolan BJ. Clinical features and prevalence of Klinefelter syndrome in transgender individuals: A systematic review. Clin Endocrinol (Oxf). 2022;97:3-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Zhang Y, Meng F, Sun X, Sun X, Hu M, Cui P, Vestin E, Li X, Li W, Wu XK, Jansson JO, Shao LR, Billig H. Hyperandrogenism and insulin resistance contribute to hepatic steatosis and inflammation in female rat liver. Oncotarget. 2018;9:18180-18197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Cui P, Hu W, Ma T, Hu M, Tong X, Zhang F, Shi J, Xu X, Li X, Shao LR, Billig H, Feng Y. Long-term androgen excess induces insulin resistance and non-alcoholic fatty liver disease in PCOS-like rats. J Steroid Biochem Mol Biol. 2021;208:105829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Martin-Grau M, Monleon D. Sex dimorphism and metabolic profiles in management of metabolic-associated fatty liver disease. World J Clin Cases. 2023;11:1236-1244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Reference Citation Analysis (1)] |
| 38. | Della Torre S. Beyond the X Factor: Relevance of Sex Hormones in NAFLD Pathophysiology. Cells. 2021;10:2502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 39. | El Mahdy Korah T, Abd Elfatah Badr E, Mohamed Emara M, Ahmed Samy Kohla M, Gamal Saad Michael G. Relation between sex hormones and hepatocellular carcinoma. Andrologia. 2016;48:948-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Song MJ, Choi JY. Androgen dysfunction in non-alcoholic fatty liver disease: Role of sex hormone binding globulin. Front Endocrinol (Lausanne). 2022;13:1053709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 41. | Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a Sexual Dimorphic Disease: Role of Gender and Reproductive Status in the Development and Progression of Nonalcoholic Fatty Liver Disease and Inherent Cardiovascular Risk. Adv Ther. 2017;34:1291-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 411] [Cited by in RCA: 397] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 42. | Ho Y, Li ZL, Shih YJ, Chen YR, Wang K, Whang-Peng J, Lin HY, Davis PJ. Integrin αvβ3 in the Mediating Effects of Dihydrotestosterone and Resveratrol on Breast Cancer Cell Proliferation. Int J Mol Sci. 2020;21:2906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Ruiz TFR, Colleta SJ, Zuccari DAPC, Vilamaior PSL, Leonel ECR, Taboga SR. Hormone receptor expression in aging mammary tissue and carcinoma from a rodent model after xenoestrogen disruption. Life Sci. 2021;285:120010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Chin YT, Yang SH, Chang TC, Changou CA, Lai HY, Fu E, HuangFu WC, Davis PJ, Lin HY, Liu LF. Mechanisms of dihydrotestosterone action on resveratrol-induced anti-proliferation in breast cancer cells with different ERα status. Oncotarget. 2015;6:35866-35879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Hickey TE, Selth LA, Chia KM, Laven-Law G, Milioli HH, Roden D, Jindal S, Hui M, Finlay-Schultz J, Ebrahimie E, Birrell SN, Stelloo S, Iggo R, Alexandrou S, Caldon CE, Abdel-Fatah TM, Ellis IO, Zwart W, Palmieri C, Sartorius CA, Swarbrick A, Lim E, Carroll JS, Tilley WD. The androgen receptor is a tumor suppressor in estrogen receptor-positive breast cancer. Nat Med. 2021;27:310-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 150] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 46. | Toniutto P, Shalaby S, Mameli L, Morisco F, Gambato M, Cossiga V, Guarino M, Marra F, Brunetto MR, Burra P, Villa E; Special Interest Group Gender in Hepatology of the Italian Association for the Study of the Liver (AISF). Role of sex in liver tumor occurrence and clinical outcomes: A comprehensive review. Hepatology. 2024;79:1141-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 47. | Vlčková R, Andrejčáková Z, Sopková D, Kozioł K, Hertelyová Z, Koziorowska A, Gancarčíková S. Effects of supplemental flaxseed on the ovarian and uterine functions of adult cycling mice. Gen Physiol Biophys. 2022;41:205-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Khalifa A, Guijarro A, Nencioni A. Advances in Diet and Physical Activity in Breast Cancer Prevention and Treatment. Nutrients. 2024;16:2262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 49. | Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol. 2018;182:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 311] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 50. | Pan J, Liu P, Yu X, Zhang Z, Liu J. The adverse role of endocrine disrupting chemicals in the reproductive system. Front Endocrinol (Lausanne). 2023;14:1324993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 51. | Saha T, Makar S, Swetha R, Gutti G, Singh SK. Estrogen signaling: An emanating therapeutic target for breast cancer treatment. Eur J Med Chem. 2019;177:116-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 52. | Reue K, Wiese CB. Illuminating the Mechanisms Underlying Sex Differences in Cardiovascular Disease. Circ Res. 2022;130:1747-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 53. | Maliqueo M, Echiburú B, Crisosto N. Perinatal androgen exposure and adipose tissue programming: is there an impact on body weight fate? Expert Rev Endocrinol Metab. 2015;10:533-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 54. | Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745-E751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1241] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 55. | Eden JA, Place J, Carter GD, Alaghband-Zadeh J, Pawson ME. The role of chronic anovulation in the polycystic ovary syndrome: normalization of sex-hormone-binding globulin levels after clomiphene-induced ovulation. Clin Endocrinol (Oxf). 1989;30:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 56. | Szukiewicz D, Trojanowski S, Kociszewska A, Szewczyk G. Modulation of the Inflammatory Response in Polycystic Ovary Syndrome (PCOS)-Searching for Epigenetic Factors. Int J Mol Sci. 2022;23:14663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 57. | Arefhosseini S, Ebrahimi-Mameghani M, Najafipour F, Tutunchi H. Non-alcoholic fatty liver disease across endocrinopathies: Interaction with sex hormones. Front Endocrinol (Lausanne). 2022;13:1032361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 58. | Dehesh T, Fadaghi S, Seyedi M, Abolhadi E, Ilaghi M, Shams P, Ajam F, Mosleh-Shirazi MA, Dehesh P. The relation between obesity and breast cancer risk in women by considering menstruation status and geographical variations: a systematic review and meta-analysis. BMC Womens Health. 2023;23:392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 59. | Dhindsa S, Ghanim H, Batra M, Kuhadiya ND, Abuaysheh S, Sandhu S, Green K, Makdissi A, Hejna J, Chaudhuri A, Punyanitya M, Dandona P. Insulin Resistance and Inflammation in Hypogonadotropic Hypogonadism and Their Reduction After Testosterone Replacement in Men With Type 2 Diabetes. Diabetes Care. 2016;39:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 200] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 60. | Ladizinski B, Lee KC, Nutan FN, Higgins HW 2nd, Federman DG. Gynecomastia: etiologies, clinical presentations, diagnosis, and management. South Med J. 2014;107:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 61. | Robeva R, Elenkova A, Zacharieva S. Causes and Metabolic Consequences of Gynecomastia in Adult Patients. Int J Endocrinol. 2019;2019:6718761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |