Published online Jan 26, 2013. doi: 10.4252/wjsc.v5.i1.26
Revised: August 23, 2012
Accepted: December 20, 2012
Published online: January 26, 2013
AIM: To study the expression of embryonal markers by fetal cardiac mesenchymal stem cells (fC-MSC) and their differentiation into cells of all the germ layers.
METHODS: Ten independent cultures of rat fC-MSC were set up from cells derived from individual or pooled fetal hearts and studies given below were carried out at passages 3, 6, 15 and 21. The phenotypic markers CD29, CD31, CD34, CD45, CD73, CD90, CD105, CD166 and HLA-DR were analyzed by flow cytometry. The expression of embryonal markers Oct-4, Nanog, Sox-2, SSEA-1, SSEA-3, SSEA-4, TRA-1-60 and TRA 1-81 were studied by immunocytochemistry. The fC-MSC treated with specific induction medium were evaluated for their differentiation into (1) adipocytes and osteocytes (mesodermal cells) by Oil Red O and Alizarin Red staining, respectively, as well as by expression of lipoprotein lipase, PPARγ2 genes in adipocytes and osteopontin and RUNX2 genes in osteocytes by reverse-transcription polymerase chain reaction (RT-PCR); (2) neuronal (ectodermal) cells by expression of neuronal Filament-160 and Glial Fibrillar Acidic Protein by RT-PCR and immunocytochemistry; and (3) hepatocytic (endodermal) cells by expression of albumin by RT-PCR and immunocytochemistry, glycogen deposits by Periodic Acid Schiff staining and excretion of urea into the culture supernatant.
RESULTS: The fC-MSC expressed CD29, CD73, CD90, CD105, CD166 but lacked expression of CD31, CD34, CD45 and HLA-DR. They expressed embryonal markers, viz. Oct-4, Nanog, Sox-2, SSEA-1, SSEA-3, SSEA-4, TRA-1-81 but not TRA-1-60. On treatment with specific induction media, they differentiated into adipocytes and osteocytes, neuronal cells and hepatocytic cells.
CONCLUSION: Our results together suggest that fC-MSC are primitive stem cell types with a high degree of plasticity and, in addition to their suitability for cardiovascular regenerative therapy, they may have a wide spectrum of therapeutic applications in regenerative medicine.
- Citation: Srikanth GVN, Tripathy NK, Nityanand S. Fetal cardiac mesenchymal stem cells express embryonal markers and exhibit differentiation into cells of all three germ layers. World J Stem Cells 2013; 5(1): 26-33
- URL: https://www.wjgnet.com/1948-0210/full/v5/i1/26.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v5.i1.26
The heart is composed of multiple cell types, including cardiomyocytes, endothelial cells, vascular smooth muscle cells, connective tissue and cells of the conduction system; hence, stem cells that have a multipotent differentiation potential with ability to give rise to cells of different germ layers would be the most suitable for efficient regeneration of heart[1,2]. Although embryonic stem cells are pluripotent, their clinical applications are restricted by their teratogenic and ethical concerns. Hence, different pre- and post-natal tissues are being extensively explored as an alternative source of pluripotent/multipotent stem cells for cardiovascular regenerative therapy[3-5].
Recently, we have identified a population of mesenchymal stem cells (MSC) from rat fetal heart and termed them as fetal cardiac MSC (fC-MSC)[6]. These stem cells possessed morphological and phenotypical characteristics of typical bone marrow derived MSC. In addition, they expressed cardiovascular markers and differentiated into all major cells of cardiovascular lineage. These cells exhibited several primitive characteristics, including extended self renewal properties, and expression of OCT-4, Nanog and Sox-2 at gene level and SSEA-1, SSEA-3 and SSEA-4 at protein level[6]. However, it remains to be determined whether the differentiation of fC-MSC is restricted to cardiovascular or mesodermal lineage or if they possess a multipotent differentiation potential with ability to differentiate into cells of other germ layers as well.
Therefore, the objective of the present study was to evaluate further the primitive characteristics of fC-MSC and we studied the expression of a wide array of embryonic/pluripotency markers by fC-MSC and their capacity to differentiate into mesodermal, ectodermal and endodermal lineages.
Heart tissues obtained from 16 d gestation age fetuses of female Sprague-Dawley rats were minced and digested with 1 mg/mL collagenase type-IV (Worthington Biochemical, United States) in serum free α-MEM medium for 30 min at 37 °C with intermittent stirring. After washing, the minced tissues were cultured under standard tissue culture conditions in 25 cm2 tissue culture flasks (Becton, Dickinson; United States) using complete culture medium consisting of α-MEM medium, 2 mg/mL of Glutamax (Gibco-Invitrogen), 16.5% fetal bovine serum (Hyclone, United States) and bacteriostatic level of penicillin-streptomycin (Gibco-Invitrogen). The semi confluent cultures of cells obtained within 72 h were harvested by trypsinization (0.05% Trypsin-EDTA) (Gibco-Invitrogen) and the cells were expanded in larger flasks up to 30 passages. Ten independent fC-MSC cultures were set up from cells derived from individual hearts or pooled from 2-3 hearts and studies given below were carried out at passages 3, 6, 15 and 21. All the procedures were performed as per guidelines of the Institutional Animal Ethics Committee of Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, India.
The cells were directly stained with pre conjugated anti-rat monoclonal antibodies to CD44-fluorescein isothyocyanate (FITC), CD90-FITC, CD45-phycoerythrin (PE) and HLA DR-PE. Indirect staining was performed using un-conjugated primary antibodies viz. mouse anti-rabbit CD29 (Abcam, United Kingdom), rabbit anti-mouse CD73 (Becton Dickinson), rabbit anti-mouse CD105 (Santa Cruz, United States), rabbit anti-mouse CD166 (LsBio, United States), rabbit anti-mouse CD34 (Santa Cruz, United States) and rabbit anti-mouse CD31 (Serotec, United Kingdom). Thereafter, cells were stained with FITC/PE conjugated polyclonal rabbit anti-mouse or mouse anti-rabbit secondary antibodies (Abcam) or isotype matched control monoclonal antibodies (Becton Dickinson). Stained cells were analyzed using FCS Express 3.0 in Flow Cytometer (FACS Calibur, Becton, and Dickinson, United States).
Expression of Oct-4, Nanog, Sox-2, SSEA-1, SSEA-3, SSEA-4, TRA-1-60 and TRA-1-81 was studied by immunocytochemistry. The fC-MSC was fixed with 4% para-formaldehyde (Sigma Aldrich) in phosphate buffered saline (PBS), pH 7.4, for 1 h at room temperature. The fixed cells were incubated overnight at 4 °C with the following primary antibodies: Oct-4, Nanog and Sox-2, SSEA-1, SSEA-3, SSEA-4, TRA-1-60 and TRA-1-81 (ES cell characterization kit; Chemicon, United States), diluted 1:50. After washing with PBS, cells were incubated with 1:500 diluted IgG (Fab) 2 FITC as the secondary antibody (Abcam) and stained with Hoechst dye. The pictures were taken using a fluorescent microscope (Nikon 80i, Japan).
Expression of lipo-protein lipase, PPARγ2, osteopontin, RUNX2, neuronal filament (NF)-160, glial fibrillar acidic protein (GFAP), albumin and GAPDH was determined by reverse-transcription polymerase chain reaction (RT-PCR). RNA of the cells was extracted using RNeasy Mini RNA isolation kit (Gibco-Invitrogen). One μg of total RNA was reverse transcribed into cDNA using random hexamers (Gibco-Invitrogen). The gene expression was analyzed for the following genes using primers from MWG Biotech, Germany (Table 1). The amplicons were resolved on 2% agarose gel (Sigma-Aldrich, United States) and pictures acquired using a gel documentation system (Alpha Imager, United States).
| Gene | Primer sequence | Product size (bp) | Accession number |
| Osteopontin | F: TCGGAGGAGAAGGCGCATTACAGC | 778 | AB001382.1 |
| R: TCCTCATGGCTGTGAAACTCGTGG | |||
| RUNX2 | F: TTCGTCAGCGTCCTATCAGTTC | 149 | NM_053470.2 |
| R: CTTCCATCAGCGTCAACACC | |||
| PPARγ2 | F: TTGATTTCTCCAGCATTTC | 125 | NM_001145366.1 |
| R: GCTCTACTTTGATCGCACT | |||
| Lipoprotein Lipase | F: GGGTCGCCTGGTCGAAGT | 450 | L03294.1 |
| R: AAAGTGCCTCCATTGGGATAAA | |||
| Neurofilament-160 | F: CTCGACTTCAGCCAGTCCTCTTCG | 550 | NM_017029.1 |
| R: TCTTTGCGCTCTACGGTGATGTGC | |||
| GFAP | F: AGCTGAACCAGCTTCGAGCCAAGG | 508 | NM_017009.2 |
| R: GGAAGCAACGTCTGTGAGGTCTGC | |||
| Albumin | F: TCGTGACAACTACGGTGAACTGGC | 640 | NM_134326.2 |
| R: TGTTCTGTCTCAGCGAGACACTGG | |||
| GAPDH | R: CCTCTCTCTTGCTCTCAGTAT | 284 | NM_017008.3 |
| F: GTATCCGTTGTGGATCTGACA |
The cells were treated with osteogenic medium consisting of DMEM medium (Gibco-Invitrogen) containing 10% FBS (Hyclone), 1 mmol/L dexamethasone, 10 mg/mL glyceraldehyde 3-phosphate and 0.1 mmol/L ascorbic acid (Osteogenesis kit, Chemicon). Control cells were treated with complete medium alone. After 21 d, the experimental and control cells were fixed with 4% para-formaldehyde and stained with Alizarin Red Stain to visualize mineralization.
The cells were treated with adipogenic medium consisting of DMEM medium (Gibco-Invitrogen) containing 10% FBS (Hyclone), 500 mmol/L isobutylmethylxanthine, 1 mmol/L dexamethasone, 10 mg/mL insulin and 100 mmol/L indomethacin (Adipogenesis kit, Chemicon). Control cells were treated with complete medium alone. Cells were fixed and stained with Oil Red O Stain to visualize fat droplets in the cells.
The cells were treated with neurogenic medium consisting of complete culture medium supplemented with 100 ng/mL of basic fibroblast growth factor, 100 ng/mL of noggin, 20 ng/mL of neurotrophin, 10 ng/mL of brain derived growth factor, 10 ng/mL of glial derived growth factor and 20 μmol/L of retinoic acid (all from (R and D systems, United States) and 1X of B-27 (Gibco-Invitrogen) and 1X of 2-mercaptoethanol (Sigma Aldrich) (experimental cells) or complete culture medium alone (control cells). The cultures were terminated after 21 d and the neuronal nature of the cells was characterized by immunocytochemistry using primary antibodies against NF-160 (ready to use) and GFAP (ready to use) (Biovision Inc., United States). After washing with PBS, cells were incubated with 1:500 diluted IgG (Fab) 2 FITC secondary antibody (Abcam) and stained with Hoechst dye (Sigma). The pictures were taken using a fluorescent microscope (Nikon).
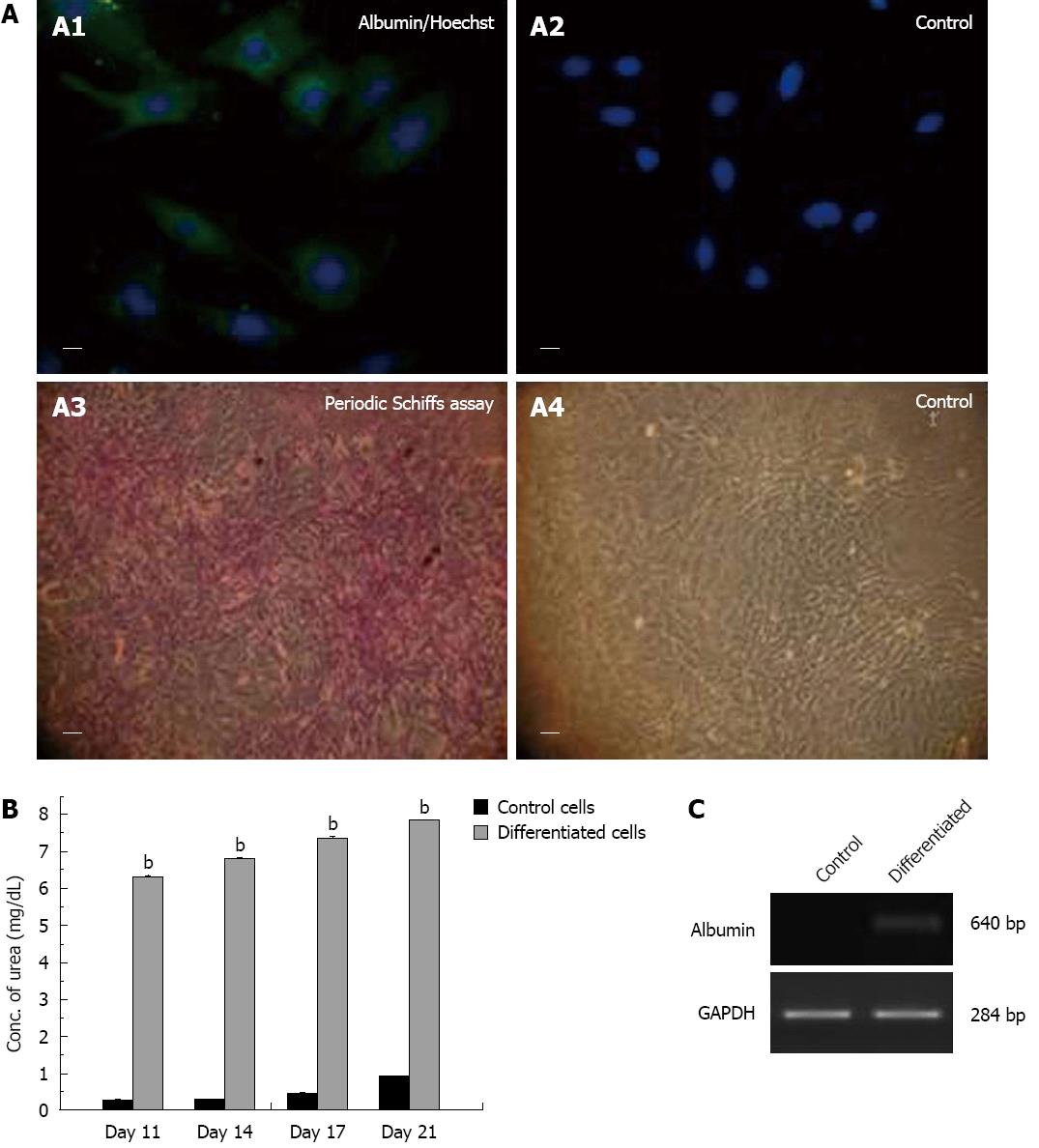
The cells were treated with hepatogenic medium consisting of complete culture medium supplemented with 50 ng/mL of hepatocyte growth factor, 50 ng/mL of fibroblast growth factor-4 (R and D systems) (experimental cells) or complete culture medium alone (control cells). Hepatocytic characterization was done by immunocytochemistry using 1:500 diluted primary antibody to albumin (Sigma-Aldrich). After washing with PBS, cells were incubated with 1:500 diluted IgG (Fab) 2 FITC as the secondary antibody (Abcam) and stained with Hoechst dye (Sigma). The pictures were taken using a fluorescent microscope (Nikon).
The hepatocytic differentiation was further confirmed by urea assay and Periodic Acid-Schiff assay.
The urea assay was performed by estimating the concentration of urea in the supernatant of experimental and control cells at 11, 14, 17 and 21 d using urea assay kit (Merck diagnostics, India), following the manufacturer’s instructions.
In the Periodic Acid-Schiff assay, experimental and control cells after fixation with 4% para-formaldehyde were oxidized in 10 g/L periodic acid for 10 min and rinsed thrice in dH2O. Thereafter, cells treated with Schiff’s reagent for 10 min were rinsed in dH2O for 10 min and stained with hematoxylin for 2 min to visualize glycogen deposits.
Results are presented as mean ± SE. Statistical significance was defined as P < 0.05 by analysis of variance using SPSS 16.0 software.
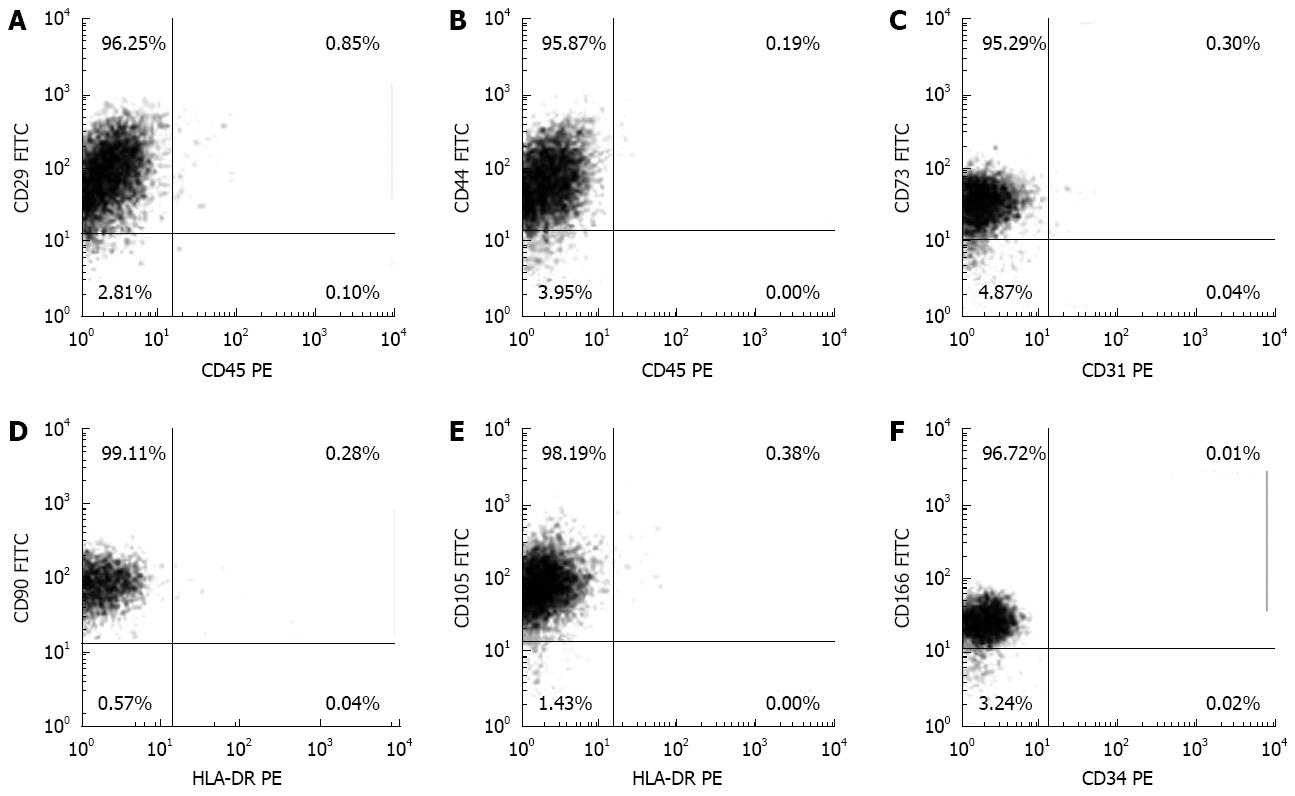
Flow cytometric analysis showed a typical mesenchymal phenotype of fC-MSC with expression of CD29, CD44, CD73, CD90, CD105 and CD166 markers and no expression of CD31, CD34, CD45 and MHC-II markers (Figure 1); this phenotype was maintained over the successive passages (Table 2).
| Markers | Primary culture | Passage 3 | Passage 6 | Passage 15 | Passage 21 |
| CD29 | 96.62 ± 0.26 | 96.54 ± 0.44 | 96.16 ± 0.58 | 96.41 ± 0.34 | 95.80 ± 0.44 |
| CD44 | 95.80 ± 0.18 | 95.96 ± 0.10 | 96.00 ± 0.30 | 96.50 ± 0.44 | 95.66 ± 0.84 |
| CD73 | 95.78 ± 0.12 | 95.10 ± 0.16 | 95.60 ± 0.24 | 95.60 ± 0.30 | 95.30 ± 0.32 |
| CD90 | 98.56 ± 0.30 | 98.90 ± 0.18 | 98.68 ± 0.28 | 98.60 ± 0.12 | 98.80 ± 0.12 |
| CD105 | 98.40 ± 0.30 | 98.00 ± 0.12 | 97.68 ± 0.18 | 97.60 ± 0.22 | 97.40 ± 0.24 |
| CD31 | 0.44 ± 0.08 | 0.50 ± 0.02 | 0.52 ± 0.02 | 0.50 ± 0.02 | 0.51 ± 0.01 |
| CD45 | 0.66 ± 0.02 | 0.64 ± 0.02 | 0.54 ± 0.02 | 0.62 ± 0.01 | 0.58 ± 0.04 |
| HLA-DR | 0.38 ± 0.02 | 0.30 ± 0.08 | 0.28 ± 0.04 | 0.34 ± 0.04 | 0.32 ± 0.02 |
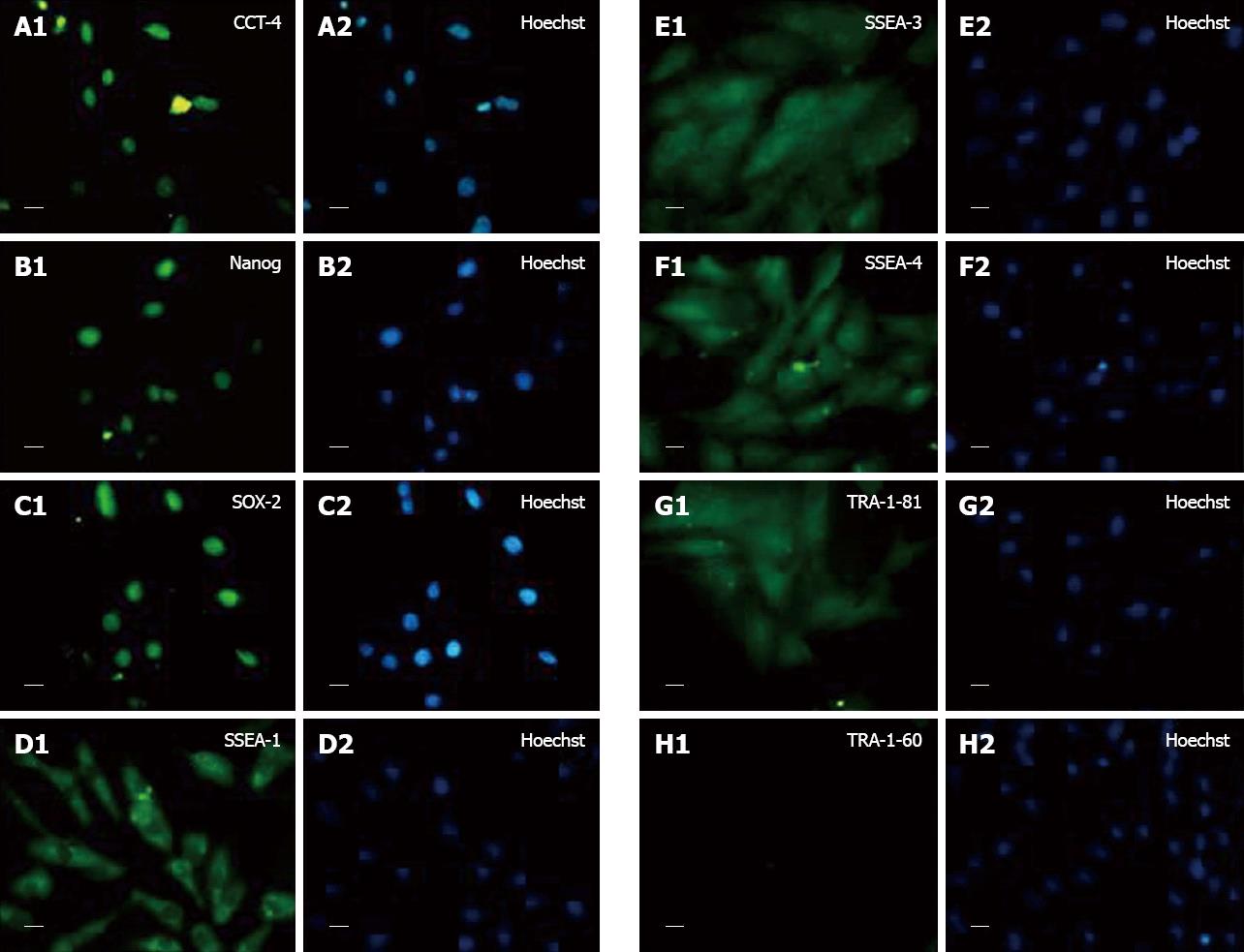
The fC-MSC expressed embryonal markers Oct-4, Nanog, Sox-2, SSEA-1, SSEA-3, SSEA-4, TRA 1-81 but not TRA 1-60, as revealed by immunocytochemistry (Figure 2).
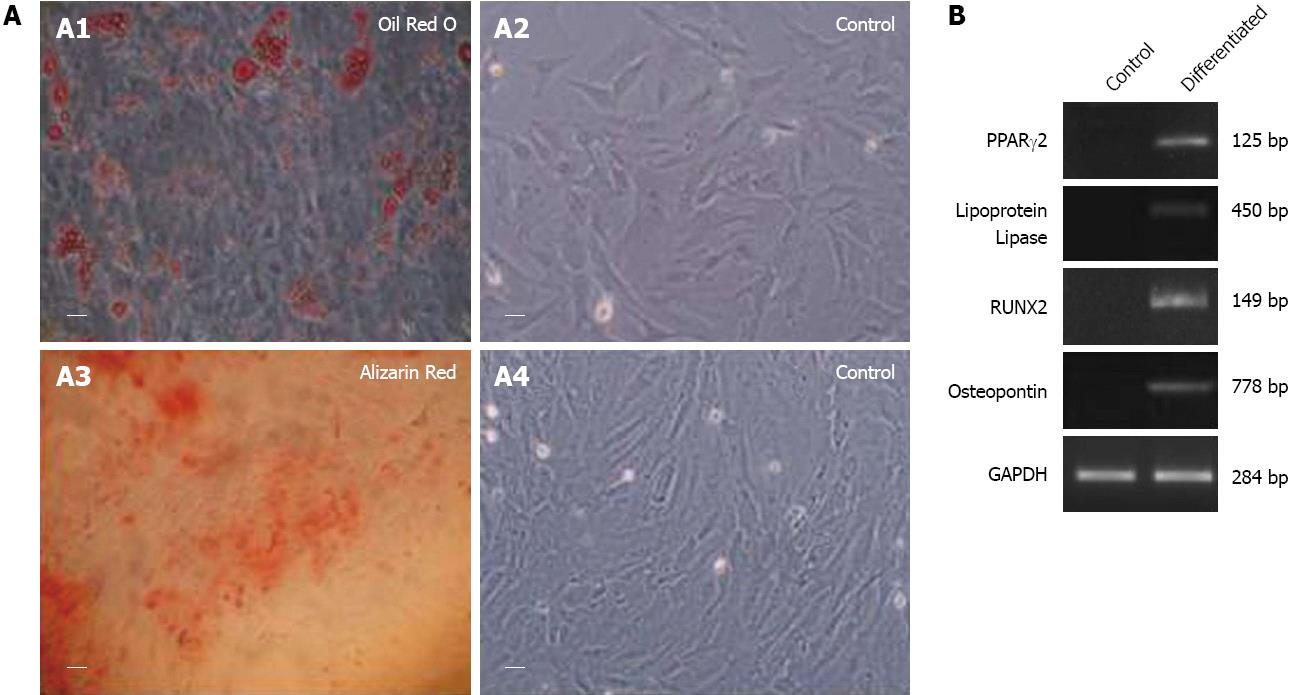
Treatment of fC-MSC with adipogenic and osteogenic induction media resulted in their differentiation into adipocytes and osteocytes (mesoderm), as demonstrated by Oil Red O and Alizarin Red staining, as well as expression of lipoprotein lipase, PPARγ2 and osteopontin and RUNX2 genes by RT-PCR, respectively (Figure 3).
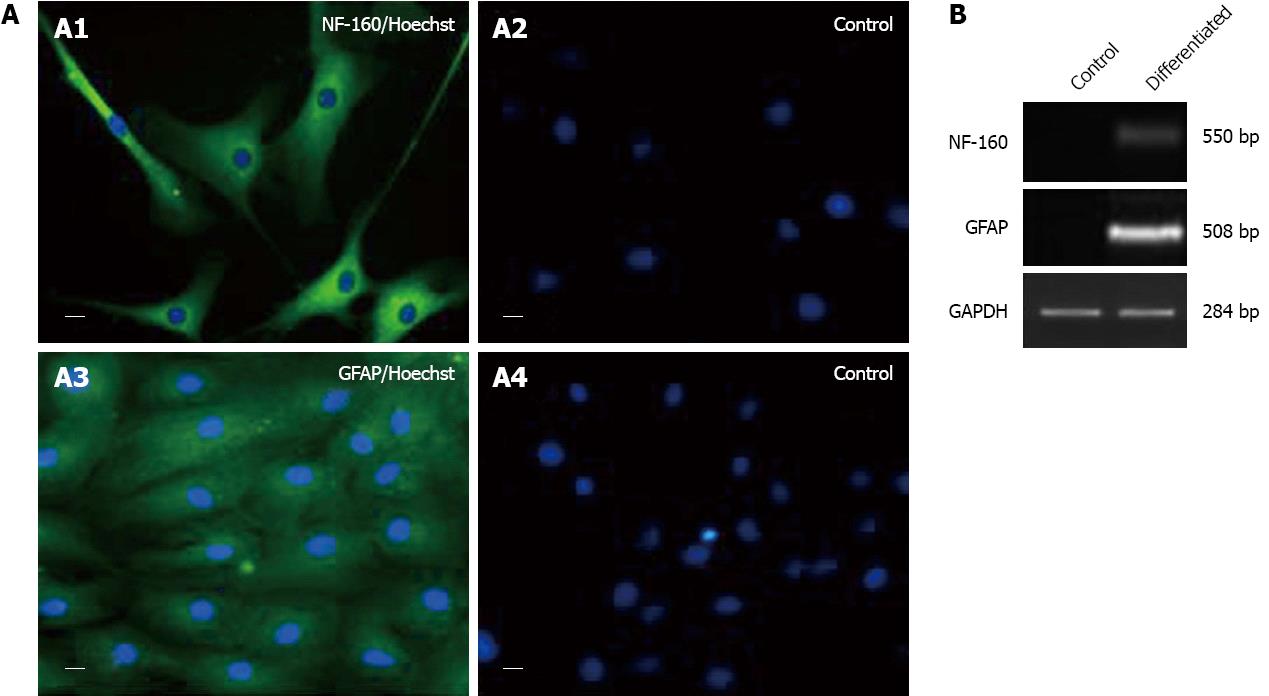
The neurogenic induction medium treated fC-MSC differentiated into neuronal cells (ectoderm), as revealed by expression of NF-160 and GFAP by RT-PCR and immunocytochemistry (Figure 4).
Similarly, on treatment with hepatogenic medium, fC-MSC exhibited differentiation into hepatocytic cells (endoderm), as demonstrated by expression of albumin by RT-PCR and immunocytochemistry, glycogen deposits by Periodic Schiffs staining and excretion of urea in the supernatant (Figure 5).
We have recently isolated a population of rat fC-MSC with typical MSC characteristics, including trigonal/spindle shaped morphology, expression of CD29, CD44, CD73, CD90 and CD105, but not of CD31, CD45 and HLA-DR, and potential to differentiate into adipogenic and osteogenic cells[6]. The fC-MSC exhibited a cardiovascular commitment, as revealed by expression of cardiovascular genes Isl-1, flk-1, Nkx2.5 and GATA-4, and differentiated into all major cardiovascular lineage cells, including cardiomyocytes, endothelial cells and smooth muscle cells. In addition, these cells expressed some embryonal markers and had an extensive expansion potential with continuous expression of telomerase reverse transcriptase and maintenance of a normal karyotype throughout the monitoring period for up to the 21st passage[6].
These primitive characteristics of fC-MSC prompted us to undertake the present study and we found that, similar to embryonic stem cells[7], the fC-MSC expressed a wide range of embryonal markers, including Oct-4, Nanog, Sox-2, SSEA-1, SSEA-3, SSEA-4 and TRA-1-81, but not TRA-1-60. In addition, on treatment with lineage specific induction medium, they differentiated into adipocytes and osteocytes (mesoderm), neural cells (ectoderm) and hepatocytic cells (endoderm). To the best of our knowledge, this is the first report showing differentiation of fC-MSC into cells of all three germ layers.
In two studies on stem cells from human fetal heart, the stem cells were demonstrated to express phenotypic markers of MSC, but they were not evaluated for expression of embryonal markers and tri-lineage differentiation potential[8,9]. Our observation on embryonic stem cell like characteristics of fC-MSC corroborates with results of a few previous studies on different fetal MSCs of rat and humans. MSC derived from rat and human amniotic membranes has been reported to exhibit expression of pluripotency markers and to differentiate into cells of ectodermal, mesodermal and endodermal lineages[10,11]. Similarly, human MSC derived from fetal lung are reported to express various embryonal markers, including Oct-4, Nanog, Sox-2, TRA-1-60, TRA-1-81 and SSEA-4, as well as to differentiate into cells of three germ layers[12]. In another human study, stem cells derived from different fetal organs have been shown to express Oct-4 and could be differentiated into cells of all three germ layers[13].
In conclusion, our study has shown that fC-MSC are primitive stem cell types that have a high degree of plasticity, as demonstrated by their expression profile of embryonal markers and potential to differentiate into cells of all the three germ layers, suggesting that, in addition to their suitability for cardiovascular regenerative therapy, they may have a wide spectrum of therapeutic applications in regenerative medicine.
We would like to express gratitude to Dr. MM Panicker, Associate Professor, Neurobiology Laboratory, National Centre for Biological Sciences, Bangalore, for helping to standardize the immunocytochemistry studies for the embryonal markers. The authors would also like to extend sincere thanks to Dr. MM Godbole, Professor, Department of Molecular Medicine and Biotechnology, SGPGIMS, Lucknow, and his research group, for extending laboratory facilities to perform immunocytochemistry experiments.
The authors’ previous observation of fetal cardiac mesenchymal stem cells (fC-MSC) expressing primitive markers and differentiation ability to cardiovascular or mesodermal lineage led us to determine multipotent differentiation potential with ability to differentiate into cells of other germ layers as well.
In this study, the authors have demonstrated that fC-MSC are primitive stem cells with capacity to differentiate into cells of all the three germ layers. Future studies on their basic biology and in vivo differentiation and function may be important for cell based therapy, generation of artificial tissues and organs and gene therapy.
To the best of our knowledge, this is the first report expressing a wide range of embryonal/pluripotent markers and showing differentiation of fC-MSC into cells of all three germ layers.
The fC-MSC with primitive stemness and multi-lineage differentiation potential might be novel stem cell types for regeneration/repair of tissues and organs derived from different primary germ layers and thus they may have a wide spectrum of therapeutic applications in regenerative medicine.
The fC-MSC are non-hematopoietic stem cells derived from fetal heart that share morphological and phenotypical characteristics of typical bone marrow derived MSC but they are very primitive nature and express cardiovascular markers, pluripotent markers and have potential to differentiate into cells of all the three germ layers.
This is an interesting study, wherein the authors have demonstrated that fC-MSC are primitive and highly multipotent stem cells and, in addition to their cardiovascular commitment, they could differentiate into cells of all the three germ layers; thus, they may have a wide spread application in regenerative therapy.
P- Reviewers Marchal JA, Valenti MT S- Editor Wen LL L- Editor Roemmele A E- Editor Zheng XM
| 1. | Epstein JA. Franklin H. Epstein Lecture. Cardiac development and implications for heart disease. N Engl J Med. 2010;363:1638-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Kimura K, Ieda M, Fukuda K. Development, maturation, and transdifferentiation of cardiac sympathetic nerves. Circ Res. 2012;110:325-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 3. | Mosna F, Annunziato F, Pizzolo G, Krampera M. Cell therapy for cardiac regeneration after myocardial infarct: which cell is the best? Cardiovasc Hematol Agents Med Chem. 2010;8:227-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Ratajczak MZ, Zuba-Surma EK, Wysoczynski M, Wan W, Ratajczak J, Wojakowski W, Kucia M. Hunt for pluripotent stem cell -- regenerative medicine search for almighty cell. J Autoimmun. 2008;30:151-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | De Kock J, Najar M, Bolleyn J, Al Battah F, Rodrigues RM, Buyl K, Raicevic G, Govaere O, Branson S, Meganathan K. Mesoderm-derived stem cells: the link between the transcriptome and their differentiation potential. Stem Cells Dev. 2012;21:3309-3323. [PubMed] |
| 6. | Srikanth GVN, Tripathy NK, Nityanand S. Isolation and Characterization of Cardiac MSCs from Rat Foetal Hearts. Int J Reg Med. 2012;1:1-8. |
| 7. | Ueda S, Kawamata M, Teratani T, Shimizu T, Tamai Y, Ogawa H, Hayashi K, Tsuda H, Ochiya T. Establishment of rat embryonic stem cells and making of chimera rats. PLoS One. 2008;3:e2800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Jiang XX, Su YF, Li XS, Zhang Y, Wu Y, Mao N. Human fetal heart-derived adherent cells with characteristics similar to mesenchymal progenitor cells. Zhongguo Shiyan Xueyexue Zazhi. 2006;14:1191-1194. [PubMed] |
| 9. | Gonzales C, Ullrich ND, Gerber S, Berthonneche C, Niggli E, Pedrazzini T. Isolation of cardiovascular precursor cells from the human fetal heart. Tissue Eng Part A. 2012;18:198-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Marcus AJ, Coyne TM, Rauch J, Woodbury D, Black IB. Isolation, characterization, and differentiation of stem cells derived from the rat amniotic membrane. Differentiation. 2008;76:130-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 2007;77:577-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 306] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 12. | Zheng C, Yang S, Guo Z, Liao W, Zhang L, Yang R, Han ZC. Human multipotent mesenchymal stromal cells from fetal lung expressing pluripotent markers and differentiating into cell types of three germ layers. Cell Transplant. 2009;18:1093-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Fang B, Li N, Song Y, Lin Q, Zhao RC. Comparison of human post-embryonic, multipotent stem cells derived from various tissues. Biotechnol Lett. 2009;31:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |