Published online Jun 26, 2025. doi: 10.4252/wjsc.v17.i6.107833
Revised: April 23, 2025
Accepted: June 9, 2025
Published online: June 26, 2025
Processing time: 88 Days and 15.2 Hours
Peripheral nerve injuries (PNI) that result in nerve gaps represent a major clinical challenge, frequently leading to long-term disability and a diminished quality of life for affected individuals. Despite advances in surgical techniques, functional recovery remains limited, highlighting the need for innovative therapeutic strategies. Mesenchymal stem cells (MSCs) have emerged as a promising avenue for nerve repair due to their regenerative, immunomodulatory, and neuroprotective properties. Thus, this review explored current approaches utilizing MSCs in the treatment of PNI, emphasizing their potential to enhance nerve rege
Core Tip: Mesenchymal stem cells (MSCs) offer a promising therapeutic approach for peripheral nerve injuries involving nerve gap by promoting axonal regeneration, modulating inflammation, secreting neurotrophic factors, and exhibiting transdifferentiation capacity. These properties position MSCs as a compelling alternative to conventional treatments. However, challenges still exist for the clinical use of MSCs, requiring further research to fully unlock their therapeutic potential and translate these advancements into effective nerve regeneration strategies.
- Citation: Ferreira LVO, Roballo KCS, Amorim RM. Mesenchymal stem cell-based therapy for peripheral nerve injuries: A promise or reality? World J Stem Cells 2025; 17(6): 107833
- URL: https://www.wjgnet.com/1948-0210/full/v17/i6/107833.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i6.107833
Peripheral nerve injuries (PNI) are relatively common and can result in debilitating consequences, including neuropathic pain, motor and sensory dysfunction in affected segments, and a substantial reduction in patients’ quality of life[1,2]. PNI can result from various etiologies, including traumatic injuries (e.g., accidents, fractures), iatrogenic causes (e.g., surgical complications), infections, chronic nerve compression, and autoimmune disorders[3,4].
Unlike the central nervous system, the peripheral nervous system (PNS) has the capacity for regeneration after injury, primarily due to the plasticity of Schwann cells (SCs)[5]. These cells proliferate, differentiate into a repair phenotype, and secrete a variety of neurotrophic factors and cytokines, creating a microenvironment conducive to neuroregeneration[6]. However, despite the inherent regenerative capacity of the PNS, spontaneous recovery is often insufficient, particularly in cases of extensive nerve damage or long-gap injuries[7].
The management of long-gap nerve injuries remains challenging, with autograft transplant as the standard approach for addressing PNI[8]. Despite advances in microsurgical techniques, this approach has inherent limitations, such as donor site morbidity and the challenge of achieving full recovery[9,10]. As a result, there is a pressing need for innovative treatments that can enhance nerve regeneration and improve functional outcomes.
In recent years several novel approaches for PNI repair have emerged, demonstrating positive effects in promoting neuroregeneration. However, fully restoring nerve function remains a challenge[11]. In this context cell-based therapies have emerged as a promising alternative, with mesenchymal stem cells (MSCs) garnering significant interest due to their regenerative potential[12]. MSCs possess properties, such as the ability to differentiate into various cell types, secrete trophic factors that promote tissue repair, and modulate the immune response, thereby creating a favorable environment for nerve regeneration[13]. Additionally, MSCs have been combined with tissue engineering techniques, which enhance their therapeutic effects in PNI repair[14]. Given these considerations, this narrative review aimed to comprehensively assess the therapeutic potential of MSCs in PNI regeneration, critically evaluating recent advancements, persisting challenges, and future directions for optimizing MSC-based therapies in nerve repair.
A comprehensive literature search was conducted using the PubMed database to identify relevant studies published up to March 2025. The search strategy incorporated keywords including “mesenchymal stem cells”, “peripheral nerve injury”, “nerve regeneration”, “extracellular vesicles”, “Schwann-like cells”, “transdifferentiation”, “nerve guidance conduits”, and “genetic engineering” as well as various combinations of these terms. The initial search was restricted to studies published between 2015 and 2025. To ensure thorough and up-to-date coverage, a subsequent broader search was performed without date limitations. Studies were considered eligible if they investigated the role, underlying mechanisms, or therapeutic potential of MSCs or their derivatives in the context of PNI. Articles that did not focus on MSC-based approaches or were unrelated to peripheral nerve repair were excluded.
PNI remains a significant cause of disability, as it can lead to the loss of motor and sensory functions, as well as neuropathic pain, compromising the quality of life of patients[15,16]. PNI can have different origins, including trauma, infections, iatrogenic injuries, tumors, and autoimmune conditions[17,18]. Every year millions of people are affected by PNI[19]. In the United States, approximately 43.8 people per million suffer traumatic nerve injuries annually[20]. A study in the country revealed that more than 2% of patients with limb trauma present with PNI[21]. Additionally, traumatic brachial plexus injuries can result in an indirect cost of over $1.1 million per patient[22].
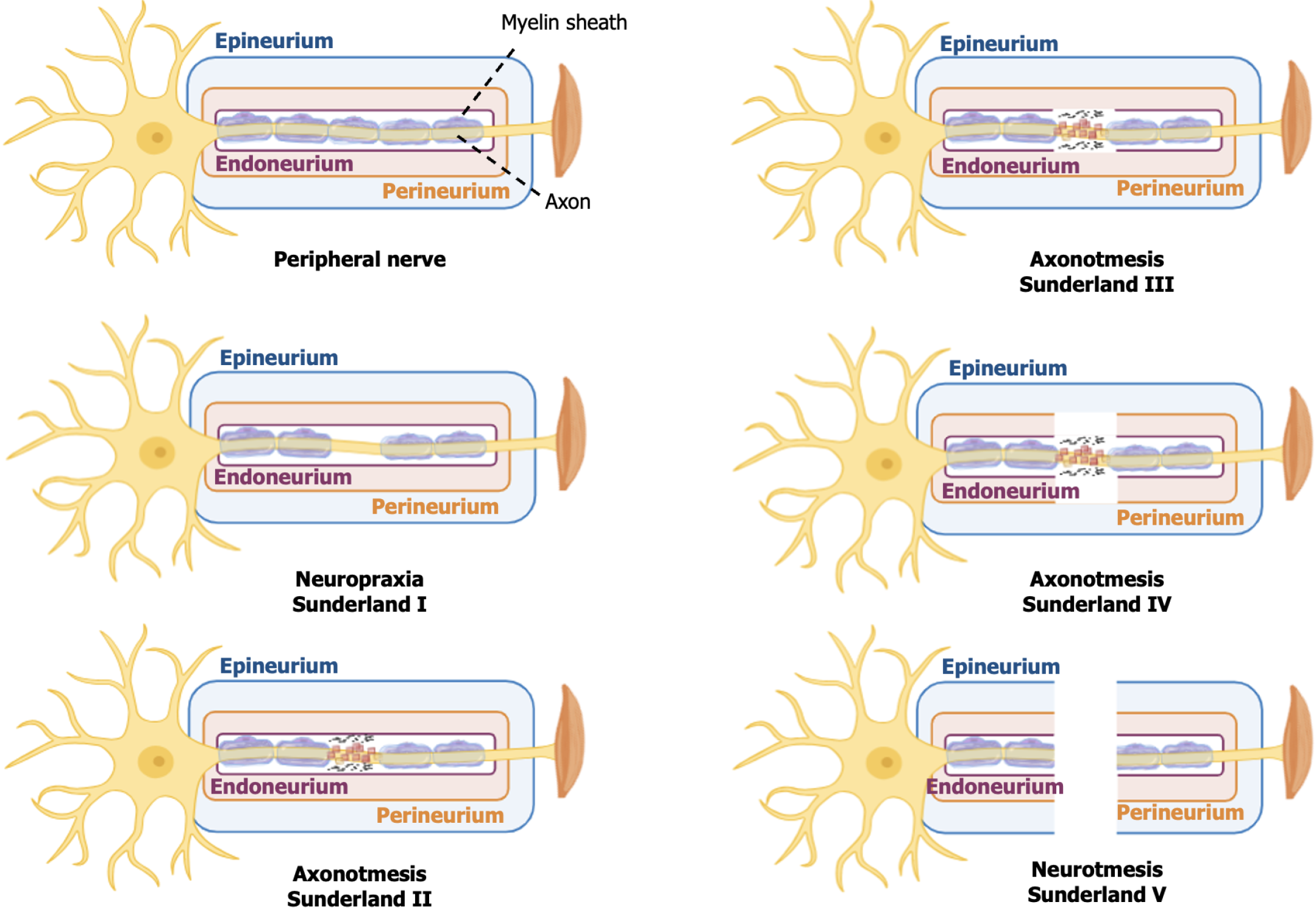
The success of PNI regeneration depends on several factors, with the severity of the injury being one of the most determining points. Traditionally, PNI are classified according to Seddon[23] in 1943 and Sunderland[24] in 1951 (Table 1 and Figure 1). Seddon’s classification consists of three categories: neuropraxia; axonotmesis; and neurotmesis. Sunderland complements this approach by subdividing the injuries into five grades based on histopathological characteristics ranging from the mildest (grade I) to the most severe (grade V). These grades correspond, respectively, to neuropraxia and neurotmesis in Seddon’s classification.
| Classification | |||||
| Seddon | Neuropraxia | Axonotmesis | Axonotmesis | Axonotmesis | Neurotmesis |
| Sunderland | I | II | III | IV | V |
| Injury | Focal demyelination | Axon and myelin damage | Axon, myelin and endoneurium damaged | Axon, myelin, endoneurium, and perineurium damaged | Complete nerve transection |
| Spontaneous recovery | Yes. Hours to a few weeks | Yes. Weeks to months | Not probable | Highly improbable | Spontaneous functional recovery is not possible |
| Surgical intervention | Normally not | Normally not | May be necessary | Necessary | Necessary |
Neuropraxia is characterized by focal demyelination without involvement of the axon or connective structures of the nerve. Grades II, III, and IV represent subdivisions of axonotmesis: In grade II, there is damage to the axon and myelin sheath; in grade III, involvement of the axon, myelin and endoneurium; and in grade IV, damage to the axon, myelin, endoneurium, and perineurium. Neurotmesis (grade V of Sunderland) involves complete nerve transection and loss of nerve continuity.
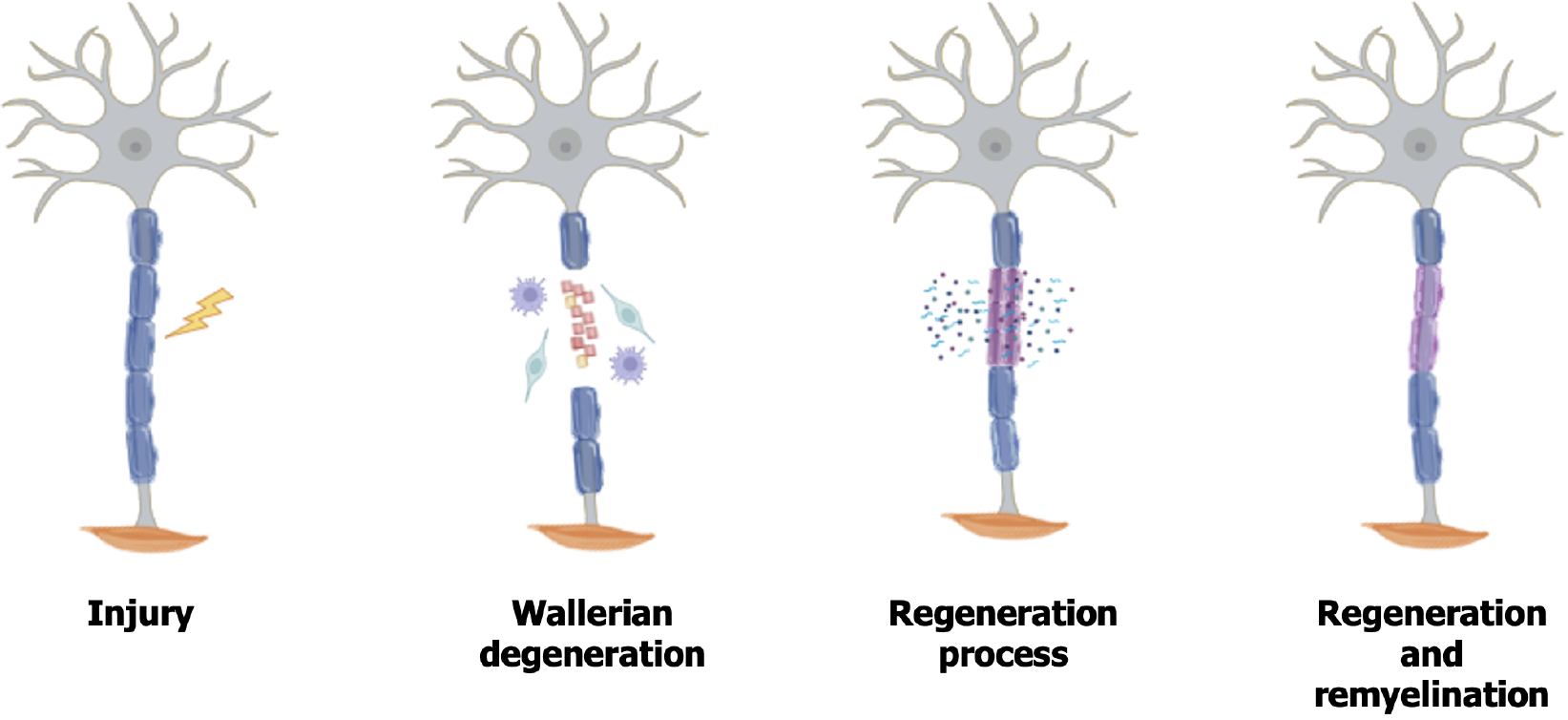
After PNI various cellular, molecular, and microstructural events occur to promote axonal regeneration[27] (Figure 2). In the distal segment of the injured axons, Wallerian degeneration begins, a process involving degradation of myelin, axonal degeneration, and proliferation of SCs, creating a microenvironment conducive to neuroregeneration[28]. Following by an influx of extracellular calcium into the axons, which results in the activation of calpain proteases, responsible for the degradation of axonal neurofilaments[11].
SCs transdifferentiate into a repair phenotype, playing a crucial role in regeneration[29,30]. They proliferate, phago
Parallel to the degeneration process, axonal regeneration begins. SCs secrete several neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), glial cell line-derived neurotrophic factor (GDNF), and nerve growth factor (NGF), which play a crucial role in neuronal regeneration[34]. Furthermore, these cells reorganize within their basal lamina, forming the Büngner bands, which act as guides for axonal growth[35]. Although the PNS has regenerative capacity, mainly due to the plasticity of SCs, regeneration is often unsatisfactory[5,36], requiring interventions to attempt a more effective recovery.
Injuries involving nerve defects are challenging and can result in severe functional deficits. Some therapeutic approaches include neurorrhaphy, nerve grafts, and pharmacological and physical therapies[11]. However, motor and sensory recovery is still limited, which drives the search for new therapeutic strategies.
Neurorrhaphy is indicated when the nerve ends can be approximated without tension or scars that would hinder regeneration[37]. However, this technique is limited to small gap defects, as excessive tension compromises axonal regeneration[38]. Autografts are the standard approach for segmental nerve defects greater than 3 cm[11,39]. Despite their effectiveness this technique has drawbacks, such as the need to remove healthy nerve tissue, limited tissue availability, and the risk of scar formation and neuroma development[40]. Allografts can be used as an alternative, employing nerves from donors or cadavers. However, this approach requires systemic immunosuppression to reduce the risk of graft rejection, limiting its clinical application[11,41].
Given the limitations of conventional methods, new strategies are being explored in PNI research, such as the use of nerve guidance conduits (NGCs) to direct axonal regeneration and reduce the need for grafts[42]. Moreover, cell therapies are gaining attention, including the use of SCs due to their essential role in nerve regeneration. Nevertheless, their clinical application still faces challenges, such as the need for a donor nerve for procurement, time required for expansion, potential low survival at the site, and susceptibility to environmental factors[1,43]. In this context MSCs have emerged as a promising alternative, offering advantages such as multipotency, plasticity, and the ability to modulate the regenerative microenvironment.
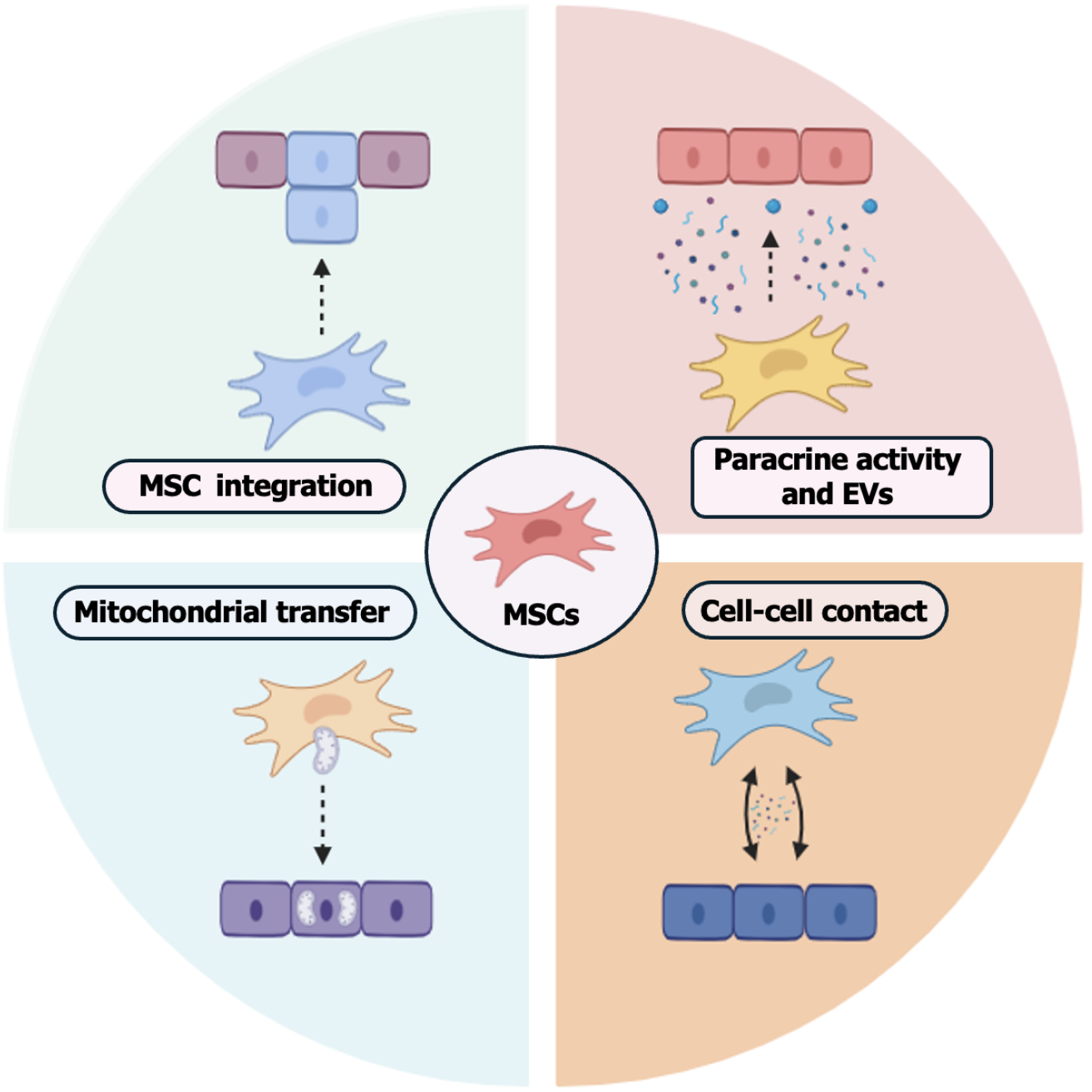
MSCs are mesodermal-origin multipotent cells that can be isolated from various adult tissues. They have a broad differentiation potential into multiple cell lineages, making them a promising option for therapeutic applications[44]. The MSCs can be obtained from different sources, including adipose tissue, bone marrow, dental pulp, amniotic membrane, umbilical cord, Wharton’s jelly, and skeletal muscles[45]. The International Society for Cellular Therapy established minimum criteria for the characterization of human MSCs, which include plastic adherence, the presence of specific markers (e.g., CD105, CD73, and CD90), the absence of others (e.g., CD34, CD45, CD79α, and HLA-DR), and the ability to differentiate into chondrogenic, osteogenic, and adipogenic lineages[46]. Some markers, such as CD271, have been adopted following this standardization, and the differentiation capacity into all three germ layers has already been demonstrated[47-49]. However, these criteria have not yet been revised. The main mechanisms of action of MSCs involve cell-cell contact, stimulation of proliferation, and differentiation of different cell types, as well as mitochondrial transfer, release of soluble factors, and extracellular vesicles (EVs)[13,50,51] (Figure 3).
MSCs have immunomodulatory, anti-inflammatory, anti-apoptotic, and neuroprotective features[52], making them a promising approach for peripheral nerve regeneration. Neuroprotection mediated by MSCs is associated with the production of factors, including NGF, BDNF, GDNF, fibroblast growth factor 2 (FGF-2), CNTF, insulin-like growth factor, and hepatocyte growth factor, and neurotrophins that promote neuronal survival and assist in functional recovery after nerve injuries[12,53,54].
In addition, the immunomodulatory effects of MSCs are attributed to the secretion of molecules such as transforming growth factor beta (TGF-β), prostaglandin E2 (PGE2), indoleamine 2,3-dioxygenase, nitric oxide, IL-6, and IL-10[55,56]. They also play an angiogenic role through the release of vascular endothelial growth factor (VEGF), inhibitor of metalloproteinase 18, and angiopoietin 1[45]. However, the immunomodulatory potential may vary between species and tissues and be influenced by the presence of inflammatory or anti-inflammatory stimuli[57].
EVs are classified based on their size and biogenesis[58]. They can be divided based on their dimensions, being categorized as small EVs and large vesicles[59]. MSCs release EVs that play an essential role in intracellular commu
The use of EVs derived from MSCs demonstrates efficacy equivalent to that of their parental cells[54,64] and offers advantages over cell therapy, including low immunogenicity, ability to cross the blood-brain barrier[65], and potential to be used as drug delivery systems[66]. Moreover, the use of these EVs has shown promising results in the treatment of PNI in laboratory animal models. Studies have demonstrated that EVs derived from different sources of MSCs, such as bone marrow-derived MSCs[67], adipose tissue-derived MSCs (AT-MSCs)[63,68], umbilical cord-derived MSCs[69,70], and gingival MSCs[71], improved nerve regeneration in vivo. However, the clinical application of EVs still faces challenges, such as the lack of standardization in collection and isolation methods, variation in their biological characteristics, and difficulties in large-scale production for therapeutic purposes[72].
MSCs exhibit immunomodulatory effects through direct contact with innate and adaptive immune cells[73]. The proximity of MSCs allows the transfer of molecules such as miRNA, peptides, and mitochondria to the cells[74]. It has been shown that MSCs can modulate the immune response by interacting with proinflammatory macrophages, promoting the conversion of the M1 phenotype to M2 and reducing T cell proliferation[75,76]. Additionally, direct contact with T lymphocytes can induce functional changes in these cells and stimulate the release of TGF-β and PGE2 [76].
Mitochondria are essential for adenosine triphosphate production, regulation of oxidative phosphorylation, and control of cellular apoptosis[77]. An in vitro study demonstrated that MSCs can transfer healthy mitochondria to neurons subjected to oxidative injury, promoting increased neuronal survival and improving cellular metabolism[78]. Addi
Several MSC delivery strategies have been explored for the treatment of PNI, aiming to maximize their regenerative efficacy (Table 2)[80-85]. Delivery routes include intramuscular and intravenous injections[80]. Intravenous administration allows systemic distribution but is limited by capillary retention in organs such as the lungs, reducing the number of MSCs that reach the injury site[80]. Additionally, intraperitoneal administration has also been investigated as an alternative route in experimental PNI in a rodent model[81]. More targeted approaches, such as epineural[82], perineural[83], and subepineural[84], administrations have been studied for offering a localized delivery, promoting direct contact with the injured nerve tissue. However, there are still controversies regarding whether microinjections may result in ultrastructural trauma and reduced cell viability[86].
| Cell | Delivery | Models | Cell numbers | Outcome | Notes | Ref. |
| AT-MSCs (canine) | Perineural | Rat sciatic nerve crush | 1 × 106 | Improved electrophysiological and motor recovery | Assessment performed for 4 weeks | [83] |
| AT-MSCs (rat) | Intraperitoneal | Rat sciatic nerve transection and suture repair | 2 × 106 | Improvement in nerve regeneration and functionality | No difference was observed in compound muscle action potential latency between the saline and MSC groups | [81] |
| BM-MSCs (rat) | IM and IV | Small gap neurorrhaphy (rat sciatic nerve) | 1 × 106 | Improvements in the sciatic function index, nerve conduction velocity, and myelin sheath thickness | The IM group showed better results compared with the IV group | [80] |
| BM-MSCs (rat) | IV and epineural | Rat sciatic nerve transection and suture repair | IV: 1 × 106. Epineural: 5 × 104 | Enhancement in the recovery rate of compound muscle action potential amplitudes and axon counts | IV administration showed a more pronounced effect on electromotor recovery, while epineural injection was more effective in increasing fiber counts | [82] |
| BM-MSCs (rat) | Local injection and IV | Rat sciatic nerve transection/repair and individual nerve transection/repair | 5 × 106 | Improvements in motor function recovery in both models | The motor function recovery was significantly more pronounced in the individual nerve transection/repair model compared with the sciatic nerve transection/repair model | [85] |
| BM-MSCs (rat) | Subepineural | Rat sciatic nerve transection and surgical coaptation | 5 × 105 | Improvements in the sciatic function index | The group treated with MSCs and immunomodulators had better functional recovery than the group treated with MSCs alone. Immunomodulation using LPS and FK506 can improve MSC survival after transplantation | [84] |
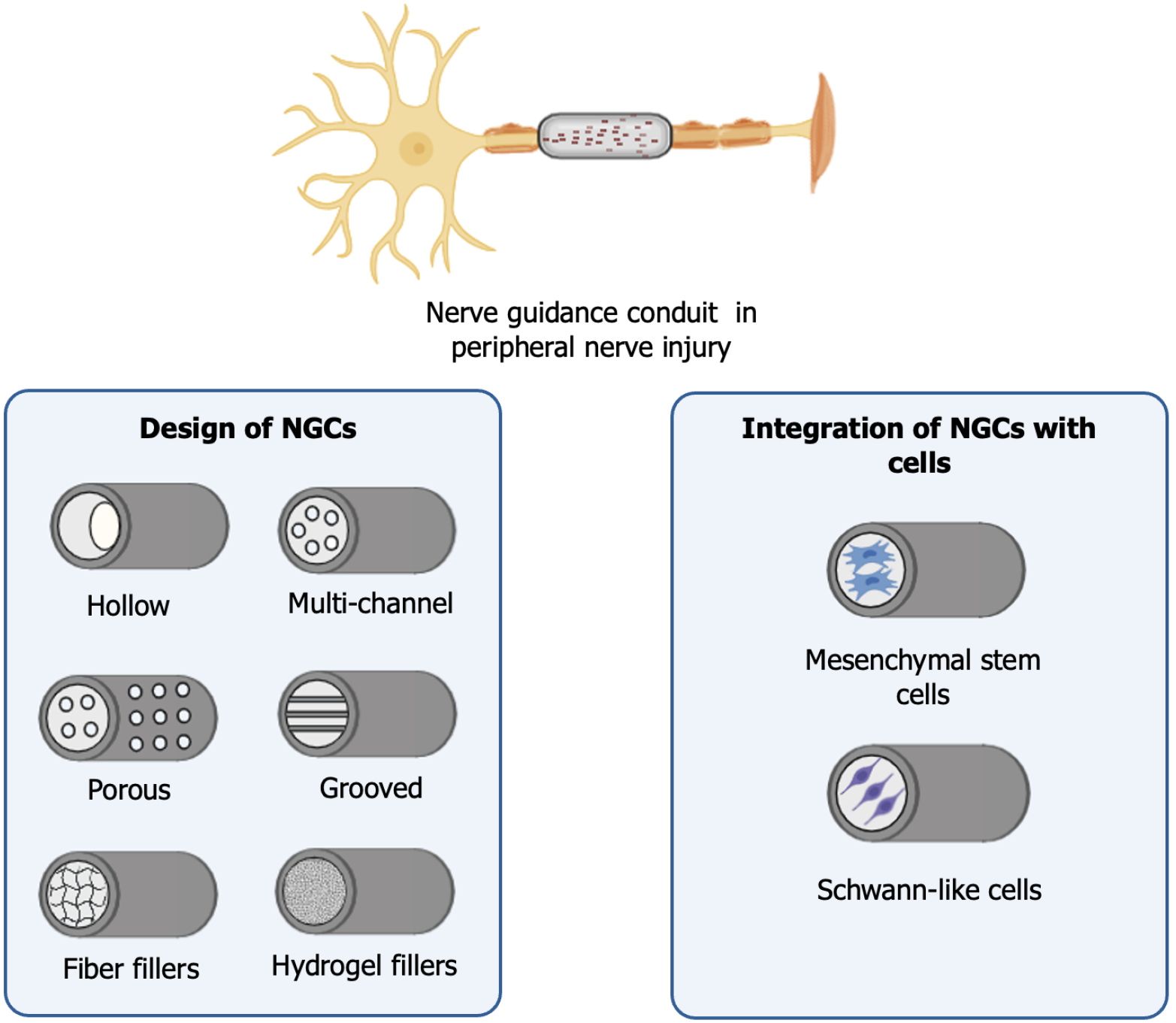
NGCs have emerged as a promising alternative for the regeneration of PNI, particularly in cases involving large gaps between the injured nerve stumps[87]. These devices provide physical support and create a favorable microenvironment for axonal regeneration, promoting the directed growth of nerve fibers[88]. NGCs can be made from autogenous and allogeneic biological materials, as well as non-biological materials[89]. The materials used can be synthetic, natural, or a combination of both, and their selection is based on properties such as biocompatibility, biodegradability, porosity, mechanical strength, and the ability to promote cellular interaction[90]. Among natural materials collagen, chitosan, fibrin, hyaluronic acid, methacrylated gelatin, and silk stand out[91]. Among synthetic materials poly(lactic acid), polyglycolic acid, poly(lactic-co-glycolic acid), and polycaprolactone show promise[91]. Besides the material selection, NGCs can present different designs, such as porous, grooved, with fiber or hydrogel fillers, hollow/non-porous, and multi-channel, which vary according to the specific needs of each application (Figure 4)[42].
Technological advances have expanded the possibilities for the design and fabrication of NGCs. Techniques such as electrospinning, freeze/dry processing, dip-coating, centrifugal casting, phase separation, and three-dimensional (3D) printing have enabled the creation of customized conduits[92]. 3D printing enables the construction of NGCs with complex architecture and adjustable mechanical properties, creating a more controlled and biomimetic environment for nerve regeneration[93].
The combination of NGCs and MSCs holds significant promise for the treatment of PNI. Research has highlighted encouraging outcomes from this approach, including enhanced axonal regeneration and improved functional recovery (Table 3). Despite the advancements in the use of NGCs and MSCs, this approach has not always been superior to the use of autografts in nerve regeneration, necessitating further research to address existing limitations.
| Cell source | Conduits | Models | Cell numbers | Outcome | Notes | Ref. |
| AT-MSCs (human) | Polycaprolactone | 15 mm gap in the rat sciatic nerve | 1 × 106 | Improvement in axonal growth and expression of factors that aid in reinnervating muscle tissue | Poloxamer hydrogel + AT-MSCs promote more axonal growth than when AT-MSCs were delivered without it | [97] |
| AT-MSCs (rat) | Fibrin gel | A 20 mm segment of the sciatic nerve was excised in rats and sutured back in the reverse direction | 3 × 106 | Enhanced remyelination, axonal regeneration, and functional recovery | The use of AT-MSCs resulted in a significant improvement compared with the autologous nerve graft group | [98] |
| AT-MSCs (rat) | Silicone tube | 10 mm gap in the rat sciatic nerve | 1 × 106 | Improvement in the recovery of walking function | The combination of AT-MSCs with platelet-rich fibrin showed better results than AT-MSCs alone | [99] |
| AT-MSCs (canine) | Polycaprolactone + heterologous fibrin biopolymer | 12 mm gap in the rat sciatic nerve | 1 × 106 | Improvement in functional motors and electrophysiological recovery | The improvements observed were not significantly different from those obtained with autografts | [100] |
| AT-MSCs (rat) | Chitosan + acellular nerve | 10 mm gap in the rat sciatic nerve | Unknown | Improvement in neurological and motor function and in the quality of the myelin sheath | At 12 weeks there was no significant difference in the degree of recovery compared with the autograft group. The electrophysiological characteristics were also similar to those of the autograft | [101] |
| BM-MSCs (rat) | Polycaprolactone + fibrin sealant | 6-7 mm gap in the rat sciatic nerve | 3 × 105 | Improvement in the regeneration process, modulation of SCs, and motor functional recovery | There was no significant difference in the total estimated number of regenerated fibers between the groups | [102] |
| BM-MSCs (rat) | Bio 3D conduits from BM-MSCs | 5 mm gap in the rat sciatic nerve | 3 × 105 | Improvements in nerve regeneration, kinematic analysis, and morphological parameters | No neuroma formation was found 8 weeks after the surgery. The Bio 3D group exhibited a higher abundance of myelinated axons compared with both the silicone NGC group and the silicone NGC with MSCs group | [96] |
| UC-MSCs (human) | Longitudinally oriented collagen conduit | A 35-mm-long segment of the dog’s sciatic nerve was removed | 1 × 106 | Improvements in axonal regeneration and functional recovery | Nerve regeneration was inferior to the autologous nerve graft group | [103] |
| UC-MSCs (human) | Bio 3D conduits from UC-MSCs | 5 mm gap in the rat sciatic nerve | 3 × 105 | Improvements in kinematic analysis, as well as in the diameters and number of myelinated axons | The Bio 3D conduit showed better results than the silicone tube and demonstrated nerve regeneration comparable with the autologous group. UC-MSCs in the Bio 3D conduit gradually diminished until week 8 | [95] |
| WJ-MSCs (human) | Acellular nerve | 10 mm gap in the rat sciatic nerve | 1 × 106 | Improvements in myelin and axon regeneration, nerve function, and muscle atrophy reduction | Evaluation at 8 weeks. Increased in both the proportion of myelin in the tissue and myelin thickness, resembling the results seen in the autograft group | [104] |
| WJ-MSCs (human) | Poly (DL-lactide-e-caprolactone) copolyester | 10 mm gap in the rat sciatic nerve | 2 × 106 | Improvements in nerve regeneration, functional recovery, and increased expression of neurotrophic and angiogenic factors | Evaluation at 12 weeks | [105] |
| OM-MSCs (rat) | Chitosan | About 10 mm gap in the rat sciatic nerve | 1 × 106 | Improved in nerve regeneration, motor performance, sciatic indexes, and lower gait dysfunction | The treated groups did not show a significant difference in the stereological results | [106] |
| GMSCs (human) | Bio 3D conduits from GMSCs | A 5 mm gap in the buccal branch of the rat facial nerve | 4 × 104 | Improvements in nerve regeneration and functional recovery | Effects comparable to the autograft group | [94] |
The integration of MSCs with NGCs can be achieved through various methods. One approach involves seeding the cells onto the conduit after fabrication, which offers flexibility but may result in low cell adhesion[107]. Alternatively, MSCs can be encapsulated in hydrogels before being integrated into 3D-printed structures or incorporated through bioprinting, which ensures better cell arrangement and loading efficiency but requires precise optimization of printing parameters[107].
Bio 3D-MSC technology refers to a cutting-edge approach that combines MSCs with 3D bioprinting to create scaffold-free NGCs. This technology enables the fabrication of complex, cell-laden structures, utilizing MSC spheroids as the unique cellular component[94]. It can be tailored to the patient’s needs using a controlled system and does not contain foreign materials[108]. Additionally, studies demonstrate that this technology promotes nerve regeneration and may serve as a promising treatment option for PNI[95,96] (Table 3). Challenges remain to be addressed regarding bioprinting. These include accurately replicating the microanatomy of nerve tissue, developing a 3D environment capable of supporting multiple cell types and achieving more precise control over the mechanical and biochemical properties of the conduit[109].
The transdifferentiation of MSCs into Schwann-like cells (SLCs) has been widely studied as an alternative to SC transplantation for the treatment of PNI. Transdifferentiation is commonly induced using chemical and growth factors, including beta-mercaptoethanol, retinoic acid, forskolin, basic FGF, platelet-derived growth factor, and heregulin[110,111]. Based on this approach, other methodologies have been developed incorporating glial growth factor-2[112], folic acid[113], and dihydrotestosterone[114] into the induction cocktails.
Moreover, there are several protocols in the literature that use neurotrophic factors, co-culture[115], glucocorticoids, insulin, progesterone[116], electrostimulation[117], cell imprinting[118], intermittent induction[119], 3D matrices[120], conditioned medium obtained from peripheral nerve explants[121], and exosomes[122]. The transdifferentiation process is regulated by various mechanisms, including transcription factors and miRNAs[72]. The involvement of miRNA-21-5p in this process has been demonstrated[123], while the overexpression of miRNA-214in SLCs may enhance their effects on nerve repair[72]. Several markers are used to characterize SLCs after transdifferentiation, as reviewed previously[124]. However, there is still no consensus on which ones should be adopted. Despite the promising therapeutic potential of SLCs derived from different sources of MSCs (Table 4), significant challenges persist. Reaching a consensus on cell characterization, ensuring cell quality and phenotypic stability, and assessing long-term safety are crucial to advancing their clinical application for PNI treatment.
| Starting cell | Delivery | Models | Method of transdifferentiation | Cell numbers | Effects | Notes | Ref. |
| AT-MSCs (rat) | Nerve fibrin conduit | 10 mm gap in the rat sciatic nerve | Chemical and growth factors | 2 × 106 | Improvement in axonal regeneration | No undifferentiated MSC transplantation group. Similar outcomes were observed between the SLCs derived from AT-MSCs and BM-MSCs 2 weeks post-transplantation | [125] |
| AT-MSCs (rat) | Nerve fibrin conduit | 10 mm gap in the rat sciatic nerve | Chemical and growth factors | 2 × 106 | Improvement in axonal and fiber diameters and reduction in muscle atrophy (gastrocnemius) | No undifferentiated MSC transplantation group. SLCs derived from AT-MSCs were more effective than those derived from BM-MSCs after 4 months | [126] |
| AT-MSCs (rat) | Silicone tube | 10 mm gap in the rat sciatic nerve | Chemical and growth factors | 1 × 106 | Improvement in axonal regeneration, sciatic function index, and myelination | AT-MSCs and SLCs exhibited a similar impact on nerve regeneration 6 months post-transplantation | [127] |
| AT-MSCs (human) | Local injection | Tibial crush in rats | Chemical and growth factors | 1 × 105 | Improvement of survival and myelin formation rates | AT-MSCs secreted neurotrophic factors, though in lower quantities compared with SLCs, and expressed glial markers p75 and GFAP even without stimulation | [128] |
| AT-MSCs (rat) | NeuraWrapTM sheath | 15 mm gap in the rat sciatic nerve | Chemical and growth factors | 4 × 106 | Improvement in axonal regeneration and myelination. The conduits containing SLCs resulted in a 3.5-fold greater proportion of axons in the distal nerve stump compared with the empty conduits after 8 weeks | No undifferentiated MSC transplantation group | [129] |
| AT-MSCs (rat) | Silicone tube | 7 mm gap in the rat facial nerve | Chemical and growth factors | 1 × 105 | Improvement in axonal regeneration and in the functional recovery of the facial nerve | AT-MSCs, SLCs, and SCs showed similar nerve regeneration potential after 13 weeks | [130] |
| AT-MSCs (ovine) | Acellular nerve allograft | 30 mm gap in the ovine peroneal nerve | Chemical and growth factors | 3 × 105 | Improvement in hindlimb function, motor recovery, and remyelination | The autograft showed better organization of the myelin sheaths and axons than acellular nerve allografts recellularized with SLCs after 12 months | [131] |
| AT-MSCs (ovine) | Acellular xenografts (human) | 20 mm gap in the ovine sciatic nerve | Chemical and growth factors | 3 × 105 | Improvement in metatarsus mobility and strength. Presence of several intrafascicular axons at the graft extremes | No difference was observed between the allograft and xenograft recellularized with SLCs groups in the biceps femoris and gastrocnemius electromyographic response after 6 months | [132] |
| BM-MSCs (rat) | Hollow fiber | 12 mm gap in the rat sciatic nerve | Chemical and growth factors | 1-2 × 107 | Motor nerve conduction velocity and sciatic nerve function improved significantly. There was an increase in the number of regenerated axons | No tumor formation was observed in the graft or the sciatic nerve segment after 6 months | [133] |
| BM-MSCs (human) | Transpermeable tube | 10 mm gap in the rat sciatic nerve | Chemical and growth factors | 1-2 × 107 | Increase in the number of regenerated axons and improvement in the sciatic function index | Intraperitoneal administration of FK506 as an immunosuppressant during the 3 weeks of evaluation | [134] |
| BM-MSCs (rat) | Chitosan conduit | 12 mm gap in the rat sciatic nerve | Induction of neurospheres, exposure to growth factors, and co-culture | 1.5 × 105 | Enhanced axonal repair and remyelination | The nerve repair and functional recovery were similar to those from sciatic nerve-derived SCs | [135] |
| BM-MSCs (rabbit) | Autogenous vein | 10 mm gap in the rabbit facial nerve buccal branch | Chemical and growth factors | 2 × 105 | Improvement in axon regeneration and remyelination | SLC group provided a faster rate of axonal extension and a larger area of myelination than the BM-MSCs group | [136] |
| BM-MSCs (human) | Chitosan conduit | 12 mm gap in the rat sciatic nerve | Induction of neurospheres, exposure to growth factors, and co-culture | 1.5 × 105 | Enhanced axonal regeneration and myelination | Subcutaneous administration of cyclosporin A for immunosuppression | [137] |
| WJ-MSCs (human) | Transpermeable tube | 8 mm gap in the rat sciatic nerve | Chemical and growth factors | 1-2 × 107 | Improvement in axonal regeneration and functional recovery | No tumor formation was observed after 3 weeks. The ability of SLCs to promote axonal regeneration was similar to that of human SCs, as evidenced by functional recovery and histological evaluation. Subcutaneous administration of FK506 for immunosuppression | [138] |
| UCB-MSCs (human) | 3D-cell spheroids | Sciatic nerve crush in rats | Chemical and growth factors | 5 × 105 | Improvement in functional and structural recovery | Subcutaneous administration of cyclosporin A for immunosuppression | [139] |
| GMSCs (human) | 3D-collagen hydrogel | Sciatic nerve crush in rats | Encapsulation in the methacrylated 3D-collagen hydrogel | 2 × 106 | Improvement in axonal regeneration and functional recovery | SLCs demonstrated immunomodulatory activity, reducing M1 macrophage activation and promoting M2 macrophage polarization | [120] |
Genetic engineering in MSCs has emerged as a promising tool to enhance their therapeutic properties through transfection with viral and non-viral vectors, enabling the targeted modification of their characteristics to meet specific needs[140]. This approach facilitates the insertion of therapeutic genes into MSCs, allowing their reprogramming to express or inhibit certain genes, overcoming limitations such as low concentration at the injury site and short survival time[44,54].
In the treatment of PNI, bone marrow MSCs from rats were transfected with BDNF and CNTF and combined with nerve transplantation to treat sciatic nerve injuries in rats, resulting in improved nerve function and increased myelin sheath thickness[141]. In another study, human bone marrow MSCs were transduced with lentivirus to overexpress VEGF, demonstrating positive effects both in vitro and in vivo in a murine sciatic nerve injury model[142]. This strategy promoted neurite outgrowth and maintained high VEGF expression for up to 14 days after transplantation[142]. Moreover, the addition of FGF-2 to rat adipose-derived MSCs, in combination with the overexpression of miR-218, showed potential to induce their transdifferentiation into the neural lineage in vitro[143]. Neuron-derived EVs have also been shown to play a key role in promoting neural differentiation of AT-MSCs, carrying synaptosomal-associated protein 25, miR-132, and miR-9[144]. Additionally, this process has demonstrated potential in vivo, where neuron-like cells derived from AT-MSCs helped reduce peripheral nerve degeneration after injury[144]. However, for clinical application, gene therapy still faces challenges such as the proper selection of target genes, maintaining stable expression in the patient, and ethical considerations[64].
Despite promising results in preclinical studies, several limitations and challenges continue to impede the clinical translation of MSC-based therapies for PNI. A major concern is the inherent heterogeneity of MSC populations, which can vary according to tissue source, donor characteristics, and culture conditions. These variations can significantly influence their differentiation potential, immunomodulatory properties, and secretion of bioactive factors, including EVs, ultimately affecting therapeutic outcomes. Furthermore, MSC survival and integration within the injured nerve environment remain suboptimal, particularly in hostile microenvironments marked by inflammation and hypoxia. Another critical challenge is the absence of standardized protocols for MSC transplantation. Variability in cell dosage, delivery routes, and treatment timelines complicates inter-study comparisons and undermines the reproducibility and scalability of therapeutic approaches.
The long-term safety of MSC-based therapies also warrants further investigation. Potential tumorigenicity must be rigorously evaluated within standardized regulatory frameworks. While strategies such as genetic modification and transdifferentiation into SLCs have shown promise in enhancing the regenerative potential of MSCs, they introduce additional complexities related to safety, stability, and ethical considerations.
In parallel, the combination of MSCs with tissue-engineered scaffolds, such as NGCs, represents a promising avenue for promoting and directing nerve regeneration. However, for these constructs to achieve clinical relevance, they must demonstrate efficacy equal to or superior to autografts, the current gold standard for bridging long-gap nerve injuries. Continued efforts to optimize the design, biocompatibility, and functionalization of NGCs remain essential for improving functional outcomes and advancing clinical translation.
MSCs exhibit great therapeutic potential for the treatment of PNI, being effective in promoting immunomodulation, neuroprotection, and neuroregeneration. The combination of MSCs with NGCs emerges as a promising strategy to optimize nerve regeneration, providing structural support and promoting the guidance of axonal growth. This combined approach has shown significant benefits in preclinical models, accelerating the regeneration of damaged peripheral nerves. However, challenges remain, such as the proper integration of MSCs with NGCs and ensuring long-term stability.
Additionally, genetic engineering, the use of EVs, and the potential for MSCs transdifferentiation represent innovative pathways aimed at maximizing therapeutic outcomes. However, substantial challenges remain to be addressed, such as ensuring stable cell expression in the patient, the ethical issues involved, and the need for global consensus on the characterization of cells after transdifferentiation. Furthermore, it is essential to overcome difficulties related to large-scale production, ensuring quality and safety for clinical application.
Although significant progress has been made, further research is needed to overcome these barriers and establish MSC therapy as an effective reality for the clinical application of PNI. While MSC-based therapies have demonstrated promising preclinical results, their translation into routine clinical practice requires overcoming key challenges, including safety, scalability, and standardization. Addressing these limitations will be crucial to establish MSCs as a viable therapeutic option for PNI patients in the near future.
| 1. | Saffari S, Saffari TM, Ulrich DJO, Hovius SER, Shin AY. The interaction of stem cells and vascularity in peripheral nerve regeneration. Neural Regen Res. 2021;16:1510-1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 2. | Tusnim J, Kutuzov P, Grasman JM. In Vitro Models for Peripheral Nerve Regeneration. Adv Healthc Mater. 2024;13:e2401605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Aman M, Zimmermann KS, Boecker AH, Thielen M, Falkner F, Daeschler S, Stolle A, Kneser U, Harhaus L. Peripheral nerve injuries in children-prevalence, mechanisms and concomitant injuries: a major trauma center's experience. Eur J Med Res. 2023;28:116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 4. | Zhai X, Wang Y. Physical modulation and peripheral nerve regeneration: a literature review. Cell Regen. 2024;13:32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Gomez-Sanchez JA, Patel N, Martirena F, Fazal SV, Mutschler C, Cabedo H. Emerging Role of HDACs in Regeneration and Ageing in the Peripheral Nervous System: Repair Schwann Cells as Pivotal Targets. Int J Mol Sci. 2022;23:2996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Liao S, Chen Y, Luo Y, Zhang M, Min J. The phenotypic changes of Schwann cells promote the functional repair of nerve injury. Neuropeptides. 2024;106:102438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 7. | Zainul Z, Ma B, Koka M, Wilkerson JL, Ortiz YT, Kerosuo L, Chandran V. Novel roles of phentolamine in protecting axon myelination, muscle atrophy, and functional recovery following nerve injury. Sci Rep. 2022;12:3344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Yao X, Xue T, Chen B, Zhou X, Ji Y, Gao Z, Liu B, Yang J, Shen Y, Sun H, Gu X, Dai B. Advances in biomaterial-based tissue engineering for peripheral nerve injury repair. Bioact Mater. 2025;46:150-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Broeren BO, Hundepool CA, Kumas AH, Duraku LS, Walbeehm ET, Hooijmans CR, Power DM, Zuidam JM, De Jong T. The effectiveness of acellular nerve allografts compared to autografts in animal models: A systematic review and meta-analysis. PLoS One. 2024;19:e0279324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Singh VK, Haq A, Tiwari M, Saxena AK. Approach to management of nerve gaps in peripheral nerve injuries. Injury. 2022;53:1308-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Lopes B, Sousa P, Alvites R, Branquinho M, Sousa AC, Mendonça C, Atayde LM, Luís AL, Varejão ASP, Maurício AC. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. Int J Mol Sci. 2022;23:918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 156] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 12. | Lavorato A, Raimondo S, Boido M, Muratori L, Durante G, Cofano F, Vincitorio F, Petrone S, Titolo P, Tartara F, Vercelli A, Garbossa D. Mesenchymal Stem Cell Treatment Perspectives in Peripheral Nerve Regeneration: Systematic Review. Int J Mol Sci. 2021;22:572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 13. | Sharifi M, Kamalabadi-Farahani M, Salehi M, Ebrahimi-Brough S, Alizadeh M. Recent perspectives on the synergy of mesenchymal stem cells with micro/nano strategies in peripheral nerve regeneration-a review. Front Bioeng Biotechnol. 2024;12:1401512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Zheng S, Wei H, Cheng H, Qi Y, Gu Y, Ma X, Sun J, Ye F, Guo F, Cheng C. Advances in nerve guidance conduits for peripheral nerve repair and regeneration. Am J Stem Cells. 2023;12:112-123. [PubMed] |
| 15. | Sarhane KA, Qiu C, Harris TGW, Hanwright PJ, Mao HQ, Tuffaha SH. Translational bioengineering strategies for peripheral nerve regeneration: opportunities, challenges, and novel concepts. Neural Regen Res. 2023;18:1229-1234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 16. | Zhu ZW, Li G, Wu GG, Zhang YJ, Bai YR, Lai BQ, Ding Y, Zeng X, Ma YH, Liu S, Wang R, Liang JH, Xu YB, He B, Zeng YS. Transplantation of peripheral nerve tissueoid based on a decellularized optic nerve scaffold to restore rat hindlimb sensory and movement functions. Biomaterials. 2025;315:122949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | André-Lévigne D, Pignel R, Boet S, Jaquet V, Kalbermatten DF, Madduri S. Role of Oxygen and Its Radicals in Peripheral Nerve Regeneration: From Hypoxia to Physoxia to Hyperoxia. Int J Mol Sci. 2024;25:2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 18. | Jiang L, Jones S, Jia X. Stem Cell Transplantation for Peripheral Nerve Regeneration: Current Options and Opportunities. Int J Mol Sci. 2017;18:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 19. | Liu Y, Zhang X, Xiao C, Liu B. Engineered hydrogels for peripheral nerve repair. Mater Today Bio. 2023;20:100668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 20. | Karsy M, Watkins R, Jensen MR, Guan J, Brock AA, Mahan MA. Trends and Cost Analysis of Upper Extremity Nerve Injury Using the National (Nationwide) Inpatient Sample. World Neurosurg. 2019;123:e488-e500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 21. | Padovano WM, Dengler J, Patterson MM, Yee A, Snyder-Warwick AK, Wood MD, Moore AM, Mackinnon SE. Incidence of Nerve Injury After Extremity Trauma in the United States. Hand (N Y). 2022;17:615-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 22. | Hong TS, Tian A, Sachar R, Ray WZ, Brogan DM, Dy CJ. Indirect Cost of Traumatic Brachial Plexus Injuries in the United States. J Bone Joint Surg Am. 2019;101:e80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Seddon HJ. Three types of nerve injury. Brain. 1943;66:237-288. [RCA] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 908] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 24. | Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74:491-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 820] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 25. | Hussain G, Wang J, Rasul A, Anwar H, Qasim M, Zafar S, Aziz N, Razzaq A, Hussain R, de Aguilar JG, Sun T. Current Status of Therapeutic Approaches against Peripheral Nerve Injuries: A Detailed Story from Injury to Recovery. Int J Biol Sci. 2020;16:116-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 26. | Maugeri G, D'Agata V, Trovato B, Roggio F, Castorina A, Vecchio M, Di Rosa M, Musumeci G. The role of exercise on peripheral nerve regeneration: from animal model to clinical application. Heliyon. 2021;7:e08281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 27. | Yuan Y, Wang Y, Wu S, Zhao MY. Review: Myelin clearance is critical for regeneration after peripheral nerve injury. Front Neurol. 2022;13:908148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 28. | Liu M, Li P, Jia Y, Cui Q, Zhang K, Jiang J. Role of Non-coding RNAs in Axon Regeneration after Peripheral Nerve Injury. Int J Biol Sci. 2022;18:3435-3446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Borger A, Stadlmayr S, Haertinger M, Semmler L, Supper P, Millesi F, Radtke C. How miRNAs Regulate Schwann Cells during Peripheral Nerve Regeneration-A Systemic Review. Int J Mol Sci. 2022;23:3440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Bosch-Queralt M, Fledrich R, Stassart RM. Schwann cell functions in peripheral nerve development and repair. Neurobiol Dis. 2023;176:105952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 96] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 31. | Gu D, Xia Y, Ding Z, Qian J, Gu X, Bai H, Jiang M, Yao D. Inflammation in the Peripheral Nervous System after Injury. Biomedicines. 2024;12:1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 32. | Zhang H, Zhang Z, Lin H. Research progress on the reduced neural repair ability of aging Schwann cells. Front Cell Neurosci. 2023;17:1228282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 33. | Wei C, Guo Y, Ci Z, Li M, Zhang Y, Zhou Y. Advances of Schwann cells in peripheral nerve regeneration: From mechanism to cell therapy. Biomed Pharmacother. 2024;175:116645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 34. | He B, Hu J, Zhu Z. Editorial: The function of Schwann cells in peripheral nervous system. Front Cell Neurosci. 2022;16:1129560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 35. | Wang Y, Du S, Liu T, Ren J, Zhang J, Xu H, Zhang H, Liu Y, Lu L. Schwann Cell Migration through Magnetic Actuation Mediated by Fluorescent-Magnetic Bifunctional Fe(3)O(4)·Rhodamine 6G@Polydopamine Superparticles. ACS Chem Neurosci. 2020;11:1359-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Pandey S, Mudgal J. A Review on the Role of Endogenous Neurotrophins and Schwann Cells in Axonal Regeneration. J Neuroimmune Pharmacol. 2022;17:398-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 37. | Nuelle JAV, Bozynski C, Stoker A. Innovations in Peripheral Nerve Injury: Current Concepts and Emerging Techniques to Improve Recovery. Mo Med. 2022;119:129-135. [PubMed] |
| 38. | Zhang M, An H, Zhang F, Jiang H, Wan T, Wen Y, Han N, Zhang P. Prospects of Using Chitosan-Based Biopolymers in the Treatment of Peripheral Nerve Injuries. Int J Mol Sci. 2023;24:12956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 39. | Yang Y, Gu W, Xu S, Wang S, Shi H, Zhang L, Meng XG, Hong F, Du Y. Treatment for peripheral nerve injury: a protocol for a systematic review and Bayesian network meta-analysis. BMJ Open. 2024;14:e090497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 40. | Meena P, Kakkar A, Kumar M, Khatri N, Nagar RK, Singh A, Malhotra P, Shukla M, Saraswat SK, Srivastava S, Datt R, Pandey S. Advances and clinical challenges for translating nerve conduit technology from bench to bed side for peripheral nerve repair. Cell Tissue Res. 2021;383:617-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 41. | Manoukian OS, Baker JT, Rudraiah S, Arul MR, Vella AT, Domb AJ, Kumbar SG. Functional polymeric nerve guidance conduits and drug delivery strategies for peripheral nerve repair and regeneration. J Control Release. 2020;317:78-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 42. | Vijayavenkataraman S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020;106:54-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 295] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 43. | Rao Z, Lin Z, Song P, Quan D, Bai Y. Biomaterial-Based Schwann Cell Transplantation and Schwann Cell-Derived Biomaterials for Nerve Regeneration. Front Cell Neurosci. 2022;16:926222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 44. | Zhang RC, Du WQ, Zhang JY, Yu SX, Lu FZ, Ding HM, Cheng YB, Ren C, Geng DQ. Mesenchymal stem cell treatment for peripheral nerve injury: a narrative review. Neural Regen Res. 2021;16:2170-2176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 45. | Babu S, Krishnan M, Panneerselvam A, Chinnaiyan M. A comprehensive review on therapeutic application of mesenchymal stem cells in neuroregeneration. Life Sci. 2023;327:121785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 46. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12677] [Article Influence: 704.3] [Reference Citation Analysis (2)] |
| 47. | Álvarez-Viejo M, Menéndez-Menéndez Y, Otero-Hernández J. CD271 as a marker to identify mesenchymal stem cells from diverse sources before culture. World J Stem Cells. 2015;7:470-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 48. | Alvites R, Branquinho M, Sousa AC, Lopes B, Sousa P, Maurício AC. Mesenchymal Stem/Stromal Cells and Their Paracrine Activity-Immunomodulation Mechanisms and How to Influence the Therapeutic Potential. Pharmaceutics. 2022;14:381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 49. | Andrzejewska A, Lukomska B, Janowski M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells. 2019;37:855-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 392] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 50. | Malekpour K, Hazrati A, Soudi S, Hashemi SM. Mechanisms behind therapeutic potentials of mesenchymal stem cell mitochondria transfer/delivery. J Control Release. 2023;354:755-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 51. | Nooshabadi VT, Mardpour S, Yousefi-Ahmadipour A, Allahverdi A, Izadpanah M, Daneshimehr F, Ai J, Banafshe HR, Ebrahimi-Barough S. The extracellular vesicles-derived from mesenchymal stromal cells: A new therapeutic option in regenerative medicine. J Cell Biochem. 2018;119:8048-8073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 52. | Margiana R, Markov A, Zekiy AO, Hamza MU, Al-Dabbagh KA, Al-Zubaidi SH, Hameed NM, Ahmad I, Sivaraman R, Kzar HH, Al-Gazally ME, Mustafa YF, Siahmansouri H. Clinical application of mesenchymal stem cell in regenerative medicine: a narrative review. Stem Cell Res Ther. 2022;13:366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 203] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 53. | Sumarwoto T, Suroto H, Mahyudin F, Utomo DN, Romaniyanto, Tinduh D, Notobroto HB, Sigit Prakoeswa CR, Rantam FA, Rhatomy S. Role of adipose mesenchymal stem cells and secretome in peripheral nerve regeneration. Ann Med Surg (Lond). 2021;67:102482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 54. | Yousefi F, Lavi Arab F, Nikkhah K, Amiri H, Mahmoudi M. Novel approaches using mesenchymal stem cells for curing peripheral nerve injuries. Life Sci. 2019;221:99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 55. | Ayala-Cuellar AP, Kang JH, Jeung EB, Choi KC. Roles of Mesenchymal Stem Cells in Tissue Regeneration and Immunomodulation. Biomol Ther (Seoul). 2019;27:25-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 56. | Ge Y, Zhang Y, Tang Q, Gao J, Yang H, Gao Z, Zhao RC. Mechanisms of the Immunomodulation Effects of Bone Marrow-Derived Mesenchymal Stem Cells on Facial Nerve Injury in Sprague-Dawley Rats. Stem Cells Dev. 2019;28:489-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Shrestha M, Nguyen TT, Park J, Choi JU, Yook S, Jeong JH. Immunomodulation effect of mesenchymal stem cells in islet transplantation. Biomed Pharmacother. 2021;142:112042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | Namini MS, Daneshimehr F, Beheshtizadeh N, Mansouri V, Ai J, Jahromi HK, Ebrahimi-Barough S. Cell-free therapy based on extracellular vesicles: a promising therapeutic strategy for peripheral nerve injury. Stem Cell Res Ther. 2023;14:254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 59. | Welsh JA, Goberdhan DCI, O'Driscoll L, Buzas EI, Blenkiron C, Bussolati B, Cai H, Di Vizio D, Driedonks TAP, Erdbrügger U, Falcon-Perez JM, Fu QL, Hill AF, Lenassi M, Lim SK, Mahoney MG, Mohanty S, Möller A, Nieuwland R, Ochiya T, Sahoo S, Torrecilhas AC, Zheng L, Zijlstra A, Abuelreich S, Bagabas R, Bergese P, Bridges EM, Brucale M, Burger D, Carney RP, Cocucci E, Crescitelli R, Hanser E, Harris AL, Haughey NJ, Hendrix A, Ivanov AR, Jovanovic-Talisman T, Kruh-Garcia NA, Ku'ulei-Lyn Faustino V, Kyburz D, Lässer C, Lennon KM, Lötvall J, Maddox AL, Martens-Uzunova ES, Mizenko RR, Newman LA, Ridolfi A, Rohde E, Rojalin T, Rowland A, Saftics A, Sandau US, Saugstad JA, Shekari F, Swift S, Ter-Ovanesyan D, Tosar JP, Useckaite Z, Valle F, Varga Z, van der Pol E, van Herwijnen MJC, Wauben MHM, Wehman AM, Williams S, Zendrini A, Zimmerman AJ; MISEV Consortium, Théry C, Witwer KW. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J Extracell Vesicles. 2024;13:e12404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1603] [Cited by in RCA: 1379] [Article Influence: 1379.0] [Reference Citation Analysis (0)] |
| 60. | Galieva LR, James V, Mukhamedshina YO, Rizvanov AA. Therapeutic Potential of Extracellular Vesicles for the Treatment of Nerve Disorders. Front Neurosci. 2019;13:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 61. | Tsiapalis D, O'Driscoll L. Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications. Cells. 2020;9:991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 215] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 62. | Yudintceva N, Mikhailova N, Fedorov V, Samochernych K, Vinogradova T, Muraviov A, Shevtsov M. Mesenchymal Stem Cells and MSCs-Derived Extracellular Vesicles in Infectious Diseases: From Basic Research to Clinical Practice. Bioengineering (Basel). 2022;9:662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 63. | Bucan V, Vaslaitis D, Peck CT, Strauß S, Vogt PM, Radtke C. Effect of Exosomes from Rat Adipose-Derived Mesenchymal Stem Cells on Neurite Outgrowth and Sciatic Nerve Regeneration After Crush Injury. Mol Neurobiol. 2019;56:1812-1824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 64. | Zou XF, Zhang BZ, Qian WW, Cheng FM. Bone marrow mesenchymal stem cells in treatment of peripheral nerve injury. World J Stem Cells. 2024;16:799-810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (3)] |
| 65. | Rezaie J, Nejati V, Mahmoodi M, Ahmadi M. Mesenchymal stem cells derived extracellular vesicles: A promising nanomedicine for drug delivery system. Biochem Pharmacol. 2022;203:115167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 66. | Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16:748-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 1130] [Article Influence: 282.5] [Reference Citation Analysis (0)] |
| 67. | Ma Y, Ge S, Zhang J, Zhou D, Li L, Wang X, Su J. Mesenchymal stem cell-derived extracellular vesicles promote nerve regeneration after sciatic nerve crush injury in rats. Int J Clin Exp Pathol. 2017;10:10032-10039. [PubMed] |
| 68. | Yin G, Yu B, Liu C, Lin Y, Xie Z, Hu Y, Lin H. Exosomes produced by adipose-derived stem cells inhibit schwann cells autophagy and promote the regeneration of the myelin sheath. Int J Biochem Cell Biol. 2021;132:105921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 69. | Ma Y, Dong L, Zhou D, Li L, Zhang W, Zhen Y, Wang T, Su J, Chen D, Mao C, Wang X. Extracellular vesicles from human umbilical cord mesenchymal stem cells improve nerve regeneration after sciatic nerve transection in rats. J Cell Mol Med. 2019;23:2822-2835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 70. | Tang H, Li J, Wang H, Ren J, Ding H, Shang J, Wang M, Wei Z, Feng S. Human umbilical cord mesenchymal stem cell-derived exosomes loaded into a composite conduit promote functional recovery after peripheral nerve injury in rats. Neural Regen Res. 2024;19:900-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 71. | Rao F, Zhang D, Fang T, Lu C, Wang B, Ding X, Wei S, Zhang Y, Pi W, Xu H, Wang Y, Jiang B, Zhang P. Exosomes from Human Gingiva-Derived Mesenchymal Stem Cells Combined with Biodegradable Chitin Conduits Promote Rat Sciatic Nerve Regeneration. Stem Cells Int. 2019;2019:2546367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 72. | Aldali F, Deng C, Nie M, Chen H. Advances in therapies using mesenchymal stem cells and their exosomes for treatment of peripheral nerve injury: state of the art and future perspectives. Neural Regen Res. 2025;20:3151-3171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Huang Y, Wu Q, Tam PKH. Immunomodulatory Mechanisms of Mesenchymal Stem Cells and Their Potential Clinical Applications. Int J Mol Sci. 2022;23:10023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 106] [Reference Citation Analysis (0)] |
| 74. | Bagno LL, Salerno AG, Balkan W, Hare JM. Mechanism of Action of Mesenchymal Stem Cells (MSCs): impact of delivery method. Expert Opin Biol Ther. 2022;22:449-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 75. | Li Y, Zhang D, Xu L, Dong L, Zheng J, Lin Y, Huang J, Zhang Y, Tao Y, Zang X, Li D, Du M. Cell-cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cell Mol Immunol. 2019;16:908-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 76. | López-García L, Castro-Manrreza ME. TNF-α and IFN-γ Participate in Improving the Immunoregulatory Capacity of Mesenchymal Stem/Stromal Cells: Importance of Cell-Cell Contact and Extracellular Vesicles. Int J Mol Sci. 2021;22:9531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 77. | Fan XL, Zhang Y, Li X, Fu QL. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 2020;77:2771-2794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 344] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 78. | Tseng N, Lambie SC, Huynh CQ, Sanford B, Patel M, Herson PS, Ormond DR. Mitochondrial transfer from mesenchymal stem cells improves neuronal metabolism after oxidant injury in vitro: The role of Miro1. J Cereb Blood Flow Metab. 2021;41:761-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 79. | Zhang Y, Xu T, Xie J, Wu H, Hu W, Yuan X. MSC-derived mitochondria promote axonal regeneration via Atf3 gene up-regulation by ROS induced DNA double strand breaks at transcription initiation region. Cell Commun Signal. 2024;22:240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 80. | Wang P, Zhang Y, Zhao J, Jiang B. Intramuscular injection of bone marrow mesenchymal stem cells with small gap neurorrhaphy for peripheral nerve repair. Neurosci Lett. 2015;585:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 81. | Yalçın MB, Bora ES, Erdoğan MA, Çakır A, Erbaş O. The Effect of Adipose-Derived Mesenchymal Stem Cells on Peripheral Nerve Damage in a Rodent Model. J Clin Med. 2023;12:6411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Cooney DS, Wimmers EG, Ibrahim Z, Grahammer J, Christensen JM, Brat GA, Wu LW, Sarhane KA, Lopez J, Wallner C, Furtmüller GJ, Yuan N, Pang J, Sarkar K, Lee WP, Brandacher G. Mesenchymal Stem Cells Enhance Nerve Regeneration in a Rat Sciatic Nerve Repair and Hindlimb Transplant Model. Sci Rep. 2016;6:31306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 83. | Rodríguez Sánchez DN, de Lima Resende LA, Boff Araujo Pinto G, de Carvalho Bovolato AL, Possebon FS, Deffune E, Amorim RM. Canine Adipose-Derived Mesenchymal Stromal Cells Enhance Neuroregeneration in a Rat Model of Sciatic Nerve Crush Injury. Cell Transplant. 2019;28:47-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 84. | Yang Z, Zheng C, Zhang F, Lin B, Cao M, Tian X, Zhang J, Zhang X, Shen J. Magnetic resonance imaging of enhanced nerve repair with mesenchymal stem cells combined with microenvironment immunomodulation in neurotmesis. Muscle Nerve. 2020;61:815-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 85. | Bingham JR, Kniery KR, Jorstad NL, Horkayne-Szakaly I, Hoffer ZS, Salgar SK. "Stem cell therapy to promote limb function recovery in peripheral nerve damage in a rat model" - Experimental research. Ann Med Surg (Lond). 2019;41:20-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 86. | Mathot F, Shin AY, Van Wijnen AJ. Targeted stimulation of MSCs in peripheral nerve repair. Gene. 2019;710:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 87. | Wu S, Shen W, Ge X, Ao F, Zheng Y, Wang Y, Jia X, Mao Y, Luo Y. Advances in Large Gap Peripheral Nerve Injury Repair and Regeneration with Bridging Nerve Guidance Conduits. Macromol Biosci. 2023;23:e2300078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 88. | Behtaj S, Ekberg JAK, St John JA. Advances in Electrospun Nerve Guidance Conduits for Engineering Neural Regeneration. Pharmaceutics. 2022;14:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 89. | Lischer M, di Summa PG, Petrou IG, Schaefer DJ, Guzman R, Kalbermatten DF, Madduri S. Mesenchymal Stem Cells in Nerve Tissue Engineering: Bridging Nerve Gap Injuries in Large Animals. Int J Mol Sci. 2023;24:7800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 90. | Parker BJ, Rhodes DI, O'Brien CM, Rodda AE, Cameron NR. Nerve guidance conduit development for primary treatment of peripheral nerve transection injuries: A commercial perspective. Acta Biomater. 2021;135:64-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 91. | Mankavi F, Ibrahim R, Wang H. Advances in Biomimetic Nerve Guidance Conduits for Peripheral Nerve Regeneration. Nanomaterials (Basel). 2023;13:2528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 92. | Zhang C, Gong J, Zhang J, Zhu Z, Qian Y, Lu K, Zhou S, Gu T, Wang H, He Y, Yu M. Three Potential Elements of Developing Nerve Guidance Conduit for Peripheral Nerve Regeneration. Adv Funct Mater. 2023;33. [DOI] [Full Text] |
| 93. | Dixon AR, Jariwala SH, Bilis Z, Loverde JR, Pasquina PF, Alvarez LM. Bridging the gap in peripheral nerve repair with 3D printed and bioprinted conduits. Biomaterials. 2018;186:44-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 94. | Zhang Q, Nguyen PD, Shi S, Burrell JC, Cullen DK, Le AD. 3D bio-printed scaffold-free nerve constructs with human gingiva-derived mesenchymal stem cells promote rat facial nerve regeneration. Sci Rep. 2018;8:6634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 95. | Iwai T, Ikeguchi R, Aoyama T, Noguchi T, Yoshimoto K, Sakamoto D, Fujita K, Miyazaki Y, Akieda S, Nagamura-Inoue T, Nagamura F, Nakayama K, Matsuda S. Nerve regeneration using a Bio 3D conduit derived from umbilical cord-Derived mesenchymal stem cells in a rat sciatic nerve defect model. PLoS One. 2024;19:e0310711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 96. | Yurie H, Ikeguchi R, Aoyama T, Tanaka M, Oda H, Takeuchi H, Mitsuzawa S, Ando M, Yoshimoto K, Noguchi T, Akieda S, Nakayama K, Matsuda S. Bio 3D Conduits Derived from Bone Marrow Stromal Cells Promote Peripheral Nerve Regeneration. Cell Transplant. 2020;29:963689720951551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 97. | Allbright KO, Bliley JM, Havis E, Kim DY, Dibernardo GA, Grybowski D, Waldner M, James IB, Sivak WN, Rubin JP, Marra KG. Delivery of adipose-derived stem cells in poloxamer hydrogel improves peripheral nerve regeneration. Muscle Nerve. 2018;58:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 98. | Saller MM, Huettl RE, Mayer JM, Feuchtinger A, Krug C, Holzbach T, Volkmer E. Validation of a novel animal model for sciatic nerve repair with an adipose-derived stem cell loaded fibrin conduit. Neural Regen Res. 2018;13:854-861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 99. | Chuang MH, Ho LH, Kuo TF, Sheu SY, Liu YH, Lin PC, Tsai YC, Yang CH, Chu CM, Lin SZ. Regenerative Potential of Platelet-Rich Fibrin Releasate Combined with Adipose Tissue-Derived Stem Cells in a Rat Sciatic Nerve Injury Model. Cell Transplant. 2020;29:963689720919438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 100. | Rodríguez-Sánchez DN, Pinto GBA, Cartarozzi LP, de Oliveira ALR, Bovolato ALC, de Carvalho M, da Silva JVL, Dernowsek JA, Golim M, Barraviera B, Ferreira RS, Deffune E, Bertanha M, Amorim RM. 3D-printed nerve guidance conduits multi-functionalized with canine multipotent mesenchymal stromal cells promote neuroregeneration after sciatic nerve injury in rats. Stem Cell Res Ther. 2021;12:303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 101. | Zhang Z, Li M, Cheng G, Wang P, Zhou C, Liu Y, Duan X, Wang J, Xie F, Zhu Y, Zhang J. A chitosan/acellular matrix-based neural graft carrying mesenchymal stem cells to promote peripheral nerve repair. Stem Cell Res Ther. 2024;15:503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 102. | Cartarozzi LP, Spejo AB, Ferreira RS Jr, Barraviera B, Duek E, Carvalho JL, Góes AM, Oliveira AL. Mesenchymal stem cells engrafted in a fibrin scaffold stimulate Schwann cell reactivity and axonal regeneration following sciatic nerve tubulization. Brain Res Bull. 2015;112:14-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 103. | Cui Y, Yao Y, Zhao Y, Xiao Z, Cao Z, Han S, Li X, Huan Y, Pan J, Dai J. Functional collagen conduits combined with human mesenchymal stem cells promote regeneration after sciatic nerve transection in dogs. J Tissue Eng Regen Med. 2018;12:1285-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 104. | Qian C, Zhang Z, Zhao R, Wang D, Li H. Effect of acellular nerve scaffold containing human umbilical cord-derived mesenchymal stem cells on nerve repair and regeneration in rats with sciatic nerve defect. Ann Transl Med. 2022;10:483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 105. | Shalaby SM, El-Shal AS, Ahmed FE, Shaban SF, Wahdan RA, Kandel WA, Senger MS. Combined Wharton's jelly derived mesenchymal stem cells and nerve guidance conduit: A potential promising therapy for peripheral nerve injuries. Int J Biochem Cell Biol. 2017;86:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 106. | Alvites RD, Branquinho MV, Sousa AC, Amorim I, Magalhães R, João F, Almeida D, Amado S, Prada J, Pires I, Zen F, Raimondo S, Luís AL, Geuna S, Varejão ASP, Maurício AC. Combined Use of Chitosan and Olfactory Mucosa Mesenchymal Stem/Stromal Cells to Promote Peripheral Nerve Regeneration In Vivo. Stem Cells Int. 2021;2021:6613029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 107. | Sousa AC, Alvites R, Lopes B, Sousa P, Moreira A, Coelho A, Santos JD, Atayde L, Alves N, Maurício AC. Three-Dimensional Printing/Bioprinting and Cellular Therapies for Regenerative Medicine: Current Advances. J Funct Biomater. 2025;16:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 108. | Ikeguchi R, Aoyama T, Tanaka M, Noguchi T, Ando M, Yoshimoto K, Sakamoto D, Iwai T, Miyazaki Y, Akieda S, Ikeya M, Nakayama K, Matsuda S. Nerve regeneration using the Bio 3D nerve conduit fabricated with spheroids. J Artif Organs. 2022;25:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 109. | Soman SS, Vijayavenkataraman S. Perspectives on 3D Bioprinting of Peripheral Nerve Conduits. Int J Mol Sci. 2020;21:5792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 110. | Fu X, Tong Z, Li Q, Niu Q, Zhang Z, Tong X, Tong L, Zhang X. Induction of adipose-derived stem cells into Schwann-like cells and observation of Schwann-like cell proliferation. Mol Med Rep. 2016;14:1187-1193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 111. | Choi SJ, Park SY, Shin YH, Heo SH, Kim KH, Lee HI, Kim JK. Mesenchymal Stem Cells Derived from Wharton's Jelly Can Differentiate into Schwann Cell-Like Cells and Promote Peripheral Nerve Regeneration in Acellular Nerve Grafts. Tissue Eng Regen Med. 2021;18:467-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 112. | Mortimer AE, Faroni A, Kilic MA, Reid AJ. Maintenance of a Schwann-Like Phenotype in Differentiated Adipose-Derived Stem Cells Requires the Synergistic Action of Multiple Growth Factors. Stem Cells Int. 2017;2017:1479137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 113. | Chen S, Ikemoto T, Tokunaga T, Okikawa S, Miyazaki K, Tokuda K, Yamada S, Saito Y, Imura S, Morine Y, Shimada M. Effective in vitro differentiation of adipose-derived stem cells into Schwann-like cells with folic acid supplementation. J Med Invest. 2021;68:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 114. | Yang X, Chen J, Xue P, Liu R, Ji W, Lu X, Liu X, Chen Z. Differentiation of bone marrow stromal cells into schwann-like cells using dihydrotestosterone combined with a classical induction method. Biotechnol Lett. 2017;39:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 115. | Hopf A, Schaefer DJ, Kalbermatten DF, Guzman R, Madduri S. Schwann Cell-Like Cells: Origin and Usability for Repair and Regeneration of the Peripheral and Central Nervous System. Cells. 2020;9:1990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 116. | Liu Y, Chen J, Liu W, Lu X, Liu Z, Zhao X, Li G, Chen Z. A Modified Approach to Inducing Bone Marrow Stromal Cells to Differentiate into Cells with Mature Schwann Cell Phenotypes. Stem Cells Dev. 2016;25:347-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 117. | Das SR, Uz M, Ding S, Lentner MT, Hondred JA, Cargill AA, Sakaguchi DS, Mallapragada S, Claussen JC. Electrical Differentiation of Mesenchymal Stem Cells into Schwann-Cell-Like Phenotypes Using Inkjet-Printed Graphene Circuits. Adv Healthc Mater. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 118. | Moosazadeh Moghaddam M, Bonakdar S, Shokrgozar MA, Zaminy A, Vali H, Faghihi S. Engineered substrates with imprinted cell-like topographies induce direct differentiation of adipose-derived mesenchymal stem cells into Schwann cells. Artif Cells Nanomed Biotechnol. 2019;47:1022-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 119. | Sun X, Zhu Y, Yin HY, Guo ZY, Xu F, Xiao B, Jiang WL, Guo WM, Meng HY, Lu SB, Wang Y, Peng J. Differentiation of adipose-derived stem cells into Schwann cell-like cells through intermittent induction: potential advantage of cellular transient memory function. Stem Cell Res Ther. 2018;9:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 120. | Zhang Q, Burrell JC, Zeng J, Motiwala FI, Shi S, Cullen DK, Le AD. Implantation of a nerve protector embedded with human GMSC-derived Schwann-like cells accelerates regeneration of crush-injured rat sciatic nerves. Stem Cell Res Ther. 2022;13:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 121. | Ferreira LVO, Kamura BDC, Oliveira JPM, Chimenes ND, Carvalho M, Santos LAD, Dias-Melicio LA, Amorim RL, Amorim RM. In Vitro Transdifferentiation Potential of Equine Mesenchymal Stem Cells into Schwann-Like Cells. Stem Cells Dev. 2023;32:422-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 122. | Zhang X, Zhang W, Sun H, Wang H. The effects of exosomes originating from different cell sources on the differentiation of bone marrow mesenchymal stem cells into schwann cells. J Nanobiotechnology. 2024;22:220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 123. | Liu YM, Wang HY, Wei CH, Li JP, Wang Y, Ma WZ, Jia H. Exploring miR-21 as a key regulator in two distinct approaches of bone marrow stromal cells differentiation into Schwann-like cells. Synapse. 2024;78:e22293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 124. | Martins Amorim R, Vinícius de Oliveira Ferreira L. Schwann-Like Cells Derived from Mesenchymal Stem Cells: Their Potential for Peripheral Nerve Regeneration. In: Stem Cell Transplantation. United Kingdom: IntechOpen, 2024. [DOI] [Full Text] |
| 125. | di Summa PG, Kingham PJ, Raffoul W, Wiberg M, Terenghi G, Kalbermatten DF. Adipose-derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg. 2010;63:1544-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 274] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 126. | di Summa PG, Kalbermatten DF, Pralong E, Raffoul W, Kingham PJ, Terenghi G. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience. 2011;181:278-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 127. | Orbay H, Uysal AC, Hyakusoku H, Mizuno H. Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. J Plast Reconstr Aesthet Surg. 2012;65:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 128. | Tomita K, Madura T, Sakai Y, Yano K, Terenghi G, Hosokawa K. Glial differentiation of human adipose-derived stem cells: implications for cell-based transplantation therapy. Neuroscience. 2013;236:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 129. | Georgiou M, Golding JP, Loughlin AJ, Kingham PJ, Phillips JB. Engineered neural tissue with aligned, differentiated adipose-derived stem cells promotes peripheral nerve regeneration across a critical sized defect in rat sciatic nerve. Biomaterials. 2015;37:242-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 130. | Watanabe Y, Sasaki R, Matsumine H, Yamato M, Okano T. Undifferentiated and differentiated adipose-derived stem cells improve nerve regeneration in a rat model of facial nerve defect. J Tissue Eng Regen Med. 2017;11:362-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 131. | Tamez-Mata Y, Pedroza-Montoya FE, Martínez-Rodríguez HG, García-Pérez MM, Ríos-Cantú AA, González-Flores JR, Soto-Domínguez A, Montes-de-Oca-Luna R, Simental-Mendía M, Peña-Martínez VM, Vílchez-Cavazos F. Nerve gaps repaired with acellular nerve allografts recellularized with Schwann-like cells: Preclinical trial. J Plast Reconstr Aesthet Surg. 2022;75:296-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 132. | Pedroza-Montoya FE, Tamez-Mata YA, Simental-Mendía M, Soto-Domínguez A, García-Pérez MM, Said-Fernández S, Montes-de-Oca-Luna R, González-Flores JR, Martínez-Rodríguez HG, Vilchez-Cavazos F. Repair of ovine peripheral nerve injuries with xenogeneic human acellular sciatic nerves prerecellularized with allogeneic Schwann-like cells-an innovative and promising approach. Regen Ther. 2022;19:131-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 133. | Mimura T, Dezawa M, Kanno H, Sawada H, Yamamoto I. Peripheral nerve regeneration by transplantation of bone marrow stromal cell-derived Schwann cells in adult rats. J Neurosurg. 2004;101:806-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 139] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 134. | Shimizu S, Kitada M, Ishikawa H, Itokazu Y, Wakao S, Dezawa M. Peripheral nerve regeneration by the in vitro differentiated-human bone marrow stromal cells with Schwann cell property. Biochem Biophys Res Commun. 2007;359:915-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 135. | Ao Q, Fung CK, Tsui AY, Cai S, Zuo HC, Chan YS, Shum DK. The regeneration of transected sciatic nerves of adult rats using chitosan nerve conduits seeded with bone marrow stromal cell-derived Schwann cells. Biomaterials. 2011;32:787-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 136. | Wang X, Luo E, Li Y, Hu J. Schwann-like mesenchymal stem cells within vein graft facilitate facial nerve regeneration and remyelination. Brain Res. 2011;1383:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 137. | Cai S, Tsui YP, Tam KW, Shea GK, Chang RS, Ao Q, Shum DK, Chan YS. Directed Differentiation of Human Bone Marrow Stromal Cells to Fate-Committed Schwann Cells. Stem Cell Reports. 2017;9:1097-1108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 138. | Matsuse D, Kitada M, Kohama M, Nishikawa K, Makinoshima H, Wakao S, Fujiyoshi Y, Heike T, Nakahata T, Akutsu H, Umezawa A, Harigae H, Kira J, Dezawa M. Human umbilical cord-derived mesenchymal stromal cells differentiate into functional Schwann cells that sustain peripheral nerve regeneration. J Neuropathol Exp Neurol. 2010;69:973-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 139. | Lin YJ, Lee YW, Chang CW, Huang CC. 3D Spheroids of Umbilical Cord Blood MSC-Derived Schwann Cells Promote Peripheral Nerve Regeneration. Front Cell Dev Biol. 2020;8:604946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 140. | Zhou T, Yuan Z, Weng J, Pei D, Du X, He C, Lai P. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 2021;14:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 422] [Article Influence: 105.5] [Reference Citation Analysis (0)] |
| 141. | Zhang YR, Ka K, Zhang GC, Zhang H, Shang Y, Zhao GQ, Huang WH. Repair of peripheral nerve defects with chemically extracted acellular nerve allografts loaded with neurotrophic factors-transfected bone marrow mesenchymal stem cells. Neural Regen Res. 2015;10:1498-1506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 142. | Man AJ, Kujawski G, Burns TS, Miller EN, Fierro FA, Leach JK, Bannerman P. Neurogenic potential of engineered mesenchymal stem cells overexpressing VEGF. Cell Mol Bioeng. 2016;9:96-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 143. | Hu F, Sun B, Xu P, Zhu Y, Meng XH, Teng GJ, Xiao ZD. MiR-218 Induces Neuronal Differentiation of ASCs in a Temporally Sequential Manner with Fibroblast Growth Factor by Regulation of the Wnt Signaling Pathway. Sci Rep. 2017;7:39427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 144. | Roballo KCS, da Silveira JC, Bressan FF, de Souza AF, Pereira VM, Porras JEP, Rós FA, Pulz LH, Strefezzi RF, Martins DDS, Meirelles FV, Ambrósio CE. Neurons-derived extracellular vesicles promote neural differentiation of ADSCs: a model to prevent peripheral nerve degeneration. Sci Rep. 2019;9:11213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |