Published online Jun 26, 2025. doi: 10.4252/wjsc.v17.i6.106520
Revised: April 2, 2025
Accepted: June 3, 2025
Published online: June 26, 2025
Processing time: 117 Days and 21 Hours
Carotid atherosclerosis is a complex disease involving multiple cellular and molecular pathways. Mesenchymal stem cells (MSCs) show therapeutic potential, but their optimal targets and efficacy are still under study. MiR-126 enhances endothelial function and promotes angiogenesis by relieving vascular endothelial growth factor signaling suppression, suggesting its potential in vascular rege
To verify if miR-126 inhibits carotid atherosclerosis via the MAPK/ERK pathway.
Rat bone marrow MSCs (product No. CP-R131, Wuhan, China) were verified by flow cytometry. The effects of miR-126 on MSCs’ proliferation, migration, apoptosis, and cytokine expression were explored using microRNA mimics and inhibitors. Fluorescence staining quantified CD31+ cells to evaluate endothelial differentiation. In vivo differentiation was assessed, and MSCs were transplanted into a rat carotid artery balloon dilatation model. Rats were randomly divided into five groups: Control, negative control mimics, miR-126 mimics, negative control inhibitor, and miR-126 inhibitor.
In vitro, MSCs treated with miR-126 mimics demonstrated enhanced proliferation, increased migration, and reduced apoptosis. These miR-126 mimics also significantly increased the secretion of vascular endothelial growth factor and basic fibroblast growth factor. Fluorescence and tissue staining indicated a higher proportion of CD31+ cells in the miR-126 mimics group. Additionally, the expression of endothelial-related genes (von Willebrand factor, endothelial nitric oxide synthase, and vascular endothelial-cadherin) was upregulated in this group. In vivo, miR-126-transfected MSCs effectively reduced neointimal thickness and promoted endothelial coverage in rats. MiR-126 stimulated MSC proliferation in a dose-dependent manner and reduced p38 and ERK1/2 phos
MiR-126 exerts significant modulatory effects on the immunoregulatory and vascular reparative functions of MSCs through the MAPK/ERK signaling pathway, promoting their differentiation into endothelial cells and thereby mitigating atherosclerosis.
Core Tip: Carotid atherosclerosis is closely linked to endothelial dysfunction, a relationship validated in rat models. As traditional drugs fall short in curing the disease and surgery carries risks, researchers are exploring gene-based therapies. MiR-126 emerges as a potential therapeutic factor. Experiments show it promotes mesenchymal stem cell differentiation into endothelial cells. Molecularly, miR-126 synergizes with the mitogen-activated protein kinases/extracellular signal-regulated kinase pathway. By modulating this pathway, miR-126 suppresses atherosclerotic lesion progression and shows promise in enhancing vascular health. These findings underscore the potential of miR-126 mimics in treating carotid atherosclerosis, laying a foundation for clinical translation.
- Citation: Ye ZQ, Meng XH, Fang X, Liu HY, Mwindadi HH. MiR-126 regulates the effect of mesenchymal stem cell vascular repair on carotid atherosclerosis through MAPK/ERK signaling pathway. World J Stem Cells 2025; 17(6): 106520
- URL: https://www.wjgnet.com/1948-0210/full/v17/i6/106520.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i6.106520
Carotid atherosclerosis is characterized by the presence of carotid artery atherosclerosis. This lesion not only affects local blood flow but also increases the risk of systemic cardiovascular events, such as increased risk of stroke[1], cognitive dysfunction[2], and increased risk of cardiovascular events[3,4]. Carotid atherosclerosis is a growing problem worldwide owing to its associated morbidity and mortality. Currently, the use of antithrombotic drugs, particularly aspirin, can be considered as first-line prevention for carotid atherosclerosis. However, the use of aspirin as first-line prevention remains highly controversial, and guidelines generally advise against the routine use of aspirin as primary prevention in adults at low risk of cardiovascular disease (CVD). For those at higher risk of CVD, the risks and benefits of aspirin use need to be assessed on an individual basis[5]. Therefore, intervention in carotid atherosclerosis from a molecular biology perspective has become an important development in current research, and the search for effective molecular markers of carotid atherosclerosis is of great clinical importance.
MicroRNAs (miRNAs) regulate gene expression at the transcriptional and post-transcriptional levels and are involved in many important physiological and pathological processes[6,7]. Studies have shown that some miRNAs may be increased in patients with carotid atherosclerosis and decreased in patients without carotid artery stenosis[8]. This indicates greater sensitivity and specificity of miRNAs in carotid atherosclerotic disease. Our results suggest that miR-126 overexpression promotes endothelial differentiation of mesenchymal stem cells (MSCs), which may be attributed to the phosphatidylinositol 3-kinase/protein kinase B and mitogen-activated protein kinases/extracellular signal-regulated kinase (MAPK/ERK) pathways that regulate MSCs, thereby causing atherogenic vascular regeneration.
MiRNAs are a class of single-stranded non-coding miRNAs, 21-23 nucleotide in length[9], that function by recognizing the 3’ untranslated region (3’-UTR) of target mRNAs, binding to their targets and post-transcriptionally inhibiting the translation levels of their targets. MiRNAs are transcribed by RNA poly II into a long transcript of the original miRNA (called pri-miRNA)[10]. Pri-miRNA is processed in the nucleus by the RNase III enzyme Drosha and the dsDNA-binding protein DGCR8/Pasha to produce a 70-100 nucleotide hairpin precursor called pre-miRNA[11]. The pre-miRNAs are then transported to the cytoplasm via the nuclear export factor exportin 5[12], where they act as substrates for Dicer (another RNase III enzyme) to produce a mature miRNA double-stranded body of approximately 20-22 base pairs. Finally, the double-stranded body dissociates and one strand is incorporated into the RNA-induced silencing complex[13], where it binds to the 3’-UTR of the target mRNA, thereby inhibiting its translation or causing its degradation.
A large body of evidence indicates that miRNAs play important roles in cellular processes such as proliferation, differentiation, and apoptosis[14,15]. Some miRNAs are expressed in a lineage-specific manner and thus have the potential to control stem cell fate decisions[16]. Recently, Ivey et al[17] showed that muscle-specific miRNA (miR-1) directs mesoderm formation in embryonic stem cells and regulates differentiation to the cardiac lineage by repressing the expression of genes in alternative lineages. Izarra et al[18] investigated the expression profiles of miR-1, miR-133, and miR-143 during myocardial differentiation of mouse ESCs and found that overexpression of these miRNAs increased the expression of cardiac genes and thus regulated myocardial differentiation. Fu et al[19] showed that miR-21 could promote proliferation and inhibit apoptosis of MSCs by targeting genes such as programmed cell death 4 and phosphatase and tensin homolog while affecting their differentiation into osteoblasts or adipocytes. Because miRNAs have the potential to control the fate of stem cells, their use in the treatment of disease will yield remarkable results. In recent years, stem cell therapy has shown that exogenous introduction of specific miRNAs can improve the proliferation and differentiation efficiency of stem cells and make them more responsive to therapeutic needs. For example, miR-34a can promote the differentiation of embryonic stem cells into specific cell types and improve the efficacy of stem cell therapy[20]. Therefore, we speculated whether miRNAs can be used as molecular markers to promote the differentiation of MSCs into endothelial cells (ECs) for the treatment of carotid atherosclerosis. This hypothesis is based on the important role of miRNAs in regulating stem cell fate decisions, particularly their potential to promote angiogenesis and EC function. The identification and use of specific miRNAs are expected to provide new therapeutic approaches to effectively ameliorate the pathology of carotid atherosclerosis.
ECs are key regulators of angiogenesis in vascular biology[21]. These cells play critical roles in vascular health and disease progression by regulating endothelial function, promoting angiogenesis, and maintaining vascular homeostasis. In atherosclerosis, ECs form a continuous barrier lining the inner surface of the blood vessels, preventing harmful substances in the bloodstream from entering the vessel wall. When the function of the endothelial barrier is impaired, lipids, inflammatory cells, and other harmful substances in the blood are more likely to enter the vessel wall, triggering an inflammatory response and lipid deposition, which is an important feature of early lesions in atherosclerosis. Therefore, prevention and treatment of atherosclerosis should focus on protecting EC function to maintain vascular health and prevent disease progression. It is well known that pluripotent stem cells can be directly differentiated into the EC lineage and give rise to transplantable, healthy, and multifunctional cells capable of repairing ischaemic tissues[22]. Increased expression of angiogenesis-associated miRNAs (miR-126, miR-130a, miR-133a, miR-133b, and miR-210) and decreased expression of anti-angiogenic miRNAs (miR-20a, miR-20b, miR-221, and miR-222) have recently been reported during angiogenesis[23,24]. However, although miRNA regulation has been demonstrated in numerous vascular biological events and in the early stages of mesoderm formation, there are no reports describing miRNAs that directly control and determine the fate of vascular EC.
Among the myriad vascular-related miRNAs, miR126 has garnered substantial attention due to its EC-specific expression and developmental relevance[25]. Zebrafish model studies have demonstrated that the synergistic action of miR-126a and miR126b at dual binding sites is crucial for proper vascular development[26]. Furthermore, miR-126 has been shown to positively regulate the response of ECs to vascular endothelial growth factor (VEGF) and promote angiogenesis, at least partially by directly suppressing negative regulators of the VEGF pathway - including sprouting-related protein 1 (SPRED1) and phosphoinositide 3-kinase regulatory subunit 2 (PIK3R2)[27]. Studies have indicated that transplantation of MSCs overexpressing miR-126 can markedly enhance neovascularization in the ischemic mouse heart, suggesting a promising strategy to improve MSC-based therapies[28]. Nevertheless, whether overexpression of miR-126 can also promote the differentiation of MSCs into the endothelial lineage warrants further investigation.
Therefore, we hypothesize that miR-126 modulates the endothelial differentiation of MSCs via the MAPK/ERK signaling pathway. This hypothesis is predicated on several observations: (1) MiR-126 plays a pivotal role in EC proliferation and vascular repair; (2) The MAPK/ERK pathway is a key regulator of cell proliferation, differentiation, and survival; and (3) Prior research suggests that miR-126 can influence the functionality and differentiation capacity of MSCs through modulation of this pathway. Thus, we postulate that miR-126 may promote MSC differentiation into ECs by activating the MAPK/ERK pathway, thereby exerting a protective effect in the treatment of carotid atherosclerosis.
Rat bone marrow-derived MSCs were procured from Procell (Product No. CP-R131, Wuhan, China), originating from the bone marrow of 3-6-week-old Wistar rats. MSCs were cultured in complete rat MSC medium (Qisai Biotechnology, QS-R207A, Wuhan, China) under standard conditions (37 °C, 5% CO2). To maintain cell viability and promote passage, cells were digested with 0.25% trypsin and subcultured at a 1:2 ratio. Human umbilical vein ECs were cultured in EC basal medium supplemented with 5%-10% fetal bovine serum (FBS), EC growth supplements [EGM-2 MV SingleQuot Kit, containing VEGF, epidermal growth factor, fibroblast growth factor (FGF), insulin-like growth factor, and steroid hormones], and 1% penicillin-streptomycin, and maintained in a humidified incubator at 37 °C, 5% CO2, and > 95% humidity. When cell confluence reached 80%-90%, cells were subcultured at a 1:2 ratio using trypsin-EDTA for digestion, followed by centrifugation, resuspension, and seeding into new culture flasks. All procedures were performed under strict aseptic conditions, with regular medium changes and continuous monitoring of cell health.
Identification of rat bone marrow-derived MSCs was performed using flow cytometry. The specific procedures were as follows: Cells were collected, and each flow cytometry tube contained 1 mL of phosphate buffered saline (PBS) with 0.5% bovine serum albumin (BSA) and 1 × 106 cells. 10 μL of each antibody was added: CD34 (Thermo Fisher, PA5-85917, CLONE#RAM34, MA, United States), CD105 (Thermo Fisher, MA1-19594, CLONE#SN6h, MA, United States), CD29 (BioLegend, 102207, CLONE#HMβ1-1, CA, United States), CD90 (BioLegend, 206105, CLONE#OX-7, CA, United States), CD44 (Thermo Fisher, MA5-17522,CLONE#IM7, MA, United States), CD45 (BioLegend, 202207, CLONE#30-F11, CA, United States), and CD31 (Thermo Fisher, MA5-16954, CLONE#390, MA, United States). After incubation at 4 °C in the dark for 30 minutes, tubes were washed twice with PBS containing 0.5% BSA. The cells were then resuspended in 0.2 mL of PBS with 0.5% BSA and analyzed using a flow cytometer (CytoFLEX, Beckman, CA, United States).
After enzymatic digestion with trypsin, the cells were resuspended in a medium containing 10% FBS and seeded into a 96-well plate at a density of 5 × 104 cells/mL. Cells were incubated overnight at 37 °C in a 5% CO2 incubator. Following cell adhesion, the cells were transfected with 30 nM of control mimics (RiboBio, miRN0000001-1-10, Guangzhou, China), miR-126 mimics (RiboBio, miR10000831-1-5, Guangzhou, China), control inhibitor (RiboBio, miR2N0000001-1-10, Guangzhou, China), and miR-126 inhibitor (RiboBio, miR20000831-1-5, Guangzhou, China) for 48 hours. Subsequently, 10 μL of CCK-8 solution (MCE, HY-K0301, Shanghai, China) was added per well, and the plate was incubated for 2 hours in the incubator. Absorbance at 450 nm was measured using a spectrophotometer.
After treatment, the cells were collected and washed twice with PBS, followed by centrifugation at 1000 rpm for 5 minutes. The procedure was conducted according to the Annexin V-FITC/PI apoptosis detection kit instructions (KeyGEN BioTECH, KGA108, Shanghai, China): 500 μL of Binding Buffer was added to resuspend the cells; 5 μL of Annexin V-FITC was added and mixed, followed by 5 μL of PI, mixed thoroughly; the reaction proceeded at room temperature in the dark for 10 minutes; apoptosis was assessed using a flow cytometer (CytoFLEX, Beckman, CA, United States).
The levels of VEGF and basic FGF (bFGF) were measured using a rat VEGF ELISA kit (MLbio, ML064294, Shanghai, China) and rat bFGF ELISA kit (MLbio, ML003037, Shanghai, China), respectively. 50 μL of a 1:1 diluted standard and 50 μL of the sample were added to the reaction wells, followed immediately by 50 μL of the biotinylated antibody. The plate was covered and gently shaken to mix, incubated at 37 °C for 1 hour. The liquid in the wells was discarded, and the wells were filled with wash buffer, shaken for 30 seconds, and then emptied; this process was repeated three times. 80 μL of 3,3’,5,5’-tetramethylbenzidine substrate was added to each well, gently shaken, and incubated at 37 °C for 30 minutes. The wells were washed as before, 50 μL of stop solution was added to each well, and optical density at 450 nm was measured immediately.
After trypsin digestion, the cells were centrifuged, the supernatant was discarded, and they were washed 1-2 times with PBS. The cells were resuspended in 1% FBS medium, and the cell density to 5 × 105/mL. A cell suspension (100 μL) was added to the upper chamber of the transwell setup, and 600 μL of medium containing 20% FBS was added to the lower chamber. After 24 hours of incubation, the transwell chambers were removed, the medium in the wells was discarded, and the chambers were washed twice with calcium-free PBS. Cells were fixed with methanol for 30 minutes and air-dried. The membrane was stained with 0.1% crystal violet for 20 minutes, and non-migrated cells were gently wiped off with a cotton swab and washed three times with PBS. Images were captured under a microscope.
Cells were lysed using RIPA buffer (Beyotime, P0013B, Shanghai, China), and protein concentration was determined with the BCA Protein Assay Kit (Beyotime, P0012S, Shanghai, China). Equal amounts of protein samples were separated by sodium-dodecyl sulfate gel electrophoresis and transferred to a polyvinylidene fluoride membrane. The membrane was then blocked with 5% non-fat milk in Tris-buffered saline with Tween at room temperature on a shaker for 2 hours. Primary antibodies were diluted in blocking solution: P-ERK1/2 (Affinity, AF1015, 1:1000, Shanghai, China), ERK1/2 (Affinity, AF0155, 1:1000, Shanghai, China), p-p38 (Affinity, AF4001, 1:1000, Shanghai, China), p38 (Affinity, AF6456, 1:1000, Shanghai, China), and GAPDH (Abcam, ab8245, 1:1000, Shanghai, China). Membranes were incubated with diluted primary antibodies overnight at 4 °C. After thorough washing with Tris-buffered saline with Tween, the membrane was incubated with HRP-conjugated secondary antibodies diluted in a blocking solution (HRP-conjugated anti-rabbit IgG, Wuhan Boshide Biotechnology Co., BA1054, 1:10000; Goat Anti-Mouse IgG (H+L) HRP, Affinity, S0002, 1:5000, Shanghai, China) at 37 °C on a shaker for 2 hours. Detection was performed using an ECL kit (Beyotime, P0018, Shanghai, China) by adding the working solution to the membrane, followed by scanning with a scanner (Canon, K10486, Shanghai, China) and analyzing the film density using BandScan software.
The MSCs were resuspended in DMEM low glucose (Procell, PM150220, Wuhan, China) supplemented with 2% FBS (Procell, 164210-50, Wuhan, China) and 50 ng/mL VEGF (Procell, PCK266, Wuhan, China). Three days post-VEGF stimulation, cells were transfected with 30 nM negative control (NC) mimics, miR-126 mimics, NC inhibitor, and miR-126 inhibitor and cultured for seven days at 37 °C, 5% CO2, with the medium being changed every two days. Cells were harvested and lysed with 1 mL of Trizol reagent, homogenized using a homogenizer, and transferred to an RNase-free 1.5 mL EP tube, lysed for 10 minutes. First-strand cDNA synthesis was performed using an EntiLink™ 1st Strand cDNA Synthesis Kit (ELK Biotechnology, EQ003, Wuhan, China). Real-time quantitative polymerase chain reaction (RT-qPCR) was conducted on a StepOne™ Real-Time PCR System (Life Technologies, CA, United States), with three replicates per sample using EnTurbo™ SYBR Green PCR SuperMix (ELK Biotechnology, EQ001, Wuhan, China) under the following conditions: 50 °C for 2 minutes, 95 °C for 10 minutes, followed by 40 cycles of 95 °C for 30 seconds and 60 °C for 30 seconds. The relative expression was calculated using the 2-ΔΔCt method. Primers used included miR-126-5p (5’-CGGCGCGTACCAAAAGT-3’, 5’-GTGCAGGGTCCGAGGT-3’), U6 (5’-CTCGCTTCGGCAGCACA-3’, 5’-AACGCTTCAC
Immunofluorescence was used to detect the expression of CD31 in cultured cells and vascular tissues. First, cryosections of transplanted vessels and cultured cells were fixed with cold acetone and blocked with 10% goat serum. The target proteins were detected using specific primary antibodies, followed by incubation with corresponding fluorescently labeled secondary antibodies. Finally, cell nuclei were counterstained with 4’,6-diamidino-2-phenylindole.
In this study, three lentiviral plasmids were constructed: PLVmiR126 for miR126 overexpression, pshRNAmiR126 for miR126 inhibition, and pLVcontrol as a control. During the cell culture phase, MSCs were maintained in DMEM supplemented with 10% FBS and cultured at 37 °C with 5% CO2. One day prior to transfection, MSCs were seeded in 6-well plates at a density of 2 × 105 cells per well to ensure 70%-80% confluency at the time of transfection. The constructed plasmids were co-transfected with packaging plasmids into 293T cells using a liposomal transfection reagent. Virus containing supernatants were collected at 48 and 72 hours post transfection, then concentrated by ultracentrifugation to obtain high titer viral particles, which were stored at -80 °C. MSCs were seeded in 24-well plates at a density of 1 × 105 cells per well. On the day of transfection, the original medium was replaced with fresh medium containing 6 μg/mL polybrene and an appropriate volume of viral particles. After 4 hours of incubation, fresh medium was added, and the cells were further cultured for 24 hours before replacing the medium with new culture medium.
Data are presented as means ± SD, based on a minimum of three independent measurements, unless specified otherwise. Statistical analyses were performed using GraphPad Prism 9 software (San Diego, CA, United States). Normality tests were performed before selecting the statistics method. For comparing data between two groups, independent unpaired two-tailed t was employed. For multiple group comparisons, one-way analysis of variance (ANOVA) with Tukey’s post hoc analysis was utilized when data were normal distribution, and Kruskal-Wallis test was utilized when data were non-normal distribution. A P value of less than 0.05 was considered statistically significant.
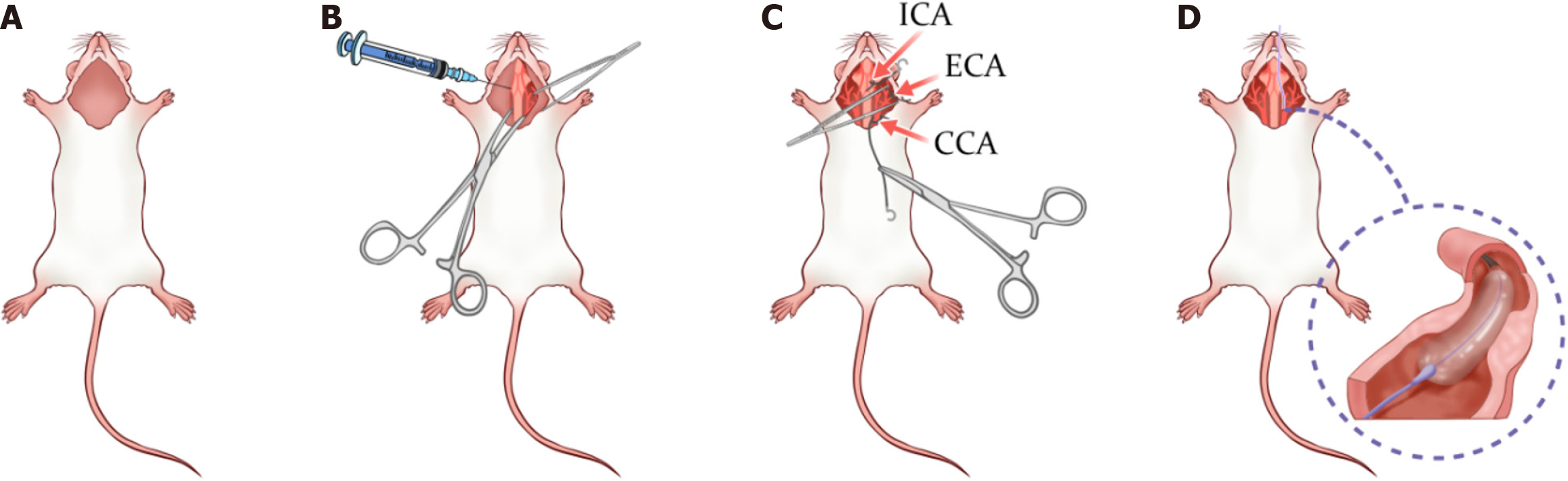
Preoperative preparation: Approximately 40 rats, procured from Shanghai Jihui Laboratory Animal Breeding Co., Ltd. [License No. SCXK(Shanghai)2022-0009], were used in the experiment. The rats, aged approximately 8 weeks, were acclimatized to controlled laboratory conditions for 2 weeks prior to the experiment. These conditions included a temperature of 23 ± 2 °C, a 12-hour light/dark cycle, and 50% humidity. The rats were given unrestricted access to standard chow and water during this period. Following acclimatization, the rats were randomly assigned into experimental groups using computer-generated coded numbers. In the experimental setup, animals were prepared for surgery as depicted in Figure 1A. Prior to surgery, animals were adequately sedated and placed supine on the surgical platform, with the head oriented toward the surgeon. The accessory tissues were retracted, and the cervical region was shaved and disinfected. All necessary surgical instruments and supplies were prepared in advance and arranged for easy access.
Tissue dissection: In the tissue dissection process (Figure 1B), a pair of sharp, blunt-sawtoothed scissors was used to make a linear incision starting directly beneath the animal’s chin and extending to the upper border of the sternum, ensuring that the scissor tips were directed upward to avoid excessive skin incision. Subsequently, medium sized hemostatic forceps were used for blunt dissection to separate the glandular tissue from the subcutaneous layer. In cases of minor vascular rupture or slight hemorrhage, cotton swabs were applied to the affected areas to achieve hemostasis, and the region was kept moist using warm, sterile PBS or other fluids. Once this step was completed, medium to large retractors were employed to maintain a clear field of view. Next, medium sized scissors were used to incise the fascia covering the glandular tissue, followed by further blunt dissection to expose the muscle layer, and careful separation of muscle tissue was performed using fine 7S forceps. For procedures involving the left common carotid artery, blunt dissection was carried out longitudinally along the left side of the central and adjacent muscle groups (e.g., sternocleidomastoid, infrahyoid muscles) to gently separate the central muscle from the parallel cervical muscles and the diagonal thin muscle band superior to the carotid vascular system, thereby facilitating visualization of the carotid vessels. Throughout the process, the instrument tips were maintained upward and all tissues were kept moist, ensuring that the left common carotid artery and its adjacent nerves and vessels were clearly visible after complete dissection and retraction.
Pre-placement of sutures: In the pre-placement of sutures (Figure 1C), it is imperative to ensure that the vascular segments are fully isolated from all adjacent tissues. At each suture placement site, all neighboring tissues were carefully dissected away via blunt dissection, ensuring that at least 2-3 millimeters of the vessel circumference was free of extraneous tissue. Specifically, one suture was placed around the proximal segment of the left common carotid artery, another loosely secured around a distal branch of the external carotid artery near the carotid bifurcation, and a third suture was loosely tied on an internal carotid branch as distally as possible from the bifurcation.
Balloon intervention: In the balloon intervention phase (Figure 1D), the procedure commenced by inserting a guidewire through the left common carotid artery to facilitate accurate placement of a balloon catheter (diameter 1.5-2.0 mm, length approximately 2 cm). Once proper catheter placement was confirmed, the balloon was inflated to a predetermined pressure (approximately 4-6 atm) and maintained for 30 seconds. To ensure sufficient endothelial injury, this balloon inflation process was repeated three times. After each inflation, the balloon was deflated, followed by re-inflation to the same pressure and a subsequent 30-second hold.
Postoperative management: Upon completion of the three balloon inflations, the balloon was fully deflated and the catheter and guidewire were carefully withdrawn from the animal. Immediate measures were then taken to close the arterial incision, typically involving the tight ligation of the opening with preplaced sutures to prevent hemorrhage. Subsequently, the muscle and skin layers were closed in a layered fashion according to standard protocols to ensure a clean wound and minimize infection risk. Postoperatively, euthanasia of the rats was performed using 40% carbon dioxide inhalation until the absence of vital signs, including breathing and heartbeat, was confirmed. This method is widely accepted and aligns with the guidelines recommended by the American Veterinary Medical Association for euthanasia. By gradually increasing the concentration of carbon dioxide, the process aims to minimize animal suffering and ensure a rapid, painless demise. The procedure was conducted by experienced personnel who closely monitored the rats’ vital signs to ensure accuracy and maximize animal welfare.
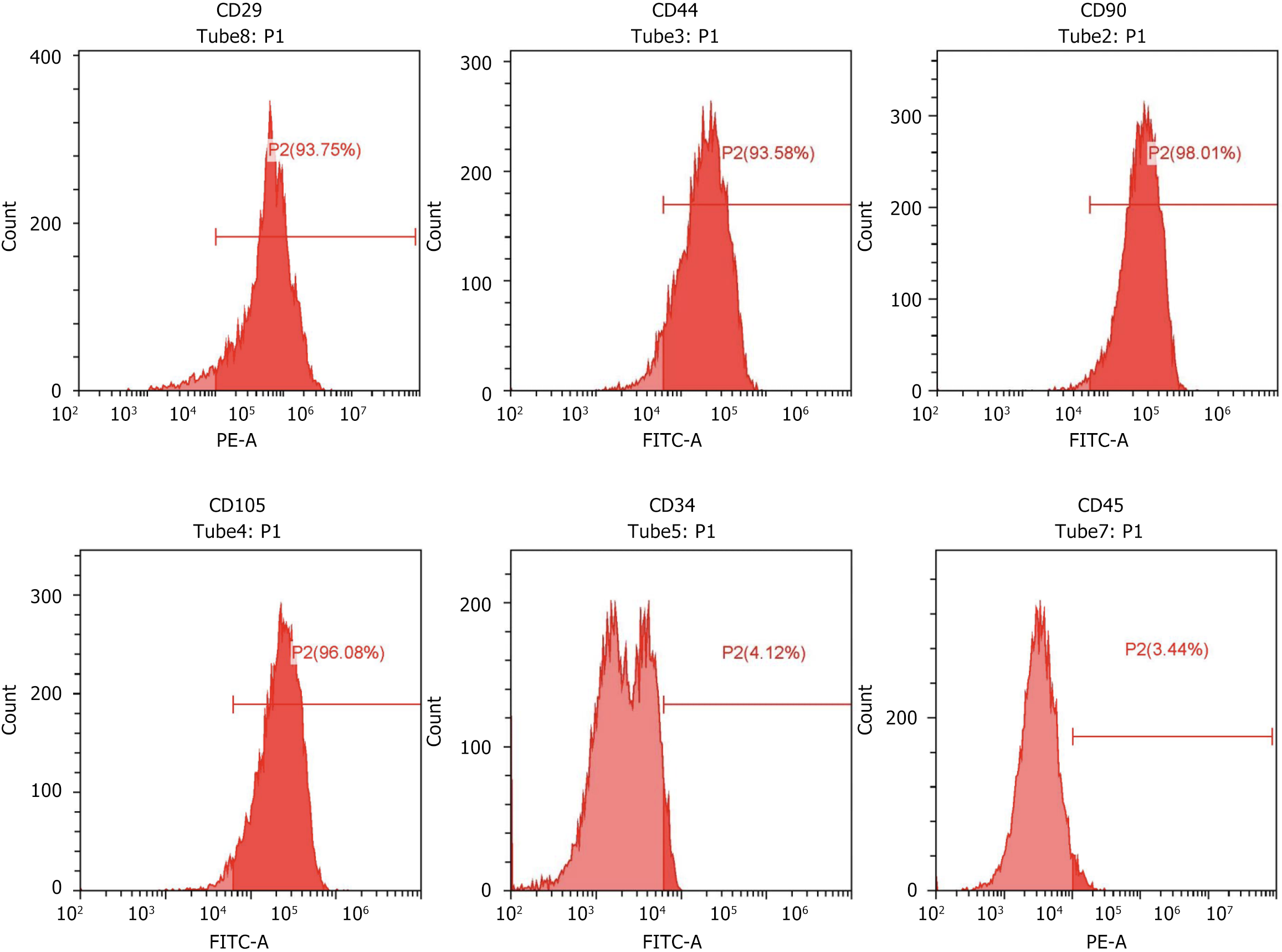
MSCs are a class of adult stem cells with multidirectional differentiation potential that can be isolated from a variety of tissues, including the bone marrow, adipose tissue, and umbilical cord blood. Surface markers of MSCs are important for their identification, isolation, and characterization[9]. According to the standards of the International Society for Cellular Therapy, MSCs should meet the expression criteria for the following surface markers: Positive expression of CD73 (also known as 5’-nucleotidase), CD90 (Thy-1), CD105 (endothelin receptor B) and negative expression of: CD14 or CD11b (predominantly expressed in monocytes and macrophages), CD34 (hematopoietic stem cell marker), CD45 (leukocyte common antigen), HLA-DR (major histocompatibility complex class II molecule)[29]. In addition to the standard markers mentioned above, other possible markers such as CD29, CD44, CD73, and CD166[29-31] have been reported in different studies; however, they are not consistently expressed by MSCs. It is worth noting that MSCs from different sources may differ in the expression of surface markers, and Petrenko et al[32] reported that human bone marrow derived-MSCs, in addition to fulfilling the International Society for Cellular Therapy criteria, expressed other MSC surface markers such as CD10, CD29, CD44, CD133, HLA-ABC, MSCA-1 and SSEA-4. In this experiment, rat bone marrow-derived MSCs were procured from Procell (product No.: CP-R131, Wuhan, China). The cells were cultured in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin, maintained at 37 °C in a humidified 5% CO2 incubator. Rat bone marrow MSCs were then phenotyped using flow cytometry to confirm their biological properties and purity. The results of this study showed that the vast majority (93.75%-98.01%) of the cell samples were CD29 (93.75%), CD44 (93.58%), CD90 (98.01%), and CD105 (96.08%) positive and CD34 (4.12%) and CD45 (3.44%) negative (Figure 2), with CD29, CD44, CD90, and CD105 being some of the hallmark MSC phenotypes, whereas CD31 and CD45 did not express hematopoietic lineage markers, indicating the successful isolation and characterization of highly pure rat bone marrow MSCs. The study showed that the rat bone marrow MSCs obtained were accurate and could be used for further studies.
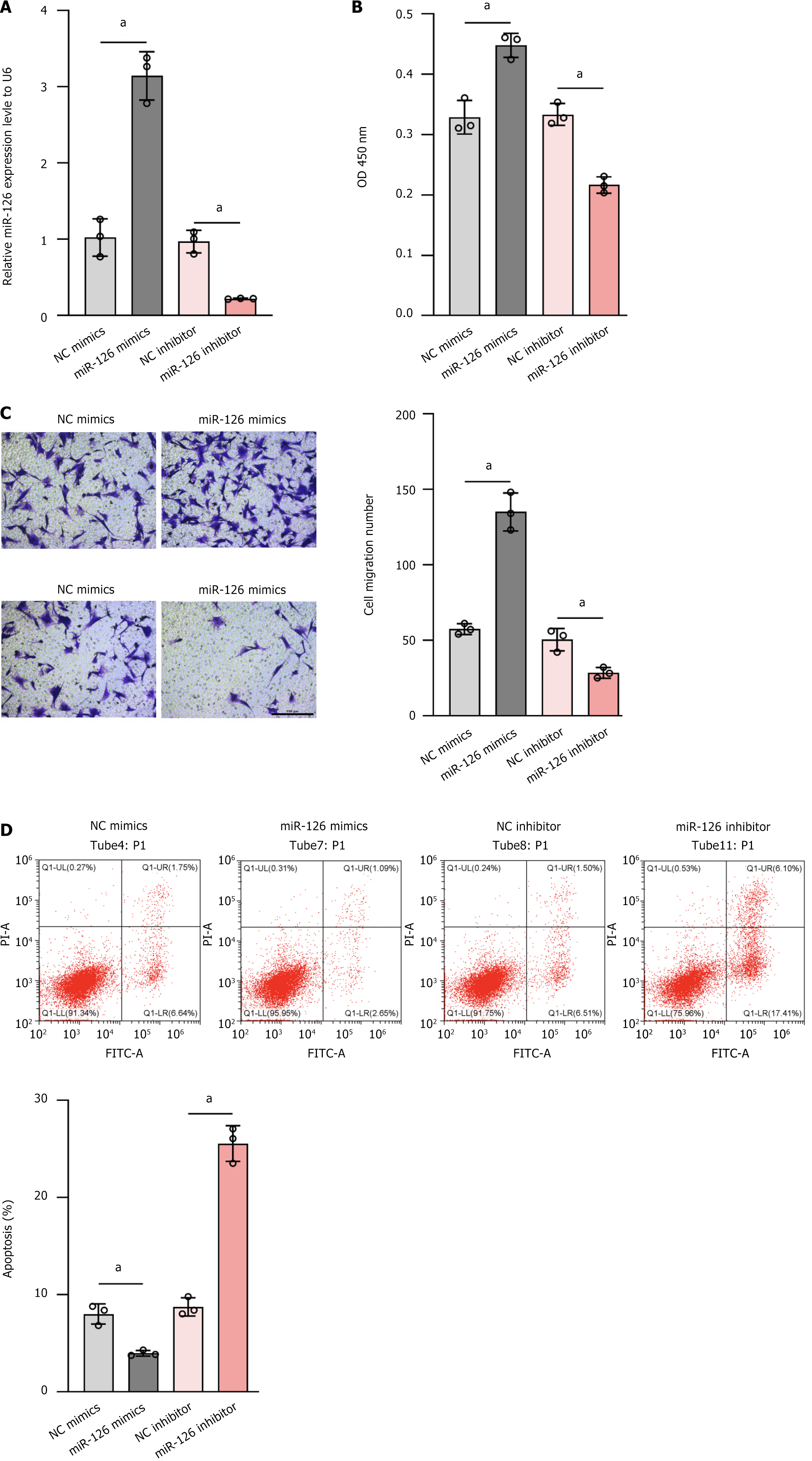
To evaluate the effect of miR-126 on MSCs in more detail, we transfected miR-126 mimics, miR-126 inhibitors, NC mimics, and NC inhibitor. The relative expression levels of miR-126 and its effect on cell proliferation ability under these conditions were analyzed by RT-qPCR and absorbance detection at 450 nm. The experimental results showed that the expression of miR-126 was more than 3-fold higher in the MSC miR-126 mimics group compared with the internal reference gene U6, while the MSC NC mimics group and the MSC NC inhibitor group had comparable expression levels with the internal reference U6 (Figure 3A). In terms of cell proliferation capacity, the MSC miR-126 mimics group showed significantly enhanced proliferation capacity (Figure 3B). Notably, the MSC miR-126 inhibitor group not only had significantly lower miR-126 expression than U6 but also had significantly weaker proliferation capacity (Figure 3A). These results confirmed the efficacy of miR-126 mimics and inhibitors in regulating miR-126 expression and suggested that miR-126 plays a key role in the processes of cell proliferation, differentiation, and apoptosis. In particular, high levels of miR-126 expression may be one of the important factors promoting the differentiation of MSCs into ECs, providing a new perspective and theoretical basis for exploring the specific role of miR-126 in cell differentiation.
It is particularly important to further investigate whether miR-126 promotes the endothelialization of MSCs, as this process is critical for the maintenance of vascular structure and function and is influenced by a variety of factors, including growth factors, cytokines, microenvironmental conditions, and mechanical forces[33]. Our study showed that miR-126 mimics were not only effective in increasing the expression of miR-126 but also in enhancing MSC activity. In addition, we observed that MSCs modified with miR-126 mimics exhibited a higher migratory ability, which may contribute to cell migration into neovascular capillaries and thus promote vascular re-endothelialization (Figure 3C). In contrast, the miR-126 inhibitor group showed the lowest cell activity and migration capacity, further highlighting the importance of miR-126 in this process.
Considering that apoptosis is a form of programmed cell death that not only reduces the number of cells but may also impair the proliferative capacity and differentiation potential of the remaining cells, which in turn may affect tissue repair and regeneration, Peck et al[34] showed that hypoxic preconditioning reduces apoptosis through activation of the hypoxia-inducible factor 1 alpha pathway and promotes the expression of genes related to endothelial differentiation, such as VEGF and eNOS[34]. Although miR-126 mimics can increase miR-126 expression and MSC activity, their overall benefit may be limited if they are associated with excessive mortality. The apoptosis assay showed that the apoptosis rate was increased in all other groups compared to the MSC miR-126 mimics group, in particular, the MSC miR-126 inhibitor group had the highest apoptosis rate (Figure 3D). However, as depicted in Figure 3B, miR-126 mimics significantly promoted cell proliferation, whereas Figure 3D indicates that the apoptosis rate was not markedly reduced. This observation is consistent with previous reports[27] demonstrating that miR-126 enhances cell proliferation by alleviating the inhibition on its target gene SPRED1 thereby augmenting ERK phosphorylation and subsequently upregulating cell cycle proteins such as cyclin D1 to drive cells into S phase.
The apoptosis rate did not significantly decrease in the miR-126 mimics group, which may be because miR-126 does not significantly regulate major apoptosis - related core genes such as members of the Caspase family and Bax/Bcl-2. Moreover, recent studies have shown that miR-126 can influence apoptosis through other mechanisms. For example, a study by Huang et al[35] found that miR-126 regulates the proliferation, migration, invasion and apoptosis of non-small lung cancer cells via the AKT2/HK2 axis, but its effect on key apoptotic mediators was not significant. Another study by Sun et al[36] demonstrated that miR-126 affects femoral fracture healing in rats through the phosphatidylinositol 3-kinase/protein kinase B signaling pathway, but it did not significantly impact the Bax/Bcl-2 ratio or caspase activity. These findings suggest that while miR-126 plays a role in cellular processes, its impact on apoptosis-related mediators may be context-dependent or indirect.
Considering the impact of cellular context and microenvironment, in conditions favoring rapid proliferation, robust survival signals (such as enhanced ERK activation) may mask apoptotic cues. Conversely, under stress conditions, such as serum deprivation, apoptotic pathways may dominate. This implies that the functional effects of miR126 are likely contingent upon the cellular state and external environmental factors. Additionally, miR-126 might indirectly modulate the local microenvironment via the secretion of factors like VEGF, although its influence on direct apoptotic regulation remains limited. Therefore, while miR126 can potentiate cell proliferation through multiple mechanisms, its direct regulatory effect on apoptosis is comparatively minimal.
To test whether miR-126-transduced MSCs activate cell differentiation via the MAPK/ERK pathway, we prepared four groups of MSC samples: Control (no treatment), miR-126 mimics group (overexpression of miR-126), miR-126 inhibitor group (inhibition of miR-126 expression) and NC. We aimed to identify the signaling pathways involved in MSC-induced VEGF upregulation.
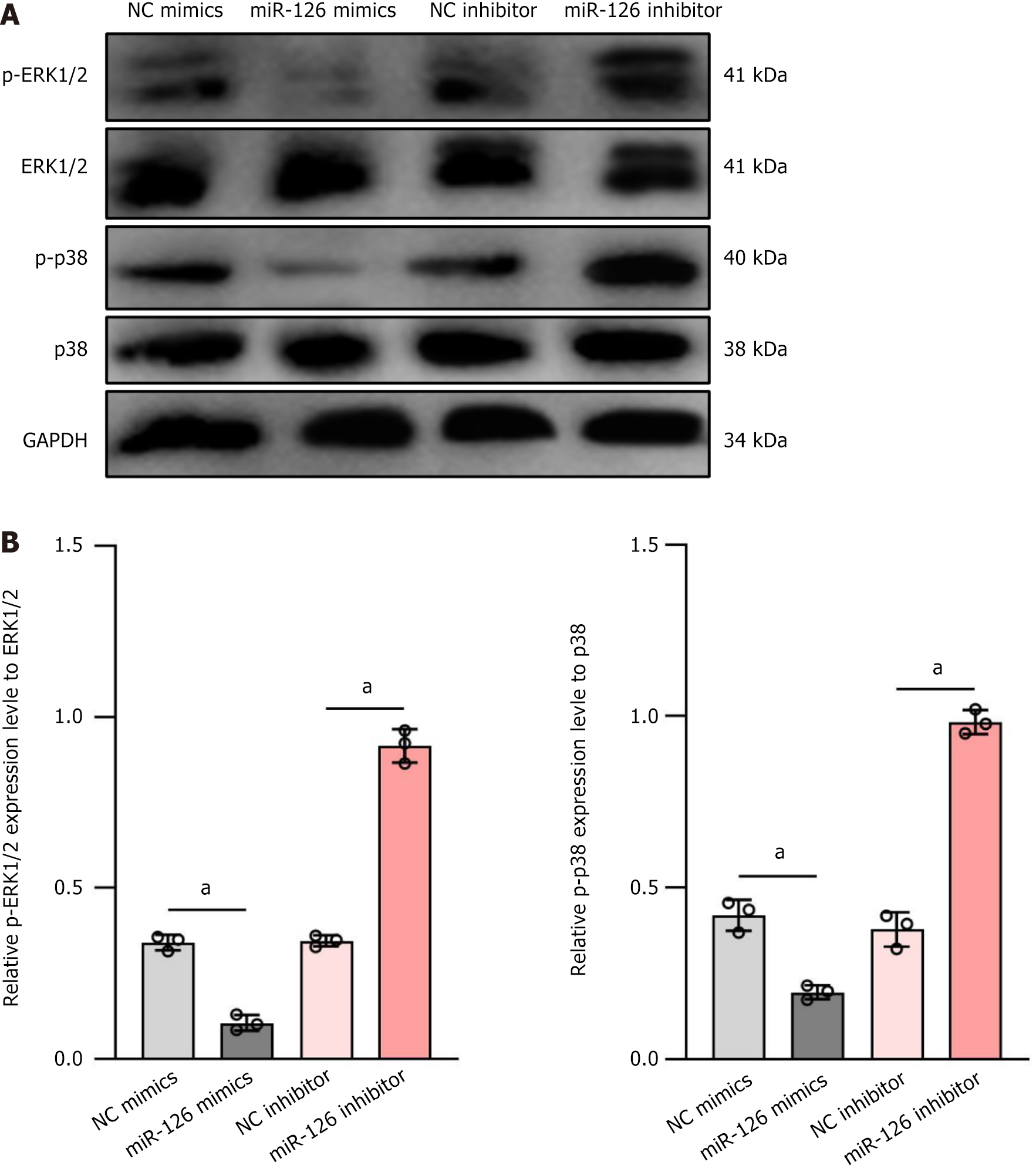
At least three different MAPK signaling pathways are known to transduce extracellular signals into the nucleus to promote cell growth, including ERK, c-Jun N-terminal kinase, and p38[37]. Of these, growth factor-induced signaling is closely associated with the ERK pathway, whereas the p38 pathway is associated with a wide range of factors including cytokines, growth factors, environmental stress, and other stimuli[38,39]. Since the MAPK/ERK1/2 signaling pathway is critical for cellular bioactivity and is closely related to VEGF regulation, we assessed p38 and ERK1/2 activities in MSC by western blot analysis. In cells transfected with miR-126 mimics, the total protein expression levels of ERK1/2 and p38 did not differ significantly compared to controls. However, at the phosphorylation level, the miR-126 mimics group exhibited significantly reduced phosphorylation of ERK1/2 and p38 (P < 0.01), whereas the miR126 inhibitor group demonstrated significantly elevated phosphorylation levels of these proteins relative to controls (P < 0.01) (Figure 4). This finding suggests that miR-126 may influence downstream signaling by modulating the phosphorylation status of ERK1/2 and thus exerting its biological effects.
From the above experimental results, it can be concluded that miR-126 can activate the MAPK/ERK1/2 signaling pathway and exert important biological effects in MSC. However, a limitation of this study is the lack of experiments to add MAPK/ERK pathway-specific chemical inhibitors (e.g., U0126) to the miR-126 mimic group. This step is crucial to confirm whether the effect of this pathway on cell differentiation is dependent on the action of miR-126. In addition, knockdown of key components (for example, MEK1/2 or ERK1/2) using small interfering RNA (siRNA) could also further validate the role of the MAPK/ERK pathway in miR-126-mediated cell differentiation.
VEGF and bFGF are important pro-angiogenic factors[40]. VEGF is a family of polypeptides including VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor, the most abundant of which is VEGF-A.VEGF usually exists in the form of homodimers and is approximately 120-200 amino acids in length. It stimulates cell proliferation and the formation of new microvessels on ECs by binding to specific receptors such as fms-like tyrosine kinase 1 (VEGFR-1) and fetal liver kinase-1 (VEGFR-2)[41]. In contrast, bFGF promotes adhesion and migration of vascular endothelial and smooth muscle cells by mediating integrin expression, which in turn contributes to neovascularization[42].
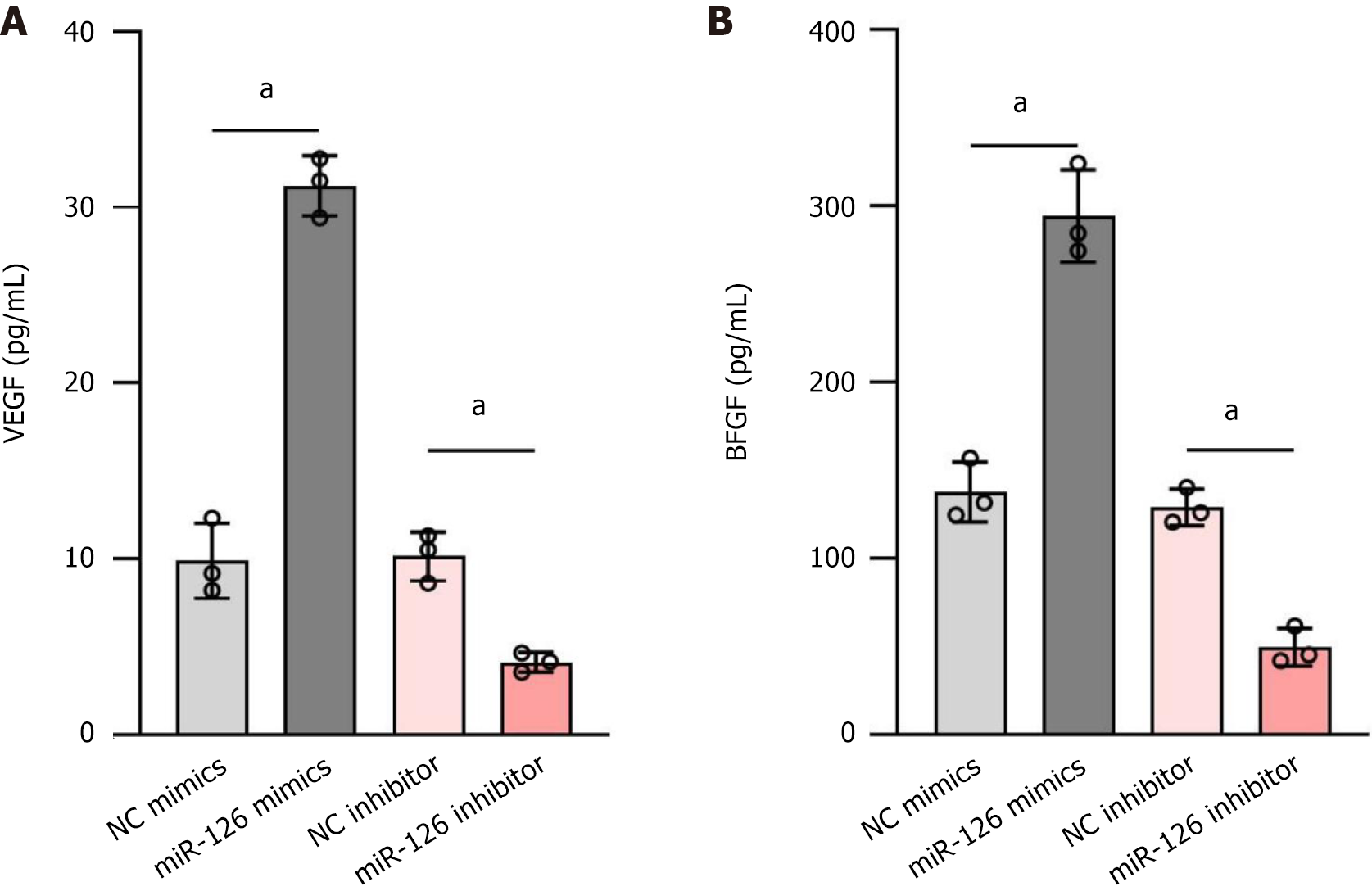
Studies have shown that miR-126 can significantly affect the function of MSCs[43]. Specifically, when miR-126 is transduced into MSCs, it enhances the paracrine effects of these cells, increasing their ability to secrete VEGF and bFGF. This effect may be achieved by direct or indirect mechanisms: On the one hand, miR-126 may directly target and inhibit pro-apoptotic genes (e.g., SPRED1, PIK3R2) (Figure 5), thereby protecting cells from apoptosis and possibly negatively regulating genes that express VEGF or bFGF; on the other hand, it may also alter internal signaling pathways within MSCs, which in turn may modulate cellular behavior and secretion patterns. To verify the relationship between miR-126 and the expression of VEGF and bFGF, we performed experiments to assess the expression levels of VEGF and bFGF in MSCs with different modifications using RT-qPCR. The results showed that the miR-126 mimics group produced the highest levels of VEGF and bFGF, while the miR-126 inhibitor group produced the lowest levels of VEGF and bFGF. Specifically, the secretion levels of VEGF and bFGF were, in descending order: MSC miR-126 mimics > MSC NC mimics > MSC NC inhibitor > MSC miR-126 inhibitor (Figure 4). This demonstrates that miR-126-transduced MSC cells indeed increase the release of VEGF and bFGF, which contributes to the improvement of the local microenvironment and promotes neovascularisation and tissue repair.
Bone marrow-derived MSCs exhibit multipotency under specific in vitro differentiation conditions; for example, they can differentiate into functional ECs. Given the early induction of miR-126 in vascular progenitor cells, we investigated whether overexpression of this EC-specific miRNA could direct the differentiation of MSCs toward an endothelial lineage. CD31, a member of the immunoglobulin superfamily and a 130 kDa transmembrane glycoprotein, is expressed in various cell types (including vascular ECs, circulating platelets, monocytes, neutrophils, and certain T-cell subpopulations) and has even been detected throughout hematopoietic stem cell development. However, CD31 is most renowned for its high specificity to ECs, making it an essential biomarker for investigating EC differentiation and function.
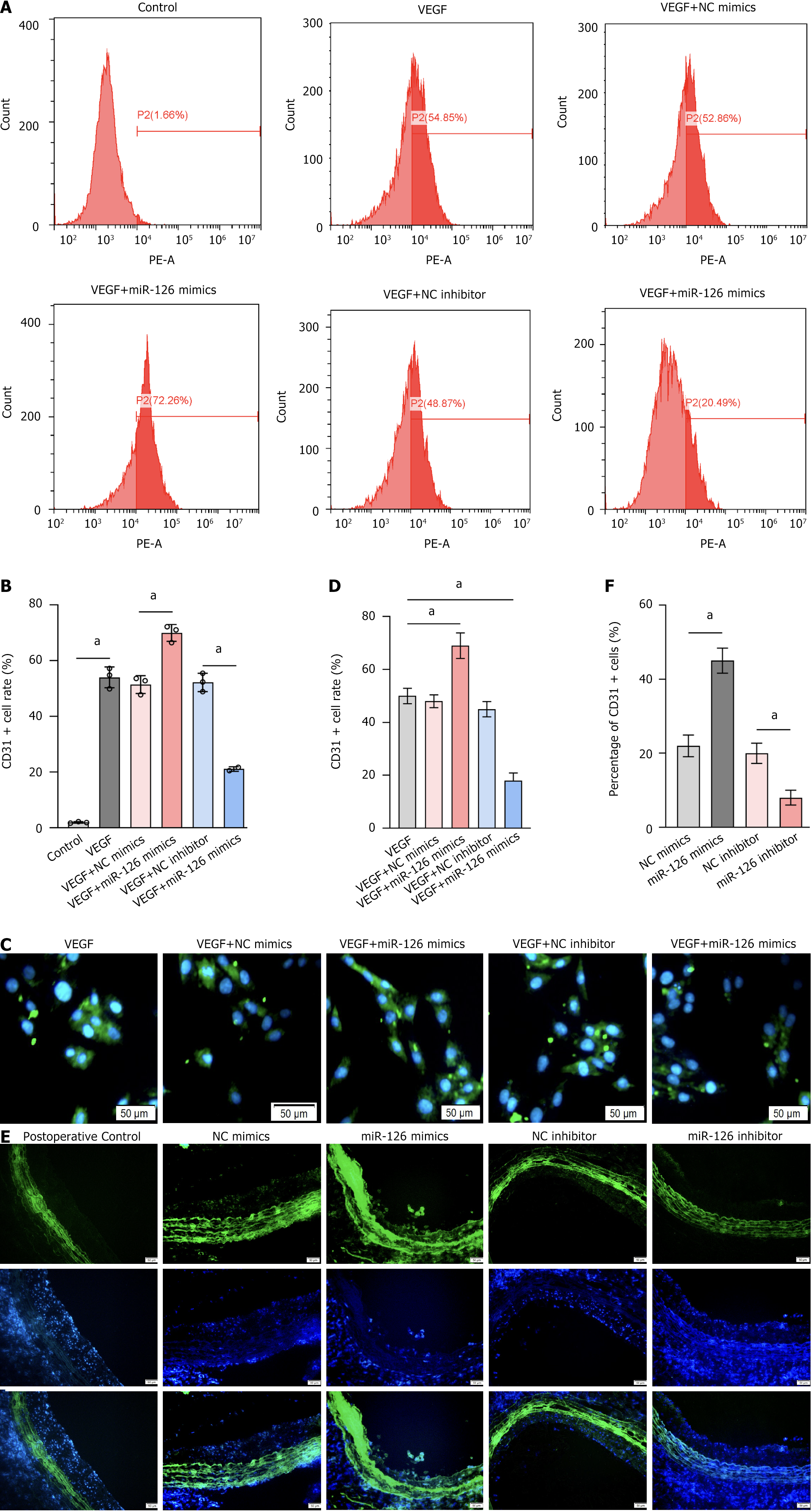
During the differentiation of bone marrow-derived MSCs into ECs, CD31 assumes particular importance. While CD31 is scarcely expressed in undifferentiated MSCs, its expression is markedly upregulated as MSCs differentiate into endothelial-like cells[44]. This significant increase not only reflects the phenotypic shift but also indicates the acquisition of functional characteristics inherent to ECs. Moreover, CD31 plays an essential role in mediating intercellular adhesion and migration among ECs, thereby facilitating angiogenesis and maintaining the integrity of the endothelial barrier. Specifically, CD31 cells have the capacity to form capillary-like structures in vitro (for example, tubular formations on Matrigel) which directly demonstrate their neovascularization potential[45]. Thus, CD31 serves both as an indicator of endothelial differentiation and as a functional marker of EC biology, including processes such as angiogenesis and barrier formation. In this study, enzyme-linked immunosorbent assay was used to compare the positivity rate of EC marker CD31 in the cytoplasm of different groups, which directly reflects the differentiation ability of transfected MSCs into ECs (Figure 6). To better evaluate the effect of miR-126 on postoperative neointimal lesion formation, VEGF was incorporated to promote cell differentiation.
Flow cytometric analysis was then performed to quantify the CD31 positivity rate of the graphs obtained after flow cytometric analysis (Figure 7A and B), the proportion of CD31-positive cells reached 72.26% in samples with the combination of VEGF and miR-126 mimics, significantly higher than the 54.85% in the VEGF-only group. In contrast, the CD31 positivity was only 20.49% in samples with the combination of VEGF and miR-126 inhibitor, significantly lower than in the VEGF-only group. Fluorescence staining of cells (Figure 7C and D) and tissues (Figure 7E and F) was also performed to evaluate the number and distribution of CD31-positive cells, as well as neointimal hyperplasia. These results further support the experimental hypothesis. However, while CD31 is a valuable marker for assessing the differentiation of MSCs into ECs, its expression may not fully capture the extent of endothelial differentiation under all conditions. CD31 levels can fluctuate in response to microenvironmental factors, and its expression is not entirely exclusive to ECs, potentially leading to an overestimation of differentiation efficiency. Additionally, high levels of CD31 expression may not comprehensively reflect endothelial functionality, as cells expressing CD31 may still lack complete endothelial properties. Under pathological conditions, the expression of CD31 may become unstable, thereby under
Therefore, to more comprehensively evaluate the biological process underlying MSC differentiation into ECs, it is advisable to employ a panel of complementary markers. vWF, a glycoprotein secreted by ECs, directly reflects endothelial functional status; eNOS, which is intimately associated with endothelial vasodilatory function, serves as an indicator of endothelial metabolic activity and vascular relaxation; and vascular endothelial-cadherin, an endothelial-specific adhesion molecule, correlates with both the maturity and functionality of ECs, thereby providing a more precise measure of MSC differentiation into functional ECs. Additionally, members of the integrin family, which are pivotal for cellular adhesion and migration, further elucidate the functional state of MSC-derived ECs. Together, these supplementary biomarkers offer a more comprehensive assessment than CD31 alone, thereby facilitating a more accurate evaluation of the completeness and functionality of MSC endothelial differentiation and providing deeper insights into the underlying biological processes.
Notably, the CD31 positivity rate was 52.86% in MSCs treated with VEGF combined with NC mimics, and 48.87% in those treated with VEGF combined with NC inhibitor. These rates are comparable to the 54.85% CD31 positivity in MSCs treated with VEGF alone. These results suggest that miR-126’s effect on MSC differentiation into ECs is not due to a basic additive effect but is likely mediated by enhanced MSC responsiveness to VEGF. In other words, miR-126 appears to sensitize MSCs to VEGF signaling, thereby more effectively promoting their differentiation into ECs.
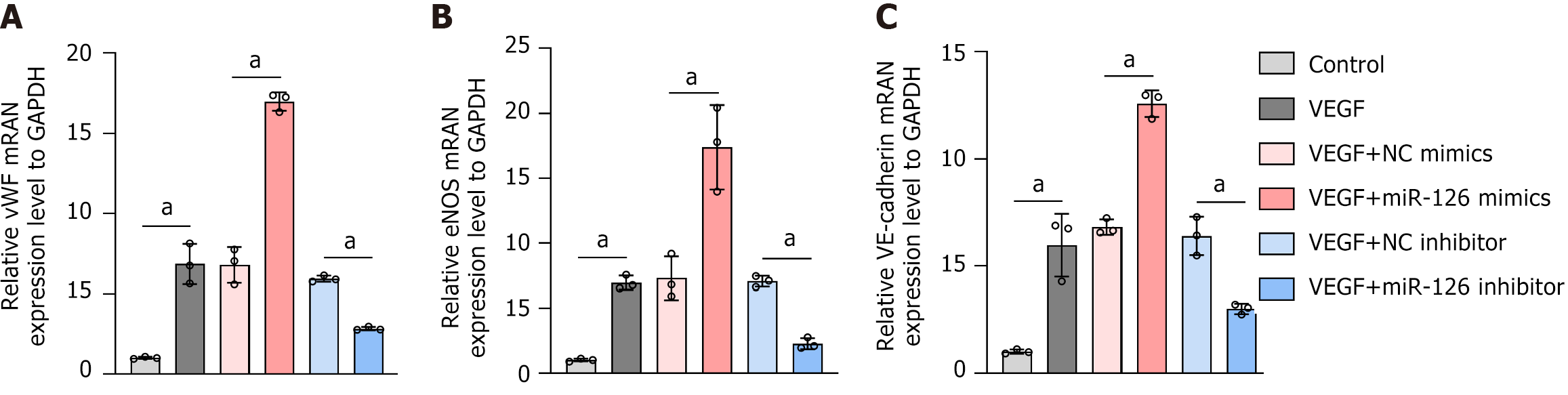
EC function is vital for maintaining homeostasis[46]. Three key molecules related to endothelial function are vWF, eNOS, and vascular endothelial-cadherin. vWF, a glycoprotein secreted by ECs, plays a core role in hemostasis and coagulation. It promotes platelet adhesion at injury sites by binding to platelet membrane glycoprotein Ib and subendothelial collagen, and also protects coagulation factor VIII from degradation. eNOS, primarily expressed in ECs, regulates vascular tone by producing nitric oxide, a potent vasodilator that relaxes vascular smooth muscle cells via multiple signaling pathways. Vascular endothelial-cadherin, a transmembrane adhesion protein specific to ECs, maintains vascular integrity by mediating cell-cell adhesion and is involved in regulating vascular permeability, angiogenesis, and remodeling, which are crucial for embryonic development, adult vascular homeostasis, and processes like inflammation and tumor angiogenesis. In our experiment, RT-qPCR showed significant increases in the mRNA expression of these genes in the miR-126 mimics group, while the miR-126 inhibitor group exhibited the opposite trend (Figure 8). This indicates that miR-126 promotes the differentiation of MSCs into functional ECs, offering new insights into its mechanism in vascular biology and underpinning its potential clinical applications.
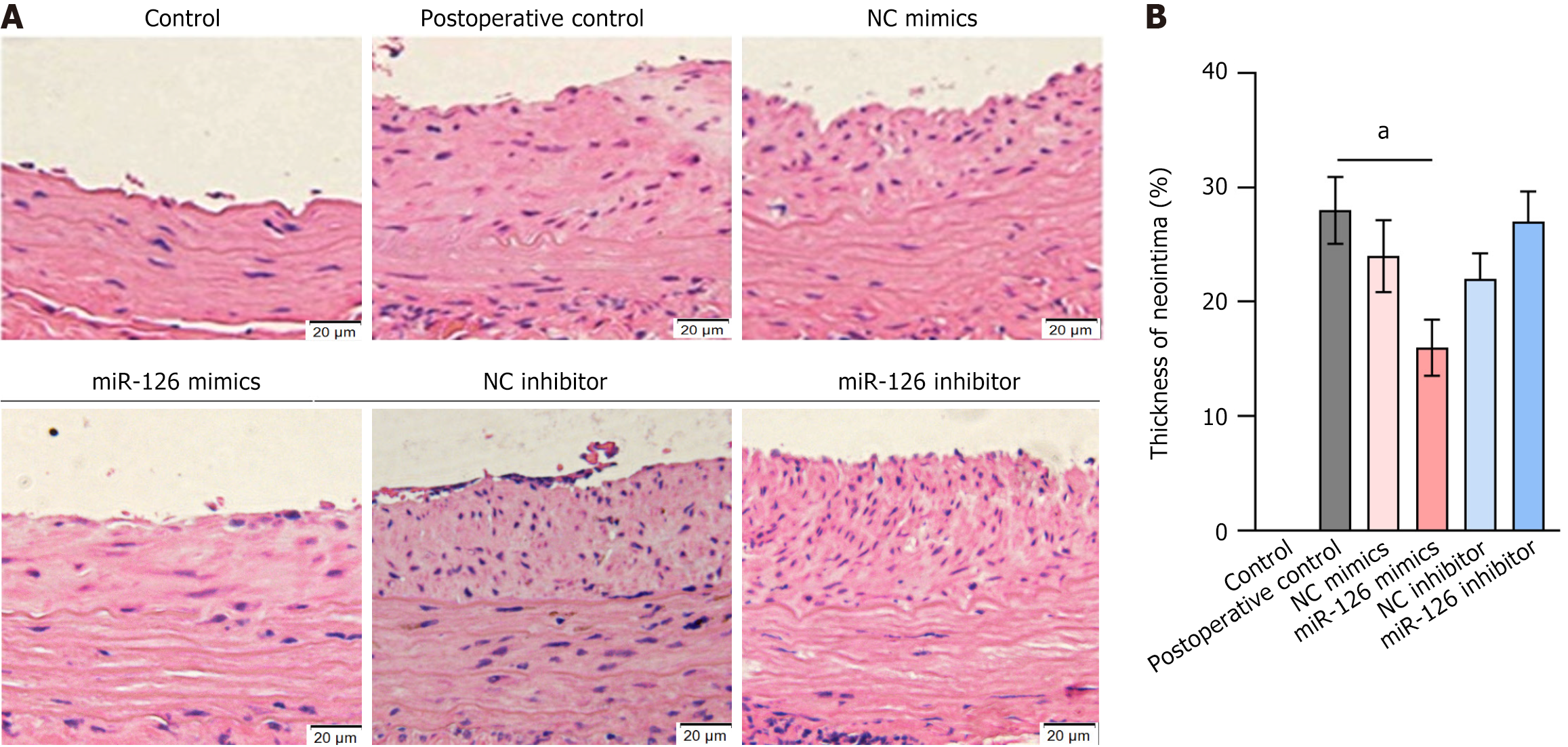
To verify the role of miR-126 in the promotion of EC growth and inhibition of endothelial proliferation, we performed a detailed study in rats. In this experiment, all rats that underwent surgery recovered successfully, and no signs of thrombosis were observed in the MSC grafts throughout the observation period. Preliminary experiments showed that MSC grafts can attenuate carotid atherosclerosis in rats by inhibiting intimal hyperplasia. To further evaluate the effect of MSC miR-126 mimics on postoperative neointimal lesion formation and luminal stenosis, we chose to infuse groups of MSCs into carotid arteries at 15-day intervals from day 28 after balloon dilatation until day 60 postoperatively, when all rats were sacrificed and carotid arteries were harvested for histopathological analysis.
Morphometric analyses showed that neointimal thickness was significantly reduced in the miR-126-modified MSC group, whereas intimal thickness was similar in the other modified groups and blank control group (Figure 9). These results suggest that miR-126-modified MSCs are effective in reducing neointimal hyperplasia, thus ameliorating carotid atherosclerosis. In addition, our study provided evidence that miR-126 regulates the vascular repair function of MSCs by activating the MAPK/ERK pathway, which in turn inhibits intimal hyperplasia and improves the effects of carotid atherosclerosis.
In conclusion, this in vivo study demonstrated that miR-126-modified MSCs not only improved the safety of cell therapy, but also significantly enhanced its therapeutic efficacy against carotid atherosclerosis, providing a solid foundation for future clinical applications. This study highlights the potential of miR-126 in the treatment of CVD and paves the way for further exploration of its potential as a therapeutic target.
Carotid atherosclerosis is a major etiological factor for stroke and poses a significant threat to human health. This condition is particularly prevalent among middle-aged and elderly populations and is characterized primarily by lipid deposition within the arterial wall - a key factor in triggering concentric neointimal formation[47]. Lipid accumulation not only provokes local inflammatory responses but also attracts macrophages and other immune cells into the arterial wall, where they transform into foam cells and further exacerbate neointimal hyperplasia. Ultimately, these pathological changes may lead to diffuse arterial stenosis or even complete occlusion. Current therapeutic strategies for carotid atherosclerosis are broadly categorized into conservative and surgical approaches. Conservative treatments, such as antiplatelet and lipid-lowering medications, can delay disease progression but fail to achieve a definitive cure. In contrast, surgical interventions - such as carotid endarterectomy and stenting - though effective, carry inherent risks. Therefore, the discovery of novel molecular targets for therapeutic intervention remains an urgent and critical challenge.
Therefore, finding new molecular targets for treatment has become an urgent problem. To find new molecular targets, researchers are exploring the following directions: (1) Inflammatory pathways: Inhibiting the nuclear factor kappa B pathway can reduce the production of inflammatory factors, thus slowing the process of atherosclerosis; targeting the Toll-like receptor 4 receptor can reduce the inflammatory response and prevent the formation of foam cells[48]; (2) Lipid metabolism: By inhibiting proprotein convertase subtilisin/kexin type 9, lowering low-density lipoprotein cholesterol levels, reducing lipid deposition in the arterial wall; e.g., ezetimibe, which can reduce intestinal absorption of cholesterol[48]; (3) Angiogenesis and endothelial proliferation: By inhibiting VEGF, angiogenesis and endothelial proliferation can be reduced; by inhibiting platelet-derived growth factor, proliferation and migration of smooth muscle cells can be reduced[49]; and (4) Gene therapy: By delivering specific siRNAs or miRNAs, the disease-causing genes can be specifically silenced to reduce the pathological process[50]. Although existing conservative and surgical treatments can manage carotid atherosclerosis to some extent, the search for new molecular targets remains key to improving therapeutic efficacy and reducing side effects.
Our study selected miR126 based on its pivotal role in regulating endothelial function and its central involvement in the pathogenesis of atherosclerosis. As an EC-specific miRNA, miR126 exerts multifaceted protective effects in maintaining vascular homeostasis: (1) It promotes EC proliferation and differentiation and enhances angiogenic capacity through activation of the VEGF/bFGF signaling axis[51]; (2) It mitigates proinflammatory cytokine secretion and reduces monocyte-endothelial adhesion (mediated by intercellular adhesion molecule 1) via inhibition of the MAPK/ERK pathway[52]; (3) It targets sphingosine-1-phosphate receptor 2 to block nuclear factor kappa B signaling, thereby synergistically suppressing inflammatory cascades such as tumor necrosis factor-alpha and interleukin (IL)-1β[53]; and (4) It modulates lipoprotein receptor-related protein 6 expression to enhance EC viability and ameliorate dysregulated cholesterol metabolism[54]. This multi-targeted regulatory capacity renders miR126 a critical node within the atherosclerosis pathological network. In addition, miR-126 is upregulated in atherosclerotic plaques to recruit immune cells and stimulate innate and adaptive immune responses[55]. MiR-126 is able to amplify immune responses by binding to the MAPK receptor, which stimulates the production of more miR-126 and further activates the MAPK/ERK pathway, creating a positive feedback loop. MiR-126 modulates the biological functions of MSCs through multiple mechanisms. MiR-126 modulates the biological functions of MSCs through multiple mechanisms, including inhibition of apoptosis, promotion of proliferation and migration, promotion of angiogenesis, modulation of inflammatory responses and promotion of differentiation. Together, these mechanisms enable miR-126 to play an important role in vascular repair and tissue regeneration in MSCs.
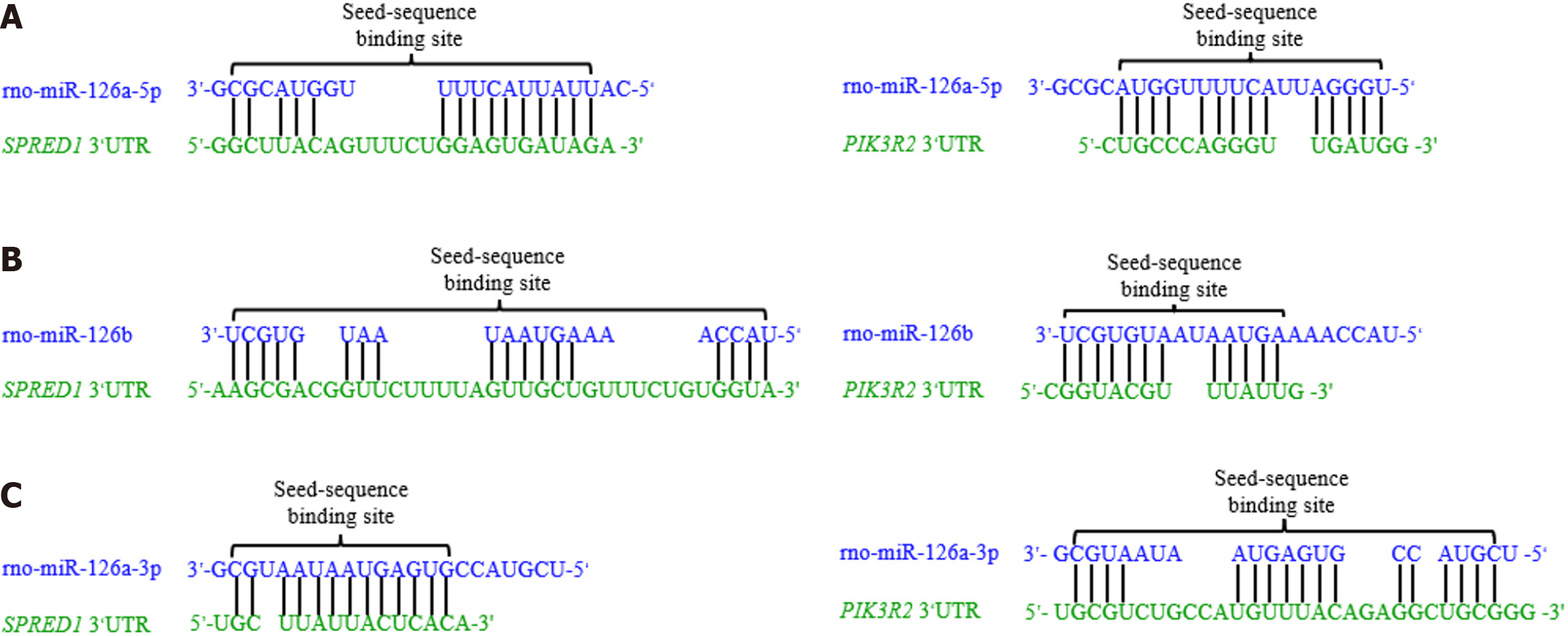
At the molecular level, the RNAhybrid tool was employed to assess the stability of miRNA-target interactions by calculating the minimum free energy (mfe)[56]. A more negative mfe value indicates a more stable binding. As shown in Figure 5: (1) The mfe between rno-miR-126a-3p and the 3’UTR of the PIK3R2 gene is -14.7 kcal/mol; (2) The mfe between rno-miR-126a-5p and the 3’UTR of the SPRED1 gene is -17.2 kcal/mol; (3) The mfe between rno-miR-126a-5p and the 3’UTR of PIK3R2 is -15.7 kcal/mol; (4) The mfe between rno-miR-126a-3p and the 3’UTR of SPRED1 is -21.9 kcal/mol; (5) The mfe between rno-miR-126b and the 3’UTR of PIK3R2 is -12.6 kcal/mol; and (6) The mfe between rno-miR-126b and the 3’UTR of SPRED1 is -16.8 kcal/mol, indicating the strongest binding stability among these interactions. The RNAhybrid analysis not only elucidated the specific complementary base-pairing patterns between these miRNAs and their target sequences but also further validated the targeting effects of rno-miR-126a-3p, rno-miR-126a-5p, and rno-miR-126b on PIK3R2 and SPRED1, and illustrated how these interactions modulate the MAPK/ERK signaling pathway.
These findings underscore the critical role of the miR-126 family in orchestrating key biological processes and elucidate the mechanism by which they execute their regulatory functions via targeted modulation of specific signaling pathways. Whether through rno-miR-126a-5p’s suppression of SPRED1 or rno-miR-126a-3p’s regulation of PIK3R2, these results highlight the central and nuanced role of miR-126 within the gene regulatory network. Collectively, this research provides a robust theoretical framework for further elucidating the roles of miRNAs under both physiological and pathological conditions and facilitates deeper exploration of their potential therapeutic applications in health and disease.
Clinical relevance studies further underscore the significance of our findings. Chen et al[57] have shown that miR-126 expression levels are significantly negatively correlated with the severity of cerebrovascular atherosclerotic lesions (r = -0.68, P < 0.01) and are markedly reduced in the plasma of patients with coronary slow flow (ΔCt values decreased 2.3-fold relative to controls). This finding suggests that miR-126 may serve as a potential biomarker for cerebrovascular atherosclerosis and coronary slow flow. Notably, gene knockout models demonstrate that miR-126 deficiency leads to disrupted vascular integrity during embryogenesis (with an increase in hemorrhage rates in zebrafish)[58] and aberrant vascular proliferation in mature vessels (with neointimal thickness in mice increasing 2.1-fold)[59], phenotypes that closely resemble the characteristics of human atherosclerosis.
Additionally, compared to other atherosclerosis-associated miRNAs, miR-126 possesses unique therapeutic advan
In this study, we first transfected rat bone marrow-derived MSCs with viral vectors carrying miR-126 to investigate its role and underlying mechanisms during MSC differentiation. Our results demonstrated that MSCs treated with miR-126 mimics exhibited enhanced proliferation, increased migration rate, and reduced apoptosis. Furthermore, during the differentiation process, the expression of endothelial markers was significantly upregulated, indicating that overexpression miR-126 effectively promote tissue remodeling and repair. Notably, the immunomodulatory capacity of MSCs plays a crucial role in cell therapy for carotid atherosclerosis, as MSCs facilitate the generation of regulatory T cells and anti-inflammatory cytokines[61]. To further validate these findings, MSCs with different treatments were infused into a rat model that underwent carotid balloon angioplasty every 15 days starting at 28 days post-surgery, and carotid tissue samples were subsequently collected for histopathological analysis at day 60. The results indicated that MSCs modified with miR-126 significantly reduced neointimal thickness, decreased macrophage infiltration, and promoted endothelial coverage. To further investigate the primary signaling pathways regulated by miR-126 in MSC differentiation, we examined its effects on the MAPK pathway. Our findings demonstrated that miR-126 promoted MSC proliferation in a dose-dependent manner, and in miR-126-modified MSCs, the phosphorylation levels of p38 and ERK1/2 were significantly reduced, whereas inhibition of miR-126 or the use of blank controls elicited the opposite effect. These observations indicate that miR-126 may modulates MSC proliferation and differentiation by regulating MAPK expression and consequently affecting the phosphorylation states of p38 and ERK1/2.
Our integrated in vitro and in vivo experiments provide complementary perspectives on the function of miR-126. In vitro, miR-126 was observed to inhibit ERK phosphorylation, whereas in vivo, it may indirectly regulate this pathway via paracrine effects on immune cells such as macrophages. This discrepancy reflects the influence of the complex in vivo microenvironment on miR-126 function, particularly with respect to its long-term effects, such as the inhibition of neointimal hyperplasia, which require interactions between cells and their surrounding milieu. In terms of target validation, our in vitro experiments using western blot and luciferase reporter assays indirectly confirmed the association between miR-126 and the MAPK/ERK signaling pathway. However, direct in vivo validation of miR-126 target genes remains necessary, potentially through the application of single-cell sequencing or tissue-specific knockout techniques.
In an innovative advance, our study provides novel insights into the treatment of coronary atherosclerosis by elucidating the role of miR126 in modulating the MAPK/ERK signaling pathway. Specifically, we delineate the mechanisms by which miR-126 regulates the proliferation, migration, apoptosis, and vascular repair functions of bone marrow derived MSCs. Our findings unequivocally demonstrate that miR-126 is critical for promoting MSC differentiation into ECs, and that its inhibition of the MAPK/ERK pathway effectively suppresses inflammatory responses and neointimal hyperplasia. This research not only deepens our understanding of miR-126’s therapeutic potential in atherosclerosis but also paves new avenues for personalized medicine, highlighting its substantial promise as a targeted treatment modality. Furthermore, by employing a rat carotid balloon angioplasty model in conjunction with MSC transplantation, our study further validates the efficacy of miR-126 in enhancing vascular health.
In summary, from molecular mechanisms to phenotypic outcomes, our study demonstrates that miR-126 directly influences MSC proliferation, differentiation, and secretory function in vitro via the MAPK/ERK signaling pathway, which translates into therapeutic effects, such as the inhibition of atherosclerosis, in vivo. These findings not only provide a mechanistic foundation for understanding the role of miR-126 but also validate its clinical potential. Both in vitro and in vivo studies consistently confirm that miR-126 exerts its effects primarily through the MAPK/ERK pathway, with increased secretion of VEGF and bFGF representing a common mechanism across models.
Although our study has explored the role of miR126 in the MAPK/ERK pathway from multiple perspectives (cell proliferation, apoptosis, and differentiation) several aspects require further refinement. First, our evaluation of carotid intimal injury relied solely on morphological assessments; future investigations should integrate advanced molecular biological and imaging techniques to enable more precise quantification of injury severity. Second, the absence of specific pathway inhibitors (e.g., U0126 for MAPK/ERK) and targeted knockdown experiments for key signaling components represents a limitation in establishing effect specificity and causality. Notably, the lack of gene silencing approaches - such as siRNA or CRISPRCas9 - to validate the function of critical molecules (e.g., ERK1/2) is a significant shortcoming. This omission may hinder the attribution of the observed effects solely to the target pathway, particularly in the context of redundant or compensatory mechanisms. Additionally, given the potential functional overlap among MAPK family members, relying solely on overexpression or inhibitor studies may be insufficient to elucidate the complexity of these regulatory networks in their entirety. Hence, correlation does not necessarily equate to causation, and the changes observed with miR126 overexpression cannot completely rule out the indirect effects of other miRNAs or paracrine factors.
Moreover, in in vivo models, the homing and differentiation of MSCs are influenced by microenvironmental factors (such as gradients of inflammatory cytokines), necessitating more refined methods - such as cell-specific knockout models - to probe these interactions in depth. Furthermore, we have yet to definitively identify the direct target genes of miR126; future studies will focus on elucidating its precise targets and mechanisms of action. Finally, although a reduction in neointimal thickness is an encouraging indicator, it does not directly equate to the inhibition of atherosclerosis. Our study did not provide data on atherosclerotic plaque area nor did it offer an exhaustive analysis of inflammatory markers (such as IL-1 and IL-6). To comprehensively understand the impact of miR126 on atherosclerosis, future work will include: (1) Detailed histological and imaging-based quantification of atherosclerotic plaque area to more accurately evaluate the therapeutic effect of miR126; (2) An expanded panel for inflammatory cytokine detection - including but not limited to IL-1, IL-6, and tumor necrosis factor-alpha - to investigate the modulatory effects of miR126 on inflammatory responses; and (3) Long-term experimental observations to assess the durability of miR126’s therapeutic efficacy and its clinical potential.
The present study demonstrated that the MAPK/ERK signaling pathway contributes to the regulation of MSC proliferation and endothelial differentiation. MiR-126-modified MSCs have better proliferation and endothelial differentiation abilities, which is beneficial for their application in the treatment of carotid atherosclerosis. MiR-126 regulates the vascular repair function of MSCs by activating the MAPK/ERK signaling pathway, which has a positive effect on carotid atherosclerosis.
The authors would like to sincerely acknowledge all the technical assistance of the Department of Vascular Surgery, Hangzhou First People’s Hospital, Zhejiang Province, China.
| 1. | Saba L, Cau R, Vergallo R, Kooi ME, Staub D, Faa G, Congiu T, Ntaios G, Wasserman BA, Benson J, Nardi V, Kawakami R, Lanzino G, Virmani R, Libby P. Carotid artery atherosclerosis: mechanisms of instability and clinical implications. Eur Heart J. 2025;46:904-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Yu P, Venkat P, Chopp M, Zacharek A, Shen Y, Ning R, Liang L, Li W, Zhang L, Landschoot-Ward J, Jiang R, Chen J. Role of microRNA-126 in vascular cognitive impairment in mice. J Cereb Blood Flow Metab. 2019;39:2497-2511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 3. | Gummesson A, Lundmark P, Chen QS, Björnson E, Dekkers KF, Hammar U, Adiels M, Wang Y, Andersson T, Bergström G, Carlhäll CJ, Erlinge D, Jernberg T, Landfors F, Lind L, Mannila M, Melander O, Pirazzi C, Sundström J, Östgren CJ, Gunnarsson C, Orho-Melander M, Söderberg S, Fall T, Gigante B. A genome-wide association study of imaging-defined atherosclerosis. Nat Commun. 2025;16:2266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Mehta A, Rigdon J, Tattersall MC, German CA, Barringer TA 3rd, Joshi PH, Sperling LS, Budoff MJ, Bertoni A, Michos ED, Blaha MJ, Stein JH, Shapiro MD. Association of Carotid Artery Plaque With Cardiovascular Events and Incident Coronary Artery Calcium in Individuals With Absent Coronary Calcification: The MESA. Circ Cardiovasc Imaging. 2021;14:e011701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | US Preventive Services Task Force, Davidson KW, Barry MJ, Mangione CM, Cabana M, Chelmow D, Coker TR, Davis EM, Donahue KE, Jaén CR, Krist AH, Kubik M, Li L, Ogedegbe G, Pbert L, Ruiz JM, Stevermer J, Tseng CW, Wong JB. Aspirin Use to Prevent Cardiovascular Disease: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327:1577-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 205] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 6. | Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234:5451-5465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1431] [Cited by in RCA: 1322] [Article Influence: 220.3] [Reference Citation Analysis (0)] |
| 7. | Brillante S, Volpe M, Indrieri A. Advances in MicroRNA Therapeutics: From Preclinical to Clinical Studies. Hum Gene Ther. 2024;35:628-648. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Tanashyan MM, Shabalina AA, Annushkin VA, Mazur AS, Kuznetsova PI, Raskurazhev AA. Circulating microRNAs in Carotid Atherosclerosis: Complex Interplay and Possible Associations with Atherothrombotic Stroke. Int J Mol Sci. 2024;25:10026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141:1202-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 1808] [Article Influence: 226.0] [Reference Citation Analysis (0)] |
| 10. | Achkar NP, Cambiagno DA, Manavella PA. miRNA Biogenesis: A Dynamic Pathway. Trends Plant Sci. 2016;21:1034-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 199] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 11. | Lee YY, Lee H, Kim H, Kim VN, Roh SH. Structure of the human DICER-pre-miRNA complex in a dicing state. Nature. 2023;615:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 58] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 12. | Kapplingattu SV, Bhattacharya S, Adlakha YK. MiRNAs as major players in brain health and disease: current knowledge and future perspectives. Cell Death Discov. 2025;11:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Iwakawa HO, Tomari Y. Life of RISC: Formation, action, and degradation of RNA-induced silencing complex. Mol Cell. 2022;82:30-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 231] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 14. | Wang W, Hao X, Lv X, Li Y, Xing W, Chen T, Si X, Shi J, Zhou Y. Overexpression of miR-99a promoted expansion and suppressed differentiation of hematopoietic stem/progenitor cells. Sci Rep. 2025;15:8890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Sadakierska-Chudy A. MicroRNAs: Diverse Mechanisms of Action and Their Potential Applications as Cancer Epi-Therapeutics. Biomolecules. 2020;10:1285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Todorov H, Weißbach S, Schlichtholz L, Mueller H, Hartwich D, Gerber S, Winter J. Stage-specific expression patterns and co-targeting relationships among miRNAs in the developing mouse cerebral cortex. Commun Biol. 2024;7:1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Ivey KN, Muth A, Arnold J, King FW, Yeh RF, Fish JE, Hsiao EC, Schwartz RJ, Conklin BR, Bernstein HS, Srivastava D. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell. 2008;2:219-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 495] [Cited by in RCA: 460] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 18. | Izarra A, Moscoso I, Cañón S, Carreiro C, Fondevila D, Martín-Caballero J, Blanca V, Valiente I, Díez-Juan A, Bernad A. miRNA-1 and miRNA-133a are involved in early commitment of pluripotent stem cells and demonstrate antagonistic roles in the regulation of cardiac differentiation. J Tissue Eng Regen Med. 2017;11:787-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Fu X, He Y, Wang X, Peng D, Chen X, Li X, Wang Q. Overexpression of miR-21 in stem cells improves ovarian structure and function in rats with chemotherapy-induced ovarian damage by targeting PDCD4 and PTEN to inhibit granulosa cell apoptosis. Stem Cell Res Ther. 2017;8:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 20. | Wu J, Yu S. [Role of microRNA-34 cluster and its therapeutic potential: a review of the literature]. Zhongguo Linchuang Yixue. 2022;29:875-884. [DOI] [Full Text] |
| 21. | Du Y, Xu XX, Yu SX, Wang YR, Liu Y, Liu F, Liu W, Li XL, Luo H, Jing G, Liu YJ. Dynamics of endothelial cells migration in nature-mimicking blood vessels. Talanta. 2024;277:126415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Xu Q, Hou W, Zhao B, Fan P, Wang S, Wang L, Gao J. Mesenchymal stem cells lineage and their role in disease development. Mol Med. 2024;30:207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 23. | Sun LL, Li WD, Lei FR, Li XQ. The regulatory role of microRNAs in angiogenesis-related diseases. J Cell Mol Med. 2018;22:4568-4587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 24. | Wang Y, Wang L, Chen C, Chu X. New insights into the regulatory role of microRNA in tumor angiogenesis and clinical implications. Mol Cancer. 2018;17:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 25. | Liao L, Tang Y, Zhou Y, Meng X, Li B, Zhang X. MicroRNA-126 (MiR-126): key roles in related diseases. J Physiol Biochem. 2024;80:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 26. | Zou J, Li WQ, Li Q, Li XQ, Zhang JT, Liu GQ, Chen J, Qiu XX, Tian FJ, Wang ZZ, Zhu N, Qin YW, Shen B, Liu TX, Jing Q. Two functional microRNA-126s repress a novel target gene p21-activated kinase 1 to regulate vascular integrity in zebrafish. Circ Res. 2011;108:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Zhang Y, Xu Y, Zhou K, Kao G, Xiao J. MicroRNA-126 and VEGF enhance the function of endothelial progenitor cells in acute myocardial infarction. Exp Ther Med. 2022;23:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Huang F, Zhu X, Hu XQ, Fang ZF, Tang L, Lu XL, Zhou SH. Mesenchymal stem cells modified with miR-126 release angiogenic factors and activate Notch ligand Delta-like-4, enhancing ischemic angiogenesis and cell survival. Int J Mol Med. 2013;31:484-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 29. | Fonseca LN, Bolívar-Moná S, Agudelo T, Beltrán LD, Camargo D, Correa N, Del Castillo MA, Fernández de Castro S, Fula V, García G, Guarnizo N, Lugo V, Martínez LM, Melgar V, Peña MC, Pérez WA, Rodríguez N, Pinzón A, Albarracín SL, Olaya M, Gutiérrez-Gómez ML. Cell surface markers for mesenchymal stem cells related to the skeletal system: A scoping review. Heliyon. 2023;9:e13464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 30. | Chen S, Liang B, Xu J. Unveiling heterogeneity in MSCs: exploring marker-based strategies for defining MSC subpopulations. J Transl Med. 2024;22:459. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Chu H, Zhang S, Zhang Z, Yue H, Liu H, Li B, Yin F. Comparison studies identify mesenchymal stromal cells with potent regenerative activity in osteoarthritis treatment. NPJ Regen Med. 2024;9:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 32. | Petrenko Y, Vackova I, Kekulova K, Chudickova M, Koci Z, Turnovcova K, Kupcova Skalnikova H, Vodicka P, Kubinova S. A Comparative Analysis of Multipotent Mesenchymal Stromal Cells derived from Different Sources, with a Focus on Neuroregenerative Potential. Sci Rep. 2020;10:4290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 33. | Qu Q, Wang L, Bing W, Bi Y, Zhang C, Jing X, Liu L. miRNA-126-3p carried by human umbilical cord mesenchymal stem cell enhances endothelial function through exosome-mediated mechanisms in vitro and attenuates vein graft neointimal formation in vivo. Stem Cell Res Ther. 2020;11:464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Peck SH, Bendigo JR, Tobias JW, Dodge GR, Malhotra NR, Mauck RL, Smith LJ. Hypoxic Preconditioning Enhances Bone Marrow-Derived Mesenchymal Stem Cell Survival in a Low Oxygen and Nutrient-Limited 3D Microenvironment. Cartilage. 2021;12:512-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 35. | Huang B, Wu G, Peng C, Peng X, Huang M, Ding J, Zhang H, Wu X. miR-126 regulates the proliferation, migration, invasion, and apoptosis of non-small lung cancer cells via AKT2/HK2 axis. IUBMB Life. 2023;75:186-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Sun Z, Liu F, Cai X, Yu W, Xu L, Yang B. MiR-126 affects femoral fracture healing in rats through PI3K/AKT signaling pathway. Panminerva Med. 2021;63:89-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Yuan W, Shi Y, Dai S, Deng M, Zhu K, Xu Y, Chen Z, Xu Z, Zhang T, Liang S. The role of MAPK pathway in gastric cancer: unveiling molecular crosstalk and therapeutic prospects. J Transl Med. 2024;22:1142. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19:1997-2007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 759] [Article Influence: 151.8] [Reference Citation Analysis (0)] |
| 39. | García-Hernández L, García-Ortega MB, Ruiz-Alcalá G, Carrillo E, Marchal JA, García MÁ. The p38 MAPK Components and Modulators as Biomarkers and Molecular Targets in Cancer. Int J Mol Sci. 2021;23:370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 40. | Han Y, Yang J, Fang J, Zhou Y, Candi E, Wang J, Hua D, Shao C, Shi Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct Target Ther. 2022;7:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 339] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 41. | Kang Y, Li H, Liu Y, Li Z. Regulation of VEGF-A expression and VEGF-A-targeted therapy in malignant tumors. J Cancer Res Clin Oncol. 2024;150:221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 42. | Zheng M, Song W, Huang P, Huang Y, Lin H, Zhang M, He H, Wu J. Drug conjugates crosslinked bioresponsive hydrogel for combination therapy of diabetic wound. J Control Release. 2024;376:701-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 43. | Huang JH, Xu Y, Yin XM, Lin FY. Exosomes Derived from miR-126-modified MSCs Promote Angiogenesis and Neurogenesis and Attenuate Apoptosis after Spinal Cord Injury in Rats. Neuroscience. 2020;424:133-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 44. | Khaki M, Salmanian AH, Abtahi H, Ganji A, Mosayebi G. Mesenchymal Stem Cells Differentiate to Endothelial Cells Using Recombinant Vascular Endothelial Growth Factor -A. Rep Biochem Mol Biol. 2018;6:144-150. [PubMed] |
| 45. | Dhumale P, Nielsen JV, Hansen ACS, Burton M, Beck HC, Jørgensen MG, Toyserkani NM, Haahr MK, Hansen ST, Lund L, Thomassen M, Sørensen JA, Andersen DC, Jensen CH, Sheikh SP. CD31 defines a subpopulation of human adipose-derived regenerative cells with potent angiogenic effects. Sci Rep. 2023;13:14401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 46. | Augustin HG, Koh GY. A systems view of the vascular endothelium in health and disease. Cell. 2024;187:4833-4858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 47. | Yuan F, Wang D, Xu K, Wang J, Zhang Z, Yang L, Yang GY, Li S. Contribution of Vascular Cells to Neointimal Formation. PLoS One. 2017;12:e0168914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 48. | Park C, Cha HJ, Lee H, Kim GY, Choi YH. The regulation of the TLR4/NF-κB and Nrf2/HO-1 signaling pathways is involved in the inhibition of lipopolysaccharide-induced inflammation and oxidative reactions by morroniside in RAW 264.7 macrophages. Arch Biochem Biophys. 2021;706:108926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 49. | Xu B, Iida Y, Glover KJ, Ge Y, Wang Y, Xuan H, Hu X, Tanaka H, Wang W, Fujimura N, Miyata M, Shoji T, Guo J, Zheng X, Gerritsen M, Kuo C, Michie SA, Dalman RL. Inhibition of VEGF (Vascular Endothelial Growth Factor)-A or its Receptor Activity Suppresses Experimental Aneurysm Progression in the Aortic Elastase Infusion Model. Arterioscler Thromb Vasc Biol. 2019;39:1652-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 50. | Alshaer W, Zureigat H, Al Karaki A, Al-Kadash A, Gharaibeh L, Hatmal MM, Aljabali AAA, Awidi A. siRNA: Mechanism of action, challenges, and therapeutic approaches. Eur J Pharmacol. 2021;905:174178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 237] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 51. | Shaw P, Dwivedi SKD, Bhattacharya R, Mukherjee P, Rao G. VEGF signaling: Role in angiogenesis and beyond. Biochim Biophys Acta Rev Cancer. 2024;1879:189079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 57] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 52. | Bahar ME, Kim HJ, Kim DR. Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies. Signal Transduct Target Ther. 2023;8:455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 270] [Article Influence: 135.0] [Reference Citation Analysis (0)] |
| 53. | Kitsou K, Kokkotis G, Rivera-Nieves J, Bamias G. Targeting the Sphingosine-1-Phosphate Pathway: New Opportunities in Inflammatory Bowel Disease Management. Drugs. 2024;84:1179-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 54. | Shay TF, Jang S, Brittain TJ, Chen X, Walker B, Tebbutt C, Fan Y, Wolfe DA, Arokiaraj CM, Sullivan EE, Ding X, Wang TY, Lei Y, Chuapoco MR, Chou TF, Gradinaru V. Human cell surface-AAV interactomes identify LRP6 as blood-brain barrier transcytosis receptor and immune cytokine IL3 as AAV9 binder. Nat Commun. 2024;15:7853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 55. | Hao XZ, Fan HM. Identification of miRNAs as atherosclerosis biomarkers and functional role of miR-126 in atherosclerosis progression through MAPK signalling pathway. Eur Rev Med Pharmacol Sci. 2017;21:2725-2733. [PubMed] |
| 56. | Krüger J, Rehmsmeier M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res. 2006;34:W451-W454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1133] [Cited by in RCA: 1429] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 57. | Chen J, Cui C, Yang X, Xu J, Venkat P, Zacharek A, Yu P, Chopp M. MiR-126 Affects Brain-Heart Interaction after Cerebral Ischemic Stroke. Transl Stroke Res. 2017;8:374-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 58. | Wu F, Yang Z, Li G. Role of specific microRNAs for endothelial function and angiogenesis. Biochem Biophys Res Commun. 2009;386:549-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 59. | Hua JY, He YZ, Xu Y, Jiang XH, Ye W, Pan ZM. Emodin prevents intima thickness via Wnt4/Dvl-1/β-catenin signaling pathway mediated by miR-126 in balloon-injured carotid artery rats. Exp Mol Med. 2015;47:e170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Lee CY, Khan G, Hyun DY, Kim SH, Park ES. Effect of umbilical cord mesenchymal stem cell-derived mitochondrial transplantation on ischemia-reperfusion injury in a rat model. Skin Res Technol. 2024;30:e70022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 61. | Mahdavi Gorabi A, Banach M, Reiner Ž, Pirro M, Hajighasemi S, Johnston TP, Sahebkar A. The Role of Mesenchymal Stem Cells in Atherosclerosis: Prospects for Therapy via the Modulation of Inflammatory Milieu. J Clin Med. 2019;8:1413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |