Published online May 26, 2025. doi: 10.4252/wjsc.v17.i5.105394
Revised: February 27, 2025
Accepted: April 15, 2025
Published online: May 26, 2025
Processing time: 125 Days and 22.6 Hours
Ischemic heart disease ranks among the foremost contributors to mortality worldwide. Myocardial infarction injury poses a prevalent challenge in current therapies. Studies have shown that mesenchymal stem cell transplantation increases cytokine release, reduces myocardial cell necrosis, and improves left ventricular function; thus, it can be used to understand protective mechanisms. Fat extract (FE) derived from mesenchymal stem cell therapy contains high levels of paracrine factors.
To study the effects of FE on myocardial injury and its mechanism of action.
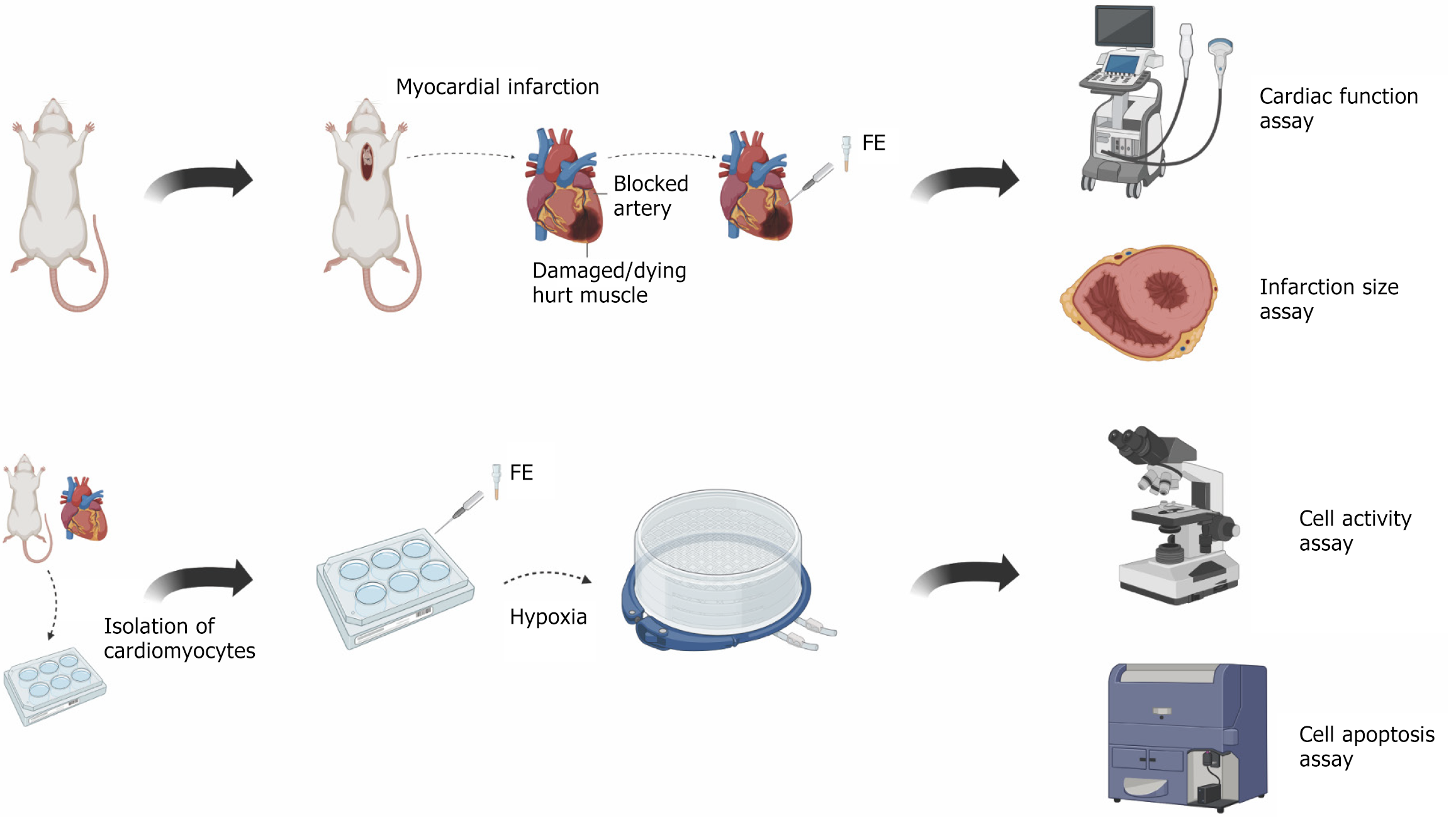
A mouse model of myocardial infarction and a hypoxic model of neonatal rat cardiomyocytes (CMs) were established to evaluate the effects of FE.
FE exhibited an inhibitory effect on CM apoptosis and improved left ventricular function. This protective effect of FE on CMs was mediated, in part, by the activation of the phosphatidylinositol 3-kinase/protein kinase B/mechanistic target of rapamycin signaling pathway.
Our findings showed that FE could be a new treatment to protect CMs in ischemic heart disease.
Core Tip: Fat extract derived from mesenchymal stem cell therapy demonstrates protective effects on cardiomyocytes under ischemic conditions by inhibiting cardiomyocyte apoptosis and improving left ventricular function. This protection is mediated through the activation of the phosphatidylinositol 3-kinase/protein kinase B/mechanistic target of rapamycin signaling pathway, suggesting fat extract as a potential new treatment for ischemic heart disease.
- Citation: Yang TY, Sun Y, Zhang WJ, Wang CQ, Zhou J. Cell-free extracts from human fat tissue attenuate ischemic injury in cardiomyocytes in a murine model. World J Stem Cells 2025; 17(5): 105394
- URL: https://www.wjgnet.com/1948-0210/full/v17/i5/105394.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i5.105394
Cardiovascular diseases represent a predominant cause of mortality worldwide, accounting for approximately 17.5 million fatalities each year[1]. The most common type of cardiovascular disease is ischemic heart disease (IHD), which is responsible for nearly 10 million fatalities each year; its prevalence is expected to rise by 46% by 2030[2]. During acute myocardial infarction (MI), necrosis occurs in myocardial cells due to ischemia, leading to scar tissue formation, progressive ventricular remodeling, and heart failure (HF). Present clinical therapeutic approaches, including percutaneous coronary intervention and coronary artery bypass grafting, have the potential to enhance coronary perfusion. The combination of β-blockers, renin-angiotensin-aldosterone system inhibitors, sodium-glucose cotransporter 2 inhibitors, along with sacubitril/valsartan can slow the progression of chronic HF after myocardial injury. However, many patients still die of HF because current treatments cannot reverse left ventricular (LV) remodeling resulting from massive myocardial cell necrosis. Since current treatments only involve improving clinical symptoms and delaying the natural progression of the disease, there is a need to explore treatment strategies to repair the damaged myocardial tissue associated with IHD.
Regenerative medicine may be vital for the effective treatment of HF through stem cell therapy to improve myocardial contractile function[3]. Of all stem cell types, mesenchymal stem cells (MSCs) have garnered significant attention in the field of IHD research due to their accessibility for isolation from various sources, including bone marrow, adipose tissue, synovial membrane, periosteum, teeth, and placenta[4]. Research indicates that MSCs primarily exert their influence via paracrine pathways[5], and cytokine delivery provides an effective alternative to traditional MSC culture because it circumvents conventional restrictions and safety complications[6].
Adipose tissue-derived stromal/stem cells (ADSCs) are garnering heightened interest as potential therapeutic agents for a variety of diseases. This growing attention can be attributed to several key factors, including the simplicity of their isolation, their relatively abundant availability, low immunogenic response, and their capacity for multipotent differentiation[6,7]. ADSCs secrete a variety of growth factors, including hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), insulin-like growth factor 1, and basic fibroblast growth factor (bFGF). These factors have the potential to regulate inflammatory and immune responses, thereby providing protection for cells[7]. Tonnard et al[8] were the first to introduce the idea of nanofat, a fluid mixture obtained from adipose tissue through mechanical emulsification and filtration, using ADSCs as a starting point[6]. After the process of centrifugation, nanofat consists of three primary components: An oil layer, a fraction that includes both cellular elements and extracellular matrix, and a liquid component[6,9]. Furthermore, the liquid component derived from nanofat is rich in various factors that closely resemble those released by ADSCs. These include brain-derived neurotrophic factor, glial cell-derived neurotrophic factor (GDNF), transforming growth factor-beta (TGF-β), HGF, bFGF, and VEGF[6]. The liquid component was designated as the cell-free fat extract (FE), which is significant in improving limb ischemia in a mouse model[6]. Compared with conventional ADSCs, FE, owing to its cell-free nature, can circumvent the instability associated with living cells, thereby achieving superior quality control. It remains unclear to researchers whether FE exerts positive effects on cardiomyocytes (CMs) under ischemic conditions.
In this study, we determined if FE administration to a mouse model of MI and a cellular model of hypoxia could protect CMs under ischemic conditions. The experiment process is shown in Figure 1. We selected FE over other stem cell derivatives, such as exosomes, because it is enriched with growth factors and exhibits unique therapeutic potential. Proteomic analysis of FE revealed that among the 1767 detected proteins, several were involved in inhibiting inflammation, resisting apoptosis, and promoting angiogenesis, suggesting that FE may exhibit multiple potential therapeutic effects for ischemic diseases.
The human liposuction aspirates were obtained from a cohort of six healthy female participants who underwent the procedure at Shanghai Ninth People’s Hospital, located in Shanghai, China, during the period spanning from October 2017 to April 2018. The participants provided written consent and had an average age of 31 years, with a range of 24 to 36 years. The Ethics Committee of Shanghai Jiao Tong University School of Medicine in Shanghai, China granted approval for the study.
The comprehensive methods employed for the isolation of FE have been elaborated in our earlier research[6]. Initially, the lipoaspirate underwent a saline wash to remove red blood cells, followed by centrifugation at 1200 × g for a duration of 3 minutes. After this initial centrifugation, the supernatant containing the oily layer and the lower fluid layer were discarded, while the central fat layer was preserved and subjected to mechanical emulsification. This emulsification process involved transferring the fat between two 10 cm3 syringes linked by a female-to-female Luer-Lok connector for 30 cycles. Subsequently, the emulsified fat was frozen at -80 °C and subsequently thawed at 37 °C to further disrupt the structural integrity of the fat tissue. Following one freeze-thaw cycle, the sample was centrifuged once more at 1200 × g for 5 minutes. This second centrifugation led to the formation of four distinct layers of fat. The uppermost oil layer, the second layer of intact fat, and the bottom debris layer were all discarded, retaining only the third aqueous layer, designated as FE. This layer was meticulously aspirated to prevent contamination from the pellet situated at the bottom. The final extract was sterilized and cleared of cellular debris by filtration through a 0.22 μm filter. The processed extract was then stored at -20 °C for future applications[6].
The research was performed in alignment with the guidelines set forth in the “Guide for the Care and Use of Laboratory Animals” as published by the United States National Institutes of Health (NIH publication No. 85-23, revised 2011) and received approval from the Animal Experiment Ethics Committee at Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine. Male C57BL/6J mice, aged six weeks, were obtained from the Shanghai Slac Experimental Animal Center located in Shanghai, China. The experimental subjects were housed in sterilized filter-top cages, maintained under controlled humidity conditions, with a 12-hour light/dark cycle at a constant temperature of 22 °C. The experimental animals were humanely euthanized by cervical dislocation one month following the surgical procedure.
Following a two-week acclimatization phase, the subjects were randomly assigned into four distinct groups: Negative control group (pre-MI), sham group (sham), control group [MI + phosphate-buffered saline (PBS)], and therapy group (MI + FE). The mice in the negative control group were healthy and underwent no surgery. The experiment unit was a single animal. Randomization was used to allocate experimental units to each group before operation. There were 6 units in each experiment group.
The animals were sedated using isoflurane (1.5%-2%). The mouse chest was incised along the midline, and the subcutaneous fascia, pectoralis major, and pectoralis minor muscles were bluntly separated using tissue forceps. Next, a tissue forceps was inserted at the fourth intercostal space on the left side, and a small opening was made to extrude and expose the heart rapidly. Ischemia was brought about by occluding the left anterior descending artery of the coronary circulation using a 5-0 suture. Following ligation, three injections of PBS (each 30 μL) were prepared and injected around the infarcted area in the control group, while diluted FE (1:1 configuration of FE with PBS) was administered in the therapy group. The mice that underwent sham operation experienced an identical procedure, with the exception of the ligation of the artery. We excluded the mouse that died during the operation.
Transthoracic echocardiography was carried out utilizing a UBM system (Vevo 2100, VisualSonics, Canada). During the examination, the mice were placed under anesthesia with 1%-3% isoflurane. The echocardiographic assessments were performed at the midpapillary muscle level, facilitated by two-dimensional long-axis imaging. Fractional shortening and ejection fractions were evaluated employing Vevo Analysis software. There were 5 units in each experiment group.
First, the heart was quickly removed and frozen with liquid nitrogen. Next, the heart was cut into five equal pieces and immersed in triphenyltetrazolium chloride (TTC) phosphate buffer solution for a duration of 30 minutes in a dark environment. The tissue was fixed in 4% paraformaldehyde. Surviving myocardium was stained red with TTC, and microscopic images of the stained cardiac tissue were captured for analysis. The measurement of infarct size was conducted utilizing ImageJ software. There were 3 units in each experiment group.
Newborn Sprague-Dawley rats, aged less than 24 hours, were euthanized via decapitation, and their hearts were promptly harvested and rinsed with pre-chilled PBS. The cardiac tissues were meticulously sectioned into 1-3 mm pieces and subjected to enzymatic digestion utilizing a solution comprising 0.25% pancreatic enzymes (Gibco, NY, United States) and 1% penicillin-streptomycin solution (Hyclone, UT, United States), followed by a 5-minute incubation at 37 °C. The resultant supernatant was gathered and transferred to Dulbecco’s Modified Eagle’s Medium (DMEM) (Hyclone, UT, United States) enriched with 10% fetal bovine serum (Gibco, NY, United States) to halt the digestion process. This enzymatic digestion procedure was performed iteratively until complete tissue digestion was achieved. Subsequent to digestion, all solutions underwent centrifugation at 1000 rpm for 5 minutes, after which the pellet was resuspended in DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin solution. Following filtration through a 100 μm mesh filter, the resulting cell suspension was inoculated into culture dishes and incubated at 37 °C in a humidified environment containing 95% O2 and 5% CO2 for 1 hour. The supernatant containing the upper cell suspension (CMs) was collected and subsequently transferred to new culture dishes, with the culture medium being refreshed every two days.
Hypoxia procedure: The growth medium was substituted with 1 mL of DMEM that lacked glucose, and subsequently incubated in an anaerobic chamber (Billups-Rothenbergh, CA, United States) for a duration of 12 hours. The hypoxia procedure was developed by referencing previous studies and subsequently refined through preliminary experiments. Air in the oxygen-free box was replaced with a gas mixture comprising 95% O2 and 5% CO2.
For the experimental groups, the following components were added to each culture well before performing the hypoxic procedure: (1) Hypoxic group, 100 μL of PBS; (2) Low-concentration treatment group, 50 μL of PBS and 50 μL of FE; (3) High-concentration treatment group, 100 μL of FE; and (4) Inhibitor group: 100 μL of FE and 0.001 μmol infigratinib. Along with this, we included a negative control group of CMs cultured at a temperature of 37 °C within an incubator that maintains an atmosphere of 95% O2 and 5% CO2.
The spontaneous beating of CMs was analyzed based on microscopic imaging. Multi-well plates containing the cultured CMs were stored at 37 °C, and videos of cell beating were captured at 60 fps using a microscope (OLYMPUS IX83, Japan). The systolic velocity and beating frequency were evaluated using the CONKLIN algorithm and quantified using the corresponding program[10].
CM apoptosis was evaluated utilizing the FITC-Annexin V Apoptosis Detection Kit (BD Biosciences, CA, United States), in accordance with the guidelines provided by the manufacturer. The stained cells were assayed using CytoFLEX S (Beckman, CA, United States) and analyzed using FlowJo10.
The CMs were utilized for the extraction of total RNA employing TRIzol Reagent (Invitrogen, CA, United States). Subsequently, reverse transcription was performed to synthesize cDNA utilizing the PrimeScript™ RT Reagent Kit (Takara, Japan). The cDNA obtained was then quantitatively amplified using TB Green Premix Ex Taq II (Takara, Japan) and processed through real-time polymerase chain reaction in triplicate on the Biosystems 6Flex platform. The gene expression levels of fibroblast growth factor receptor 1 (FGFR1), MET, tropomyosin receptor kinase B (TRKB), GFR1, VEGF receptor 2 (VEGFR2), and TGF-beta receptor (TGFBR) are presented relative to that of GAPDH. The sequences of the primers utilized for amplification, both forward and reverse, were as detailed below: VEGFR2, forward, 5’-TTCACAGTCGGGTTACAGGC-3’; reverse, 5’-CTGCCGACGTTCCTCTCTTT-3’; GAPDH, forward, 5’-AGTTCAACGGCACAGTCAAG-3’; reverse, 5’-TACTCAGCACCAGCATCACC-3’; TGFBR, forward, 5’-TAGGAGCCCCCATTTGGTTC-3’, reverse, 5’-CCAGCACTCGGTCAAAGTCT-3’; GFR-α1, forward, 5’- TGTCTTTCTGATAATGATTACGGA-3’; reverse, 5’-CTACGATGTTTCTGCCAATGATA-3’; TRKB, forward, 5’-AGCAATCGGGAGCATCTCT-3’; reverse, 5’-TACCCAT
Proteins were extracted from the conditioned media (CMs) and myocardial tissues, subsequently subjected to sodium-dodecyl sulfate gel electrophoresis for separation and then transferred onto a polyvinylidene fluoride membrane. The membranes underwent a blocking step using 5% milk in Tris-buffered saline with Tween buffer, followed by incubation at 4 °C overnight with primary antibodies. These included a mouse polyclonal antibody against GAPDH (1:5000, 60004-1-Ig, Proteintech, IL, United States), a polyclonal antibody targeting mechanistic target of rapamycin (mTOR) (1:1000, #2983, Cell Signaling Technology, MA, United States), a polyclonal antibody for phospho-mTOR (Ser2448) (1:1000, #5536, Cell Signaling Technology, MA, United States), a monoclonal antibody against protein kinase B (AKT) (1:1000, 60203-2-Ig, Proteintech, IL, United States), and a recombinant antibody for phospho-AKT (Ser473) (1:1000, 80455-1-RR, Proteintech, IL, United States). Following this, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies and visualized using an enhanced chemiluminescence detection system. Densitometric analysis was performed utilizing Scion Image software sourced from the United States.
We assigned at least three mice to each experiment group so that we could conduct statistical analyses. All experimental results are expressed as the mean accompanied by the standard error of the mean. The statistical significance of differences between two groups was assessed using a two-tailed Student’s t-test. For comparisons involving multiple groups, a one-way analysis of variance followed by Tukey’s multiple comparison test was employed. A P value of less than 0.05 was deemed statistically significant. Data were analyzed using GraphPad Prism. The data met the assumptions of normality and homogeneity of variance.
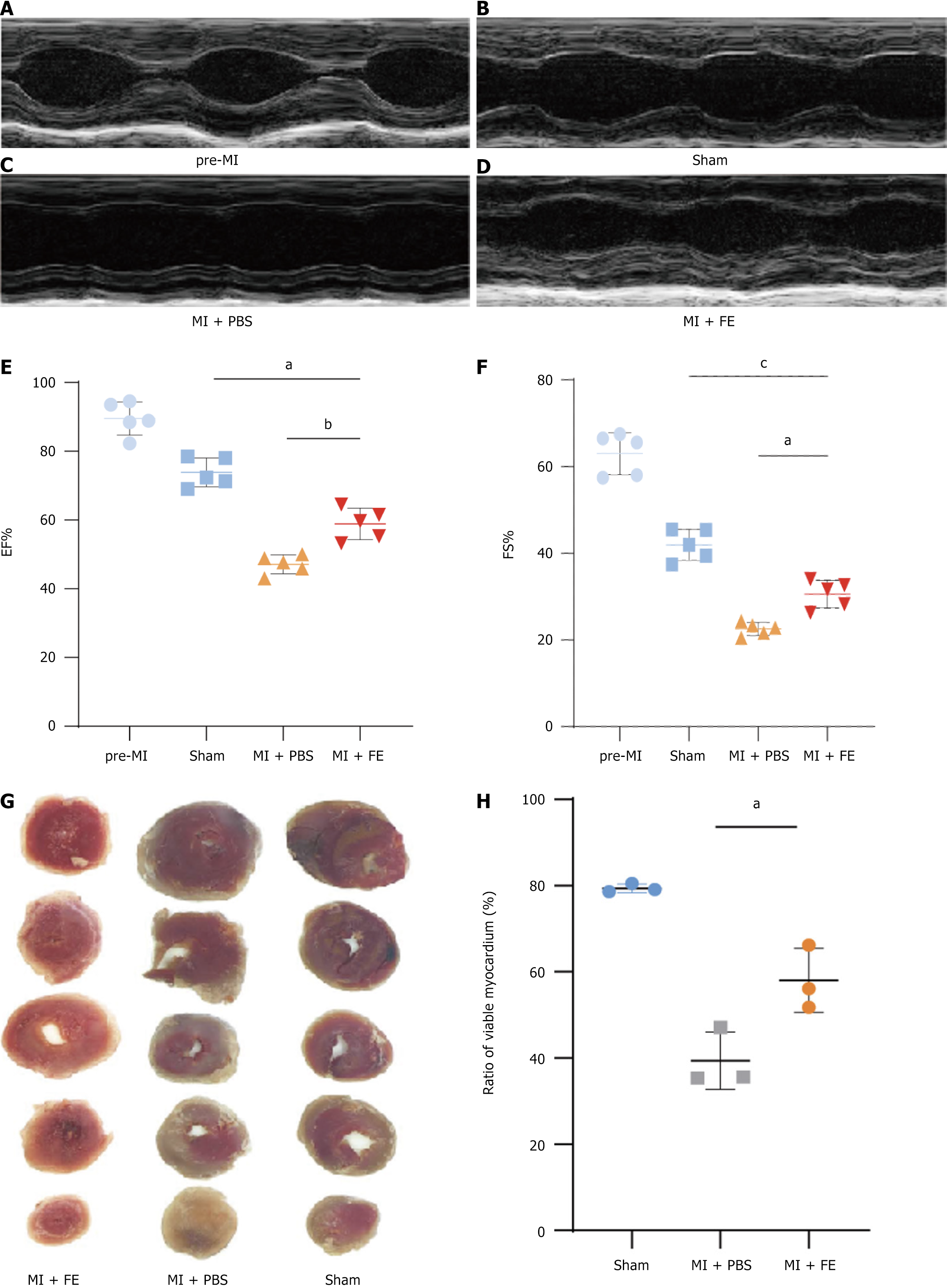
To assess the therapeutic effect of FE on cardiac function after MI in adult mice, we generated an MI model and monitored LV systolic function through two-dimensional echocardiography. Compared to that in the healthy group, LV systolic function was significantly reduced in the sham-operated group and MI groups (Figure 2A-D). The LV ejection fraction was lower in the control group than in the treatment group (Figure 2C-E, P = 0.002). Similarly, the control group showed a lower LV shortening fraction than that in the treatment group (Figure 2F, P = 0.011). Notably, the sham-operated group had slightly poorer cardiac function than that in the healthy group, possibly due to pneumothorax caused by the open-chest operation. The echocardiography results suggested that FE could improve cardiac function after MI in mice. We assessed the infarct size of the heart in mice using TTC staining. The infarct was smaller in the treatment group than in the control group (Figure 2G and H, P = 0.018), supporting the premise that FE could improve cardiac function after MI in mice.
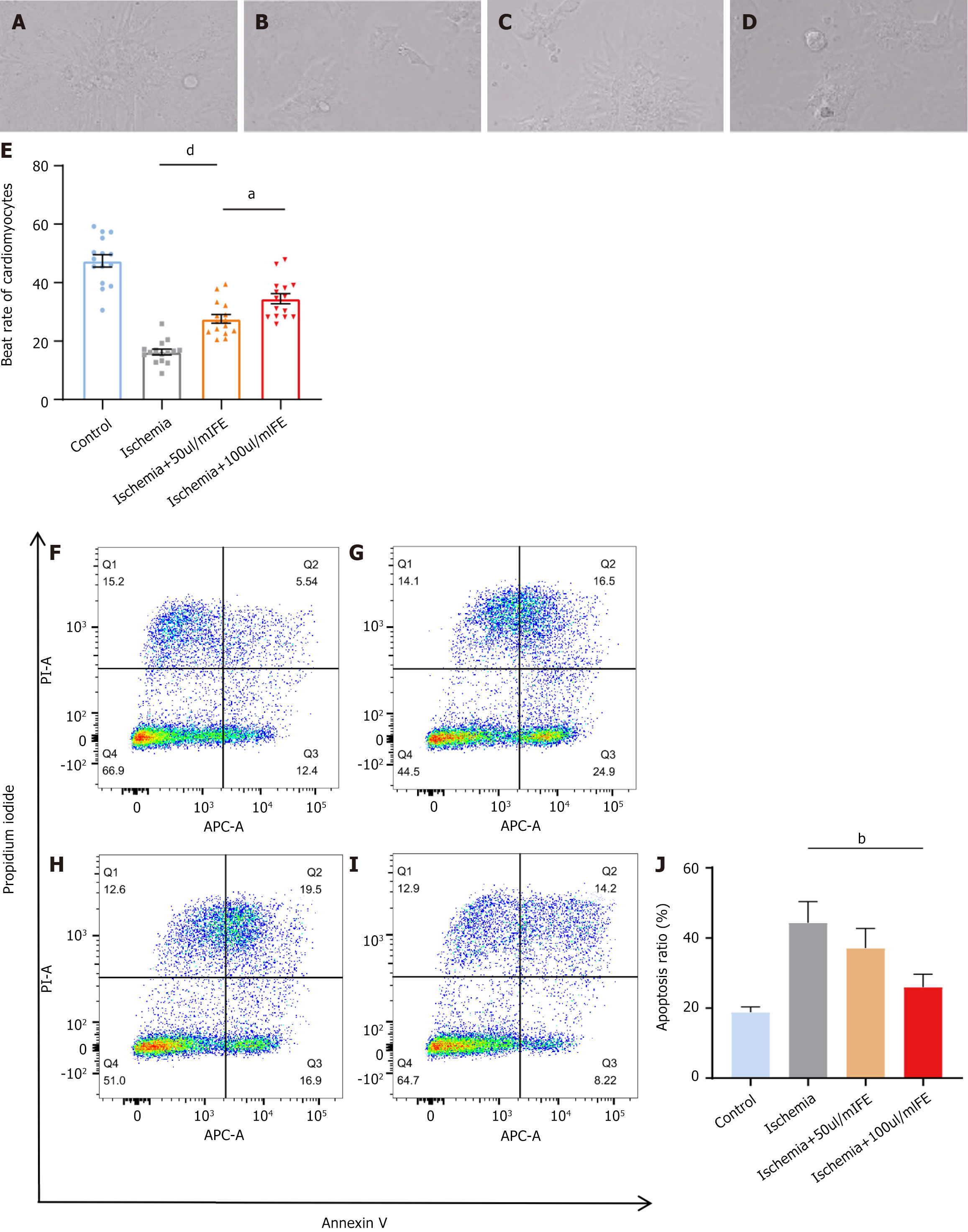
To assess the therapeutic effect of FE at the cellular level after ischemic injury, we generated a hypoxic model of neonatal rat CMs and calculated the beating rate of CMs by microscopy; this enabled the assessment of cell viability. Neonatal rat CMs showed a significantly lower beating rate after 12 hours of hypoxic culture than that in the negative controls (Figure 3A-D). Three groups were set up as follows: An ischemia group, 50 μL/mL treatment group, and 100 μL/mL treatment group. The degree of cell viability recovery showed a positive correlation with the FE concentration (Figure 3E; ischemia group vs 50 μL/mL treatment group, P < 0.0001; 50 μL/mL treatment group vs 100 μL/mL treatment group, P = 0.022).
We then assessed apoptosis levels in each group using flow cytometry. We discovered that the percentage of apoptotic CMs in the ischemia group rose from 18.8% ± 1.5% to 44.4% ± 6.0% relative to that in the negative control group; the percentage of apoptotic CMs in the 50 μL/mL treatment group decreased to 37.1% ± 5.6% compared with that in the ischemia group, which did not demonstrate a statistically significant difference (Figure 3F-J, P > 0.05). The percentage of apoptotic cells in the 100 μL/mL treatment group further decreased to 26.0% ± 3.6% compared with that in the ischamia group (Figure 3F-J, P = 0.005). These data suggested that FE could produce a therapeutic effect on hypoxic rat CMs, and this effect tended to be stronger with increasing concentrations.
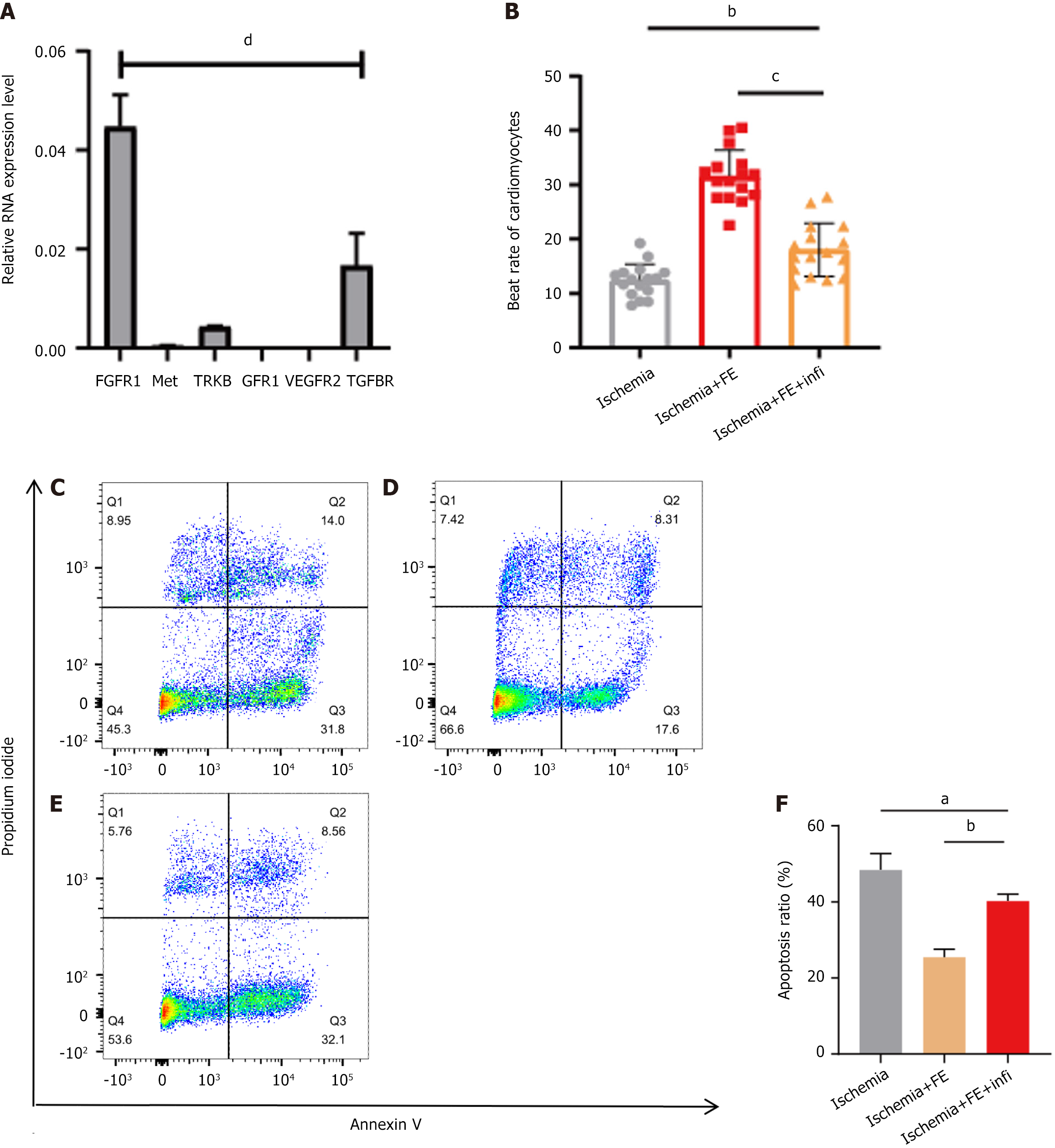
FE contains a variety of active cytokines. To determine the cytokines that contribute to the inhibition of CM apoptosis under hypoxic conditions, we selected the top six cytokines in FE, brain-derived neurotrophic factor, GDNF, TGF-β, HGF, bFGF, and VEGF. We identified receptors for each cytokine that have been reported in ischaemic injury: TRKB, TGFBR, Met, FGFR1, and VEGFR2. Since GDNF has not been reported in myocardial ischemia, we chose GFR1, a receptor of GDNF, which has been studied more extensively in the neurological field. Next, we determined the relative mRNA expression levels of the above six receptors (relative to GAPDH) by quantitative polymerase chain reaction in neonatal rat CMs. We discovered that FGFR1, the bFGF receptor, showed high expression (Figure 4A). We hypothesized that bFGF plays a significant role in CM apoptosis inhibition by FE.
Infigratinib is an inhibitor of the FGFR family and partially inhibits the therapeutic effects of FE (Figure 4B-F). The beating rate of neonatal rat CMs in the infigratinib-treated group was lower than that in the FE group (Figure 4B, P < 0.001) but higher than that in the ischemia group (Figure 4B, P = 0.002). The percentage of apoptotic CMs in the infigratinib group was higher than in the FE group (Figure 4D-F, P = 0.002) but lower than that in the ischemia group (Figure 4C, E, and F, P = 0.029).
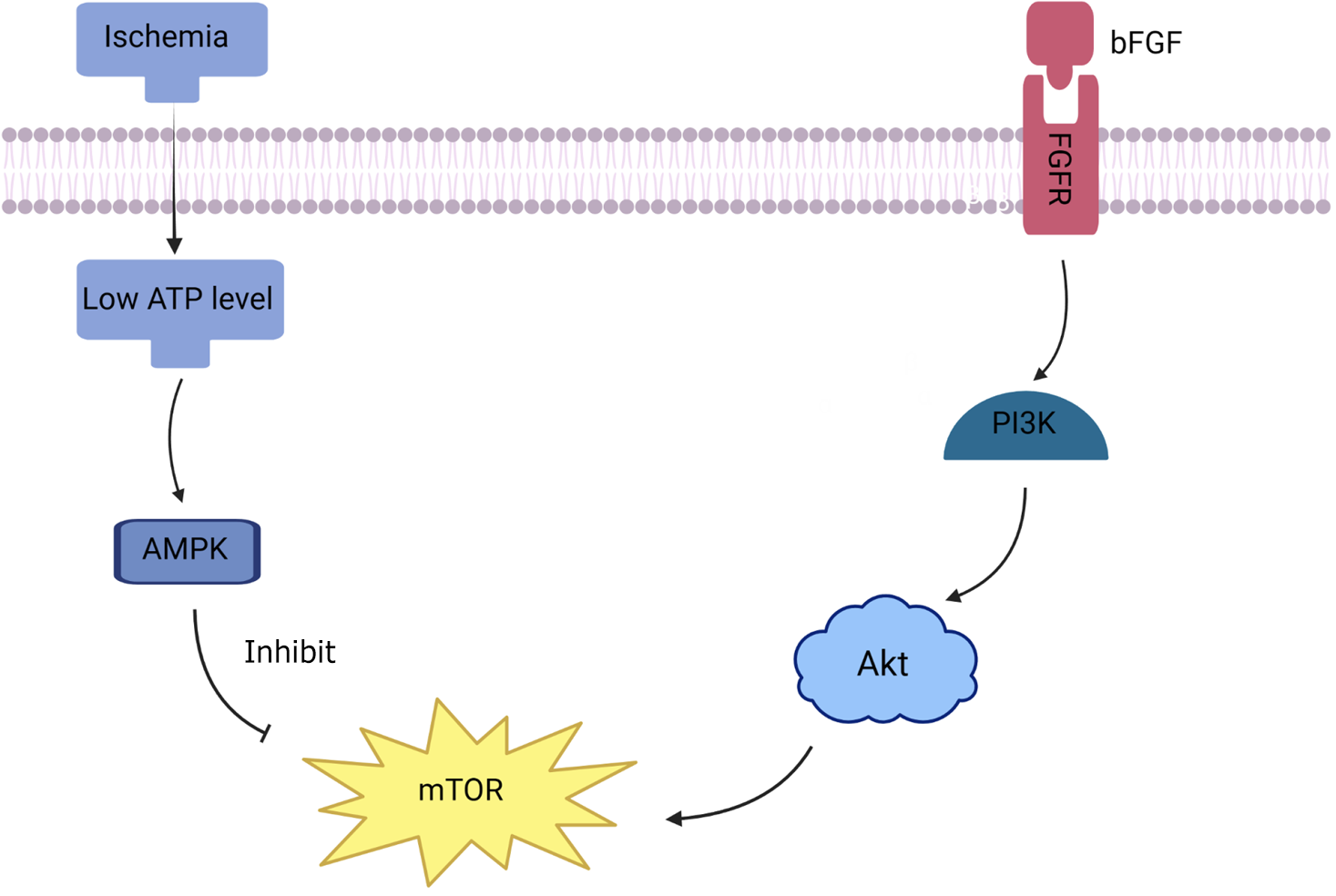
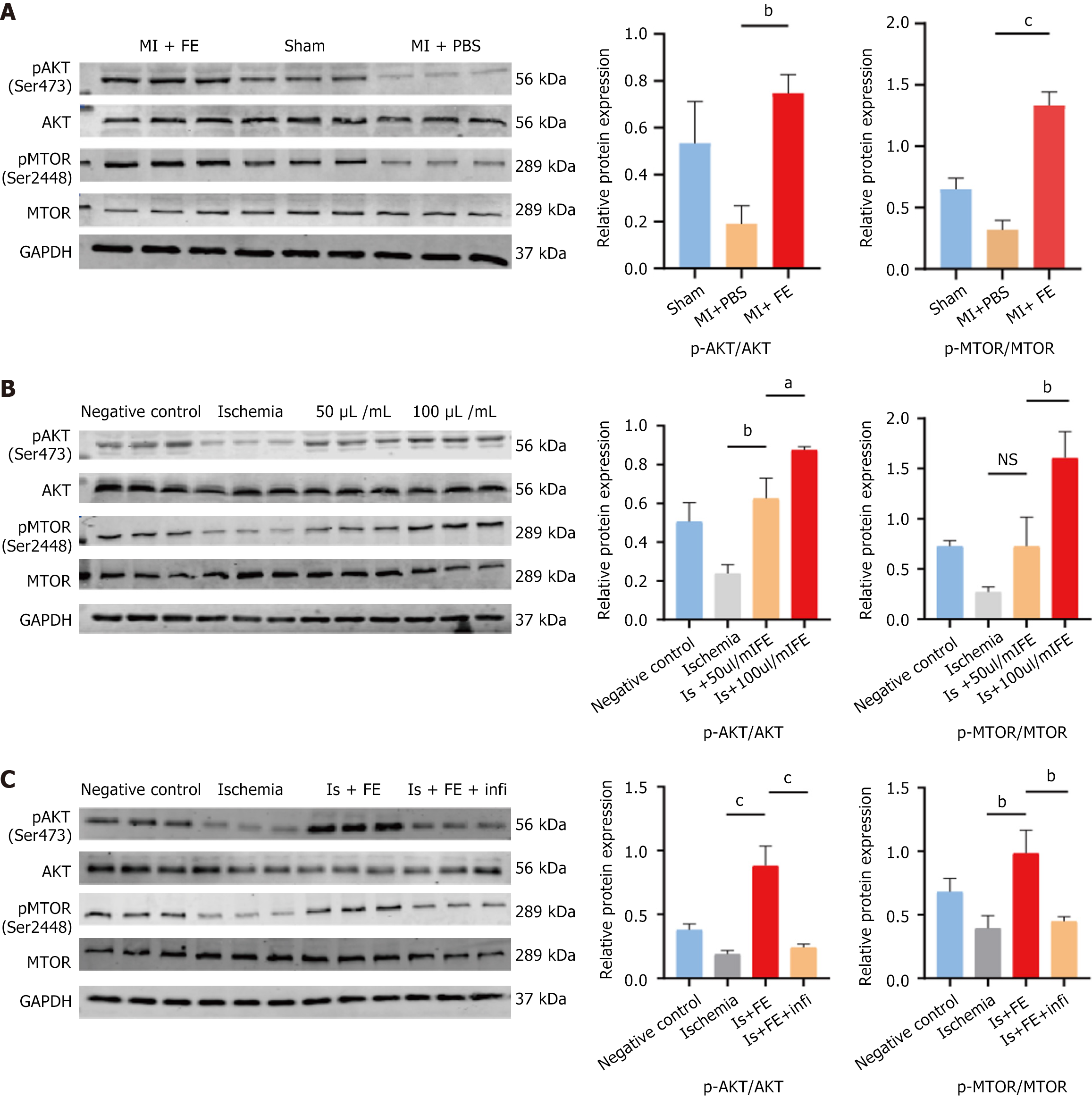
The phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR signaling pathway regulates cell survival and proliferation and plays an essential role in CM protection after ischemic injury. Recent evidence suggests that bFGF mediates the Akt/mTOR signaling pathway in myocardial ischaemia/reperfusion injury; therefore, we hypothesized that FE, rich in growth factors, would protect CM under ischemic conditions by activating the PI3K/Akt/mTOR signaling pathway (Figure 5). We assessed the phosphorylation levels of Akt and mTOR in our MI model in vivo and in a hypoxic model in vitro. A western blot analysis demonstrated that Akt/mTOR phosphorylation levels decreased significantly after MI in mice and were higher after FE treatment than in the untreated MI group (Figure 6A). At the cellular level, similarly, the phosphorylation levels of Akt and mTOR increased in the 50 μL/mL FE and 100 μL/mL FE groups and the degree of enhancement increased with the FE concentration (Figure 6B). Furthermore, the phosphorylation levels of Akt and mTOR were significantly lower after treatment with FGFR family inhibitor than in the FE treatment group (Figure 6C). These data suggest that recovery from MI with FE treatment may, in part, involve the activation of the Akt/mTOR signaling pathway.
MSCs are a type of adult stem cell characterized by their capacity for self-renewal and multidirectional differentiation. We expected MSCs to be valuable for myocardial repair based on their potential to differentiate into CMs. Research conducted in 2001 demonstrated that the localized administration of bone marrow cells has the potential to create new myocardial tissue, thereby enhancing the prognosis of patients with coronary artery disease[11]. However, controversial results regarding whether pluripotent adult progenitor cells can differentiate into CMs were subsequently reported. In a rat model of chronic MI, researchers discovered that multipotent adult progenitor cells neither improved global pump function nor differentiated into CMs[12].
The primary mechanisms by which MSC therapy improves LV function include the following: (1) Large amounts of secreted cytokines and paracrine factors counteract inflammatory responses, activate cytoprotective pathways in reversibly damaged myocardial cells, and prevent fibrosis and LV remodeling; (2) Differentiation into endothelial and smooth muscle cells promotes angiogenesis; and (3) The proliferation and differentiation of remaining stem cells are stimulated[4,13-15].
Although the paracrine effect of MSCs has been confirmed in many preclinical studies on heart repair[16-19], they present some limitations. The poor survival of MSCs after simple delivery to ischemic myocardial tissues may be due to microenvironmental hypoxia, inflammation, and high oxidative stress[20]. The retention time of MSCs in the target area after transplantation also remains unclear.
According to a study published in 2015, MSCs are detectable between weeks 3 and 10 after injection in the myocardial tissue of porcine hearts with MI[21]. In addition, the tumorigenicity of MSCs remains controversial; Røsland et al[22] reported that human bone marrow-derived MSCs exhibit spontaneous malignant transformation, including hyperdifferentiation and morphological transformation in vitro. However, Bernardo et al[23] revealed that human bone marrow-derived MSCs did not show telomerase activity, telomerase reverse transcriptase activity, altered telomere lengths, or chromosomal irregularities that suggest the onset of cancer following prolonged culture in vitro.
FE derived from MSCs contains high levels of paracrine factors; FE is a cell-free liquid fraction of nanofat. These properties indicate that FE can theoretically circumvent the problems of low graft viability and potential tumorigenicity of MSCs. Our study showed that FE could improve the survival of CMs in a hypoxic environment and improve cardiac function after MI. Additionally, our study revealed that the mechanism underlying FE-mediated protection involves several components, and the activation of the PI3K/Akt/mTOR signaling pathway is crucial. This signaling pathway regulates an extensive range of cellular activities involved in cell survival, proliferation, and metabolism. PI3K is part of a lipid kinase family known for its distinctive function of phosphorylating the inositol ring at the 3’-OH position on inositol phospholipids. Activation of receptor tyrosine kinases by growth factors leads to PI3K activation and the production of phosphatidylinositol-(3,4,5)-trisphosphate, which binds and activates Akt[24]. mTOR, which is phosphorylated by Akt, plays important roles in cell growth, proliferation, survival, autophagy, metabolism, and protein synthesis[25]. The potential clinical application of FE lies in its ability to serve as an adjunctive therapy for existing treatments of IHD, such as percutaneous coronary intervention. The mode of delivery of FE could be improved, as direct injections of FE can lead to myocardial damage, inhomogeneous distribution, and a short residence time. Therefore, a carrier that achieves a longer sustainable release, such as a hydrogel or cardiac tonic agent, may be necessary.
Overall, this study has a few limitations. First, we investigated only one component of FE. The effects of different FE concentrations on composites remain to be explored. Second, FE was sourced from a restricted pool of donors; it is not clear whether the effect of FE could differ among women from different age groups. Third, the introduction of FE may complicate the mechanisms of the PI3K/Akt/mTOR signaling pathway. Fourth, in our study, we did not conduct a comparative analysis of the efficacy and safety between FE and other stem cell derivatives, like exosomes. Finally, owing to its complex composition, FE may exhibit potential off-target effects. FE contains TGF-β and other profibrotic factors; however, this study did not evaluate the impact of FE on myocardial fibrosis.
Our findings suggest that FE, through its rich growth factor content and activation of the PI3K/Akt/mTOR pathway, holds promise as a novel therapeutic strategy for IHD.
| 1. | Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56-e528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4294] [Cited by in RCA: 5881] [Article Influence: 980.2] [Reference Citation Analysis (5)] |
| 2. | Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67-e492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4163] [Cited by in RCA: 4806] [Article Influence: 686.6] [Reference Citation Analysis (1)] |
| 3. | Golpanian S, Wolf A, Hatzistergos KE, Hare JM. Rebuilding the Damaged Heart: Mesenchymal Stem Cells, Cell-Based Therapy, and Engineered Heart Tissue. Physiol Rev. 2016;96:1127-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 254] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 4. | Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1203] [Cited by in RCA: 1173] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 5. | Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 964] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 6. | Yu Z, Cai Y, Deng M, Li D, Wang X, Zheng H, Xu Y, Li W, Zhang W. Fat extract promotes angiogenesis in a murine model of limb ischemia: a novel cell-free therapeutic strategy. Stem Cell Res Ther. 2018;9:294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Harasymiak-Krzyżanowska I, Niedojadło A, Karwat J, Kotuła L, Gil-Kulik P, Sawiuk M, Kocki J. Adipose tissue-derived stem cells show considerable promise for regenerative medicine applications. Cell Mol Biol Lett. 2013;18:479-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Tonnard P, Verpaele A, Peeters G, Hamdi M, Cornelissen M, Declercq H. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg. 2013;132:1017-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 398] [Article Influence: 33.2] [Reference Citation Analysis (1)] |
| 9. | Yao Y, Dong Z, Liao Y, Zhang P, Ma J, Gao J, Lu F. Adipose Extracellular Matrix/Stromal Vascular Fraction Gel: A Novel Adipose Tissue-Derived Injectable for Stem Cell Therapy. Plast Reconstr Surg. 2017;139:867-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 10. | Huebsch N, Loskill P, Mandegar MA, Marks NC, Sheehan AS, Ma Z, Mathur A, Nguyen TN, Yoo JC, Judge LM, Spencer CI, Chukka AC, Russell CR, So PL, Conklin BR, Healy KE. Automated Video-Based Analysis of Contractility and Calcium Flux in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Cultured over Different Spatial Scales. Tissue Eng Part C Methods. 2015;21:467-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 223] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 11. | Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3891] [Cited by in RCA: 3550] [Article Influence: 147.9] [Reference Citation Analysis (0)] |
| 12. | Agbulut O, Mazo M, Bressolle C, Gutierrez M, Azarnoush K, Sabbah L, Niederlander N, Abizanda G, Andreu EJ, Pelacho B, Gavira JJ, Perez-Ilzarbe M, Peyrard S, Bruneval P, Samuel JL, Soriano-Navarro M, García-Verdugo JM, Hagège AA, Prósper F, Menasché P. Can bone marrow-derived multipotent adult progenitor cells regenerate infarcted myocardium? Cardiovasc Res. 2006;72:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Ratajczak MZ, Kucia M, Jadczyk T, Greco NJ, Wojakowski W, Tendera M, Ratajczak J. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia. 2012;26:1166-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 263] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 14. | Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011;50:280-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 379] [Cited by in RCA: 349] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 15. | Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 611] [Cited by in RCA: 541] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 16. | Du YY, Zhou SH, Zhou T, Su H, Pan HW, Du WH, Liu B, Liu QM. Immuno-inflammatory regulation effect of mesenchymal stem cell transplantation in a rat model of myocardial infarction. Cytotherapy. 2008;10:469-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Chen J, Liu Z, Hong MM, Zhang H, Chen C, Xiao M, Wang J, Yao F, Ba M, Liu J, Guo ZK, Zhong J. Proangiogenic compositions of microvesicles derived from human umbilical cord mesenchymal stem cells. PLoS One. 2014;9:e115316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Li X, Zhao H, Qi C, Zeng Y, Xu F, Du Y. Direct intercellular communications dominate the interaction between adipose-derived MSCs and myofibroblasts against cardiac fibrosis. Protein Cell. 2015;6:735-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Miao C, Lei M, Hu W, Han S, Wang Q. A brief review: the therapeutic potential of bone marrow mesenchymal stem cells in myocardial infarction. Stem Cell Res Ther. 2017;8:242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 20. | Liu XB, Wang JA, Ji XY, Yu SP, Wei L. Preconditioning of bone marrow mesenchymal stem cells by prolyl hydroxylase inhibition enhances cell survival and angiogenesis in vitro and after transplantation into the ischemic heart of rats. Stem Cell Res Ther. 2014;5:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Tao B, Cui M, Wang C, Ma S, Wu F, Yi F, Qin X, Liu J, Wang H, Wang Z, Ma X, Tian J, Chen Y, Wang J, Cao F. Percutaneous intramyocardial delivery of mesenchymal stem cells induces superior improvement in regional left ventricular function compared with bone marrow mononuclear cells in porcine myocardial infarcted heart. Theranostics. 2015;5:196-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Røsland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R, Lønning PE, Bjerkvig R, Schichor C. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331-5339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 480] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 23. | Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, Montagna D, Maccario R, Villa R, Daidone MG, Zuffardi O, Locatelli F. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142-9149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 509] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 24. | Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1407] [Cited by in RCA: 1786] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 25. | Shi B, Ma M, Zheng Y, Pan Y, Lin X. mTOR and Beclin1: Two key autophagy-related molecules and their roles in myocardial ischemia/reperfusion injury. J Cell Physiol. 2019;234:12562-12568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |