Copyright
©The Author(s) 2025.
World J Stem Cells. Jun 26, 2025; 17(6): 106520
Published online Jun 26, 2025. doi: 10.4252/wjsc.v17.i6.106520
Published online Jun 26, 2025. doi: 10.4252/wjsc.v17.i6.106520
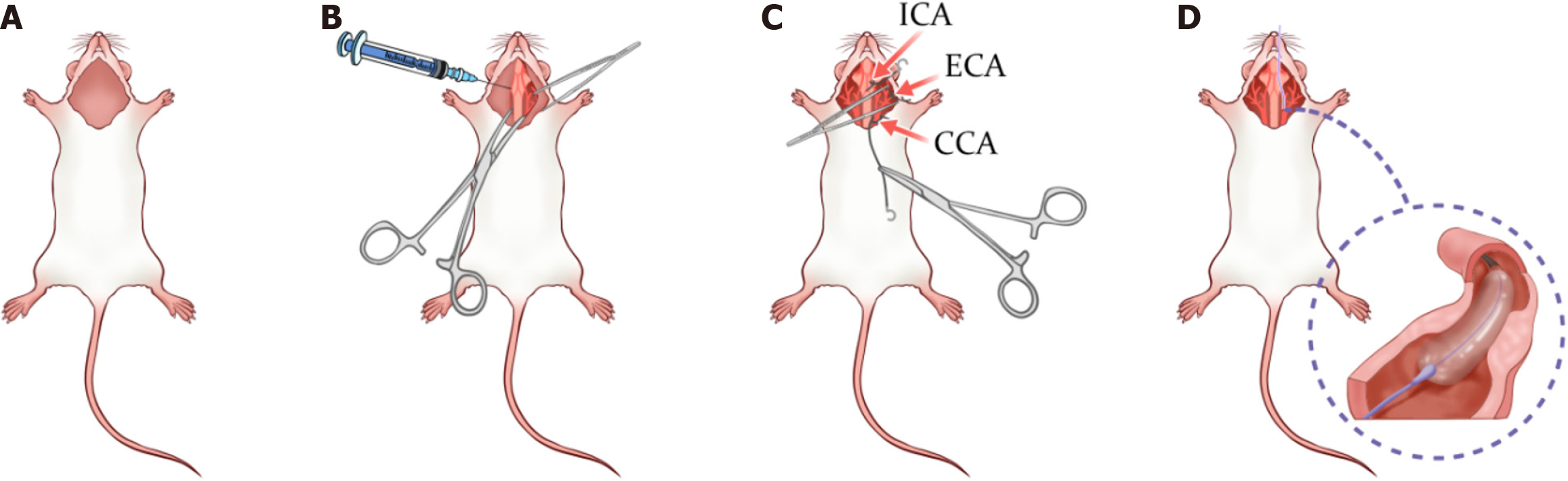
Figure 1 Rat carotid artery balloon injury surgery simulation.
A: Preoperative preparation phase: At this stage, the rat has been adequately anesthetized and secured supine on the operating table. Preoperative hair shaving and cleaning have been completed; B: Tissue dissection: In this step, surgical scissors and hemostatic forceps are used to incise the neck skin and dissect the glandular tissue to expose the underlying vascular structures; C: Further tissue dissection and vascular exposure: The image labels the internal carotid artery, external carotid artery, and common carotid artery, which are key areas of surgery. This step involves a combination of blunt and sharp dissection techniques to fully expose the carotid artery system; D: Balloon injury phase: In this step, a catheter is inserted through the common carotid artery and, after accurate positioning, the balloon dilation procedure is performed to induce endothelial injury. ICA: Internal carotid artery; ECA: External carotid artery; CCA: Common carotid artery.
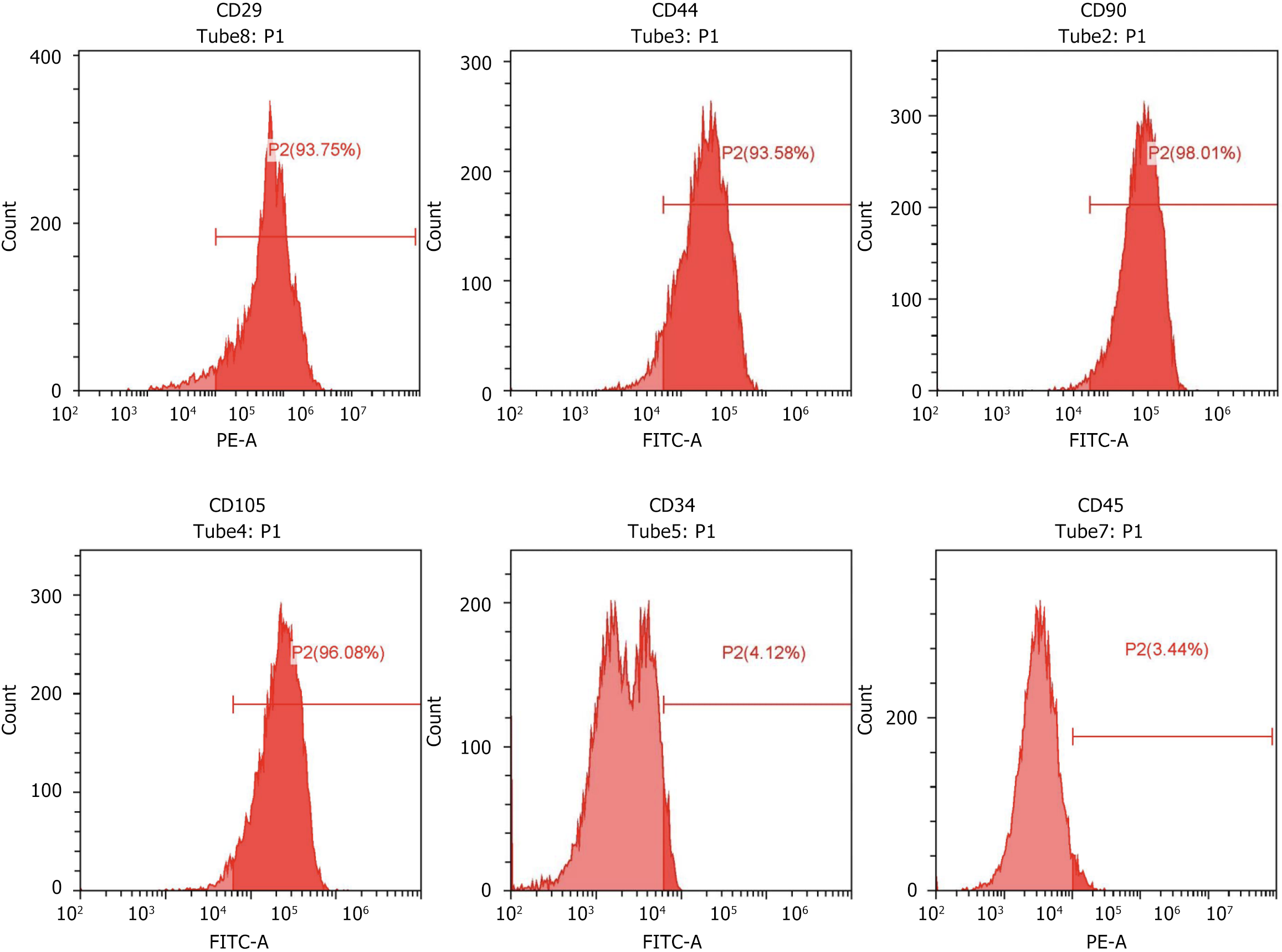
Figure 2 Flow cytometry.
Flow cytometry was used to identify surface markers of rat bone marrow mesenchymal stem cells, confirming positive expression of CD29 (93.75%), CD44 (93.58%), CD90 (98.01%), CD105 (96.08%), and negative expression of CD34 (4.12%), and CD45 (3.44%), verifying the mesenchymal stem cell identity.
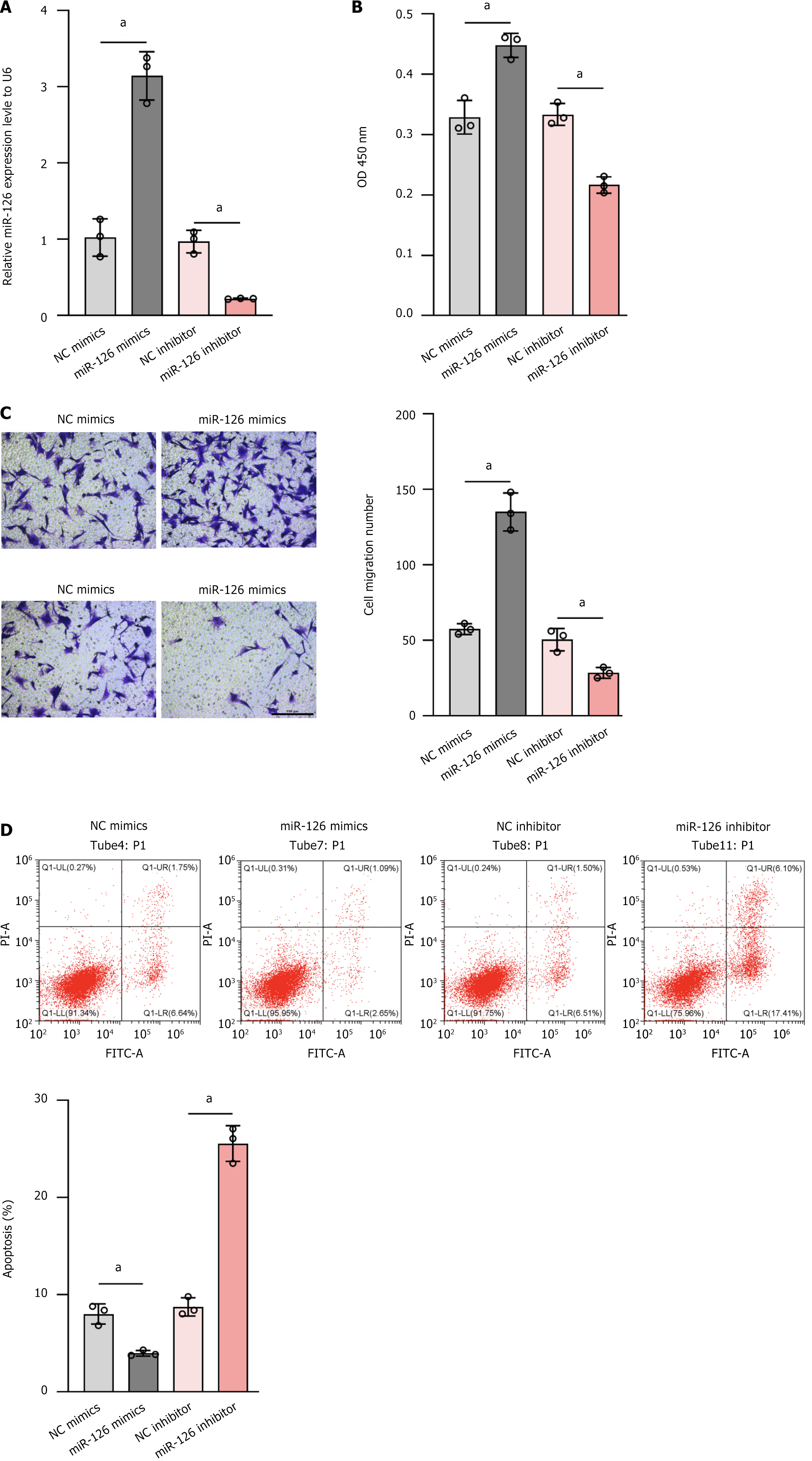
Figure 3 To assess the relative expression level of miR-126, cell proliferation, cell migration ability, and apoptosis.
A: Relative expression level of miR-126 was determined by quantitative polymerase chain reaction. MiR-126 mimics significantly increase miR-126 expression, while miR-126 inhibitor reduces it; B: Cell proliferation was assessed by measuring optical density at 450 nm. Compared with the control group (negative control mimics), the miR-126 mimics group showed enhanced cell proliferation (higher optical density value); C: Cell migration ability was assessed by wound healing assay. The number of migrated cells in the miR-126 mimics group was significantly higher than that in the negative control mimics group. The bar chart quantified the number of migrated cells, further supporting these observations; D: Cells were assessed by flow cytometry apoptosis. MiR-126 mimics reduced apoptosis, while miR-126 inhibitor significantly increased it. The bar chart quantified the apoptosis rate, further supporting these observations. Data are presented as the mean ± SD, and differences between groups were calculated using one-way ANOVA. aP < 0.01. OD: Optical density; NC: Negative control.
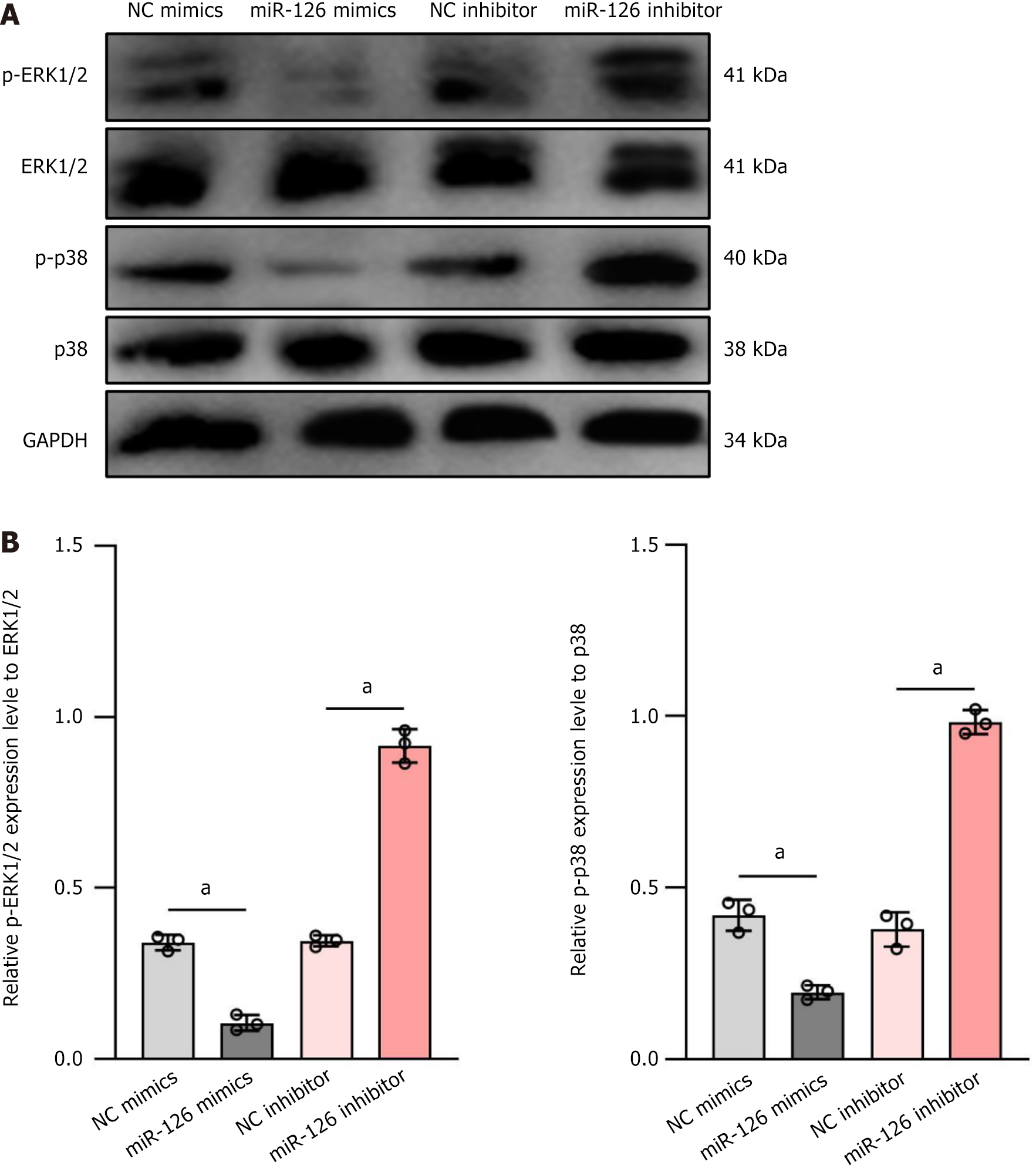
Figure 4 On the effects of miR-126 on the phosphorylation of 1 extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinases signaling pathways.
A: Western blot analysis was used to evaluate the expression levels of p-extracellular signal-regulated kinase (ERK)1/2, ERK1/2, p-p38, and p38 under four conditions: Negative control mimics, miR-126 mimics, negative control inhibitor, and miR-126 inhibitor. The results showed that in the miR-126 mimics group, the expression of p-ERK1/2 and p-p38 was significantly decreased, while in the miR-126 inhibitor group, the phosphorylation levels of these proteins were markedly increased; B: The bar charts quantified the relative expression levels of p-ERK1/2 and p-p38, further supporting the above results. Thus, miR-126 may influence cell functions, particularly the differentiation of mesenchymal stem cells into functional endothelial cells, by modulating the ERK1/2 and p38 signaling pathways. Data are presented as mean ± SD, and differences between groups were calculated using one-way ANOVA. aP < 0.01. ERK: Extracellular signal-regulated kinase; NC: Negative control.
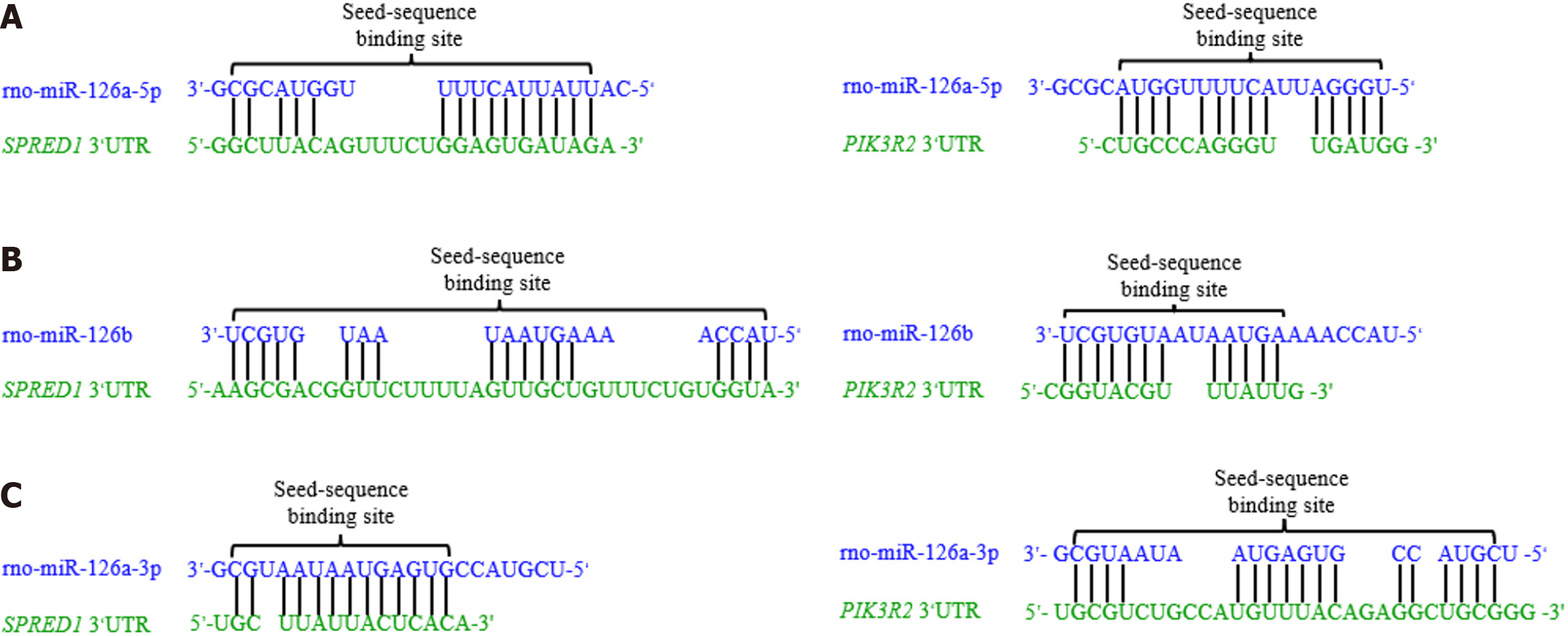
Figure 5 The complementary pairing between microRNA sequences and their target gene 3’-untranslated region.
A: The seed sequence of rno-miR-126a-5p is fully complementary to the 3’-untranslated region (3’UTR) of sprouting-related protein 1 (SPRED1) and phosphatidylinositol 3-kinase regulatory subunit 2 (PIK3R2); B: The seed sequence of rno-miR-126b is partially complementary to the 3’UTR of SPRED1 and PIK3R2, with some mismatches in the binding sites; C: The seed sequence of rno-miR-126a-3p shows partial complementary pairing with the 3’UTR of SPRED1 and PIK3R2, including additional mismatches in the PIK3R2 binding site. Data from the RNAhybrid database. 3’UTR: 3’-untranslated region; SPRED1: Sprouting-related protein 1; PIK3R2: Phosphatidylinositol 3-kinase regulatory subunit 2.
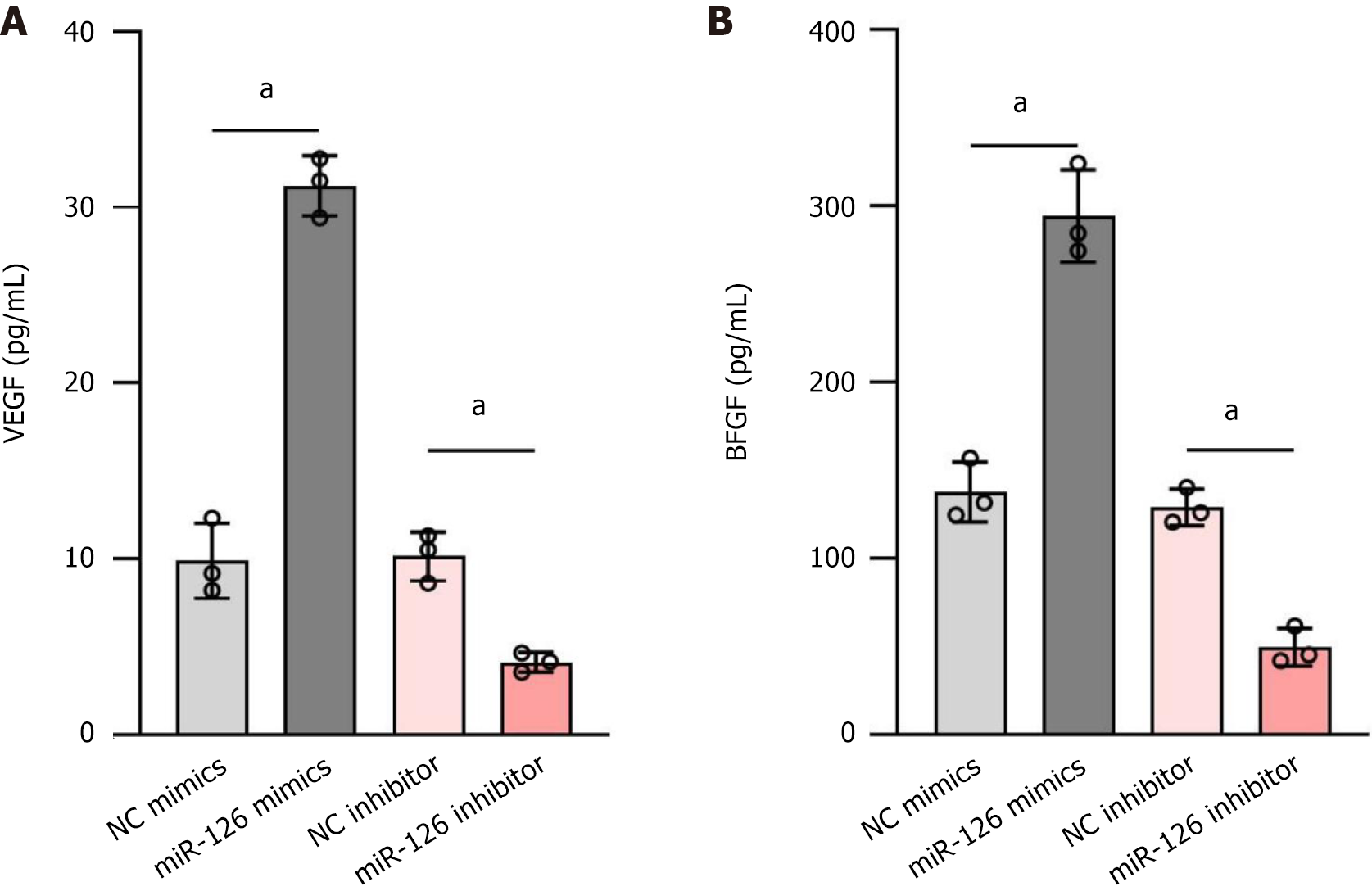
Figure 6 Enzyme-linked immunosorbent assay to detect the effect of miR-126 mimic on the levels of vascular endothelial growth factor and basic fibroblast growth factor in mesenchymal stem cells.
A: The changes in the concentration of vascular endothelial growth factor (VEGF) under different treatment conditions; B: The changes in the concentration of basic fibroblast growth factor (bFGF) under different treatment conditions. The up-regulation of miR-126 significantly increased VEGF and bFGF secretion, whereas the down-regulation of miR-126 led to a marked decrease in the secretion of both VEGF and bFGF. Data are presented in mean ± SD and differences between groups were statistically calculated using one-way ANOVA. aP < 0.01. NC: Negative control; VEGF: Vascular endothelial growth factor; bFGF: Basic fibroblast growth factor.
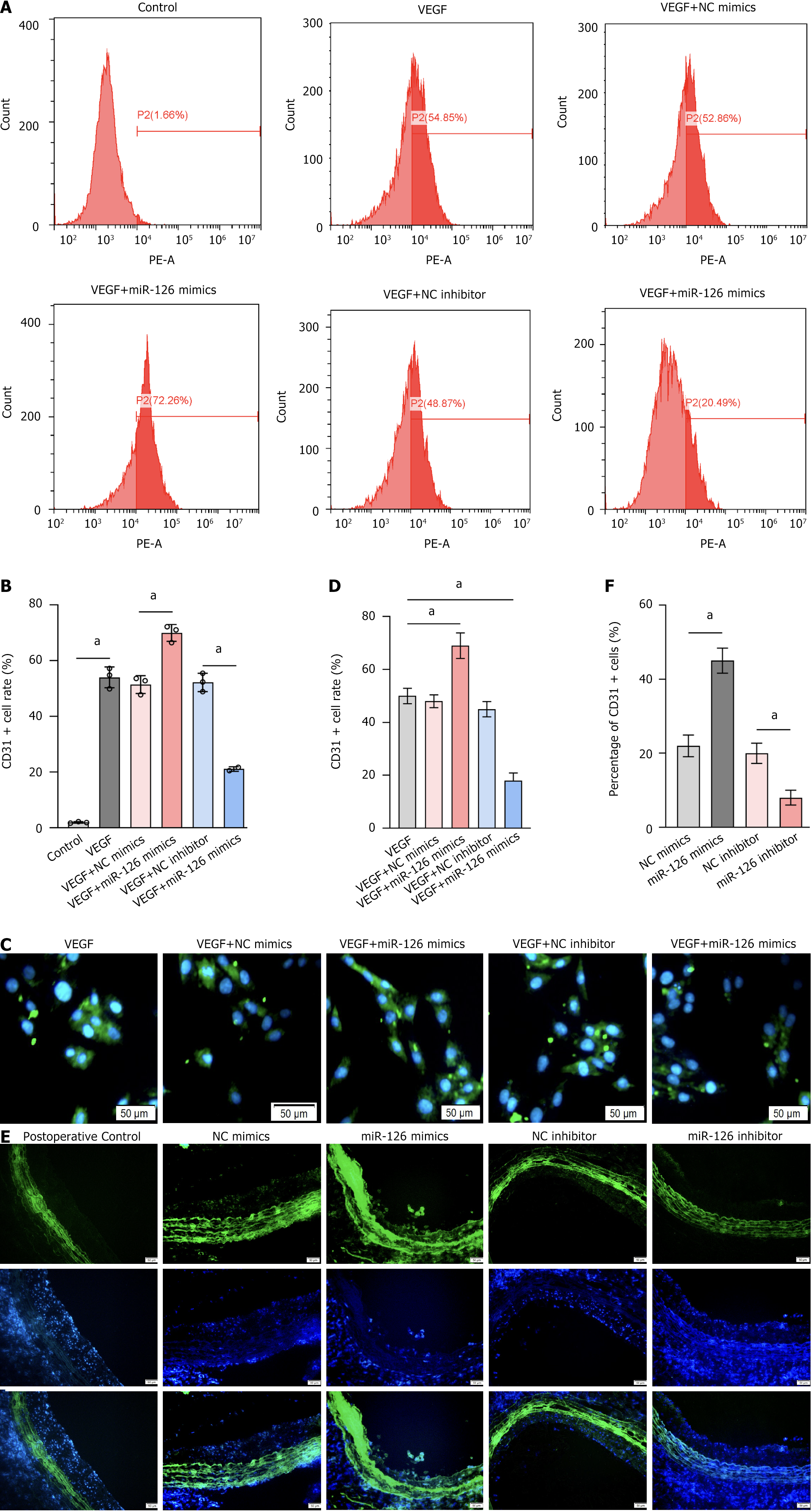
Figure 7 MiR-126 plays a crucial role in promoting the differentiation of mesenchymal stem cells into endothelial cells.
A and B: CD31 positivity rates across different treatment groups. The proportion of CD31-positive cells reached 72.26% in samples with the combination of vascular endothelial growth factor (VEGF) and miR-126 mimics, significantly higher than the 54.85% in the VEGF-only group. In contrast, the CD31 positivity was only 20.49% in samples with the combination of VEGF and miR-126 inhibitor, significantly lower than in the VEGF-only group; C: Cellular immunofluorescence sections showing the effects of miR-126 mimics and inhibitor on CD31 positivity; D: Bar chart corresponding to Figure 7C; E: Tissue immunofluorescence sections showing the effects of miR-126 mimics and inhibitor on CD31 positivity; F: Bar chart corresponding to Figure 7E. They demonstrate that miR-126 mimics significantly increased the proportion of CD31+ cells, whereas miR-126 inhibitor markedly reduced the proportion of CD31+ cells. Data are presented as the mean ± SD, and differences between groups were analyzed using one-way ANOVA. aP < 0.01. NC: Negative control; VEGF: Vascular endothelial growth factor.
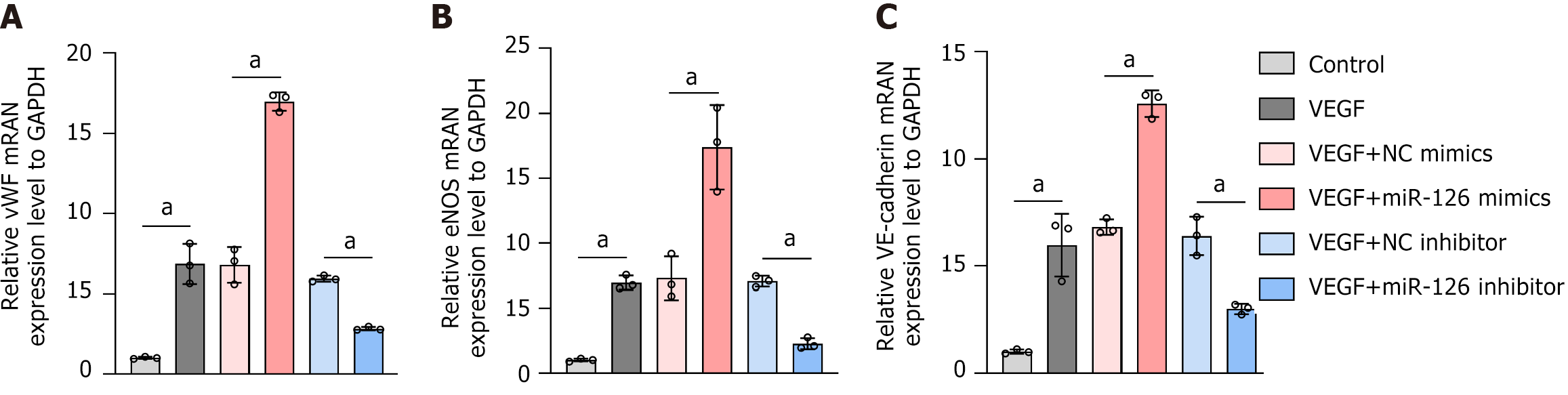
Figure 8 The mRNA levels of von Willebrand factor, endothelial nitric oxide synthase, and vascular endothelial-cadherin in vascular grafts from the group of mesenchymal stem cell grafts with different modifications were detected by real-time quantitative polymerase chain reaction.
A: The mRNA levels of von Willebrand factor; B: The mRNA levels of endothelial nitric oxide synthase; C: The mRNA levels of vascular endothelial-cadherin. The results showed that vascular endothelial growth factor significantly increased the mRNA levels of these genes in mesenchymal stem cells. Furthermore, miR-126 mimics enhanced these effects, while miR-126 inhibitors reversed them. This indicates that miR-126 can modulate the expression of endothelial-related genes in mesenchymal stem cells when combined with vascular endothelial growth factor. Data are presented as the mean ± SD, and differences between groups were analyzed using one-way ANOVA. aP < 0.01. vWF: von Willebrand factor; eNOS: Endothelial nitric oxide synthase; VE: Vascular endothelial; NC: Negative control; VEGF: Vascular endothelial growth factor.
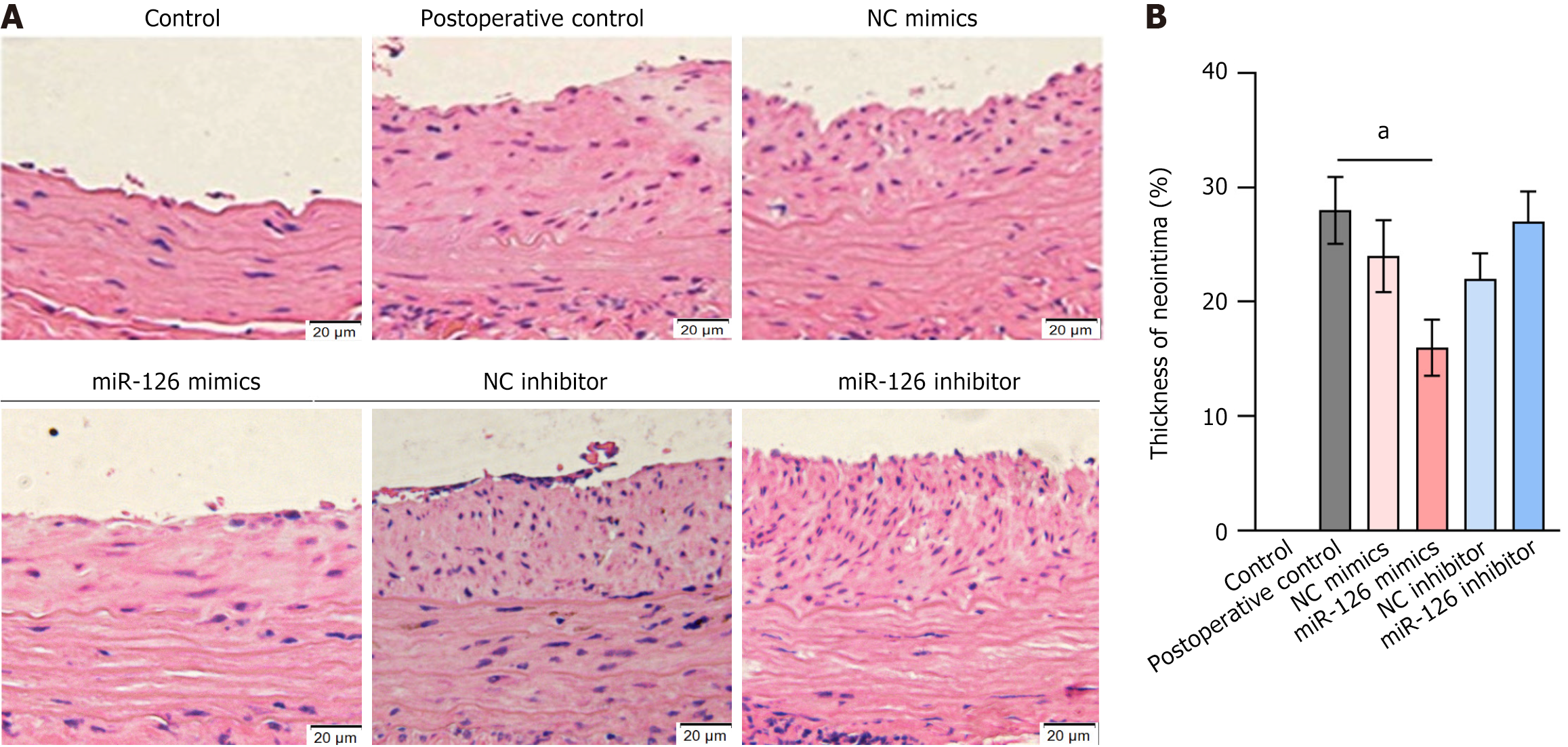
Figure 9 The therapeutic effect of miR-126 mimics-transfected mesenchymal stem cell transplantation on carotid neointimal thickening was evaluated.
A: Cross-sectional vessel slices were stained with hematoxylin and eosin to assess neointimal formation (5-6 mice per group); B: Neointimal thickness was expressed as a percentage relative to the control group. Results showed that transplantation of miR-126 mimics-transfected mesenchymal stem cells (MSCs) significantly inhibited neointimal thickening compared to the preoperative control group. In contrast, transplantation of miR-126 inhibitor-transfected MSCs did not show a significant effect on neointimal thickening. These findings underscore the therapeutic potential of transplanting miR-126 mimics-transfected MSCs in reducing neointimal hyperplasia. Data are presented as the mean ± SD, and differences between groups were analyzed using one-way ANOVA. aP < 0.01. NC: Negative control.
- Citation: Ye ZQ, Meng XH, Fang X, Liu HY, Mwindadi HH. MiR-126 regulates the effect of mesenchymal stem cell vascular repair on carotid atherosclerosis through MAPK/ERK signaling pathway. World J Stem Cells 2025; 17(6): 106520
- URL: https://www.wjgnet.com/1948-0210/full/v17/i6/106520.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i6.106520