Published online Jun 28, 2025. doi: 10.3748/wjg.v31.i24.104437
Accepted: June 3, 2025
Published online: June 28, 2025
Processing time: 188 Days and 12.6 Hours
We previously identified miR-10b-5p as a key regulator of gastrointestinal (GI) motility through its essential role in the development and function of interstitial cells of Cajal (ICC), the pacemaker cells of the gut. Loss of miR-10b-5p in ICC im
To investigate the roles of miR-10a-5p and miR-10b-5p in age-associated intestinal dysmotility and assess the therapeutic potential of restoring their expression.
We employed aged mice, mir-10a and mir-10b single and double knockout (KO) models, and human plasma and colon samples across age groups. GI and colonic transit, ICC network integrity, and expression levels of miR-10a/b-5p were eva
Aged mice exhibited delayed GI and colonic transit, reduced fecal output, and diminished expression of miR-10a-5p and miR-10b-5p, which peaked during late embryonic and early postnatal stages and declined with age. This decline para
miR-10a-5p and miR-10b-5p are essential for ICC maintenance and colonic motility, and their age-related decline contributes to GI dysmotility in both mice and humans. Restoring their levels offers a promising therapeutic stra
Core Tip: Gastrointestinal (GI) dysmotility is strongly associated with aging, driven by functional changes in the gut. We previously identified miR-10b-5p as a key regulator of GI motility disorders. However, the specific roles of miR-10a-5p and miR-10b-5p in age-related GI dysmotility remain unexplored. This study presents compelling evidence supporting the miR-10a-5p and miR-10b-5p as critical regulators of interstitial cells of Cajal growth and function, playing essential roles in maintaining GI motility in aged mice and humans. Restoration of these microRNAs through miR-10a-5p and miR-10b-5p mimics in aged mice effectively alleviated GI dysmotility, particularly constipation, offering a promising therapeutic strategy for age-related GI motility disorders.
- Citation: Baek G, Singh R, Ha SE, Cho H, Padmanabhan S, Vishwanath V, Kim MS, Seon D, You J, Lee MY, Ro S. miR-10a-5p and miR-10b-5p restore colonic motility in aged mice. World J Gastroenterol 2025; 31(24): 104437
- URL: https://www.wjgnet.com/1007-9327/full/v31/i24/104437.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i24.104437
Gastrointestinal (GI) dysmotility is strongly associated with aging, driven by functional changes in the digestive system[1]. Constipation, a prevalent issue among older adults, results from a combination of physiological, lifestyle, and medical factors[2]. Among these, age-related changes in the GI tract, particularly the slowing of colonic transit, play a significant role[3]. Colonic motility, primarily regulated by interstitial cells of Cajal (ICC)[4], is impaired in aging due to a decline in both the number and function of ICC, as observed in aged mice and humans[5-7]. A reduction in ICC numbers is directly linked to constipation[4,8].
ICC are pacemaker cells that generate electrical slow waves, driving phasic contractions in the GI tract[9]. Subtypes of ICC include myenteric plexus ICC (ICC-MY) and submucosal plexus ICC (ICC-SMP), both of which are critical for the rhythmic electrical activity that coordinates digestive tract contractions[5]. The growth and function of ICC are regulated by the receptor tyrosine kinase KIT[10]. KIT signaling is essential for ICC development, maintenance, and pacemaker activity[11].
MicroRNAs (miRNAs) are small non-coding RNAs, approximately 22 nucleotides in length, that are pivotal regulators of gene expression through mRNA targeting and translational repression. miRNAs influence various cellular processes, including proliferation, differentiation, and apoptosis[12]. During miRNA biogenesis, miRNAs are processed into duplexes consisting of 5’ and 3’ strands, both of which can be functional[13]. However, most miRNAs exhibit a 5’ and 3’ strand bias, where one strand is preferentially incorporated into the RNA-induced silencing complex, while the other is typically degraded[14].
In our previous studies, we demonstrated that mir-10b is essential for ICC growth and function in the GI tract[15]. mir-10a and mir-10b belong to the same family, with miR-10a-5p and miR-10b-5p being the predominantly generated strands from their respective genes[14]. These miRNAs target KLF11, a transcriptional repressor that negatively regulates KIT expression in ICC[15]. A deficiency of miR-10b-5p in mice leads to impaired GI motility due to reduced KIT expression and subsequent ICC loss[15].
In this study, we investigated the roles of miR-10a-5p and miR-10b-5p in colonic dysmotility associated with aging in mice and humans.
This study utilized C57BL/6J (ranging from embryo day 6 to 25-month-old male mice), as well as mir-10a knockout (KO)[16], mir-10b KO[17] and mir-10a/b double KO mice. All animals were housed and maintained in the centralized animal care facility at the University of Nevada, Reno (UNR) Animal Resources. All processes involving animal subjects were approved by the Institutional Animal Care and Use Committee at UNR, which is fully accredited by the American Asso
Mouse body weight was measured biweekly. Fasting blood glucose levels were assessed every two weeks following a 6-hour fast. Blood samples were obtained from the tail vein using a small needle prick and analyzed with a blood glucose monitoring system.
GI motilities were assessed in overnight-fasted mice by measuring total GI transit time (TGITT) and colonic transit time (CTT)[17]. For TGITT, mice received 0.1 mL of a semiliquid solution containing 5% Evans blue dye in 0.9% NaCl with 0.5% methylcellulose via oral gavage. The time from gavage to the appearance of the first blue fecal pellet was recorded, representing the dye’s passage through the entire GI tract. For CTT, a 3 mm glass bead was gently inserted into the colon approximately 3 cm from the anus using a lubricated disposable Pasteur pipette. CTT was defined as the time elapsed from bead insertion to its expulsion, reflecting colonic motility.
To assess defecation patterns, fecal pellets were collected weekly from each mouse. During collection, an alpha pad (LBS Biotechnology; London, United Kingdom) replaced standard corn cob bedding to facilitate retrieval of fresh fecal matter. Over a 24-hour period, all fecal pellets were collected, weighed, and counted. Fecal pellet output was determined by multiplying the total pellet count by their average weight (g), providing a quantitative measure of defecation over 24 hours.
Mouse proximal colonic tissue and human colonic tissue were analyzed using whole-mount or frozen section staining techniques. Tissues were incubated at 4 °C with anti-KIT antibody (R&D Systems; MN, United States) for 48 hours, followed by a 2-hour incubation with a secondary antibody at room temperature. After washing with 1x TBS, samples were mounted with Fluoroshield mounting medium containing DAPI (Abcam; Cambridge, United Kingdom). Images were acquired using Fluoview FV10-ASW 3.1 Viewer software (Olympus; Tokyo, Japan) with an Olympus FV1000 con
The miR-10a-5p and miR-10b-5p fragments were amplified by RT-qPCR and purified using the QIAquick® PCR Puri
Small RNAs were isolated using the mirVana™ miRNA Isolation Kit (Thermo Fisher Scientific; MA, United States) following the manufacturer's instructions. RNA quality and quantity were assessed using a Nanodrop 2000 Spectrophotometer (Thermo Fisher Scientific; MA, United States). Polyadenylation of RNAs was performed using Poly (A) Polyme
| Name | Sequences (5' to 3') | Sense | Product (bp) | Spices | Application |
| miRTQ | CGAATTCTAGAGCTCGAGGCAGGCG | Reverse | cDNA | ||
| miRTQ-rev | CGAATTCTAGAGCTCGAGGCAGG | Reverse | qPCR | ||
| miR-10a | TACCCTGTAGATCCGAATTTG | Forward | 123 | Mouse/human | qPCR |
| miR-10b | TACCCTGTAGAACCGAATTTG | Forward | 123 | Mouse/human | qPCR |
| Snord55 | GGTAATGCTGCATACTCCCGAG | Forward | Human | qPCR | |
| Snord66 | CTGAGACCACATGATGGGATTG | Forward | Mouse | qPCR | |
| M13 | CAGGAAACAGCTATGAC | Forward | 289 | Cloning | |
| M13-rev | ACTGGCCGTCGTTTTAC | Reverse | 289 | Cloning |
The cloned miR-10a-5p and miR-10b-5p PCR products were purified, eluted, and sequenced using M13 forward and reverse primers at the Nevada Genomic Center. The resulting chromatograms were analyzed to confirm the DNA sequences of miR-10a-5p and miR-10b-5p in the cloned PCR products.
Chemically synthesized miR-10a-5p and miR-10b-5p mimics (Thermo Fisher Scientific, MA, United States) were com
| Name | Sequence (5' to 3') | Sense | Spices | Application |
| miR-10a mimic | UACCCUGUAGAUCCGAAUUUGUG | Sense | Mouse/human | In vivo |
| TTAUGGGACAUCUAGGCUUAAAC | Antisense | |||
| miR-10b mimic | UACCCUGUAGAACCGAAUUUGUG | Sense | Mouse/human | In vivo |
| TTAUGGGACAUCUUGGCUUAAAC | Antisense |
Human plasma samples were obtained from 26 healthy volunteers (aged 21-80 years) at Wonkwang University Hospital, South Korea. Colon tissue samples, specifically marginal tissue distant from cancer lesions, were collected from patients undergoing colonic resection for neoplasms at the Wonkwang University Hospital Biobank. Informed consent was obtained from all participants, and the study protocol was approved by the Wonkwang University Institutional Review Board.
Proteins were extracted from mouse and human colon tissues, and Western blotting was conducted as previously described[15]. Primary antibodies used included anti-KIT (goat, 1:100, R&D Systems; MN, United States) and anti-GAPDH (Rabbit, 1:2500, Cell signaling; MA, United States). Blot images were acquired using the Vilber Fusion Solo.6S EDGE V0.70 imaging system (Vilber; Paris, France), and band quantification was conducted using ImageJ software.
Experimental data are presented as mean ± SEM. Intergroup comparisons were conducted using one-way analysis of variance with appropriate corrections for multiple comparisons. Data analysis was performed using GraphPad Prism (version 8.0, GraphPad Software). Statistical significance was defined as P values less than 0.05 for all tests.
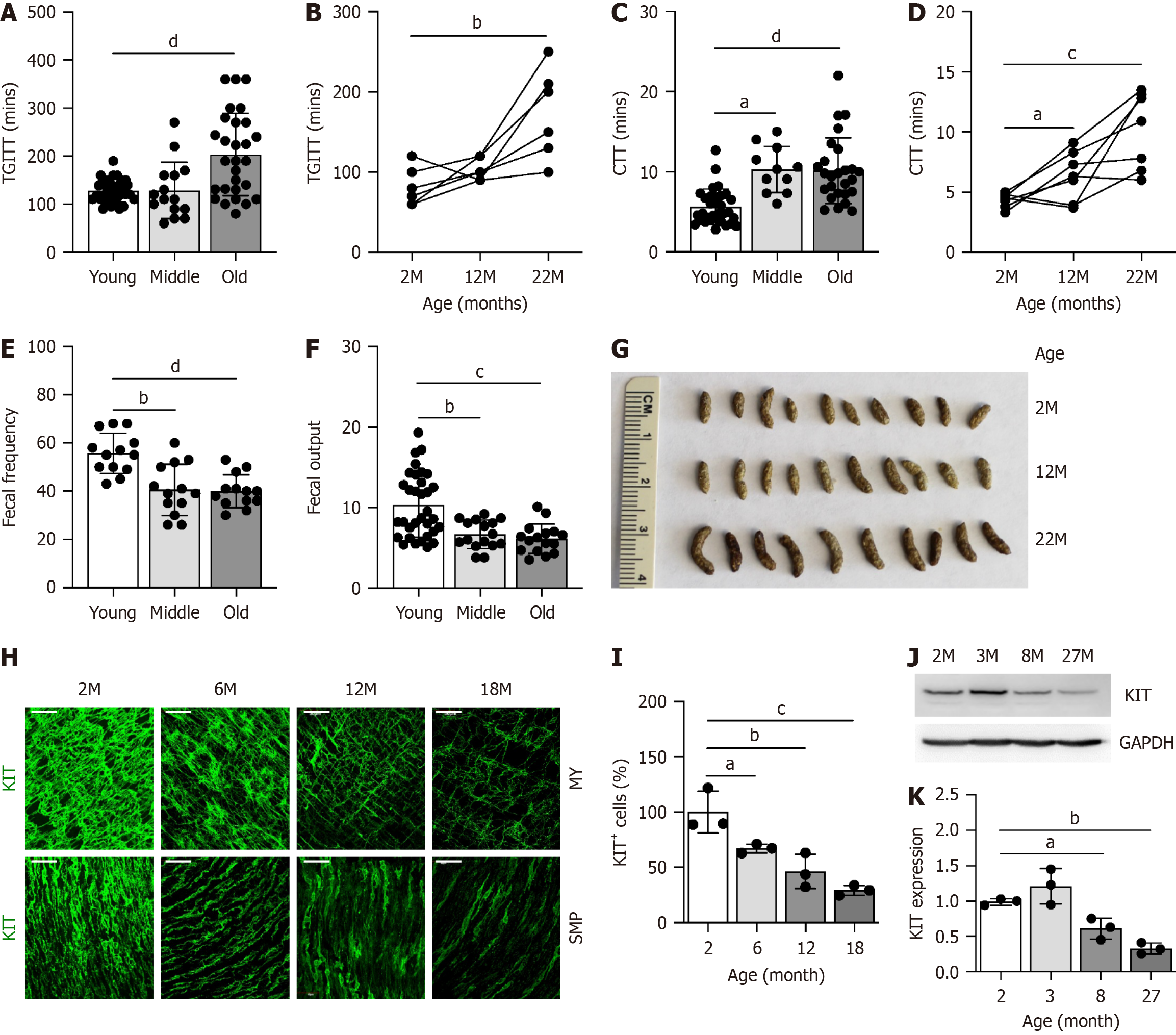
To examine the impact of aging on GI function, we assessed GI motility and analyzed fecal pellets in three age groups: Young (2-6 months), middle-aged (8-14 months), and old (18-30 months) mice (Table 3). TGITT was significantly delayed in old mice compared to young mice, both overall (Figure 1A) and at the individual level (Figure 1B). Similarly, CTT was prolonged in middle-aged mice and further delayed in old mice (Figure 1C and D). Fecal analyses revealed reduced fecal frequency and output in middle-aged and old mice compared to their young counterparts (Figure 1E and F). These changes were accompanied by an increase in fecal pellet size in the middle-aged and old groups, a hallmark of age-associated constipation (Figure 1G). We also examined ICC, which play a critical role in GI motility[18,19]. The expression of KIT, a key marker for ICC[20] and their function[21], showed an age-related decline. ICC subpopulations in the proximal colon, specifically ICC-MY and ICC-SMP, were fully mature in young mice at 2 months but progressively diminished with age (Figure 1H and I). Furthermore, KIT protein levels were significantly reduced in the proximal colonic muscle tissue of middle- and old-aged mice (Figure 1J and K). These findings suggest that the decline in ICC with aging contributes to colonic dysmotility, particularly constipation, in aged mice.
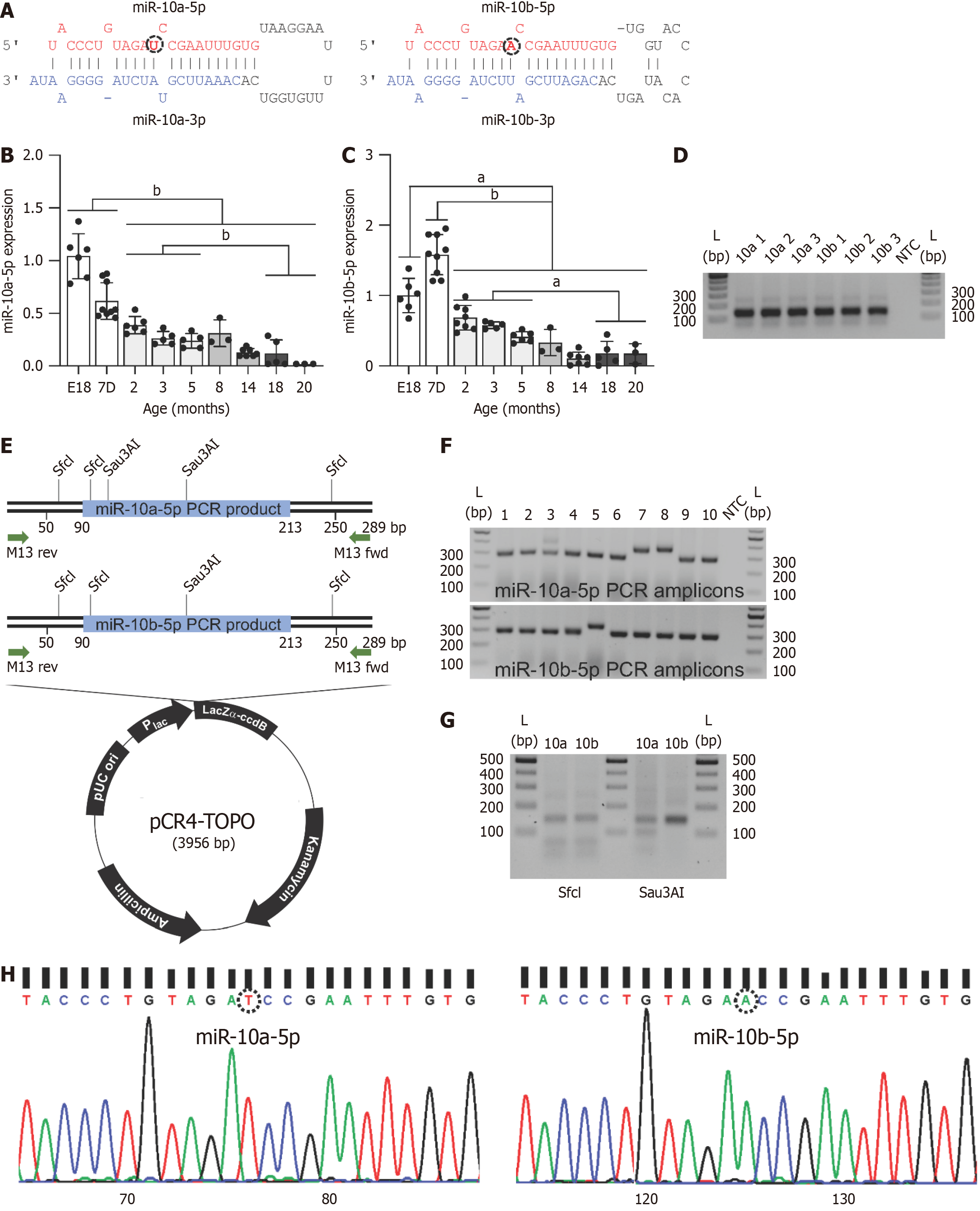
Our previous study demonstrated that miR-10b-5p deficiency in ICC leads to reduced KIT expression and subsequent GI dysmotility[15]. Notably, miR-10b-5p is abundantly co-expressed with its family member, miR-10a-5p, making them the most highly expressed miRNAs in ICC[15]. miR-10a-5p and miR-10b-5p (collectively referred to as miR-10a/b-5p) belong to the same family but are derived from two distinct precursors and differ by a single nucleotide in their central region (Figure 2A). To investigate age-related changes in miR-10a/b-5p expression, their levels were quantified by RT-qPCR in mice from embryonic day 18 (E18) to 20 months. miR-10a-5p exhibited high expression at E18, followed by a gradual decline with age (Figure 2B). Similarly, miR-10b-5p displayed comparable levels at E18, peaked at 7 days postpartum (7D), and subsequently decreased over time (Figure 2C). These findings suggest that miR-10a/b-5p levels decrease with aging in mice.
To validate the specificity of our RT-qPCR assays for miR-10a-5p and miR-10b-5p, the amplicons were analyzed on an agarose gel, revealing single bands of approximately 120 bp (Figure 2D). These bands were eluted, cloned into the pCR4-TOPO vector, and amplified using M13 forward and reverse primers (Figure 2E). Clones of the expected size (289 bp) were selected for further analysis (Figure 2F). Digestion of the PCR products with restriction enzymes confirmed the specificity of the assay. Both miR-10a-5p and miR-10b-5p amplicons were digested by SfcI, which recognizes a shared cleavage site, producing fragments of 30 bp, 40 bp, 65 bp, and 154 bp (Figure 2E and G). However, Sau3AI digestion yielded distinct fragment patterns: miR-10a-5p produced fragments of 50 bp, 99 bp, and 140 bp, whereas miR-10b-5p produced fragments of 140 bp and 149 bp (Figure 2E and G). Finally, sequencing of the PCR products confirmed that the RT-qPCR selectively amplified miR-10a-5p and miR-10b-5p, further validating the assay's specificity (Figure 2H).
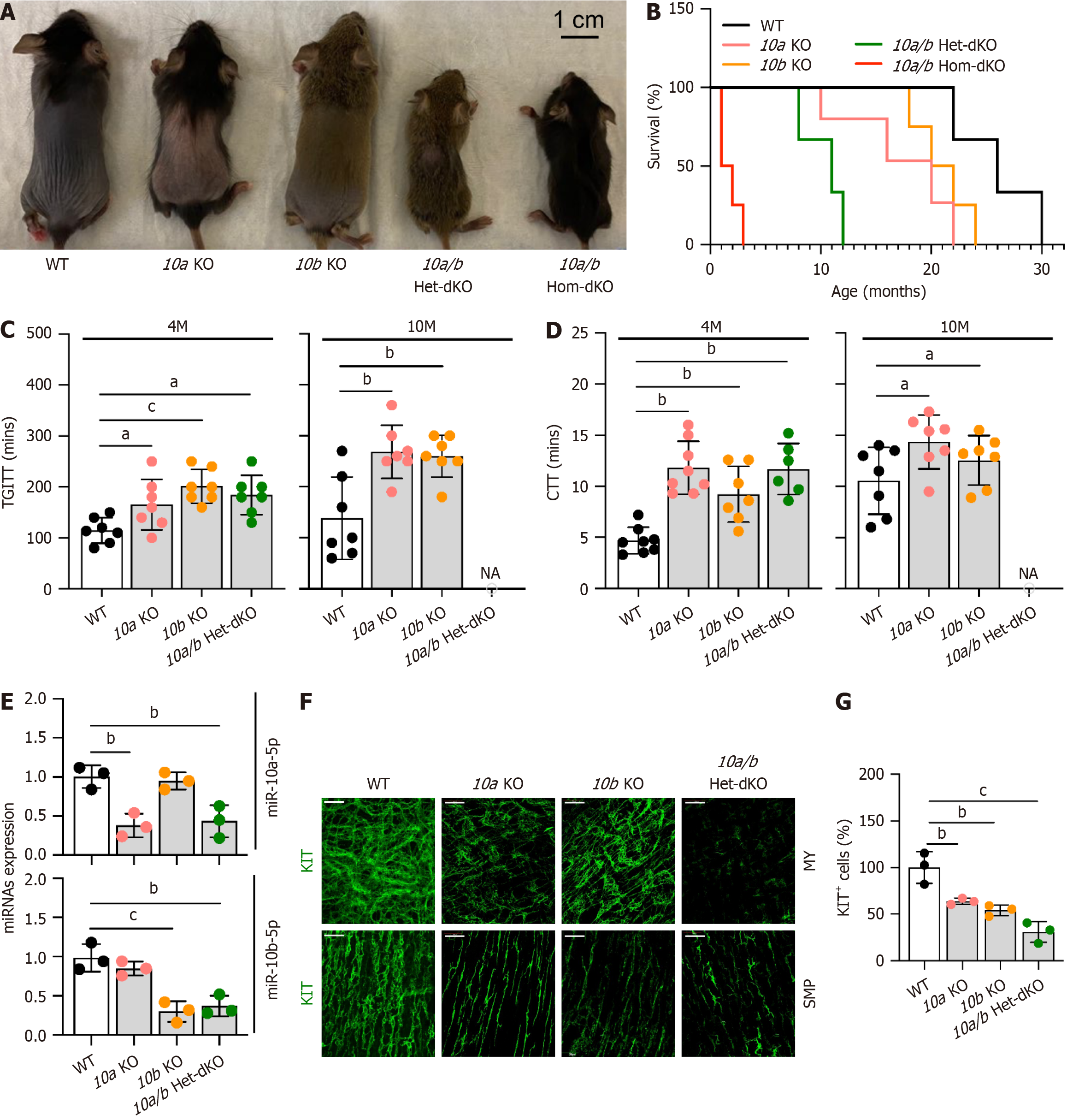
To examine whether miR-10a-5p and/or miR-10b-5p deficiency impacts the GI phenotype in mice, we generated mir-10b KO mice[17], obtained mir-10a KO mice[16], and created double mir-10a/b KO mice. Gross anatomical images of 1-month-old mir-10a/b single and double KO and wild-type (WT) mice are shown in Figure 3A. The mir-10b single KO mice were similar in size to the WT mice. In contrast, the mir-10a single KO mice were noticeably smaller. Notably, the mir-10a/b heterozygous and homozygous double mice were significantly smaller than either the mir-10a or mir-10b single KO mice (Figure 3A). The survival rates of the mir-10a/b single and double KO mice highlighted the lethal effects of dual gene deletion (Figure 3B). While mir-10a and mir-10b single KO mice survived up to 22 and 24 months, respectively, their WT counterparts lived up to 30 months. However, the mir-10a/b heterozygous and homozygous double KO mice showed drastically reduced lifespans, surviving up to 12 months and 3 months, respectively.
The mir-10a/b single and double KO mice displayed marked GI dysmotility, as evidenced by significantly delayed TGITT and CTT at 4 months, which became further prolonged by 10 months (Figure 3C and D). We confirmed a signi
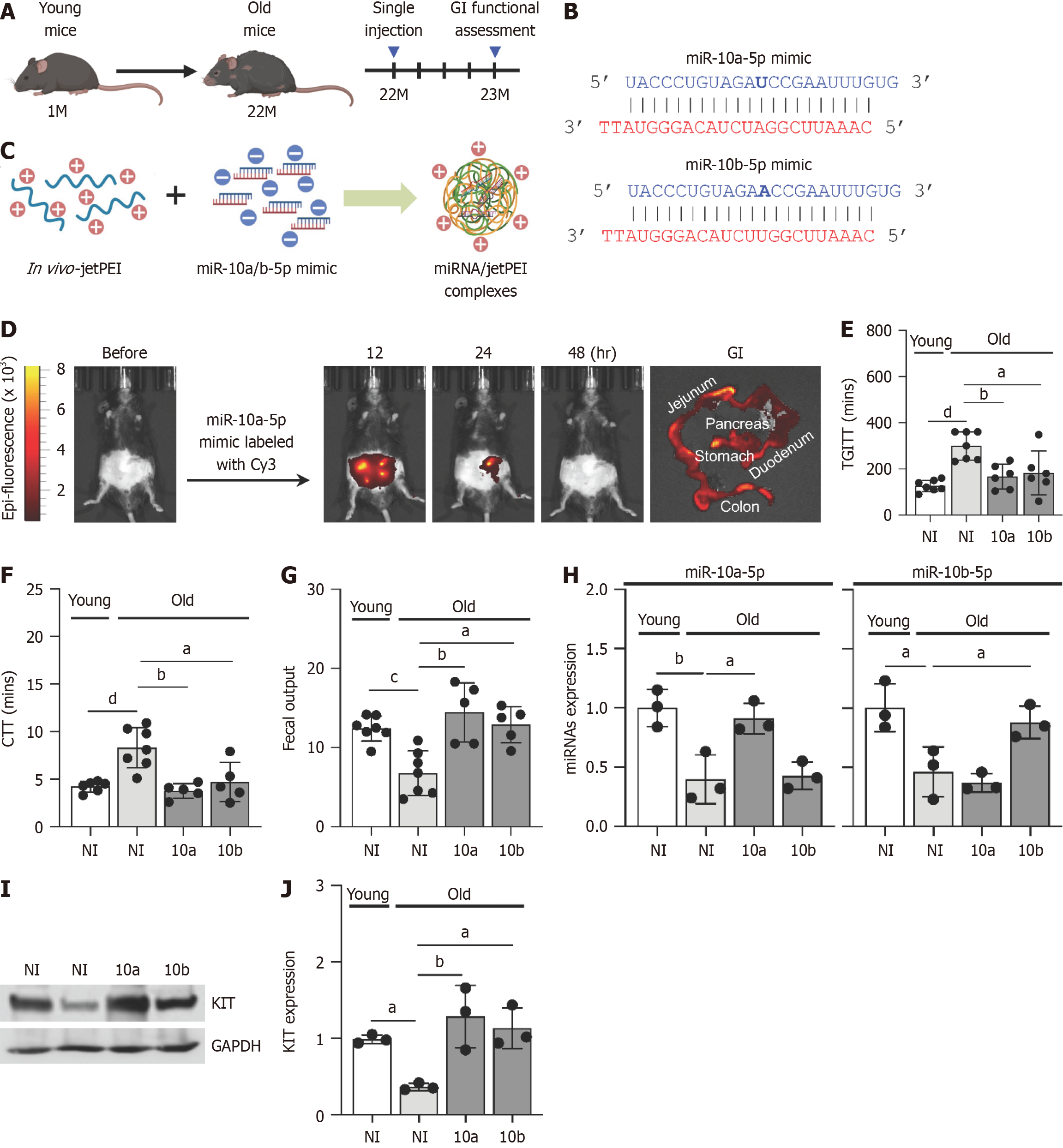
To assess whether miR-10a-5p and miR-10b-5p mimics could restore delayed GI motility in aged mice, we injected miR-10a-5p or miR-10b-5p mimics into 22-month-old mice with GI dysmotility (Figure 4A). These mimics, structured as RNA duplexes with two TT overhangs, replicate endogenous miR-10a-5p and miR-10b-5p, enhancing their stability and preferential strand selection (Figure 4B). The negatively charged miRNA duplexes were encapsulated with positively charged polymers using in vivo-jetPEI, forming miRNA/jetPEI complexes (Figure 4C).
To verify effective delivery, the miR-10a-5p mimic labeled with Cy3 was administered to mice. The mimic was successfully delivered to multiple organs, including the stomach, small intestine, colon, and pancreas. Peak detection occurred at 12 hours post-injection, decreased noticeably by 24 hours, and was undetectable at 48 hours (Figure 4D).
Both miR-10a-5p mimic and miR-10b-5p mimic effectively alleviated GI dysmotility in aged mice after 4 weeks post-single injection, as evidenced by significantly improved TGITT and CTT (Figure 4E and F). Moreover, fecal output was markedly restored in aged mice to levels comparable to those of young mice following miR-10a/b-5p mimic injection (Figure 4G). We further assessed miR-10a and miR-10b levels in colonic smooth muscle in aged mice and confirmed their restoration in colonic tissues following miR-10a/b-5p mimic injection (Figure 4H). Notably, KIT expression was signifi
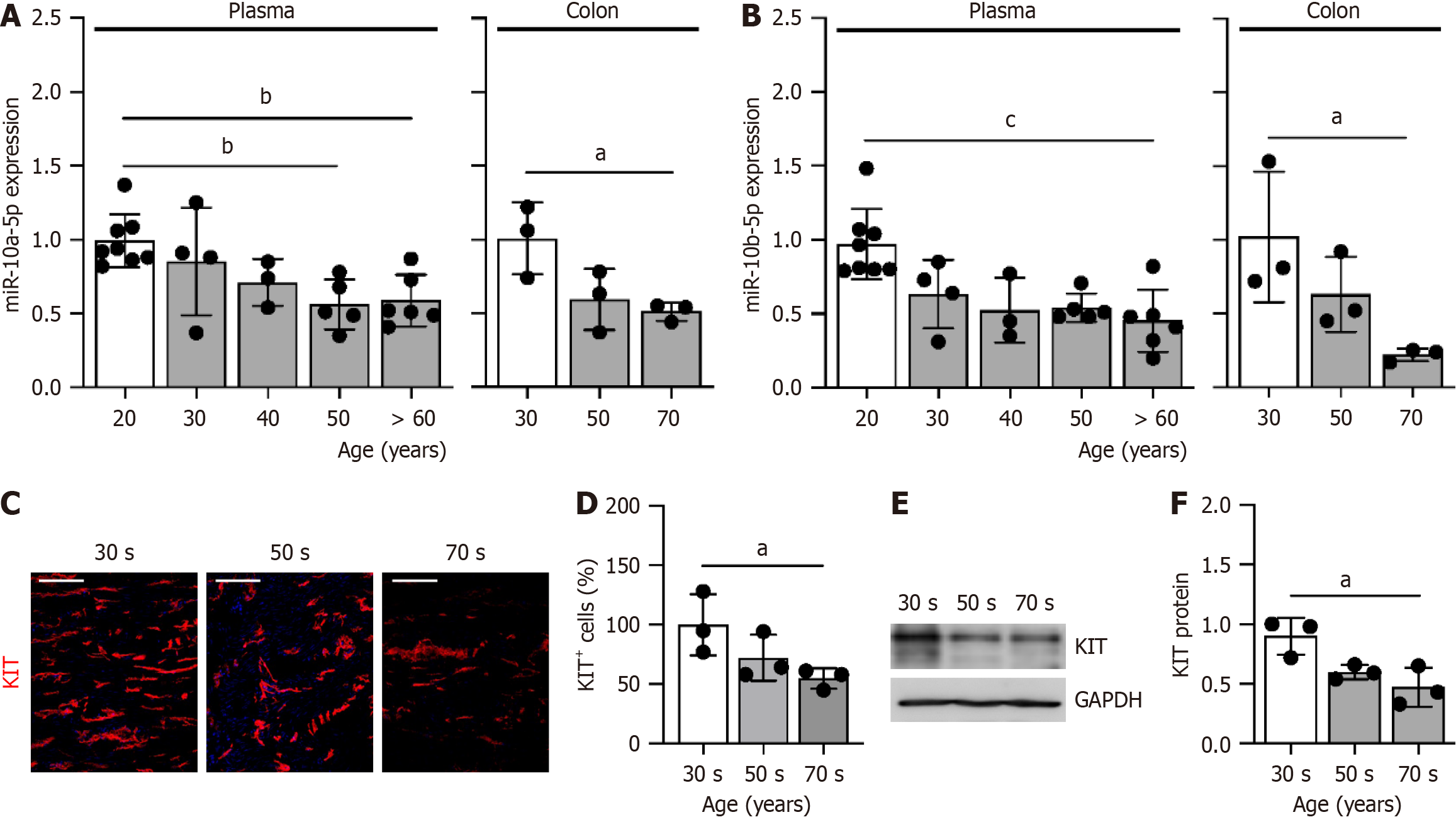
To validate the findings from the mouse models, we measured the levels of miR-10a/b-5p and KIT expression in human samples across different age groups. Both miR-10a-5p and miR-10b-5p were abundantly present in plasma and colonic tissue at 20 and 30 years of age, but their levels progressively declined in individuals aged 60 and 70 years (Figure 5A and B). Similarly, the number of ICC in the colon was significantly reduced in individuals in their 50 years, with a further decline observed in those in their 70 years, compared to those of 30 years (Figure 5C and D). Consistent with these trends, KIT protein levels also showed a marked age-related decrease in individuals in their 50 years and 70 years (Figure 5E and F). These findings collectively suggest that the age-associated decline in miR-10a/b-5p levels both in the plasma and colonic tissue contributes to the reduced expression of KIT, which may underlie the loss of ICC in the colon.
GI dysmotility, such as constipation, is associated with a decline in ICC in the colon of aged humans[8]; however, the precise molecular mechanisms mediated by miRNAs underlying ICC loss with aging remain unclear. This study reveals that miR-10a-5p and miR-10b-5p are crucial regulators of ICC growth and function, playing essential roles in maintaining GI motility in both aged mice and humans. Restoration of these miRNAs through miR-10a-5p and miR-10b-5p mimics in aged mice effectively alleviated GI dysmotility, particularly constipation, offering a promising therapeutic strategy for age-related GI motility disorders.
We confirmed the critical roles of miR-10a-5p and miR-10b-5p in regulating GI phenotypes using mir-10a/b single and double KO mice. miRNAs often exhibit functional redundancy in targeting mRNAs, as multiple miRNAs can regulate the same mRNA[22]. As a result, single miRNA knockouts typically do not produce pronounced phenotypes[23]. Addi
GI dysmotility observed in mir-10a/b single and double KO mice correlates with the high expression levels of these miRNAs in jejunal and colonic ICC[15]. Notably, mir-10a KO mice exhibited a more severe phenotype than mir-10b KO mice, consistent with the higher expression levels of miR-10a in both jejunal and colonic ICC[15]. miR-10a-5p is the most abundantly expressed miRNA in jejunal ICC and the third most highly expressed in colonic ICC, with its levels being twice as high in jejunal ICC compared to colonic ICC[15]. While colonic dysmotility is directly associated with constipa
GI motility is primarily regulated by ICC[28]. Dysfunction or loss of ICC in the GI tract has been associated with constipation in both animal models and humans[4,15]. Additionally, ICC decline with aging has been observed in mice and humans[29,30]. We previously demonstrated that miR-10b-5p is essential for ICC growth and function[15]. This miRNA enhances ICC growth and functionality by targeting KLF11, a transcriptional repressor of the KIT gene, thereby relieving KLF11-mediated suppression and enhancing KIT expression[15]. Notably, the miR-10b-5p target site in KLF11 is identical in mice and humans to that of miR-10a-5p[15], indicating that both miRNAs regulate KIT expression in ICC via KLF11.
miR-10a-5p and miR-10b-5p mimics significantly improved GI dysmotility in aged mice. Our previous studies demonstrated that these mimics effectively alleviated dysmotility symptoms, including delayed gastric emptying, TGITT, and CTT, in high-fat and high-sucrose-induced diabetic mice, as well as in mir-10b global and ICC-specific KO mice[15]. This study further validated that miR-10a-5p and miR-10b-5p mimics, encapsulated using the positively charged polymer jetPEI, were efficiently delivered to the stomach, small intestine, and colon. A growing number of miRNA-based therapeutics have entered clinical development to treat various diseases, including cancer[31]. The field of miRNA the
Consistent with findings in mice, miR-10a/b-5p levels, KIT expression, and the number of ICC in the colon decline with age in humans. Previously, we observed a significant reduction in miR-10b-5p levels in patients with constipation-predo
The authors would like to thank Benjamin J. Weigler, DVM, and Walt Mandeville, DVM, for their professional animal services provided to mice.
| 1. | Stillhart C, Asteriadis A, Bocharova E, Eksteen G, Harder F, Kusch J, Tzakri T, Augustijns P, Matthys C, Vertzoni M, Weitschies W, Reppas C. The impact of advanced age on gastrointestinal characteristics that are relevant to oral drug absorption: An AGePOP review. Eur J Pharm Sci. 2023;187:106452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 2. | Schuster BG, Kosar L, Kamrul R. Constipation in older adults: stepwise approach to keep things moving. Can Fam Physician. 2015;61:152-158. [PubMed] |
| 3. | Dumic I, Nordin T, Jecmenica M, Stojkovic Lalosevic M, Milosavljevic T, Milovanovic T. Gastrointestinal Tract Disorders in Older Age. Can J Gastroenterol Hepatol. 2019;2019:6757524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 4. | Huizinga JD, Hussain A, Chen JH. Interstitial cells of Cajal and human colon motility in health and disease. Am J Physiol Gastrointest Liver Physiol. 2021;321:G552-G575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 5. | Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev. 2014;94:859-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 357] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 6. | Gomez-Pinilla PJ, Gibbons SJ, Sarr MG, Kendrick ML, Shen KR, Cima RR, Dozois EJ, Larson DW, Ordog T, Pozo MJ, Farrugia G. Changes in interstitial cells of cajal with age in the human stomach and colon. Neurogastroenterol Motil. 2011;23:36-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Sun T, Li D, Hu S, Huang L, Sun H, Yang S, Wu B, Ji F, Zhou D. Aging-dependent decrease in the numbers of enteric neurons, interstitial cells of Cajal and expression of connexin43 in various regions of gastrointestinal tract. Aging (Albany NY). 2018;10:3851-3865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | He CL, Burgart L, Wang L, Pemberton J, Young-Fadok T, Szurszewski J, Farrugia G. Decreased interstitial cell of cajal volume in patients with slow-transit constipation. Gastroenterology. 2000;118:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 286] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 9. | Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 447] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 10. | Friedmacher F, Rolle U. Interstitial cells of Cajal: clinical relevance in pediatric gastrointestinal motility disorders. Pediatr Surg Int. 2023;39:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 11. | Torihashi S, Nishi K, Tokutomi Y, Nishi T, Ward S, Sanders KM. Blockade of kit signaling induces transdifferentiation of interstitial cells of cajal to a smooth muscle phenotype. Gastroenterology. 1999;117:140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 227] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122:6-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1002] [Cited by in RCA: 1091] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 13. | Shang R, Lee S, Senavirathne G, Lai EC. microRNAs in action: biogenesis, function and regulation. Nat Rev Genet. 2023;24:816-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 390] [Article Influence: 195.0] [Reference Citation Analysis (0)] |
| 14. | Kozomara A, Birgaoanu M, Griffiths-Jones S. miRBase: from microRNA sequences to function. Nucleic Acids Res. 2019;47:D155-D162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1834] [Cited by in RCA: 2980] [Article Influence: 596.0] [Reference Citation Analysis (0)] |
| 15. | Singh R, Ha SE, Wei L, Jin B, Zogg H, Poudrier SM, Jorgensen BG, Park C, Ronkon CF, Bartlett A, Cho S, Morales A, Chung YH, Lee MY, Park JK, Gottfried-Blackmore A, Nguyen L, Sanders KM, Ro S. miR-10b-5p Rescues Diabetes and Gastrointestinal Dysmotility. Gastroenterology. 2021;160:1662-1678.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 16. | Stadthagen G, Tehler D, Høyland-Kroghsbo NM, Wen J, Krogh A, Jensen KT, Santoni-Rugiu E, Engelholm LH, Lund AH. Loss of miR-10a activates lpo and collaborates with activated Wnt signaling in inducing intestinal neoplasia in female mice. PLoS Genet. 2013;9:e1003913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Zogg H, Singh R, Ha SE, Wang Z, Jin B, Ha M, Dafinone M, Batalon T, Hoberg N, Poudrier S, Nguyen L, Yan W, Layden BT, Dugas LR, Sanders KM, Ro S. miR-10b-5p rescues leaky gut linked with gastrointestinal dysmotility and diabetes. United European Gastroenterol J. 2023;11:750-766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Hwang SJ, Kwon JG, Beckett EAH, Kim M, Herbert T, Sanders KM, Ward SM. Functional roles of interstitial cells of Cajal in the GI tract of rats. Am J Physiol Gastrointest Liver Physiol. 2025;328:G677-G695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Chen H, Ordög T, Chen J, Young DL, Bardsley MR, Redelman D, Ward SM, Sanders KM. Differential gene expression in functional classes of interstitial cells of Cajal in murine small intestine. Physiol Genomics. 2007;31:492-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Zhou J, O'Connor MD, Ho V. The Potential for Gut Organoid Derived Interstitial Cells of Cajal in Replacement Therapy. Int J Mol Sci. 2017;18:2059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Torihashi S, Ward SM, Nishikawa S, Nishi K, Kobayashi S, Sanders KM. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995;280:97-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 139] [Article Influence: 4.6] [Reference Citation Analysis (2)] |
| 22. | Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25833] [Cited by in RCA: 27840] [Article Influence: 1325.7] [Reference Citation Analysis (0)] |
| 23. | Vidigal JA, Ventura A. The biological functions of miRNAs: lessons from in vivo studies. Trends Cell Biol. 2015;25:137-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 398] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 24. | Gurtan AM, Sharp PA. The role of miRNAs in regulating gene expression networks. J Mol Biol. 2013;425:3582-3600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 334] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 25. | Dinning PG, Di Lorenzo C. Colonic dysmotility in constipation. Best Pract Res Clin Gastroenterol. 2011;25:89-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Jackman L, Arpe L, Thapar N, Rybak A, Borrelli O. Nutritional Management of Pediatric Gastrointestinal Motility Disorders. Nutrients. 2024;16:2955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Bildstein T, Charbit-Henrion F, Azabdaftari A, Cerf-Bensussan N, Uhlig HH. Cellular and molecular basis of proximal small intestine disorders. Nat Rev Gastroenterol Hepatol. 2024;21:687-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility--insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol. 2012;9:633-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 312] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 29. | Taheri N, Choi EL, Nguyen VTT, Zhang Y, Huynh NM, Kellogg TA, van Wijnen AJ, Ordog T, Hayashi Y. Inhibition of EZH2 Reduces Aging-Related Decline in Interstitial Cells of Cajal of the Mouse Stomach. Cell Mol Gastroenterol Hepatol. 2024;18:101376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Patejdl R. Gastrointestinal Motility Function and Dysfunction in the Elderly Patient: What Are the Effects of Aging? Visc Med. 2024;40:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 31. | Seyhan AA. Trials and Tribulations of MicroRNA Therapeutics. Int J Mol Sci. 2024;25:1469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 129] [Reference Citation Analysis (0)] |
| 32. | Yang W, Chen L, Xu L, Bilotta AJ, Yao S, Liu Z, Cong Y. MicroRNA-10a Negatively Regulates CD4(+) T Cell IL-10 Production through Suppression of Blimp1. J Immunol. 2021;207:985-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |