Published online Jan 7, 2023. doi: 10.3748/wjg.v29.i1.171
Peer-review started: September 7, 2022
First decision: October 19, 2022
Revised: November 1, 2022
Accepted: December 5, 2022
Article in press: December 5, 2022
Published online: January 7, 2023
Processing time: 118 Days and 22.4 Hours
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a severe threat to human health. Polygonum multiflorum (PM) has been proven to remedy mitochondria and relieve MAFLD, but the main pharmacodynamic ingredients for mitigating MAFLD remain unclear.
To research the active ingredients of PM adjusting mitochondria to relieve high-fat diet (HFD)-induced MAFLD in rats.
Fat emulsion-induced L02 adipocyte model and HFD-induced MAFLD rat model were used to investigate the anti-MAFLD ability of PM and explore their action mechanisms. The adipocyte model was also applied to evaluate the activities of PM-derived constituents in liver mitochondria from HFD-fed rats (mitochondrial pharmacology). PM-derived constituents in liver mitochondria were confirmed by ultra-high-performance liquid chromatography/mass spectrometry (mitocho
PM repaired mitochondrial ultrastructure and prevented oxidative stress and energy production disorder of liver mitochondria to mitigate fat emulsion-induced cellular steatosis and HFD-induced MAFLD. PM-derived constituents that entered the liver mitochondria inhibited oxidative stress damage and improved energy production against cellular steatosis. Eight chemicals were found in the liver mitochondria of PM-administrated rats. The anti-steatosis ability of one monomer and the anti-MAFLD activity of the monomer group were validated.
PM restored mitochondrial structure and function and alleviated MAFLD, which may be associated with the remedy of oxidative stress and energy production. The identified eight chemicals may be the main bioactive ingredients in PM that adjusted mitochondria to prevent MAFLD. Thus, PM provides a new approach to prevent MAFLD-related mitochondrial dysfunction. Mitochondrial pharmacology and pharmacochemistry further showed efficient strategies for determining the bioactive ingredients of Chinese medicines that adjust mitochondria to prevent diseases.
Core Tip: We found that Polygonum multiflorum (PM) protected the mitochondrial ultrastructure and prevented oxidative stress and energy production disorder in the liver mitochondria to mitigate metabolic dysfunction-associated fatty liver disease (MAFLD). Eight chemicals were identified from the liver mitochondria of the PM-treated rats using a novel strategy based on mitochondrial pharmacology and pharmacochemistry. The constituents identified regulated mitochondria to alleviate MAFLD. Our results indicate that PM restored mitochondrial structure and function and alleviated MAFLD, which may be related to oxidative stress and energy production. The eight substances may be the main pharmacodynamic ingredients in PM that regulate mitochondria to prevent MAFLD.
- Citation: Yu LP, Li YJ, Wang T, Tao YX, Zhang M, Gu W, Yu J, Yang XX. In vivo recognition of bioactive substances of Polygonum multiflorum for protecting mitochondria against metabolic dysfunction-associated fatty liver disease. World J Gastroenterol 2023; 29(1): 171-189
- URL: https://www.wjgnet.com/1007-9327/full/v29/i1/171.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i1.171
Metabolic dysfunction-associated fatty liver disease (MAFLD) was considered a better definition of nonalcoholic fatty liver disease. In addition to causing cirrhosis and hepatocellular carcinoma, MAFLD is also closely related to the occurrence of a range of metabolic diseases. MAFLD affects the health of 30% of adults worldwide and represents a heavy clinical and economic burden on patients and society[1]. To date, no pharmacotherapy targeting MAFLD is available, except for preventative lifestyle improvements and physical exercise to lose weight[2,3]. Thus, it is urgent to find a cure for this complex liver disease[4].
The study found that mitochondrial dysfunction plays a significant role in the development of MAFLD. The oxidative damage of mitochondrial fatty acids and the decline of mitochondrial quality are considered to be extremely important motivations in the development of MAFLD[5]. Thus, modulation of mitochondrial function may be an effective strategy to alleviate MAFLD.
Polygonum multiflorum (PM) has been used as a traditional medicine for many centuries in China. Its main chemical components include anthraquinones, flavonoids, phenols, and phospholipids. Recent studies have shown that PM has many pharmacological activities, including hepatoprotective, antihyperlipidemic, anti-inflammatory, antioxidant, anticancer, immunomodulatory, and neuroprotective effects[6]. PM extracts can remedy mitochondrial dysfunction in the liver to alleviate MAFLD, such as the promotion of β-oxidation of fatty acids and the activity of carnitine palmitoyltransferase 1[7,8]. In addition, PM regulated lipid metabolism by reducing very low-density lipoprotein and diacylglycerol acyltransferase levels, downregulating hydroxyl methyl-glutaryl coenzyme A reductase, and upregulating cholesterol 7-alpha-hydroxylase or cytochrome P450 7A[9]. Although PM shows treatment efficacy against MAFLD through regulating mitochondria, the bioactive substances involved are still unclear, which acutely limits the development and utilization of PM.
In our previous study, a new strategy of mitochondrial pharmacology and pharmacochemistry for the investigation of pharmacodynamic substances in traditional Chinese medicines (TCMs) was proposed. Using this strategy, the main active ingredients in PM that adjust mitochondria to relieve MAFLD were identified. The results indicated that this strategy could be applied for the efficient search for in vivo mitochondria-regulated constituents in TCMs[10].
Here, we investigated the bioactive substances of PM extracts remedying mitochondria to alleviate MAFLD induced by a high-fat diet (HFD) through mitochondrial pharmacology and pharmacochemistry, after determining the efficacy and potential principle of PM on MAFLD and mitochondria in vitro and in vivo pharmacodynamic tests. The results showed that PM relieved HFD-induced MAFLD by improving oxidative stress and energy production. The five substances recognized by the proposed method may be the main active ingredients in PM that adjust mitochondria to relieve MAFLD. Thus, PM represents a promising drug for the treatment of MAFLD-related mitochondrial dysfunction to relieve MAFLD. Additionally, mitochondrial pharmacology and pharmacochemistry were efficient strategies for identifying the bioactive ingredients of TCMs regulating mitochondria to prevent human disorders.
Standards including resveratrol [(RES), ≥ 98%], emodin [(ED), ≥ 98%], aloe-emodin [(AE), ≥ 98%], emodin 8-O-glucoside [(EG), ≥ 98%], 2,3,5,4’-tetrahydroxy stilbene 2-Ο-β-D-glucoside [(THSG), ≥ 98%], and physcion [(PN), ≥ 98%] were obtained from Chengdu Pufei De Biotech Co., Ltd. (Chengdu, China). Torachrysone [(TCS), ≥ 98%] was purchased from Yunnan Xili biology science and technology co., ltd (Kunming, China). The dried root of PM was collected from the Luoshiwan Chinese Medicine Market (Kunming, China). Dried PM decoction pieces were verified by Professor Jie Yu. PM credential specimens (No. 19080322) were stored in the Key Laboratory of Southern Medicine Utilization, Yunnan University of Chinese Medicine (Kunming, China). The other chemicals and reagents used were described in our previous report[10].
After PM was processed according to the method specified in the 2020 Chinese Pharmacopoeia and decocted with 10 times the amount of 90% ethanol for 110 min. The filtrate was collected after filtration. Dregs were decocted with 10 times 50% ethanol for 110 min and filtered. Then, two filtrates were mixed and condensed at 65 °C. Finally, the concentrate was freeze-dried into a powder.
The chemical characteristics of PM extract were assayed according to the regulation described in the 2020 Chinese Pharmacopoeia. PM was assayed by Shimadzu LC-30A ultra-high performance liquid chromatography (UHPLC). Analytical conditions were as follows: (1) Shimadzu UHPLC column (Shim-pack XR-ODS C8, 100 mm × 2.0 mm I.D., 2.2 μm); (2) Mobile phase for the separation of ED and PN: methanol and 0.1% aqueous phosphoric acid solution (80:20 v/v); (3) Mobile phase for the separation of THSG: Acetonitrile and water solution (25:75 v/v); (4) Detection wavelength: 254 nm for ED and PN; 320 nm for THSG; (5) Temperature: 30 ºC; (6) Flow rate: 0.2 mL/min; and (7) Volume: 10 μL. References of ED, PN, and THSG were dissolved in methanol to yield contents of 0.08 mg/mL, 0.04 mg/mL, and 0.2 mg/mL, respectively. For the detection of THSG and for the detection of ED and PN, 0.1 g and 0.02 g, respectively, of PM powder was dissolved in 1.0 mL of methanol. Concentrations of free anthraquinone (ED and PN) and THSG in the PM extract were 0.57% and 0.71%, respectively.
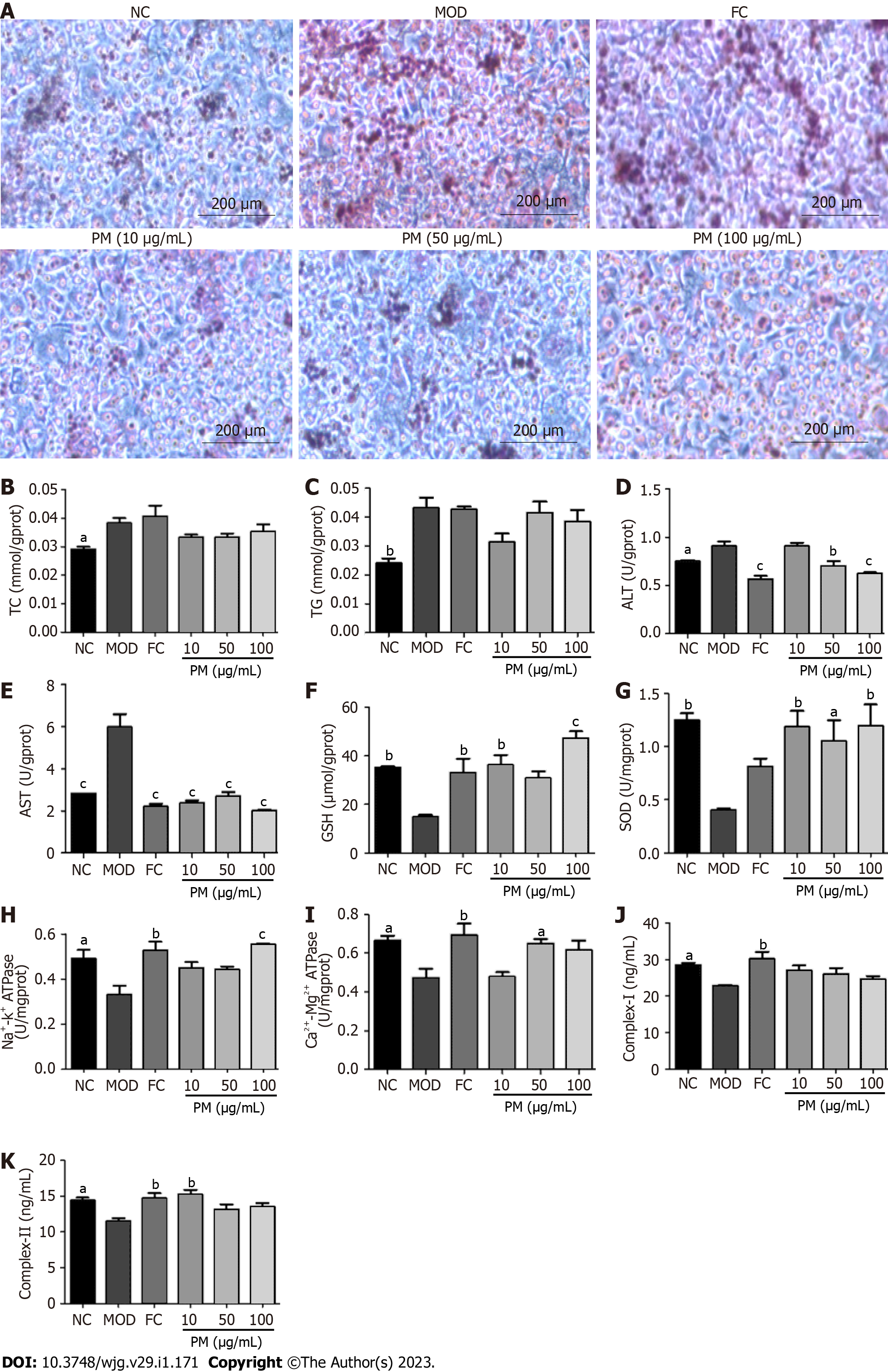
L02 cells were grown in RPMI 1640 medium containing 10% fetal bovine serum and 1% penicillin-streptomycin at 37 ºC and 5% CO2. After growing to about 80%, the cells were treated with lipid emulsion. Then, the cells were stimulated by PM extracts (10, 50, and 100 μg/mL) and fenofibrate capsules (150 μmol/L) or a media (control) for 24 h. Bicinchoninic acid (BCA) assay was used to analyze protein concentrations in cell lysates. Triglyceride (TG), total cholesterol (TC), aspartate transaminase (AST), alanine transaminase (ALT), superoxide dismutase (SOD), glutathione (GSH), ATP synthase (ATPase), complex I, and complex II levels were determined using the kits. All data were obtained by the microplate reader. Furthermore, for the detection of intracellular neutral lipid accumulation, L02 cells were seeded into 6-well chambers. The collected cells were washed twice with ice-cold phosphate-buffered saline and fixed with 2.5% paraformaldehyde for 30 min. Afterward, the cells were added with 60% isopropanol and cultured for 5 min. Then, cells were stained with 0.2% Oil red O solution for 30 min. After washing twice with phosphate-buffered saline, cells were stained with hematoxylin for 3 min. Finally, images were obtained using a CX31 Olympus Imaging System.
Experimental animals: Healthy male Sprague Dawley rats (200 ± 50 g) were provided by Dashuo Biotech. Co., Ltd. (Chengdu, China). All animals were raised in conditions of 22 ± 1 ºC, a humidity of 60% ± 10%, and a light/dark cycle of 12 h with free access to food and water. The experiments in this study were approved by the Institutional Ethical Committee on Animal Care and Experimentations of Yunnan University of Chinese Medicine (R-06201965).
Grouping and administration of treatment: After 5-7 d of adaptive feeding, the rats were randomly divided into six groups (n = 6): (1) Normal control group (NC); (2) Model group (MOD); (3) RES group (40 mg/kg); (4) Low-dose PM group (2 g/kg); (5) Middle-dose PM group (4 g/kg); and (6) High-dose PM group (8 g/kg). Rats were given the test samples by intragastric administration once a day for 3 mo. Except for the NC group, rats in all groups were fed an HFD (10% egg yolk, 10% refined lard, 79% basic diet, and 1% cholesterol) to induce MAFLD in rats for 3 mo.
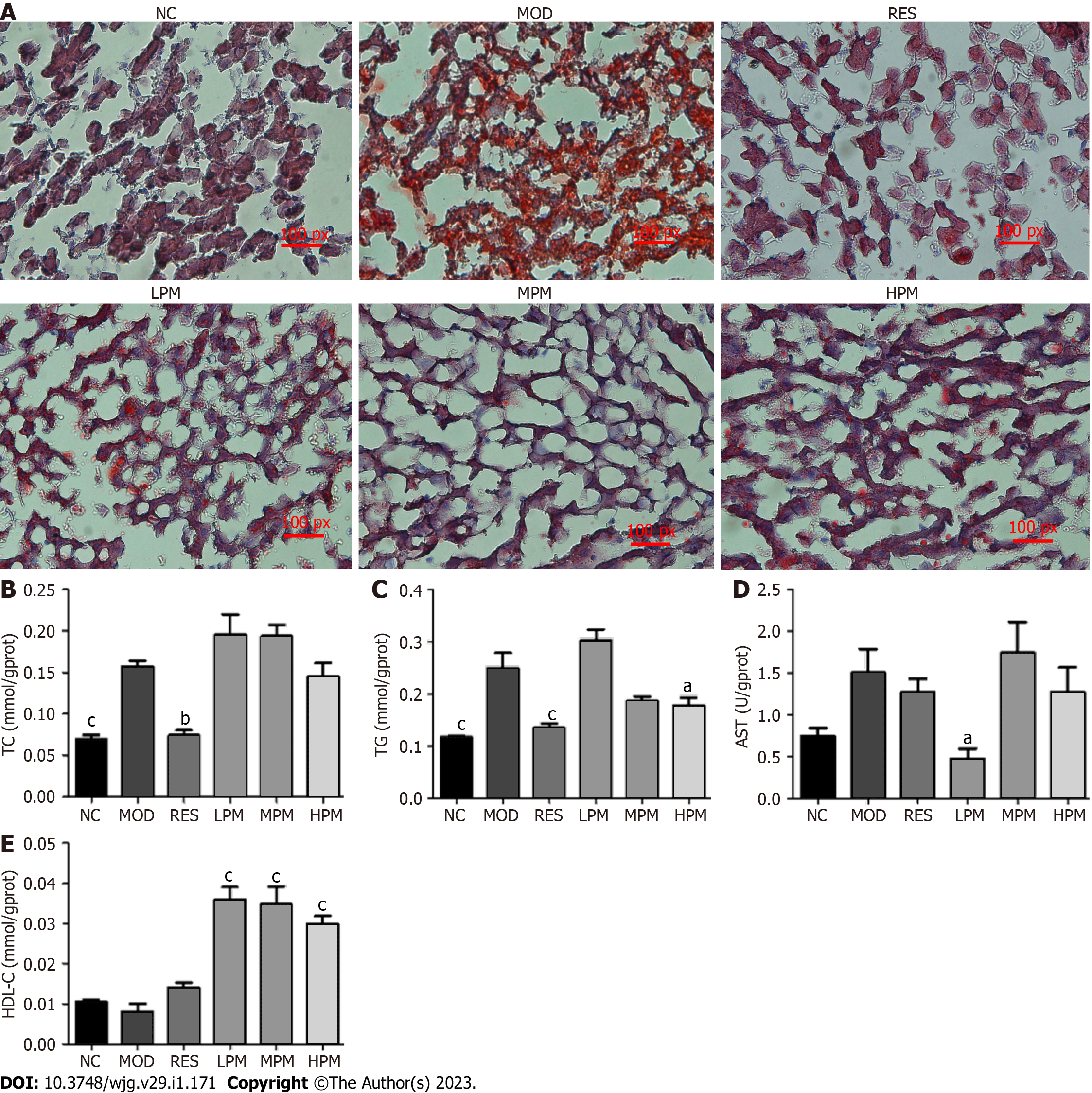
Sample collection: The daily food intake of all rats was recorded, and weight was recorded once a week. At the end of the experiment, the rats were anesthetized by intraperitoneal injection with 10% sodium pentobarbital. Blood samples were obtained from the abdominal aorta and coagulated at 4 ºC. Blood was then centrifuged at 3500 rpm for 15 min to acquire serum. The liver, kidneys, and spleen were weighed. A portion of the liver was applied for Oil red O staining and transmission electron microscopy examination as previously described[10], and the remaining portion was stored at -80 °C.
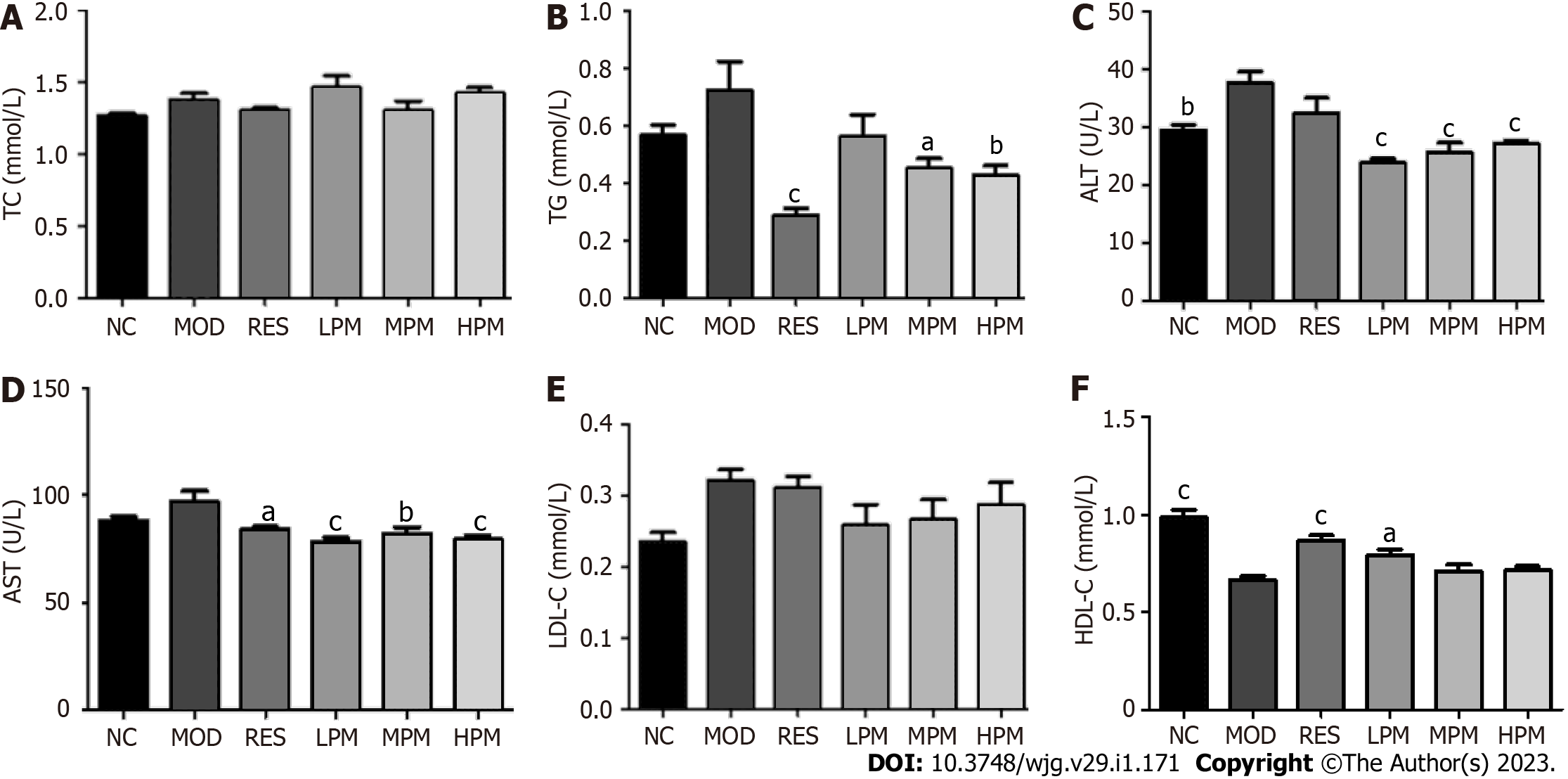
Measurement of biochemical indicators in the serum and liver: Serum levels of TC, TG, low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), ALT, and AST were determined following the kit instructions. In addition, liver homogenates were prepared with nine volumes of normal saline and were centrifuged at 2000 r/min for 10 min. The levels of TC, TG, LDL-C, HDL-C, ALT, and AST in the liver supernatant were detected according to the instructions of the kits. BCA assay was used to determine protein concentration in the liver. All data were collected by a microplate reader.
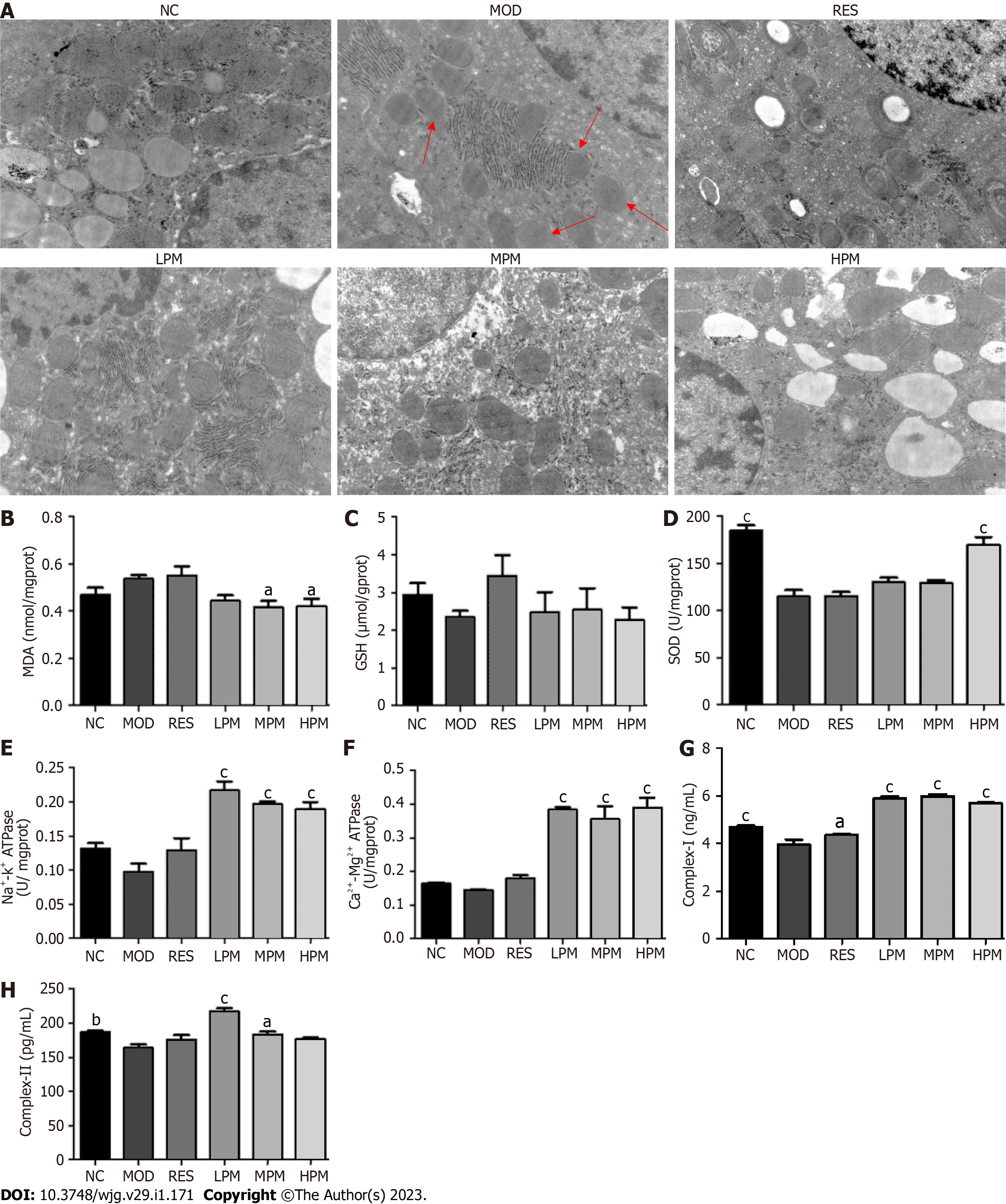
Detection of liver mitochondrial indices: Liver mitochondria were obtained as in previous studies[11] and resuspended in saline. BCA assay was used to determine protein concentration. The levels of malondialdehyde (MDA), SOD, GSH, ATPase, and complex I and II in the suspension of mitochondria were detected according to the instructions of the kit. All data were collected by a microplate reader.
Production of liver mitochondrial extracts: The mitochondria of rat livers in the MOD group and HPM group were separated as previously reported[11]. The obtained mitochondria were suspended in chromatographic methanol and then ultrasonicated for 20 min followed by centrifugation at 10000 × g at 4 ºC for 25 min. The supernatant was collected and dried with mild nitrogen, and mitochondrial extracts were stored at -80 °C.
In vitro assay of anti-steatosis ability of PM-derived constituents in mitochondria: L02 cells were stimulated with 5% fat emulsion for 24 h and then cultured with fenofibrate capsules (150 μmol/L) and mitochondrial extracts (50, 100, and 200 μg/mL) of rats in the MOD group and HPM group for 24 h. Cells were collected after being washed twice with phosphate-buffered saline. BCA assay was used to determine the protein concentration in the cell lysate. TC, TG, SOD, GSH, complex I, complex II, and ATPase levels in the cell were detected according to the instructions of the kits. All data were collected by a microplate reader. Additionally, cells stained with Oil red O were examined as in the aforementioned method.
Structural assignment of PM-derived constituents in liver mitochondria: PM extracts and liver mitochondrial extracts isolated from the MOD and HPM groups were redissolved in HPLC-grade methanol and filtered through a 0.22 μm Millipore filter (Bedford, MA, United States). The filtrate was analyzed by a UHPLC Dionex Ultimate 3000 system coupled with a Thermo Scientific Q-Exactive TM hybrid quadrupole-orbitrap mass spectrometry (MS) (Thermo Fisher Scientific, San Jose, CA, United States). The detailed analytical conditions of UHPLC and MS were described in our previous report[10].
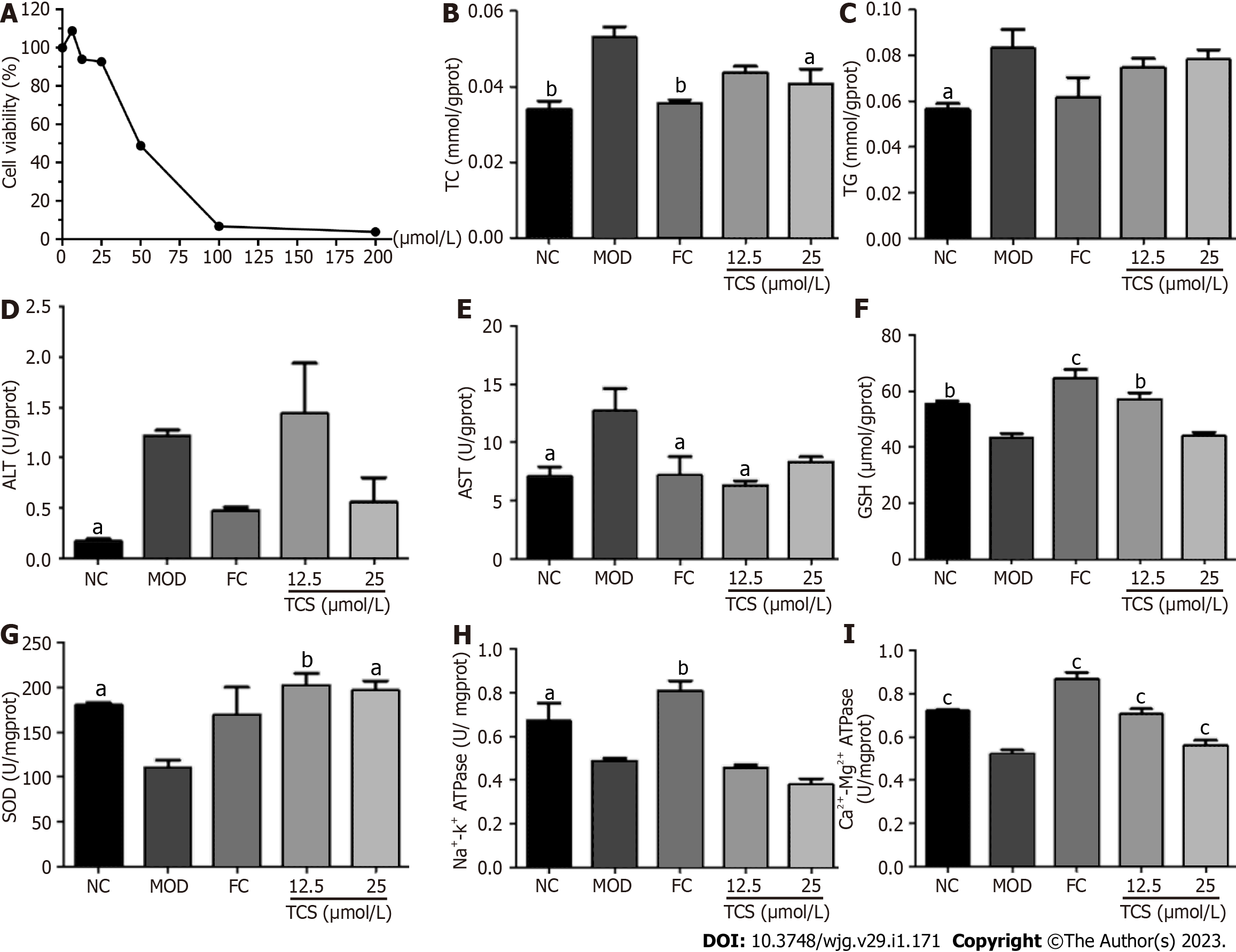
In vitro assay of anti-steatosis ability of PM-derived monomer in mitochondria: The anti-steatosis activity of TCS, a PM-derived monomer, on L02 cells induced by fat emulsion was measured. First, the cell activity of TCS at different concentrations (0, 6.25, 12.5, 25, 50, 100, and 200 μmol/L) was measured according to the CCK-8 kit’s instructions. Subsequently, fat emulsion-treated steatosis cells were introduced with TCS (12.5 and 25 μmol/L). BCA assay was used to analyze the protein concentration of the cell lysate. The levels of TG, TC, AST, ALT, SOD, GSH, and ATPase were measured on the microplate reader according to the instructions of the kits.
In vivo determination of anti-MAFLD activity of the PM-derived monomer group in liver mitochondria: Effects of the PM-derived monomer group on MAFLD were evaluated on HFD-induced mice. The monomer group consisted of ED, AE, EG, and THSG in a ratio of 14.87:1:10.03:18.37. This ratio was the concentration ratio of these molecules in the PM extract counted according to the areas of the chromatographic peak obtained in the UHPLC/MS assay of PM. Male C57BL/6J mice (20 ± 2 g) were provided from Hunan SJA Laboratory Animal Co., Ltd. (Hunan, China). After 5-7 d of adaptive feeding, mice were fed with a normal chow diet as the control group (n = 6), and other groups were fed an HFD for 8 wk to induce MAFLD. Mice in the treatment groups (n = 6) were intraperitoneally injected as follows: (1) A low-dose PM-derived monomer group in the mitochondria (30 mg/kg/d); (2) High-dose PM-derived monomer group in the mitochondria (90 mg/kg/d); and (3) RES (18 mg/kg/d). MAFLD was induced by an HFD for 3 mo. Starting from the 9th wk, mice received an intraperitoneal injection with the test samples once a day for 4 wk. The methods of sample collection and serum, liver, and mitochondrial indices detection were the same as the experiment of PC extract administered to rats described above.
All data were represented as the mean ± SD. Statistical analysis was performed on GraphPad Prism version 8.0 software for Windows (San Diego, CA, United States). One-way analysis of variance was used for comparison between groups. Statistical significance is displayed as P < 0.05.
The inhibitory activity of PM extracts was assessed by 5% fat emulsion-induced intracellular lipid accumulation in L02 cells. Oil red O staining revealed that fat emulsion-treated cells had higher intracellular lipid levels compared to untreated L02 cells (NC). However, treatment with PM extracts greatly blocked fat emulsion-induced lipid accumulation (Figure 1A). As shown in Figures 1B-K, after 24 h of stimulation with fat emulsion, TG, TC, AST, and ALT levels remarkably increased, and GSH, SOD, Na+-K+-ATPase, Ca2+-Mg2+-ATPase, complex I, and complex II levels declined. However, after PM incubation, ALT and AST levels decreased, and SOD, GSH, Na+-K+-ATPase, Ca2+-Mg2+-ATPase, and complex II levels markedly increased. The levels of TC and TG showed a decreasing trend, and the level of complex I showed an increasing trend. Together, these findings suggested that PM extract relieved the steatosis of L02 cells.
Effect of PM on body weight, food consumption, and organ indices of rats: As shown in Table 1, without affecting food intake, rats fed an HFD for 3 mo gained weight at the end of the study. Moreover, after 3 mo of HFD feeding, the liver index of rats increased remarkably, and body weight, kidney index, and spleen index showed an increasing trend. However, the administration of PM extract significantly decreased the spleen index, and body weight, liver index, and kidney index showed a decreasing trend. Thus, these data suggested that PM relieved HFD-induced weight gain and organ enlargement.
| NC | MOD | RES | LPM | MPM | HPM | |
| Initial body weight (g) | 263.67 ± 23.43 | 263.83 ± 28.54 | 261.50 ± 16.99 | 275.83 ± 6.05 | 268.83 ± 12.42 | 246.00 ± 15.09 |
| Final body weight (g) | 458.67 ± 46.43 | 470.17 ± 89.14 | 424.67 ± 25.97 | 454.67 ± 16.98 | 479.83 ± 33.88 | 432.83 ± 47.94 |
| Body weight gain (g) | 195.00 ± 23.00 | 206.33 ± 60.60 | 163.17 ± 8.98 | 178.83 ± 10.93 | 211.00 ± 21.46 | 186.83 ± 32.86 |
| Food intake (g) | 15852.54 | 14023.10 | 14265.09 | 13828.25 | 13081.76 | 13645.74 |
| Liver index (%) | 2.15 ± 0.09b | 2.64 ± 0.22 | 2.32 ± 0.12a | 2.55 ± 0.11 | 2.49 ± 0.16 | 2.49 ± 0.20 |
| Kidney index (%) | 0.52 ± 0.02 | 0.54 ± 0.07 | 0.52 ± 0.02 | 0.50 ± 0.02 | 0.49 ± 0.03 | 0.54 ± 0.04 |
| Spleen index (%) | 0.1589 ± 0.01 | 0.1618 ± 0.02 | 0.1432 ± 0.01 | 0.1267 ± 0.01b | 0.1427 ± 0.01 | 0.1345 ± 0.01a |
Effects of PM extract on serum parameters: As displayed in Figures 2A-F, after 3 mo of an HFD, ALT levels dramatically increased, while HDL-C levels markedly decreased. Additionally, the levels of TC, TG, AST, and LDL-C showed an increasing trend. After administration with PM extract, TG, ALT, and AST levels decreased obviously, and HDL-C level markedly increased. LDL-C levels showed a decreasing trend. Taken together, these data suggested that PM relieved HFD-induced dyslipidemia.
Effects of PM extract on liver status: Figure 3A shows the massive accumulation of lipid droplets, diffuse hepatocellular edema, and degeneration in the liver of rats after 3 mo of HFD feeding. However, after administration with PM extract and RES, the lipid droplets in the liver cells of rats reduced, and liver status tended to be normal. Additionally, Figures 3B-E shows that the levels of TC and TG improved obviously after 3 mo of HFD feeding. The level of AST showed an increasing trend, and the level of HDL-C showed a decreasing trend. After treatment with PM extract, TG and AST levels dramatically declined, and HDL-C levels dramatically increased. Thus, these findings suggested that PM extract reduced lipid accumulation in the liver of HFD-fed rats.
Effects of PM extract on mitochondrial status: Figure 4A shows that after the feeding of an HFD, the liver mitochondria swelled. The inner and outer membranes and ridges were blurred, and the matrix was unclear, as indicated by the red arrow in the MOD group. However, the ultrastructural appearance of mitochondria was reversed by PM treatment. Additionally, Figures 4B-H shows that after the feeding of an HFD, SOD, complex I, and complex II levels in the mitochondria decreased obviously. Furthermore, GSH, Na+-K+-ATPase, and Ca2+-Mg2+-ATPase levels in liver mitochondria showed a decreasing trend, and the MDA level in the liver mitochondria showed an increasing trend. After administration with PM extract, SOD, Na+-K+-ATPase, Ca2+-Mg2+-ATPase, complex I, and complex II levels remarkably increased, and the level of MDA remarkably decreased. The level of GSH showed an increasing trend. Combined, these indices suggested that PM prevented damage to mitochondrial structure and functions to alleviate MAFLD.
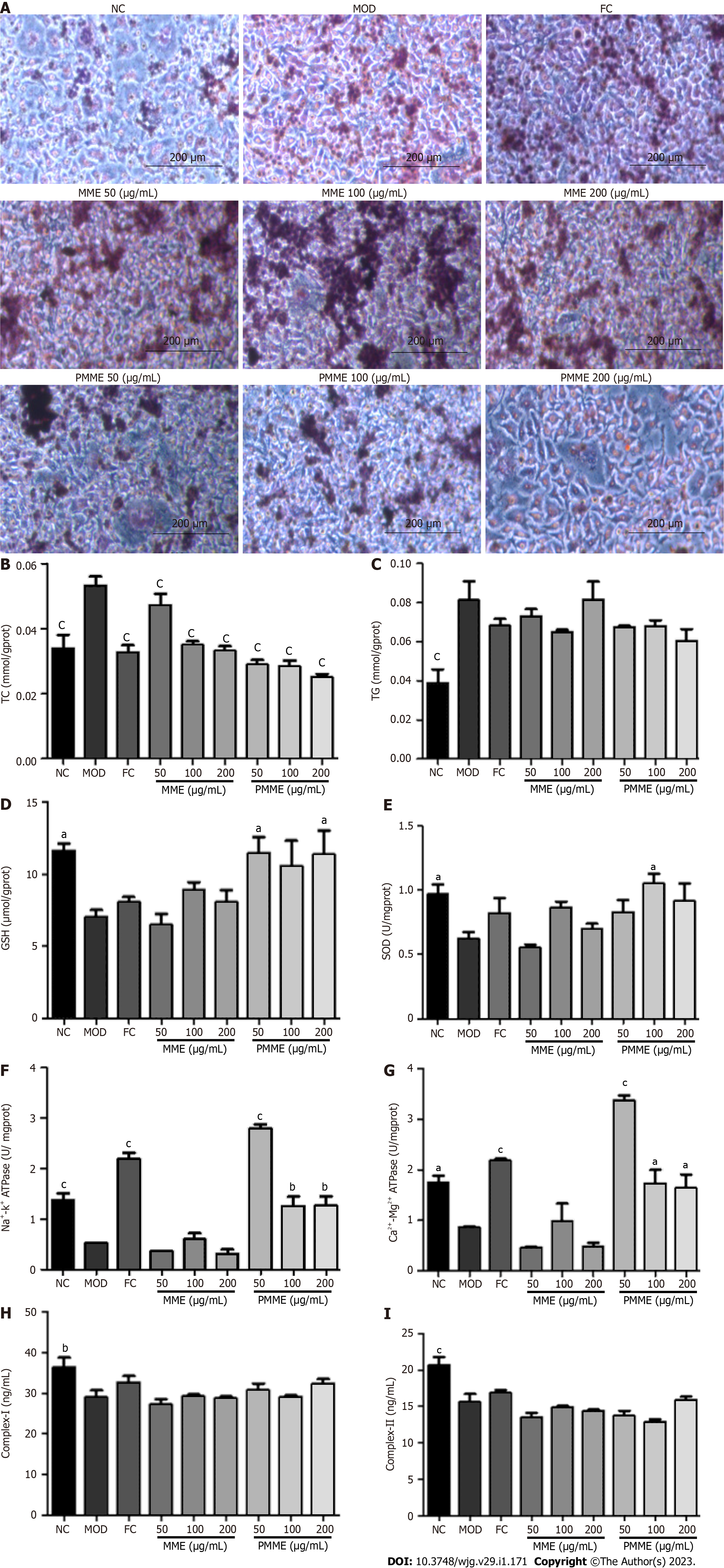
Oil red O staining revealed that fat emulsion-triggered L02 cells had higher intracellular lipid levels compared to untreated L02 cells (NC). After liver mitochondrial extracts from HPM treatment, intracellular lipid accumulation was prevented and was not prevented by liver mitochondrial extracts from MOD treatment (Figure 5A). As shown in Figures 5B-I, after incubation with fat emulsion, the levels of TC and TG increased, and GSH, SOD, Na+-K+-ATPase, Ca2+-Mg2+-ATPase, complex I, and complex II levels decreased. After incubation with liver mitochondrial extracts from HPM, TC levels decreased, and GSH, SOD, Na+-K+-ATPase, and Ca2+-Mg2+-ATPase levels increased. Furthermore, the TG level showed a downward trend, and levels of complex I and complex II displayed an increasing trend. However, liver mitochondrial extracts from MOD treatment did not reverse the pathological changes induced by fat emulsion, except for the significantly reversed TC level. These results showed that PM-derived constituents in the liver mitochondrial extracts ameliorated cellular steatosis by regulating oxidative stress and energy production.
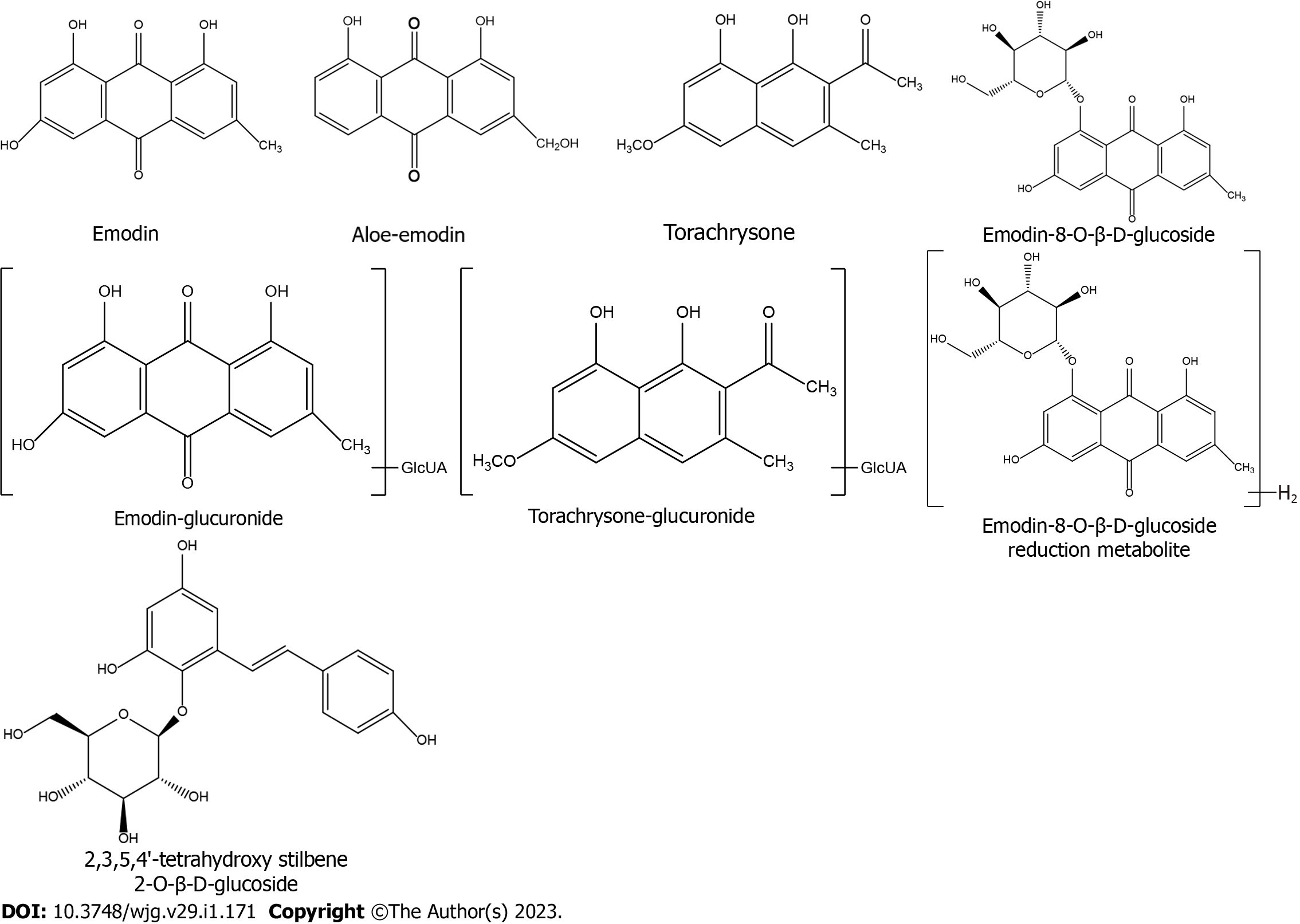
After analyzing accurate high-resolution MSn data provided by UHPLC/MS (Table 2) and comparing the data with antecedently described[12-19] and standards, eight PM-derived monomers, including five prototype monomers and three of their metabolites (Figure 6), were screened in mitochondrial liver extracts of PM-treated rats. Among the identified monomers, there were one stilbene, five anthraquinones, and two naphthols. Prototype monomers consisted of ED, AE, EG, THSG, and TCS. The metabolites comprised emodin-glucuronide, torachrysone-glucuronide, and emodin-8-O-β-D-glucopyranoside reduction metabolite, which originated from ED, THCS, and EG, respectively.
| No | TR (min) | (M-H)- (m/z) | Molecular formula | Predicted (m/z) | Measured (m/z) | Diff (ppm) | ESI-MSn(-), m/z (abundance) | Assigned identification | Ref. |
| 1 | 18.87 | 269.0454 | C15H10O5 | 269.0450 | 269.0454 | 3.457 | 269.04541 (100.00), 241.05089 (2.18), 225.14899 (1.98) | Aloe-emodin1 | [12-14] |
| 2 | 15.90 | 431.0981 | C21H20O10 | 431.0978 | 431.0981 | 2.011 | 431.109811 (34.23), 311.05627 (4.32), 269.04553 (100.00), 240.04503 (0.69) | Emodin-8-O-glucoside1 | [14-16] |
| 3 | 33.91 | 269.0457 | C15H10O5 | 269.0450 | 269.0457 | 4.461 | 269.04553 (100), 241.05331 (0.18), 225.05482 (1.37) | Emodin1 | [15-19] |
| 4 | 18.80 | 445.0778 | C21H18O11 | 445.0771 | 445.0778 | 2.881 | 269.04553 (100.00), 225.05527 (0.19) | Emodin-glucuronide | [17] |
| 5 | 9.06 | 405.1190 | C20H22O9 | 405.1186 | 405.1190 | 2.422 | 243.06613 (62.27) | 2,3,5,4’-Tetrahydroxy stilbene-1 | [17] |
| 6 | 13.17 | 433.1136 | C21H22O10 | 433.1135 | 433.1136 | 1.539 | 257.08191 (100.00), 175.02435 (8.79), 152.99582 (3.58), 113.02423 (59.47) | Emodin-8-O-β-D-glucopyranoside reduction metabolite | [17] |
| 7 | 13.46 | 245.0820 | C14H14O4 | 245.0814 | 245.0820 | 4.834 | 245.08208 (87.57), 230.05850 (9.07) | Torachrysone1 | [17] |
| 8 | 15.15 | 421.1142 | C20H22O10 | 421.1135 | 421.1142 | 3.032 | 245.08179 (76.39), 230.05852 (4.02) | Torachrysone-glucuronide | [17] |
As shown in Figure 7, after incubation with fat emulsion, the levels of TC, TG, ALT, and AST dramatically increased, and GSH, SOD, Na+-K+-ATPase, and Ca2+-Mg2+-ATPase levels dramatically decreased. After TCS stimulation, TC and AST levels decreased, and the levels of GSH, SOD, and Ca2+-Mg2+-ATPase increased. The levels of TG and ALT showed a decreasing trend. These data suggested that TCS mitigated fat emulsion-triggered cellular steatosis.
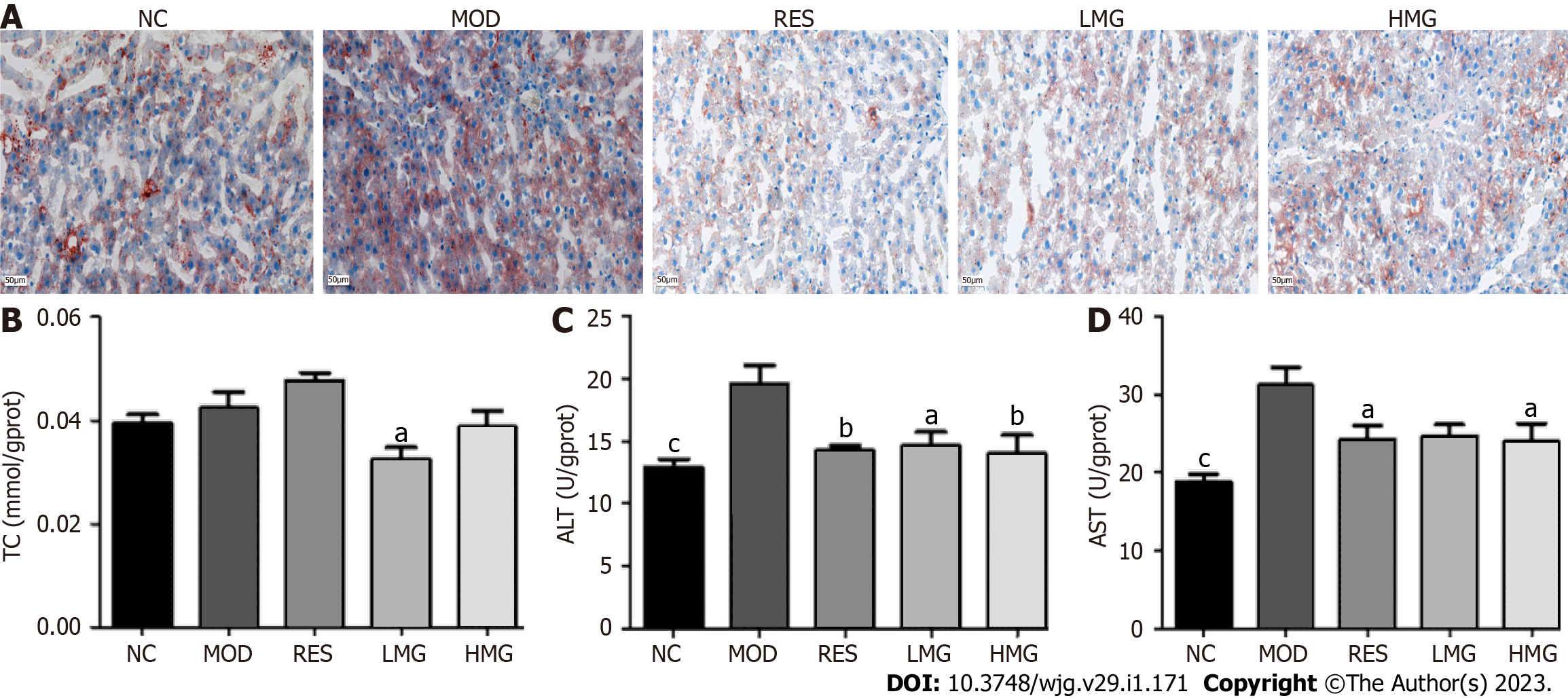
Effects of PM-derived monomer group on body weight, food consumption, and organ index of MAFLD mice: As shown in Table 3, without affecting food intake, mice fed an HFD for 3 mo gained weight at the end of the study. The liver and kidney index increased obviously, and the spleen index showed an increasing trend. After treatment with the PM-derived monomer group, the liver index decreased. Body weight and the kidney index showed a decreasing trend, whereas the spleen index level increased in the HMG group. Thus, these data indicated that the PM-derived monomer group reduced weight gain and organ index swelling caused by HFD.
| NC | MOD | RES | LMG | HMG | |
| Initial body weight (g) | 20.03 ± 0.54 | 20.38 ± 0.32 | 20.40 ± 0.59 | 19.83 ± 0.92 | 20.33 ± 1.00 |
| Final body weight (g) | 22.90 ± 0.56b | 25.68 ± 1.12 | 24.22 ± 1.29 | 25.20 ± 1.74 | 24.37 ± 1.50 |
| Body weight gain (g) | 2.87 ± 0.02 | 5.30 ± 0.79 | 3.82 ± 0.70 | 5.37 ± 0.82 | 4.03 ± 0.50 |
| Food intake (g) | 2274.20 | 2268.50 | 2655.80 | 2473.30 | 2290.60 |
| Liver index (%) | 3.52 ± 0.05c | 4.24 ± 0.10 | 3.17 ± 0.11c | 3.16 ± 0.11c | 3.50 ± 0.21c |
| Kidney index (%) | 1.00 ± 0.57c | 1.20 ± 0.05 | 1.11 ± 0.08 | 1.15 ± 0.08 | 1.15 ± 0.08 |
| Spleen index (%) | 0.21 ± 0.03 | 0.31 ± 0.06 | 0.31 ± 0.04 | 0.30 ± 0.06 | 0.48 ± 0.22a |
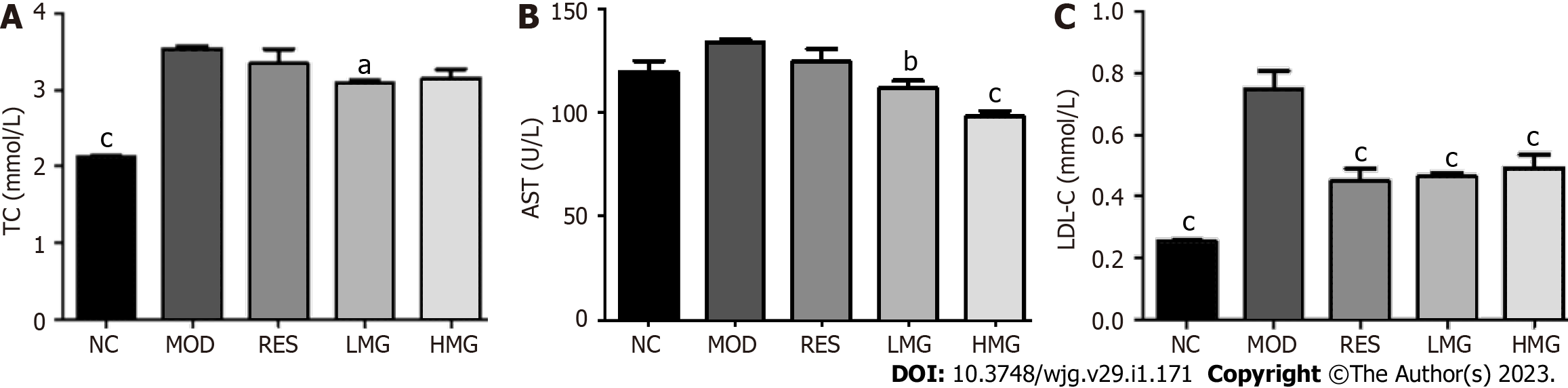
Effects of the PM-derived monomer group on serum indicators: Figures 8A-C show that after 3 mo of HFD feeding, TC and LDL-C levels increased obviously, and the level of AST showed an increasing trend. After treatment with the PM-derived monomer group, the levels of TC, AST, and LDL-C were decreased. Thus, the results show that the PM-derived monomer group alleviated HFD-induced dyslipidemia.
Effects of the PM-derived monomer group on the liver lipid content: As displayed in Figure 9A, after 3 mo of HFD feeding, the livers exhibited lipid accumulation, diffuse hepatocellular edema, and degeneration. However, this status of the livers was restored to normal after administration with PM-derived monomer groups. Figures 9B and 9D shows that after 3 mo of HFD consumption, ALT and AST levels increased, and the level of TC showed an increasing trend. After administration with the PM-derived monomer group, levels of TC, ALT, and AST decreased obviously. These data suggested that the PM-derived monomer group reduced lipid accumulation in the livers of HFD mice.
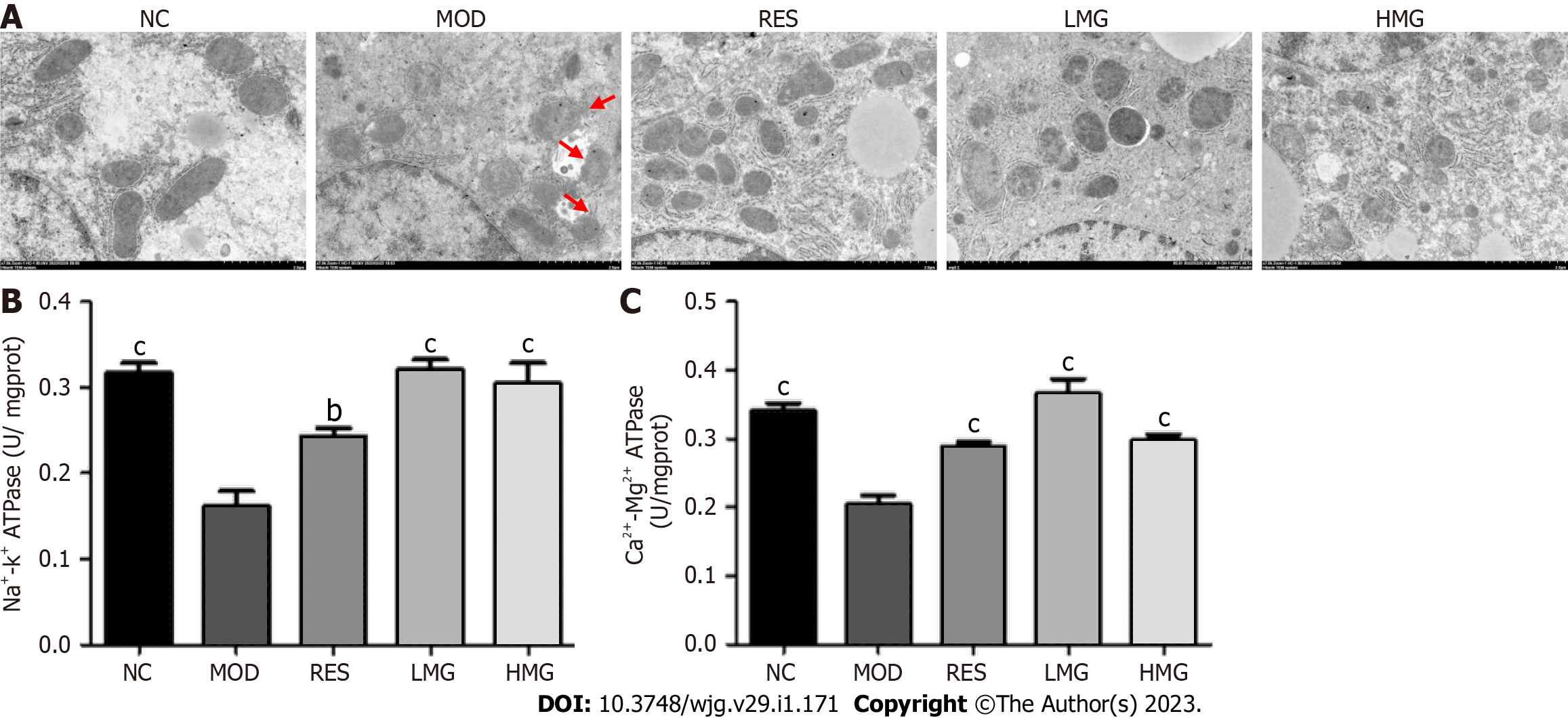
Effects of PM-derived monomer group on the status of liver mitochondria: As shown in Figure 10A, after 3 mo of HFD feeding, the liver mitochondria swelled. The inner and outer membranes and ridges were blurred, and the matrix was unclear, as indicated by the red arrows in the MOD group. However, treatment with the PM-derived monomer group markedly improved the ultrastructure of mitochondria. Figures 10B and 10C displays that after 3 mo of HFD feeding, Na+-K+-ATPase and Ca2+-Mg2+-ATPase levels decreased obviously. However, after treatment with the PM-derived monomer group, the levels of Na+-K+-ATPase and Ca2+-Mg2+-ATPase increased. The results showed that the PM-derived monomer group regulated the state of mitochondria to alleviate MAFLD.
In this study, fat emulsion-induced L02 adipocytes and HFD-induced MAFLD rats were established to evaluate the efficacy and underlying mechanism of action of PM on MAFLD and mitochondria. MAFLD primarily manifests as excessive liver fat accumulation, increased serum and liver TC, TG, ALT, AST, LDL-C levels, and decreased HDL-C level[20]. TC, TG, ALT, and AST in L02 cell levels were markedly increased by the induction of fat emulsion, which could be alleviated by PM extracts. Our data also showed that the contents of TC, TG, ALT, AST, and LDL-C in the serum and liver of rats fed with HFD increased, while the level of HDL-C decreased, which could be recovered after PM treatment. Moreover, significant lipid droplet accumulation was observed in the liver of rats fed an HFD, which could be recovered after PM treatment. Together, these findings indicated that PM effectively prevented lipid metabolism disorders in MAFLD.
Mitochondria regulate a range of important physiological and biochemical cellular processes, such as energy production, apoptosis, oxidative stress, lipid metabolism, and calcium homeostasis. Multiple studies have shown evidence of molecular, biochemical, and biophysical mitochondrial abnormalities in MAFLD initiation and progression[21]. In this study, fat emulsion decreased GSH, SOD, Ca2+-Mg2+-ATPase, Na+-K+-ATPase, complex I, and complex II levels in hepatic cells, which could be recovered to a certain extent by PM treatment. Similarly, the mitochondria of MAFLD rats fed an HFD were swollen and damaged. The levels of SOD, GSH, ATPase, and complex I and II in liver mitochondria of MAFLD rats were greatly reduced, and the level of MDA increased, which could be reversed by PM treatment. These results suggested that PM extract significantly improved mitochondrial structure and function in the MAFLD process.
In our previous study, the strategy of mitochondrial pharmacology and pharmacochemistry was confirmed to possess the capability to screen the main active constituents of the TCMs regulating the mitochondria to prevent human disease[10]. In the present study, this strategy was used to reveal the pharmacodynamic substances in PM extract remedying mitochondria against MAFLD. Mitochondrial pharmacology confirmed that the PM-derived constituents that regulate oxidative stress and energy production to relieve steatosis of hepatocytes entered the liver mitochondria. Moreover, a total of eight PM-derived monomers that entered the mitochondria of MAFLD rats treated with PM extract were successfully identified by mitochondrial pharmacochemistry, including five prototype components (AE, ED, EG, THSG, and TCS), and three of their metabolites (emodin-glucuronide, emodin-8-O-β-D-glucopyranoside reduction metabolite, and torachrysone-glucuronide). Four monomers have been reported to cure MAFLD, and include AE[22,23], ED[22,24,25], EG[26], and THSG[27]. Our data also show that TCS improved oxidative stress and energy production to prevent steatosis in L02 adipocytes induced by fat emulsion. Furthermore, it was found that the PM-derived monomer group identified from liver mitochondria regulated the state of mitochondria to alleviate MAFLD in HFD-fed mice. Together, these results indicated that the eight substances were potentially major active constituents of PM against MAFLD, thereby validating the capability of mitochondrial pharmacology and pharmacochemistry for efficiently identifying in vivo mitochondria-regulated constituents in TCMs.
It is noted that the effects of PM extracts and their components on the detected indices did not show dose-dependence, which is similar to many other TCMs[20]. This may be caused by the complicated substances and action mechanisms of the TCMs, such as multi-components, multiple acting targets, and multiple ways[20]. Additionally, the doses of PM extract and its ingredients used in this study were likely not within the dose-dependent range. In addition, due to the insufficient liver samples, the depth analysis of mechanisms that PM extract and its monomer group regulated mitochondrial status to alleviate MAFLD in HFD-fed animals was not performed, which merits further investigation.
The data obtained in this study showed that PM extract restored mitochondrial structure and function and alleviated MAFLD caused by HFD, which may be related to the mitigation of oxidative stress-induced damage and the improvement of energy production. The eight substances obtained by the strategy may be the main bioactive ingredients in PM that regulate mitochondria to relieve MAFLD. Thus, PM treatment provided a new method to prevent MAFLD-related mitochondrial dysfunction from alleviating MAFLD. Mitochondrial pharmacology and pharmacochemistry were shown to be efficient strategies for recognizing the bioactive ingredients of TCMs that alleviate mitochondria to prevent disease.
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a serious threat to human health. Mitochondrial dysfunction is a mechanism involved in MAFLD. Modulation of mitochondrial function may become a novel strategy for the treatment of MAFLD. For centuries, Polygonum multiflorum (PM) has been used as a common traditional Chinese medicine and nutritional ingredient in China and has been proven to remedy mitochondria and further relieve MAFLD.
To date, the main pharmacodynamic ingredients of PM for regulating mitochondria against MAFLD remain unclear.
To investigate the pharmacodynamic ingredients for the mitochondrial remedy action of PM against high-fat diet (HFD)-induced MAFLD in rats.
Fat emulsion-induced L02 adipocyte model and HFD-induced MAFLD rat model were used to evaluate the anti-MAFLD ability of PM and the mechanism of action involved. The adipocyte model was also used to determine the activities of PM-derived constituents in liver mitochondria from HFD-fed rats (mitochondrial pharmacology). PM-derived constituents in liver mitochondria were recognized by ultra-high performance liquid chromatography/mass spectrometry (mitochondrial pharmacochemistry). The abilities of the PM-derived monomer and the monomer group were evaluated by adipocyte model and MAFLD mouse model, respectively.
PM repaired mitochondrial ultrastructure and prevented oxidative stress and energy production disorder of mitochondria to mitigate fat emulsion-induced cellular steatosis and HFD-induced MAFLD. PM-derived constituents that entered the mitochondria inhibited oxidative stress damage and improved energy production against cellular steatosis. Eight chemicals were found in the mitochondria of PM-administrated rats. The anti-steatosis ability of one monomer and the anti-MAFLD activity of the monomer group were validated.
PM restored mitochondrial structure and function and alleviated MAFLD, which may be associated with the remedy of oxidative stress and energy production. The identified eight chemicals may be the main bioactive ingredients in PM that adjust mitochondria to prevent MAFLD.
PM treatment provided a new approach to prevent MAFLD-related mitochondrial dysfunction from alleviating MAFLD. Mitochondrial pharmacology and pharmacochemistry are efficient strategies for identifying the bioactive ingredients of traditional Chinese medicines regulating mitochondria to prevent disease.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Masaki N, Japan; Xia B, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Zhao S
| 1. | Shao YL, Fan JG. [Prevalence and harm of nonalcoholic fatty liver disease]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:10-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Wang PX, Ji YX, Zhang XJ, Zhao LP, Yan ZZ, Zhang P, Shen LJ, Yang X, Fang J, Tian S, Zhu XY, Gong J, Zhang X, Wei QF, Wang Y, Li J, Wan L, Xie Q, She ZG, Wang Z, Huang Z, Li H. Corrigendum: Targeting CASP8 and FADD-like apoptosis regulator ameliorates nonalcoholic steatohepatitis in mice and nonhuman primates. Nat Med. 2017;23:1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Xu X, Poulsen KL, Wu L, Liu S, Miyata T, Song Q, Wei Q, Zhao C, Lin C, Yang J. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH). Signal Transduct Target Ther. 2022;7:287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 193] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 4. | Chiang H, Lu HF, Chen JC, Chen YH, Sun HT, Huang HC, Tien HH, Huang C. Adlay Seed (Coix lacryma-jobi L.) Extracts Exhibit a Prophylactic Effect on Diet-Induced Metabolic Dysfunction and Nonalcoholic Fatty Liver Disease in Mice. Evid Based Complement Alternat Med. 2020;2020:9519625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Ramanathan R, Ali AH, Ibdah JA. Mitochondrial Dysfunction Plays Central Role in Nonalcoholic Fatty Liver Disease. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 85] [Reference Citation Analysis (0)] |
| 6. | Lin L, Ni B, Lin H, Zhang M, Li X, Yin X, Qu C, Ni J. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: a review. J Ethnopharmacol. 2015;159:158-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 268] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 7. | Fei-Fei HU, Zhan-Xia H, Shao-Bo Z, Yu-Chen S, Li-Li JI. [Study on improvement provided by water extracts of Polygoni Multiflori Radix and Polygoni Multiflori Radix Praeparata on non-alcoholic steatohepatitis in mice induced by MCD]. Zhongguo Zhong Yao Za Zhi. 2020;45:4732-4739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Lin L, Hao Z, Zhang S, Shi L, Lu B, Xu H, Ji L. Study on the protection of water extracts of Polygoni Multiflori Radix and Polygoni Multiflori Radix Praeparata against NAFLD and its mechanism. J Ethnopharmacol. 2020;252:112577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Lin P, He YR, Lu JM, Li N, Wang WG, Gu W, Yu J, Zhao RH. In vivo lipid regulation mechanism of polygoni multiflori radix in high-fat diet fed rats. Evid Based Complement Alternat Med. 2014;2014:642058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Yu LP, Li YQ, Li YJ, Zi L, Tao YX, Hao JJ, Zhang M, Gu W, Zhang F, Yu J, Yang XX. In vivo identification of the pharmacodynamic ingredients of Polygonum cuspidatum for remedying the mitochondria to alleviate metabolic dysfunction-associated fatty liver disease. Biomed Pharmacother. 2022;156:113849. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Yang XX, Xu F, Wang D, Yang ZW, Tan HR, Shang MY, Wang X, Cai SQ. Development of a mitochondria-based centrifugal ultrafiltration/Liquid chromatography/mass spectrometry method for screening mitochondria-targeted bioactive constituents from complex matrixes: Herbal medicines as a case study. J Chromatogr A. 2015;1413:33-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | 12.Wang L, Sang M, Liu E, Banahene PO, Zhang Y, Wang T, Han L, Gao X. Rapid profiling and pharmacokinetic studies of major compounds in crude extract from Polygonum multiflorum by UHPLC-Q-TOF-MS and UPLC-MS/MS. J Pharm Biomed Anal. 2017;140:45-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Wu Z, Wang X, Chen M, Hu H, Cao J, Chai T, Wang H. A Study on Tissue-Specific Metabolite Variations in Polygonum cuspidatum by High-Resolution Mass Spectrometry-Based Metabolic Profiling. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Wang X, Qin Y, Li GQ, Chen S, Ma JQ, Guo YL, Luo WZ. Study on Chemical Constituents in Polygoni Cuspidati Folium and its Preparation by UPLC-ESI-Q-TOF-MS/MS. J Chromatogr Sci. 2018;56:425-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Tang D, Zhu JX, Wu AG, Xu YH, Duan TT, Zheng ZG, Wang RS, Li D, Zhu Q. Pre-column incubation followed by fast liquid chromatography analysis for rapid screening of natural methylglyoxal scavengers directly from herbal medicines: case study of Polygonum cuspidatum. J Chromatogr A. 2013;1286:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Xu H, Qu J, Wang J, Han J, Han K, Li Q, Bi W, Liu R. Discovery of pulmonary fibrosis inhibitor targeting TGF-β RI in Polygonum cuspidatum by high resolution mass spectrometry with in silico strategy. JPA. 2020;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Guo ZH, Jia ZX, Chen KK, Liu J, Xu WJ, Duan FP, Xian Z, Wei ZY, Chen LM, Xiao HB. [Studies on constituents of Polygonum multiflorum extract in vivo and in vitro based on UPLC-Q-TOF-MS]. Zhongguo Zhong Yao Za Zhi. 2018;43:2796-2805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Pan Z, Liang H, Liang C, Xu W. [An ultra-high-pressure liquid chromatography/linear ion trap-Orbitrap mass spectrometry method coupled with a diagnostic fragment ions-searching-based strategy for rapid identification and characterization of chemical components in Polygonum cuspidatum]. Se Pu. 2015;33:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Liang L, Xu J, Zhou WW, Brand E, Chen HB, Zhao ZZ. Integrating Targeted and Untargeted Metabolomics to Investigate the Processing Chemistry of Polygoni Multiflori Radix. Front Pharmacol. 2018;9:934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Mu JK, Zi L, Li YQ, Yu LP, Cui ZG, Shi TT, Zhang F, Gu W, Hao JJ, Yu J, Yang XX. Jiuzhuan Huangjing Pills relieve mitochondrial dysfunction and attenuate high-fat diet-induced metabolic dysfunction-associated fatty liver disease. Biomed Pharmacother. 2021;142:112092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Liang L, Li FJ, Liu X, Mu JK, Wang X, Dong JC, Zeng LX, Gu W, Li JP, Yang XX, Yu J. Identification of Mitochondrial Ligands with Hepatoprotective Activity from Notopterygii Rhizoma et Radix Using Affinity Ultrafiltration/Liquid Chromatography/Mass Spectrometry. Biomed Res Int. 2019;2019:5729263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Wu C, Bian Y, Lu B, Wang D, Azami NLB, Wei G, Ma F, Sun M. Rhubarb free anthraquinones improved mice nonalcoholic fatty liver disease by inhibiting NLRP3 inflammasome. J Transl Med. 2022;20:294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Su ZL, Hang PZ, Hu J, Zheng YY, Sun HQ, Guo J, Liu KY, Du ZM. Aloe-emodin exerts cholesterol-lowering effects by inhibiting proprotein convertase subtilisin/kexin type 9 in hyperlipidemic rats. Acta Pharmacol Sin. 2020;41:1085-1092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Shen C, Pan Z, Wu S, Zheng M, Zhong C, Xin X, Lan S, Zhu Z, Liu M, Wu H, Huang Q, Zhang J, Liu Z, Si Y, Tu H, Deng Z, Yu Y, Liu H, Zhong Y, Guo J, Cai J, Xian S. Emodin palliates high-fat diet-induced nonalcoholic fatty liver disease in mice via activating the farnesoid X receptor pathway. J Ethnopharmacol. 2021;279:114340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Wang S, Li X, Guo H, Yuan Z, Wang T, Zhang L, Jiang Z. Emodin alleviates hepatic steatosis by inhibiting sterol regulatory element binding protein 1 activity by way of the calcium/calmodulin-dependent kinase kinase-AMP-activated protein kinase-mechanistic target of rapamycin-p70 ribosomal S6 kinase signaling pathway. Hepatol Res. 2017;47:683-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Mishra SK, Tiwari S, Shrivastava A, Srivastava S, Boudh GK, Chourasia SK, Chaturvedi U, Mir SS, Saxena AK, Bhatia G, Lakshmi V. Antidyslipidemic effect and antioxidant activity of anthraquinone derivatives from Rheum emodi rhizomes in dyslipidemic rats. J Nat Med. 2014;68:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Wang W, He Y, Lin P, Li Y, Sun R, Gu W, Yu J, Zhao R. In vitro effects of active components of Polygonum Multiflorum Radix on enzymes involved in the lipid metabolism. J Ethnopharmacol. 2014;153:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |