Published online Aug 21, 2022. doi: 10.3748/wjg.v28.i31.4310
Peer-review started: February 17, 2022
First decision: April 16, 2022
Revised: April 30, 2022
Accepted: June 24, 2022
Article in press: June 24, 2022
Published online: August 21, 2022
Processing time: 179 Days and 20.5 Hours
Individuals within specific risk groups for pancreatic ductal adenocarcinoma (PDAC) [mucinous cystic lesions (MCLs), hereditary risk (HR), and new-late onset diabetes mellitus (NLOD)] represent an opportunity for early cancer detection. Endoscopic ultrasound (EUS) is a premium image modality for PDAC screening and precursor lesion characterization. While no specific biomarker is currently clinically available for this purpose, glypican-1 (GPC1) is overexpressed in the circulating exosomes (crExos) of patients with PDAC compared with healthy subjects or those harboring benign pancreatic diseases.
To evaluate the capacity of GPC1+ crExos to identify individuals at higher risk within these specific groups, all characterized by EUS.
This cross-sectional study with a prospective unicentric cohort included 88 subjects: 40 patients with MCL, 20 individuals with HR, and 20 patients with NLOD. A control group (CG) was submitted to EUS for other reasons than pancreatic pathology, with normal pancreas and absence of hereditary risk factors (n = 8). The inclusion period was between October 2016 and January 2019, and the study was approved by the Ethics Committee of Centro Hospitalar Universitário de São João, Porto, Portugal. All patients provided written informed consent. EUS and blood tests for quantification of GPC1+ crExos by flow cytometry and carbohydrate antigen 19-9 (CA 19-9) levels by ELISA were performed in all subjects. EUS-guided tissue acquisition was done whenever necessary. For statistical analysis, SPSS® 27.0 (IBM Corp., Armonk, NY, United States) version was used. All graphs were created using GraphPad Prism 7.00 (GraphPad Software, San Diego, CA, United States).
Half of MCLs harbored worrisome features (WF) or high-risk stigmata (HRS). Pancreatic abnormalities were detected by EUS in 10.0% and 35.0% in HR and NLOD individuals, respectively, all considered non-malignant and “harmless.” Median levels of GPC1+ crExos were statistically different: MCL [99.4%, interquartile range (IQR): 94.9%-99.8%], HR (82.0%, IQR: 28.9%-98.2%), NLOD (12.6%, IQR: 5.2%-63.4%), and CG (16.2%, IQR: 6.6%-20.1%) (P < 0.0001). Median levels of CA 19-9 were within the normal range in all groups (standard clinical cut-off of 37 U/mL). Within HR, individuals with a positive history of cancer had higher median levels of GPC1+ crExos (97.9%; IQR: 61.7%-99.5%), compared to those without (59.7%; IQR: 26.3%-96.4%), despite no statistical significance (P = 0.21). Pancreatic cysts with WF/HRS were statistically associated with higher median levels of GPC1+ crExos (99.6%; IQR: 97.6%-99.8%) compared to those without (96.5%; IQR: 81.3%-99.5%) (P = 0.011), presenting an area under the receiver operating characteristic curve value of 0.723 (sensitivity 75.0% and specificity 67.7%, using a cut-off of 98.5%; P = 0.012).
GPC1+ crExos may act as biomarker to support the diagnosis and stratification of PDAC precursor lesions, and in signaling individuals with genetic predisposition in the absence of EUS abnormalities.
Core Tip: Patients with mucinous cystic lesions (MCLs), hereditary risk (HR), and new-late onset diabetes mellitus represent a target for early pancreatic ductal adenocarcinoma (PDAC) detection. Within these groups, we evaluated the capacity of glypican-1 positive (GPC1+) circulating exosomes (crExos) to identify individuals at higher risk, all characterized by endoscopic ultrasound (EUS). High levels of GPC1+ crExos were present in MCL and in individuals with HR (predominantly in those with history of cancer), even in the absence of EUS abnormalities. GPC1 may represent a biomarker to support the diagnosis and stratification of PDAC precursor lesions, and indicate genetic predisposition.
- Citation: Moutinho-Ribeiro P, Batista IA, Quintas ST, Adem B, Silva M, Morais R, Peixoto A, Coelho R, Costa-Moreira P, Medas R, Lopes S, Vilas-Boas F, Baptista M, Dias-Silva D, Esteves AL, Martins F, Lopes J, Barroca H, Carneiro F, Macedo G, Melo SA. Exosomal glypican-1 is elevated in pancreatic cancer precursors and can signal genetic predisposition in the absence of endoscopic ultrasound abnormalities. World J Gastroenterol 2022; 28(31): 4310-4327
- URL: https://www.wjgnet.com/1007-9327/full/v28/i31/4310.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i31.4310
Pancreatic ductal adenocarcinoma (PDAC) is considered one of the deadliest malignant diseases around the world, and is estimated to become the second leading cause of cancer-related deaths in the United States in 2030[1]. Most patients present with advanced disease at diagnosis, with only 20% being candidates for surgical treatment, the only chance for cure[2,3].
In its early stages, PDAC usually develops with few or no symptoms, and current sectional imaging modalities are inadequate to detect early small lesions[3]. Endoscopic ultrasound (EUS) is a highly accurate diagnostic technique for pancreatic lesions, with its role majored by the possibility of performing EUS-guided tissue acquisition[4-6].
Among PDAC precursor lesions are pancreatic intraepithelial neoplasias (PanINs) and mucinous cystic lesions (MCLs)[7,8]. While the former are very difficult to identify by available imaging modalities, the latter can be more clearly detected and characterized, specially by magnetic resonance imaging (MRI)/magnetic resonance cholangiopancreatography (MRCP) and EUS[5,9].
Along with the improvement of imaging diagnostic accuracy, the search for a biomarker that could adequately identify PDAC at early stages or its high-risk precursor lesions is a top research priority. The only biomarker approved for clinical use is carbohydrate antigen 19-9 (CA 19-9), but it lacks sensitivity and specificity for PDAC early detection, being mostly reserved for monitoring response to therapy and disease progression[10]. In recent years, several molecules have been tested to serve this purpose[11,12]. Among these, membrane-anchored proteoglycan glypican-1 (GPC1) has been shown to be a good candidate. We previously studied GPC1 in circulating exosomes (crExos) and found that it could identify PDAC patients among healthy individuals with perfect accuracy[13]. In addition, GPC1+ crExos correlated with tumor burden and patients’ survival. Moreover, in a genetically engineered mouse model of PDAC, GPC1 was overexpressed in crExos even before the tumor could be detected by MRI[13]. Finally, we also showed higher levels of GPC1+ crExos in MCL compared to controls, although the number of patients was very limited[13]. Several studies have demonstrated the role of GPC1 in PDAC[14-18]. Considering the distinct methodologies that some of the studies adopted to study GPC1 in circulation, some support the potential of GPC1+ crExos as a biomarker for early detection of PDAC[14,15,19], while others only demonstrate their correlation with disease burden[16-18].
At present, while 90% of PDAC cases are sporadic, only individuals harboring increased hereditary risk (HR), either kindreds of familial pancreatic cancer (FPC) (7%) or belonging to other cancer syndromes with increased risk of PDAC (3%), are candidates for screening[20-22]. Nevertheless, there are other well-defined PDAC-associated risk groups that deserve special attention and can constitute a refined population to be surveilled in order to increase the rate of early detection and improve overall survival. One of these groups is composed by individuals older than 50 years with a recent (< 36 mo) diagnosis of type II diabetes mellitus (DM). Increasing epidemiological, clinical, and experimental evidence shows that new-onset DM can be a clinical manifestation of asymptomatic PDAC or harbinger the disease and offers the promise for early detection in these individuals[3,23-26]. In fact, although the complex and multidirectional relationship between the two entities is not fully understood, new-late onset DM (NLOD) has been recognized as an entity signaling a 6- to 8-fold increased risk of developing PDAC in 3 years[27].
We previously showed that levels of GPC1+ crExos discriminate PDAC from chronic pancreatitis (CP) with high accuracy[28]. In this work, we aimed to determine the capacity of GPC1+ crExos to identify individuals at higher risk of developing PDAC, comparing its levels with EUS pancreatic abnormalities (PA) within specific risk groups: MCL, HR, and NLOD.
This cross-sectional study with a prospective unicentric cohort included 88 subjects: 40 patients with MCLs, 20 individuals with HR, 20 patients with NLOD, and 8 individuals in the control group (CG). The inclusion period was between October 2016 and January 2019, and the study was approved by the Ethics Committee of Centro Hospitalar Universitário de São João (CHUSJ) (ID No. CES 327-15), Porto, Portugal. All patients provided written informed consent and underwent blood sample collection at the time of EUS.
In the MCL group, we considered for inclusion both intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasms (MCNs). For its diagnosis, we used both imagiological and cyst fluid analysis criteria. We have assumed IPMN etiology when EUS and/or MRCP clearly showed communication of the cyst(s) with the pancreatic ductal system. In this situation, branch-duct type (BD-IPMN) were presumed if multiple and no dominant main pancreatic duct (MPD) dilation was seen. Main-duct IPMN (MD-IPMN) was considered if a segmental or diffuse dilatation of the main duct > 5 mm was observed without any other cause of obstruction. Mixed-type IPMN (MT-IPMN) was defined when features of MD-IPMN and BD-IPMN coexisted[29]. MCN was assumed when a mucin-producing cyst forming epithelial neoplasia of the pancreas with a distinctive ovarian-type stroma was present, typically with no communication with the ductal system[30].
We performed EUS-guided fine needle aspiration (FNA) in almost all cystic lesions included. Mucinous nature was supported by a fluid carcinoembryonic antigen (CEA) > 192 ng/mL and/or fluid glucose < 50 mg/dL[31]. In relation to amylase content, if > 250 U/L, a diagnosis of IPMN was likely, whereas levels < 250 U/L suggested MCN. If the aspirated content was sufficient, a sample was also evaluated by experienced cytopathologists.
In the HR group, we considered for inclusion FPC (family history of PDAC in at least two first-degree, or in three or more first- and second-degree relatives) and PDAC susceptibility gene mutation carriers[20,22,32,33]. All of these individuals had a clinically and genetically established diagnosis and have been followed in a dedicated consultation for hereditary digestive cancers in our institution. They underwent detailed evaluation of family history, and verification of cancer diagnoses by review of medical records and genetic testing.
In the NLOD group, we included patients aged ≥ 50 years who had been diagnosed with type II DM within a period of 36 mo[23,24]. Diagnosis of type II DM was made according to the American Diabetes Association and consisted of: Fasting plasma glucose level ≥ 126 mg/dL, a 2-h plasma glucose level of ≥ 200 mg/dL during a 75-g oral glucose tolerance test, a random plasma glucose level of ≥ 200 mg/dL in a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, or a hemoglobin A1c level of ≥ 6.5%[34].
In the CG, individuals who underwent EUS for other reasons than pancreatic pathology were included with a normal pancreas and absence of hereditary/familial risk factors. The exclusion criteria were patients unable or unwilling to give informed consent, individuals younger than 18 years of age, pregnancy or breast feeding, contraindications to endoscopic procedures or contrast administration, contraindications to computed tomography (CT) or MRI, coagulopathy (prothrombin time > 50% of control, activated partial thromboplastin time > 50 s, or international normalized ratio > 1.5), patients on chronic anticoagulation, platelet count < 50000/μL, and inability to tolerate sedated upper endoscopy due to cardiopulmonary instability or other contraindication to endoscopic procedures.
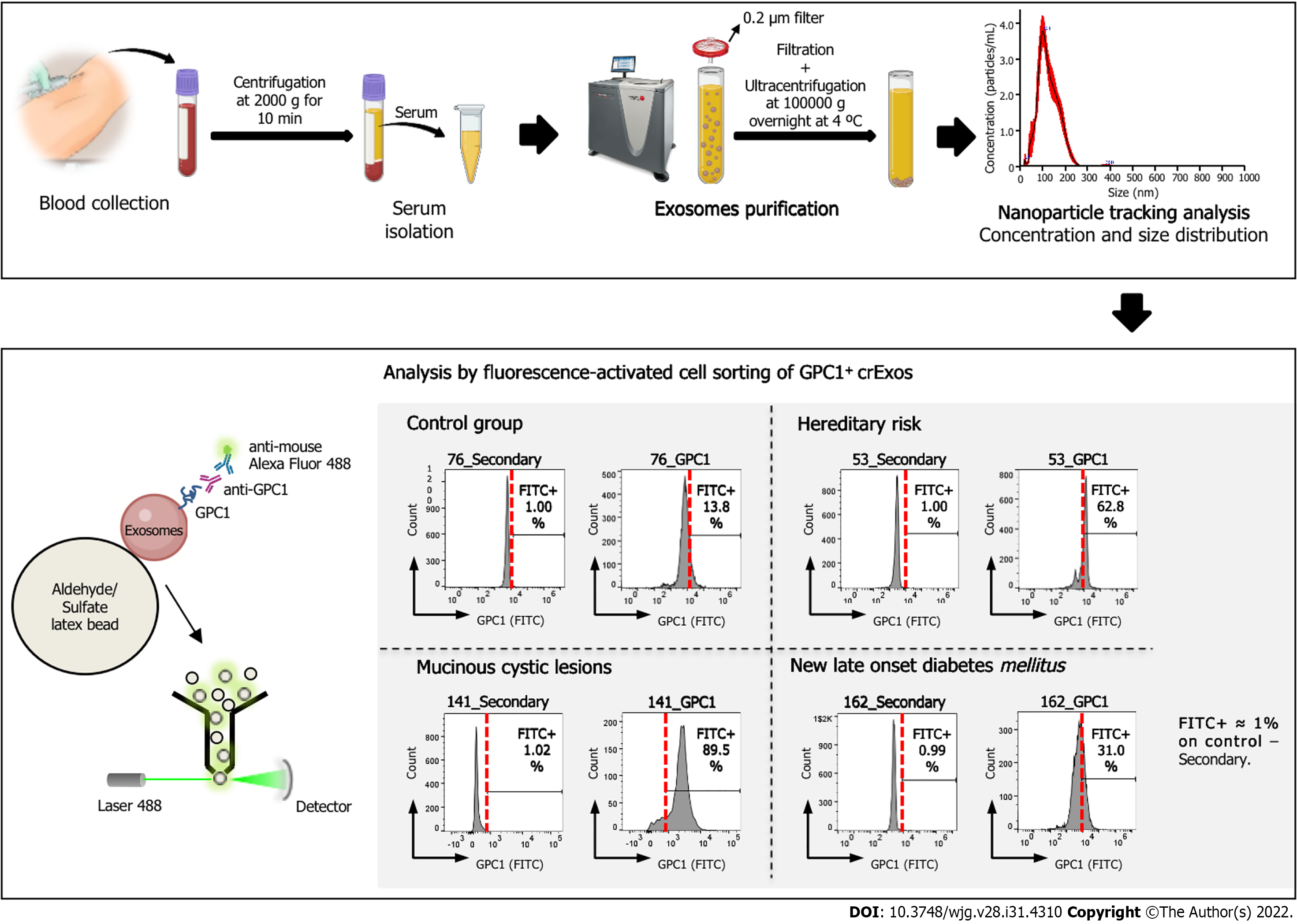
At the time of inclusion, immediately before EUS examination, a blood sample was collected for complete blood count as well as renal, liver, and pancreatic chemistry at the CHUSJ laboratory. A separate blood sample collected at the same time was used to quantify CA 19-9 serum levels (ab108642, CA 19-9 Human ELISA Kit; Abcam, Cambridge, MA, United States) and GPC1 expression in crExos by fluorescence-activated cell sorting (FACS) (Figure 1). For CA 19-9, the standard clinical cut-off of 37 U/mL was used. A complete personal and family clinical history was registered, and demographic data was recorded [age, body mass index (BMI)], previous history of pancreatic disease, smoking and drinking habits, history of diabetes and duration since diagnosis, digestive and systemic symptoms. Laboratory values and previous results of cross-sectional imaging (CT, MRI, or magnetic resonance cholangiopancreatography) were registered. The results of the EUS evaluation at the time of inclusion in the study as well as transechoendoscopic or surgically collected specimens were also recorded.
All subjects were observed, examined, and followed-up at the Department of Gastroenterology of CHUSJ (Porto, Portugal) until death or July 2021.
All EUS evaluations were performed by three experienced endoscopists under deep sedation (propofol or pethidine plus midazolam as assisted by an anesthesiologist). Linear scopes from Olympus® (GT-UCT140, GT-UCT180; Tokyo, Japan) and Pentax® (EG-3670UTK, EG-3870UTK; Tokyo, Japan) along with Olympus® EU-ME2 and Hitachi Avius® (Tokyo, Japan) image processors were used.
PA were classified into major and minor changes. Major changes comprised cystic lesions, CP-like parenchymal changes, and solid lesions. Minor ones constituted changes in pancreatic echogenicity compatible with lipomatous transformation[35,36]. EUS high-risk stigmata (HRS) and worrisome features (WF) were categorized according to the International consensus of Fukuoka guidelines for the management of IPMN of the pancreas[29]. A main duct dilatation ≥ 10 mm or an enhancing mural nodule > 5 mm were classified as HRS, whereas a main duct dilatation between 5 and 9 mm, an enhancing mural nodule < 5 mm, a cyst diameter > 3 cm, the presence of thickened/enhancing cyst walls, an abrupt change in pancreatic duct caliber with distal pancreatic atrophy, and lymphadenopathy were considered WF.
Doppler, elastography, and contrast-enhanced EUS image acquisition (sulphur hexafluoride microbubbles; SonoVue®, Bracco, Italy) were used for assistance in the characterization of mural nodules and thickened cyst walls.
EUS-FNA was performed in almost all cystic lesions, preferably with a 22 G needle (Wilson-Cook Medical, Winston, NC, United States and Boston Scientific, Natick MA, United States) in a single pass, and along with antibiotic prophylaxis (single intravenous infusion of 200 mg ciprofloxacin). The fluid collected was sent for amylase, glucose, and CEA quantification, and also, if in enough volume, for cytological examination. Solid or indeterminate lesions, mural nodules, or suspicious thickenings of the cyst wall or septa were punctured in a distinct pass, preferably with a 22 G fine needle biopsy. Adverse events related to EUS procedures were registered and monitored.
Microscopic examinations of specimens resulting from EUS-FNA/B and/or surgical resection were analyzed by experienced pathologists in pancreatic diseases and contextualized to patients’ clinical history and imaging findings. Cytological evaluation was performed according to the Papanicolaou Society of Cytopathology System for Reporting Pancreaticobiliary Cytology[37].
At the same time of EUS performance, blood samples were collected at the Department of Gastroenterology of CHUSJ. Serum samples were obtained by centrifugation of the whole blood sample at 2000 g for 10 min at 4 °C and collection of the supernatant. The resultant serum samples were then aliquoted and stored at -80 °C prior to analysis.
Human serum samples were allowed to thaw on ice with periodic agitation to avoid degradation. A volume of 200 μL serum was retrieved into a new tube and centrifuged at 10000 rpm for 2 min at 4 ºC. The recovered supernatant was diluted in 200 μL NaCl prior to filtration into 14 mL Open-Top Thinwall Ultra-Clear Tubes (Beckman Coulter, Inc., Brea, CA, United States) using a 0.2 μm pore filter (Whatman International Ltd., England, United Kingdom). The tubes were filled with NaCl, and the samples were ultracentrifuged at 100000 g overnight at 4 °C. The next day, the supernatant was carefully and thoroughly discarded and the exosomes’ pellet was resuspended in 300 μL of 1 × phosphate-buffered saline (PBS). To determine particle concentration and size distribution, nanoparticle tracking (NTA) (NanoSight NS300) was performed using 10 μL of the exosomes sample dissolved in 1 × PBS at a 1:100 dilution. The remaining exosomes sample was saved at -20 ºC for downstream analysis.
Using the exosomes concentration previously obtained by NTA analysis as a reference, an equal number of exosomes was used for downstream FACS analysis. A total of 3.0 × 109 exosomes were coupled to 4 μm aldehyde/sulfate latex beads (A37304; Thermo Fisher Scientific, Waltham, MA, United States) that had been previously equilibrated at room temperature (through resuspension of 5 μL beads in 100 μL of 1 × PBS and rotation at room temperature for 15 min). The sample volume was adjusted to 300 μL with 1 × PBS. Upon incubation in a rotator at room temperature for 15 min and then at 4 ºC for 30 min to allow the formation of exosomes-bead complexes, 300 μL glycine 1 M in 1 × PBS was added to the sample, followed by incubation at room temperature for 1 h with continuous rotation. Samples were centrifuged at 12000 rpm for 2 min and the supernatant was discarded. The pellet containing the exosomes-bead complexes was subjected to a blocking step with 100 μL of 10% bovine serum albumin (BSA) in 1 × PBS and then incubated with continuous rotation at room temperature for 45 min. The samples were centrifuged at 12000 rpm for 2 min and divided into two tubes: Control and GPC1-incubated samples. Control samples were incubated with a solution of 2% BSA in 1 × PBS, whereas experimental samples were incubated with anti-GPC1 (1:240 dilution in a solution of 2% BSA in 1 × PBS, MAB8351; Abnova, Taipei, Taiwan) overnight in a rotator at 4 ºC. The next morning, the samples were spun at 12000 rpm for 2 min and the pellet was washed twice with 2% BSA in 1 × PBS, with centrifugation at 12000 rpm for 2 min between washes. Following incubation with an Alexa-488-tagged secondary antibody (anti-mouse A-21202; Thermo Fisher Scientific) for 30 min with continuous rotation at room temperature of both control and experimental samples, samples were washed again twice with 2% BSA in 1 × PBS. Finally, samples were resuspended in 300 μL of 2% BSA in 1 × PBS for BD Accuri C6 or BD FACS Canto II analysis (BD Biosciences, Haryana, IN, United States). Using the control samples of each patient (i.e. exosomes-bead complexes from each patient only incubated with secondary antibody) as a reference, the fluorescein isothiocyanate (FITC) voltage was adjusted until the percentage of FITC+ beads was 1%. Then, using the same gate and FITC voltage, the percentage of beads bound with GPC1+ crExos in the experimental sample was determined for each patient separately. Data were analyzed using FlowJo software.
Categorical variables are described as absolute and relative frequencies and continuous variables as mean and standard deviation, median, percentiles, minimum, and maximum. Hypotheses were tested regarding the distribution of continuous variables via the independent samples t-test/one-way analysis of variance (ANOVA) or nonparametric Mann-Whitney and Kruskal-Wallis test depending on normal or non-normal distribution, respectively, and considering the nature of the hypothesis. The chi-squared and ANOVA tests were used for categorical variables analysis. Pearson's correlation coefficient was used to assess the statistical relationship/association between two continuous variables. The diagnostic accuracy of GPC1+ crExos was assessed by receiver operating characteristic curve (ROC) considering 95% confidence intervals. Area under the ROC curve (AUROC) was calculated. SPSS® 27.0 (IBM Corp., Armonk, NY, United States) version was used. All graphs were created using GraphPad Prism 7.00 (GraphPad Software, San Diego, CA, United States). The statistical review of the study was performed by a biomedical statistician.
The baseline demographic characteristics of the 88 individuals included in the study (40 MCL, 20 HR, 20 NLOD, and 8 CG) are summarized in Table 1. The overall female:male ratio was 1.6:1 and did not differ statistically among groups. The mean age of the population was 60.3 ± 12.6 years. The older individuals belonged to the MCL group, with a median age of 67.2 ± 8.9 years, whereas the younger individuals were in the HR group, with a median age of 48.7 ± 10.8 years (P < 0.001). Patients in the NLOD group presented with a significantly higher BMI (29.9 ± 4.5 kg/m2; P < 0.001).
| Cohort characteristics | MCL, n = 40 | HR, n = 20 | NLOD, n = 20 | CG, n = 8 | Total, n = 88 | P value |
| Sex | NS | |||||
| Male | 14 (35.0) | 9 (45.0) | 9 (45.0) | 2 (25.0) | 34 (38.6) | |
| Female | 26 (65.0) | 11 (55.0) | 11 (55.0) | 6 (75.0) | 54 (61.4) | |
| Age in yr | 67.2 ± 8.9 | 48.7 ± 10.8 | 60.8 ± 7.4 | 53.8 ± 18.7 | 60.3 ± 12.6 | < 0.001 |
| BMI in kg/m2 | 24.9 ± 3.8 | 24.2 ± 3.9 | 29.9 ± 4.5 | 25.0 ± 4.0 | 25.9 ± 4.5 | < 0.001 |
| Smoking habits | NS | |||||
| Never | 27 (67.5) | 13 (65.0) | 12 (60.0) | 7 (87.5) | 59 (67.1) | |
| Ex-smoker, > 5 yr | 8 (20.0) | 4 (20.0) | 7 (35.0) | 1 (12.5) | 20 (22.7) | |
| Active | 5 (12.5) | 3 (15.0) | 1 (5.0) | 0 (0.0) | 9 (10.2) | |
| Alcohol habits | NS | |||||
| No | 22 (55.0) | 11 (55.0) | 10 (50.0) | 7 (87.5) | 50 (56.8) | |
| Yes | 18 (45.0) | 9 (45.0) | 10 (50.0) | 1 (12.5) | 38 (43.2) | |
| Family history of PDAC | < 0.001 | |||||
| No | 34 (85.0) | 7 (35.0) | 18 (90.0) | 8 (100.0) | 67 (76.1) | |
| Yes | 6 (15.0) | 13 (65.0) | 2 (10.0) | 0 (0.0) | 21 (23.9) | |
No statistically significant differences were observed in relation to smoking or drinking habits among the studied groups. Overall, 10.2% of the population were active smokers and 22.7% ex-smokers (more than 5 years of abstinence). In relation to alcohol consumption, 43.2% were considered active drinkers (average ingesting amounts of > 30 g or > 40 g alcohol per day, in case of female or male individuals, respectively).
Family history of PDAC was present in 23.9% of the entire population, with the highest proportion observed in the HR group (65.0%; P < 0.001). In the HR group, 17 (85.0%) individuals were diagnosed with Lynch Syndrome, 1 (5.0%) with Peutz-Jeghers syndrome, and the remaining 2 (10%) with FPC. Six individuals with Lynch Syndrome had a previous personal history of cancer, mostly colorectal (n = 5), but all were disease-free at the time of inclusion. Detailed information about this group, including the type of harbored mutation(s), can be found in Table 2. In the NLOD group, the mean time between inclusion in the study and establishment of DM diagnosis was 20.9 ± 8.6 mo.
| Lynch syndrome, n = 17 (85.0%) | Peutz-Jeghers syndrome, n = 1 (5.0%) | FPC, n = 2 (10.0%) | |
| Germline mutation(s) | |||
| MLH1 | 7 (41.2) | - | - |
| MSH2 | 6 (35.3) | - | - |
| MLH1 + MSH2 | 1 (5.9) | - | - |
| MSH2 + MSH6 | 2 (11.7) | - | - |
| MSH6 | 1 (5.9) | - | - |
| STK11 | - | 1 (100.0) | - |
| Not identified | - | 2 (100.0) | |
| Personal history of cancer | |||
| No | 11 (64.7) | 1 (100.0) | 2 (100.0) |
| Yes | 6 (35.3) | 0 (0.0) | 0 (0.0) |
| Type of cancer | |||
| Colon and rectum | 5 (83.3%) | - | - |
| Endometrium | 1 (16.7) | - | - |
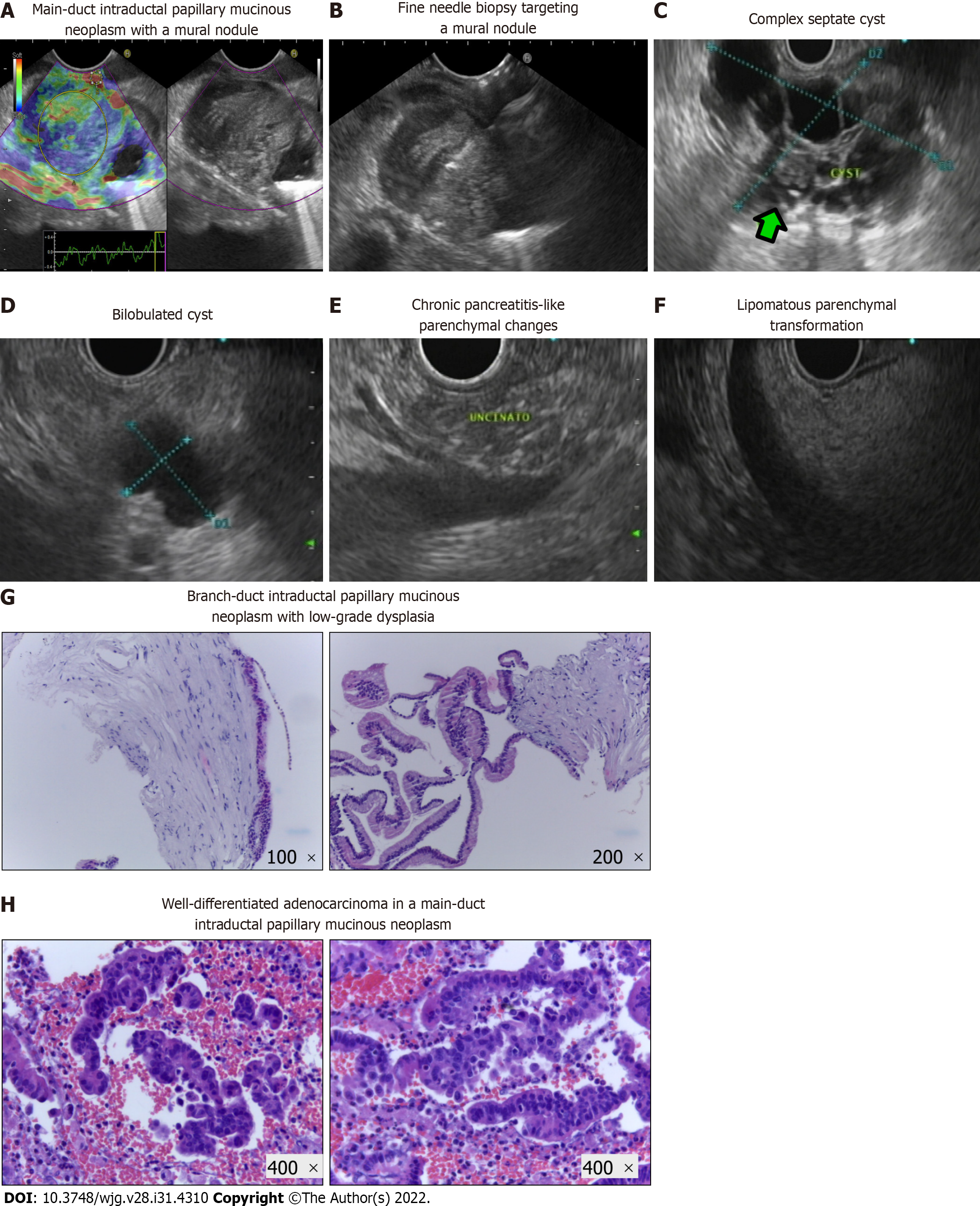
Main PA detected by EUS is illustrated in Figure 2A-F and constituted cystic lesions (with or without WF/HRS), CP-like parenchymal changes, and lipomatous transformation of pancreatic parenchyma. The EUS features of cystic lesions in the MCL group are described in Table 3. Of the 40 cases included, 39 (97.5%) were IPMNs, and most (89.8%) were classified as BD-IPMNs. Sixty percent of patients presented with multiple cysts, with a mean size of the dominant lesion of 28.1 ± 14.1 mm. In respect to WF and/or HRS, mural nodules, wall thickening, and a dilated MPD were identified in 10%, 7.5%, and 15% of the lesions, respectively. Nineteen of the forty MCL patients (47.5%) underwent additional study with MRCP.
| Type of mucinous cyst, n (%) | MCN | 1 (2.5) |
| IPMN | 39 (97.5) | |
| Type of IPMN, n (%) | MD-IPMN | 2 (5.1) |
| BD-IPMN | 35 (89.8) | |
| MT-IPMN | 2 (5.1) | |
| Number of cysts, n (%) | Single | 16 (40.0) |
| Multiple | 24 (60.0) | |
| Main cyst size in mm, mean ± SD | 28.1 ± 14.4 | |
| Main cyst size in mm, median (IQR) | 21.5 (16.3-40.5) | |
| Septa, n (%) | Absent | 10 (25.0) |
| Present | 30 (75.0) | |
| Mural nodules, n (%) | Absent | 36 (90.0) |
| Present | 4 (10.0) | |
| Wall thickening, n (%) | Absent | 37 (92.5) |
| Present | 3 (7.5) | |
| MPD caliber, n (%) | Normal | 34 (85.0) |
| Dilated | 6 (15.0) | |
The clinicopathological characteristics of the study population, particularly the type of PA found in EUS among the different groups, the results of EUS-guided tissue acquisition, surgical treatment and specimen analysis, as well as information on cancer detection during follow-up and mortality rate, are summarized in Table 4. Besides the MCL group, where PA in the form of cyst(s) were present in all patients by definition, PA were also detected during the EUS exam in 10.0% and 35.0% of the subjects belonging to HR and NLOD groups, respectively. Half of the lesions in the MCL group were considered to harbor WF or HRS, in contrast to the PA found in the other groups that were all “harmless” in terms of ultrasonographic appearance (CP-like parenchymal changes, lipomatous transformation and infracentimetric simple cystic lesions). EUS-FNA/B was performed only in the MCL group in 35 of the 40 patients (87.5%), either to confirm the diagnosis or in the presence of WF or HRS (Figure 2G and H). Malignancy was detected in 3 patients; in 1, the malignancy was detected in a subsequent EUS exam performed during follow-up (this case was previously reported by our team[38]). The result of cytological exam was considered inconclusive in 37.1% of the procedures. Of the 88 patients of the cohort, 15 (17.0%) were submitted to surgical resection, and all belonged to the MCL group. These 15 patients either harbored MD or MT-IPMNs or presented a cyst with WF/HRS with suspicious or positive cytology. Malignancy was confirmed in the surgical specimen in all 3 patients with a previous positive cytological exam, and the remaining 12 had a definitive histopathological diagnosis of a low grade dysplastic MCL.
| MCL, n = 40 | HR, n = 20 | NLOD, n = 20 | CG, n = 8 | Total, n = 88 | |
| PA in EUS | |||||
| No | 0 (0.0) | 18 (90.0) | 13 (65.0) | 8 (100.0) | 39 (44.3) |
| Yes | 40 (100.0) | 2 (10.0) | 7 (35.0) | 0 (0.0) | 49 (55.7) |
| Type of PA | |||||
| Cystic lesions | 40 (100.0) | 2 (100.0) | 2 (28.6) | - | 44 (89.8) |
| CP-like parenchymal changes | 0 (0.0) | 0 (0.0) | 2 (28.6) | - | 2 (4.1) |
| Solid lesions | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | 0 (0.0) |
| Lipomatous transformation | 0 (0.0) | 0 (0.0) | 3 (42.8) | - | 3 (6.1) |
| PA with HRS / WF | |||||
| No | 20 (50.0) | 2 (100.0) | 7 (100.0) | - | 29 (59.2) |
| Yes | 20 (50.0) | 0 (0.0) | 0 (0.0) | - | 20 (40.8) |
| EUS FNA/B | |||||
| No | 5 (12.5) | 20 (100.0) | 20 (100) | 8 (100) | 53 (60.2) |
| Yes | 35 (87.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 35 (39.8) |
| Inconclusive | 13 (37.1) | - | - | - | 13 (37.1) |
| Benign | 19 (54.3) | - | - | - | 19 (54.3) |
| Malignant | 3 (8.6)1 | - | - | - | 3 (8.6)1 |
| Surgery | |||||
| No | 25 (62.5) | 20 (100) | 20 (100) | 8 (100) | 73 (83.0) |
| Yes | 15 (37.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 15 (17.0) |
| Benign/Malignant | 12 (80.0)/3 (20.0) | -/- | -/- | -/- | 12 (80.0)/3 (20.0) |
| F-up in mo | 33.3 ± 12.8 | 34.9 ± 7.1 | 27.7 ± 4.7 | 36.1 ± 5.1 | 32.6 ± 10.0 |
| Cancer detection during F-up | 1 (2.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.1) |
| Mortality | 5 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (5.7) |
The mean follow-up period of the entire cohort was 32 ± 8.6 mo and did not differ statistically among groups (P = NS). Five of the 88 individuals (5.7%) died during follow-up, but only one of these due to cancer progression. Two of the other deaths were related to post-operative complications in a 74-year-old and 75-year-old patients, one was related to a pulmonary carcinoma that developed 1 year after inclusion in a 72-year-old patient, and the remaining one corresponded to an 85-year-old patient that did not survive to an infectious respiratory insufficiency. The 2 other patients submitted to surgical resection of malignant cysts were alive and disease-free after 45 mo of follow-up.
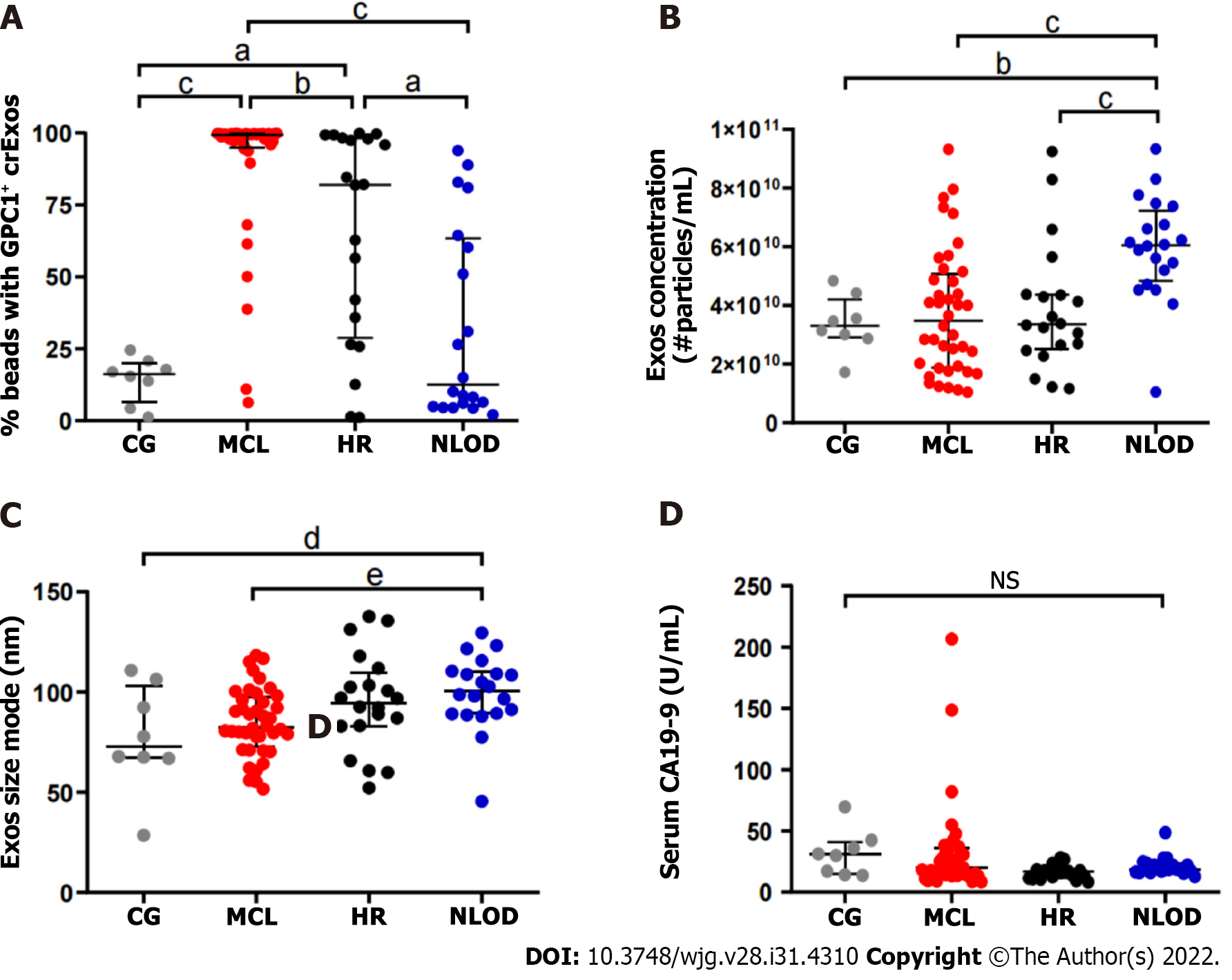
The overall median levels of GPC1+ crExos coupled to beads were statistically different among the studied groups: MCL [99.4%, interquartile range (IQR): 94.9%-99.8%], HR (82.0%, IQR: 28.9%-98.2%), and NLOD and CG groups (12.6%, IQR: 5.2%-63.4% and 16.2%, IQR: 6.6%-20.1%, respectively) (P < 0.0001) (Figure 3A and Table 5).
| Biomarker | MCL, n = 40 | HR, n = 20 | NLOD, n = 20 | CG, n = 8 | Total, n = 88 | P value |
| GPC1+ crExos, % | < 0.001 | |||||
| Median | 99.4 | 82.0 | 12.6 | 16.2 | 86.8 | |
| Min/Max | 6.4/99.9 | 1.1/99.0 | 2.1/93.9 | 1.2/24.5 | 1.1/99.9 | |
| IQR | 94.9-99.8 | 28.9-98.2 | 5.2-63.4 | 6.6-20.1 | 18.6-99.4 | |
| CA 19-9 in U/mL | NS | |||||
| Median | 20.4 | 16.9 | 18.8 | 30.6 | 18.4 | |
| Min/Max | 8.5/206.6 | 8.3/28.0 | 12.8-48.7 | 13.7-69.6 | 8.3/206.6 | |
| IQR | 14.0-35.8 | 11.7-18.1 | 16.6-23.4 | 15.0-40.8 | 14.2-27.7 | |
The crExos concentration and their median size were significantly higher in NLOD patients (6.05E+10/mL; IQR: 4.83-7.22 and 100.7 nm; IQR: 89.2-110.1, respectively) compared to all other groups (P < 0.001 and P = 0.012, respectively) (Figure 3B and C and Table 6).
| MCL, n = 40 | HR, n = 20 | NLOD, n = 20 | CG, n = 8 | Total, n = 88 | P value | |
| Size in nm | 0.012 | |||||
| Median | 82.5 | 94.7 | 100.7 | 72.8 | 90.5 | |
| IQR | 72.8-97.6 | 83.0-109.7 | 89.2-110.1 | 67.0-102.8 | 77.9-103.2 | |
| Concentration as particles E + 10/mL | < 0.001 | |||||
| Median | 3.48 | 3.36 | 6.05 | 3.30 | 4.10 | |
| IQR | 1.88-5.08 | 2.51-4.37 | 4.83-7.22 | 2.91-4.20 | 2.60-5.69 | |
In turn, overall median levels of CA 19-9 were 18.4 U/mL (IQR: 8.3-206) and did not differ statistically between groups: MCL: 20.4 U/mL (IQR: 14.0-35.8), HR: 16.9 U/mL (IQR: 11.7-18.1), NLOD: 18.8 U/mL (IQR: 12.8-48.7) and CG: 30.6 U/mL (IQR: 13.7-69.6) (P = NS) (Figure 3D and Table 5). All CA 19-9 median levels were considered in the normal range considering the clinical standard cut-off (37 U/mL).
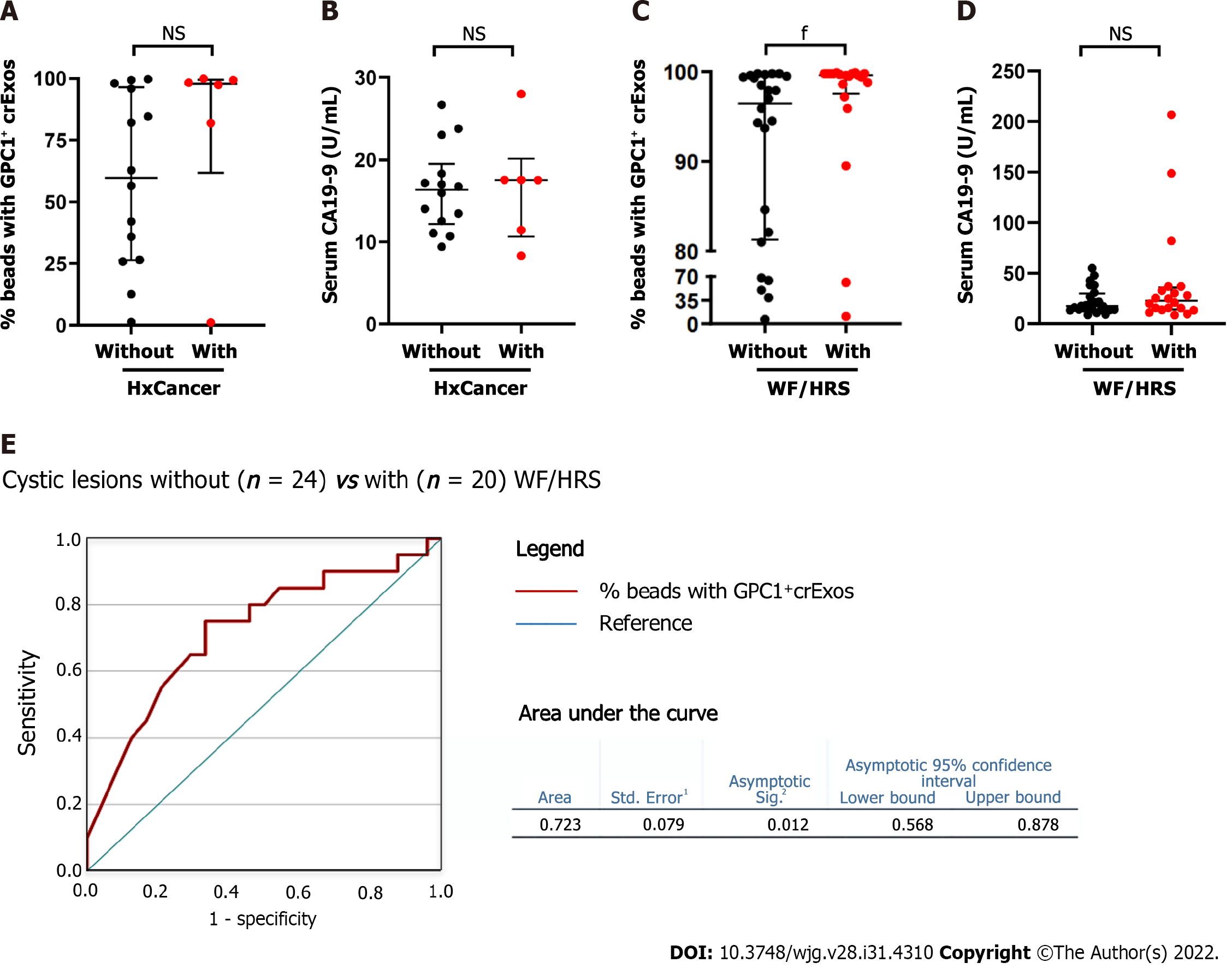
We further investigated the profile of both biomarkers in a sub-analysis in two distinct settings. First, among the HR group, we evaluated GPC1+ crExos and CA 19-9 levels according to the presence or absence of personal cancer history (Figure 4A and B). In the individuals with a positive history of cancer (5 colorectal and 1 cancer of the endometrium), although not statistically significant, we observed higher median levels of GPC1+ crExos (97.9%; IQR: 61.7%-99.5%) compared to those with a negative history (n = 14) (59.7%; IQR: 26.3%-96.4%) (P = 0.21). In relation to CA 19-9 median levels, they did not differ among these two subgroups (17.5 U/mL; IQR: 10.7%-20.1% and 16.3 U/mL; IQR: 12.2%-19.5%, respectively) (P = NS).
Second, in the total number of patients harboring pancreatic cystic lesions (40 in the MCL group, 2 diagnosed in the HR group, and 2 in the NLOD group), we studied the levels of GPC1+ crExos and CA 19-9 according to the presence or absence of WF/HRS in the EUS examination (Figure 4C and D). When WF or HRS were present (n = 20), patients presented with statistically significant higher median levels of GPC1+ crExos (99.6%; IQR: 97.6%-99.8%) compared to those with endosonographic “harmless” lesions (n = 24) (96.5%; IQR: 81.3%-99.5%) (P = 0.011) (Figure 4C). The levels of GPC1+ crExos presented an AUROC value of 0.723 in differentiating the group of cystic lesions with WF/HRS from those without (sensitivity 75.0% and specificity 67.7% for a cut-off of 98.5% (P = 0.012) (Figure 4E). CA 19-9 median levels did not statistically differ between these two subgroups (22.9 U/mL; IQR: 14.1-35.8 and 17.4 U/mL; IQR: 13.7-29.8, respectively) (P = NS) (Figure 4D).
MCLs are established as PDAC precursor lesions, with different risks considering morphologic appearance as well as cytological findings resulting from guided tissue acquisition. While the diagnostic and management of pancreatic cysts differs between international guidelines[29,39-42], they all agree that the risk of malignancy should be based, essentially, in the assessment of the classical WF/HRS, which was initially defined in the Fukuoka consensus[43].
Our cohort mainly constituted IPMNs, most of which were BD-IPMN type and half of the lesions presented with WF and/or HRS[29]. Malignancy was suspected by EUS in two advanced lesions with main duct involvement, which were confirmed by cytology. Interestingly, the third confirmed malignant cyst corresponded to a case we have previously reported[38], and was diagnosed during a follow-up examination of an apparently “inoffensive” cystic lesion in the index EUS. Our results have confirmed the limited utility of the use of cytology alone for cyst etiological characterization[44,45], as 37% of the results of EUS-FNA of the cyst fluid were considered inconclusive by pathologists. This limitation of cyst fluid cytological analysis tends to be overwhelmed by the referral for surgical resection of indeterminate suspicious lesions. In fact, 15 of the 40 patients with MCL proposed for surgical treatment, after evaluation by a multidisciplinary team, corresponded to 3 cases with a positive cytology for malignancy and 12 patients with indeterminate/inconclusive results of EUS-FNA. The surgical specimen confirmed malignancy in all 3 cases with previous diagnosis and in the remaining 12 revealed benign IPMNs with low grade dysplasia. The overtreatment of some of these patients is an important issue, as the morbidity and mortality of pancreatic surgery is not negligible[46,47]. In the present cohort, 2 of the 5 deaths recorded were precisely due to complications after surgery, both in patients harboring benign lesions. This observation supports the drive of research, justifying the need to evaluate the role of upcoming biomarkers, like exosomal GPC1, in complement with EUS evaluation, for the stratification of MCL, ultimately contributing to refine the criteria for surgical treatment or continued surveillance. In this setting, our preliminary results initially reported were promising, as the levels of GPC1+ crExos were equally elevated in patients with PDAC and with MCLs[48,49]. The findings in the present study confirm these observations, as the median levels of GPC1+ crExos observed in the MCL group (significantly higher than in the other studied groups) were in the same magnitude as the ones registered for PDAC patients, that we have recently reported using the same methodology[28]. Moreover, when we analyzed the levels GPC1+ crExos among all pancreatic cystic lesions (MCL group plus those detected in the screening EUS in HR and NLOD groups), we found that they were statistically more elevated when WF/HRS were present, suggesting that this biomarker can, in fact, have a role in the risk stratification of these PDAC precursor lesions.
This is the first study to access the profile of GPC1+ crExos in HR and NLOD individuals, and our results showed that the median levels of this biomarker were statistically more elevated among the HR group when compared to NLOD patients and controls. Considering our previous published observations[28], these levels of GPC1+ crExos in HR individuals were also higher than those registered among patients with CP (28.4%), but not as elevated as in those with PDAC (99.7%). Interestingly, our results also showed a tendency of the individuals with a history of previous cancer (mainly colorectal) to present higher levels of GPC1+ crExos when compared to those without. While PA were detected in only 10% among HR individuals, all representing simple cystic lesions, these observations may suggest a potential role of GPC1+ crExos as a marker of genetically determined predisposition for cancer development, even in the absence of “harmful” pancreatic lesions.
Important studies have recently been published[24-26] and prospective investigational projects are ongoing[50] demonstrating the importance to access the magnitude of the risk to develop PDAC among NLOD patients and to define PDAC incidence during long-term follow-up. This justified the inclusion of NLOD patients in our investigation cohort. We found PA in EUS in 35% of the NLOD patients, comprising simple cystic lesions, CP-like changes and lipomatous parenchymal transformations, all of them considered “harmless”, not requiring further investigation. Despite the highest median values of exosomes’ size and particle concentration being registered in this group, the percentage of crExos positive for GPC1 was low and not statistically different from the CG. This finding is in accordance with the lack of sufficient evidence, at the present, to include these patients in regular screening programs for PDAC early detection.
Some limitations of this study should be pointed out, namely the sample size, the possibility of a referral bias giving the nature of our tertiary center, the relatively short period of patients’ follow-up, and the cut-off found to stratify patients with cystic lesions and WF/HRS from those without. Also, the impossibility to include PanIN lesions in the study that would certainly power the analysis of GPC1 profile among PDAC pathological precursors. Lastly, the technique of exosomes isolation and analysis can still hamper its translation to clinical use. Despite these restraints, our results open the door for future studies to determine the value of this biomarker in the stratification of risk groups.
In summary, we demonstrate that GPC1+ crExos levels are elevated in MCL, in the same magnitude of PDAC patients. These levels were statistically higher in cysts harboring WF/HRS. High levels were also registered among individuals with HR for PDAC (predominantly in those with history of previous cancer). Longitudinal studies will clarify the potential of exosomal GPC1 as a biomarker for the diagnosis and stratification of PDAC precursor lesions, as well as in signaling individuals with genetic predisposition for this neoplasia, ultimately contributing to refine screening and surveillance strategies.
Most of the patients with pancreatic ductal adenocarcinoma (PDAC) are diagnosed with advanced disease at which stage treatment is inefficient. In order to improve survival, new strategies to identify and stratify individuals at risk of developing PDAC [i.e. individuals with hereditary risk (HR), mucinous cystic lesions (MCLs) and new-late onset diabetes mellitus (NLOD)] are urgently needed. Endoscopic ultrasound (EUS) is one of the imaging modalities with better performance for the study of the pancreas. In turn, carbohydrate antigen 19-9 (CA 19-9), the only biomarker approved for clinical use, is still imperfect as it lacks sensitivity and specificity. Glypican-1 (GPC1) represents a promising candidate since it has been demonstrated to be overexpressed in circulating exosomes (crExos) of patients with PDAC, possibly allowing early detection.
To contribute to further evaluate the capacity of GPC1+ crExos to act as a potential biomarker for the investigation of individuals at risk for PDAC development, allowing for the early detection of the disease or its high-grade precursor lesions. Ultimately, we aim to contribute to improve patient’s survival.
We aimed to determine the capacity of GPC1+ crExos to identify individuals at higher risk to develop PDAC by comparing EUS pancreatic abnormalities (PA) with its levels within specific risk groups: HR, MCL and NLOD.
We conducted a cross-sectional study with a prospective unicentric cohort including individuals with HR, MCL and NLOD, along with a control group (CG). All subjects were submitted to EUS at time of inclusion, with detailed characterization of detected PA. At the same time, blood samples were analyzed for GPC1+ crExos and CA 19-9 by flow cytometry and ELISA, respectively. EUS-guided tissue acquisition was performed whenever necessary. SPSS® 27.0 version was used for statistical analysis and all graphs were created using GraphPad Prism 7.
We found that: Individuals harboring MCL globally presented with the highest levels of GPC1+ crExos; these levels were increased in the presence of worrisome features and or high-risk stigmata characterized by EUS; GPC1+ crExos levels were also elevated among individuals with HR for PDAC (predominantly in those with personal history of cancer), even in absence of harmful PA in EUS examination; and NLOD patients presented with GPC1+ crExos levels as low as those observed in the CG.
Our study supports the role of GPC1+ crExos in the diagnosis and stratification of PDAC precursor lesions, namely MCL, and its eventual capacity in signaling individuals with genetic predisposition for this neoplasia, even when no harmful PA are detected by EUS.
Our data encourage longitudinal studies to confirm the potential of exosomal GPC1 as a biomarker to be used in the future in the management of individuals at risk for PDAC.
The authors acknowledge the support of the Translational Cytometry i3S Scientific Platform and the collaboration of Catarina Meireles MD, Elvira Sampaio MD, Liliana Silva MD and Mariana Santos MD in patient management.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Portugal
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen SY, China; Villa E, United States S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64217] [Article Influence: 16054.3] [Reference Citation Analysis (174)] |
| 2. | Moutinho-Ribeiro P, Coelho R, Giovannini M, Macedo G. Pancreatic cancer screening: Still a delusion? Pancreatology. 2017;17:754-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (2)] |
| 3. | Singhi AD, Koay EJ, Chari ST, Maitra A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology. 2019;156:2024-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 503] [Article Influence: 83.8] [Reference Citation Analysis (1)] |
| 4. | Moutinho-Ribeiro P, Iglesias-Garcia J, Gaspar R, Macedo G. Early pancreatic cancer - The role of endoscopic ultrasound with or without tissue acquisition in diagnosis and staging. Dig Liver Dis. 2019;51:4-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Bhutani MS, Koduru P, Joshi V, Saxena P, Suzuki R, Irisawa A, Yamao K. The role of endoscopic ultrasound in pancreatic cancer screening. Endosc Ultrasound. 2016;5:8-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Luz LP, Al-Haddad MA, Sey MS, DeWitt JM. Applications of endoscopic ultrasound in pancreatic cancer. World J Gastroenterol. 2014;20:7808-7818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Hruban RH, Maitra A, Kern SE, Goggins M. Precursors to pancreatic cancer. Gastroenterol Clin North Am. 2007;36:831-849, vi. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Pereira SP, Oldfield L, Ney A, Hart PA, Keane MG, Pandol SJ, Li D, Greenhalf W, Jeon CY, Koay EJ, Almario CV, Halloran C, Lennon AM, Costello E. Early detection of pancreatic cancer. Lancet Gastroenterol Hepatol. 2020;5:698-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 299] [Article Influence: 59.8] [Reference Citation Analysis (1)] |
| 9. | Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS, Macari M, Megibow AJ, Miller FH, Mortele KJ, Merchant NB, Minter RM, Tamm EP, Sahani DV, Simeone DM. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the society of abdominal radiology and the american pancreatic association. Gastroenterology. 2014;146:291-304.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 10. | Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol. 2012;3:105-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 351] [Reference Citation Analysis (3)] |
| 11. | Yadav DK, Bai X, Yadav RK, Singh A, Li G, Ma T, Chen W, Liang T. Liquid biopsy in pancreatic cancer: the beginning of a new era. Oncotarget. 2018;9:26900-26933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Buscail E, Maulat C, Muscari F, Chiche L, Cordelier P, Dabernat S, Alix-Panabières C, Buscail L. Liquid Biopsy Approach for Pancreatic Ductal Adenocarcinoma. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2108] [Cited by in RCA: 2187] [Article Influence: 218.7] [Reference Citation Analysis (0)] |
| 14. | Lewis JM, Vyas AD, Qiu Y, Messer KS, White R, Heller MJ. Integrated Analysis of Exosomal Protein Biomarkers on Alternating Current Electrokinetic Chips Enables Rapid Detection of Pancreatic Cancer in Patient Blood. ACS Nano. 2018;12:3311-3320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 220] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 15. | Xiao D, Dong Z, Zhen L, Xia G, Huang X, Wang T, Guo H, Yang B, Xu C, Wu W, Zhao X, Xu H. Combined Exosomal GPC1, CD82, and Serum CA19-9 as Multiplex Targets: A Specific, Sensitive, and Reproducible Detection Panel for the Diagnosis of Pancreatic Cancer. Mol Cancer Res. 2020;18:300-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Qian JY, Tan YL, Zhang Y, Yang YF, Li XQ. Prognostic value of glypican-1 for patients with advanced pancreatic cancer following regional intra-arterial chemotherapy. Oncol Lett. 2018;16:1253-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Zhou CY, Dong YP, Sun X, Sui X, Zhu H, Zhao YQ, Zhang YY, Mason C, Zhu Q, Han SX. High levels of serum glypican-1 indicate poor prognosis in pancreatic ductal adenocarcinoma. Cancer Med. 2018;7:5525-5533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Frampton AE, Prado MM, López-Jiménez E, Fajardo-Puerta AB, Jawad ZAR, Lawton P, Giovannetti E, Habib NA, Castellano L, Stebbing J, Krell J, Jiao LR. Glypican-1 is enriched in circulating-exosomes in pancreatic cancer and correlates with tumor burden. Oncotarget. 2018;9:19006-19013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 19. | Lucien F, Lac V, Billadeau DD, Borgida A, Gallinger S, Leong HS. Glypican-1 and glycoprotein 2 bearing extracellular vesicles do not discern pancreatic cancer from benign pancreatic diseases. Oncotarget. 2019;10:1045-1055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley JW, Kamel I, Nio Y, Schulick RS, Bassi C, Kluijt I, Levy MJ, Chak A, Fockens P, Goggins M, Bruno M; International Cancer of Pancreas Screening (CAPS) Consortium. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62:339-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 564] [Article Influence: 47.0] [Reference Citation Analysis (1)] |
| 21. | Goggins M, Overbeek KA, Brand R, Syngal S, Del Chiaro M, Bartsch DK, Bassi C, Carrato A, Farrell J, Fishman EK, Fockens P, Gress TM, van Hooft JE, Hruban RH, Kastrinos F, Klein A, Lennon AM, Lucas A, Park W, Rustgi A, Simeone D, Stoffel E, Vasen HFA, Cahen DL, Canto MI, Bruno M; International Cancer of the Pancreas Screening (CAPS) consortium. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut. 2020;69:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 392] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 22. | Aslanian HR, Lee JH, Canto MI. AGA Clinical Practice Update on Pancreas Cancer Screening in High-Risk Individuals: Expert Review. Gastroenterology. 2020;159:358-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 23. | Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134:981-987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 469] [Cited by in RCA: 420] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 24. | Sharma A, Smyrk TC, Levy MJ, Topazian MA, Chari ST. Fasting Blood Glucose Levels Provide Estimate of Duration and Progression of Pancreatic Cancer Before Diagnosis. Gastroenterology. 2018;155:490-500.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 25. | Sharma A, Kandlakunta H, Nagpal SJS, Feng Z, Hoos W, Petersen GM, Chari ST. Model to Determine Risk of Pancreatic Cancer in Patients With New-Onset Diabetes. Gastroenterology. 2018;155:730-739.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 237] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 26. | Boursi B, Finkelman B, Giantonio BJ, Haynes K, Rustgi AK, Rhim AD, Mamtani R, Yang YX. A Clinical Prediction Model to Assess Risk for Pancreatic Cancer Among Patients With New-Onset Diabetes. Gastroenterology. 2017;152:840-850.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 27. | Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, Petersen GM. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129:504-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 368] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 28. | Moutinho-Ribeiro P, Adem B, Batista I, Silva M, Silva S, Ruivo CF, Morais R, Peixoto A, Coelho R, Costa-Moreira P, Lopes S, Vilas-Boas F, Durães C, Lopes J, Barroca H, Carneiro F, Melo SA, Macedo G. Exosomal glypican-1 discriminates pancreatic ductal adenocarcinoma from chronic pancreatitis. Dig Liver Dis. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1150] [Article Influence: 143.8] [Reference Citation Analysis (1)] |
| 30. | Reddy RP, Smyrk TC, Zapiach M, Levy MJ, Pearson RK, Clain JE, Farnell MB, Sarr MG, Chari ST. Pancreatic mucinous cystic neoplasm defined by ovarian stroma: demographics, clinical features, and prevalence of cancer. Clin Gastroenterol Hepatol. 2004;2:1026-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 159] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | McCarty TR, Garg R, Rustagi T. Pancreatic cyst fluid glucose in differentiating mucinous from nonmucinous pancreatic cysts: a systematic review and meta-analysis. Gastrointest Endosc. 2021;94:698-712.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 32. | Overbeek KA, Levink IJM, Koopmann BDM, Harinck F, Konings ICAW, Ausems MGEM, Wagner A, Fockens P, van Eijck CH, Groot Koerkamp B, Busch ORC, Besselink MG, Bastiaansen BAJ, van Driel LMJW, Erler NS, Vleggaar FP, Poley JW, Cahen DL, van Hooft JE, Bruno MJ; Dutch Familial Pancreatic Cancer Surveillance Study Group. Long-term yield of pancreatic cancer surveillance in high-risk individuals. Gut. 2022;71:1152-1160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 104] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 33. | DaVee T, Coronel E, Papafragkakis C, Thaiudom S, Lanke G, Chakinala RC, Nogueras González GM, Bhutani MS, Ross WA, Weston BR, Lee JH. Pancreatic cancer screening in high-risk individuals with germline genetic mutations. Gastrointest Endosc. 2018;87:1443-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S15-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1098] [Cited by in RCA: 1859] [Article Influence: 464.8] [Reference Citation Analysis (0)] |
| 35. | Lorenzo D, Rebours V, Maire F, Palazzo M, Gonzalez JM, Vullierme MP, Aubert A, Hammel P, Lévy P, de Mestier L. Role of endoscopic ultrasound in the screening and follow-up of high-risk individuals for familial pancreatic cancer. World J Gastroenterol. 2019;25:5082-5096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, Topazian M, Takahashi N, Fletcher J, Petersen G, Klein AP, Axilbund J, Griffin C, Syngal S, Saltzman JR, Mortele KJ, Lee J, Tamm E, Vikram R, Bhosale P, Margolis D, Farrell J, Goggins M; American Cancer of the Pancreas Screening (CAPS) Consortium. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796-804; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 486] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 37. | Pitman MB, Centeno BA, Ali SZ, Genevay M, Stelow E, Mino-Kenudson M, Castillo CF, Schmidt CM, Brugge WR, Layfield LJ. Standardized terminology and nomenclature for pancreatobiliary cytology: The Papanicolaou Society of Cytopathology Guidelines. Cytojournal. 2014;11:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Moutinho-Ribeiro P, Costa-Moreira P, Adem B, Batista I, Almeida M, Barroca H, Lopes J, Carneiro F, Melo SA, Macedo G. Exosomal glypican-1 for risk stratification of pancreatic cystic lesions: A case of pathological progression in the absence of any suspicious imaging finding. Pancreatology. 2020;20:571-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Vege SS, Ziring B, Jain R, Moayyedi P; Clinical Guidelines Committee; American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819-22; quize12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 755] [Article Influence: 75.5] [Reference Citation Analysis (1)] |
| 40. | Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 420] [Article Influence: 60.0] [Reference Citation Analysis (1)] |
| 41. | Megibow AJ, Baker ME, Morgan DE, Kamel IR, Sahani DV, Newman E, Brugge WR, Berland LL, Pandharipande PV. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol. 2017;14:911-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 215] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 42. | European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 884] [Article Influence: 126.3] [Reference Citation Analysis (1)] |
| 43. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K; International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1613] [Article Influence: 124.1] [Reference Citation Analysis (0)] |
| 44. | Thornton GD, McPhail MJ, Nayagam S, Hewitt MJ, Vlavianos P, Monahan KJ. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: a meta-analysis. Pancreatology. 2013;13:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 45. | Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 900] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 46. | Nimptsch U, Krautz C, Weber GF, Mansky T, Grützmann R. Nationwide In-hospital Mortality Following Pancreatic Surgery in Germany is Higher than Anticipated. Ann Surg. 2016;264:1082-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 172] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 47. | Kimura W, Miyata H, Gotoh M, Hirai I, Kenjo A, Kitagawa Y, Shimada M, Baba H, Tomita N, Nakagoe T, Sugihara K, Mori M. A pancreaticoduodenectomy risk model derived from 8575 cases from a national single-race population (Japanese) using a web-based data entry system: the 30-day and in-hospital mortality rates for pancreaticoduodenectomy. Ann Surg. 2014;259:773-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 300] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 48. | Moutinho-Ribeiro P, Santos A, Batista I, Adem B, Silva S, Morais R, Coelho R, Lopes S, Vilas-Boas F, Baptista M, Barroca H, Machado J, Carneiro F, Melo SA, Macedo G. Exosomal Glypican-1 and Pancreatic Adenocarcinoma: Prime Time for Early Diagnosis? Am J Gastroenterol. 2018;113:S44. [DOI] [Full Text] |
| 49. | Moutinho-Ribeiro P, Silva S, Adem B, Silva M, Lopes S, Vilas-Boas F, Melo S, Macedo G. Glipican-1 circulating exosomes levels are higher in pancreatic adenocarcinoma and cystic mucinous neoplasms than in other associated risk groups for pancreatic cancer: Clues for early diagnosis? Pancreatology. 2017;17:S63-S64. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 50. | Maitra A, Sharma A, Brand RE, Van Den Eeden SK, Fisher WE, Hart PA, Hughes SJ, Mather KJ, Pandol SJ, Park WG, Feng Z, Serrano J, Rinaudo JAS, Srivastava S, Chari ST; Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer (CPDPC). A Prospective Study to Establish a New-Onset Diabetes Cohort: From the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas. 2018;47:1244-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |