Published online Feb 14, 2018. doi: 10.3748/wjg.v24.i6.693
Peer-review started: September 14, 2017
First decision: October 24, 2017
Revised: November 6, 2017
Accepted: November 28, 2017
Article in press: November 28, 2017
Published online: February 14, 2018
Processing time: 144 Days and 23.7 Hours
To elucidate the mechanism of patchouli alcohol (PA) in treatment of rat models of diarrhea-predominant irritable bowel syndrome (IBS-D).
We studied the effects of PA on colonic spontaneous motility using its cumulative log concentration (3 × 10−7 mol/L to 1 × 10−4 mol/L). We then determined the responses of the proximal and distal colon segments of rats to the following stimuli: (1) carbachol (1 × 10−9 mol/L to 1 × 10−5 mol/L); (2) neurotransmitter antagonists including Nω-nitro-
PA exerted a concentration-dependent inhibitory effect on the spontaneous contraction of the colonic longitudinal smooth muscle, and the half maximal effective concentration (EC50) was 41.9 μmol/L. In comparison with the KCl-treated IBS-D group, the contractile response (mg contractions) in the PA + KCl-treated IBS-D group (11.87 ± 3.34) was significantly decreased in the peak tension (P < 0.01). Compared with CCh-treated IBS-D rat colon, the cholinergic contractile response of IBS-D rat colonic smooth muscle (EC50 = 0.94 μmol/L) was significantly decreased by PA (EC50 = 37.43 μmol/L) (P < 0.05). Lack of nitrergic neurotransmitter release in stress-induced IBS-D rats showed contraction effects on colonic smooth muscle. Pretreatment with PA resulted in inhibitory effect on
PA application may serve as a new therapeutic approach for IBS-D.
Core tip: We reported the results from an isolated colonic smooth muscle experiment in a chronic wrap-restraint stress-induced rat model of diarrhea-predominant irritable bowel syndrome (IBS-D). The model enabled us to study the possible mechanisms underlying IBS-D and the inhibitory effects of patchouli alcohol (PA) on an isolated IBS-D rat colon. This study demonstrated for the first time that the PA was involved in cholinergic and nonadrenergic, noncholinergic neurotransmitter regulation in the enteric nervous system (ENS) in vitro. PA acts as a neurotransmitter agent in ENS. The results suggest that PA is a new treatment option for IBS-D.
- Citation: Zhou TR, Huang JJ, Huang ZT, Cao HY, Tan B. Inhibitory effects of patchouli alcohol on stress-induced diarrhea-predominant irritable bowel syndrome. World J Gastroenterol 2018; 24(6): 693-705
- URL: https://www.wjgnet.com/1007-9327/full/v24/i6/693.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i6.693
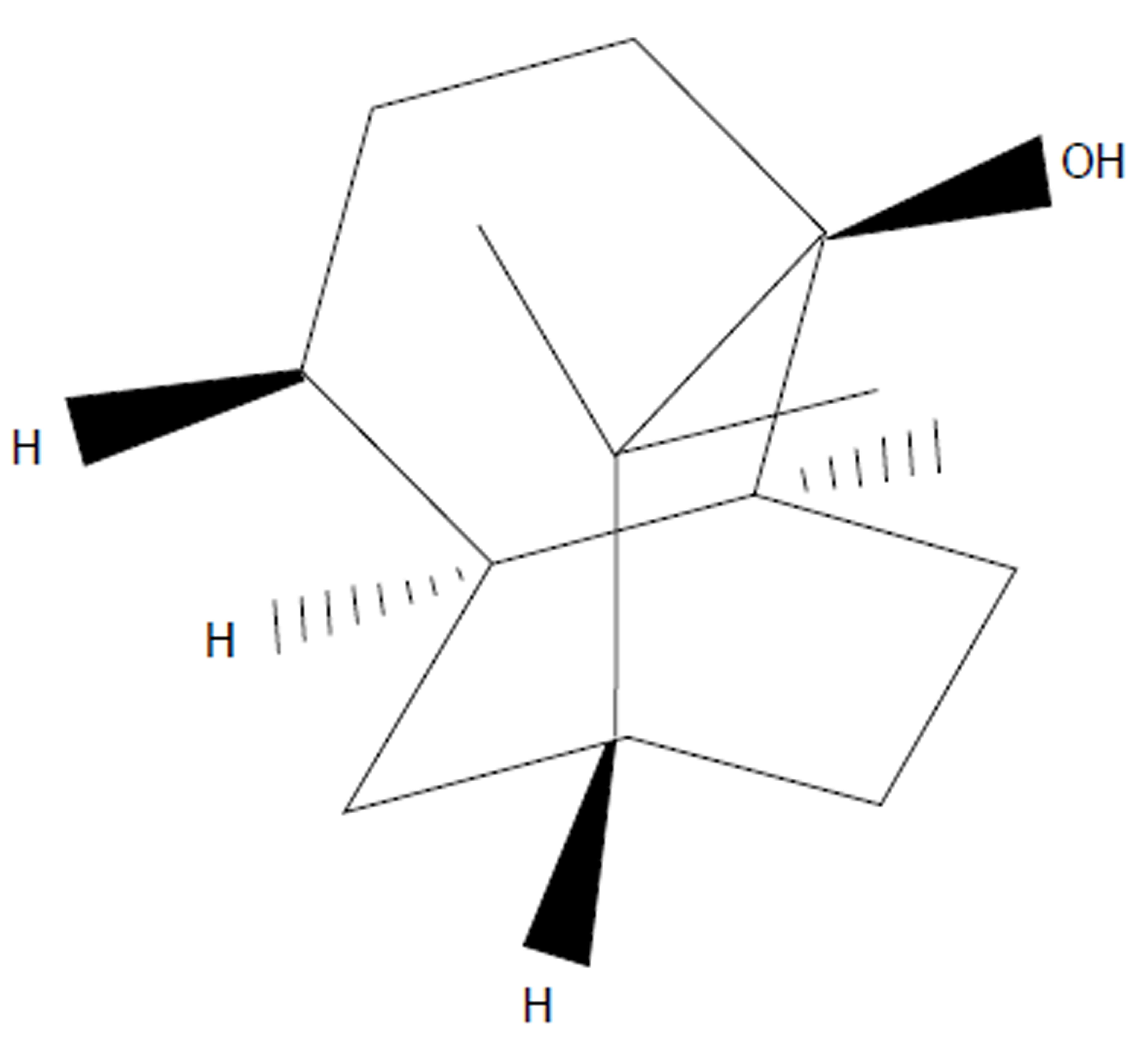
Pogostemonis Herba is the dry aerial part of Pogostemon cablin (Blanco) Benth, which is a well-known medicinal herb in Asian countries and has been widely used to treat functional gastrointestinal disorders[1]. Patchouli alcohol (PA; its structure is shown in Figure 1) is a tricyclic sesquiterpene extracted from Pogostemonis Herba; this compound is also the major active ingredient of Pogostemonis Herba. In European countries, PA is widely used in products for daily use and cosmetics[2]. Previous studies on PA have shown its component’s pharmacological effects, including immunomodulatory, anti-inflammatory, antioxidative, antimicrobial, and antitumor activities; however, the use of PA in the medical field has not been reported[3]. In two previous works[4,5], PA affects the calcium ion antagonism and exerts antiemetic properties by ameliorating the excessive contraction in the digestive organ smooth muscle. These results implied that PA might play a role in neurotransmission regulation of the digestive system smooth muscle. However, no further research on the pharmacological effects of PA in neurotransmission regulation has been reported.
Irritable bowel syndrome (IBS) is a prevalent functional bowel disorder, and diarrhea-predominant IBS (IBS-D) is a major subtype of this disease. This subtype not only inflicts a significant socioeconomic burden[6], but also severely decreases the quality of life in patients with this condition[7]. At present, the precise pathophysiological mechanism of IBS-D remains unclear. Psychosocial stress, neuroendocrine abnormality, and disturbed gastrointestinal motility are potential modes of pathogenesis[8]. Recent studies[9,10] have shown that the abnormal changes in peripheral nerve factors can directly affect the normal movement and secretion of the gastrointestinal tract and sensitivity of gastrointestinal wall to mechanical or chemical stimuli. The myenteric plexus in the intestinal tract regulates the intestinal motility. Neuropsychological factors have been given serious attention in clinical and basic medical studies on IBS-D. Several drugs that target the neurotransmitter receptors, such as loperamide, eluxadoline, alosetron, and some antidepressants, are considered as the treatment options for patients with IBS-D[11]. Additionally, medical food, medications, and psychological therapies can alleviate the symptoms of IBS-D to a certain extent[12]. However, a standard treatment algorithm has not been established for IBS-D[13]. The present in vitro study was based on our in vivo model. Our in vivo study showed that compared with the control group, significant visceral hypersensitivity in rat model rats was observed by the abdominal withdrawal reflex score on the 21st day to the 28th day of the model establishment. The frequency of defecation in model rats increased significantly from the 14th day to the 28th day of model establishment. The stool of the model rats was mostly mushy. HE staining indicated that the intestine tissue samples in model rats showed no evident pathological changes compared with rats in the control group.
Therefore, based on the literature and our previous work, we hypothesize that the action mechanism of PA to treat IBS-D is related to the drug regulation of the neural pathways in the enteric nervous system (ENS) via its influence on neurotransmitter release. Therefore, the present study aims to further explore the effects of PA on isolated IBS-D rat colon in vitro. Our results also indicated that PA plays a specific role in neurotransmitter release regulation in ENS.
Male Sprague-Dawley (SD) rats aged four weeks and weighing 100 ± 10 g were purchased and cared for in strict compliance with the Guide to Animal Use and Care published by the Research Center for Laboratory Animals (Guangzhou University of Chinese Medicine, China). Rats were maintained under a constant 12 h/12 h light/dark cycle at an environmental temperature of 20 °C to 25 °C and humidity of 50% to 70%. The Animal Care and Use Committee of the Guangzhou University of Chinese Medicine approved all procedures used in this study.
After the 7-day adaptation, the male SD rats were randomly divided into two groups as follows: control and model groups. Model group rats were subjected to a 2 h wrap-restraint period daily for 14 d. Then, these rats underwent a fourteen-day rest period.
In our previous studies, we confirmed PA (purity > 99.0%) by its melting point, infrared spectrometry, 1H and 13C nuclear magnetic resonance, and mass spectrometry analyses[14,15]. The following drugs were used. Dimethyl sulfoxide (DMSO) was used as medium to dissolve PA (DMSO < 0.2% in all experiments). Carbachol (CCh; Sigma-Aldrich, United States), Nω-Nitro-
After euthanasia by CO2 asphyxiation, the rats were weighed, and colons and jejuna were excised and placed in 37 °C Kreb’s solution (NaCl, 120 mmol/L; KCl, 5.9 mmol/L; NaHCO3, 25 mmol/L; Na2HPO4·12H2O, 1.2 mmol/L; MgCl2·6H2O, 1.2 mmol/L; CaCl2, 2.5 mmol/L; and dextrose, 11.5 mmol/L). At the end of the experiments, the weight of each tissue from the colon and jejunum was recorded after blotting on filter paper.
Full-thickness colonic and jejunal segments were isolated from the control and IBS-D model rats. Hung in the direction of the longitudinal muscle, one end of each tissue was attached to a fixed hook, whereas the other end of each strip was attached to a flexible hook with surgical suture. Each surgical suture was affixed in a force transducer that measured the isometric tension (Harvard Apparatus, United States). Afterward, the tissues were transferred to an organ bath containing Kreb’s solution, maintained at 37 °C, and continuously gassed with carbogen (950 mL/L O2 + 50 mL/L CO2). After a tension-free adaptive treatment for 0.5 h, the tissues from the colon and jejunum were stretched under 1.4 g of tension and equilibrated for 1 h. Force measurements were displayed on a strip chart recorder (Harvard Apparatus, United States; LabChart, New Zealand) and were digitally acquired by computer (LabChart software).
After equilibration, PA of various concentrations (3 × 10−7 mol/L to 1 × 10−4 mol/L) were added to each bath to observe the drug effects on spontaneous contraction of the colonic longitudinal smooth muscle. At the end of each experiment, the colonic contractile responses to KCl (120 mmol/L) in the control and IBS-D model rats were compared.
In this in vitro study, CCh (1 × 10−9 mol/L to 1 × 10−5 mol/L) was added to the organ bath, and the contractile responses in the control and IBS-D model rats were compared. After the contractile response of CCh reached a plateau, the strips were washed thrice and equilibrated for 1 h in the next experiment.
After equilibration, electrical field stimulation (EFS; 40 V, 2 Hz to 30 Hz, 0.5 ms pulse duration, 10 s) was performed to elicit the nerve-mediated contraction of the colonic tissue strips. EFS was achieved through the platinum electrodes connected to a stimulator (Harvard Apparatus, United States). The inhibitory or excitatory neurotransmitter agents, such as
Tissues were pretreated at a single PA concentration of 100 μmol/L to observe its drug effects on the functions of the neurotransmitter agents, including CCh (10−9 mol/L to 10−5 mol/L),
After EFS, Kreb’s solution in each bath was collected (400 μL), frozen immediately in liquid nitrogen, and then stored at -80 °C until assay for ATP. The ATP levels in samples were determined by the luciferin-luciferase ATP bioluminescence assay kit (ATP Determination Kit, Thermo Fisher, United States). To calculate ATP release, we corrected the amounts detected in samples using the standard curve.
Data are expressed as means ± SEs around the mean. Non-pairwise comparisons were performed using the Student’s t test. ANOVA was used to test three or more variables for statistical significance. Nonlinear and linear regression analyses were also utilized as appropriate. Calculations were performed using SPSS 20.0 based on the number of individual tissue segments. A P value of < 0.05 was considered statistically significant.
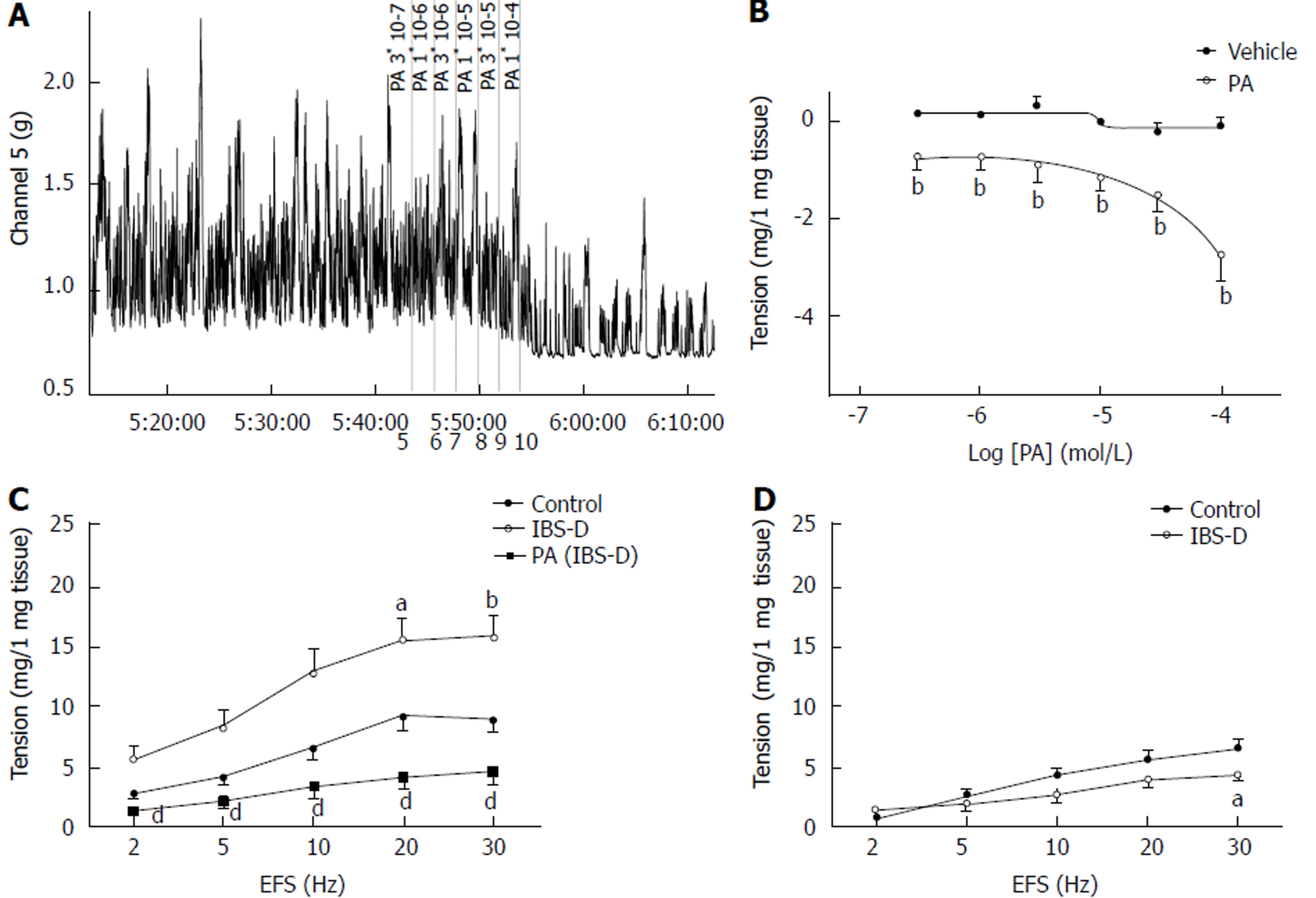
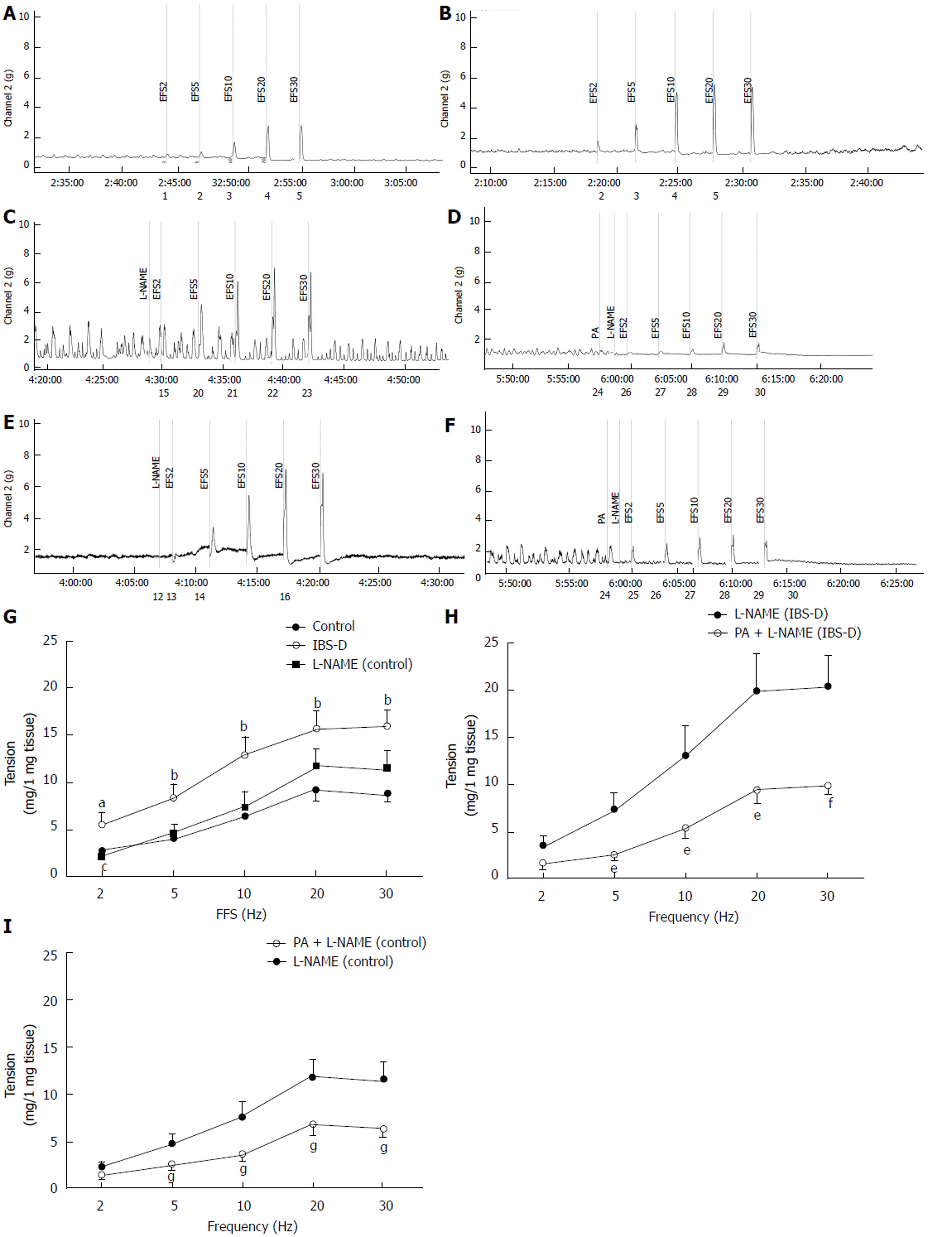
After equilibration, colonic muscle strips developed spontaneous basal tension for several minutes. A total of 6 cumulative PA concentrations (3 × 10−7 mol/L to 1 × 10−4 mol/L) caused a notable concentration-dependent decrease in the basal tension of the isolated rat colonic longitudinal muscle, and the half maximal effective concentration (EC50) was 41.9 μmol/L (P < 0.01). PA (3 × 10−6 to 1 × 10−4 mol/L) lowered the amplitude of the spontaneous contraction in the longitudinal smooth muscles of the control rats relative to that in the vehicle strips; however, the difference was insignificant (P > 0.05). The effect of PA on the frequency of the spontaneous contraction of the colonic smooth muscle did not show significant differences between groups (P > 0.05). Compared with the control group, the EFS-induced contraction of the colonic longitudinal smooth muscles in the IBS-D group significantly increased at 20 and 30 Hz (P < 0.05). In contrast, the colonic tissues of the PA-pretreated group (100 µmol/L, 1 min) showed a significantly lower EFS-induced contraction than that of the IBS-D group (P < 0.01). Meanwhile, the jejunal tissue tension was significantly lower at 30 Hz in the IBS-D group than in the control group (P < 0.05) (Figure 2).
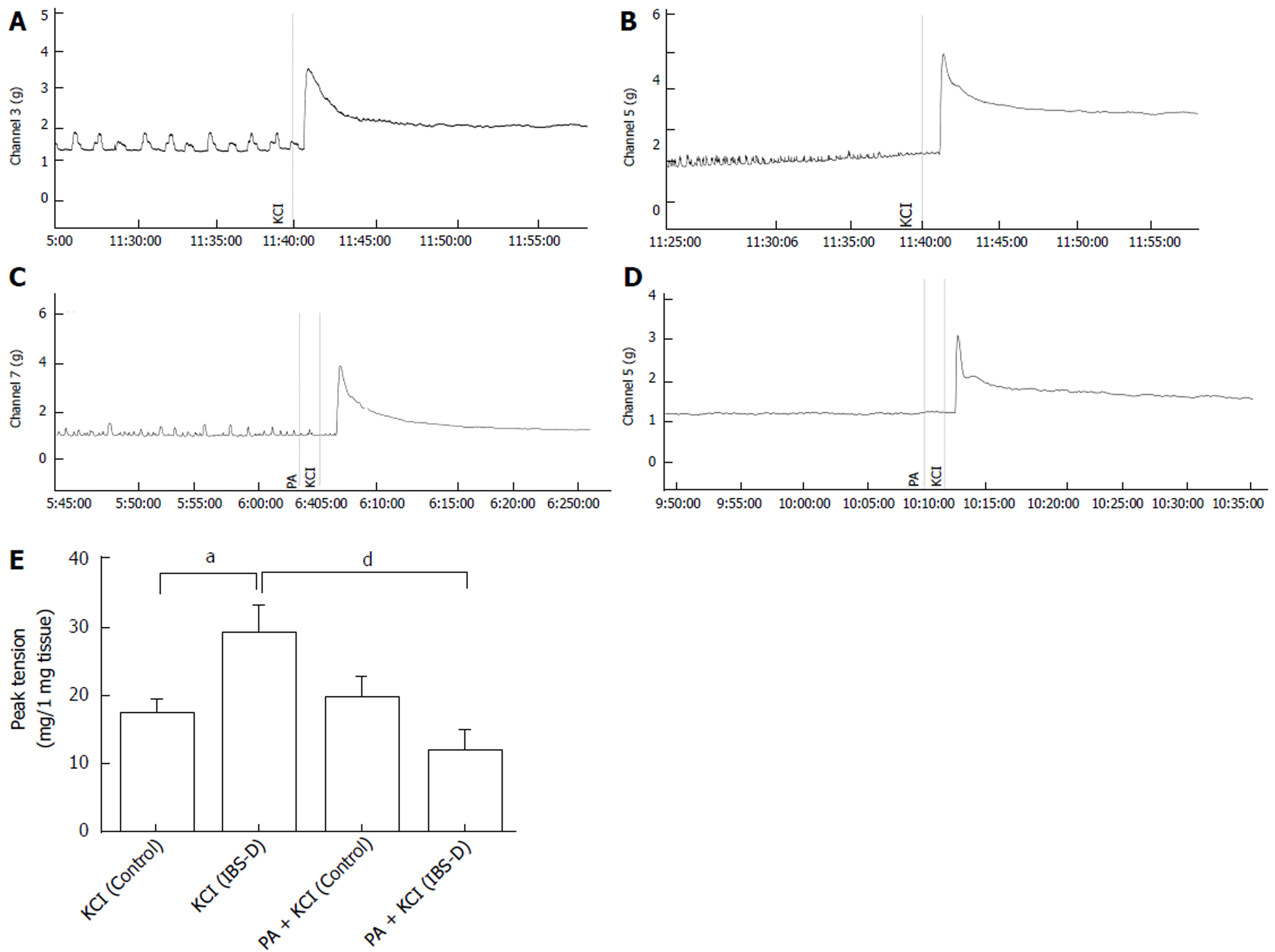
Contractile responses to KCl (120 mmol/L) were tested at the end of each experiment; the results showed significant differences between the control and IBS-D groups. The KCl peak contractile responses (mg contractions) reached 17.05 ± 2.47 and 29.00 ± 4.38 in the control and IBS-D rat colons, respectively (P < 0.05). To observe the effect of PA on the isolated colonic segment contraction induced by high extracellular KCl level, we tested the peak contractile responses of the tissues that were pretreated with PA (100 μmol/L, 1 min) before KCl administration (120 mmol/L) at the end of each experiment. KCl- and PA + KCl-treated control groups showed no significant differences (P > 0.05). However, in comparison with the KCl-treated IBS-D group, the contractile responses (mg contractions) of the PA + KCl-treated IBS-D group (11.87 ± 3.34) showed significant decrease in the peak tension (P < 0.01) (Figure 3).
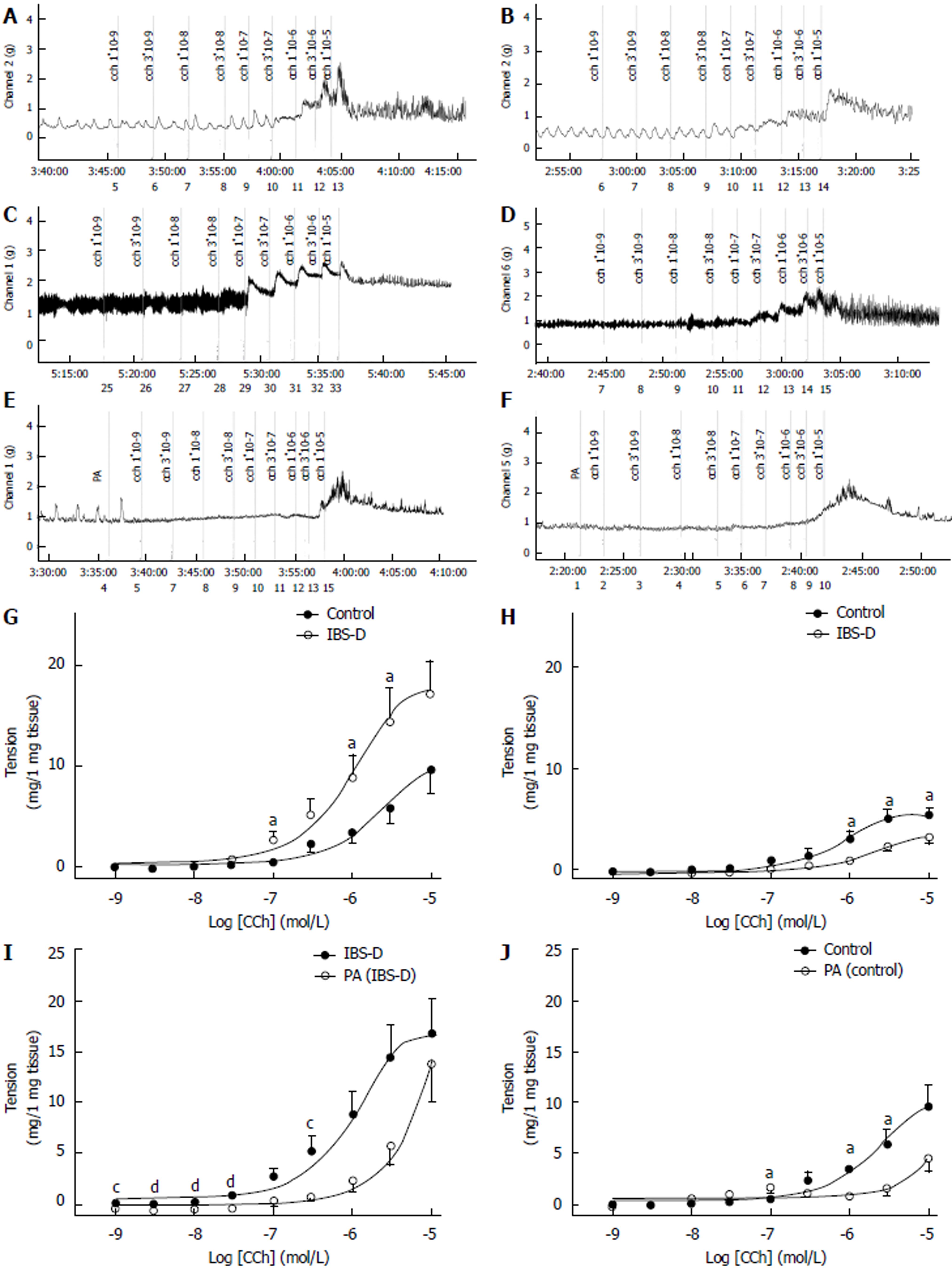
Cholinergic neurotransmission produced an inverse alteration in the colon and jejunum of IBS-D rats. The contractile force of the colon smooth muscle was significantly higher in the IBS-D group than in the control group (P < 0.05). EC50 in the IBS-D group (EC50 = 0.94 μmol/L) that was obtained in the presence of CCh was lower than that in the control group (EC50 = 2.1 μmol/L). Compared with the control group, the contractile force of the jejunal smooth muscle was significantly lower in the IBS-D group (P < 0.05). EC50 in the IBS-D group (EC50 = 1.82 μmol/L) that was obtained in the presence of CCh was higher than that in the control group (EC50 = 0.78 μmol/L). The CCh-induced contraction in the colon was abolished by the pretreatment with a single concentration of PA (100 μmol/L). The IBS-D colon pretreated at a single PA concentration of 100 μmol/L significantly decreased the tension of CCh-induced (1 × 10−9 mol/L to 3 × 10−7 mol/L) contraction (P < 0.01) than that in the IBS-D group. Compared with the control group, the PA-pretreated control colon presented significantly decreased tension during the CCh-induced contraction (1 × 10−7 mol/L to 3 × 10−6 mol/L) (P < 0.01). However, at higher CCh concentrations, PA (100 μmol/L) treatment exhibited nonsignificant blocking effect on the colonic CCh-induced contractions, compared with the control group (P > 0.05). Similarly, compared with the control group, the pretreatment of IBS-D colon in 100 μmol/L of PA significantly decreased the tension of the CCh-induced contraction (1 × 10−9 mol/L to 3 × 10−8 mol/L) (P < 0.05); it also showed no significant difference at higher CCh concentrations of 1 × 10−7 mol/L to 1 × 10−5 mol/L (P > 0.05) (Figure 4).
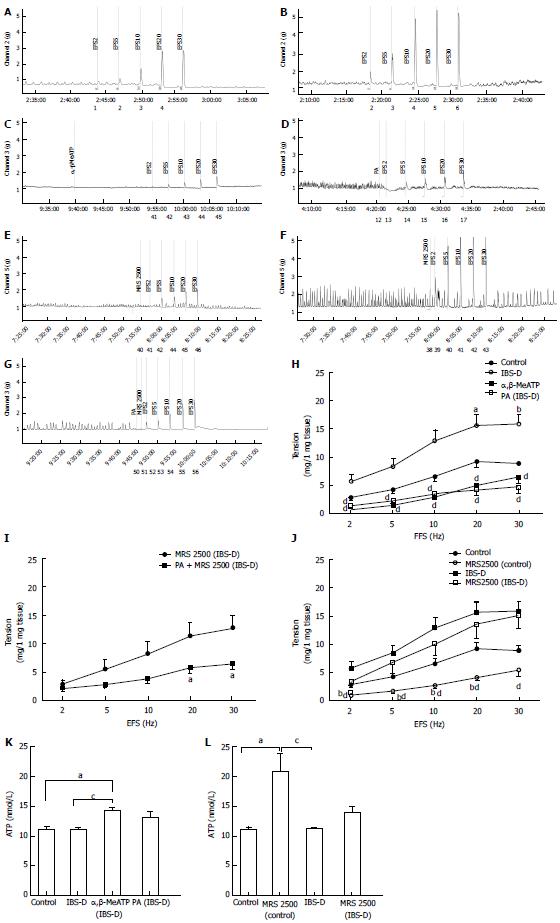
Compared with the control group, the EFS-induced contraction significantly increased in the colonic longitudinal smooth muscle in the IBS-D group at 2 Hz to 30 Hz (P < 0.05). Compared with IBS-D group, the EFS-induced contraction in the
EFS-induced contractions of the colonic longitudinal smooth muscle were significantly decreased by the P2-purinoceptor agonist α,β-MeATP (100 µmol/L) in the IBS-D colon at 2 Hz to 30 Hz compared with that in the untreated IBS-D group (P < 0.01). Meanwhile, no significant difference was observed in the EFS-induced contractions between the PA-treated (100 µmol/L) IBS-D and α,β-MeATP-treated IBS-D groups (P > 0.05). The EFS-induced contractions were significantly decreased by the coapplication of PA (100 µmol/L) and P2Y1 receptor antagonist MRS 2500 in the IBS-D colon at 20 and 30 Hz compared with that in the IBS-D group treated with MRS 2500 only (1 µmol/L) (P < 0.05). The EFS-induced contraction was significantly decreased by MRS 2500 relative to that in IBS-D group (1 µmol/L) (P < 0.01). The ATP assay yielded the following results. No significant difference was detected in the ATP levels between the IBS-D and control groups (P > 0.05). However, α,β-MeATP significantly increased the ATP level of the IBS-D rat colon compared with those of the control (P < 0.05) and IBS-D groups (P < 0.05). MRS 2500 significantly increased the ATP level of the control rat colon compared with that in the control (P < 0.05) and IBS-D groups (P < 0.05). The ATP level did not show significant difference between the MRS 2500-treated and untreated IBS-D groups (P > 0.05) (Figure 6).
Due to the lack of standard treatment algorithm for IBS-D and the limited drugs used to alleviate the symptoms instead of the treatment of disease, alternative therapeutic medications for patients with IBS-D are urgently needed. Pogostemonis Herba is a vital aromatic damp-resolving agent that is often prescribed to treat vomiting and diarrhea. Previous studies[16,17] have demonstrated the antidiarrheal effects of Pogostemonis Herba. PA is a major active ingredient of this herbal medicine, which exhibits notable bioactivities and involves wide applications. The present study aimed to investigate the neurogenic response of PA on an isolated stress-induced IBS-D rat model to identity the potential mechanisms of PA in IBS-D treatment. Given that IBS-D is closely related to motility disorders of the colon rather than that of the small intestine[18,19], The present study focused on the colonic contractile responses.
ENS coordinates the movement patterns in the gastrointestinal tract[20]. The receptors on the neurons and neuroglia in ENS mediate the essential gastrointestinal functions[21,22], including gastrointestinal muscle control. Our study clearly demonstrated that PA (3 × 10−7 mol/L to 1 × 10−4 mol/L) causes concentration-dependent relaxation of the spontaneous contraction of the colonic longitudinal smooth muscles in vitro at EC50 of 41.9 µmol/L. This study is the first to directly demonstrate the inhibition of PA in rat colon motility. However, the mechanism of this acute response to PA remains unknown. Thus, we further investigated whether this acute response to PA was due to the alteration of the neurotransmissions in ENS using the neuropharmacological and electrophysiological technologies to contract the isolated longitudinal smooth muscles of rat colons. We also determined whether this property of PA could be applied to regulate the neurotransmission disorders in IBS-D rats.
The voltage-gated potassium channel (VGKC) function is determined by the membrane potential. The increased extracellular potassium levels result in the depolarization of the intestinal smooth muscle cells through decreasing the resting membrane potential, followed by muscle contractions at the suprathreshold stimulus. VGKCs are primarily regulated by extracellular drugs and neurotransmitters[23]. Previous studies have shown that the release of neurotransmitters, such as nitric oxide (NO) or acetylcholine, may be induced by KCl at concentrations higher than 60 mmol/L[24]. High sensitivity to KCl-induced contractions was noted in our IBS-D rat colons, which is consistent with a previous study[25]. In the present study, the contractile responses induced by high extracellular KCl levels (120 mmol/L) were abolished by PA in the IBS-D rat colon segments. This result suggests that PA may have functions similar to potassium channel blockers. Although further study is required to confirm this assumption, the involvement of the PA-mediated hyperpolarization on the colonic relaxation of IBS-D rat colons has been shown.
Under normal conditions, the enteric smooth muscle, which is innervated by the autonomic nervous system, contracts and relaxes rhythmically[26]. Previous studies confirmed that the colon motility dysfunction and abnormal neurotransmission changes[27] are important mechanisms underlying the IBS-D development. Neuropathic changes in ENS most possibly generated the symptomatic bowel diseases, including IBS-D[28]. In ENS, the cholinergic excitatory motor neurons exert excitatory effects on the gastrointestinal tract. PA inhibited the CCh-induced contractions; thus, PA may regulate acetylcholine transmission by antagonizing the muscarinic receptor or nicotinic acetylcholine receptor. Cholinergic nerves were excited in colons of stress-induced IBS-D rats, which is consistent with the published data[29]. Pretreatment with a single PA concentration strongly inhibited the CCh-induced (1 × 10−9 mol/L to 3 × 10−7 mol/L) contractions in the IBS-D rat colon in vitro. Our study is the first to demonstrate that PA exerts an inhibitory effect on the cholinergic nerves of the IBS-D rat colons. However, whether this inhibitory effect against the acetylcholine operates on the muscarinic receptors, nicotinic acetylcholine receptors, or both, needs further research. In contrast, the isolated jejunum from IBS-D rats exhibited inhibitory effect on the cholinergic nerves. The wrap-restraint stress results in decreased small intestinal transit, whereas it resulted in increased large intestinal transit in rats[30]. Thus, these acetylcholine disorders may result from the wrap-restraint stress. However, further results are needed to confirm this finding. IBS-D treatment may also contribute to the cholinergic receptor-blocking effect of PA on the isolated colon but not the jejunum.
NO is a vital non-adrenergic, non-cholinergic inhibitory transmitter in the gastrointestinal tract. NO is involved in both the colonic relaxation and contractions[31]; however, it typically participates in the nitrergic relaxation. Subsequently, mechanisms of the nitrergic nerves in the gut remain unclear, and the modulation of the nitrergic and cholinergic transmission in the nerve-muscle pathway[32,33] requires further study. NO synthases utilize
ATP is a purine inhibitory neurotransmitter that mediates the non-adrenergic, non-cholinergic relaxation in the gastrointestinal smooth muscles[35,36]. Studies showed that in wrap-restraint stress rat model, ATP participates in the intestinal motility[37]. However, the effect and mechanism of ATP in the IBS-D rat colon still need clarification. On the other hand, α,β-MeATP is a P2-purinoreceptor agonist. Our results demonstrated that excess contraction of the IBS-D rat colon can be inhibited by α,β-MeATP. Under EFS conditions, PA produced an inhibitory effect on the coadministration of MRS 2500 (1 µmol/L) in the IBS-D rat colon. PA affected the muscle tone and caused ATP-induced relaxation, indicating that PA may be involved in the activating effect of ATP on the IBS-D rat colon. However, this observation requires further investigation. P2Y1 receptor is involved in ENS control and coordination of intestinal motility[38]. P2Y1 receptor functions as a receptor for extracellular ATP, which can be activated by the endogenous ligands of ATP and ADP. Recent studies suggested that highly potent and selective P2Y1 receptor antagonist MRS 2500 inhibited the ATP-induced relaxation. However, when we further tested the effect of MRS 2500 on the rat longitudinal smooth muscle, 1 µmol/L of MRS 2500 produced a significant relaxation effect in the control rats in vitro but not in the stress-induced IBS-D rats. A previous work[39] reported an unexplained inhibitory effect of MRS 2500 (1 µmol/L) on spontaneous motility in vitro. Thus, ATP may play a complementary role in regulating colonic motility of IBS-D rats. In our continuing study, ATP assay results agreed with the conclusions mentioned above. Subsequently, we cannot explain the MRS 2500-induced relaxation of the nerve-mediated contraction elicited by EFS in vitro. Thus, additional in vivo and in vitro studies are necessary to clarify this phenomenon.
Electrophysiological methods were used for our neuropharmacological study. This method shows the effects of PA on the structural and physiological components of the biopsychosocial model of IBS-D; the latter may enable the development for clinical research and application. However, the following limitations must be considered. First, we cannot identify the pathway dominating the IBS-D colon response to PA treatment. These inhibitory effects by PA may be related to the changes in one upstream switch. Nevertheless, our results strongly confirmed the inhibitory effects of PA on the spontaneous, CCh-induced, and EFS-induced colonic contractions. Second, animal models and mechanisms of different IBS-D models vary. The wrap-restraint stress model mimics but cannot fully represent the physiological and pathological conditions of patients with IBS-D. Thus, whether the PA treatment is appropriate for other IBS-D models or patients requires further study. Third, the role of ATP in the wrap-restraint stress IBS-D model or the involvement of ATP in mechanism of PA treatment of IBS-D rats is unclear. Fourth, the nature of our study limits our conclusion because a direct correlation between colonic contractions and electrical signaling cannot be completely confirmed. However, the present study provides evidence regarding the involvement of PA in neuromediated relaxation of the longitudinal smooth muscle in rat colon.
In conclusion, PA exerts inhibitory effects on the IBS-D rat colon, which supports our hypothesis. In addition, related responses possibly involve cholinergic, nitrergic, and K+ channel pathways. ATP may not be the dominant pathway for participation of PA in the colonic relaxation of the stress-induced IBS-D rats. PA is a potential new candidate to effectively treat IBS-D. The findings in this study may help extend the pharmacological applications of PA. PA may be responsible for the antidiarrheal effect of Pogostemonis Herba. Additional in vivo and in vitro investigations on the effect of PA are needed, and potential pharmacological target protein for PA in treatment of IBS-D rat colon needs to be studied.
Irritable bowel syndrome (IBS) is a prevalent functional bowel disorder that inflicts a significant socioeconomic burden and decreases the patient quality of life. In China, the percentage of patients with diarrhea-predominant IBS (IBS-D) is 74.1%. Therefore, the neuropsychological factors have gained much attention in the clinical and basic studies on IBS-D. However, a standard treatment algorithm has not been established for this condition. Pogostemonis Herba is used in Asian countries to treat functional gastrointestinal disorders; patchouli alcohol is the major active ingredient of Pogostemonis Herba. Previous studies have indicated that patchouli alcohol (PA) may participate in the neurotransmission regulation of the smooth muscles in the digestive system. However, no research on the pharmacological effects of PA in the neurotransmission regulation has been published.
This study aimed to investigate the effects of PA on the isolated IBS-D rat colon and its related mechanisms. The findings in this work can help extend the pharmacological applications of PA.
The main objective of this study was to test our hypothesis that the mechanism of PA in treatment of IBS-D is related to the drug regulation of the neural pathways in the enteric nervous system via its influence on the neurotransmitter release with focus on PA research. Additional in vivo and in vitro investigations on the effect of PA and the identification of the potential pharmacological target protein in PA to treat IBS-D rat colon are needed.
In this in vitro study, the effect of PA on colonic spontaneous motility was studied using the cumulative log concentration (3 × 10−7 mol/L to 1 × 10−4 mol/L). Responses to CCh (10−9 mol/L to 10−5 mol/L) and neurotransmitter antagonists, including
In this study, PA exerted a concentration-dependent inhibitory effect on the spontaneous contraction of the colonic longitudinal smooth muscle. Pretreatment of PA could inhibit the peak tension of high extracellular concentration of the KCl-induced contraction of the IBS-D rat colon. The cholinergic contractile response in the colonic smooth muscle of IBS-D rat, which was induced by CCh, was reduced by the pretreatment of PA. Lack of nitrergic neurotransmitter, which was released in the stress-induced IBS-D rat, showed contraction effects on the colonic smooth muscle. Pretreatment of PA resulted in the relaxant effects on the
PA exerts inhibitory effects on the IBS-D rat colon, which supports our hypothesis. In addition, related responses possibly involve cholinergic, nitrergic, and K+ channel pathways. ATP may not be the dominant pathway for participation of PA in the colonic relaxation of the stress-induced IBS-D rats. PA is a potential new candidate to effectively treat IBS-D. The findings in this study may help extend the pharmacological applications of PA. PA may be responsible for the antidiarrheal effect of Pogostemonis Herba. Additional in vivo and in vitro investigations on the effect of PA are needed, and potential pharmacological target protein for PA in treatment of IBS-D rat colon needs to be studied.
Our results strongly confirmed the inhibitory effects of PA on the spontaneous, CCh-induced, and EFS-induced colonic in vitro contractions. PA acts as a neurotransmitter agent in ENS, and is thus considered to be a new treatment option for IBS-D. However, more questions will be addressed in the future studies. For instance, the pathways dominating the IBS-D colon in response to PA treatment; and the structural and functional changes in the potential target proteins under the effect of PA. The relaxation effects of PA may be related to changes in one upstream switch. Our further studies will focus on the effect of PA on the potential target proteins in IBS-D rats both in vivo and in vitro. Patch-clamp methods will be used to measure the K+ current, and immunofluorescence will be used to investigate the expression and colocalization of the target proteins. Furthermore, Western blot and qPCR will be performed to evaluate the expression of the target proteins.
The authors would like to thank Dr. Paul He for the technical support.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kamiya T S- Editor: Chen K L- Editor: Ma JY E- Editor: Ma YJ
| 1. | Lim CY, Kim BY, Lim SH, Cho SI. Effects of Pogostemon cablin Blanco extract on hypoxia induced rabbit cardiomyocyte injury. Pharmacogn Mag. 2015;11:311-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Bhatia SP, Letizia CS, Api AM. Fragrance material review on patchouli alcohol. Food Chem Toxicol. 2008;46 Suppl 11:S255-S256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Hu G, Peng C, Xie X, Zhang S, Cao X. Availability, Pharmaceutics, Security, Pharmacokinetics, and Pharmacological Activities of Patchouli Alcohol. Evid Based Complement Alternat Med. 2017;2017:4850612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Ichikawa K, Kinoshita T, Sankawa U. The screening of Chinese crude drugs for Ca2+ antagonist activity: identification of active principles from the aerial part of Pogostemon cablin and the fruits of Prunus mume. Chem Pharm Bull (Tokyo). 1989;37:345-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Yang Y, Kinoshita K, Koyama K, Takahashi K, Tai T, Nunoura Y, Watanabe K. Anti-emetic principles of Pogostemon cablin (Blanco) Benth. Phytomedicine. 1999;6:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Slattery SA, Niaz O, Aziz Q, Ford AC, Farmer AD. Systematic review with meta-analysis: the prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment Pharmacol Ther. 2015;42:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 7. | Singh P, Staller K, Barshop K, Dai E, Newman J, Yoon S, Castel S, Kuo B. Patients with irritable bowel syndrome-diarrhea have lower disease-specific quality of life than irritable bowel syndrome-constipation. World J Gastroenterol. 2015;21:8103-8109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (4)] |
| 8. | Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. 2012;367:1626-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 229] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 9. | Gwynne RM, Bornstein JC. Synaptic transmission at functionally identified synapses in the enteric nervous system: roles for both ionotropic and metabotropic receptors. Curr Neuropharmacol. 2007;5:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Peiris M, Hockley JRF, Reed DE, Smith ES, Bulmer DC, Blackshaw LA. Peripheral K(V)7 channels regulate visceral sensory function in mouse and human colon. Mol Pain. 2017;13. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Lacy BE. Diagnosis and treatment of diarrhea-predominant irritable bowel syndrome. Int J Gen Med. 2016;9:7-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Lucak S, Chang L, Halpert A, Harris LA. Current and emergent pharmacologic treatments for irritable bowel syndrome with diarrhea: evidence-based treatment in practice. Therap Adv Gastroenterol. 2017;10:253-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Corsetti M, Whorwell P. New therapeutic options for IBS: the role of the first in class mixed µ- opioid receptor agonist and δ-opioid receptor antagonist (mudelta) eluxadoline. Expert Rev Gastroenterol Hepatol. 2017;11:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Su ZQ, Wu XL, Bao MJ, Li CW, Kong SZ, Su ZR, Lai XP, Li YC, Chen JN. Isolation of (-)-Patchouli Alcohol from Patchouli Oil by Fractional Distillation and Crystallization. Tropical Journal of Pharmaceutical Research. 2014;359. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Xu YF, Lian DW, Chen YQ, Cai YF, Zheng YF, Fan PL, Ren WK, Fu LJ, Li YC, Xie JH. In Vitro and In Vivo Antibacterial Activities of Patchouli Alcohol, a Naturally Occurring Tricyclic Sesquiterpene, against Helicobacter pylori Infection. Antimicrob Agents Chemother. 2017;61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Chen X, He B, Li X, Luo J. [Effects of herba Pogostemonis on gastrointestinal tract]. Zhong Yao Cai. 1998;21:462-466. [PubMed] |
| 17. | He B, Chen X, Luo J. [Effects of five different polar extracts from Herba pogostemonis being gotten rid of volatile oil on gastrointestinal tract]. Zhong Yao Cai. 2001;24:422-424. [PubMed] |
| 18. | Kanazawa M, Palsson OS, Thiwan SI, Turner MJ, van Tilburg MA, Gangarosa LM, Chitkara DK, Fukudo S, Drossman DA, Whitehead WE. Contributions of pain sensitivity and colonic motility to IBS symptom severity and predominant bowel habits. Am J Gastroenterol. 2008;103:2550-2561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Manabe N, Wong BS, Camilleri M, Burton D, McKinzie S, Zinsmeister AR. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil. 2010;22:293-e82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 20. | Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 1059] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 21. | Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2012;9:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 286] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 22. | Burns AJ, Pachnis V. Development of the enteric nervous system: bringing together cells, signals and genes. Neurogastroenterol Motil. 2009;21:100-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Hafting T, Haug TM, Ellefsen S, Sand O. Hypotonic stress activates BK channels in clonal kidney cells via purinergic receptors, presumably of the P2Y subtype. Acta Physiol (Oxf). 2006;188:21-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Araujo CB, Bendhack LM. High concentrations of KCl release noradrenaline from noradrenergic neurons in the rat anococcygeus muscle. Braz J Med Biol Res. 2003;36:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Zhang M, Leung FP, Huang Y, Bian ZX. Increased colonic motility in a rat model of irritable bowel syndrome is associated with up-regulation of L-type calcium channels in colonic smooth muscle cells. Neurogastroenterol Motil. 2010;22:e162-e170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Schemann M. Control of gastrointestinal motility by the “gut brain”--the enteric nervous system. J Pediatr Gastroenterol Nutr. 2005;41 Suppl 1:S4-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Callahan MJ. Irritable bowel syndrome neuropharmacology. A review of approved and investigational compounds. J Clin Gastroenterol. 2002;35:S58-S67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Vanner S, Greenwood-Van Meerveld B, Mawe G, Shea-Donohue T, Verdu EF, Wood J, Grundy D. Fundamentals of Neurogastroenterology: Basic Science. Gastroenterology. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 29. | Guarino MP, Barbara G, Cicenia A, Altomare A, Barbaro MR, Cocca S, Scirocco A, Cremon C, Emerenziani S, Stanghellini V. Supernatants of irritable bowel syndrome mucosal biopsies impair human colonic smooth muscle contractility. Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Williams CL, Villar RG, Peterson JM, Burks TF. Stress-induced changes in intestinal transit in the rat: a model for irritable bowel syndrome. Gastroenterology. 1988;94:611-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 182] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Barthó L, Lefebvre RA. Nitric oxide-mediated contraction in enteric smooth muscle. Arch Int Pharmacodyn Ther. 1995;329:53-66. [PubMed] |
| 32. | Baccari MC, Calamai F, Staderini G. Modulation of cholinergic transmission by nitric oxide, VIP and ATP in the gastric muscle. Neuroreport. 1994;5:905-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Senbel AM, Hashad A, Sharabi FM, Daabees TT. Activation of muscarinic receptors inhibits neurogenic nitric oxide in the corpus cavernosum. Pharmacol Res. 2012;65:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Mizuta Y, Takahashi T, Owyang C. Nitrergic regulation of colonic transit in rats. Am J Physiol. 1999;277:G275-G279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509-581. [PubMed] |
| 36. | Manzini S, Maggi CA, Meli A. Further evidence for involvement of adenosine-5’-triphosphate in non-adrenergic non-cholinergic relaxation of the isolated rat duodenum. Eur J Pharmacol. 1985;113:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Van Crombruggen K, Lefebvre RA. Nitrergic-purinergic interactions in rat distal colon motility. Neurogastroenterol Motil. 2004;16:81-98. [PubMed] |
| 38. | Wood JD. The enteric purinergic P2Y1 receptor. Curr Opin Pharmacol. 2006;6:564-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Gil V, Gallego D, Grasa L, Martín MT, Jiménez M. Purinergic and nitrergic neuromuscular transmission mediates spontaneous neuronal activity in the rat colon. Am J Physiol Gastrointest Liver Physiol. 2010;299:G158-G169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |