Published online Feb 7, 2015. doi: 10.3748/wjg.v21.i5.1531
Peer-review started: May 20, 2014
First decision: June 27, 2014
Revised: August 6, 2014
Accepted: October 15, 2014
Article in press: October 15, 2014
Published online: February 7, 2015
Processing time: 266 Days and 5.5 Hours
AIM: To investigate the effect of interleukin (IL)-22 on hepatic fibrosis in mice and the possible mechanism involved.
METHODS: Liver fibrosis was induced in male BALB/c mice by CCl4. Recombinant IL-22 (rmIL-22) was administered intraperitoneally in CCl4-treated mice. Fibrosis was assessed by histology and Masson staining. The activation of hepatic stellate cells (HSCs) was investigated by analysis of α-smooth muscle actin expression. The frequencies of T helper (Th) 22 cells, Th17 cells and Th1 cells, the expression of inflammatory cytokines [IL-22, IL-17A, interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), IL-6, IL-1β] and transcription factors [aryl hydrocarbon receptor (AHR), RAR-related orphan receptor (RORγt), T-bet] mRNA in the liver were investigated. In addition, the plasma levels of IL-22, IL-17A, IFN-γ, TNF-α, IL-6 and IL-1β were evaluated.
RESULTS: Significant elevations in circulating Th22 cells, Th17 cells, Th1 cells, IL-22, IL-17A, and IFN-γ were observed in the hepatic fibrosis group compared with the control group (P < 0.01). Treatment with rmIL-22 in mice with hepatic fibrosis ameliorated the severity of hepatic fibrosis, which was confirmed by lower hepatic fibrosis pathological scores (P < 0.01). RmIL-22 decreased the frequencies of Th22 cells (6.71% ± 0.97% vs 8.09% ± 0.74%, P < 0.01), Th17 cells (4.34% ± 0.37% vs 5.71% ± 0.24%, P < 0.01), Th1 cells (3.09% ± 0.49% vs 4.91% ± 0.73%, P < 0.01), and the levels of IL-22 (56.23 ± 3.08 vs 70.29 ± 3.01, P < 0.01), IL-17A (30.74 ± 2.77 vs 45.68 ± 2.71, P < 0.01), and IFN-γ (74.78 ± 2.61 vs 124.89 ± 2.82, P < 0.01). Down-regulation of IL-22, IL-17A, IFN-γ, TNF-α, IL-6, IL-1β, AHR RORγt, and T-bet gene expression in the liver was observed in the rmIL-22 group (P < 0.01).
CONCLUSION: The frequencies of Th22, Th17 and Th1 cells are elevated in hepatic fibrosis. RmIL-22 can attenuate HSC activation and down-regulate the levels of inflammatory cytokines, thereby ameliorating liver fibrogenesis.
Core tip: T helper (Th)-22 cells are involved in tissue inflammation and immunity. The presence of Th22 cells and the role of interleukin (IL)-22 in hepatic fibrosis have seldom been reported. The present study examined the frequency of Th22 cells, investigated the expression of IL-22, and determined the effects of recombinant IL-22 on hepatic fibrosis in mice. The aim of this preliminary study was to determine the presence of Th22 cells and the functional characteristics of IL-22 in hepatic fibrosis. The frequency of Th22 cells was elevated in mice with hepatic fibrosis. Recombinant IL-22 ameliorated liver fibrogenesis via attenuation of hepatic stellate cell activation and down-regulation of inflammatory cytokines.
- Citation: Lu DH, Guo XY, Qin SY, Luo W, Huang XL, Chen M, Wang JX, Ma SJ, Yang XW, Jiang HX. Interleukin-22 ameliorates liver fibrogenesis by attenuating hepatic stellate cell activation and downregulating the levels of inflammatory cytokines. World J Gastroenterol 2015; 21(5): 1531-1545
- URL: https://www.wjgnet.com/1007-9327/full/v21/i5/1531.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i5.1531
Liver fibrosis is a major cause of morbidity and mortality worldwide due to chronic liver injury. Activation of hepatic stellate cells (HSCs) is considered the most important event in the production of extracellular matrix (ECM) and collagens, thereby leading to hepatic fibrosis[1]. Despite advances in the characterization of the fibrotic process, the exact molecular mechanisms of the disease are poorly understood. Persistent immunological responses and inappropriate release of immune mediators have been linked to injury, damage, and scar formation in host tissues and identified as crucial pathological processes. However, the fundamental mechanisms responsible for liver fibrosis have not been completely clarified.
Recently, emerging evidence has shown that CD4+T cells play an important role in the development of liver inflammation and fibrosis[2-4]. CD4+T helper cells have recently been subdivided into four major subsets, largely based on their expression profile of transcription factors and secreted cytokines: T helper (Th) cell type 1, Th2, Th17 and regulatory T cells[5,6]. Th1 cells are characterized by the secretion of interferon-γ (IFN-γ), a proinflammatory cytokine which is necessary for the activation of macrophages and is involved in immunity against intracellular pathogens. Studies have shown that injection of IFN-γ decreased total liver collagen content in rats with dimethylnitrosamine-induced fibrosis[7]. Nevertheless, IFN-γ inhibits HSC proliferation and activation, and down-regulates the expression of ECM in these cells. Th17 cells are a more recently discovered subset of CD4+ T-helper cells characterized by the production of their signature cytokine IL-17. They represent another subtype of proinflammatory T-helper cells that differs from Th1 and Th2 cells in development and function. Differentiation of Th17 cells requires the combined actions of transforming growth factor beta (TGF-β), IL-6, and IL-21 in mice. These cytokines induce the expression of the orphan nuclear receptor RAR-related orphan receptor (RORγt) (mice) or RORc (human). Th17 cells have been demonstrated to play a critical role in inflammatory diseases, autoimmune diseases and graft-versus-host diseases by secreting IL-17A and other cytokines. Several studies have revealed that Th17 cells are involved in the pathogenesis of liver diseases[8,9].
Th22 cells have been recognized as a novel Th cell subset, which are characterized by abundant production of IL-22, but not IL-17 or IFN-γ[10,11]. Th22 cells express the chemokine receptors CCR6, CCR4 and CCR10, and have high expression of the key transcription factor, aryl hydrocarbon receptor (AHR), but low or undetectable expression of T-bet and ROR-γt[10]. In addition, the differentiation toward the Th22 phenotype from naive T cells occurs in the presence of IL-6 and tumor necrosis factor-α (TNF-α)[10]. Developing literature has demonstrated that Th22 cells are associated with psoriasis, rheumatoid arthritis, systemic sclerosis, and human immunodeficiency virus infection. However, the presence of Th22 cells and the role of Th22 in hepatic fibrosis are unknown. IL-22 is a member of the IL-10 cytokine family and is produced primarily by Th22 cells, Th17 cells and Th1 cells. IL-22 can exert its effects via a heterodimeric transmembrane receptor complex consisting of IL-10R2 and IL-22R1. It is thought that IL-22 may play an important role in regulating inflammatory disease-related inflammatory responses. The relationship between IL-22 and IL-17, as well as between IL-17 and IFN-γ, is of particular interest, and their expression is often linked to proinflammatory processes. IL-22 exerts functions similar to those of IL-17, both contributing to the control of extracellular bacterial infection. However, IL-22 has specific functions, such as induction of wound-healing responses and tissue repair protecting against myocarditis or liver disease[12], where IL-17 is not implicated. In particular, a recent study indicated that IL-22 treatment prevented T cell hepatitis[13], stimulated liver regeneration[14], improved fatty liver[15], and induced senescence of HSCs, thereby ameliorating liver fibrogenesis[15].
It is well known that Th17 and Th1 cells are involved in hepatic fibrosis. However, the existence and underlying mechanisms of Th22 cells in hepatic fibrosis are still unclear. Therefore, in the present study, we investigated the distribution of Th22 cells in relation to Th17 and Th1 cells in CCl4-induced hepatic fibrosis in mice, and examined the expression of IL-22, IL-17 and IFN-γ. Mice with hepatic fibrosis treated with recombinant IL-22 (rmIL-22) were examined with the aim of determining the presence of Th22 cells and the functional characteristics of IL-22 in hepatic fibrosis.
Specific pathogen-free male BALB/c mice aged 6 wk and weighing 18-20 g were purchased from the Laboratory Animal Center (Guangxi Medical University, China, No. SCXKG 2010-0002). All experimental procedures on mice were approved by the Committee on the Ethics of Animal Experiments of Guangxi Medical University. The animals were kept in a pathogen-free mouse room (12 h light/12 h dark; temperature, 22-24 °C), and received water ad libitum by the Animal Care Facility Service (Guangxi Medical University, China). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
A total of 72 mice were randomly divided into the hepatic fibrosis group (n = 40, 10 mice/subgroup) and the control group (n = 32, 8 mice/subgroup). Each group was divided into four subgroups (weeks 0, 4, 8 and 12). The hepatic fibrosis group was treated with 2 mL/kg body weight of 20% CCl4 (Shanghai Yangtze River Chemical Company, Shanghai, China) intraperitoneally for 12 wk. Control mice received olive oil (Sigma, United States) intraperitoneally. All surviving animals were sacrificed on weeks 0, 4, 8 and 12, 72 h after the last injection. Livers and spleens were removed aseptically as fresh specimens for measurement, blood was harvested and the plasma was prepared for analysis.
A total of 16 mice were treated with 2 mL/kg body weight of 20% CCl4 (Shanghai Yangtze River Chemical Company; Shanghai, China) intraperitoneally for 8 wk, and then randomly divided into two groups. The mice were given 300 μg/kg of rmIL-22 (0.3 μg/g; R and D Systems, Inc., Minneapolis, MN, United States) daily by intraperitoneal injection for 14 d before being sacrificed (n = 8, rmIL-22 group). The control groups were injected with a carrier solution of 0.5% BSA (in PBS, pH 7.0) (n =8, carrier group). All surviving animals were sacrificed on week 10 after CCl4 injection. The livers, spleens and plasma were collected.
The harvested liver tissues were fixed in 10% neutral buffered formalin and embedded in paraffin. Slices 4 μm thick were prepared and stained with hematoxylin/eosin (HE) and Masson’s trichrome staining according to standard procedures, and histopathological changes were observed under light microscopy (Nikon Eclipse E800 Microscope; Kawasaki, Kanagawa, Japan). The Ishak fibrosis score was used as the scoring system for measuring the degree of liver fibrosis. A score of 0 indicated no fibrosis, scores of 1 and 2 indicated degrees of portal fibrosis, and scores of 3 and 4 indicated bridging fibrosis. A score of 5 indicated nodular formation and incomplete cirrhosis, and 6 indicated established cirrhosis. Histological assessment was performed by two clinical pathologists without knowledge of the experimental design.
The liver tissue sections were dewaxed, hydrated and subjected to heat-induced antigen retrieval. Sections were blocked and incubated overnight at 4 °C with mouse anti-alpha-smooth muscle actin (α-SMA) antibody (Sigma-Aldrich, St. Louis, MO) at 1:300 dilution. Negative-control antibodies consisted of species-matched, and where appropriate, IgG subclass-matched Ig, used at the same dilution. The sections were subsequently washed and incubated with HRP-conjugated goat anti-mouse IgG secondary antibodies, followed by incubation for 5-10 min with 3,3′-diaminobenzidine tetrachloride and visualization of specific staining under light microscopy. Stained slides were analyzed by light microscopy, and high-power field images (magnification × 400) of the livers were taken of each mouse (Olympus BX53; Tokyo, Japan). α-SMA deposition in the cytoplasm was evaluated semi-quantitatively according to the extent and intensity of staining using Image-Pro Plus Version 6.0 (Media Cybernetics, Bethesda, MD, United States). Five fields from each slice were randomly selected by two pathology experts, who were unaware of the groups.
Splenic cells were gently dispersed through a nylon mesh into a single-cell suspension and washed with RPMI 1640 (GIBCO; California, United States). Single cell suspensions were prepared by lysing red blood cells using red blood cell lysing buffer (BD Biosciences; San Diego, CA). These splenic mononuclear cells were then resuspended in RPMI 1640 medium with 10% FCS (GIBCO; United States), stimulated with phorbol myristate acetate (25 ng/mL, Sigma-Aldrich) and ionomycin (1 μg/mL, Sigma-Aldrich) in the presence of GolgiPlug (1 μL/106 cells, BD Biosciences) at 37 °C, 5% CO2 in a 24-well culture plate. After 5 h incubation, the cells were harvested and stained with PerCP-Cy5.5 conjugated anti-mouse CD4 (BD Biosciences). Then the cells were stained intracellularly with anti-IL-22-PE (eBioscience, San Diego, CA, United States), anti-IL-17-APC (eBioscience, United States), and anti-IFN-γ-Alexa- Fluor®488 (BD Biosciences) monoclonal antibodies after fixation and permeabilization (BD Biosciences). Isotype-matched control antibodies were used to determine the level of background staining and help set a gate. Stained cells were analyzed by a FACS-Calibur flow cytometer (BD Bioscience) and FlowJo software 7.6.1 (Tristar, El Segundo, CA, United States). Th22, Th17, and Th1 cells were defined as IL-22+IL-17-IFN-γ-CD4+, IL-17+CD4+, and IFN-γ+CD4+ T cells, respectively.
Total RNA of homogenized heart tissues was extracted with TRIZOL Reagent W (Invitrogen, California, United States), and then reverse transcribed into cDNA with a Reverse Transcription kit (Ferma, CA, United States) according to the manufacturer’s instructions. Primers for IL-22, IL-17A, IFN-γ, TNF-α, IL-1β, IL-6 T-bet, RORγt, AHR, and the housekeeping gene β-actin were designed by Primer Premier 5.0. Real-time reverse transcription-polymerase chain reaction (RT-PCR) was performed using an ABI 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, United States) using SYBR green. After an initial denaturation step for 3 min at 94 °C, a three-step cycling procedure (denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 60 s) was used for 35 cycles. The templates and primers were as follows: mouse IL-22 (Forward, F) 5′-CGA TTG GGG AAC TGG ACC TG-3′ and (Reverse, R) 5′-GGA CGT TAG CTT CTC ACT TT-3′; IL-17A, (F) 5′- GTG TCT CTG ATG CTG TTG -3′ and (R) 5′-AAC GGT TGA GGT AGT CTG-3′; IFN-γ, (F) 5′-CTC AAG TGG CAT AGA TGT GGA AG-3′ and (R) 5′-GCT GGA CCT GTG GGT TGT TGA-3′; AHR (F) 5′-ATG GAG GCC AGG ACC AGT GTA G-3′ (R) 5′-TGC TGT GAC AAC CAG CAC AAA G-3′; RORγt, (F) 5′-GCG GAG CAG ACA CAC TTA CA-3′ and (R) 5′-TTG GCA AAC TCC ACC ACA TA-3′; T-bet, (F) 5′-TTC CCA TTC CTG TCC TTC AC-3′ and (R) 5′-CCT CTG GCT CTC CAT CAT TC-3′; TNF-α, (F) 5′-AGT CCG GGC AGG TCT ACT TT-3′ and (R) 5′-TTG GAC CCT GAG CCA TAA TC -3′; IL-6, (F) 5′-ACA GAA GGA GTG GCT AAG GAC C-3′ and (R) 5′-TAG GCA TAA CGC ACT AGG TTT -3′; IL-1β, (F) 5′-CAG GAT GAG GAC ATG AGC ACC -3′ and (R) 5′-CTC TGC AGA CTC AAA CTC CAC-3′; β-actin, (F) 5′-AAT TCC ATC ATG AAG TGT GA -3′ and (R) 5′-ACT CCT GCT TGC TGA TCC AC -3′. The relative gene expressions were normalized to the level of β-actin transcripts and quantified by the △△CT method using 7500 System Sequence Detection software (Applied Biosystems). All reactions were performed at least twice for each sample.
Cytokine content in plasma was determined by enzyme-linked immunosorbent assays. The amount of IL-22 was detected using the Quantikine Mouse IL-22 IL-17A Immunoassay (eBioscience, United States). The levels of IFN-γ, TNF-α, IL-6, and IL-1β in mice were determined by enzyme linked immunosorbent assay (ELISA) kits (Boster, China), according to the manufacturer’s instructions. The sensitivity of ELISA kits for IL-22, IL-17A, IFN-γ, TNF-α, IL-6 and IL-1β, was 5, 5, 7, 4, 7 and 7 pg/mL, respectively, and no cross-reactivity was observed during these determinations. All samples were measured in triplicate.
Data are summarized as mean ± SD based on experiments repeated in triplicate. Comparisons were carried out using the Student’s t test for two-group comparisons, or one-way ANOVA test for multiple comparisons. The differences between pathological scores were evaluated using the Mann-Whitney U test. All data were analyzed with SPSS 16.0. P values less than 0.05 were considered statistically significant.
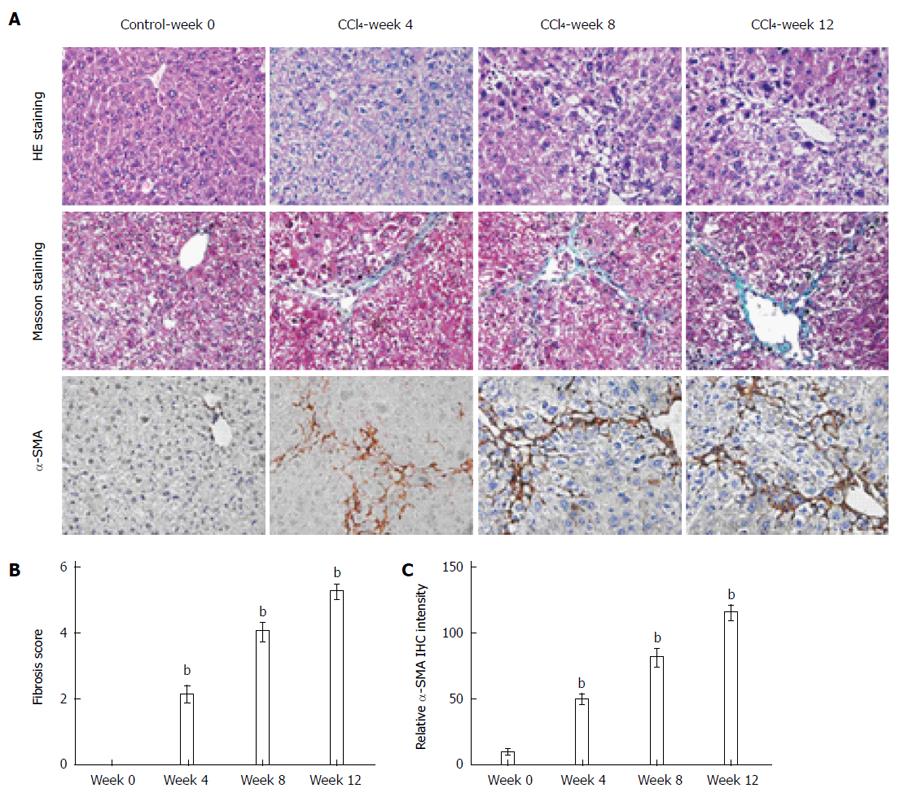
CCl4 administration caused significant hepatic inflammation, including hepatocyte ballooning, necrosis and regeneration. The severity of hepatic fibrosis gradually increased. Progressive necroinflammation and perisinusoidal and portal fibrosis were observed, and irregular necrosis and bridging fibrosis emerged at week 8 (Figure 1A). The extension of fiber cable and formation of hepatic lobules were observed and very few areas of healthy hepatocytes and collagen deposition with septa bridging portal regions were detected at the end of 12 wk (Figure 1A).
The degree of liver fibrosis was evaluated by Masson staining (Figure 1A), a qualitative assessment of liver fibrosis, and the hepatic pathological scores peaked at week 12 (Figure 1B). In contrast, the control group showed no inflammatory cell infiltration, necrosis or fibrosis in livers. HSCs are the main collagen-producing cells in liver fibrosis, and we analyzed activated HSCs by α-SMA expression via immunohistochemical staining (Figure 1A). Following CCl4 administration, increased α-SMA staining was observed in the livers of mice from weeks 0 to 12 (Figure 1C). α-SMA spread to the portal area, demonstrating increased activation of HSCs.
As shown in Figure 2, the frequencies of splenic Th22 cells (IL-22+IL-17-IFN-γ- CD4+ cells) in the hepatic fibrosis group from weeks 0 to 12 were 1.98% ± 0.34%, 18.2% ± 3.1%, 12.45% ± 1.6%, and 8.62% ± 1.01%, respectively. In the control group, the frequencies of splenic Th22 cells from weeks 0 to 12 were 1.95% ± 0.25%, 1.93% ± 0.25%, 2.01% ± 0.22%, and 2.05% ± 0.24%, respectively. These data indicated that the time-course of Th22 cells was significantly higher than the control group from week 4 (P < 0.01), and peaked at week 4 (P < 0.01) (Figure 2A, 2D). The frequencies of Th17 cells (IL-17+/CD4+T cells) in the hepatic fibrosis group from weeks 0 to 12 were 0.73% ± 0.15%, 7.22% ± 0.67%, 6.11% ± 0.74%, and 5.57% ± 0.68%, respectively. In the control group, the frequencies of Th17 cells from weeks 0 to 12 were 0.69% ± 0.1%, 0.71% ± 0.09%, 0.73% ± 0.07%, and 0.77% ± 0.09%, respectively. The percentage of Th17 cells in the hepatic fibrosis group was significantly higher than that in the control group (P < 0.01), and peaked at week 4 (P < 0.01) (Figure 2B and E). Similarly, the frequencies of Th1 cells (IFN-r+/CD4+T cells) in the hepatic fibrosis group from weeks 0 to 12 were 0.87% ± 0.18%, 6.63% ± 1.4%, 4.64% ± 1.23%, and 4.53% ± 0.59%, respectively. In the control group, the frequencies of Th1 cells from weeks 0 to 12 were 0.94% ± 0.17%, 0.87% ± 0.22%, 1.01% ± 0.21%, and 1.02% ± 0.19%, respectively. A significantly increased level of Th1 cells was observed in the hepatic fibrosis group compared with the control group (P < 0.01), and peaked at week 4 (P < 0.01) (Figure 2C and F). The number of Th22 cells in the hepatic fibrosis group was positively correlated with the number of Th17 and Th1 cells, and the number of Th17 and Th1 cells was positively correlated (both P < 0.01, Figure 2G-I).
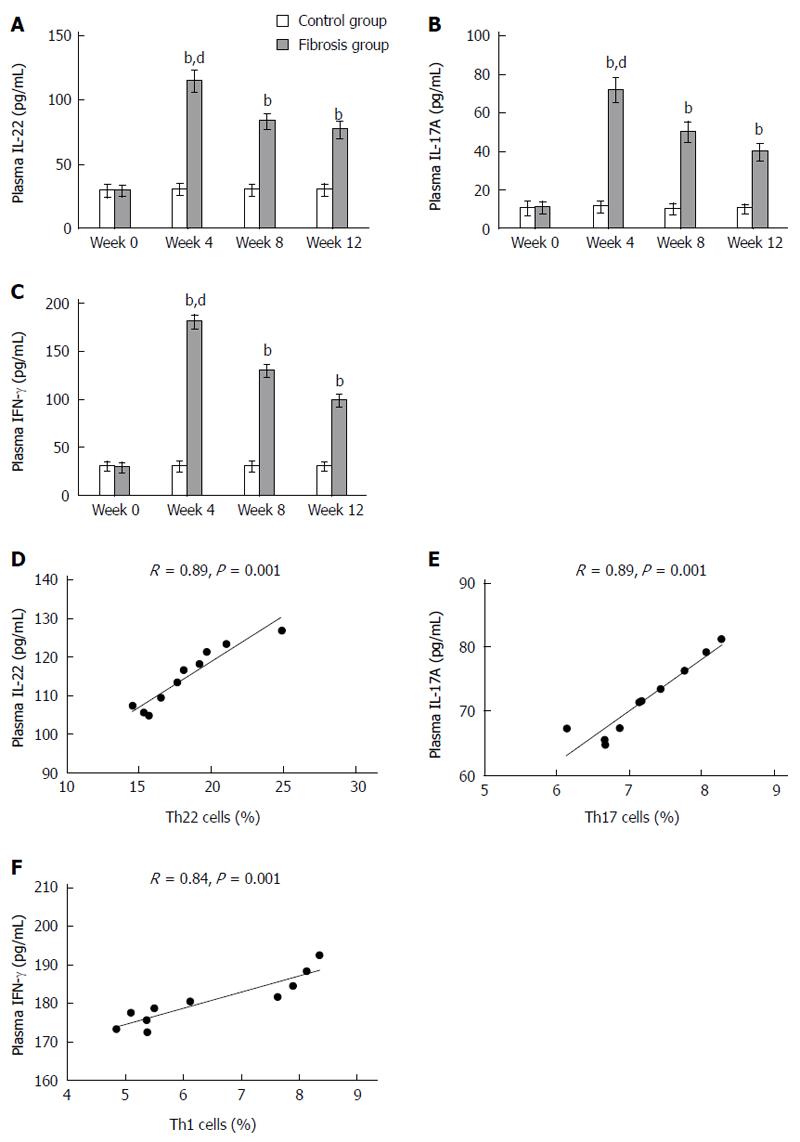
Peripheral blood levels of IL-22 in the hepatic fibrosis group from weeks 0 to 12 were 29.23 ± 3.68, 114.79 ± 7.8, 83.19 ± 5.41, and 76.89 ± 5.69, respectively. In the control group, peripheral blood levels of IL-22 from weeks 0 to 12 were 29.31 ± 4.35, 30.45 ± 3.66, 29.79 ± 3.9, and 29.89 ± 3.93, respectively. In accordance with flow cytometry analysis of Th22 cells, IL-22 was up-regulated in mice with hepatic fibrosis from week 4 and peaked at week 4 (P < 0.01) (Figure 3A). The levels of IL-17A in the hepatic fibrosis group from weeks 0 to 12 were 10.79 ± 2.6, 71.9 ± 5.72, 50.03 ± 4.84, and 39.62 ± 3.84, respectively. In the control group, the levels of IL-17A were 10.4 ± 3.27, 11.16 ± 2.55, 10.31 ± 2.35, and 9.81 ± 2.33, respectively. In accordance with flow cytometry analysis of Th17 cells, IL-17A was up-regulated in mice with hepatic fibrosis from week 4 and peaked at week 4 (P < 0.01) (Figure 3B). Similarly, the levels of IFN-γ in the hepatic fibrosis group from weeks 0 to 12 were 29.56 ± 4.15, 180.51 ± 6.44, 129.86 ± 5.56, and 99.21 ± 5.22. In the control group, the levels of IFN-γ were 30.54 ± 4.2, 30.49 ± 4.93, 30.6 ± 4.64, and 30.48 ± 3.6, respectively. In accordance with flow cytometry analysis of Th1 cells, IFN-γ was up-regulated in mice with hepatic fibrosis from week 4 and peaked at week 4 (P < 0.01) (Figure 3C). The numbers of Th22, Th17 and Th1 cells in the hepatic fibrosis group were positively correlated with the production of IL-22, IL-17A and IFN-γ protein, respectively (P < 0.01, Figure 3D-F).
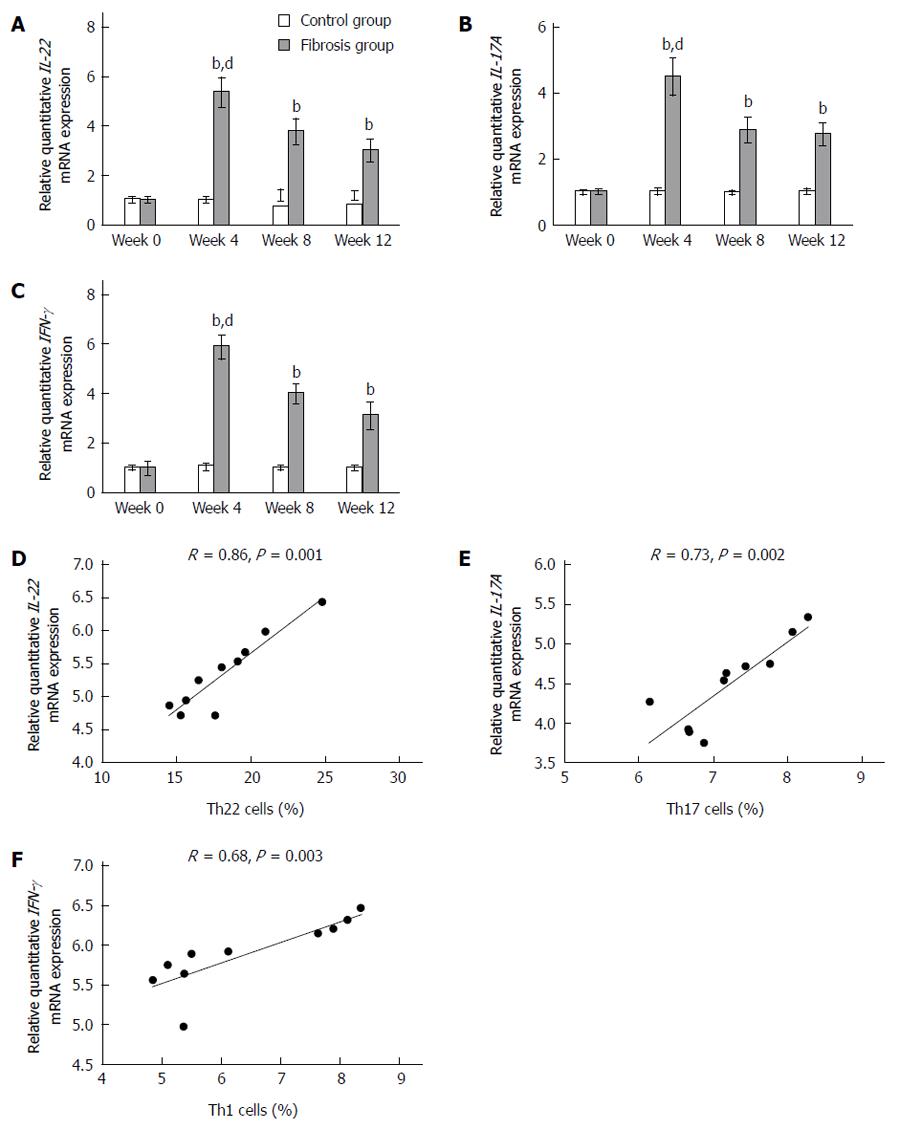
As shown in Figure 4, the intensity of hepatic IL-22 mRNA normalized to β-actin in the hepatic fibrosis group from weeks 0 to 12 and the levels were 1.02 ± 0.08, 5.36 ± 0.57, 3.77 ± 0.49, and 3.03 ± 4.42, respectively. In the control group, these levels from weeks 0 to 12 were 1.03 ± 0.08, 1.02 ± 0.08, 0.99 ± 0.09, and 1.01 ± 0.06, respectively. In accordance with flow cytometry analysis of Th22 cells, IL-22 mRNA was up-regulated in mice with hepatic fibrosis from week 4 and peaked at week 4 (P < 0.01) (Figure 4A). The intensity of IL-17A mRNA normalized to β-actin in the hepatic fibrosis group from weeks 0 to 12 and the levels were 1.01 ± 0.05, 4.5 ± 0.54, 2.88 ± 0.35, and 2.76 ± 0.31, respectively. In the control group, these levels from weeks 0 to 12 were 1 ± 0.05, 1.02 ± 0.07, 0.99 ± 0.04, and 1.02 ± 0.06, respectively. In accordance with flow cytometry analysis of Th17 cells, IL-17A mRNA was up-regulated in mice with hepatic fibrosis from week 4 and peaked at week 4 (P < 0.01) (Figure 4B). Similarly, the intensity of hepatic IFN-γ mRNA normalized to β-actin in the hepatic fibrosis group from weeks 0 to 12 and the levels were 0.98 ± 0.25, 5.89 ± 0.44, 4 ± 0.36, and 3.12 ± 0.52, respectively. In the control group, these levels from weeks 0 to 12 were: 1.02 ± 0.06, 1.06 ± 0.1, 1.01 ± 0.05, and 1.01 ± 0.05, respectively. In accordance with flow cytometry analysis of Th1 cells, IFN-γ mRNA was up-regulated in mice with hepatic fibrosis from week 4 and peaked at week 4 (P < 0.01) (Figure 4C). The numbers of Th22, Th17 and Th1 cells in the hepatic fibrosis group were positively correlated with the production of IL-22, IL-17A and IFN-γ mRNA, respectively (P < 0.01) (Figure 4D-F).
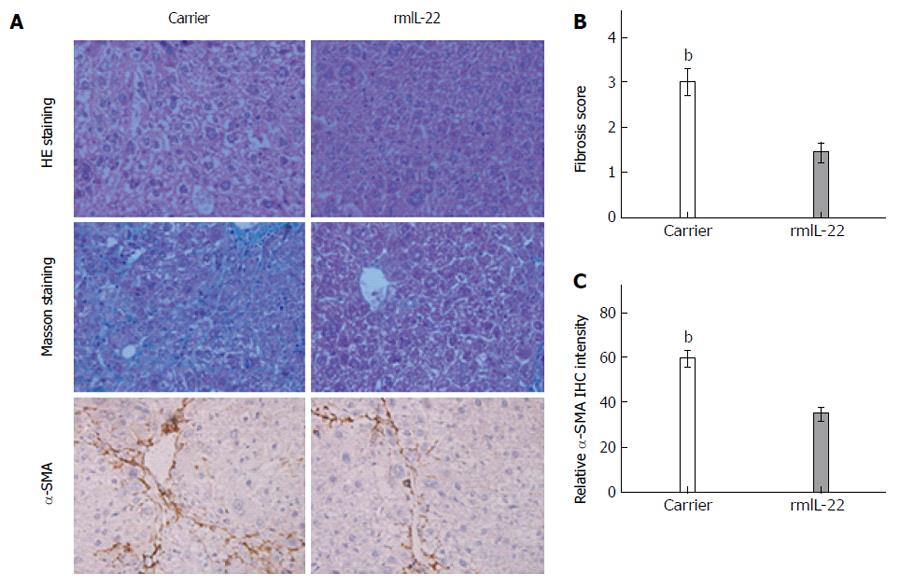
As shown in Figure 5, rmIL-22 significantly attenuated hepatic inflammation, necrosis and fibrosis compared with the carrier group (Figure 5A). The degree of liver fibrosis in the two groups of mice was investigated using Masson staining. The fibrosis scores in the rmIL-22 group were significantly lower than those in the carrier group (P < 0.01) (Figure 5B). Compared with the carrier group, there was a reduction in the number of α-SMA positive cells in the rmIL-22 group (P < 0.01) (Figure 5C). These results indicated that rmIL-22 improved CCl4-induced liver fibrosis.
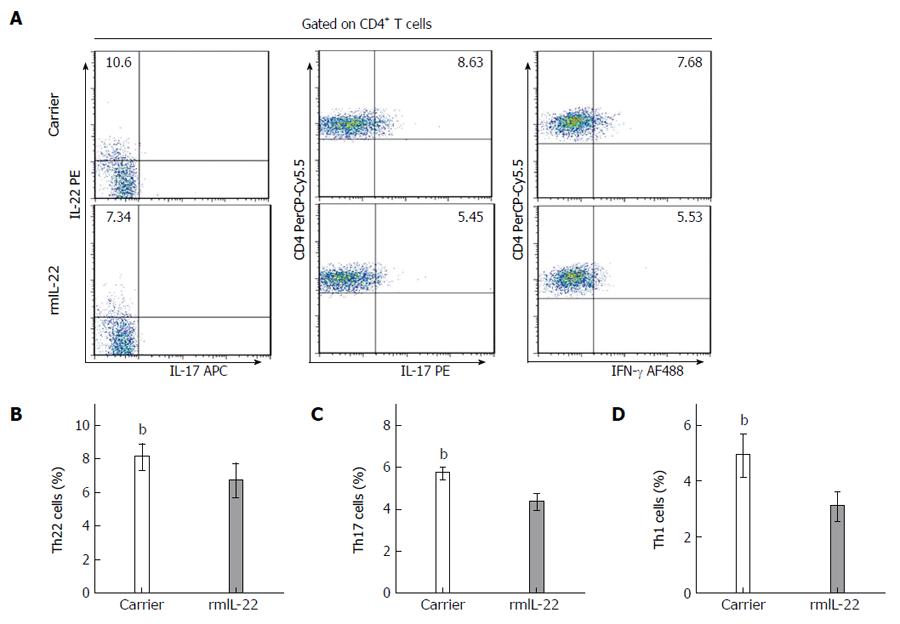
As shown in Figure 6A, the alterations in Th22, Th17 and Th1 cells in the rmIL-22 group and the carrier group were investigated by flow cytometry. The percentage of Th22 cells in the rmIL-22 group was less than that in the carrier group (6.71% ± 0.97% vs 8.09% ± 0.74%, P < 0.01) (Figue 6B). The percentage of Th17 cells in the rmIL-22 group was lower than that in the carrier group (4.34% ± 0.37% vs 5.71% ± 0.24%, P < 0.01) (Figure 6C). Similarly, the percentage of Th1 cells in the rmIL-22 group was lower than that in the carrier group (3.09% ± 0.49% vs 4.91% ± 0.73%, P < 0.01) (Figure 6D).
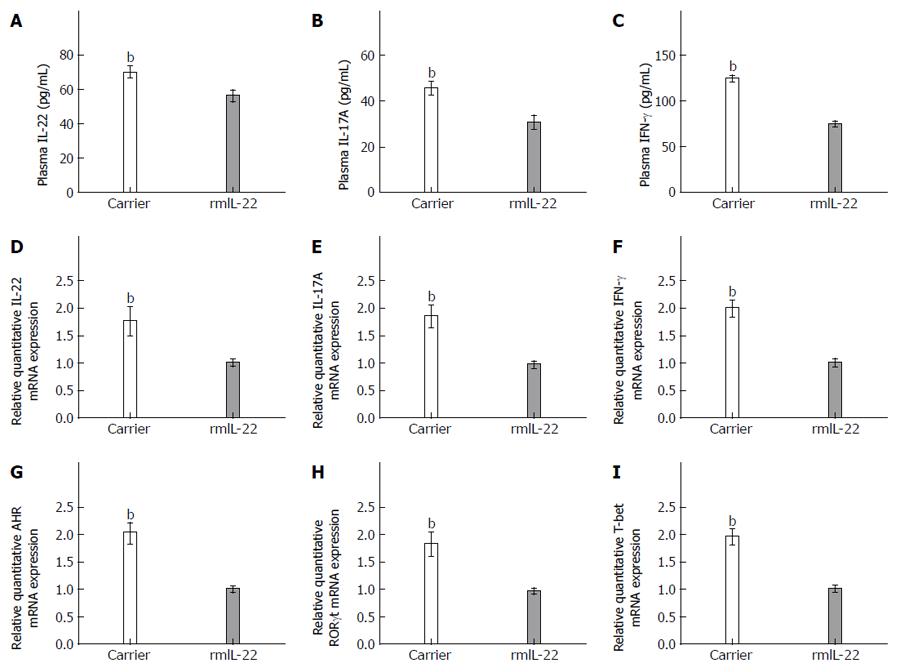
To confirm the finding that rmIL-22 suppressed Th22, Th17 and Th1 responses, we examined the effect of rmIL-22 on the expression of Th22 cell-, Th17 cell- and Th1 cell-related cytokines. The hepatic mRNA expression of inflammatory cytokines such as IL-22, IL-17A and IFN-γ was detected by RT-PCR. Moreover, their protein production in plasma was measured by ELISA. As shown in Figure 7A-C, compared with those in the carrier group, the levels of total circulating IL-22, IL-17A and IFN-γ were markedly decreased in the rmIL-22 group (56.23 ± 3.08 vs 70.29 ± 3.01, 30.74 ± 2.77 vs 45.68 ± 2.71, 74.78 ± 2.61 vs 124.89 ± 2.82, P < 0.01). Similarly, rmIL-22 reduced the level of IL-22, IL-17A and IFN-γ mRNA, which was much lower than that in the carrier group (1.01 ± 0.06 vs 1.77 ± 0.25, 0.98 ± 0.05 vs 1.86 ± 0.19, 1.01 ± 0.06 vs 2 ± 0.14, P < 0.01) (Figure 7D-F). Notably, the expression of AHR, RORγt and T-bet in liver tissues, the transcription factors controlling differentiation of Th22, Th17 and Th1 cells, respectively, were 1.5-2-fold lower in the rmIL-22 group than in the carrier group (Figure 7G-I).
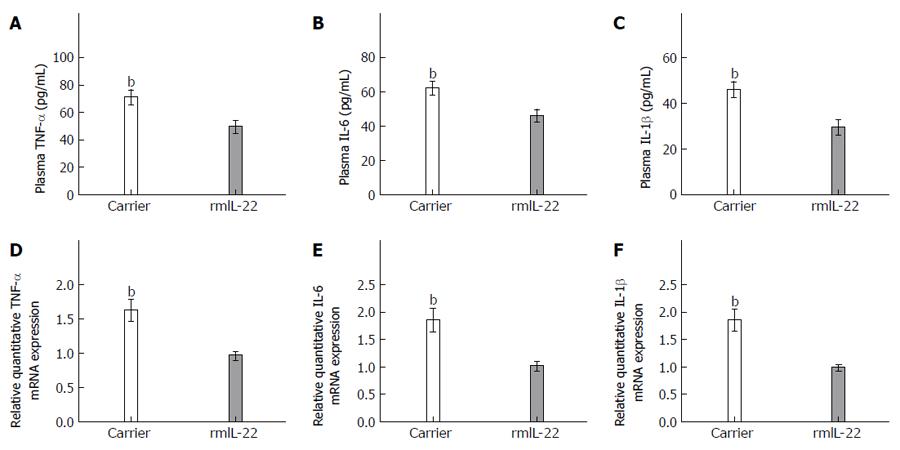
Immune cells have been shown to infiltrate liver lesions, some of which probably produce inflammatory cytokines, and possibly orchestrate Th22, Th17 and Th1 responses. To determine the status of these inflammatory cytokines in response to rmIL-22 treatment, we also examined protein production in plasma measured by ELISA. Compared with those in the carrier group, the levels of TNF-α, IL-6 and IL-1β proteins were markedly decreased in the rmIL-22 group (P < 0.01) (Figure 8A-C). In addition, hepatic mRNA expression of TNF-α, IL-6 and IL-1β was measured by RT-PCR. We observed significantly lower mRNA levels of TNF-α, IL-6 and IL-1β in liver tissues from the rmIL-22 group compared with the carrier group (P < 0.01) (Figure 8D-F).
CD4+ helper T cells adapt and amplify their responses to match different categories of infections and coordinate the many types of immune cells that can affect fibrosis[16,17]. There is growing evidence that T cell activation and its cytokine expression play a key role in the pathogenesis of hepatic fibrosis, such as IFN-γ-producing Th1 cells and IL-17-producing Th17 cells[18,19]. However, the potential role of Th22 cells in hepatic fibrosis is still not clear. In this study, we examined the frequency of splenic Th22, Th17 and Th1 cells, the concentrations of plasma IL-22, IL-17A and IFN-γ, and the expression of liver IL-22, IL-17A and IFN-γ mRNA in mice with CCl4-induced hepatic fibrosis. To further investigate the effects of IL-22, mice with hepatic fibrosis treated with rmIL-22 were examined. Our data showed that the percentages of splenic Th22, Th17 and Th1 cells were significantly higher in mice with CCl4-induced hepatic fibrosis than in control mice, and peaked at week 4. The concentrations of plasma IL-22, IL-17A and IFN-γ, and the expression of liver IL-22, IL-17A and IFN-γ mRNA in mice with CCl4-induced hepatic fibrosis were significantly higher than those in control mice, and peaked at week 4. Treatment with rmIL-22 in mice with hepatic fibrosis ameliorated the severity of hepatic fibrosis, which was confirmed by lower hepatic fibrosis pathological scores. rmIL-22 decreased the frequencies of Th22 cells, Th17 cells, Th1 cells and the levels of IL-22, IL-17, IFN-γ, TNF-α, IL-6, and IL-1β. Down-regulation of IL-22, IL-17, IFN-γ, TNF-α, IL-6, IL-1β, AHR, RORγt, and T-bet gene expression in the liver was observed in the rmIL-22 group.
Th1 cells play important roles in the pathogenic process of hepatic fibrosis. Shi et al[20] indicated that an expansion of IFN-γ-producing Th1 cells in C57BL/6 mice resulted in comparatively minimal fibrosis, whereas BALB/c mice, that have a predominant Th2 response, developed severe fibrosis in response to CCl4[20]. Our study demonstrated that the percentage of splenic Th1 cells significantly increased in mice with CCl4-induced hepatic fibrosis compared with control mice, and peaked at week 4, consistent with a previous observation[21]. However, in disagreement with our data, other researchers found that disease progression in mice with CCl4-induced hepatic fibrosis was associated with increased IL-4 and decreased IFN-γ, produced by CD4+Th2 and CD4+Th1 cells, respectively[22]. This difference may stem from the different genetic backgrounds of these mice. In addition, we also detected significantly higher levels of plasma IFN-γ and expression of liver IFN-γ mRNA in mice with CCl4-induced hepatic fibrosis, which peaked at week 4. Moreover, we found that the concentration of plasma IFN-γ and the expression of liver IFN-γ mRNA were positively correlated with the percentages of splenic Th1 cells in mice with CCl4-induced hepatic fibrosis. These results confirm that IFN-γ is predominantly secreted by Th1 cells in mice with CCl4-induced hepatic fibrosis and that the increased number of Th1 cells and higher level of IFN-γ may participate in the pathogenesis of hepatic fibrosis.
Th17 cells have been shown to be involved in the pathogenesis of hepatic fibrosis. The frequency of Th17 cells in the diseased liver correlates with liver fibrosis in patients with viral hepatitis[23,24], autoimmune hepatitis[25], and alcoholic liver disease[26]. We found that the percentage of splenic Th17 cells significantly increased in mice with CCl4-induced hepatic fibrosis compared with control mice, and peaked at week 4, consistent with a previous observation[21]. However, in disagreement with our data, other researchers found that the frequency of Th17 cells gradually increased in mice with CCl4-induced hepatic fibrosis. The difference between the week 4 subgroup and the controls was not significant. The frequency of Th17 cells was significantly increased in both the weeks 8 and 12 subgroups compared with the controls[27]. This difference may stem from the different genetic backgrounds of these mice. In addition, we also detected significantly higher levels of plasma IL-17A and expression of liver IL-17A mRNA in mice with CCl4-induced hepatic fibrosis, which peaked at week 4. Some researchers have demonstrated that IL-17A and IL-17RA deficiency protects mice from liver fibrosis induced by CCl4 and bile duct ligation[28-30]. Tan et al[29] recently reported that activation of HSCs and the production of collagen in CCl4-induced liver fibrosis are IL-17A dependent. Therefore, IL-17A neutralization may be a promising approach for anti-fibrotic therapy in patients with chronic liver diseases. Moreover, we found that the concentration of plasma IL-17A and the expression of liver IL-17A mRNA were positively correlated with the percentage of splenic Th17 cells in mice with CCl4-induced hepatic fibrosis. These findings confirm that IL-17A is predominantly secreted by Th17 cells in mice with CCl4-induced hepatic fibrosis and that the increased number of Th17 cells and higher level of IL-17A may participate in the pathogenesis of hepatic fibrosis.
Th22 cells play a complicated and important role in inflammatory and autoimmune diseases. Recently, it was shown that Th22 cells may play a role in the pathogenesis of atopic dermatitis, immune thrombocytopenia, tuberculous pleural effusion, and viral myocarditis. However, the potential role of Th22 cells in hepatic fibrosis is still not clear. This study showed that splenic Th22 cells were highly expressed in mice with CCl4-induced hepatic fibrosis compared with control mice and peaked at week 4. These findings suggest that inflammation and the immune microenvironment of hepatic fibrosis are suitable for the differentiation and proliferation of Th22 cells. We also detected significantly higher levels of plasma IL-22 and expression of liver IL-22 mRNA in mice with CCl4-induced hepatic fibrosis, which peaked at week 4. Moreover, we found that the concentration of plasma IL-22 and the expression of liver IL-22 mRNA were positively correlated with the percentage of splenic Th22 cells in mice with CCl4-induced hepatic fibrosis. These results confirm that IL-22 is predominantly secreted by Th22 cells in mice with CCl4-induced hepatic fibrosis and that the increased number of Th22 cells and higher level of IL-22 may participate in the pathogenesis of hepatic fibrosis. In addition, positive correlations were observed between Th22 cells and Th1 cells, Th22 cells and Th17 cells, Th17 cells and Th1 cells in mice with CCl4-induced hepatic fibrosis. These positive correlations suggest that these T cell subsets may play a synergistic role in the development of hepatic fibrosis.
IL-22, a crucial cytokine in Th22 cells, mediates its effects via IL-22R and propagates downstream signals including the JAK/STAT, ERK, JNK, PI-3K and p38 MAP kinase pathways. For example, other researchers have previously demonstrated that IL-22 plays an important role in protecting against liver injury[31-33], ameliorating fatty liver disease[15,34] and promoting liver regeneration[34]. In the present study, we identified a protective function of IL-22 in hepatic fibrosis. This protective role of IL-22 was observed in various animal models to different extents. Our study showed that rmIL-22 significantly attenuated hepatic inflammation, necrosis and fibrosis compared with the carrier group. The fibrosis scores of the rmIL-22 group were significantly lower than those in the carrier group, as assessed by the ISHAK fibrosis scoring system and computerized morphometric quantification of Masson’s staining. Compared with the carrier group, rmIL-22 markedly decreased the areas of α-SMA staining, which indicated a reduced number of activated HSCs. As HSCs activation is responsible for ECM production, intrahepatic angiogenesis and vascular remodeling in cirrhotic liver[1], this suggests that rmIL-22 treatment ameliorates liver fibrosis by targeting HSCs in a murine model of CCl4-induced liver fibrosis[16].
In our experimental study, we demonstrated that rmIL-22 not only reduced liver inflammation, but significantly suppressed the activation of HSCs. These changes were accompanied by significant down-regulation of Th22, Th17 and Th1 cells infiltration in the spleen compared with the carrier group. Treatment with rmIL-22 decreased the proportions of Th22, Th17 and Th1 cells. Our data indicated that rmIL-22 exerts its therapeutic effects partly by regulating the differentiation of Th22, Th17 and Th1 cells. In addition, we found that rmIL-22 reduced the level of total circulating IL-22, IL-17A and IFN-γ protein, which was much lower than that in the carrier group. Similarly, compared with those in the carrier group, the levels of IL-22, IL-17A, IFN-γ, AHR, RORγt and T-bet mRNA were markedly decreased in the rmIL-22 group.
The differentiation of Th22, Th17 and Th1 cells is largely dependent on cytokines produced by the immune system. We found that rmIL-22 decreased both the protein and mRNA concentrations of the proinflammatory cytokines TNF-α, IL-6 and IL-1β in mice with CCl4-induced hepatic fibrosis. This observation suggested that rmIL-22 modulated the immune response in hepatic fibrosis. Previous evidence has demonstrated that rmIL-22 significantly down-regulated TNF-α and other genes in the lipogenic mouse liver[35]. Following injection of anti-IL-22 antibodies, the mRNA and protein levels of TNF-α and IL-6 were markedly up-regulated in mice with acute viral myocarditis[36]. Recent evidence has indicated that the development of T helper cells is largely determined by their cytokine environment. The cytokine, IL-1β, enhances both Th1 and Th17 responses[37,38], IL-6 plus TGF-β induce the Th17 response[39,40], whereas IL-6 plus TNF-α induce the Th22 response[10]. TNF-α is a potential contributor by equipping dendritic cells with the ability to induce T cells toward Th1 and Th17 phenotypes[41]. Accordingly, rmIL-22 inhibits Th22, Th17 and Th1 responses most likely by modulating the release of cytokines (TNF-α, IL-6 and IL-1β).
In conclusion, this study showed that the numbers of Th22, Th17 and TH1 cells were significantly increased in mice with CCl4-induced hepatic fibrosis. On the other hand, we demonstrated that IL-22 has a profound anti-fibrosis effect in mice with hepatic fibrosis. IL-22 restricts the development of Th22, Th17 and Th1 responses by suppressing the secretion of TNF-α, IL-6 and IL-1β. IL-22-mediated release of cytokines appears to be a promising therapeutic target for the treatment of hepatic fibrosis, although this speculation requires further stringent investigation.
Liver fibrosis is a common chronic progressive liver disease that threatens human health, therefore, it is important to prevent its occurrence. However, to date, the pathogenesis of liver fibrosis has not been entirely elucidated. T helper (Th)-22, Th17 and Th1 cells are T lymphocyte subgroups. Some studies have reported that Th22, Th17 and Th1 cells are closely related to many autoimmune diseases. However, the functional characteristics of Th22 cells and interleukin (IL)-22 in hepatic fibrosis have seldom been reported.
Th22, Th17 and Th1 cells are closely related to a variety of autoimmune diseases. IL-22 restricts the development of Th22, Th17 and Th1 responses by suppressing the secretion of tumour necrosis factor-α, IL-6 and IL-1β, and preventing the occurrence of liver fibrosis.
This is believed to be the first study to determine the effect of Th22 cells and IL-22 in CCl4-induced liver fibrosis in mice and its potential mechanisms. This study showed that the percentage of Th22 cells is high in hepatic fibrosis, and IL-22 significantly suppresses the activation of hepatic stellate cells.
IL-22-mediated release of cytokines appears to be a promising therapeutic target for the treatment of hepatic fibrosis.
Th22 (IL-22-producing CD4+T helper cells), Th17 (IL-17-producing CD4+T helper cells) and Th1 (interferon gamma-producing CD4+T helper cells) are described as three additional subsets of T lymphocytes that can orchestrate host immune responses by releasing distinct cytokine profiles.
This was a good descriptive study in which the authors analyzed the effect of Th22, Th17 and Th1 cells on CCl4-induced liver fibrosis in mice. The results are interesting and suggest that IL-22 is a potential therapeutic target that could be used for preventing the occurrence of liver fibrosis.
P- Reviewer: Gao B, Khattab MA, Xu Y S- Editor: Gou SX L- Editor: O’Neill M E- Editor: Liu XM
| 1. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2165] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 2. | Hashimoto N, Shimoda S, Kawanaka H, Tsuneyama K, Uehara H, Akahoshi T, Kinjo N, Taketomi A, Shirabe K, Akashi K. Modulation of CD4⁺ T cell responses following splenectomy in hepatitis C virus-related liver cirrhosis. Clin Exp Immunol. 2011;165:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Heymann F, Hammerich L, Storch D, Bartneck M, Huss S, Rüsseler V, Gassler N, Lira SA, Luedde T, Trautwein C. Hepatic macrophage migration and differentiation critical for liver fibrosis is mediated by the chemokine receptor C-C motif chemokine receptor 8 in mice. Hepatology. 2012;55:898-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | Karlmark KR, Wasmuth HE, Trautwein C, Tacke F. Chemokine-directed immune cell infiltration in acute and chronic liver disease. Expert Rev Gastroenterol Hepatol. 2008;2:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 827] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 6. | Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1059] [Cited by in RCA: 1074] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 7. | Baroni GS, D’Ambrosio L, Curto P, Casini A, Mancini R, Jezequel AM, Benedetti A. Interferon gamma decreases hepatic stellate cell activation and extracellular matrix deposition in rat liver fibrosis. Hepatology. 1996;23:1189-1199. [PubMed] |
| 8. | Zhang JY, Song CH, Shi F, Zhang Z, Fu JL, Wang FS. Decreased ratio of Treg cells to Th17 cells correlates with HBV DNA suppression in chronic hepatitis B patients undergoing entecavir treatment. PLoS One. 2010;5:e13869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Niu Y, Liu H, Yin D, Yi R, Chen T, Xue H, Zhang S, Lin S, Zhao Y. The balance between intrahepatic IL-17(+) T cells and Foxp3(+) regulatory T cells plays an important role in HBV-related end-stage liver disease. BMC Immunol. 2011;12:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 822] [Cited by in RCA: 828] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 11. | Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 788] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 12. | Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647-659. [PubMed] |
| 13. | Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332-1342. [PubMed] |
| 14. | Ren X, Hu B, Colletti LM. IL-22 is involved in liver regeneration after hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2010;298:G74-G80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Yang L, Zhang Y, Wang L, Fan F, Zhu L, Li Z, Ruan X, Huang H, Wang Z, Huang Z. Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J Hepatol. 2010;53:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 16. | Kong X, Feng D, Wang H, Hong F, Bertola A, Wang FS, Gao B. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology. 2012;56:1150-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 358] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 17. | Holt AP, Salmon M, Buckley CD, Adams DH. Immune interactions in hepatic fibrosis. Clin Liver Dis. 2008;12:861-882, x. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Weng HL, Feng DC, Radaeva S, Kong XN, Wang L, Liu Y, Li Q, Shen H, Gao YP, Müllenbach R. IFN-9 inhibits liver progenitor cell proliferation in HBV-infected patients and in 3,5-diethoxycarbonyl-1,4-dihydrocollidine diet-fed mice. J Hepatol. 2013;59:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Gao B, Waisman A. Th17 cells regulate liver fibrosis by targeting multiple cell types: many birds with one stone. Gastroenterology. 2012;143:536-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Shi Z, Wakil AE, Rockey DC. Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses. Proc Natl Acad Sci USA. 1997;94:10663-10668. [PubMed] |
| 21. | Tu CT, Li J, Wang FP, Li L, Wang JY, Jiang W. Glycyrrhizin regulates CD4+T cell response during liver fibrogenesis via JNK, ERK and PI3K/AKT pathway. Int Immunopharmacol. 2012;14:410-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Bot A. Immunoglobulin deficient mice generated by gene targeting as models for studying the immune response. Int Rev Immunol. 1996;13:327-340. [PubMed] |
| 23. | Sun HQ, Zhang JY, Zhang H, Zou ZS, Wang FS, Jia JH. Increased Th17 cells contribute to disease progression in patients with HBV-associated liver cirrhosis. J Viral Hepat. 2012;19:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Ge J, Wang K, Meng QH, Qi ZX, Meng FL, Fan YC. Implication of Th17 and Th1 cells in patients with chronic active hepatitis B. J Clin Immunol. 2010;30:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Longhi MS, Liberal R, Holder B, Robson SC, Ma Y, Mieli-Vergani G, Vergani D. Inhibition of interleukin-17 promotes differentiation of CD25⁻ cells into stable T regulatory cells in patients with autoimmune hepatitis. Gastroenterology. 2012;142:1526-1535.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | Lemmers A, Moreno C, Gustot T, Maréchal R, Degré D, Demetter P, de Nadai P, Geerts A, Quertinmont E, Vercruysse V. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49:646-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 27. | Sun XF, Gu L, Deng WS, Xu Q. Impaired balance of T helper 17/T regulatory cells in carbon tetrachloride-induced liver fibrosis in mice. World J Gastroenterol. 2014;20:2062-2070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, Cong M, Iwaisako K, Liu X, Zhang M. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143:765-776.e1-e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 563] [Article Influence: 43.3] [Reference Citation Analysis (1)] |
| 29. | Tan Z, Qian X, Jiang R, Liu Q, Wang Y, Chen C, Wang X, Ryffel B, Sun B. IL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activation. J Immunol. 2013;191:1835-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 30. | Hara M, Kono H, Furuya S, Hirayama K, Tsuchiya M, Fujii H. Interleukin-17A plays a pivotal role in cholestatic liver fibrosis in mice. J Surg Res. 2013;183:574-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Mastelic B, do Rosario AP, Veldhoen M, Renauld JC, Jarra W, Sponaas AM, Roetynck S, Stockinger B, Langhorne J. IL-22 Protects Against Liver Pathology and Lethality of an Experimental Blood-Stage Malaria Infection. Front Immunol. 2012;3:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Chestovich PJ, Uchida Y, Chang W, Ajalat M, Lassman C, Sabat R, Busuttil RW, Kupiec-Weglinski JW. Interleukin-22: implications for liver ischemia-reperfusion injury. Transplantation. 2012;93:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Xing WW, Zou MJ, Liu S, Xu T, Gao J, Wang JX, Xu DG. Hepatoprotective effects of IL-22 on fulminant hepatic failure induced by d-galactosamine and lipopolysaccharide in mice. Cytokine. 2011;56:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291-1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 367] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 35. | Park O, Wang H, Weng H, Feigenbaum L, Li H, Yin S, Ki SH, Yoo SH, Dooley S, Wang FS. In vivo consequences of liver-specific interleukin-22 expression in mice: Implications for human liver disease progression. Hepatology. 2011;54:252-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 215] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 36. | Kong Q, Wu W, Yang F, Liu Y, Xue Y, Gao M, Lai W, Pan X, Yan Y, Pang Y. Increased expressions of IL-22 and Th22 cells in the coxsackievirus B3-Induced mice acute viral myocarditis. Virol J. 2012;9:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, Dinarello CA, Paul WE. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci USA. 2009;106:7119-7124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 456] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 38. | Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1001] [Cited by in RCA: 984] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 39. | Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179-189. [PubMed] |
| 40. | Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967-974. [PubMed] |