Published online Dec 7, 2015. doi: 10.3748/wjg.v21.i45.12822
Peer-review started: April 18, 2014
First decision: June 19, 2015
Revised: July 2, 2015
Accepted: September 2, 2015
Article in press: September 2, 2015
Published online: December 7, 2015
Processing time: 233 Days and 23.2 Hours
AIM: To investigate whether heat shock pretreatment (HSP) improves mesenchymal stem cell (MSC) repair via autophagy following hepatic ischemia-reperfusion injury (HIRI).
METHODS: Apoptosis of MSCs was induced by 250 mM hydrogen peroxide (H2O2) for 6 h. HSP was carried out using a 42 °C water bath for 1, 2 or 3 h. Apoptosis of MSCs was analyzed by flow cytometry, and Western blot was used to detect Bcl-2, Bax and cytochrome C expression. Autophagy of MSCs was analyzed by flow cytometry and transmission electron microscopy, and the expression of beclin I and LC3-II was detected by Western blot. MSCs were labeled in vivo with the fluorescent dye, CM-Dil, and subsequently transplanted into the portal veins of rats that had undergone HIRI. Liver levels of proliferating cell nuclear antigen (PCNA) were quantified by fluorescent microscopy. Serum aminotransferase activity and the extent of HIRI were also assessed at each time point.
RESULTS: HSP for 2 h reduced apoptosis of MSCs induced by H2O2 as seen by a decrease in apoptotic rate, a decrease in Bax and cytochrome C expression and an increase in Bcl-2 expression (P < 0.001). In addition, HSP for 2 h induced autophagy of MSCs exposed to H2O2 as shown by an increase in acidic vesicular organelle-positive cells, beclin 1 and LC3-II expression, and autophagosome formation (P < 0.05). Treatment with 3-methyladenine attenuated HSP-induced autophagy and abolished the protective effects of HSP on the apoptosis of MSCs. Rapamycin failed to have additional effects on either autophagy or apoptosis compared with HSP alone. The phosphorylation of p38MAPK was significantly elevated and the phosphorylation of mTOR was downregulated in heat shock pretreated MSCs. Treatment with the p38MAPK inhibitor, SB203580, reduced HSP-induced autophagy in MSCs. In vivo studies showed that the transplantation of HSP-MSCs resulted in lower serum aminotransferase levels, lower Suzuki scores, improved histopathology and an increase in PCNA-positive cells (P < 0.05).
CONCLUSION: HSP effectively induces autophagy following exposure to H2O2via the p38MAPK/mTOR pathway, which leads to enhanced MSC survival and improved MSC repair following HIRI in rats.
Core tip: We investigated the interaction between autophagy and apoptosis in mesenchymal stem cells (MSCs) exposed to H2O2. We found that heat shock pretreatment (HSP)-induced autophagy served as a protective mechanism. HSP for 2 h improved the therapeutic potential of MSCs in the treatment of ischemia-reperfusion (I/R) injury in rats and enhanced autophagy via the p38MAPK/mTOR pathway, which is involved in the protective effects of HSP on H2O2-induced MSC apoptosis. Systemic administration led to an increase in HSP-MSCs homing to I/R liver cells compared with MSCs, resulting in a significant improvement in liver function, accelerated mitogenic response and alleviation of histopathological damage.
-
Citation: Qiao PF, Yao L, Zhang XC, Li GD, Wu DQ. Heat shock pretreatment improves stem cell repair following ischemia-reperfusion injury
via autophagy. World J Gastroenterol 2015; 21(45): 12822-12834 - URL: https://www.wjgnet.com/1007-9327/full/v21/i45/12822.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i45.12822
During surgical trauma, particularly liver transplantation, hepatic ischemia-reperfusion injury (HIRI) may occur, which is associated with a significant reduction in liver function[1,2]. Effective treatment strategies aimed at reducing HIRI may therefore offer major benefits in hepatic surgery and liver transplantation. A previous study demonstrated the specific involvement of bone marrow mesenchymal stem cells (MSCs) in the repair of HIRI[3]. However, due to local hypoxia, inflammation, and especially oxidative stress in the targeted tissue, the transplanted MSCs did not withstand the difficult microenvironment caused by ischemia-reperfusion (I/R) injury. Thus, low cell survival reduced the therapeutic effect[4]. It was also reported that < 1% of transplanted MSCs survived to the fourth day in an immunodeficient mouse heart model[5]. The poor MSC survival rate was also observed after transplantation into lungs and kidneys with I/R injury[6,7]. Therefore, it is imperative to protect MSCs from oxidative stress and other pro-apoptotic factors to improve their therapeutic effect.
Heat shock pretreatment (HSP) is known to activate certain types of self-protective proteins and protects cells in vitro from various environmental insults[8-10]. Several reports have shown that HSP of transplanted cells enhanced their survival in a heart model both in vivo and in vitro[11,12]. Thus, we hypothesized that HSP of MSCs could enhance their survival following transplantation into the liver after I/R injury. Recently, the induction of autophagy was shown to be a novel method of protecting MSCs from apoptosis[13,14]. Several reports have shown that heat shock treatment can activate autophagy in multiple cell types[15,16]. However, it is unknown whether autophagy can be activated by heat shock treatment or how it affects MSCs.
Autophagy is an essential cellular mechanism that occurs in eukaryotic cells[17,18]. In recent years, it has been found that autophagy plays a vital role in cell apoptosis and its role depends on cell type and cellular conditions. Autophagy can lead to pro-survival pathways, while inappropriate autophagy can induce cell death[19]. Under ischemia or hypoxia/serum deprivation (H/SD), autophagy can protect MSCs from apoptosis by eliminating reactive oxygen species and damaged organelles to provide energy[13,20]. Moreover, H/SD-induced autophagy has also been demonstrated to induce apoptosis in some cell types. Autophagy can also directly promote type II programmed cell death[21]. However, the functional role of autophagy in oxidative stress-induced apoptosis in MSCs has not been fully elucidated.
Mitogen-activated protein kinase (p38MAPK) is a positive regulator of autophagy and is regulated by heat shock treatment to improve cardiac cell survival[8]. p38MAPK can be activated in response to exogenous stress such as hypoxia, starvation and heat shock, which in turn activates mitogen-activated protein kinase kinases (MKK)-3/4/6 and their effector kinases to stimulate autophagy[22]. However, little is known about the function of the p38MAPK pathway in regulating the activation of autophagy in MSCs following heat shock treatment.
The aim of this study was to examine the function of autophagy in MSC apoptosis induced by oxidative stress injury. Further, we investigated whether HSP activates autophagy via the p38MAPK/mTOR pathway to protect MSCs against apoptosis.
Thirty-two male Sprague-Dawley rats (about 220 g; 10 wk old) from the Animal Center of the Second Affiliated Hospital, Harbin Medical University were used in this study. The rats were cared for in accordance with the guidelines published by the US National Institutes of Health. All study procedures were approved by the Harbin Medical University Institutional Animal Care and Use Committee. The study was conducted in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Academy Press.
MSCs were collected as previously described[3], and density centrifugation was performed to isolate MSCs[23]. The femurs and tibias from male Wistar rats aged 4 wk were flushed, and bone marrow cells were collected and then fractionated in Lymphoprep™ density solution. Following centrifugation at 800 ×g for 20 min, the cells at the interface were collected and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, United States) containing 10% fetal bovine serum and 1% penicillin/streptomycin. Cells were incubated at 37 °C with 95% humidity and 5% CO2. After 48 h, the culture medium was changed to remove non-adherent cells. After the fourth passage, MSCs were washed with phosphate buffered saline (PBS), exposed to HSP for different time periods (1, 2 or 3 h) in a 42 °C water bath and then incubated at 37 °C in a humidified atmosphere containing 5% CO2 for 24 h (HSP-MSC group). Control cells were cultured under normal conditions without HSP (MSC group). To simulate tissue I/R microenvironment in vitro, MSCs were treated with 250 mM H2O2 (Sigma-Aldrich, United States) for 6 h[24,25]. The autophagy inhibitor, 3-methyladenine (3-MA; 5 mM; Sigma-Aldrich, United States), the autophagy promoter, rapamycin (10 nM; Cell Signaling Technology, United States), or the p38MAPK inhibitor, SB203580 (5 mM; Beyotime, China), was added to further examine the role of autophagy on MSC apoptosis.
Cell apoptosis was examined using the Annexin V-FITC/PI Kit (Becton-Dickinson, United States). Briefly, MSCs were collected in 200 mL medium. Following resuspension, approximately 10 mL of Annexin V solution were added and incubated for 15 min at room temperature in the dark. Then, 300 mL medium buffer and 5 mL propidium iodide (PI) were added and the cell suspension was incubated for 15 min at room temperature in the dark. The cell suspension was then immediately analyzed by flow cytometry (Becton-Dickinson, United States). Cell Quest software was used to analyze 104 cells.
Cell autophagy was examined by detecting acidic vesicular organelles (AVO) using acridine orange (AO) stain (Solarbio, China) according to published protocols[26]. Briefly, cells were stained with 1 mg/mL AO for 15 min and collected in PBS. In AO-stained cells, the cytoplasm fluoresces bright green, whereas AVOs, including lysosomes and autolysosomes, fluoresce bright red. The green (510-530 nm) and red (650 nm) fluorescence emission from 104 cells illuminated with blue (488 nm) excitation light was measured by flow cytometry using Cell Quest software.
MSCs were harvested and fixed with 2.5% glutaraldehyde at 4 °C for 2 h. Cells were then suspended in PBS containing 1% osmic acid at 4 °C for 2 h, Following dehydrating and embedding[13], ultrathin sections were prepared on uncoated copper grids using an Ultrotome (Leica, Reichert Ultracuts) and stained with uranyl acetate and lead citrate. Images were captured using a transmission electron microscope (JEM1230; JEOL).
Protein lysates were separated using SDS-PAGE and transferred to nitrocellulose membranes (Amersham Pharmacia Biotech, United States). Membranes were probed with the appropriate primary antibodies (Supplemental Table 1). Alexa Fluor® 680 donkey anti-mouse IgG (H + L) or Alexa Fluor® 680 donkey anti-rabbit IgG (H + L) were used as secondary antibodies (1:5000; Invitrogen, United States). Fluorophores were detected using the Odyssey™ Infrared Imaging System (Li-Cor, Lincoln, NE, United States).
The transplanted MSCs were labeled with 10 μmol/L CM-Dil (Invitrogen, United States) according to published protocols[27].
HIRI in the rat model was performed as previously described[3]. Briefly, a midline laparotomy was performed following anesthesia administration with intraperitoneal sodium pentobarbital (60 mg/kg). The left lateral and medial lobes of the liver were then clamped at their bases using an atraumatic clip. Ischemia was induced in 70% of the segmental liver and prevented ischemia in the mesenteric veins[28]. Throughout the administration of anesthesia, body temperature was monitored by a rectal probe and maintained at 37 °C by a heating lamp. The clamp was removed after 60 min, and 1 × 106 CM-Dil-labeled MSCs or HSP-MSCs suspended in 200 μL PBS were immediately transplanted into the portal vein using a 30-gauge needle, in the MSC group and HSP-MSC group, respectively. The control group underwent laparotomy only and received 200 μL PBS. The 32 rats were randomly divided into 4 groups. At 24 h after transplantation, 2 mL blood was harvested from the inferior vena cava before the animals were sacrificed by cervical spine dislocation. Livers were harvested immediately.
The chest was opened following tracheal intubation and the rats were perfused with 4% paraformaldehyde (Sigma-Aldrich, United States) in 0.01 M PBS following an overdose of anesthesia (sodium pentobarbital; 100 mg/kg, intraperitoneal) for 2 min[29]. Harvested livers were cryopreserved in 30% sucrose at 4 °C overnight, embedded in optimal cutting temperature (OCT) compound, and cut into 4 μm-thick sections using a cryostat. The sections were rinsed twice with PBS, fixed in 4% paraformaldehyde for 20 min at room temperature, and washed three times with PBS. After permeabilization with 0.2% Triton X-100, the sections were blocked at 4 °C overnight in 1% BSA/0.05% Triton X-100. Sections were then incubated with an antibody against PCNA (1:200) at 37 °C for 2 h. After washing three times with PBS, the sections were incubated with Alexa Fluor® 488-conjugated Affinipure goat anti-rabbit IgG (H + L) secondary antibody (1:200; ZSGB-Bio, China) for 1 h at room temperature. After extensive washing, the sections were examined under a fluorescence microscope[30].
To evaluate the severity of HIRI, the serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured by an automatic analyzer (Hitachi, Japan) as described previously[31].
Tissue sections of 1.5 cm × 1.5 cm × 2 mm were subjected to immunohistochemical staining to assess PCNA 24 h after cell transplantation. Immunohistochemical staining of sections for PCNA expression was performed by a standard streptavidin-biotin peroxidase complex method[32]. Tissue sections (4 mm) were deparaffinized and rehydrated by standard protocols, autoclaved at 95 °C for 20 min, and cooled to 30 °C. Normal rabbit serum (10%) was used to block non-specific binding sites. Sections were then incubated with anti-PCNA primary antibody (1:100) in PBS containing 1% bovine serum albumin at 4 °C overnight. The sections were washed in PBS, incubated with biotinylated anti-rabbit IgG for 30 min at room temperature, and then a streptavidin-biotin peroxidase complex solution (Nichirei, Japan). The chromogen, 3, 3’-diaminobenzidine tetra-hydrochloride, was used as a 0.02% solution containing 0.005% H2O2 in 50 mmol/L ammonium acetate-citrate acid buffer (pH 6.0). Sections were counterstained with Mayer’s hematoxylin and mounted. Negative controls were established by replacing the primary antibody with normal rabbit serum. No staining was detected in the negative controls.
The degree of HIRI was assessed by histological analysis as previously described[3].
The data were expressed as the mean ± SD, and representative results were from at least three independent experiments. For quantitative continuous data, the differences between two groups were examined and the data were analyzed using t-tests. When multiple comparisons were possible, ANOVA coupled with Tukey’s post-hoc test correction was used. P < 0.05 was considered statistically significant. Statistical analyses were carried out using SPSS version 21 (SPSS Inc., Chicago, IL, United States) or the GraphPad Prism 5.0 software package (GraphPad Software, Inc., La Jolla, CA, United States).
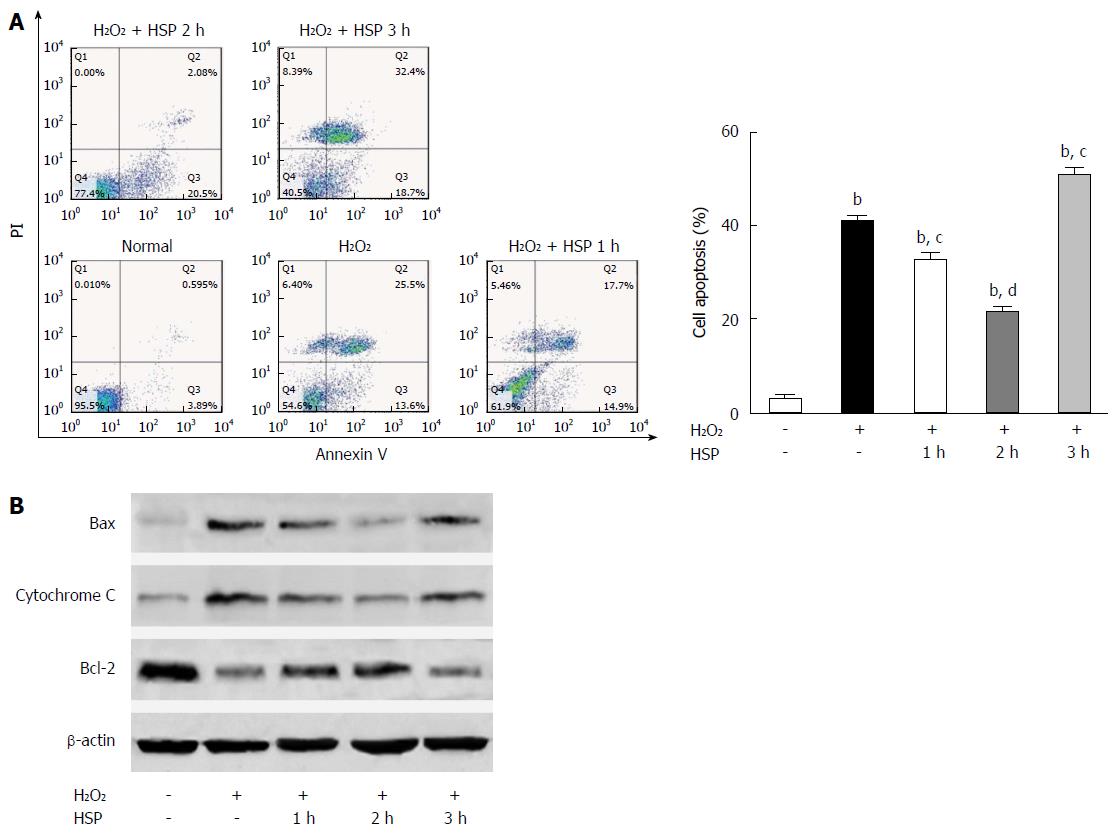
The apoptotic rate and levels of the pro-apoptotic proteins, Bax and cytochrome C, were all reduced. The anti-apoptotic protein, Bcl-2, was increased in the HSP1h and HSP2h groups compared to the control and H2O2 group (Figure 1; P < 0.01). However, in the HSP3h group, the apoptotic rate and expression of Bax and cytochrome C were increased, while Bcl-2 expression was reduced (Figure 1; P < 0.01). These results suggest that 2 h of HSP protected MSCs from H2O2-induced apoptosis.
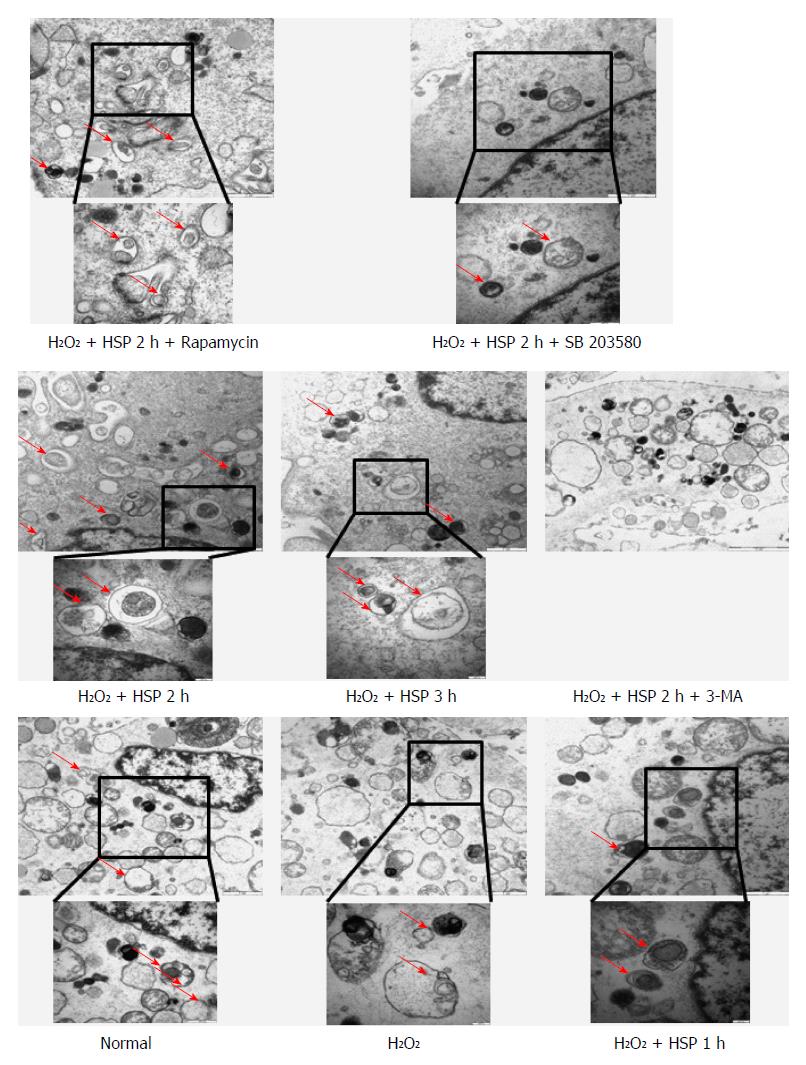
To examine whether HSP activated autophagy in MSCs, the cells were pretreated with heat shock for 1, 2 or 3 h, and then exposed to H2O2 for 6 h. The number of AVO-positive cells identified by flow cytometry was increased in the HSP group compared with the control group (Figure 2A; P < 0.05). Different durations of HSP led to a time-dependent increase in the action of autophagy in MSCs exposed to H2O2, which peaked in the HSP3h group (P < 0.01). HSP-MSCs showed a significant time-dependent increase in the expression of LC3B-II and the autophagic marker, beclin 1, compared to the control group (Figure 2D). Autophagosomes observed in HSP-MSCs exposed to H2O2 are shown in Figure 3. These results suggest that HSP promoted autophagic activity in MSCs exposed to H2O2 in a time-dependent manner.
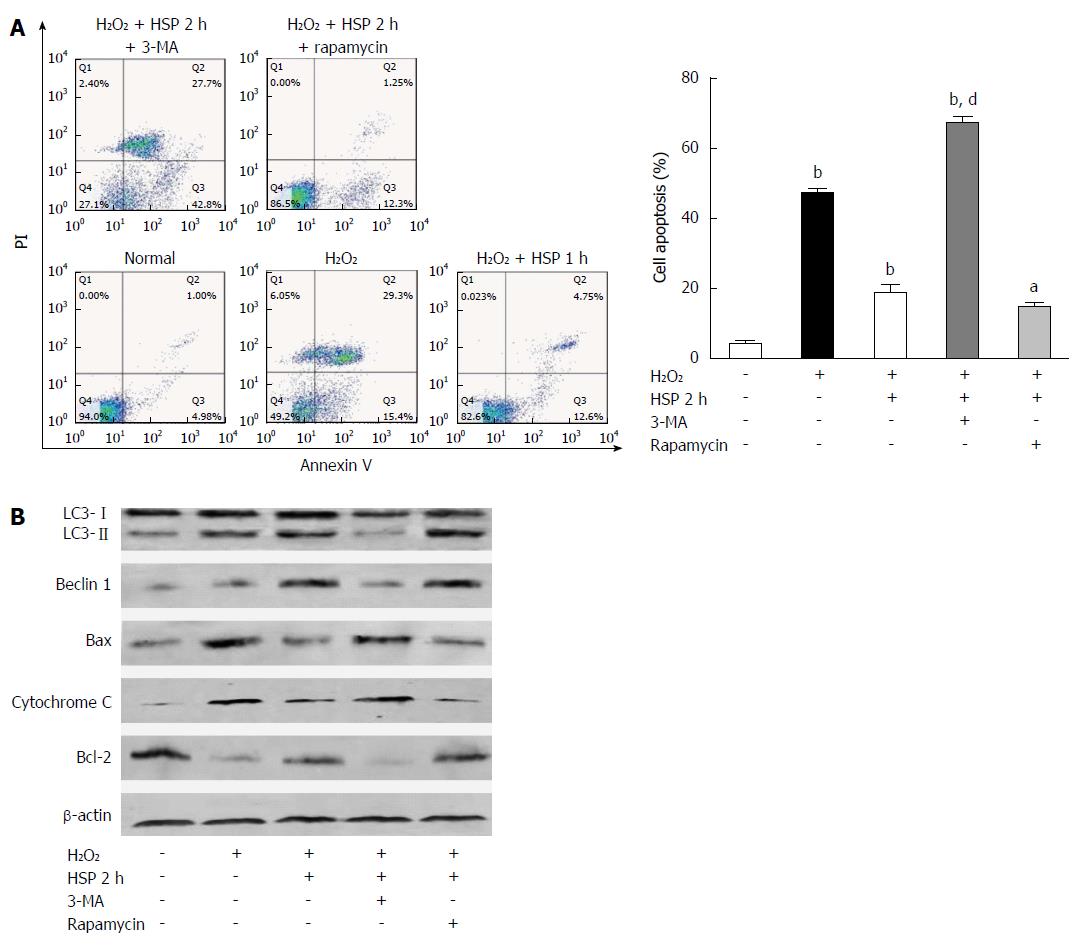
We found that HSP2h achieved the greatest protective effect against H2O2-induced apoptosis using flow cytometry and Western blot (Figure 1). To determine the role of autophagy in MSCs, we exposed cells to HSP for 2 h with 3-MA or rapamycin and H2O2 treatment for 6 h, and assessed autophagy and the apoptotic rate. Following 6 h of H2O2 treatment, 3-MA attenuated both the activation of autophagy and the anti-apoptotic capacity in MSCs treated with heat shock for 2 h, as shown by fewer AVO-positive MSCs (Figure 2C), lower expression of LC3-II and beclin 1 (Figure 4B) and fewer autophagosomes in MSCs (P < 0.01; Figure 3). In addition, a higher apoptotic rate (Figure 4A), increased expression of Bax and cytochrome C, and decreased expression of Bcl-2 (Figure 4B) were found compared with the control group (P < 0.01) and the HSP2h group (P < 0.05). In addition, rapamycin failed to have any effect on autophagic activity and the apoptotic rate in MSCs pretreated with heat shock for 2 h. These results indicated that activation of autophagy by HSP for 2 h may serve as a protective mechanism against apoptosis in MSCs exposed to H2O2.
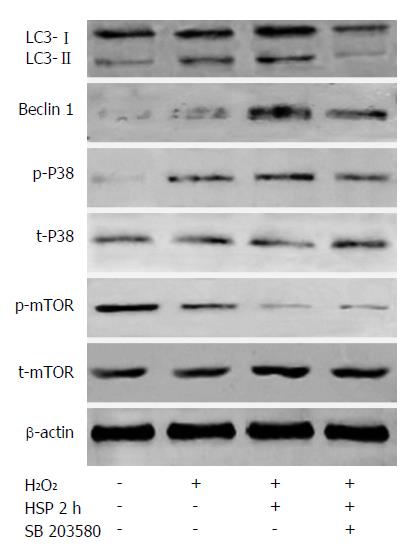
To investigate whether HSP induced autophagy by activating the p38MAPK pathway, the p38MAPK inhibitor, SB203580, was used and the levels of autophagy were evaluated in HSP-MSCs exposed to H2O2. The results revealed that the expression of p38MAPK and mTOR did not significantly change in any of the groups. However, the phosphorylation of p38MAPK was upregulated and the phosphorylation of mTOR was downregulated in the HSP2h group compared with the control group (Figure 5). SB203580 reduced autophagy in the HSP2h group, as shown by a decrease in the number of AVO-positive MSCs (P < 0.05) (Figure 2C), expression of LC3-II and beclin 1 (Figure 5) and autophagosome formation (Figure 3). Furthermore, treatment with SB203580 abrogated the effects of p38MAPK phosphorylation, but failed to have any effect on the phosphorylation of mTOR. These data suggested that the p38MAPK/mTOR signaling pathway had a stimulatory role in the effects of HSP on MSC autophagy under H2O2 conditions.
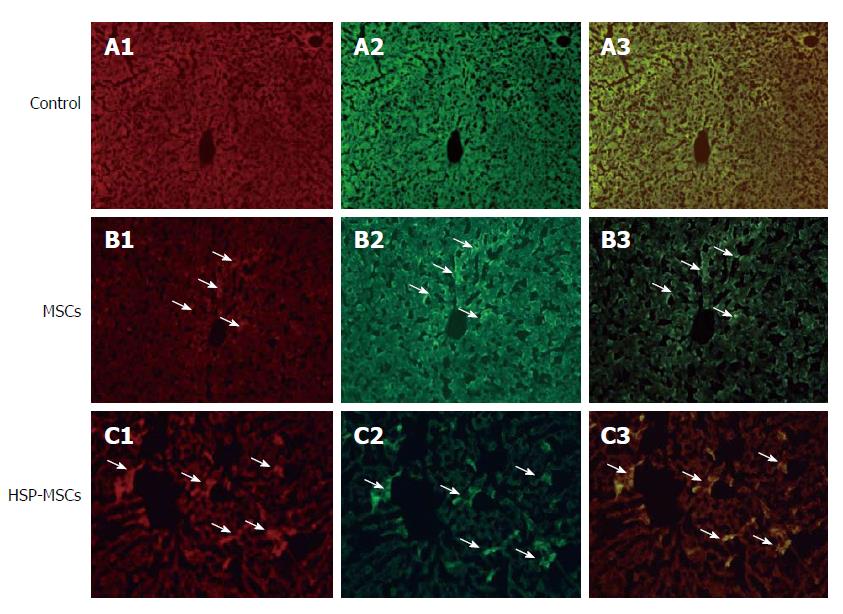
We then investigated the survival rate and homing of transplanted MSCs to livers. Representative fluorescence microscopic images of MSCs after transplantation are shown in Figure 6. CM-Dil-labeled cells were detected only in sections that received transplanted MSCs. The total number of double-positive MSCs labeled by CM-Dil and PCNA in the HSP-MSC-treated group was higher than that in the MSC-treated group (P < 0.05). CM-Dil-labeled MSCs also showed PCNA reactivity.
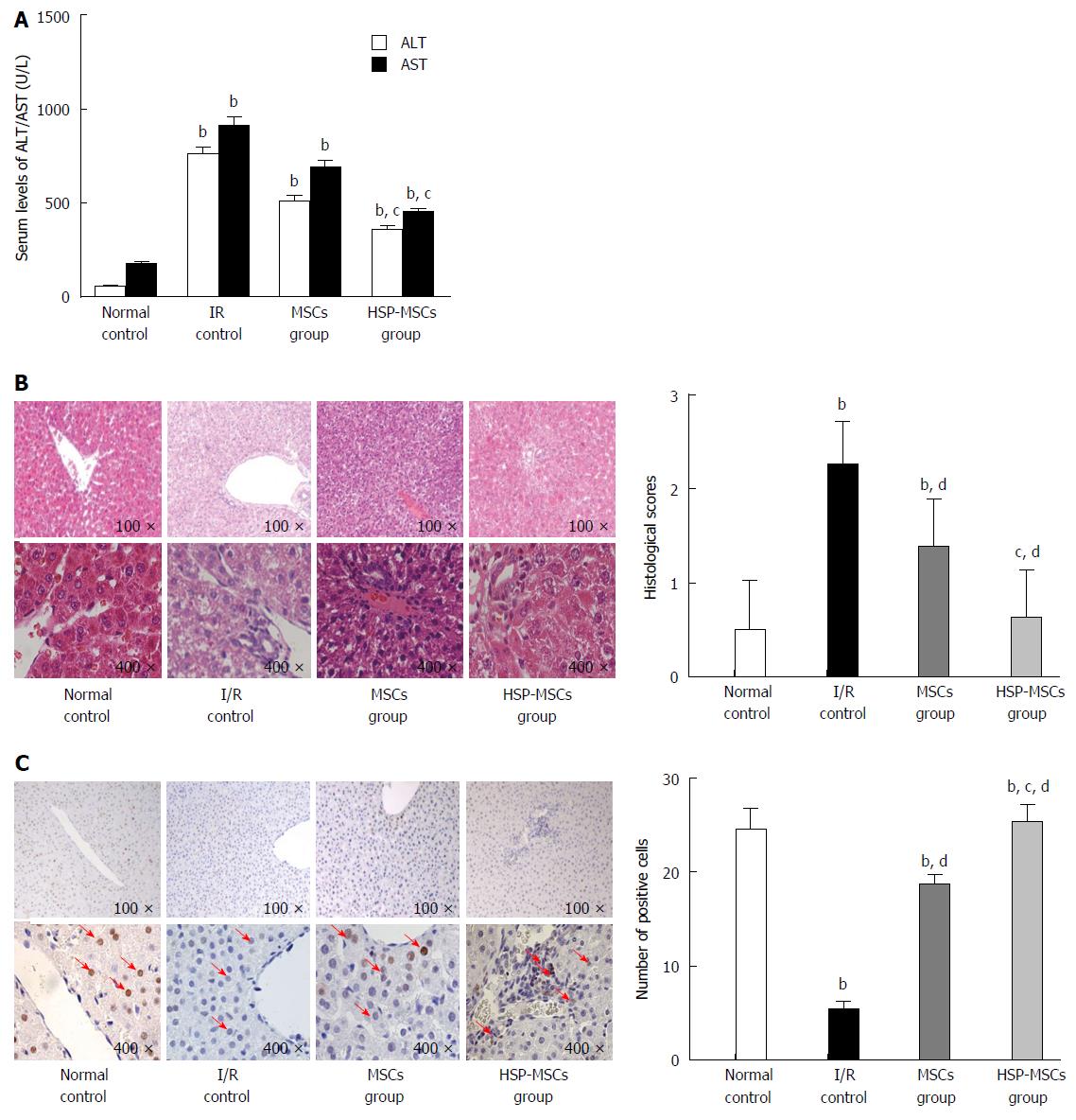
Twenty-four hours after MSC transplantation, liver function was assessed by serum AST and ALT levels. Compared with the control group, transplantation of MSCs improved liver function in rats. However, HSP-MSC-treated rats had lower AST and ALT levels compared with MSC-treated animals (Figure 7A; P < 0.05). A histological score was then assigned to the liver and the expression of PCNA was examined 24 h after transplantation. As expected, all I/R-induced livers showed sinusoidal congestion, cytoplasmic vacuolization and focal necrosis, which are indicative of severe damage. When compared with the I/R control group and the MSC-treated group, the HSP-MSC-treated group showed significantly improved histopathology and lower Suzuki scores 24 h after transplantation (Figure 7B). Moreover, compared with the I/R control group and PBS-treated rats, the livers from HSP-MSC-treated and MSC-treated rats showed a significantly increased number of PCNA-positive cells. Interestingly, the number of PCNA-positive cells in livers from HSP-MSC-treated rats was significantly increased compared with MSC-treated rats (Figure 7C; P < 0.05).
In the present study, we investigated the interaction between autophagy and apoptosis and the protective mechanism of autophagy activation by HSP in MSCs exposed to H2O2. Our results show that HSP for 2 h improves the therapeutic potential of MSCs in the treatment of HIRI in rats and enhances autophagy via the p38MAPK/mTOR pathway, which partly acted in the protective role of HSP on MSC apoptosis induced by H2O2. When administered systemically, more viable HSP-MSCs homed to the I/R liver compared with MSCs, which led to a significant improvement in liver function, an accelerated mitogenic response and alleviation of histopathological damage in the rat model.
In a previous study, we found that transplanted MSCs attenuated HIRI by suppressing oxidative stress and inhibiting apoptosis in rats[3]. However, the I/R microenvironment is detrimental to graft cells and induces cell death, thereby attenuating the therapeutic effect of stem cell transplantation[5-7]. Implanted MSCs must have a long life to ensure long-term MSC-based therapy in I/R tissues. It has been reported that short-term HSP can significantly improve the viability of transplanted cells and thus enhance their tissue repairing capabilities in I/R tissue[10,11]. As H2O2 was previously shown to be a critical mediator of I/R-induced cell death[24,32], we induced a HIRI microenvironment by treating MSCs with H2O2 to investigate the function of HSP in vitro. We found that HSP for 2 h resulted in the most significant anti-apoptotic effects in MSCs exposed to H2O2 compared to the other groups. In addition, H2O2-induced apoptosis of MSCs was aggravated in the HSP3h group (Figure 1). More importantly, exposure to HSP for 2 h before transplantation enhanced the survival rate and therapeutic outcome of MSCs in vivo. These data suggest that HSP at 42 °C for 2 h was the optimal period for improving the effect of MSCs transplantation in the repair of HIRI in rats. The HSP procedure is a simple method to improve implanted cell survival with little risk and can be performed not only in the liver, but also other organs.
Autophagy has been implicated in many processes, including cell differentiation, growth, development and survival[33]. Autophagy can be activated by various stresses involved in mediating cell survival or death[25]. In the present study, we found that HSP ranging from 1 to 3 h leads to a time-dependent increase in the action of autophagy in MSCs exposed to H2O2 (Figure 2A and D; Figure 3). In addition to the anti-apoptotic effect of HSP in MSCs, these findings suggest that autophagy induced by HSP for 2 h results in the most significant anti-apoptotic effect in MSCs exposed to H2O2. We therefore performed HSP for 2 h to examine the effect of H2O2-induced apoptosis and the protective effect of autophagy against apoptosis in MSCs. The protective effect of autophagy against apoptosis has previously been reported in models of I/R injury[34], including a model using H2O2. One well-established view is that appropriate autophagy is essential for cell survival[35]. More recently, Herberg et al[20] reported that the SDF-1/CXCR4 axis plays a key role in mediating MSC survival exposed to H2O2 by activating autophagy. Consistent with these results, our data show that the autophagy inhibitor, 3-MA, abrogates the anti-apoptotic effect observed in the HSP2h group, and the autophagy inducer, rapamycin, does not reduce apoptosis of MSCs exposed to H2O2. These data suggest that moderate activation of autophagy mediated by HSP for 2 h may play a critical role in HSP to improve the survival of MSCs exposed to H2O2. It is known that autophagy is considered a double-edged sword in terms of cell survival. Moreover, we found that the activation of autophagy by HSP in MSCs is not paralleled by a corresponding increase in tolerance to H2O2-induced apoptosis. HSP for 1 and 2 h induced autophagy, which was an anti-apoptosis mechanism rather than a pro-apoptosis pathway in MSCs exposed to H2O2. Prolonged or excessive autophagy, which was mediated by HSP for 3 h, may digest essential components and lead to cell death. Thus, activation of autophagy may be a new mechanism in the process of HSP protecting MSCs from H2O2-induced apoptosis.
p38MAPK appears to have a dual role in that it has a positive or negative role in autophagy depending on conditions, cell type or type of cell stress[36-39]. In the present study, we assessed p38MAPK/mTOR pathway activation levels to determine the mechanisms underlying HSP-induced autophagy in MSCs exposed to H2O2. Interestingly, we found that HSP for 2 h increases p38MAPK activation and correspondingly alleviates mTOR activation. Moreover, p38MAPK inhibition abrogates autophagy induced by HSP for 2 h, but does not significantly impair mTOR suppression. In addition, our results indicate that treatment with rapamycin does not further induce autophagy of MSCs compared with HSP alone in the presence of H2O2, indicating that HSP may be involved in the same mechanism as rapamycin to activate autophagy in MSCs. These data suggest that the p38MAPK/mTOR signaling pathway may be involved in the mechanism of HSP-induced autophagy in MSCs exposed to H2O2.
To confirm the observations in the in vitro assay, we investigated the protective effect of HSP on MSCs in vivo. We determined the extent of MSCs localized in I/R livers of the recipient group by counting the number of CM-Dil fluorescent-labeled cells. It is well established that PCNA, which is synthesized in the cell nucleus, is a nuclear antigen related to the cell life cycle. PCNA is expressed in the G1 and S phases, and performs the essential function of providing replicative DNA polymerases in eukaryotic cells. The level of PCNA in resting cells is low, but is substantially increased in multiplying and transformed cells[40,41]. As shown in Figure 6, the HSP-MSCs group show more double-positive cells labeled by CM-Dil and PCNA than the MSCs group, which indicates that more HSP-MSCs subsequently underwent cell division and that HSP enhances the survival rate of transplanted MSCs in the liver. Furthermore, a marked decrease in serum aminotransferase levels, improved histopathology, lower Suzuki scores and an increased number of PCNA-positive cells in response to transplantation of HSP-MSCs were observed compared with the MSC group and the control group (Figure 7). These results indicate that HSP increases the homing and survival rate of transplanted MSCs, and thus improves the therapeutic potential of MSCs in the treatment of HIRI in vivo.
In summary, we found, for the first time, that HSP effectively enhances the homing and survival rate of MSCs, and thereby improves the therapeutic outcome of MSCs in the treatment of HIRI. The activation of autophagy via the p38MAPK/mTOR pathway may be a novel mechanism of HSP to improve the survival of MSCs exposed to H2O2. Activation of autophagy by HSP may be an attractive method of preventing apoptosis of MSCs and promoting their application in cellular therapies in regenerative medicine.
Mesenchymal stem cells (MSCs) exert a protective effect in hepatic ischemia-reperfusion injury (HIRI). However, due to local hypoxia, inflammation, and particularly oxidative stress in the targeted tissue, the transplanted MSCs do not withstand the difficult microenvironment due to ischemia-reperfusion (I/R) injury and low cell survival reduces the therapeutic effect. Autophagy is a complex ‘‘self-eating’’ process and can reduce apoptosis of MSCs exposed to H2O2. Heat shock pretreatment (HSP) is known to protect cells from various environmental insults and has been shown to induce autophagy in some cell lines. Previous studies show that HSP can regulate mitogen-activated protein kinase (p38MAPK), a positive modulator of autophagy in MSCs. Therefore, the authors designed this study to determine the role of HSP in autophagy activation via the p38MAPK/mTOR pathway to protect MSCs against apoptosis induced by oxidative stress injury.
Autophagy is an evolutionarily conserved process that occurs in all eukaryotic cells. Evidence suggests that under hypoxia/serum deprivation (H/SD) conditions, autophagy can protect MSCs by providing energy or eliminating reactive oxygen species and damaged organelles, and can reduce apoptosis. In addition, several reports show that HSP increases survival rate following cell transplantation in the heart. However, it is unknown whether autophagy can be activated by HSP or its effect and exact mechanism in MSCs.
This study shows that activation of autophagy was a protective mechanism of HSP in MSCs. The results show that HSP for 2 h improves the therapeutic potential of MSCs in the treatment of HIRI in rats and enhances autophagy via the p38MAPK/mTOR pathway, which mediates, at least partly, the protective effects of HSP on MSC apoptosis exposed to H2O2. When administered systemically, more viable HSP-MSCs home to the I/R liver compared with MSCs, which leads to a significant improvement in liver function, an accelerated mitogenic response and the alleviation of histopathological damage in the rat model.
This study indicates that HSP effectively enhances MSCs homing and survival rate, and thus improves the therapeutic outcome of MSCs in the treatment of HIRI in rats. The activation of autophagy via the p38MAPK/mTOR pathway may be a novel mechanism of HSP to enhance the survival of MSCs exposed to H2O2. The regulation of autophagy by HSP may be an attractive strategy in preventing apoptosis of MSCs, thus promoting their application in cellular therapies in regenerative medicine.
HIRI is an inevitable event and occurs in a number of clinical settings, including liver surgery, hemorrhagic shock with subsequent fluid resuscitation, sepsis, hepatic artery ligation, trauma, and some vascular lesions, and especially in liver transplantation. Autophagy is an evolutionarily conserved process that occurs in all eukaryotic cells and is considered a double-edged sword in relation to cell survival. Heat shock pretreatment involves short-term exposure to mild hyperthermia that can significantly enhance cell tolerance and viability.
The work presented here is an interesting contribution that demonstrates the interaction of autophagy with apoptosis on MSCs under H2O2 conditions, and the activation of autophagy as a protective mechanism of HSP on MSCs.
P- Reviewer: Keyashian K, Kamiya T, Tsujikawa T S- Editor: Yu J L- Editor: Filipodia E- Editor: Wang CH
| 1. | Montalvo-Jave EE, Escalante-Tattersfield T, Ortega-Salgado JA, Piña E, Geller DA. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J Surg Res. 2008;147:153-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 289] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 2. | Walsh KB, Toledo AH, Rivera-Chavez FA, Lopez-Neblina F, Toledo-Pereyra LH. Inflammatory mediators of liver ischemia-reperfusion injury. Exp Clin Transplant. 2009;7:78-93. [PubMed] |
| 3. | Jin G, Qiu G, Wu D, Hu Y, Qiao P, Fan C, Gao F. Allogeneic bone marrow-derived mesenchymal stem cells attenuate hepatic ischemia-reperfusion injury by suppressing oxidative stress and inhibiting apoptosis in rats. Int J Mol Med. 2013;31:1395-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Wang X, Jameel MN, Li Q, Mansoor A, Qiang X, Swingen C, Panetta C, Zhang J. Stem cells for myocardial repair with use of a transarterial catheter. Circulation. 2009;120:S238-S246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1647] [Cited by in RCA: 1548] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 6. | Atashi F, Modarressi A, Pepper MS. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem Cells Dev. 2015;24:1150-1163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 490] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 7. | Mias C, Trouche E, Seguelas MH, Calcagno F, Dignat-George F, Sabatier F, Piercecchi-Marti MD, Daniel L, Bianchi P, Calise D. Ex vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem Cells. 2008;26:1749-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 156] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | Yamashita N, Hoshida S, Nishida M, Igarashi J, Taniguchi N, Tada M, Kuzuya T, Hori M. Heat shock-induced manganese superoxide dismutase enhances the tolerance of cardiac myocytes to hypoxia-reoxygenation injury. J Mol Cell Cardiol. 1997;29:1805-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Venkatakrishnan CD, Tewari AK, Moldovan L, Cardounel AJ, Zweier JL, Kuppusamy P, Ilangovan G. Heat shock protects cardiac cells from doxorubicin-induced toxicity by activating p38 MAPK and phosphorylation of small heat shock protein 27. Am J Physiol Heart Circ Physiol. 2006;291:H2680-H2691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Jiang B, Xiao W, Shi Y, Liu M, Xiao X. Heat shock pretreatment inhibited the release of Smac/DIABLO from mitochondria and apoptosis induced by hydrogen peroxide in cardiomyocytes and C2C12 myogenic cells. Cell Stress Chaperones. 2005;10:252-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Suzuki K, Smolenski RT, Jayakumar J, Murtuza B, Brand NJ, Yacoub MH. Heat shock treatment enhances graft cell survival in skeletal myoblast transplantation to the heart. Circulation. 2000;102:III216-III221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Bhora FY, Wise RM, Foster AH. Heat shock protects cardiomyocytes from hypoxia-mediated apoptosis by attenuation of nitric oxide. In Vivo. 2000;14:597-602. [PubMed] |
| 13. | Zhang Q, Yang YJ, Wang H, Dong QT, Wang TJ, Qian HY, Xu H. Autophagy activation: a novel mechanism of atorvastatin to protect mesenchymal stem cells from hypoxia and serum deprivation via AMP-activated protein kinase/mammalian target of rapamycin pathway. Stem Cells Dev. 2012;21:1321-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 14. | Hou J, Han ZP, Jing YY, Yang X, Zhang SS, Sun K, Hao C, Meng Y, Yu FH, Liu XQ. Autophagy prevents irradiation injury and maintains stemness through decreasing ROS generation in mesenchymal stem cells. Cell Death Dis. 2013;4:e844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 15. | Zhao Y, Gong S, Shunmei E, Zou J. Induction of macroautophagy by heat. Mol Biol Rep. 2009;36:2323-2327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Han J, Xu X, Qin H, Liu A, Fan Z, Kang L, Fu J, Liu J, Ye Q. The molecular mechanism and potential role of heat shock-induced p53 protein accumulation. Mol Cell Biochem. 2013;378:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Virgin HW, Levine B. Autophagy genes in immunity [J]. Nature immunology. 2009;10:461-470. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 350] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 18. | Chen W, Zhang L, Zhang K, Zhou B, Kuo ML, Hu S, Chen L, Tang M, Chen YR, Yang L. Reciprocal regulation of autophagy and dNTP pools in human cancer cells. Autophagy. 2014;10:1272-1284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1461] [Cited by in RCA: 1372] [Article Influence: 91.5] [Reference Citation Analysis (0)] |
| 20. | Herberg S, Shi X, Johnson MH, Hamrick MW, Isales CM, Hill WD. Stromal cell-derived factor-1β mediates cell survival through enhancing autophagy in bone marrow-derived mesenchymal stem cells. PLoS One. 2013;8:e58207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Azad MB, Chen Y, Henson ES, Cizeau J, McMillan-Ward E, Israels SJ, Gibson SB. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy. 2008;4:195-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 286] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 22. | Filippi-Chiela EC, Bueno e Silva MM, Thomé MP, Lenz G. Single-cell analysis challenges the connection between autophagy and senescence induced by DNA damage. Autophagy. 2015;11:1099-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Zhang XM, Du F, Yang D, Yu CJ, Huang XN, Liu W, Fu J. Transplanted bone marrow stem cells relocate to infarct penumbra and co-express endogenous proliferative and immature neuronal markers in a mouse model of ischemic cerebral stroke. BMC Neurosci. 2010;11:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Liu H, Liu S, Li Y, Wang X, Xue W, Ge G, Luo X. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS One. 2012;7:e34608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 25. | Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2022] [Cited by in RCA: 2068] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 26. | Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, Domingo D, Yahalom J. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439-444. [PubMed] |
| 27. | Tong J, Ding J, Shen X, Chen L, Bian Y, Ma G, Yao Y, Yang F. Mesenchymal stem cell transplantation enhancement in myocardial infarction rat model under ultrasound combined with nitric oxide microbubbles. PLoS One. 2013;8:e80186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Nauta RJ, Tsimoyiannis E, Uribe M, Walsh DB, Miller D, Butterfield A. Oxygen-derived free radicals in hepatic ischemia and reperfusion injury in the rat. Surg Gynecol Obstet. 1990;171:120-125. [PubMed] |
| 29. | Sheikh AY, Lin SA, Cao F, Cao Y, van der Bogt KE, Chu P, Chang CP, Contag CH, Robbins RC, Wu JC. Molecular imaging of bone marrow mononuclear cell homing and engraftment in ischemic myocardium. Stem Cells. 2007;25:2677-2684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Kim HJ, Moradi H, Yuan J, Norris K, Vaziri ND. Renal mass reduction results in accumulation of lipids and dysregulation of lipid regulatory proteins in the remnant kidney. Am J Physiol Renal Physiol. 2009;296:F1297-F1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Katoh K, Nakanishi Y, Akimoto S, Yoshimura K, Takagi M, Sakamoto M, Hirohashi S. Correlation between laminin-5 gamma2 chain expression and epidermal growth factor receptor expression and its clinicopathological significance in squamous cell carcinoma of the tongue. Oncology. 2002;62:318-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol. 2007;18:2439-2446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 206] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 33. | Mathew R, White E. Autophagy in tumorigenesis and energy metabolism: friend by day, foe by night. Curr Opin Genet Dev. 2011;21:113-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 34. | Wang D, Ma Y, Li Z, Kang K, Sun X, Pan S, Wang J, Pan H, Liu L, Liang D. The role of AKT1 and autophagy in the protective effect of hydrogen sulphide against hepatic ischemia/reperfusion injury in mice. Autophagy. 2012;8:954-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 35. | Zhou J, Wu S, Chen Y, Zhao J, Zhang K, Wang J, Chen S. microRNA-143 is associated with the survival of ALDH1+CD133+ osteosarcoma cells and the chemoresistance of osteosarcoma. Exp Biol Med (Maywood). 2015;240:867-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Webber JL, Tooze SA. Coordinated regulation of autophagy by p38alpha MAPK through mAtg9 and p38IP. EMBO J. 2010;29:27-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 37. | Comes F, Matrone A, Lastella P, Nico B, Susca FC, Bagnulo R, Ingravallo G, Modica S, Lo Sasso G, Moschetta A. A novel cell type-specific role of p38alpha in the control of autophagy and cell death in colorectal cancer cells. Cell Death Differ. 2007;14:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Doyle A, Zhang G, Abdel Fattah EA, Eissa NT, Li YP. Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin-proteasome and autophagy-lysosome pathways. FASEB J. 2011;25:99-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 39. | Matsuzawa T, Kim BH, Shenoy AR, Kamitani S, Miyake M, Macmicking JD. IFN-γ elicits macrophage autophagy via the p38 MAPK signaling pathway. J Immunol. 2012;189:813-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 40. | Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci. 2003;116:3051-3060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 837] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 41. | Shizhu J, Xiangwei M, Xun S, Mingzi H, Bingrong L, Dexia K, Xinghong W, Fenghua P. Bone marrow mononuclear cell transplant therapy in mice with CCl4-induced acute liver failure. Turk J Gastroenterol. 2012;23:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |