Published online Nov 21, 2015. doi: 10.3748/wjg.v21.i43.12498
Peer-review started: May 11, 2015
First decision: June 2, 2015
Revised: June 20, 2015
Accepted: August 25, 2015
Article in press: August 25, 2015
Published online: November 21, 2015
Processing time: 194 Days and 15.1 Hours
Intraductal papillary neoplasm of the bile duct (IPNB) is a rare bile duct neoplasm mostly found in far eastern nations where hepatolithiasis and clonorchiasis infections are endemic. In western countries, it is very rare and the etiology is unknown. In this article, we report the first IPNB patient we encountered in our clinic and a literature review. The patient is a 38-year-old female with a history of choledocholithiasis who presented with obstructive jaundice. She was found to have a papillary mass at the junction of the right hepatic duct and common hepatic duct with six masses in the liver parenchyma. The immunophenotypic and histologic features of the tumor are consistent with IPNB, gastric subtype. The patient had a partial hepatectomy and has been receiving palliative chemotherapy. In a search of PubMed database, we collected 354 IPNB patients reported in 22 articles. In these patients, 52.8% were from Japan and 27.7% were from western countries including the United States (11.0%). The age of the patients ranged from 35 to 80 years old with an average of 64.6. Male/female ratio was 1.5. Macroscopically, 57.5% of the tumors were in the left lobe and 29.5% were in the right lobe. The average size of the tumor were 4.2 cm at the time of diagnosis. Histologically, pancreato-biliary subtype accounted for 41.8%, intestinal 28.0%, gastric 13.5% and oncocytic 16%. An invasive component is most often present in the pancreato-biliary and gastric subtypes. Despite recent advanced technologies, diagnosis of IPNB is still challenging, especially in western countries due to its rarity. Defined clinico-pathologic features are in demand for the accurate diagnosis and proper treatment.
Core tip: Intraductal papillary neoplasm of the bile duct (IPNB) is very rare in the United States. In this article, we reported the first IPNB patient we encountered. The patient is a 38-year-old female who is one of the youngest patients that have been reported. She was found to have intraductal papillary masses in the common hepatic duct and liver parenchyma. The diagnosis was IPNB, gastric subtype. In a search of PubMed database, we collected 354 IPNB patients from 22 articles and summarized the clinico-pathologic features including geographic distribution, age, gender, symptoms, location, microscopic subtypes, differential diagnosis, pathogenesis and therapeutic options.
- Citation: Tan Y, Milikowski C, Toribio Y, Singer A, Rojas CP, Garcia-Buitrago MT. Intraductal papillary neoplasm of the bile ducts: A case report and literature review. World J Gastroenterol 2015; 21(43): 12498-12504
- URL: https://www.wjgnet.com/1007-9327/full/v21/i43/12498.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i43.12498
Intraductal papillary neoplasm of the bile duct (IPNB) is a bile duct neoplasm characterized by a predominantly papillary growth pattern in dilated bile ducts. An invasive component is present in approximately 40%-80% of reported cases. IPNB is a rare tumor, mostly found in far eastern nations like Taiwan, Japan, and North and South Korea where hepatolithiasis and clonorchiasis are endemic[1-5]. In western countries, it is sporadic and the etiology is unknown. As a precursor lesion of cholangiocarcinoma, IPNB was recently included in the World Health Organization (WHO) classification of the bile duct tumors to include the previous categories of biliary papilloma and papillomatosis. IPNBs are considered the biliary counterpart of pancreatic intraductal papillary mucinous neoplasms (IPMNs). These two types of neoplasms show a mucin-secreting columnar epithelium with varying degrees of atypia and are similarly classified into four subtypes based on the histomorphology and immunophenotypical profile: pancreato-biliary, intestinal, gastric, and oncocytic subtypes (Table 1)[3,6]. However, pancreato-biliary is the most common one for IPNBs, while gastric subtype is the most common one for IPMNs. IPMNs have better prognosis with a lower frequency of an invasive component.
| MUC1 | MUC2 | MUC5AC | |
| Pancreato-biliary | + | - | + |
| Intestinal | - | + | + |
| Gastric | - | - | + |
| Oncocytic | + (focal) | + (focal) | + |
As a new entity, the characteristics of IPNB, including the clinico-pathologic features, the prognostic factors and oncogenic pathways are still ill-defined, especially of those of the sporadic neoplasms seen in western countries. Its identification can represent a diagnostic challenge. In this case report, we describe the cytologic and histopathologic features of IPNB with invasive adenocarcinoma in a very young female patient.
A 38-year-old female presented to an outside hospital in December 2012 due to one-week of acholic stools, jaundice, pruritus and dark colored urine. Her past medical history was significant for choledocholithiasis. Blood workup was consistent with obstructive jaundice. She underwent endoscopic retrograde cholangiopancreatogram (ERCP), percutaneous biliary drainage and stone extraction. Subsequently, the patient’s symptoms resolved. Three months later, the patient developed recurrent symptoms and presented to the same hospital. Another ERCP was performed and a biliary stent was placed. While placing the stent, a mucoid mass in the common hepatic duct was identified and biopsied. It was diagnosed as adenocarcinoma with papillary architecture. The patient was referred to our institution for further management.
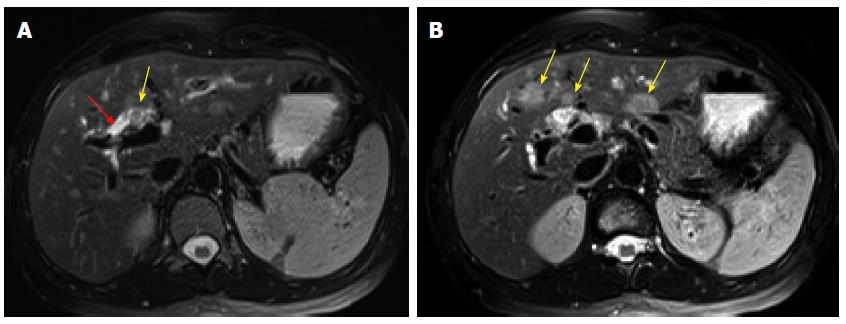
In April 2013, the patient presented to our institution. Her laboratory tests demonstrated a total bilirubin 2.0 mg/dL (reference range: 0.2-1.3 mg/dL), direct bilirubin 1.5 mg/dL (reference range: 0-0.4 mg/dL), alkaline phosphatase 195 UI/L (reference range: 38-126 UI/L), AST 83 UI/L (reference range: 15-46 UI/L), ALT 187 UI/L (reference range: 9-52 UI/L), and CA19-9 37.2 UI/mL (reference range: 0-35 UI/mL). Serology was negative for hepatitis A, B and C infection. Magnetic resonance imaging revealed an ill-defined soft tissue fullness at the bifurcation of the common hepatic duct causing biliary occlusion. The left and right hepatic ducts were also involved. Additionally, there were three ill-defined but suspicious intrahepatic masses in segments IVB and III that measured up to 1.8 cm. They were considered as metastases (Figure 1). Based on the radiologic findings, the patient was considered as a poor candidate for resection; therefore, chemotherapy was initiated using Cisplatin 25 mg/m2 IV at day #1 with Gemcitabine 1000 mg/m2 IV at day #1 and day #8 for total of 4 to 6 cycles. After initiation of the treatment, the patient showed signs of improvement including decreased CA19-9 level and stable radiologic findings. During the sixth to seventh cycle of the chemotherapy in October 2013, the patient complained of mild right upper quadrant discomfort. At this time, laboratory tests showed a total bilirubin 0.6 mg/dL, alkaline phosphatase 86 UI/L, AST 40 UI/L, ALT 39 UI/L, and CA19-9 161.3 UI/mL. Contrast enhanced computed tomography (CT) demonstrated interval increased size of the previously identified intrahepatic lesions and an additional new liver lesion (Figure 2). A decision of salvage surgery was made after positron emission tomography (PET)/CT confirmed that the tumor was confined to the liver and bile ducts. In December 2013, the patient underwent a left hepatic lobectomy with cholecystectomy and hepaticojejunostomy.
Gross examination of the 438-g partial hepatectomy specimen revealed a 1.5 cm × 1.5 cm × 1.0 cm papillary friable tan mass at the junction of the right hepatic duct and common hepatic duct. The mass was confined to the dilated bile duct. Additionally, there were six well-circumscribed homogenous tan masses ranging from 1.0 to 6.0 cm in the liver parenchyma. One of these masses was immediately adjacent to the papillary mass in the bile duct.
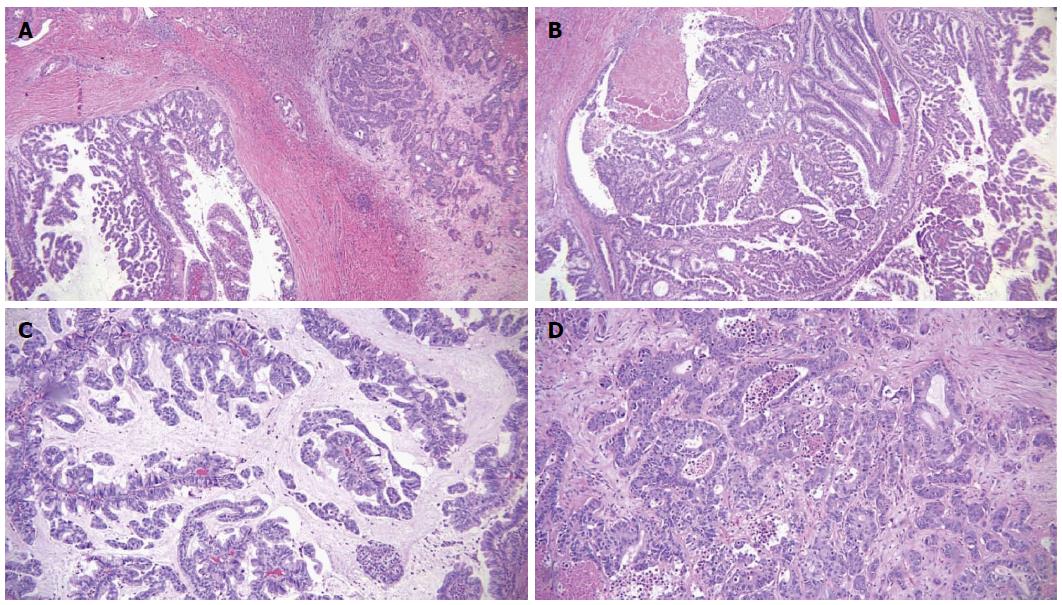
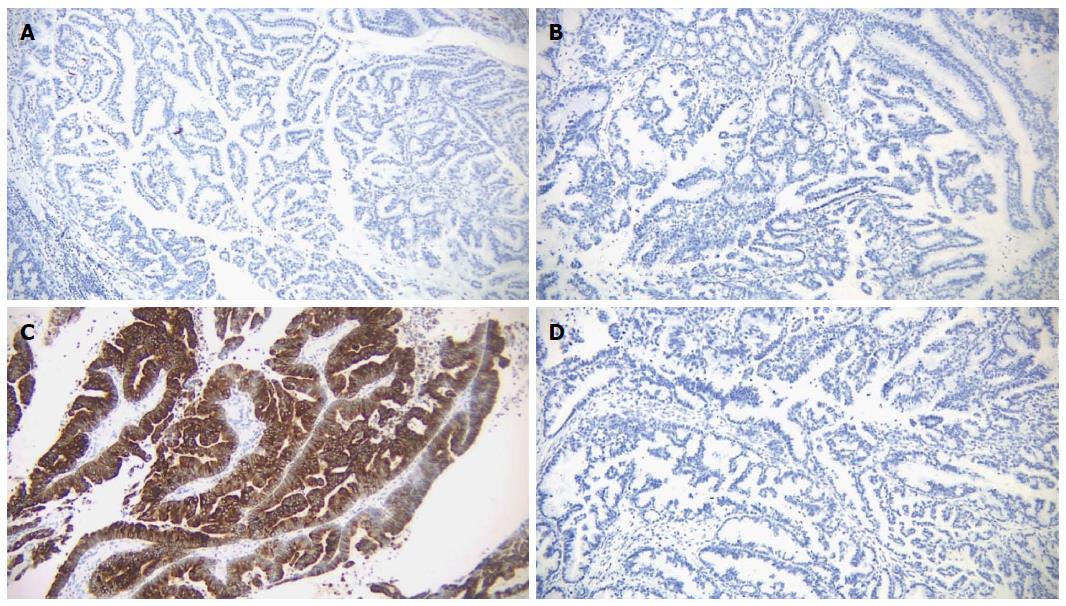
Microscopic examination of the biliary mass showed a papillary mass dilating the bile duct lumen and adjacent to infiltrative adenocarcinoma (Figure 3A). The papillary fronds had fine vascular cores and were lined by foveolar-type epithelial cells with mucinous cytoplasm and different degrees of dysplasia (Figure 3B). Mucin production was evident (Figure 3C). The hepatic masses showed similar morphology with infiltrative features (Figure 3D). There was focal necrosis and sclerosis (5%) due to chemotherapy. Ovarian type stroma was not identified. By immunohistochemistry, the tumor was positive for mucin core protein (MUC)5 and negative for MUC1, MUC2 and CDX2, which is consistent with gastric subtype (Figure 4). The hepatic resection margins were negative for tumor, however, the tumor was present at less than 1 mm from the margin.
Postoperatively, the patient did well. Her CA19-9 dropped to 37.4 UI/mL one month after the surgery. She received postoperative adjuvant chemotherapy with FOLFOX for 3 mo followed by concurrent chemo-radiation. However, in May, 2014, she was found to have left periaortic and gastrohepatic lymphadenopathy and biopsy confirmed metastatic carcinoma, morphologically similar to the invasive IPNB. Since then the patient has been receiving palliative chemotherapy with FOLFIRI and has been stable.
IPNB is a rare tumor, initially described in 1976 as multicentric biliary papillomatosis associated with invasive adenocarcinoma. In 2006, Zen et al[5] reported ten cases of papillary biliary tumors, described the histopathologic features and classified the tumor cells into three subtypes including pancreatico-biliary, intestinal and gastric subtype. Oncocytic type was believed to be a variant of the pancreatico-biliary type. In this article the name of intraductal papillary neoplasm of bile duct (IPNB) was given for the first time to this distinct new entity which included biliary papilloma, papillomatosis and papillary adenocarcinoma. In 2010, IPNB was included in the WHO classification of the bile duct tumors.
In a search of PubMed database, we collected 22 articles including case reports and clinical studies on IPNB patients[2,6-26]. In total 354 patients were reported. Most of the patients (52.8%) were from Japan; 19.5% of these patients were from other far eastern countries including China, Korea and Taiwan; 27.7% were from western countries including the United Kingdom (4.0%), the United States (11.0%) and Germany (12.7%). The age of the patients ranged from 35 to 80 years old with an average of 64.6. Male patients were predominant with a male/female ratio of 1.5. Twenty patients had choledocholithiasis or clonorchiasis and all of them were from far eastern countries.
Similar to other bile duct tumors, most IPNB patients present with right hypochondria (35%-88.5%), recurrent acute cholangitis (5%-59%) and obstructive jaundice (20%-36%). Up to 5% of the patients can be asymptomatic[2,3]. IPNB can be found in the extrahepatic and intrahepatic bile ducts[6]. Of the 354 patients we analyzed, 57.5% of the tumors were in the left lobe, 29.5% were in the right lobe and 13.4% were in the other locations including the common hepatic duct.
Grossly, most IPNBs are papillary or solid intraductal tumors with grape-like appearance. Cystic lesions and papillary mural nodules are common[6]. Histologically, there are four subtypes: pancreato-biliary, intestinal, gastric and oncocytic. An invasive component is most often present in the pancreato-biliary and gastric subtypes. As seen in Table 1, mucin core proteins are characteristic markers for the IPBN subtypes. In the reported 354 patients, the average size of the tumors were 4.2 cm. Pancreatobiliary subtype represented 41.8%, intestinal subtype 28.0%, oncocytic subtype 16% and gastric subtype 13.5%. An invasive component was present in 36.4% of all cases.
The pathogenesis of IPNB is still not clear. Most of the studies, especially the largest studies, exclusively enrolled Asian patients, a considerable proportion of them with hepatolithiasis or clonorchiasis infection. In western countries, this association has not been identified[2]. Schlitter et al[6] investigated the common molecular pathways in the development of IPNB in 45 patients from European countries. As shown in Table 1, while MUC1, MUC2 and MUC5AC have been very consistent with the histologic subtype, MUC6 and CDX2 were seen in all the subtypes with a predominant expression of MUC6 in pancreatobiliary subtype and CDX2 in intestinal subtype. Genetic tests of these patients suggested a stepwise progression from low-grade intraductal papillary dysplasia at its beginning to invasive adenocarcinoma at its end. Mutated KRAS, overexpression of TP53 and loss of p16 are most commonly involved in this process, whereas loss of SMAD4 was found in late phases of tumor development. Alterations of HER2, EGFR, β-catenin and GNAS were rare events[6]. To interpret these genetic and molecular changes and to investigate the pathogenesis of IPNB, more clinical studies are needed.
The differential diagnosis of IPBN includes two entities with different histomorphology and prognosis: hepatic mucinous cystic neoplasm (HMCN) and cholangiocarcinoma (CCA). HMCN is defined as a cyst-forming epithelial neoplasm with typical ovarian-type stroma but with no communication with the bile ducts. Zen et al[7] studied 29 HMCNs and found that all HMCNs were seen in female and younger patients with an age ranging from 21 to 69 (median: 45). The masses were larger and predominantly located in segment IV. They showed multilocular cysts with septation or a cyst-in-cyst appearance. Microscopically, the single layered epithelium was benign biliary-type with occasional mucin-containing cells and minimal atypia. They were negative for cytokeratin 20, MUC2, MUC5AC and MUC6. The prognosis was excellent. All the patients had resection and had no recurrence up to 132 mo.
Cholangiocarcinoma patients are older with a median age of 67 and a male predominance (male:female ratio is 3:1)[6]. CCA arises from biliary epithelial cells or hepatic progenitor cells and is classified anatomically as intrahepatic CCA, perihilar CCA and distal CCA. IPNB is the precursor lesion of dCCA. Grossly, the tumor shows solitary, multinodular or diffuse small nodules. Microscopically, the tumor is moderate to well-differentiated adenocarcinoma with glandular and tubular structures, mucin production and dense desmoplasia. The prognosis is poor; the patients have a median survival of 24 mo after diagnosis.
For IPNB patients without metastasis, surgical intervention is still the first choice of treatment including pancreaticoduodenectomy (31%), hemihepatectomy (28%), bile duct resection (18%), segmental liver resection (15%) and liver transplant (5%)[6]. When major surgery is not indicated, palliative treatments including chemotherapy, percutaneous transhepatic biliary drainage, percutaneous cholangioscopic laser ablation and iridium-192 intraluminal therapy are recommended[3]. The overall survival of IPNB patients is better than CCA patient with a median survival of 62 mo after diagnosis. The survival is related to the percentage and depth of the invasive component, lymphovascular invasion and cellular atypia. Sex, age, location and the epithelial subtype are not associated to the survival[2].
Despite recent advances in diagnosis, such as improved imaging, serology, cytology and molecular techniques including FISH, diagnosis of HMCN, IPNB and CCA is still challenging. However, accurate distinction of IPBN is needed to recognize patients with better prognosis and to further investigate the natural history of this precursor of cholangiocarcinoma.
In summary, we are reporting an IPNB in a very young patient, who is one of the youngest reported. The patient is a Hispanic female and was found to have choledocholithiasis. She underwent hepatic lobectomy after chemotherapy. Histology of the tumor shows foveolar appearance with MUC5AC positive immunostain, consistent with a gastric subtype. Postoperatively, she was found to have metastatic carcinoma to the left periaortic and gastrohepatic lymph nodes, morphologically similar to the invasive IPNB. Now she is receiving palliative chemotherapy and has been stable.
Thirty-eight-year-old female presented with one-week of acholic stools, jaundice, pruritus and dark colored urine.
Clinical impression was obstructive jaundice.
Differential diagnosis includes hepatic mucinous cystic neoplasm and cholangiocarcinoma which can be differentiated from Intraductal papillary neoplasm of the bile ducts (IPNBs) by the presence of bile duct communication and ovarian-type stroma.
At the time of presenting at our hospital, the patient’s laboratory tests demonstrated a total bilirubin 2.0 mg/dL, direct bilirubin 1.5 mg/dL, alkaline phosphatase 195 UI/L, AST 83 UI/L, ALT 187 UI/L, and CA19-9 37.2 UI/mL.
Computed tomography and magnetic resonance imaging were performed at the time of presenting at our hospital and revealed an ill-defined soft tissue fullness at the bifurcation of the common hepatic duct involving the left and right hepatic ducts as well as three intrahepatic masses which were suspicious for metastasis.
By macro- and microscopic examination the pathological diagnosis was invasive intraductal papillary neoplasm (adenocarcinoma) of bile ducts, 3 cm, gastric subtype, with multiple intrahepatic metastases up to 6.0 cm.
The patient underwent a left hepatic lobectomy with cholecystectomy and hepatojejunostomy followed by postoperative adjuvant chemotherapy with FOLFOX for months and then concurrent chemo-radiation.
This patient has a reported history of choledocholithiasis and underwent stone extraction through endoscopic retrograde cholangiopancreatogram at an outside hospital. Postoperatively, although she was receiving chemo-radiation, she was found to have left periaortic and gastrohepatic lymphadenopathy and biopsy confirmed metastatic carcinoma, morphologically similar to the invasive IPNB.
IPNB is very rare in the United States and caution is needed in order to differentiate this entity from other bile duct tumors and hepatic tumors, especially cholangiocarcinoma and hepatic mucinous cystic neoplasm since they have similar morphology but different in prognosis and treatment.
Authors try to present a rare patient with intraductal papillary neoplasm of bile duct which was commonly combined with hepatolithiasis in Asian countries. After reviewing this manuscript, positive information of this article is worth to the readers.
P- Reviewer: Ker CG S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Jung G, Park KM, Lee SS, Yu E, Hong SM, Kim J. Long-term clinical outcome of the surgically resected intraductal papillary neoplasm of the bile duct. J Hepatol. 2012;57:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Rocha FG, Lee H, Katabi N, DeMatteo RP, Fong Y, D’Angelica MI, Allen PJ, Klimstra DS, Jarnagin WR. Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology. 2012;56:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (2)] |
| 3. | Wan XS, Xu YY, Qian JY, Yang XB, Wang AQ, He L, Zhao HT, Sang XT. Intraductal papillary neoplasm of the bile duct. World J Gastroenterol. 2013;19:8595-8604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 108] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Yeh TS, Tseng JH, Chiu CT, Liu NJ, Chen TC, Jan YY, Chen MF. Cholangiographic spectrum of intraductal papillary mucinous neoplasm of the bile ducts. Ann Surg. 2006;244:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, Kurumaya H, Katayanagi K, Kawashima A, Masuda S. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 284] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 6. | Schlitter AM, Born D, Bettstetter M, Specht K, Kim-Fuchs C, Riener MO, Jeliazkova P, Sipos B, Siveke JT, Terris B. Intraductal papillary neoplasms of the bile duct: stepwise progression to carcinoma involves common molecular pathways. Mod Pathol. 2014;27:73-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Zen Y, Pedica F, Patcha VR, Capelli P, Zamboni G, Casaril A, Quaglia A, Nakanuma Y, Heaton N, Portmann B. Mucinous cystic neoplasms of the liver: a clinicopathological study and comparison with intraductal papillary neoplasms of the bile duct. Mod Pathol. 2011;24:1079-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Sasaki M, Matsubara T, Nitta T, Sato Y, Nakanuma Y. GNAS and KRAS mutations are common in intraductal papillary neoplasms of the bile duct. PLoS One. 2013;8:e81706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Kubota K, Nakanuma Y, Kondo F, Hachiya H, Miyazaki M, Nagino M, Yamamoto M, Isayama H, Tabata M, Kinoshita H. Clinicopathological features and prognosis of mucin-producing bile duct tumor and mucinous cystic tumor of the liver: a multi-institutional study by the Japan Biliary Association. J Hepatobiliary Pancreat Sci. 2014;21:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Naito Y, Kusano H, Nakashima O, Sadashima E, Hattori S, Taira T, Kawahara A, Okabe Y, Shimamatsu K, Taguchi J. Intraductal neoplasm of the intrahepatic bile duct: clinicopathological study of 24 cases. World J Gastroenterol. 2012;18:3673-3680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Yang J, Wang W, Yan L. The clinicopathological features of intraductal papillary neoplasms of the bile duct in a Chinese population. Dig Liver Dis. 2012;44:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Onishi I, Kitagawa H, Harada K, Maruzen S, Sakai S, Makino I, Hayashi H, Nakagawara H, Tajima H, Takamura H. Intraductal papillary neoplasm of the bile duct accompanying biliary mixed adenoneuroendocrine carcinoma. World J Gastroenterol. 2013;19:3161-3164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Watanabe A, Suzuki H, Kubo N, Araki K, Kobayashi T, Sasaki S, Wada W, Arai H, Sakamoto K, Sakurai S. An Oncocytic Variant of Intraductal Papillary Neoplasm of the Bile Duct that Formed a Giant Hepatic Cyst. Rare Tumors. 2013;5:e30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Shimoda T, Yoshida H, Hirakata A, Makino H, Yokoyama T, Maruyama H, Ueda J, Tanno M, Naito Z, Uchida E. Surgical resection of cystic intraductal papillary adenocarcinoma of the bile duct: report of a case. J Nippon Med Sch. 2013;80:234-239. [PubMed] |
| 15. | Lim JH, Zen Y, Jang KT, Kim YK, Nakanuma Y. Cyst-forming intraductal papillary neoplasm of the bile ducts: description of imaging and pathologic aspects. AJR Am J Roentgenol. 2011;197:1111-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Zen Y, Amarapurkar AD, Portmann BC. Intraductal tubulopapillary neoplasm of the bile duct: potential origin from peribiliary cysts. Hum Pathol. 2012;43:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Dong A, Dong H, Zhang L, Zuo C. F-18 FDG uptake in borderline intraductal papillary neoplasms of the bile duct. Ann Nucl Med. 2012;26:594-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Takanami K, Hiraide T, Kaneta T, Hayashi H, Unno M, Fujishima F, Fukuda H, Yamada S, Takahashi S. FDG PET/CT findings in malignant intraductal papillary mucinous neoplasm of the bile ducts. Clin Nucl Med. 2010;35:83-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Nakanishi Y, Nakanuma Y, Ohara M, Iwao T, Kimura N, Ishidate T, Kijima H. Intraductal papillary neoplasm arising from peribiliary glands connecting with the inferior branch of the bile duct of the anterior segment of the liver. Pathol Int. 2011;61:773-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Kim BS, Joo SH, Lim SJ, Joo KR. Intrahepatic biliary intraductal papillary mucinous neoplasm with gallbladder agenesis: case report. Surg Laparosc Endosc Percutan Tech. 2012;22:e277-e280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Kakisaka T, Kamiyama T, Yokoo H, Nakanishi K, Wakayama K, Tsuruga Y, Kamachi H, Mitsuhashi T, Taketomi A. An intraductal papillary neoplasm of the bile duct mimicking a hemorrhagic hepatic cyst: a case report. World J Surg Oncol. 2013;11:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Tsuchida K, Yamagata M, Saifuku Y, Ichikawa D, Kanke K, Murohisa T, Tamano M, Iijima M, Nemoto Y, Shimoda W. Successful endoscopic procedures for intraductal papillary neoplasm of the bile duct: a case report. World J Gastroenterol. 2010;16:909-913. [PubMed] |
| 23. | Jhuang JY, Hsieh MS. Pseudomyxoma peritonei (mucinous carcinoma peritonei) preceded by intraductal papillary neoplasm of the bile duct. Hum Pathol. 2012;43:1148-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Makino I, Yoshimitsu Y, Sakuma H, Nakai M, Ueda H. A large cystic tumor with bile duct communication originating around the hepatic hilum. J Gastrointestin Liver Dis. 2010;19:77-80. [PubMed] |
| 25. | Oki H, Hayashida Y, Namimoto T, Aoki T, Korogi Y, Yamashita Y. Usefulness of gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance cholangiography for detecting mucin retention in bile ducts: a rare intraductal papillary mucinous neoplasm of the bile duct. Jpn J Radiol. 2011;29:590-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Sohn WJ, Jo S. A huge intraductal papillary mucinous carcinoma of the bile duct treated by right trisectionectomy with caudate lobectomy. World J Surg Oncol. 2009;7:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |